- 1Department of Translational and Clinical Research, School of Chemical and Life Sciences, Jamia Hamdard, New Delhi, India
- 2Department of Hemato-Oncology and Bone Marrow Transplant, Rajiv Gandhi Cancer Institute and Research Centre, Rohini, India
- 3Department of Translational and Clinical Research, School of Chemical and Life Sciences, Jamia Hamdard, New Delhi, India
Objective: Depression is the most commonly observed psychological manifestation experienced by individuals diagnosed with cancer. The purpose of the study was to investigate the association between levels of IL-4, BDNF, neopterin, and depressive symptoms in lymphoma patients receiving consecutive cycles of chemotherapy.
Methods: Newly diagnosed lymphoma patients scheduled to receive R-CHOP chemotherapy were enrolled. Effects of R-CHOP on circulatory biomarkers and depressive symptoms were assessed at three-time points [baseline assessment 7 days before the first dose of chemotherapy (TP1), interim assessment after the third cycle of chemotherapy (TP2), and follow-up assessment after the 6th cycle of chemotherapy (TP3)].
Results: Seventy lymphoma patients, with a mean age of 44.17 ± 13.67 years, were enrolled. Patients receiving R-CHOP were found significantly increased neopterin levels between given time points TP1 vs. TP2, TP1 vs. TP3, and TP2 vs. TP3 (p < 0.001). However, IL-4 and BDNF levels significantly decreased with consecutive cycles of chemotherapy (p < 0.001). On Patient Health Questionnaire assessment (PHQ-9), scores of items like loss of interest, feeling depressed, sleep problems, loss of energy, and appetite problems were found significantly affected with consecutive cycles of chemotherapy (p < 0.001). The study found weak negative correlations between IL-4, BDNF, and neopterin levels and changes in PHQ-9 scores at both TP2 and TP3, suggesting a potential inverse relationship between these markers and depression symptoms.
Conclusion: In conclusion, the present study suggests a potential link between elevated neopterin levels, decreased IL-4, and BDNF levels, and the presence of depression in lymphoma patients receiving R-CHOP chemotherapy. This study provides valuable insights into understanding the emotional challenges faced by cancer patients, offering information for more personalized interventions and comprehensive support approaches within the oncology setting.
1 Introduction
Lymphoma is a malignant neoplasm of immune cells that originates from B and T lymphocytes as well as natural killer cells. It includes a wide range of tumors with varying occurrence rates, etiology, pathogenesis, pathologic features, and clinical outcomes, and is commonly categorized as non-Hodgkin lymphoma (NHL) or Hodgkin lymphoma (HL) (1, 2). NHL was the 11th most frequently diagnosed cancer, with approximately 545,000 new cases, and the 11th leading contributor to cancer-related mortality in 2020, with an estimated 260,000 deaths (3). Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) regimen is the existing standard of treatment for more than 60% of NHL patients (4). While advancements in treatment have improved survival rates, the impact of lymphoma extends beyond the real world, often influencing the psychosocial well-being of affected individuals (5). Numerous neuropsychiatric symptoms, such as depression, are present in cancer patients, and this condition is linked to a lower quality of life (6–8). About 25% of cancer patients experience depression related to their illness, and 6–13% of cancer patients cross-sectionally meet the diagnostic criteria for major depression (9). The interplay between these symptoms and biologic stressors, such as pain and the burden of physical symptoms, often impacts cancer progression and survival of the patients (10). Consequently, recognizing and effectively managing these disorders emerges as a crucial concern within the field of oncology practice (11, 12).
Depression is considered to be among the most commonly observed psychological manifestations experienced by individuals diagnosed with cancer (13). Its severity tends to increase during the administration of chemotherapy and persists for a significant duration following the completion of treatment. Moreover, it becomes evident in the reoccurrence of the disease and ultimately represents an independent prognostic determinant for mortality (14). Evidence indicates that biological mechanisms may be significant in addition to the evident emotional and psychosocial aspects of depression in cancer (15). Depression is recognized as a psychoneuroimmunological disorder, where a variety of behavioral, neuroendocrine, and neurochemical changes linked to these psychological disorders are believed to be caused by peripheral immune activation via the release of proinflammatory cytokines (16–19). Highlighting the potential role of the immune system in the onset of depression is likely to unveil new pathways in the field of psychopathology.
In addition to its robust correlation with depression, inflammation plays pivotal roles throughout all phases of tumor development (20). Within the tumor microenvironment, various cell types, including infiltrated inflammatory cells, endothelial cells, tumor-associated fibroblasts, and predominantly epithelial cells, including tumor cells, generate proinflammatory cytokines (21). While markers such as IL-6, TNF-alpha, and CRP have well-established relationships with depression in cancer patients (22), the present study focuses on IL-4, BDNF, and neopterin due to their unique and potentially significant roles in the inflammatory and neurotrophic pathways. IL-4 is a cytokine with known anti-inflammatory properties that might modulate immune responses differently from the pro-inflammatory markers like IL-6 and TNF-alpha (22, 23). In the context of cancer, the intricate interplay between the immune system and mental health is increasingly recognized. Studies suggest that IL-4 may play a role in regulating the immune response to cancer cells, but its influence on the psychological well-being of cancer patients, particularly in relation to depression, is complex (24). In previous studies, a negative relationship between psychological symptoms and IL-4 in cancer patients has been observed (19, 25). By investigating IL-4, we aim to understand the association between pro- and anti-inflammatory processes in depression associated with cancer. BDNF, a neurotrophin widely found in the central nervous system, is prominently distributed in regions such as the prefrontal cortex and hippocampus (26). BDNF has been involved in the pathophysiology of depression in cancer patients. Studies have shown that decreased BDNF levels are associated with cognitive impairment and depression in patients with cancer (27). BDNF plays a crucial role in synaptic activity and long-term synaptic memories, and changes in its levels can influence learning, memory, and depression-like behaviors (28). Additionally, BDNF has been found to be a potent protective factor against neurodegeneration, including in Alzheimer’s disease (29). Its involvement in depression is well-documented in other contexts, and its levels might provide insights into neurobiological changes specific to cancer patients undergoing chemotherapy. Therefore, BDNF and its association with depression in cancer patients is an important area of research that can contribute to the understanding and treatment of depression in this population.
Neopterin, a biomarker of immune system activation and oxidative stress, which is increasingly recognized as an important marker in the pathophysiology of depression. Elevated neopterin levels might reflect a distinct pathway linking immune activation to depressive symptoms in cancer patients (30). Wachter et al., first discovered high neopterin concentrations in the urine of cancer and viral infection patients (31). Following the initial discovery of elevated levels of neopterin in the urine of cancer patients (31), researchers have explored the presence of neopterin in the blood or urine of individuals with various types of cancer. Extensive research has been conducted on the levels of neopterin in the blood and urine of individuals diagnosed with gynecological tumors (32). Numerous investigations have recognized neopterin as a predictive indicator in gynecological cancers, such as cervical carcinoma (33) and ovarian cancer (34). Elevated levels of neopterin have been observed in individuals with hematological malignancies (35–37). In patients with lymphoma, increased concentrations of neopterin in urine have shown a correlation with response to therapy (38). Elevated neopterin levels have been documented not only following the administration of immune-activating agents such as cytokines but also after undergoing chemotherapy (39). Numerous studies have explored the blood levels of neopterin in patients with schizophrenia (SZ) and major depressive disorder (MDD) to date, revealing inconsistent findings. Studies have shown elevated neopterin plasma levels in individuals experiencing depression (40, 41).
There is paucity of data regarding mental health disorders among patients with hematologic malignancies (42–46), despite the fact that efforts to examine mental health disorders in oncology have focused primarily on solid malignancies. Although these molecules show promise as potential predictors of depression in cancer patients, it is important to note that the relationship between these biomarkers and depression is complex and multifaceted. However, the specific relationship between these biomarkers and depressive symptoms in the context of lymphoma remains relatively unexplored. This gap in knowledge is particularly pertinent given the unique challenges faced by individuals with lymphoma, including the impact of the disease itself, treatment-related side effects, and the psychological stress of living with a life-threatening illness. By focusing on these biomarkers, our study aims to uncover novel pathways and mechanisms that might be targeted for therapeutic interventions, thereby contributing to a more comprehensive understanding of depression in the oncology setting.
This study aims to address this gap by investigating the association between IL-4, BDNF, neopterin and depressive symptoms in lymphoma patients. Understanding the potential links between immune dysregulation and depressive symptomatology in this specific population holds implications for both clinical practice and theoretical frameworks that seek to elucidate the multifaceted connections between the immune system and mental health. Moreover, insights derived from this research may contribute to the development of targeted interventions aimed at mitigating depressive symptoms and improving the overall well-being of individuals navigating the complex landscape of lymphoma.
2 Methods
2.1 Study design and settings
This prospective longitudinal study recruited 70 newly diagnosed lymphoma patients between September 2020 and December 2021, following approval from the Institutional Review Board (IRB) of Rajiv Gandhi Cancer Institute and Research Centre, New Delhi, India. The aim was to study the impact of chemotherapy on depressive symptoms and its correlation with IL-4, BDNF, neopterin. The study focused on patients who were undergoing R-CHOP chemotherapy (comprising 375 mg/m2 rituximab, 750 mg/m2 cyclophosphamide; 50 mg/m2 doxorubicin; 1.4 mg/m2 vincristine, up to maximal dose of 2 mg on day 1; 40 mg/m2 prednisolone) as part of their treatment regimen. Written informed consent was obtained from every eligible patient, and strict measures were implemented to safeguard patient confidentiality.
2.2 Participants
The study recruited patients from a tertiary care hospital, focusing on individuals who met specific eligibility criteria. Inclusion criteria comprised newly diagnosed lymphoma patients confirmed through histological and cytological assessments, scheduled to undergo R-CHOP treatment, chemotherapy-naïve, and aged between 18 and 65 years. Exclusion criteria encompassed individuals below 18 years, patient with prior history of depression, a history of schizophrenia or other neuropsychiatric disorders, substance abuse, use of anti-inflammatory drugs, incompetence for interview, and lack of willingness to participate and provide consent for the study.
2.3 Assessment of depression using PHQ-9 in cancer patients
The Patient Health Questionnaire (PHQ-9), validated, self-report screening tool to measure the severity of depressive symptoms. Distinguishing itself from other depression scales, the PHQ-9 comprises nine items aligned with the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-V) criteria for MDD (47). The questionnaire evaluates the frequency with which the participants were disturbed by any of the nine items in the 2 weeks prior. Each item on the PHQ-9 is rated on a scale of 0 to 3 (0 = not at all; 1 = several days; 2 = more than a week; 3 = nearly every day). The total PHQ-9 score ranges from 0 to 27, with scores of 5–9 categorized as mild depression, 10–14 as moderate depression, 15–19 as moderately severe depression, and ≥ 20 as severe depression (48). While the PHQ-9 total score provides a comprehensive assessment of depression severity, its single-score nature may obscure the differential impact of specific symptoms. By analyzing individual PHQ-9 items, we sought to explore potential differential relationships between specific symptoms (e.g., anhedonia, fatigue, concentration difficulties) and biomarkers in lymphoma patients undergoing chemotherapy. This approach allows us to identify potential symptom-specific effects that may not be apparent when considering the overall depression score.
2.4 Evaluation of IL-4, BDNF and neopterin levels in cancer patients
Before the initiation of each chemotherapy cycle, 5-mL of blood sample was withdrawn at each time-point (TP1, TP2 and TP3). The samples underwent centrifugation for 15 min at 4000 rpm to facilitate serum separation. The resulting supernatant serum was then transferred into two separate aliquots and stored at −80°C until analysis. Serum interleukin IL-4, BDNF and neopterin levels were quantified for each 50 μL of serum sample using a highly sensitive ELISA kits from Bioassay Technology Laboratory (Shanghai Korain Biotech Co., Ltd., Shanghai, China).
2.5 Statistical analysis
The statistical analysis was conducted using IBM SPSS software (version 25.0, IBM Corp., Armonk, NY, USA). Categorical variables were defined in terms of frequencies and corresponding proportions, while continuous variables were expressed as mean ± SD/Median (IQR). The Kolmogorov–Smirnov test was employed to assess the distribution of the collected data. Given the non-normal distribution, non-parametric tests were applied. Demographic data is presented based on the distribution of the respective data. Friedman’s Two-way Analysis of Variance by Ranks test was employed to analyze the impact of treatment modality and time interval on factors such as IL-4, BDNF, neopterin and PHQ-9 score in lymphoma patients, adjusting for covariates such as age, gender, and level of education to control for potential confounding effects of these demographic variables. Post-hoc analysis was done using Kruskal Wallis One-way ANOVA test. Statistical significance was set at a p-value less than 0.05, and the significance level was adjusted for multiple comparisons using Bonferroni’s correction to reduce the risk of Type I error. Associations that remained robust after Bonferroni adjustment for three comparisons (alpha = 0.0017) were duly noted. Correlation analyses were carried out using Spearman’s rank correlation coefficient rho (ρ). Percentage change was computed using the formula [% change = (Final value − Initial value) × 100/Initial value], with the median value at TP1 considered as the initial value and the median value at TP3 as the final value, to provide a clear measure of change over the treatment period. A post-hoc power analysis was conducted to justify the adequacy of the sample size, using G*Power software (version 3.1.9.4). We based our calculations on the following parameters: effect Size (f) = 0.403 calculated based on partial eta squared (η2) = 0.14; type-I error (α) = 0.05; power (1 − β): Targeted at 0.80, which is a commonly accepted threshold in clinical research.
3 Results
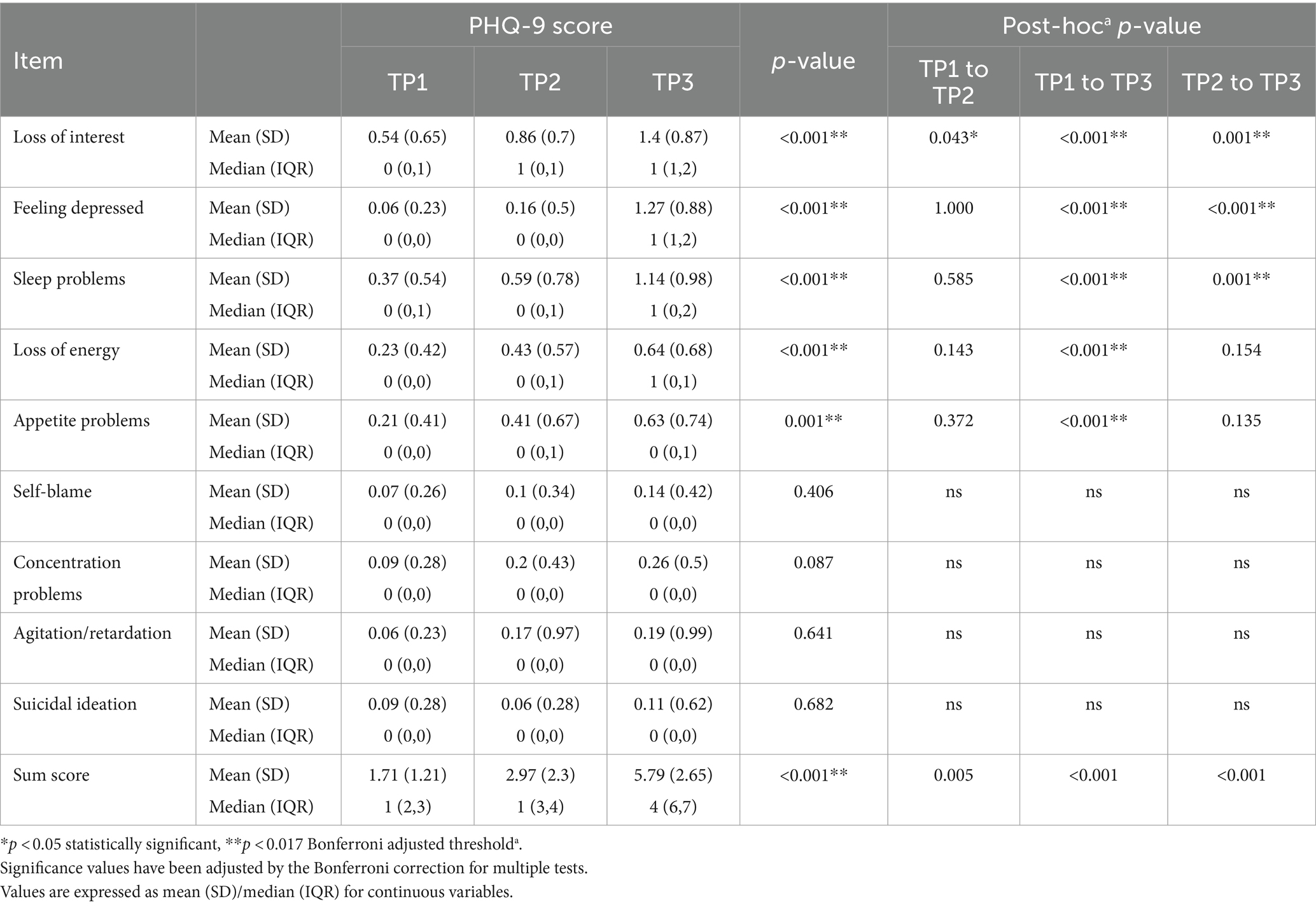
The present study included 70 lymphoma patients, 62.85% of them were male (44) and 37.15% were females (26), with a mean age of 44.17 ± 13.67 years. All the patients received R-CHOP as the main chemotherapy regimen. Table 1 displays the clinical and demographic features of the study participants.
3.1 Effect of R-CHOP on IL-4, BDNF and neopterin levels in lymphoma patients
Patients who received R-CHOP chemotherapy were found with significantly decreased levels of IL-4 and BDNF between given time-points TP1 vs. TP2, TP1 vs. TP3 and TP2 vs. TP3 (p < 0.001). However, levels of neopterin were significantly elevated with consecutive cycles of chemotherapy (p < 0.001). For, BDNF and neopterin, the changes in the levels for TP2 to TP3 were not significant as shown in Table 2.
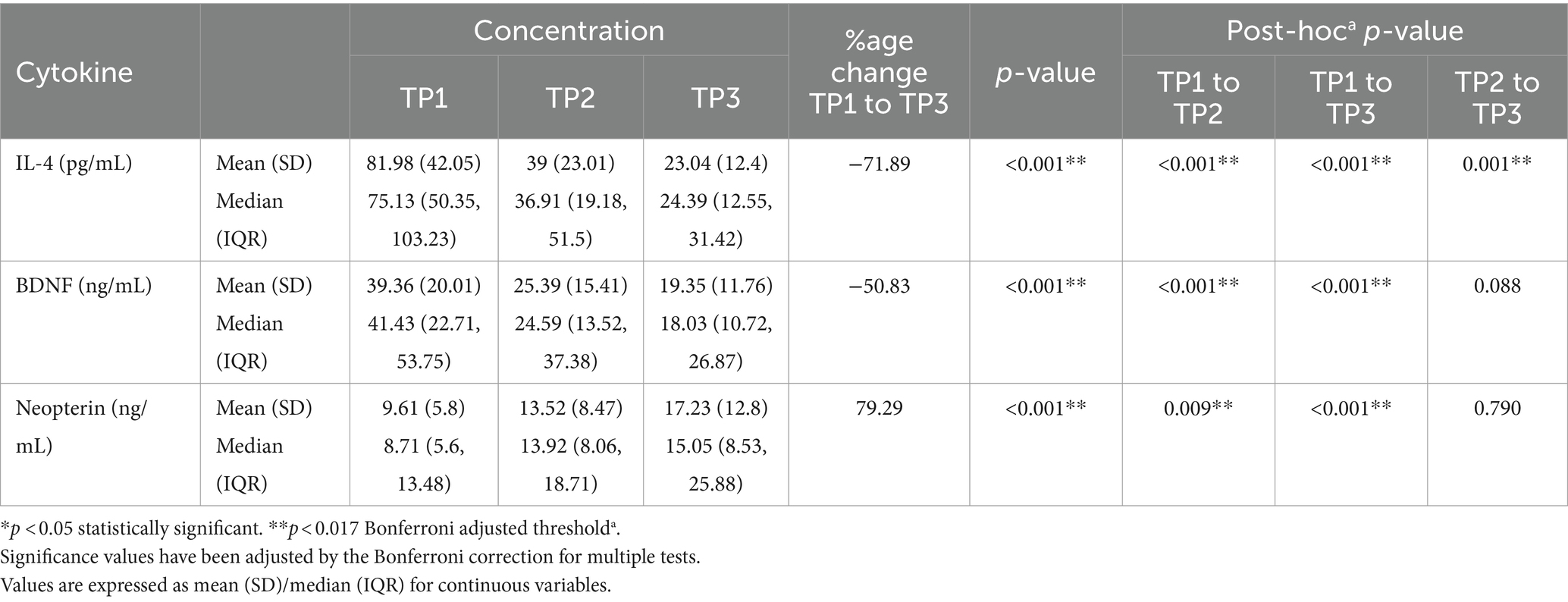
Table 2. The effect of different cycles of R-CHOP on IL-4, BDNF and neopterin levels of all lymphoma patients (n = 70).
3.2 Effect of R-CHOP on PHQ-9 scale
Scores of PHQ-9 items like Loss of interest (p < 0.001), feeling depressed (p < 0.001), sleep problems (p < 0.001), loss of energy (p < 0.001), and appetite problems (p < 0.001) were found significantly affected with consecutive cycles of chemotherapy (Table 3). The scores recorded at TP3 were significantly increased from the baseline scores. However, no change from TP1 to TP3 was observed in the scores of items like self-blame, concentration problem, agitation/retardation and suicidal ideation. The overall change in the total PHQ-9 score from TP1 to TP2 (p = 0.005), TP1 to TP3 (p < 0.001) and TP2 to TP3 (<0.001) were found significantly affected.
3.3 Correlation coefficient for IL-4, BDNF and neopterin levels and PHQ-9 items
The correlation of IL-4, BDNF, and neopterin levels with the PHQ-9 score changes was assessed at TP2 and TP3 as shown in Figure 1. The results indicate weak negative associations for all markers with the changes in PHQ-9 scores at both time points, though none of these correlations were statistically significant. IL-4 shows a weak negative correlation with changes in PHQ-9 scores at both TP2 (ρ = −0.209) and TP3 (ρ = −0.223). A negative correlation indicates that as IL-4 levels increase, the PHQ-9 score decreases slightly, suggesting a potential inverse relationship between IL-4 and depression symptoms. However, the associations were not significant (p-values >0.05). BDNF also exhibits a weak negative correlation with changes in PHQ-9 scores at TP2 (ρ = −0.225) and TP3 (ρ = −0.212). Similar to IL-4, the negative correlation suggests that higher BDNF levels might be associated with lower PHQ-9 scores. The lack of statistical significance (p-values >0.05). Neopterin shows a weak negative correlation with changes in PHQ-9 scores at TP2 (ρ = −0.225) and TP3 (ρ = −0.203). Despite the weak negative correlation, an increase in neopterin levels with an increase in PHQ-9 scores was noted, which suggests a contradiction, as the weak negative correlation implies higher neopterin levels might generally be associated with lower PHQ-9 scores.
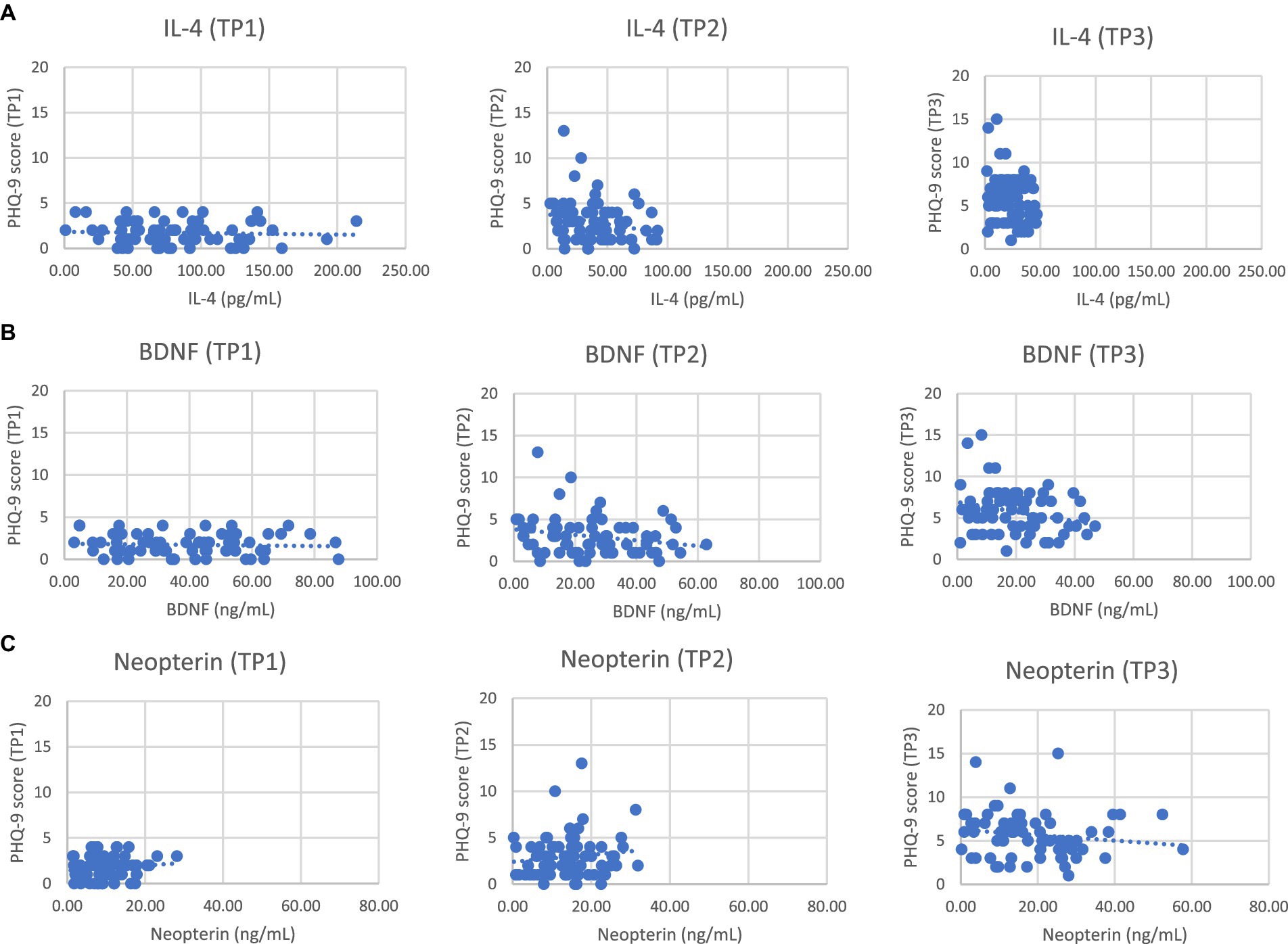
Figure 1. Scatter plots between PHQ-9 scores and serum levels of IL-4 (A) or BDNF (B) and neopterin (C) in NHL patients at different time points.
4 Discussion
In the present study, we investigated association between levels of IL-4, BDNF, neopterin and depression in lymphoma patients receiving consecutive cycles of chemotherapy. We observed significant alterations in the levels of these immune response markers throughout the treatment. Patients evaluated before chemotherapy presented higher concentrations of IL-4 and BDNF, which decreased following the cycles of chemotherapy. Conversely, low baseline neopterin levels increased post-chemotherapy treatment. A weak negative association of IL-4, BDNF and neopterin was observed with PHQ-9 scores. Additionally, significantly poorer performance in PHQ-9 items like loss of interest, feeling depressed, sleep problems, loss of energy, and appetite problems was found after consecutive cycles of chemotherapy.
The PHQ-9 is extensively employed in primary care settings to screen for clinical depression (48, 49). However, its application in clinical oncology groups has been limited (50–52). In clinical oncology research, the emphasis has primarily been on evaluating the PHQ-9’s diagnostic precision in identifying MDD, rather than profiling a group of cancer patients exhibiting depressive symptoms identified by the PHQ-9. A cross-sectional study conducted on cancer patients undergoing initial cycles of chemotherapy, both at day 0 and 28 days into the treatment, revealed an increase in PHQ-9 scores following the chemotherapy cycles (53). Similar findings have been observed in the present study, where we found an increase in the PHQ-9 item scores after receiving cycles of chemotherapy. Notable changes were observed in several PHQ-9 items in the present study, specifically in items like loss of interest, feeling depressed, sleep problems, loss of energy, and appetite problems (p < 0.001). These items, reflecting core symptoms of depression, were significantly affected by the consecutive cycles of R-CHOP chemotherapy. These findings can be explained by the facts that prolonged treatment durations, repeated admissions to the hospital, and the adverse effects of chemotherapy significantly affect patients’ mental and emotional health. This is likely due to the prolonged chemotherapy and repeated hospital visits negatively impacting their psychological health. Subsequent studies have reported deterioration in the psychological condition and occurrence of depression following cycles of chemotherapy in cancer patients (53, 54). The worsening of specific depressive symptoms is consistent with existing literature on inflammation-mediated depression. Inflammatory cytokines such as IL-6 and TNF-alpha are closely linked to anhedonia and depressed mood by disrupting the brain’s reward pathways (55). Elevated levels of IL-6 and CRP have been associated with sleep disturbances, as inflammation impacts the sleep–wake cycle and reduces sleep quality (55). Fatigue and loss of energy, common features of inflammatory depression, are driven by cytokine-induced sickness behavior, leading to lethargy and decreased motivation (56). Additionally, cytokines can affect hypothalamic function, contributing to changes in appetite, either increased or decreased (57). However, no changes were observed in items related to self-blame, concentration problems, agitation/retardation, and suicidal ideation. This might be due to the multifactorial nature of these symptoms, which are influenced by various biological, psychological, and social factors beyond inflammation alone. These symptoms may not be as directly linked to inflammatory pathways as the core symptoms of depression observed in our study. The observations highlight the critical role of inflammation in shaping various depressive symptoms, reinforcing the need to consider inflammatory pathways in the diagnosis and treatment of depression. Understanding these mechanisms may guide more targeted therapeutic approaches for managing depression, particularly in populations with increased inflammation. The PHQ-9 scores in the present study were comparatively low compared to those reported in similar studies involving cancer patients. Previous research has demonstrated varying rates of depression among cancer patients, with some studies indicating higher average PHQ-9 scores. For example, Walker et al. (58) found that 13% of cancer patients met the criteria for major depression based on the PHQ-9, with mean scores significantly exceeding those observed in the present study. Similarly, studies on patients undergoing chemotherapy frequently report higher PHQ-9 scores. Hartung et al. (51), for instance, recorded mean PHQ-9 scores ranging from moderate to severe depression levels in chemotherapy patients. The lower scores in our sample might be due to differences in patient demographics, disease stages, and the specific chemotherapy treatment administered.
Extensive evidence substantiates the bidirectional communication between the immune system and the central nervous system, often referred as “neuroimmune axis” (59, 60). The “cytokine hypothesis” of depression etiology suggests that proinflammatory cytokines produced by cells in the tumor microenvironment cause behavioral changes observed in cancer patients, which primarily affect the central nervous system function (57). Cytokines bind to specific receptors on nerve cells and can influence serotonin metabolism by altering the metabolism of tryptophan impacting the hypothalamic–pituitary–adrenal axis, leading to an increased inflammatory response that interrupts the functioning of glucocorticoid receptors. These cytokines may also modify neural plasticity, affecting mood regulation and contributing to depressive symptoms (12, 16).
IL-4 is a cytokine involved in regulating the immune system, mainly secreted by mast cells, Th2 cells, eosinophils and basophils (23). It stimulates the proliferation of activated B-cells and T-cells, as well as the differentiation of B-cells into plasma cells, playing a crucial role in the regulation of both the humoral and adaptive immune system. IL-4 has anti-inflammatory properties and can inhibit the production of Th1 cells, macrophages, and interferon-gamma (IFN-γ) (61). Research indicates that a person suffering from depression triggers the compensatory immunoregulatory response system (CIRS). This activation is a countermeasure against their hyperactive inflammatory response system (IRS), which helps maintain the immunological balance (62). Depressed patients exhibit elevated levels of pro-inflammatory cytokines, including IL-6, IL-1, TNF-α. To counterbalance the exaggerated inflammatory response induced by these pro-inflammatory cytokines, IL-4 is produced in large quantities. These anti-inflammatory cytokines perform their regulatory role by either inhibiting the production of pro-inflammatory cytokines or by promoting the development of phenotypes linked to M2 macrophages (63). The evidence supporting the link between IL-4 and depression is conflicting. Studies have shown elevated IL-4 levels in patients with severe depression (61, 64) while, others have demonstrated reduced IL-4 levels in acute depression (65, 66). A cohort study done in in early-stage breast cancer patients investigated the changes in cytokine (IL-1β, TNF-α and IL-4) levels before and after chemotherapy demonstrated higher IL-4 levels in patients after receiving the chemotherapy treatment (67). Furthermore, a notable reduction in the levels of IL-4 has been observed in aggressive NHL patients following the chemotherapy treatment (68). The present study revealed a significantly decreased IL-4 levels following cycles of chemotherapy and a negative correlation with PHQ-9 scores align with studies suggesting that major depression in cancer patients may be linked to inadequately controlled inflammation related to the disease.
Brain-derived neurotrophic factor (BDNF) is a protein primarily secreted by neurons within the brain, distributed across various regions (69). Notably, the hippocampus exhibits the highest concentrations of BDNF. This protein plays a crucial role in promoting neurogenesis and influencing neuroplasticity in the brain. BDNF can cross the BBB contributing to detectable levels in the blood (70). There is evidence to suggest that the pathophysiology of MDD is associated with low serum concentrations of BDNF (71, 72). Lower BDNF levels have been linked with chemotherapy-related cognitive impairment (73, 74) but the association with depression in cancer patients remains unclear (73, 75). According to preclinical studies, neurogenesis and proliferating neural stem cells in the hippocampus are markedly reduced when exposed to cytotoxic agents (76, 77). This chemotherapy-induced decrease in neurogenic and oligogenic populations in the brain was associated with reduced BDNF and TrkB activity in the hippocampus (78, 79). The decrease in BDNF levels may possibly be the consequence of cytokines inhibiting BDNF expression in the brain. Extensive studies on BDNF lend support to the “neurotrophic hypothesis” of depression. This hypothesis emphasizes that a reduction in BDNF levels is central to the onset of depression, leading to neuroplastic changes. These changes include the loss of neurons, diminished neurogenesis in the hippocampus, and a reduction in glial cells, all contributing to depressive symptoms (80).
In addition to the general role of BDNF in depression, genetic variations such as the Val66Met polymorphism have been identified as potential vulnerability factors, particularly in the context of inflammation. The Val66Met polymorphism, which involves the substitution of valine (Val) with methionine (Met) at codon 66, affects BDNF secretion and has been linked to various neuropsychiatric conditions, including depression (81). A cohort study demonstrated that women with the Met allele exhibited greater depressive symptoms in the presence of elevated inflammation compared to those with the Val/Val genotype, suggesting increased susceptibility to depression. This interaction between BDNF genotype and inflammation may explain why some cancer patients, particularly those undergoing chemotherapy, are more prone to depression (55). Although the present study did not assess BDNF genotypes, a significant decline in BDNF levels post-R-CHOP chemotherapy and an inverse relationship with PHQ-9 scores was noted suggesting the pathophysiological role of BDNF in chemotherapy-related depression. Future research incorporating genetic factors like the Val66Met polymorphism could refine our understanding and management of depression in lymphoma patients.
Neopterin is an aromatic pteridine produced by monocytes and macrophages following stimulation by IFN-γ, released by activated T-cells. It is proposed that neopterin serves as an indicative marker for the activation of the immune system. It plays a role in the growth, differentiation, and progression of tumors as well as the activation of oncogenes (82). Elevated neopterin levels have been observed in individuals with depression (40, 83). Furthermore, a meta-analysis of case–control studies revealed higher blood neopterin concentrations in individuals with depression compared to healthy controls (84). These findings show an association between neopterin levels and depression. Elevated neopterin levels have been observed in various conditions linked to immune activation. Research has shown that neopterin levels increase in various disorders associated with immune system activation, including cancer (30). Elevated neopterin levels have been observed after chemotherapy administration (39). Neopterin released by immune cells or adipose tissue can penetrate the CNS, triggering local immune activation. Activated immune cells such as monocytes, macrophages, and T cells can migrate from the periphery to the brain, where they produce cytokines. Chronic immune activation can cause microglia to release inflammatory mediators that affect neurotransmitter systems and neuronal health. Inflammatory cytokines are known to impact neuronal development, apoptosis, and brain-derived neurotrophic factor (BDNF) receptor (TrkB) phosphorylation, disrupting BDNF signaling. Neopterin released by peripheral immune cells or adipose tissue can access the CNS to initiate local immune activation, and activated immune cells such as monocytes/macrophages and T cells can be recruited from the periphery to the brain parenchyma, and these cells can in turn produce cytokines in the brain. Under conditions of chronic immune activation, microglia can become a source of inflammatory mediators that may influence brain neurotransmitter systems and neuronal integrity (85). The present study also demonstrated elevated neopterin levels following consecutive cycles of chemotherapy, Along with a weak correlation between neopterin levels and PHQ-9 scores. The strength of the present study was that it evaluated inflammation and depression in patients undergoing chemotherapy for up to six cycles, using a design specifically intended to evaluate these outcomes in patients with lymphoma. Measuring cytokines before and after each chemotherapy cycle helped to strengthen the study by revealing changes in the patients over time.
The present study has several limitations. First, the relatively small sample size and single-center design, which may limit the generalizability of the study findings. Although the post-hoc power analysis suggests that the sample size of 70 patients is sufficient to detect medium effect sizes, smaller effect sizes may not be identified with the same level of confidence. Future studies with larger sample size and multi-center cohorts could provide more robust and generalizable data. It was difficult to ascertain the connection between changes in the immune response markers, depression and the progression of cancer since the study did not include an NHL control group. In order to draw better comparisons, it could be more beneficial for future research to use both healthy and NHL controls who are not undergoing chemotherapy, as references. Including both kinds of controls aids in determining how chemotherapy and cancer affect depressive symptoms and the levels of immune markers. Despite the limitations of this study, we believe it has important clinical and research implications. Targeting biomarkers such as neopterin, IL-4, and BDNF may help in the management of depression in lymphoma patients undergoing chemotherapy. Neopterin levels can be reduced through anti-inflammatory therapies and immunomodulatory agents, potentially alleviating depressive symptoms. IL-4 modulation via regular physical activity, dietary interventions, and cytokine therapy may promote an anti-inflammatory state, contributing to improved mood and immune function. BDNF levels can be elevated through the use of antidepressants, structured exercise regimens, psychotherapy, and nutritional supplements like vitamin D, and omega-3 fatty acids, which support neuroplasticity and mitigate depression. These biomarker-focused interventions present a promising approach to personalized depression management, warranting further clinical validation.
5 Conclusion
In conclusion, our study provides valuable insights into the potential association between IL-4, BDNF, neopterin, and depressive symptoms in lymphoma patients undergoing R-CHOP chemotherapy. The results imply that these biomarkers might be involved in the mechanisms underlying depression in lymphoma patients undergoing R-CHOP chemotherapy. This has important implications for the management of psychological well-being in this patient population. Further research is warranted to elucidate the underlying mechanisms and to explore the potential of these biomarkers as therapeutic targets or prognostic indicators. Our work contributes to the growing body of evidence on the complex relationship between biological factors and depressive symptoms in cancer patients, and highlights the need for comprehensive support strategies in the oncology setting.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Rajiv Gandhi Cancer Institute and Research Centre Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
PM: Conceptualization, Writing – review & editing, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Writing – original draft. DB: Data curation, Supervision, Validation, Visualization, Writing – review & editing. Nidhi: Conceptualization, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Swerdlow, S, Campo, E, Harris, N, Pileri, S, Stein, H, and Thiele, J. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. Lyon: IARC (2016).
2. Aladily, TN, Khreisat, W, Ashukhaibi, O, Alkhatib, SM, Annab, H, Tarawneh, MS, et al. The epidemiology of lymphoma in Jordan: a nationwide population study of 4189 cases according to World Health Organization classification system. Hematol Oncol Stem Cell Ther. (2021) 14:336–42. doi: 10.1016/j.hemonc.2020.10.002
3. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clinicians. (2021) 71:209–49. doi: 10.3322/caac.21660
4. Spinner, MA, and Advani, RH. Current frontline treatment of diffuse large B-cell lymphoma. Oncology. (2022) 36:51–8. doi: 10.46883/2022.25920940
5. Van Der Kruk, SR, Butow, P, Mesters, I, Boyle, T, Olver, I, White, K, et al. Psychosocial well-being and supportive care needs of cancer patients and survivors living in rural or regional areas: a systematic review from 2010 to 2021. Support Care Cancer. (2022) 30:1021–64. doi: 10.1007/s00520-021-06440-1
6. Derogatis, LR. The prevalence of psychiatric disorders among cancer patients. JAMA. (1983) 249:751. doi: 10.1001/jama.1983.03330300035030
7. Liu, Y, Tian, S, Ning, B, Huang, T, Li, Y, and Wei, Y. Stress and cancer: the mechanisms of immune dysregulation and management. Front Immunol. (2022) 13:1032294. doi: 10.3389/fimmu.2022.1032294
8. National Cancer Institute. Adjustment to cancer: anxiety and distress. National Cancer Institute. (2023). Available at: https://www.cancer.gov/about-cancer/coping/feelings/anxiety-distress-hp-pdq (Accessed January 3, 2024).
9. McFarland, DC, Doherty, M, Atkinson, TM, O’Hanlon, R, Breitbart, W, Nelson, CJ, et al. Cancer-related inflammation and depressive symptoms: systematic review and meta-analysis. Cancer. (2022) 128:2504–19. doi: 10.1002/cncr.34193
10. Hulbert-Williams, N, Neal, R, Morrison, V, Hood, K, and Wilkinson, C. Anxiety, depression and quality of life after cancer diagnosis: what psychosocial variables best predict how patients adjust? Psycho Oncol. (2012) 21:857–67. doi: 10.1002/pon.1980
11. Alacacioglu, A, Binicier, O, Gungor, O, Oztop, I, Dirioz, M, and Yilmaz, U. Quality of life, anxiety, and depression in Turkish colorectal cancer patients. Support Care Cancer. (2010) 18:417–21. doi: 10.1007/s00520-009-0679-2
12. Miranda, DO, Anatriello, E, Azevedo, LR, Cordeiro, JFC, Peria, FM, Flória-Santos, M, et al. Elevated serum levels of proinflammatory cytokines potentially correlate with depression and anxiety in colorectal cancer patients in different stages of the antitumor therapy. Cytokine. (2018) 104:72–7. doi: 10.1016/j.cyto.2017.09.030
13. So, WKW, Marsh, G, Ling, WM, Leung, FY, Lo, JCK, Yeung, M, et al. Anxiety, depression and quality of life among Chinese breast cancer patients during adjuvant therapy. Eur J Oncol Nurs. (2010) 14:17–22. doi: 10.1016/j.ejon.2009.07.005
14. Polikandrioti, M, Evaggelou, ZS, Zerdila, M, Koukoularis, D, and Kyritsi, E. Evaluation of depression in patients undergoing chemotherapy. Health Sci J. (2008) 2:162–72.
15. Young, K, and Singh, G. Biological mechanisms of cancer-induced depression. Front Psych. (2018) 9:299. doi: 10.3389/fpsyt.2018.00299
16. Miller, AH, Maletic, V, and Raison, CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. (2009) 65:732–41. doi: 10.1016/j.biopsych.2008.11.029
17. Felger, JC, and Lotrich, FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. (2013) 246:199–229. doi: 10.1016/j.neuroscience.2013.04.060
18. Huang, T-L, and Lin, C-C. Advances in biomarkers of major depressive disorder. Adv Clin Chem. (2015) 68:177–204. doi: 10.1016/bs.acc.2014.11.003
19. Li, M, Kouzmina, E, McCusker, M, Rodin, D, Boutros, P, Paige, CJ, et al. Cytokines and depression in cancer patients and caregivers. NDT. (2017) 13:2903–11. doi: 10.2147/NDT.S144774
20. Grivennikov, SI, Greten, FR, and Karin, M. Immunity, inflammation, and Cancer. Cell. (2010) 140:883–99. doi: 10.1016/j.cell.2010.01.025
21. Quante, M, Varga, J, Wang, TC, and Greten, FR. The gastrointestinal tumor microenvironment. Gastroenterology. (2013) 145:63–78. doi: 10.1053/j.gastro.2013.03.052
22. Shi, R, Gwee, X, Chua, DQ, Tan, CT, Yap, KB, Larbi, A, et al. Inflammatory markers and incident depression: evidence in a population-based prospective study. Psychoneuroendocrinology. (2022) 142:105806. doi: 10.1016/j.psyneuen.2022.105806
23. Gadani, SP, Cronk, JC, Norris, GT, and Kipnis, J. IL-4 in the brain: a cytokine to remember. J Immunol. (2012) 189:4213–9. doi: 10.4049/jimmunol.1202246
24. Kwaśniak, K, Czarnik-Kwaśniak, J, Maziarz, A, Aebisher, D, Zielińska, K, Karczmarek-Borowska, B, et al. Scientific reports concerning the impact of interleukin 4, interleukin 10 and transforming growth factor on cancer cells. Cent Eur J Immunol. (2019) 44:190–200. doi: 10.5114/ceji.2018.76273
25. Li, M, Kouzmina, E, McCusker, M, Rodin, D, Boutros, PC, Paige, CJ, et al. Pro- and anti-inflammatory cytokine associations with major depression in cancer patients. Psycho-Oncology. (2017) 26:2149–56. doi: 10.1002/pon.4316
26. Ng, T, Lee, YY, Chae, J, Yeo, AHL, Shwe, M, Gan, YX, et al. Evaluation of plasma brain-derived neurotrophic factor levels and self-perceived cognitive impairment post-chemotherapy: a longitudinal study. BMC Cancer. (2017) 17:867. doi: 10.1186/s12885-017-3861-9
27. Almeida, S, Camacho, M, Barahona-Corrêa, JB, Oliveira, J, Lemos, R, Da Silva, DR, et al. Criterion and construct validity of the Beck depression inventory (BDI-II) to measure depression in patients with cancer: the contribution of somatic items. Int J Clin Health Psychol. (2023) 23:100350. doi: 10.1016/j.ijchp.2022.100350
28. Biracyaza, E, Habimana, S, and Rusengamihigo, D. Psychometric properties of the Beck Depression Inventory (BDI-II) in cancer patients: cancer patients from Butaro ambulatory cancer center, Rwanda. PRBM. (2021) 14:665–74. doi: 10.2147/PRBM.S306530
29. Wang, Z, Wang, S, Liu, Y, Gao, S, Yu, Y, and Hu, Z. Serum levels of BDNF in patients with adenoma and colorectal cancer. Dis Markers. (2021) 2021:1–6. doi: 10.1155/2021/8867368
30. Melichar, B, Spisarová, M, Bartoušková, M, Krčmová, LK, Javorská, L, and Študentová, H. Neopterin as a biomarker of immune response in cancer patients. Ann Transl Med. (2017) 5:280–10. doi: 10.21037/atm.2017.06.29
31. Wachter, H, Hausen, A, and Grassmayr, K. Increased urinary excretion of neopterin in patients with malignant tumors and with virus diseases (author’s transl). Hoppe Seylers Z Physiol Chem. (1979) 360:1957–60.
32. Melichar, B, Solichova, D, and Freedman, RS. Neopterin as an indicator of immune activation and prognosis in patients with gynecological malignancies. Int J Gynecol Cancer. (2006) 16:240–52. doi: 10.1111/j.1525-1438.2006.00294.x
33. Reibnegger, GJ, Bichler, AH, Dapunt, O, Fuchs, DN, Fuith, LC, Hausen, A, et al. Neopterin as a prognostic indicator in patients with carcinoma of the uterine cervix. Cancer Res. (1986) 46:950–5.
34. Reibnegger, G, Hetzel, H, Fuchs, D, Fuith, LC, Hausen, A, Werner, ER, et al. Clinical significance of neopterin for prognosis and follow-up in ovarian cancer. Cancer Res. (1987) 47:4977–81.
35. Hausen, A, Fuchs, D, Grünewald, K, Huber, H, König, K, and Wechter, H. Urinary neopterine as marker for haematological neoplasias. Clin Chim Acta. (1981) 117:297–305. doi: 10.1016/0009-8981(81)90117-0
36. Hausen, A, Fuchs, D, Grünewald, K, Huber, H, König, K, and Wachter, H. Urinary neopterine in the assessment of lymphoid and myeloid neoplasia, and neopterine levels in haemolytic anaemia and benign monoclonal gammopathy. Clin Biochem. (1982) 15:34–7. doi: 10.1016/S0009-9120(82)90403-9
37. Caenazzo, A, Pietrogrande, F, Sgarabotto, D, Dazzi, F, Sartori, D, Vattamattathil, KP, et al. Serum neopterin levels in haematological malignancies. Haematologica. (1993) 78:225–9.
38. Piccinini, L, Zironi, S, Federico, M, Pini, LA, and Luppi, G. Urinary Neopterin in malignant lymphoma. Int J Biol Markers. (1991) 6:231–6. doi: 10.1177/172460089100600403
39. Melichar, B, Urbánek, L, Krcmová, L, Kalábová, H, Melicharová, K, Malírová, E, et al. Urinary neopterin, hemoglobin and peripheral blood cell counts in breast carcinoma patients treated with dose-dense chemotherapy. Anticancer Res. (2008) 28:2389–96.
40. Maes, M, Scharpé, S, Meltzer, HY, Okayli, G, Bosmans, E, D’Hondt, P, et al. Increased neopterin and interferon-gamma secretion and lower availability of L-tryptophan in major depression: further evidence for an immune response. Psychiatry Res. (1994) 54:143–60. doi: 10.1016/0165-1781(94)90003-5
41. Hoekstra, R, Fekkes, D, Van De Wetering, BJM, Pepplinkhuizen, L, and Verhoeven, WMA. Effect of light therapy on biopterin, neopterin and tryptophan in patients with seasonal affective disorder. Psychiatry Res. (2003) 120:37–42. doi: 10.1016/S0165-1781(03)00167-7
42. Prasad, SM, Eggener, SE, Lipsitz, SR, Irwin, MR, Ganz, PA, and Hu, JC. Effect of depression on diagnosis, treatment, and mortality of men with clinically localized prostate cancer. JCO. (2014) 32:2471–8. doi: 10.1200/JCO.2013.51.1048
43. Ravi, P, Karakiewicz, PI, Roghmann, F, Gandaglia, G, Choueiri, TK, Menon, M, et al. Mental health outcomes in elderly men with prostate cancer. Urol Oncol. (2014) 32:1333–40. doi: 10.1016/j.urolonc.2014.05.005
44. Iglay, K, Santorelli, ML, Hirshfield, KM, Williams, JM, Rhoads, GG, Lin, Y, et al. Diagnosis and treatment delays among elderly breast cancer patients with pre-existing mental illness. Breast Cancer Res Treat. (2017) 166:267–75. doi: 10.1007/s10549-017-4399-x
45. Haskins, CB, McDowell, BD, Carnahan, RM, Fiedorowicz, JG, Wallace, RB, Smith, BJ, et al. Impact of preexisting mental illness on breast cancer endocrine therapy adherence. Breast Cancer Res Treat. (2019) 174:197–208. doi: 10.1007/s10549-018-5050-1
46. Sathianathen, NJ, Fan, Y, Jarosek, SL, Konety, I, Weight, CJ, Vinogradov, S, et al. Disparities in bladder cancer treatment and survival amongst elderly patients with a pre-existing mental illness. Eur Urol Focus. (2020) 6:1180–7. doi: 10.1016/j.euf.2019.02.007
47. Bains, N, and Abdijadid, S. Major depressive disorder. Treasure Island (FL): StatPearls Publishing (2024).
48. Kroenke, K, Spitzer, RL, and Williams, JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
49. Spitzer, RL. Validation and utility of a self-report version of PRIME-MDThe PHQ primary care study. JAMA. (1999) 282:1737–44. doi: 10.1001/jama.282.18.1737
50. Hinz, A, Mehnert, A, Kocalevent, R-D, Brähler, E, Forkmann, T, Singer, S, et al. Assessment of depression severity with the PHQ-9 in cancer patients and in the general population. BMC Psychiatry. (2016) 16:22. doi: 10.1186/s12888-016-0728-6
51. Hartung, TJ, Friedrich, M, Johansen, C, Wittchen, H, Faller, H, Koch, U, et al. The hospital anxiety and depression scale (HADS) and the 9-item patient health questionnaire (PHQ-9) as screening instruments for depression in patients with cancer. Cancer. (2017) 123:4236–43. doi: 10.1002/cncr.30846
52. Wagner, LI, Pugh, SL, Small, W, Kirshner, J, Sidhu, K, Bury, MJ, et al. Screening for depression in cancer patients receiving radiotherapy: feasibility and identification of effective tools in the NRG oncology RTOG 0841 trial. Cancer. (2017) 123:485–93. doi: 10.1002/cncr.29969
53. Charonpongsuntorn, C, Tiranaprakij, P, Wangtawesap, S, and Muangnoi, P. 1566P sleep quality and depression in cancer patients receiving systemic chemotherapy: a prospective study. Ann Oncol. (2022) 33:S1263. doi: 10.1016/j.annonc.2022.07.1660
54. Bhattacharyya, S, Bhattacherjee, S, Mandal, T, and Das, D. Depression in cancer patients undergoing chemotherapy in a tertiary care hospital of North Bengal, India. Indian J Public Health. (2017) 61:14. doi: 10.4103/0019-557X.200252
55. Dooley, LN, Ganz, PA, Cole, SW, Crespi, CM, and Bower, JE. Val66Met BDNF polymorphism as a vulnerability factor for inflammation-associated depressive symptoms in women with breast cancer. J Affect Disord. (2016) 197:43–50. doi: 10.1016/j.jad.2016.02.059
56. Dantzer, R, and Kelley, KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. (2007) 21:153–60. doi: 10.1016/j.bbi.2006.09.006
57. Dantzer, R, O’Connor, JC, Freund, GG, Johnson, RW, and Kelley, KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. (2008) 9:46–56. doi: 10.1038/nrn2297
58. Walker, J, Hansen, CH, Martin, P, Symeonides, S, Ramessur, R, Murray, G, et al. Prevalence, associations, and adequacy of treatment of major depression in patients with cancer: a cross-sectional analysis of routinely collected clinical data. Lancet Psychiatry. (2014) 1:343–50. doi: 10.1016/S2215-0366(14)70313-X
59. Dantzer, R. Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur J Pharmacol. (2004) 500:399–411. doi: 10.1016/j.ejphar.2004.07.040
60. Morimoto, K, and Nakajima, K. Role of the immune system in the development of the central nervous system. Front Neurosci. (2019) 13:916. doi: 10.3389/fnins.2019.00916
61. Ogłodek, E. Changes in the serum levels of cytokines: IL-1β, IL-4, IL-8 and IL-10 in depression with and without posttraumatic stress disorder. Brain Sci. (2022) 12:387. doi: 10.3390/brainsci12030387
62. Maes, M, and Carvalho, AF. The compensatory immune-regulatory reflex system (CIRS) in depression and bipolar disorder. Mol Neurobiol. (2018) 55:8885–903. doi: 10.1007/s12035-018-1016-x
63. Sarmin, N, Roknuzzaman, ASM, Mouree, TZ, Islam, MDR, and Al Mahmud, Z. Evaluation of serum interleukin-12 and interleukin-4 as potential biomarkers for the diagnosis of major depressive disorder. Sci Rep. (2024) 14:1652. doi: 10.1038/s41598-024-51932-9
64. Al-Hakeim, HK, Al-Kufi, SN, Al-Dujaili, AH, and Maes, M. Serum interleukin levels and insulin resistance in major depressive disorder. CNSNDDT. (2018) 17:618–25. doi: 10.2174/1871527317666180720155300
65. Goldsmith, DR, Rapaport, MH, and Miller, BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. (2016) 21:1696–709. doi: 10.1038/mp.2016.3
66. Osimo, EF, Pillinger, T, Rodriguez, IM, Khandaker, GM, Pariante, CM, and Howes, OD. Inflammatory markers in depression: a meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav Immun. (2020) 87:901–9. doi: 10.1016/j.bbi.2020.02.010
67. Zhao, J, Zuo, H, Ding, K, Zhang, X, Bi, Z, and Cheng, H. Changes in plasma IL-1β, TNF-α and IL-4 levels are involved in chemotherapy-related cognitive impairment in early-stage breast cancer patients. Am J Transl Res. (2020) 12:3046–56.
68. Soydinc, HO, Guney, N, Basaran, M, Duranyildiz, D, and Yasasever, V. Clinical significance of interleukin-4 and interleukin-18 levels in aggressive non-Hodgkin’s lymphoma patients. Genet Mol Res. (2016) 15:gmr.15038590. doi: 10.4238/gmr.15038590
69. Pan, W, Banks, WA, Fasold, MB, Bluth, J, and Kastin, AJ. Transport of brain-derived neurotrophic factor across the blood–brain barrier. Neuropharmacology. (1998) 37:1553–61. doi: 10.1016/S0028-3908(98)00141-5
70. Chauhan, VS, Khan, SA, and Kulhari, K. Correlation of brain-derived neurotrophic factor with severity of depression and treatment response. Med J Armed Forces India. (2023) 79:451–7. doi: 10.1016/j.mjafi.2020.09.014
71. Polyakova, M, Stuke, K, Schuemberg, K, Mueller, K, Schoenknecht, P, and Schroeter, ML. BDNF as a biomarker for successful treatment of mood disorders: a systematic & quantitative meta-analysis. J Affect Disord. (2015) 174:432–40. doi: 10.1016/j.jad.2014.11.044
72. Kishi, T, Yoshimura, R, Ikuta, T, and Iwata, N. Brain-derived neurotrophic factor and major depressive disorder: evidence from meta-analyses. Front Psych. (2018) 8:308. doi: 10.3389/fpsyt.2017.00308
73. Jehn, CF, Becker, B, Pfeiffer, S, Krebs, M, Possinger, K, Flath, B, et al. Cognitive dysfunction, brain-derived neurotrophic factor and IL-6 levels in cancer patients with depression. JCO. (2006) 24:8632–2. doi: 10.1200/jco.2006.24.18_suppl.8632
74. Ng, DQ, Cheng, I, Wang, C, Tan, CJ, Toh, YL, Koh, YQ, et al. Brain-derived neurotrophic factor as a biomarker in cancer-related cognitive impairment among adolescent and young adult cancer patients. Sci Rep. (2023) 13:16298. doi: 10.1038/s41598-023-43581-1
75. Jehn, CF, Becker, B, Flath, B, Nogai, H, Vuong, L, Schmid, P, et al. Neurocognitive function, brain-derived neurotrophic factor (BDNF) and IL-6 levels in cancer patients with depression. J Neuroimmunol. (2015) 287:88–92. doi: 10.1016/j.jneuroim.2015.08.012
76. Christie, L-A, Acharya, MM, Parihar, VK, Nguyen, A, Martirosian, V, and Limoli, CL. Impaired cognitive function and hippocampal neurogenesis following cancer chemotherapy. Clin Cancer Res. (2012) 18:1954–65. doi: 10.1158/1078-0432.CCR-11-2000
77. Wigmore, P. The effect of systemic chemotherapy on neurogenesis, plasticity and memory In: C Belzung and P Wigmore, editors. Neurogenesis and neural plasticity. Current topics in behavioral neurosciences. Berlin, Heidelberg: Springer Berlin Heidelberg (2012). 211–40.
78. Mustafa, S, Walker, A, Bennett, G, and Wigmore, PM. 5-fluorouracil chemotherapy affects spatial working memory and newborn neurons in the adult rat hippocampus. Eur J Neurosci. (2008) 28:323–30. doi: 10.1111/j.1460-9568.2008.06325.x
79. Park, H-S, Kim, C-J, Kwak, H-B, No, M-H, Heo, J-W, and Kim, T-W. Physical exercise prevents cognitive impairment by enhancing hippocampal neuroplasticity and mitochondrial function in doxorubicin-induced chemobrain. Neuropharmacology. (2018) 133:451–61. doi: 10.1016/j.neuropharm.2018.02.013
80. Chakrapani, S, Eskander, N, De Los Santos, LA, Omisore, BA, and Mostafa, JA. Neuroplasticity and the biological role of brain derived neurotrophic factor in the pathophysiology and management of depression. Cureus. (2020) 12:e11396. doi: 10.7759/cureus.11396
81. Porter, GA, and O’Connor, JC. Brain-derived neurotrophic factor and inflammation in depression: pathogenic partners in crime? WJP. (2022) 12:77–97. doi: 10.5498/wjp.v12.i1.77
82. Yildirim, Y, Gunel, N, Coskun, U, Pasaoglu, H, Aslan, S, and Cetin, A. Serum neopterin levels in patients with breast cancer. Med Oncol. (2008) 25:403–7. doi: 10.1007/s12032-008-9054-2
83. Celik, C, Erdem, M, Caycı, T, Ozdemir, B, Ozgur Akgul, E, Kurt, YG, et al. The association between serum levels of neopterin and number of depressive episodes of major depression. Prog Neuro Psychopharmacol Biol Psychiatry. (2010) 34:372–5. doi: 10.1016/j.pnpbp.2010.01.002
84. Cavaleri, D, Bartoli, F, Capogrosso, CA, Guzzi, P, Moretti, F, Riboldi, I, et al. Blood concentrations of neopterin and biopterin in subjects with depression: a systematic review and meta-analysis. Prog Neuro-Psychopharmacol Biol Psychiatry. (2023) 120:110633. doi: 10.1016/j.pnpbp.2022.110633
Keywords: depression, PHQ-9, BDNF, IL-4, neopterin, lymphoma, chemotherapy
Citation: Mishra P, Bhurani D and Nidhi (2024) Elevated neopterin and decreased IL-4, BDNF levels and depression in lymphoma patients receiving R-CHOP chemotherapy. Front. Neurol. 15:1392275. doi: 10.3389/fneur.2024.1392275
Edited by:
Erwin Chiquete, National Institute of Medical Sciences and Nutrition Salvador Zubirán, MexicoReviewed by:
Serge Ely Dibakou, Centre International de Recherches Médicales de Franceville, GabonZev Nakamura, University of North Carolina at Chapel Hill, United States
Copyright © 2024 Mishra, Bhurani and Nidhi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nidhi, bmlkaGkuYmhhcmFsQGdtYWlsLmNvbQ==
 Pinki Mishra
Pinki Mishra Dinesh Bhurani
Dinesh Bhurani Nidhi
Nidhi