- 1Department of Emergency, The Fifth Affiliated Hospital of Sun Yat-sen University, Zhuhai, China
- 2Department of Neurovascular Disease, The Fifth Affiliated Hospital of Sun Yat-sen University, Zhuhai, China
Objectives: This study aims to present the first comprehensive meta-analysis assessing the effectiveness and safety of drug-eluting stents (DES) versus bare-metal stents (BMS) in treating intracranial and vertebral artery stenosis.
Methods: A comprehensive examination was undertaken to compare the effectiveness and safety of DES and BMS in individuals experiencing symptomatic stenosis in the intracranial and vertebral arteries through an in-depth analysis of clinical research. We conducted an extensive search across multiple databases including PubMed, Embase, Web of Science, and the Cochrane Library up to September 2024. The emphasis of our investigation was on various outcomes including rates of in-stent restenosis, symptomatic occurrences of in-stent restenosis, incidence of stroke, procedural success, mortality rates, complications associated with the procedure, and any adverse events.
Results: Our analysis included 12 studies with a total of 1,243 patients (562 in the DES group and 681 in the BMS group). The findings demonstrated a significantly lower rate of in-stent restenosis in the DES group for both intracranial [odds ratio (OR): 0.23; 95% confidence interval (CI): 0.13 to 0.41; p < 0.00001] and vertebral artery stenosis (OR: 0.38; 95% CI: 0.20 to 0.72; p = 0.003) compared to the BMS group. Additionally, the DES group showed a significantly reduced rate of postoperative strokes in vertebral artery stenosis cases (OR: 0.38; 95% CI: 0.16 to 0.90; p = 0.03), with no significant differences noted in the intracranial artery stenosis comparison (OR: 0.63; 95% CI: 0.20 to 1.95; p = 0.42). The study also revealed no significant disparities in symptomatic in-stent restenosis, procedural success, mortality, adverse effects, and perioperative complications between the two groups across the conditions studied.
Conclusion: The comparison indicates that DES significantly reduces the risk of in-stent restenosis and postoperative strokes in patients with vertebral artery stenosis, compared to BMS. For both intracranial and vertebral artery stenosis, DES and BMS exhibit comparable safety profiles.
Systematic review registration:
1 Introduction
Strokes’ prevalence globally is significantly impacted by intracranial artery stenosis, with 8 to 10% of strokes in North America and a substantial 30 to 50% in Asia being attributed to it (1–5). Responsible for 15 to 20% of posterior circulation strokes is vertebral artery stenosis (6, 7). Not only does this condition impede cerebral blood flow, leading to cerebral hypoperfusion, but it also serves as a potential source of arterial embolisms. Evidence from several studies suggests a link between vertebral artery stenosis and an increased likelihood of recurrent strokes (6, 8–10). Aggressive medical management is recommended as the cornerstone of stroke prevention for patients with intracranial and vertebral artery stenosis according to current guidelines (11). In substantially reducing the recurrence of strokes in these patients, this approach has been effective (2, 12, 13). Despite aggressive medical therapy, some patients still face a high risk of stroke recurrence (14, 15). When aggressive medical therapy fails, surgery and endovascular therapy become reasonable options. Due to the unique location of the vertebral and intracranial arteries, surgical revascularization is often challenging, with relevant perioperative complications and mortality related (16, 17).
Intravascular stents coated with anti-vascular endotheliocytes proliferation drugs on the surface or inside are known as drug-eluting stents (DES) (18). The sustained release of drugs on the surface of DES, compared with bare metal stents (BMS), can restrict the proliferation and migration of vascular smooth muscle cells in the stent, thereby restraining intravascular thrombosis and preventing in-stent restenosis. Mainly applied currently is DES to treat symptomatic stenosis in intracranial and vertebral arteries. Wu et al. (19) reported in a meta-analysis that drug-coated balloon angioplasty is a safe and effective method for the treatment of vertebral artery stenosis. Additionally, clinical studies have found that DES have a lower rate of restenosis for the treatment of intracranial and vertebral artery stenosis compared to BMS (18, 20–23). At present, the superiority of DES over BMS remains inconclusive due to factors such as the small sample size, short follow-up time, and variations in arterial stenosis sites. The first meta-analysis comparing the efficacy and safety of DES and BMS for intracranial and vertebral artery stenosis was reported.
2 Methods
2.1 Literature search
According to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) 2020 statement (24) and has been prospectively registered in the PROSPERO (CRD42023439967). Until September 2024, we meticulously searched through PubMed, Embase, Web of Science, and Cochrane databases to identify clinical studies comparing the efficacy and safety of DES with BMS in individuals suffering from symptomatic stenosis in intracranial and vertebral arteries. Through the following terms, we searched the literature extensively: “drug-eluting stents,” “bare-metal stent,” “intracranial,” “vertebral,” and “stenosis.” The detailed search strategies are as follows: (((“Drug-Eluting Stents”[Mesh]) OR (((((((((((((Drug Eluting Stents) OR (Stents, Drug-Eluting)) OR (Stents, Drug Eluting)) OR (Drug-Eluting Stent)) OR (Drug Eluting Stent)) OR (Stent, Drug-Eluting)) OR (Drug-Coated Stents)) OR (Drug Coated Stents)) OR (Stents, Drug-Coated)) OR (Stents, Drug Coated)) OR (Drug-Coated Stent)) OR (Drug Coated Stent)) OR (Stent, Drug-Coated))) AND ((bare-metal stent) OR (bare metal stent))) AND (((intracranial) OR (vertebral)) AND (stenosis)). Furthermore, we manually screened the bibliography lists of all included RCTs. Two authors independently gathered eligible articles and evaluated them. Any discrepancies in literature retrieval were resolved through discussion.
2.2 Inclusion and exclusion criteria
Eligible articles had to meet the following criteria:
P: patients diagnosed with symptomatic intracranial and vertebral artery stenosis.
I: DES.
C: BMS.
O: at least one outcome (such as in-stent restenosis, symptomatic in-stent restenosis, stroke, technical success, mortality, periprocedural complications, and adverse events) was measured; (5) data were available to analyze either odds ratios (OR) or weighted mean differences (WMD).
S: randomized controlled trial (RCT), cohort study, or case-control study.
Exclusions were made for study protocols, unpublished research, non-original studies (including letters, comments, abstracts, corrections, and replies), studies lacking adequate data, and review articles.
2.3 Data abstraction
Two authors independently conducted data abstraction, with any discrepancies resolved by a third author. We abstracted following information from eligible studies: first author name, published year, research period, study region, study design, intervention/exposure, control/non-exposure, sample size, age, gender, follow-up time, body mass index (BMI), comorbidity, stent length, in-stent restenosis, symptomatic in-stent restenosis, stroke, technical success, mortality, periprocedural complications, and adverse events. If the continuous data in the article was presented as median plus range or median plus interquartile range (IQR), we reanalysed the mean ± standard deviation (SD) via the methods reported by Wan et al. (25) and Luo et al. (26). Corresponding authors were contacted for full data if available, in cases where the research data is insufficient.
2.4 Quality evaluation
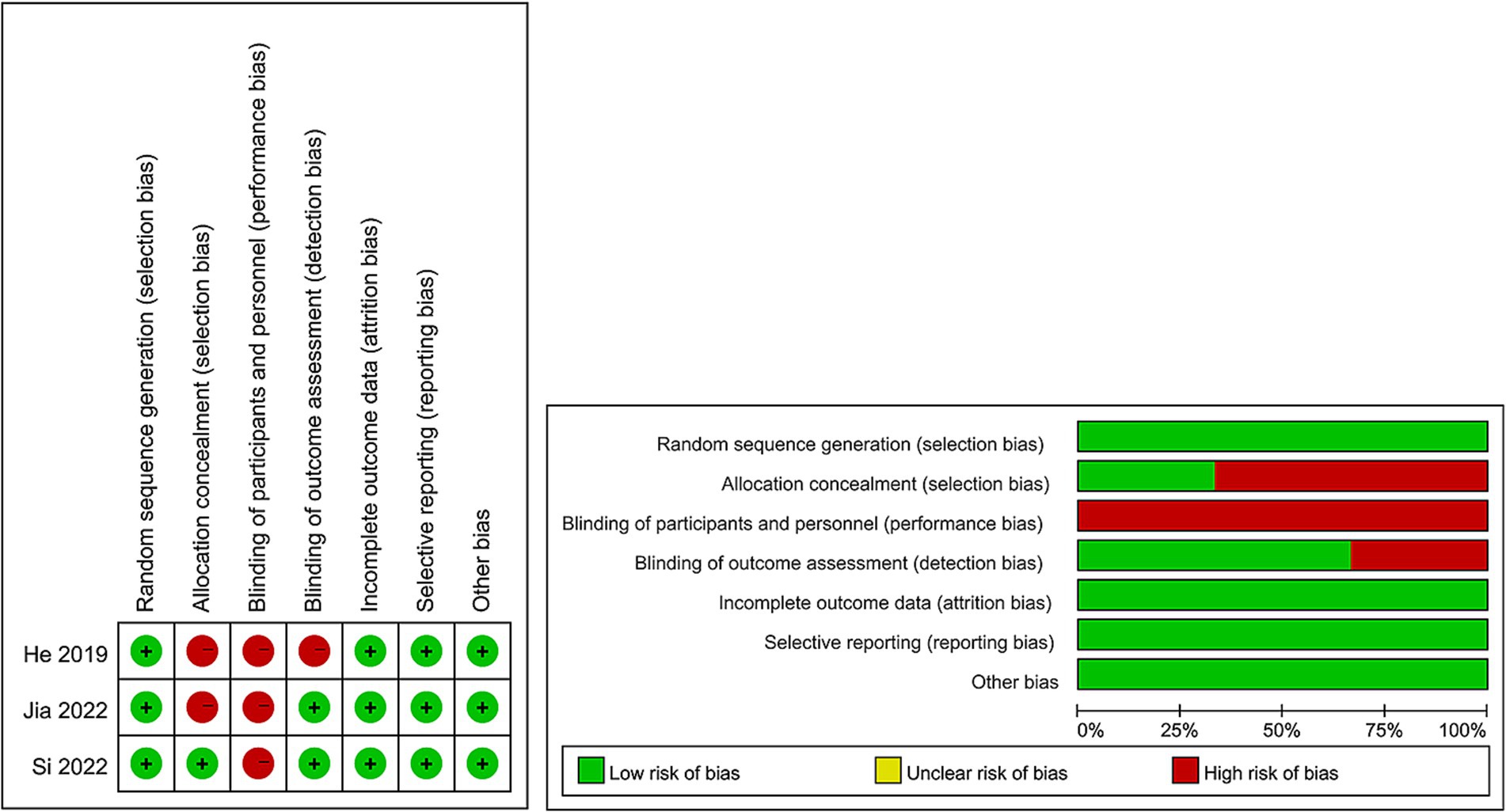
For evaluating the quality of eligible cohort studies, the Newcastle–Ottawa Scale (NOS) was utilized (27), with high quality being attributed to studies scoring 7–9 points (28). The quality assessment of eligible RCTs was conducted following the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 based on seven terms: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias (29). Every study aspect was assigned one of three evaluation outcomes: low risk, high risk, or unclear risk. Higher quality was attributed to studies with more evaluations indicating “low risk” bias. The quality of all included studies was independently assessed by two authors, with any disagreements settled through discussion.
2.5 Statistical analysis
The analysis utilized Review Manager 5.4.1 edition. Continuous data were synthesized using the WMD, while dichotomous data synthesis employed OR. Each metric was accompanied by 95% confidence intervals (CIs). To evaluate the heterogeneity of each outcome, the Cochran’s Q test (chi-squared test, χ2) and the inconsistency index (I2) were applied (30). χ2 p-value less than 0.1 or I2 more than 50% were regarded as high heterogeneity. The total WMD or OR for outcomes with significant heterogeneity (χ2 p-value less than 0.1 or I2 more than 50%) was calculated using the random-effects model. Otherwise, the fixed-effects model was applied. Additionally, subgroup analyses were conducted for outcomes with two or more included studies to evaluate possible confounders, if data were sufficient. Furthermore, we performed sensitivity analyses to evaluate the impact of each individual study on the overall WMD or OR when there were more than two studies included. Additionally, potential publication bias was assessed by generating funnel plots using Review Manager 5.4.1 and conducting Egger’s regression tests using Stata 15.1 edition (Stata Corp, College Station, Texas, United States) for outcomes involving more than two studies. A p-value less than 0.05 was deemed indicative of statistically significant publication bias.
3 Results
3.1 Literature retrieval, study characteristics, and baseline
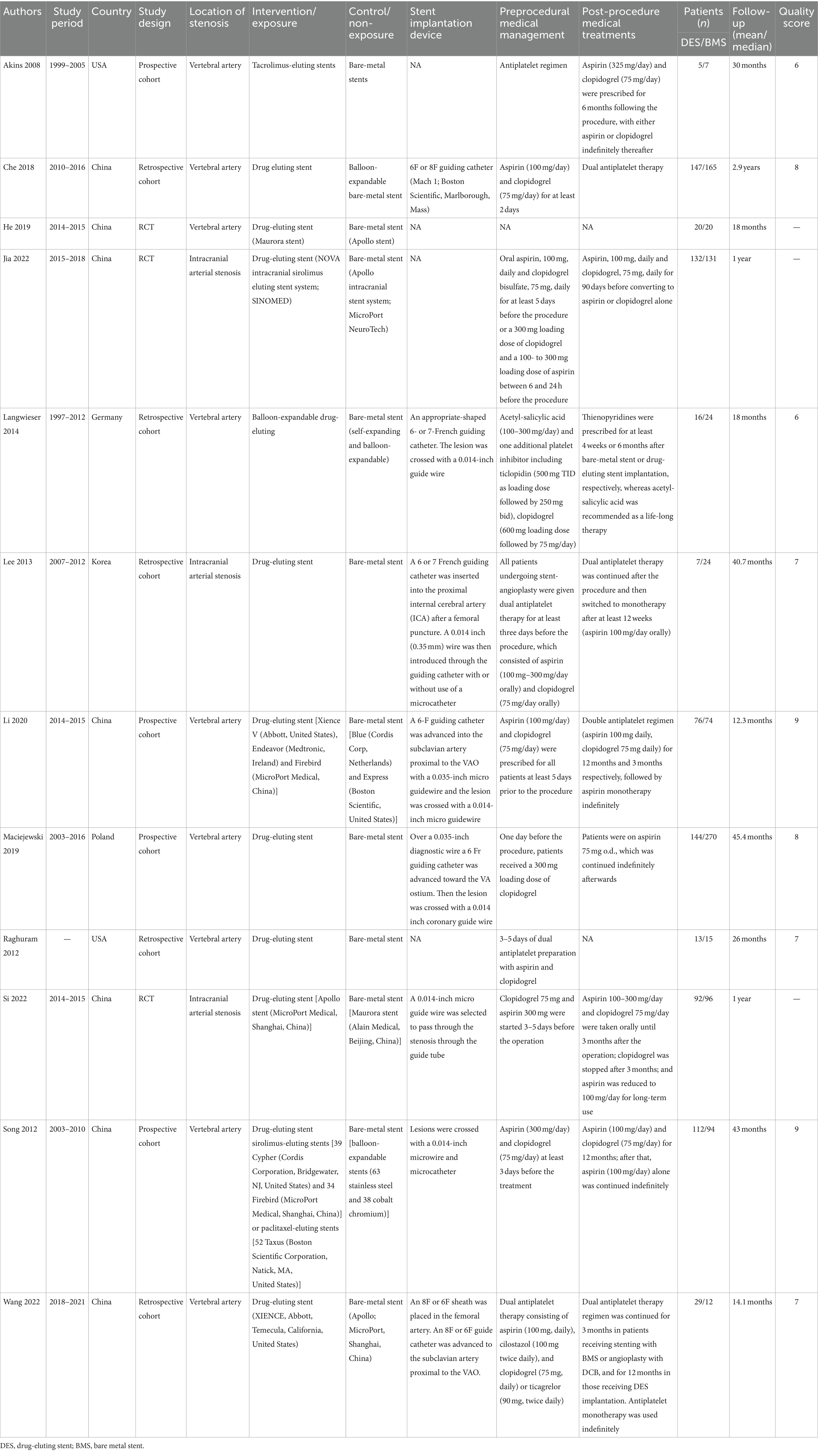
The flowchart of the literature retrieval and selection process is illustrated in Figure 1. Through systematic literature searches, a total of 178 related studies were identified in PubMed (n = 39), Embase (n = 75), Web of Science (n = 55), and Cochrane (n = 9). The detailed search strategy is presented in Supplementary Table S1. After the elimination of duplicate studies, 105 titles and abstracts underwent evaluation. Eventually, a meta-analysis was conducted, encompassing 12 studies and involving 1,243 patients (562 in the DES group versus 681 in the BMS group) (18, 20–23, 31–38). Presented in Table 1 are the characteristics and quality assessment of each eligible study. The details of quality evaluation for all included RCTs are shown in Figure 2, and Supplementary Table S2 displays the details of quality evaluation for all included cohorts. For intracranial artery stenosis, the two groups were comparable in age (WMD: 0.67; 95% CI: −1.04, 2.38; p = 0.44), gender (male) (OR: 1.01; 95% CI: 0.65, 1.57; p = 0.96), proportion of hypertension (OR: 0.74; 95% CI: 0.39, 1.41; p = 0.36), and proportion of coronary artery disease (OR: 1.03; 95% CI: 0.60, 1.77; p = 0.92) (Table 2). For cases of vertebral artery stenosis, both groups showed similar distributions in terms of gender (male) (OR: 0.92; 95% CI: 0.66, 1.26; p = 0.59), prevalence of hypertension (OR: 0.77; 95% CI: 0.36, 1.67; p = 0.51), prevalence of diabetes mellitus (OR: 1.05; 95% CI: 0.79, 1.40; p = 0.74), prevalence of coronary artery disease (OR: 1.30; 95% CI: 0.98, 1.74; p = 0.07), stent length (WMD: −0.25; 95% CI: −0.57, 0.08; p = 0.14), and BMI (WMD: −0.04; 95% CI: −1.14, 1.06; p = 0.94). The age of the DES group, however, was significantly lower than that of the BMS group (WMD: −1.29; 95% CI: −2.50, −0.09; p = 0.04) (Table 3).
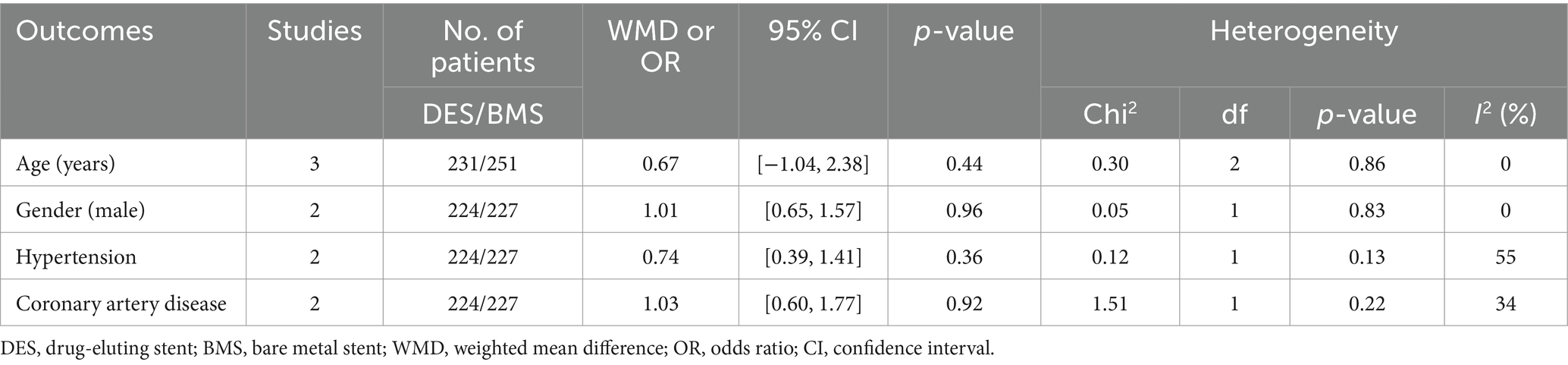
Table 2. Demographics and clinical characteristics of included studies for intracranial artery stenosis.
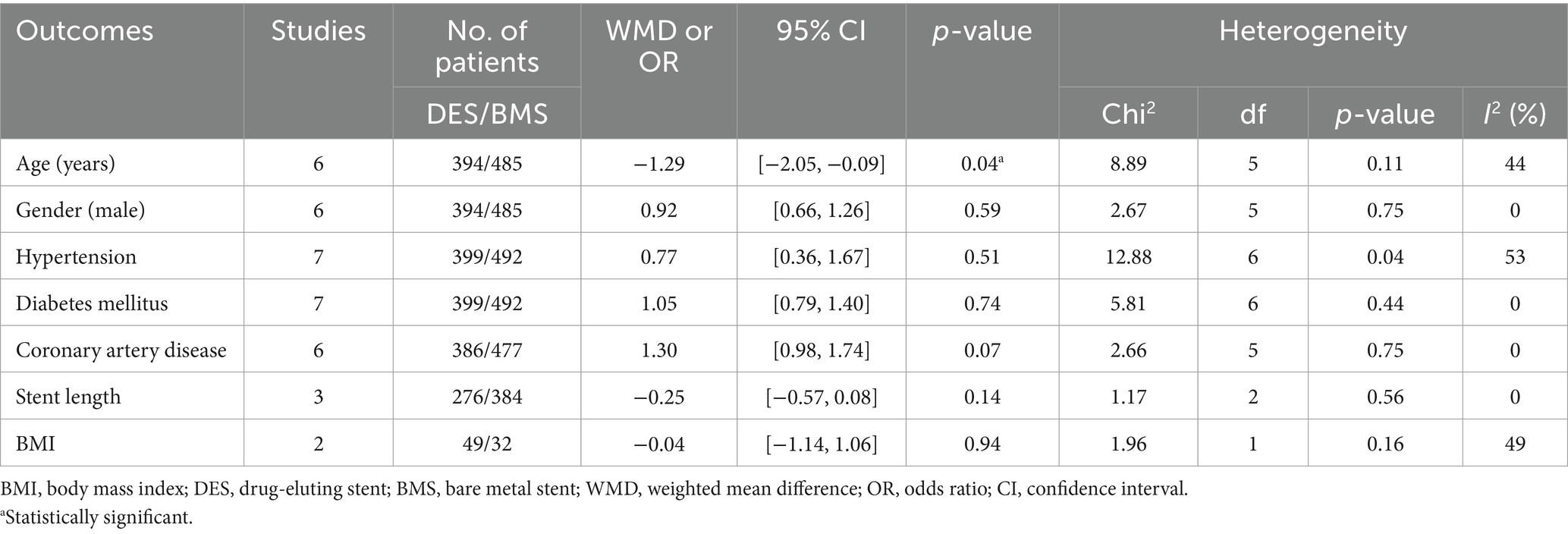
Table 3. Demographics and clinical characteristics of included studies for vertebral artery stenosis.
3.2 In-stent restenosis
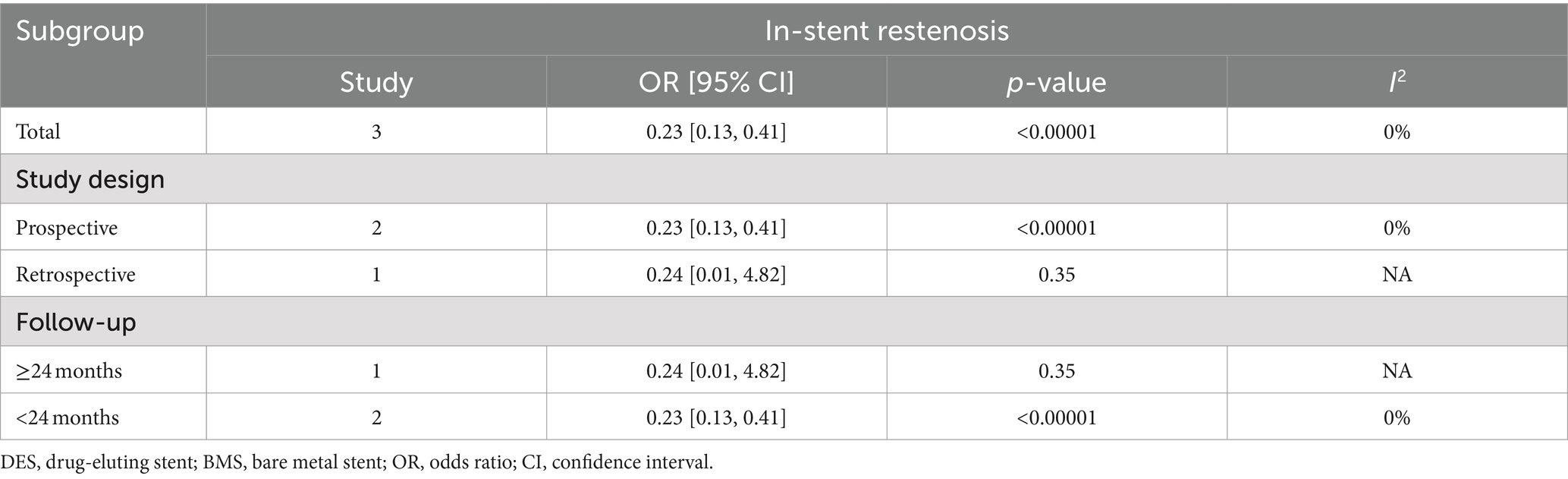
Intracranial artery stenosis, in-stent restenosis results were synthesized from 3 studies, including 375 patients (181 DES versus 194 BMS) (18, 21, 34). A significant lower in-stent restenosis rate in the DES group was revealed by meta-analysis (OR: 0.23; 95% CI: 0.13, 0.41; p < 0.00001), without significant heterogeneity observed (I2 = 0%, p = 0.98) (Figure 3A). Subgroup analysis unveiled that significance endured in prospective studies and those with a follow-up period of less than 24 months, yet dissipated in retrospective studies and those spanning 24 months or more in duration (Table 4).
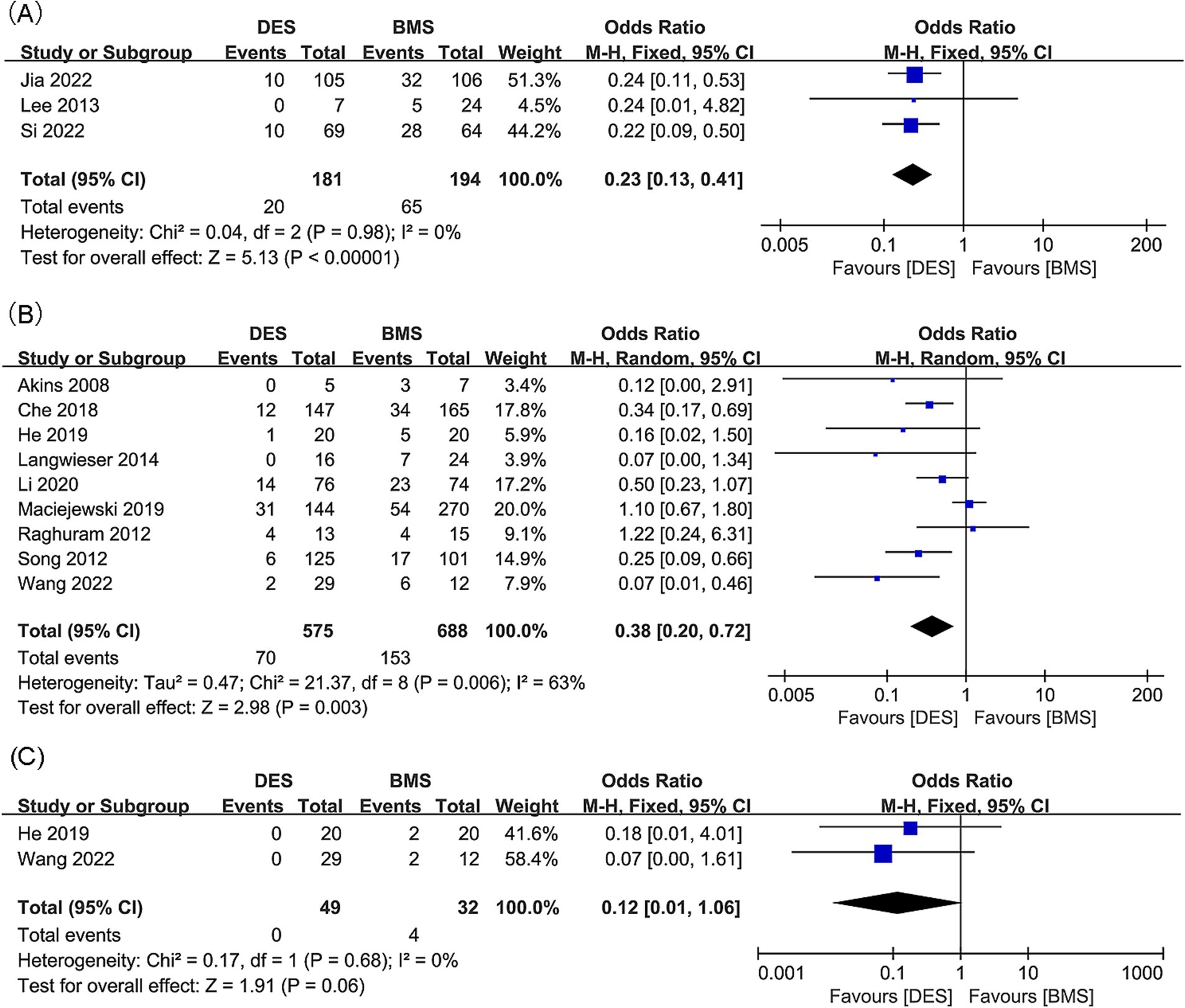
Figure 3. Forest plots of (A) in-stent restenosis (intracranial artery stenosis), (B) in-stent restenosis (vertebral artery stenosis), and (C) symptomatic in-stent restenosis (vertebral artery stenosis).
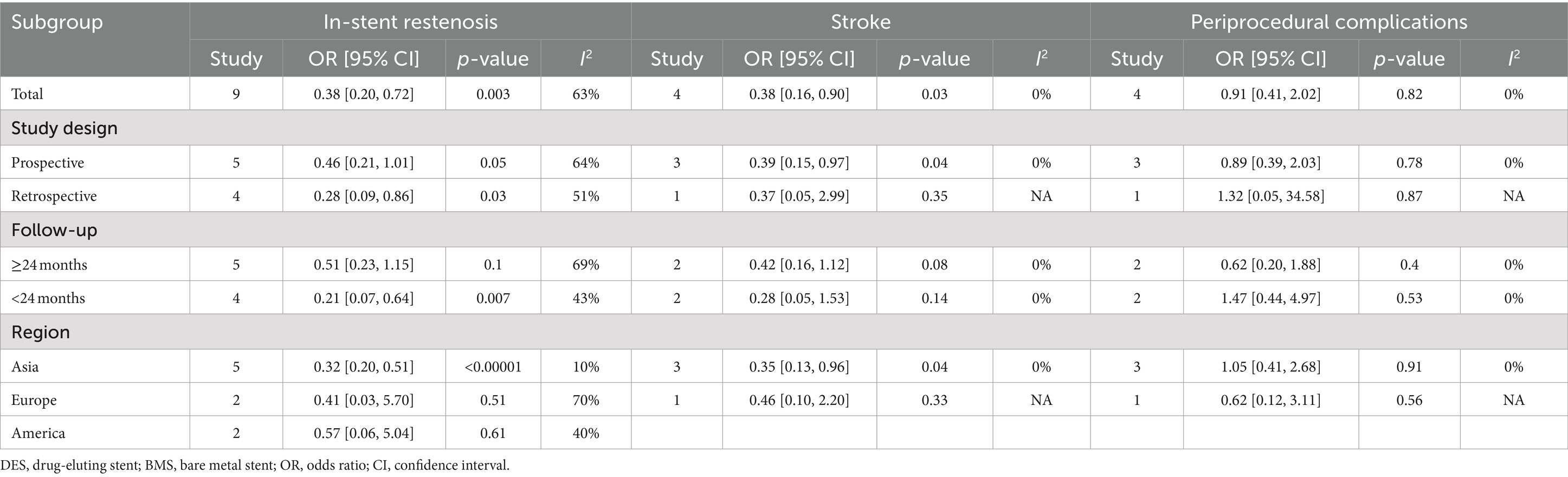
Findings regarding in-stent restenosis for vertebral artery stenosis were synthesized from 9 studies, which included 1,263 patients (575 DES versus 688 BMS) (20, 22, 23, 31–33, 35–37). A significant reduction in the rate of in-stent restenosis within the DES group was revealed by the meta-analysis (OR: 0.38; 95% CI: 0.20, 0.72; p = 0.003), along with significant heterogeneity (I2 = 63%, p = 0.006) (Figure 3B). Significance persisted in prospective studies, retrospective studies, and studies with a follow-up period <24 months, as well as in Asian studies according to subgroup analysis. However, it disappeared in studies with a follow-up period ≥24 months, as well as in European and American studies (Table 5).
3.3 Symptomatic in-stent restenosis
Two studies, including 81 patients (49 with DES and 32 with BMS), were conducted to synthesize data on symptomatic in-stent restenosis for vertebral artery stenosis (20, 32). No statistically significant difference was detected in the rate of symptomatic in-stent restenosis between the DES and BMS groups (OR: 0.12; 95% CI: 0.01, 1.06; p = 0.06), with no notable heterogeneity observed (I2 = 0%, p = 0.68) (Figure 3C).
3.4 Technical success
Results of technical success for intracranial artery stenosis were synthesized from two studies, which included 429 patients (214 treated with DES and 215 with BMS) (18, 21). No significant difference was found between the DES and BMS group for technical success rate, with an odds ratio of 1.56 (95% CI: 0.62, 3.91; p = 0.34). Additionally, no significant heterogeneity was observed (I2 = 12%, p = 0.29) (Figure 4A).
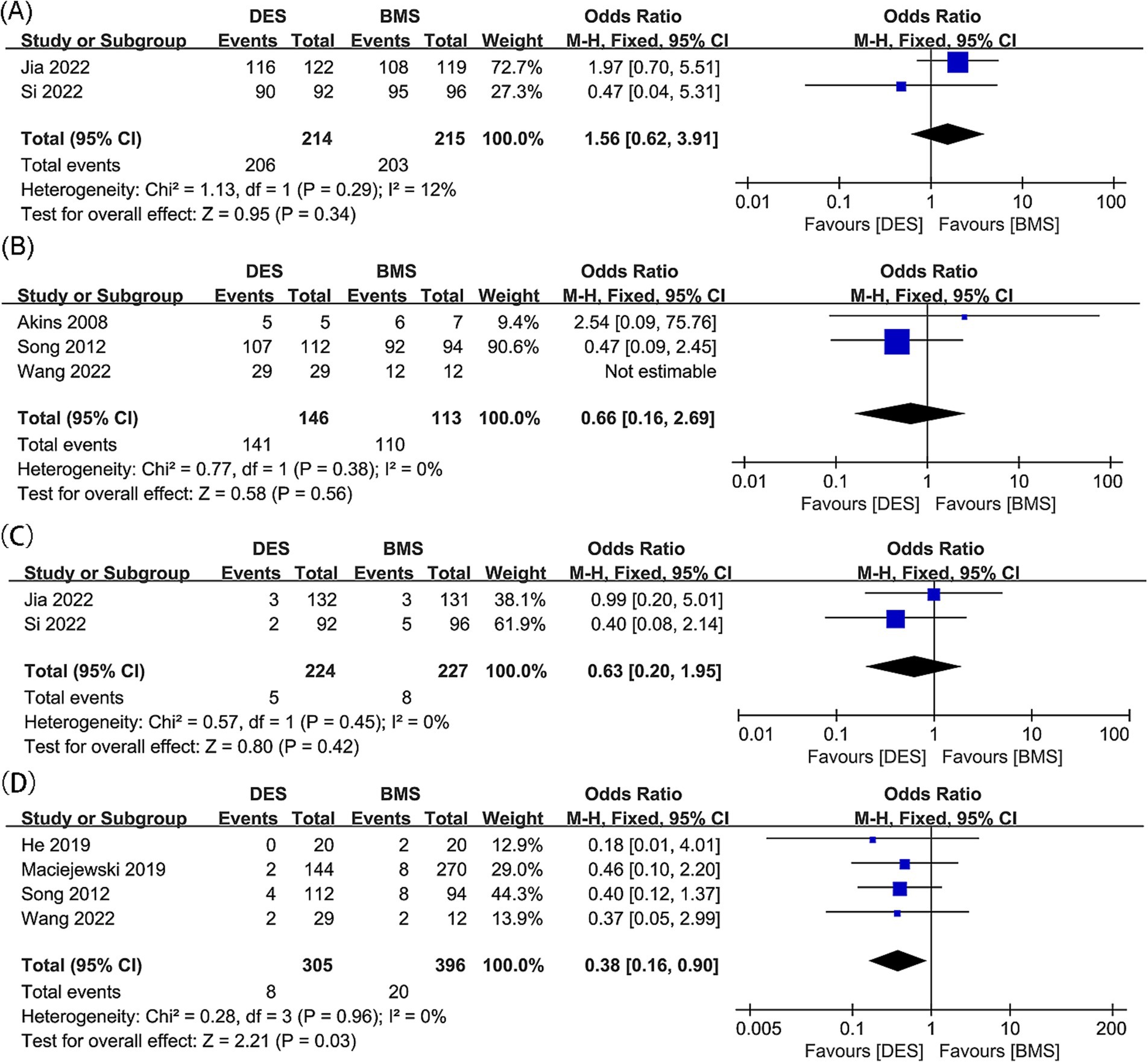
Figure 4. Forest plots of (A) technical success (intracranial artery stenosis), (B) technical success (vertebral artery stenosis), (C) postoperative stroke (intracranial artery stenosis), and (D) postoperative stroke (vertebral artery stenosis).
Technical success outcomes for vertebral artery stenosis were synthesized from three studies, with 259 patients included (146 DES versus 113 BMS) (20, 23, 31). No significant difference was found between the DES and BMS groups for technical success rate (OR: 0.66; 95% CI: 0.16, 2.69; p = 0.56), and no significant heterogeneity was observed (I2 = 0%, p = 0.38) (Figure 4B).
3.5 Postoperative stroke
Postoperative stroke outcomes for intracranial artery stenosis were collated from two studies, which included 451 patients (224 treated with DES versus 227 with BMS) (18, 21). There was no significant difference observed in the postoperative stroke rates between the DES and BMS groups (OR: 0.63; 95% CI: 0.20, 1.95; p = 0.42). Additionally, no significant heterogeneity was detected (I2 = 0%, p = 0.45) (Figure 4C).
No significant difference was found between the DES and BMS groups for postoperative stroke rate, with no significant heterogeneity observed (OR: 0.63; 95% CI: 0.20, 1.95; p = 0.42; I2 = 0%, p = 0.45) (20, 23, 32, 36). In the DES group, meta-analysis revealed a significant lower postoperative stroke rate (OR: 0.38; 95% CI: 0.16, 0.90; p = 0.03) without significant heterogeneity (I2 = 0%, p = 0.96) (Figure 4D). Subgroup analysis revealed that significance persisted in prospective and Asian studies, while it diminished in retrospective studies, as well as studies with follow-up periods of both less than 24 months and 24 months or more. Additionally, the significance was absent in European and American studies (Table 5).
3.6 Mortality
Mortality outcomes for intracranial artery stenosis were synthesized from two studies, which included 451 patients (224 with DES versus 227 with BMS) (18, 21). No significant difference was found in mortality between the DES and BMS groups (OR: 0.75; 95% CI: 0.17, 3.40; p = 0.71), and no significant heterogeneity was observed (I2 = 0%, p = 0.78) (Figure 5A).

Figure 5. Forest plots of (A) mortality (intracranial artery stenosis), (B) mortality (vertebral artery stenosis), (C) adverse events (intracranial artery stenosis), (D) adverse events (vertebral artery stenosis), and (E) periprocedural complications (vertebral artery stenosis).
Results of mortality for vertebral artery stenosis were synthesized from 2 studies, which included 218 patients (117 DES versus 101 BMS) (23, 31). No significant difference in mortality rates between the DES and BMS groups was observed (OR: 0.67; 95% CI: 0.29, 1.52; p = 0.34), and there was no significant heterogeneity noted (I2 = 0%, p = 0.44) (Figure 5B).
3.7 Adverse events
Adverse event outcomes related to intracranial artery stenosis were synthesized from two studies, which included 451 patients (224 with DES versus 227 with BMS) (18, 21). No significant difference was found between the DES and BMS groups for adverse events (OR: 0.63; 95% CI: 0.20, 1.95; p = 0.42), and no significant heterogeneity (I2 = 0%, p = 0.45) was observed (Figure 5C).
Results of adverse events for vertebral artery stenosis were synthesized from two studies, which included 80 patients (36 receiving DES versus 44 receiving BMS) (32, 33). In the DES group, a meta-analysis revealed a significantly lower rate of adverse events (OR: 0.08; 95% CI: 0.02, 0.38; p = 0.001), with no significant heterogeneity detected (I2 = 0%, p = 0.83) (Figure 5D).
3.8 Periprocedural complications
Four studies, comprising 811 patients (361 with DES and 450 with BMS), were analyzed to synthesize data on periprocedural complications related to vertebral artery stenosis (20, 23, 35, 36). No significant difference in periprocedural complications rate was observed between the DES and BMS groups (OR: 0.91; 95% CI: 0.41, 2.02; p = 0.82), with no significant heterogeneity detected (I2 = 0%, p = 0.78) (Figure 5E). Within all subgroups, the results consistently showed insignificance in the subgroup analysis (Table 5).
3.9 Publication bias
For in-stent restenosis (intracranial artery stenosis), in-stent restenosis (vertebral artery stenosis), postoperative stroke (vertebral artery stenosis), and periprocedural complications (vertebral artery stenosis), we examined potential publication bias using funnel plots and Egger’s regression tests. Both statistical (Egger’s test) and visual (funnel plots) analyses detected no evidence of publication bias for in-stent restenosis in the intracranial artery (Egger’s test p = 1.000) (Figure 6A), in-stent restenosis in the vertebral artery (Egger’s test p = 0.050) (Figure 6B), postoperative stroke in the vertebral artery (Egger’s test p = 0.202) (Figure 6C), and periprocedural complications in the vertebral artery (Egger’s test p = 0.944) (Figure 6D).
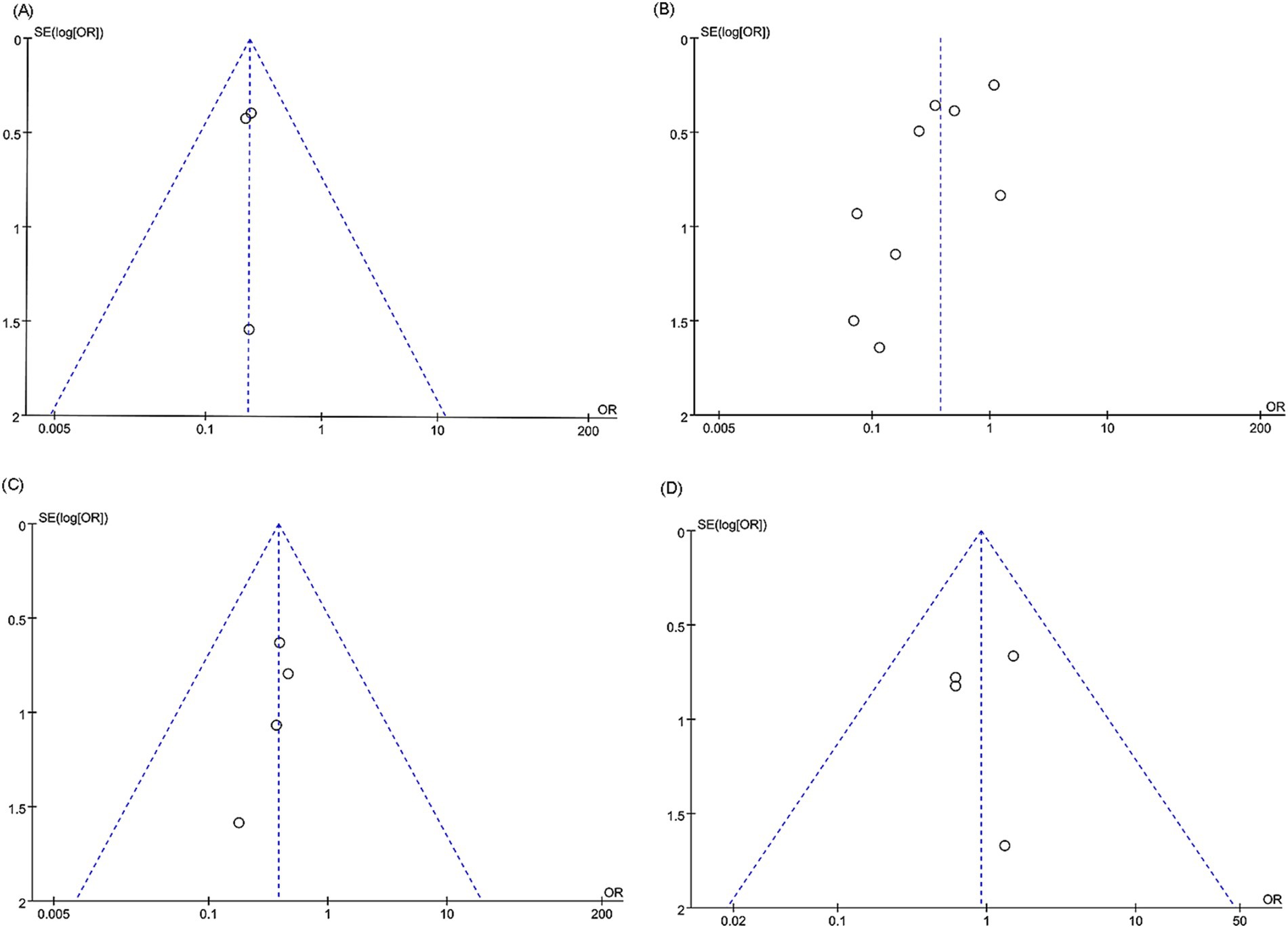
Figure 6. Funnel plots of (A) in-stent restenosis (intracranial artery stenosis), (B) in-stent restenosis (vertebral artery stenosis), (C) postoperative stroke (vertebral artery stenosis), and (D) periprocedural complications (vertebral artery stenosis).
3.10 Sensitivity analysis
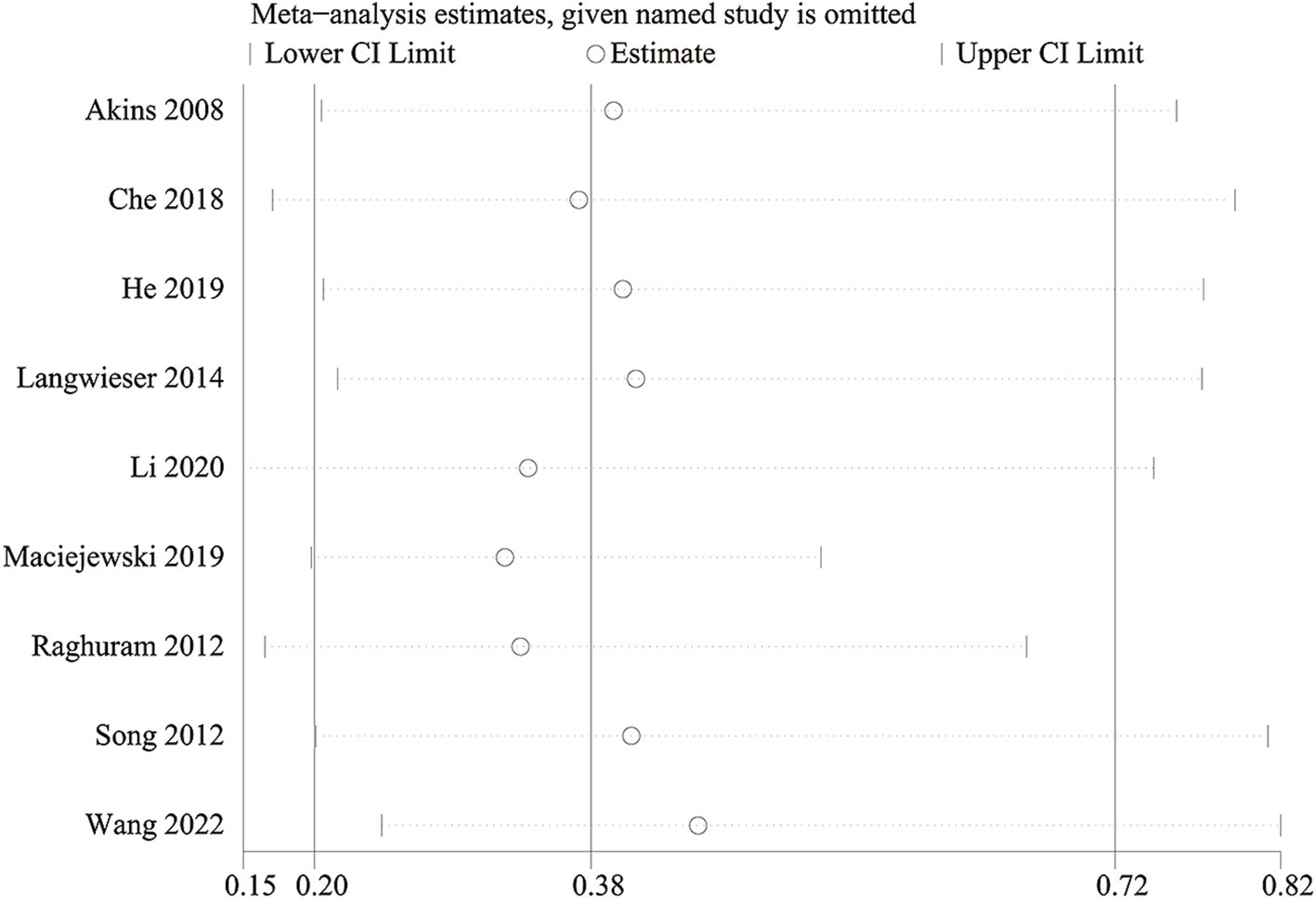
We conducted a sensitivity analysis to evaluate the impact of each study on the overall OR for in-stent restenosis (specifically focusing on vertebra artery stenosis) by systematically excluding eligible studies one at a time. Our analysis revealed that even with the removal of each individual study, the total OR for in-stent restenosis (vertebral artery stenosis) remained consistent (Figure 7). However, upon removal of the study reported by Maciejewski et al. (36), the heterogeneity of in-stent restenosis (specifically, vertebral artery stenosis) decreased from 63 to 16%, suggesting that this paper might have been the main contributor to the significant heterogeneity observed in in-stent restenosis (vertebral artery stenosis).
4 Discussion
Stenting is considered inferior to aggressive medical therapy as a potential management strategy for intracranial or vertebral artery stenosis with impaired blood flow, primarily due to the high adverse event rates linked with stenting (13, 17, 39). In-stent restenosis presents another major obstacle to successful stenting, often resulting in non-procedural ischemic events (40, 41). In patients receiving the recent standard of self-expanding or balloon-installed BMS, in-stent restenosis appears within 1 year in 15 to 33% of cases (42–45). After stent implantation, DES reduce in-stent restenosis by restraining the proliferation and migration of endothelial cells and smooth muscle cells (46). In the treatment of arterial stenosis, DES has changed the status by reducing in-stent restenosis and associated ischemic events (47, 48). DES has been recommended as the standard device for percutaneous coronary intervention rather than BMS recently (49). However, the debate continues regarding whether DES outperforms BMS in terms of efficacy and safety for intracranial and vertebral artery stenosis.
To our knowledge, this is the first meta-analysis comparing DES and BMS for intracranial and vertebral artery stenosis. Despite differences in the design and type of stent used, similar results to some previous studies were shown in this study (18–21, 35). Among patients with stenosis in the intracranial and vertebral arteries, restenosis has been reported as a key factor affecting the long-term efficacy of treatment (20). Our study findings show a significant reduction in in-stent restenosis rates among patients with intracranial or vertebral artery stenosis who underwent treatment with DES compared to those who received BMS. The findings of our study are consistent with those of two previously published meta-analyses, which suggest that patients with symptomatic extracranial vertebral artery stenosis had a higher incidence of restenosis in the BMS group compared to DES (50, 51). Additionally, in two large case series, a higher risk of restenosis was found to be related to BMS in patients with vertebral artery stenosis (22, 52). The mechanism for the higher rate of restenosis in the BMS group is unclear. Studies have shown that the two main causes of restenosis in BMS may be recoil and intimal hyperplasia (53, 54). It is worth mentioning that, unlike DES, BMS does not apply antiproliferative medications that restrict smooth muscle cell proliferation. Therefore, it can be speculated that the deficiency of inhibition of intimal hyperplasia in the BMS group resulted in the most severe stenosis advancement and the highest restenosis rate during follow-up in the BMS group.
Furthermore, our meta-analysis found a significant lower postoperative stroke rate in the DES group for vertebral artery stenosis, while no significant difference was observed between the DES and BMS group for intracranial artery stenosis. Despite all four articles addressing postoperative stroke (vertebral artery stenosis) and reporting non-significant differences between DES and BMS, the outcomes of the studies were all skewed in favor of the DES group. After conducting pooled analysis, it was ultimately determined that the DES cohort exhibited a reduced incidence of postoperative stroke, with no notable heterogeneity observed as a result. This discovery could be attributed to the reduced incidence of in-stent stenosis observed in the DES cohort. Our study found no significant difference, generally, in mortality, adverse effects, and perioperative complications between the DES group and the BMS group. Despite the meta-analysis indicating a significant reduction in adverse events rates within the DES group compared to the BMS group for vertebral artery stenosis, it’s important to acknowledge that only two original studies contributed to this finding. Therefore, further research is essential to corroborate these results. In addition, subgroup analysis found that the advantage of DES in restenosis rate disappeared when the follow-up time exceeded 24 months. This finding may be related to the characteristics of DES. As time goes by, the dose of drugs released by DES will gradually decrease, and its average service life is 3–10 years, so its long-term treatment effect may not be significantly better than BMS (55). In addition, although DES can reduce the restenosis rate, long-term drug release and polymer stimulation may increase the risk of thrombosis, which will also affect the long-term efficacy (55).
We must acknowledge several limitations of this meta-analysis. Firstly, all included RCTs reported a high risk in the blinding of participants and personnel because of the deficiency of feasibility of blinding for the type of stents used, and only 1 of 3 included RCTs had low risk in the allocation concealment, which may lead to some bias. Secondly, most of the original studies on intracranial arterial stenosis included in this meta-analysis did not consider the separation of patients with anterior and posterior circulation. Due to the different natural history of arterial stenosis in the anterior and posterior circulation, the operation method, clinical prognosis and restenosis rate after stenting are also different. Thirdly, we conducted subgroup analysis on some outcomes according to the study design, follow-up time and region, and the subgroup analysis found that the results were not stable in different subgroups, suggesting that this study still had a certain degree of heterogeneity, although most the results are reported as non-significant Cochran’s Q p-values. In addition, the drug eluting inhibitors involved in this article mainly include tacrolimus, sirolimus and paclitaxel, which may be one of the sources of heterogeneity. However, there are few literatures reporting specific drug eluting inhibitors, and we cannot perform subgroup analysis based on this factor to explore its impact on the results. Besides, in all included literature, the most common pre- and post-implantation treatment drugs included aspirin, clopidogrel, and ticlopidin. However, because the patients included in each study had different disease courses and characteristics, the pre- and post-implantation drug doses and treatment courses were very heterogeneous among the studies, and subgroup analysis could not be performed, although we believe that different antiplatelet regimens will affect the treatment success rate and long-term prognosis of patients. Despite several limitations of this meta-analysis, we conducted the first meta-analysis comparing DES and BMS for intracranial and vertebral artery stenosis. Results of this meta-analysis validated the superiority of the DES intracranial and vertebral artery stenosis reported by previous studies.
5 Conclusion
Pooled analyses have revealed that DES, when compared to BMS, exhibit a significant reduction in the risk of in-stent restenosis (including intracranial and vertebral artery stenosis) as well as postoperative stroke (particularly in cases of vertebral artery stenosis). However, this superiority appears to be limited to a relatively short follow-up period. Generally, DES and BMS demonstrate similar safety profiles for intracranial and vertebral artery stenosis. To further evaluate the efficacy and safety of drug-eluting stents versus bare-metal stents in patients with symptomatic intracranial and vertebral artery stenosis, additional large-scale, multi-center, double-blind RCTs are warranted.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YZ: Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – original draft. WL: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. LZ: Conceptualization, Formal analysis, Funding acquisition, Project administration, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Funds of China (Grant No. 81971098) to LZ.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1389254/full#supplementary-material
References
1. Leng, X, Hurford, R, Feng, X, Chan, KL, Wolters, FJ, Li, L, et al. Intracranial arterial stenosis in Caucasian versus Chinese patients with TIA and minor stroke: two contemporaneous cohorts and a systematic review. J Neurol Neurosurg Psychiatry. (2021) 92:590–7. doi: 10.1136/jnnp-2020-325630
2. Hurford, R, Wolters, FJ, Li, L, Lau, KK, Küker, W, and Rothwell, PM. Prevalence, predictors, and prognosis of symptomatic intracranial stenosis in patients with transient ischaemic attack or minor stroke: a population-based cohort study. Lancet Neurol. (2020) 19:413–21. doi: 10.1016/S1474-4422(20)30079-X
3. Wang, Y, Zhao, X, Liu, L, Soo, YO, Pu, Y, Pan, Y, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke. (2014) 45:663–9. doi: 10.1161/STROKEAHA.113.003508
4. Qureshi, AI, and Caplan, LR. Intracranial atherosclerosis. Lancet. (2014) 383:984–98. doi: 10.1016/S0140-6736(13)61088-0
5. Holmstedt, CA, Turan, TN, and Chimowitz, MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol. (2013) 12:1106–14. doi: 10.1016/S1474-4422(13)70195-9
6. Thompson, MC, Issa, MA, Lazzaro, MA, and Zaidat, OO. The natural history of vertebral artery origin stenosis. J Stroke Cerebrovasc Dis. (2014) 23:e1–4. doi: 10.1016/j.jstrokecerebrovasdis.2012.12.004
7. Wityk, RJ, Chang, HM, Rosengart, A, Han, WC, DeWitt, LD, Pessin, MS, et al. Proximal extracranial vertebral artery disease in the New England Medical Center Posterior Circulation Registry. Arch Neurol. (1998) 55:470–8. doi: 10.1001/archneur.55.4.470
8. Kim, YJ, Lee, JH, Choi, JW, Roh, HG, Chun, YI, Lee, JS, et al. Long-term outcome of vertebral artery origin stenosis in patients with acute ischemic stroke. BMC Neurol. (2013) 13:171. doi: 10.1186/1471-2377-13-171
9. Gulli, G, Marquardt, L, Rothwell, PM, and Markus, HS. Stroke risk after posterior circulation stroke/transient ischemic attack and its relationship to site of vertebrobasilar stenosis: pooled data analysis from prospective studies. Stroke. (2013) 44:598–604. doi: 10.1161/STROKEAHA.112.669929
10. Bamford, J, Sandercock, P, Dennis, M, Burn, J, and Warlow, C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. (1991) 337:1521–6. doi: 10.1016/0140-6736(91)93206-O
11. Kleindorfer, DO, Towfighi, A, Chaturvedi, S, Cockroft, KM, Gutierrez, J, Lombardi-Hill, D, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. (2021) 52:e364–467. doi: 10.1161/STR.0000000000000375
12. Liu, L, Wong, KS, Leng, X, Pu, Y, Wang, Y, Jing, J, et al. Dual antiplatelet therapy in stroke and ICAS: subgroup analysis of CHANCE. Neurology. (2015) 85:1154–62. doi: 10.1212/WNL.0000000000001972
13. Chimowitz, MI, Lynn, MJ, Derdeyn, CP, Turan, TN, Fiorella, D, Lane, BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. (2011) 365:993–1003. doi: 10.1056/NEJMoa1105335
14. Leng, X, Lan, L, Ip, HL, Abrigo, J, Scalzo, F, Liu, H, et al. Hemodynamics and stroke risk in intracranial atherosclerotic disease. Ann Neurol. (2019) 85:752–64. doi: 10.1002/ana.25456
15. Sangha, RS, Naidech, AM, Corado, C, Ansari, SA, and Prabhakaran, S. Challenges in the medical management of symptomatic intracranial stenosis in an urban setting. Stroke. (2017) 48:2158–63. doi: 10.1161/STROKEAHA.116.016254
16. Wabnitz, AM, Derdeyn, CP, Fiorella, DJ, Lynn, MJ, Cotsonis, GA, Liebeskind, DS, et al. Hemodynamic markers in the anterior circulation as predictors of recurrent stroke in patients with intracranial stenosis. Stroke. (2018) 50:143–7. doi: 10.1161/STROKEAHA.118.020840
17. Derdeyn, CP, Chimowitz, MI, Lynn, MJ, Fiorella, D, Turan, TN, Janis, LS, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet. (2014) 383:333–41. doi: 10.1016/S0140-6736(13)62038-3
18. Si, JH, Ma, N, Gao, F, Mo, DP, Luo, G, and Miao, ZR. Effect of a drug-eluting stent vs. bare metal stent for the treatment of symptomatic intracranial and vertebral artery stenosis. Front Neurol. (2022) 13:854226. doi: 10.3389/fneur.2022.854226
19. Wu, S, Yin, Y, Li, Z, Li, N, Ma, W, and Zhang, L. Using drug-coated balloons for symptomatic vertebral artery origin stenosis: a systematic review and meta-analysis. J Clin Neurosci. (2023) 107:98–105. doi: 10.1016/j.jocn.2022.12.004
20. Wang, Z, Ling, Y, Zhao, H, Mao, Y, Dong, Q, and Cao, W. A comparison of different endovascular treatment for vertebral artery origin stenosis. World Neurosurg. (2022) 164:e1290–7. doi: 10.1016/j.wneu.2022.06.026
21. Jia, B, Zhang, X, Ma, N, Mo, D, Gao, F, Sun, X, et al. Comparison of drug-eluting stent with bare-metal stent in patients with symptomatic high-grade intracranial atherosclerotic stenosis: a randomized clinical trial. JAMA Neurol. (2022) 79:176–84. doi: 10.1001/jamaneurol.2021.4804
22. Che, WQ, Dong, H, Jiang, XJ, Peng, M, Zou, YB, Xiong, HL, et al. Clinical outcomes and influencing factors of in-stent restenosis after stenting for symptomatic stenosis of the vertebral V1 segment. J Vasc Surg. (2018) 68:1406–13. doi: 10.1016/j.jvs.2018.02.042
23. Song, L, Li, J, Gu, Y, Yu, H, Chen, B, Guo, L, et al. Drug-eluting vs. bare metal stents for symptomatic vertebral artery stenosis. J Endovasc Ther. (2012) 19:231–8. doi: 10.1583/11-3718.1
24. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
25. Wan, X, Wang, W, Liu, J, and Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
26. Luo, D, Wan, X, Liu, J, and Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27:1785–805. doi: 10.1177/0962280216669183
27. Wells, G, Shea, B, O’Connell, D, Peterson, J, Welch, V, Losos, M, et al. (2011) The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. (Accessed December 28, 2020)
28. Kim, SR, Kim, K, Lee, SA, Kwon, SO, Lee, JK, Keum, N, et al. Effect of red, processed, and white meat consumption on the risk of gastric cancer: an overall and dose-response meta-analysis. Nutrients. (2019) 11:826. doi: 10.3390/nu11040826
29. Cumpston, M, Li, T, Page, MJ, Chandler, J, Welch, VA, Higgins, JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. (2019) 10:ED000142. doi: 10.1002/14651858.ED000142
30. Higgins, JP, and Thompson, SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
31. Akins, PT, Kerber, CW, and Pakbaz, RS. Stenting of vertebral artery origin atherosclerosis in high-risk patients: bare or coated? A single-center consecutive case series. J Invasive Cardiol. (2008) 20:14–20.
32. He, Y, Li, T, Bai, W, Zhu, L, Wang, M, and Zhang, Y. Cerebrovascular drug-eluting stent versus bare-metal stent in the treatment of vertebral artery stenosis: a non-inferiority randomized clinical trial. J Stroke. (2019) 21:101–4. doi: 10.5853/jos.2018.00479
33. Langwieser, N, Prothmann, S, Buyer, D, Poppert, H, Schuster, T, Fusaro, M, et al. Safety and efficacy of different stent types for the endovascular therapy of extracranial vertebral artery disease. Clin Res Cardiol. (2014) 103:353–62. doi: 10.1007/s00392-013-0659-x
34. Lee, JH, Jo, SM, Jo, KD, Kim, MK, Lee, SY, and You, SH. Comparison of drug-eluting coronary stents, bare coronary stents and self-expanding stents in angioplasty of middle cerebral artery stenoses. J Cerebrovasc Endovasc Neurosurg. (2013) 15:85–95. doi: 10.7461/jcen.2013.15.2.85
35. Li, L, Wang, X, Yang, B, Wang, Y, Gao, P, Chen, Y, et al. Validation and comparison of drug eluting stent to bare metal stent for restenosis rates following vertebral artery ostium stenting: a single-center real-world study. Interv Neuroradiol. (2020) 26:629–36. doi: 10.1177/1591019920949371
36. Maciejewski, DR, Pieniazek, P, Tekieli, L, Paluszek, P, Przewlocki, T, Tomaszewski, T, et al. Comparison of drug-eluting and bare metal stents for extracranial vertebral artery stenting. Postepy Kardiol Interwencyjnej. (2019) 15:328–37. doi: 10.5114/aic.2019.87887
37. Raghuram, K, Seynnaeve, C, and Rai, AT. Endovascular treatment of extracranial atherosclerotic disease involving the vertebral artery origins: a comparison of drug-eluting and bare-metal stents. J Neurointerv Surg. (2012) 4:206–10. doi: 10.1136/neurintsurg-2011-010051
38. Egger, M, Davey Smith, G, Schneider, M, and Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
39. Zaidat, OO, Fitzsimmons, BF, Woodward, BK, Wang, Z, Killer-Oberpfalzer, M, Wakhloo, A, et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA. (2015) 313:1240–8. doi: 10.1001/jama.2015.1693
40. Derdeyn, CP, Fiorella, D, Lynn, MJ, Turan, TN, Cotsonis, GA, Lane, BF, et al. Nonprocedural symptomatic infarction and in-stent restenosis after intracranial angioplasty and stenting in the SAMMPRIS trial (Stenting and Aggressive Medical Management for the Prevention of Recurrent Stroke in Intracranial Stenosis). Stroke. (2017) 48:1501–6. doi: 10.1161/STROKEAHA.116.014537
41. Jin, M, Fu, X, Wei, Y, Du, B, Xu, XT, and Jiang, WJ. Higher risk of recurrent ischemic events in patients with intracranial in-stent restenosis. Stroke. (2013) 44:2990–4. doi: 10.1161/STROKEAHA.113.001824
42. Kang, K, Zhang, Y, Shuai, J, Jiang, C, Zhu, Q, Chen, K, et al. Balloon-mounted stenting for ICAS in a multicenter registry study in China: a comparison with the WEAVE/WOVEN trial. J Neurointerv Surg. (2021) 13:894–9. doi: 10.1136/neurintsurg-2020-016658
43. Peng, G, Zhang, Y, and Miao, Z. Incidence and risk factors of in-stent restenosis for symptomatic intracranial atherosclerotic stenosis: a systematic review and meta-analysis. AJNR Am J Neuroradiol. (2020) 41:1447–52. doi: 10.3174/ajnr.A6689
44. Zhang, L, Huang, Q, Zhang, Y, Liu, J, Hong, B, Xu, Y, et al. Wingspan stents for the treatment of symptomatic atherosclerotic stenosis in small intracranial vessels: safety and efficacy evaluation. AJNR Am J Neuroradiol. (2012) 33:343–7. doi: 10.3174/ajnr.A2772
45. Levy, EI, Turk, AS, Albuquerque, FC, Niemann, DB, Aagaard-Kienitz, B, Pride, L, et al. Wingspan in-stent restenosis and thrombosis: incidence, clinical presentation, and management. Neurosurgery. (2007) 61:644–51. doi: 10.1227/01.NEU.0000290914.24976.83
46. Inoue, T, and Node, K. Molecular basis of restenosis and novel issues of drug-eluting stents. Circ J. (2009) 73:615–21. doi: 10.1253/circj.CJ-09-0059
47. Stone, GW, Ellis, SG, Cox, DA, Hermiller, J, O'Shaughnessy, C, Mann, JT, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. (2004) 350:221–31. doi: 10.1056/NEJMoa032441
48. Moses, JW, Leon, MB, Popma, JJ, Fitzgerald, PJ, Holmes, DR, O'Shaughnessy, C, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. (2003) 349:1315–23. doi: 10.1056/NEJMoa035071
49. Neumann, FJ, Sousa-Uva, M, Ahlsson, A, Alfonso, F, Banning, AP, Benedetto, U, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. (2019) 40:87–165. doi: 10.1093/eurheartj/ehy394
50. Tank, VH, Ghosh, R, Gupta, V, Sheth, N, Gordon, S, He, W, et al. Drug eluting stents versus bare metal stents for the treatment of extracranial vertebral artery disease: a meta-analysis. J Neurointerv Surg. (2016) 8:770–4. doi: 10.1136/neurintsurg-2015-011697
51. Langwieser, N, Buyer, D, Schuster, T, Haller, B, Laugwitz, KL, and Ibrahim, T. Bare metal vs. drug-eluting stents for extracranial vertebral artery disease: a meta-analysis of nonrandomized comparative studies. J Endovasc Ther. (2014) 21:683–92. doi: 10.1583/14-4713MR.1
52. Li, J, Hua, Y, Needleman, L, Forsberg, F, Eisenbray, JR, Li, Z, et al. Arterial occlusions increase the risk of in-stent restenosis after vertebral artery ostium stenting. J Neurointerv Surg. (2019) 11:574–8. doi: 10.1136/neurintsurg-2018-014243
53. Stayman, AN, Nogueira, RG, and Gupta, R. A systematic review of stenting and angioplasty of symptomatic extracranial vertebral artery stenosis. Stroke. (2011) 42:2212–6. doi: 10.1161/STROKEAHA.110.611459
54. Albuquerque, FC, Fiorella, D, Han, P, Spetzler, RF, and McDougall, CG. A reappraisal of angioplasty and stenting for the treatment of vertebral origin stenosis. Neurosurgery. (2003) 53:607–16. doi: 10.1227/01.NEU.0000079494.87390.28
Keywords: drug-eluting stent, bare-metal stent, intracranial artery stenosis, vertebral artery stenosis, meta-analysis
Citation: Zhang Y, Li W and Zhang L (2024) Efficacy and safety of drug-eluting stents versus bare-metal stents in symptomatic intracranial and vertebral artery stenosis: a meta-analysis. Front. Neurol. 15:1389254. doi: 10.3389/fneur.2024.1389254
Edited by:
Yin Huang, Sichuan University, ChinaReviewed by:
Marie-Sophie Schüngel, University Hospital in Halle, GermanyDehong Cao, Sichuan University, China
Copyright © 2024 Zhang, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Zhang, emhhbmdsOTJAc3lzdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Yidan Zhang1†
Yidan Zhang1† Wenbin Li
Wenbin Li