- 1Department of Experimental Medicine, University of Campania “Luigi Vanvitelli”, Naples, Italy
- 2Regional Center of Pharmacovigilance and Pharmacoepidemiology of Campania Region, Naples, Italy
- 3Eu2P Programme, University Bordeaux, Bordeaux, France
- 4Multiple Sclerosis Regional Center, “A. Cardarelli” Hospital, Naples, Italy
- 5Neurological Clinic and Stroke Unit, “A. Cardarelli” Hospital, Naples, Italy
Introduction: Disease modifying therapies (DMTs) used to treat multiple sclerosis (MS) can be associated to the occurrence of hematological disorders. This systematic review aims to provide an overview of these events occurring in real-life conditions, by describing case reports and series published in the literature.
Methods: A literature search of all publications up to January 5th 2024 on the Medline and Embase databases was carried out. The results were presented both in the text and in tables.
Results: Sixty-seven case reports/series were included in this review, of which more than half related to alemtuzumab, natalizumab and ocrelizumab. The publication date of included studies ranged from 2006 to 2024. The majority of case reports and series described the occurrence of late-onset hematological disorders (events that occurred more than 30 days after the first DMT administration), mainly represented by case of neutropenia, autoimmune hemolytic anemia and immune thrombocytopenia. All cases reported a favorable outcome, apart one case report that described a fatal case. Among included cases, 4 articles, all related to natalizumab, described the occurrence of myeloid disorders in 13 newborns from mother receiving the DMT.
Discussion: Considering the limitations identified in the majority of included studies, further ad hoc studies are strongly needed to better evaluate the hematological disorders of DMTs. Meantime, the strict monitoring of treated patients for the occurrence of these toxicities should be highly recommended.
1 Introduction
Multiple sclerosis (MS) is a chronic, autoimmune disease affecting the central nervous system (CNS) which is the result of an immune dysregulation associated with genetic and environmental factors (1). Clinically isolated syndrome (CIS) refers to a first episode of neurologic symptoms (that might include optic neuritis, vertigo, weakness in the arms and legs, difficulty with coordination, balance, walking, speaking and ataxia) caused by inflammation in the CNS that could become MS if additional activity occurs (2). According to the 2017 revision of McDonald criteria (3), the diagnosis of MS can be made in patients with CIS and clinical or MRI demonstration of dissemination in space, and in presence of Cerebrospinal Fluid (CSF)-specific oligoclonal bands. Once MS is diagnosed, different subtypes can be distinguished, including relapsing remitting MS (RRMS), primary progressive MS (PPMS) and secondary progressive MS (SPMS). Among these forms, RRMS is the most common subtype (4). Based on this classification, RRMS is characterized by periods between relapses that are free of worsening, while MS progressive forms present a period during which patients exhibit continuous decline of neurological functions. However, as recently reported by Granziera et al. (5) steady progression independent of relapse activity (PIRA) is a frequently identified in RRMS. Recent data from an open-source global compendium on MS epidemiology reported that 2.8 million people are estimated to live with MS worldwide (35.9 per 100,000 population) and that since 2013 MS prevalence has increased globally (including among pediatric population). The pooled incidence rate is 2.1 per 100,000 persons/year (6). The mean age at diagnosis is 32 years, and the combined incidence rate among the 75 reporting nations is 2.1 per 100,000 people/year. The disease is twice as common in women as it is in men, although the ratio of women to men is as high as 4:1 in some countries (6, 7).
The pharmacological management of MS foresees the use of disease-modifying therapies (DMTs) that are able to reduce the number of relapses and delay disease’s progression (8). These drugs, which act on different biological pathways and show distinct efficacy/safety profiles, are classified as low/moderate- or high-efficacy treatment. Among low/moderate efficacy therapies are IFNBs, glatiramer acetate (GA), teriflunomide, and dimethyl fumarate (DMF). These therapies are generally safer than higher efficacy agents. On the other hand, monoclonal antibodies (ocrelizumab, natalizumab, alemtuzumab and ofatumumab), Sphingosine 1-phosphate receptor (S1PR) modulators (fingolimod, siponimod, ozanimod, and ponesimod), cladribine and mitoxantrone show higher efficacy profiles but they are also associated with greater risks of adverse drug reactions (ADRs) (9, 10). DMTs have distinct pharmacodynamics properties, resulting in immunomodulatory and anti-inflammatory response, and consequently impact individual efficacy and tolerability profiles. A recent study carried out by Barbieri MA et al. on data from the Italian Pharmacovigilance database highlighted that the most reported DMTs-induced ADRs were general and administration site conditions, followed by nervous, skin and blood disorders (11). Among blood disorders, myelosuppression, also defined as myelotoxicity or bone marrow suppression, represents a rare idiosyncratic ADR that can be associated with any drug, including DMTs. Myelotoxicities, which include anemia, leucopenia and thrombocytopenia, are potentially life-threatening events due to infection and bleeding complications of neutropenia and thrombocytopenia (12). As reported by Schweitzer et al., DMTs are able to selectively suppress or modulate the immune system leading to unwanted ADRs affecting leukocytes in peripheral blood (13).
In order to provide an overview of DMTs-induced hematological disorders in real-life conditions, we carried out an extensive systematic review of published case reports and case series. Our aims are to describe the main characteristics of DMTs-induced hematological disorders in terms of patients’ demographic, their medication history, suspected DMTs, seriousness, management and outcome of the event and time of onset of selected ADRs and to provide general evidence regarding the hematological profile of DMTs currently used to treat MS.
2 Materials and methods
2.1 Search strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guideline (14) and the Cochrane Handbook for Systematic Reviews of Interventions (v6.4) (15) were used to perform a standardized data search, extraction, reporting, and presentation.
Two authors (CS and VL) independently performed a literature search of all publications up to January 5th, 2024 on the Medline and Embase databases using the following keywords: multiple sclerosis AND (aplastic anemia OR anemia OR neutropenia OR thrombocytopenia OR myelosuppression OR pancytopenia) AND (disease-modifying therapies OR DMT OR DMTs OR alemtuzumab OR interferon beta-1b OR cladribine OR teriflunomide OR glatiramer acetate OR ofatumumab OR peginterferon beta-1a OR fingolimod OR siponimod OR ozanimod OR natalizumab OR ponesimod OR ocrelizumab OR dimethyl fumarate OR diroximel fumarate OR interferon beta-1a) AND (case report OR case series OR clinical case OR clinical cases).
2.2 PICOS/study selection
Population: patients diagnosed with MS according to Mc Donald diagnostic criteria (3); Interventions: treatment with a DMT approved for the MS (thus, excluding DMTs used in off-label conditions, such as rituximab); Comparators: none; Outcomes: occurrence of myelo-lymphoid ADRs such as aplastic anemia, anemia, neutropenia, thrombocytopenia, myelosuppression, pancytopenia (including hematologic autoimmune conditions such as Autoimmune hemolytic anemia - AIHA - and immune thrombocytopenia - ITP) in patients receiving a DMT; Study designs: case reports or case series.
Screening was based on reading the titles, abstracts and full-texts of the publications. Articles not in English language were excluded. We also excluded meta-analyses, reviews, meeting/conference abstracts, clinical trials and observational studies.
2.3 Data extraction and analysis
Data on selected articles were imported into MS Excel. Data were collected from the full-text publications relevant to DMTs-induced myeloid ADRs, including patient demographics, medication history for MS, time to event [(TTE), the intervening period of time from the first DMT administration to the occurrence of myeloid ADR], ADRs’ signs and symptoms, including relevant laboratory findings related to myeloid toxicity, seriousness and outcome.
2.4 Risk of bias assessment of individual studies
A quality assessment of the included studies was performed using the Joanna Briggs Institute (JBI) critical appraisal checklist for case reports and series (16). For neonatal cases the risk of bias assessment was not performed. Any discrepancies were resolved through discussion.
3 Results
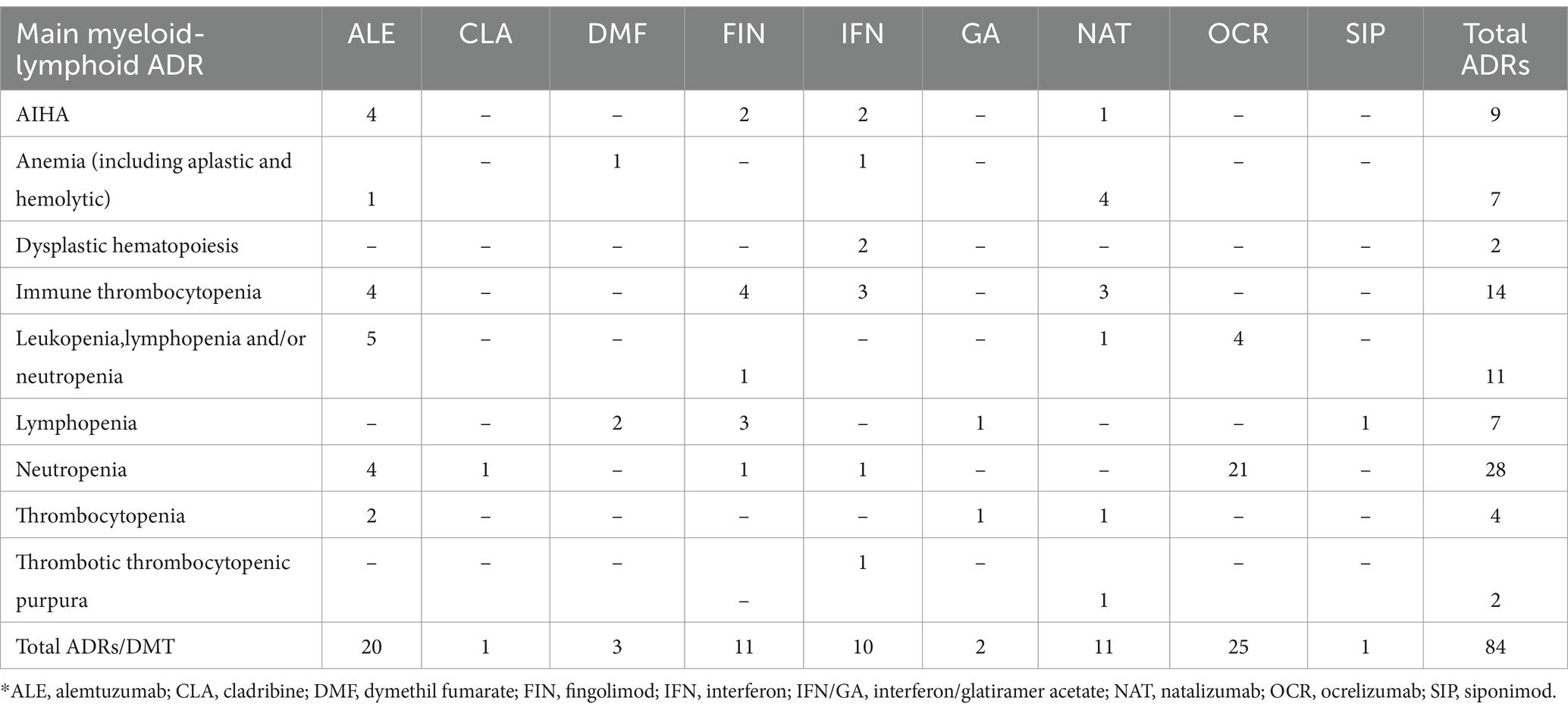
Our initial search yielded 135 results from Medline and 517 results from Embase. Following the screening of title, abstract and a more thorough examination of full text, 67 articles were included in this review (17–83), of which 56 case reports and 11 case series concerning overall 97 patients (84 adults and 13 newborns). Of included studies, 18 were related to alemtuzumab (n = 20 patients), 13 to natalizumab (n = 11 adults; n = 13 newborns), 13 to ocrelizumab (n = 25), 9 to IFN (n = 10), 7 to fingolimod (n = 11), 3 to DMF (n = 3), 1 to cladribine (n = 1), 1 to siponimod (n = 1), 1 to glatiramer acetate (n = 1) and 1 to the combined therapy GA and IFN-β 1a (n = 1) (Figure 1; Tables 1–3). Among these cases, 4 articles (29, 49, 50, 55), all related to natalizumab, described the occurrence of myelotoxicities in newborns from mother receiving the DMT. In the Supplementary Table S1 an overview of DMTs, including their routes and frequencies of administration, mechanisms of actions and data from clinical trials on the occurrence of hematological disorders are reported.
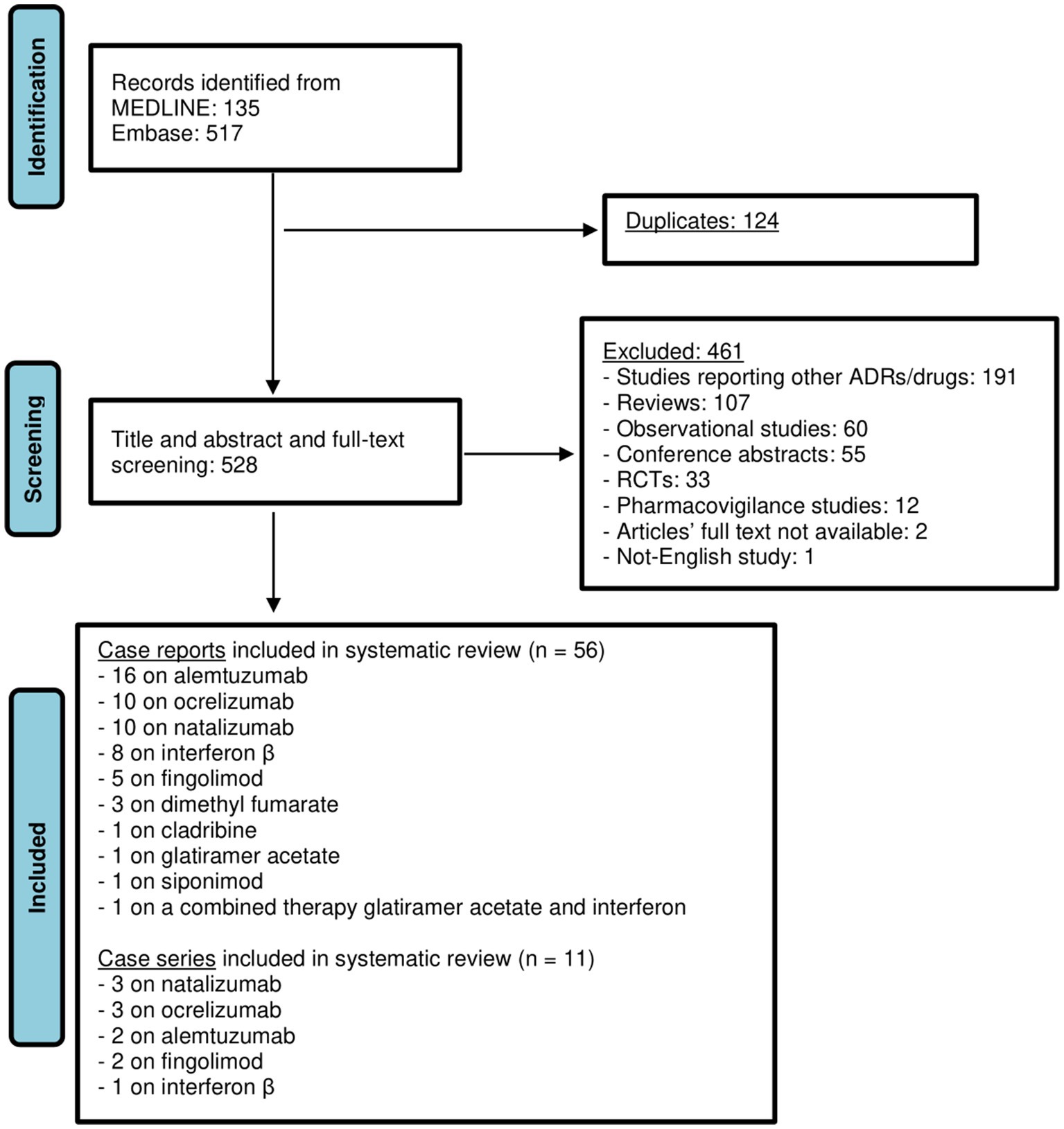
Figure 1. Flow chart illustrating literature search outcomes. From: Page MJ, McKenzia JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021:372:n71. doi: 10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/.

Table 3. Case reports describing the occurrence of myelotoxicities in newborns from mothers who received natalizumab during pregnancy.
The publication date of the included studies ranged from 2006 to 2023. The results of risk of bias assessment of included studies are shown in Supplementary Tables S2, S3 (for case reports and case series, respectively). Neonatal cases (29, 49, 50, 55) were not included in the risk of bias assessment. Fifty-three case reports and 10 case series were evaluated using the risk of bias assessment tool. Regarding case reports, 8 studies (33, 37, 42, 58, 67, 70, 75, 81) had the highest methodological quality, together with 3 other studies (30, 43, 56) that similarly reported high quality information. For the remaining case reports, the risk of bias evaluation revealed mainly the lack of data on patients’ race and patients’ history presented as timeline. Regarding the risk of bias assessment for case series, we found that out of 8 studies, only one (20) had a high methodological quality; for the remaining studies the lack of data mainly concerned the clear identification of inclusion/exclusion criteria, the consecutive and complete inclusion of patients and the reporting of presenting sites and demographic information.
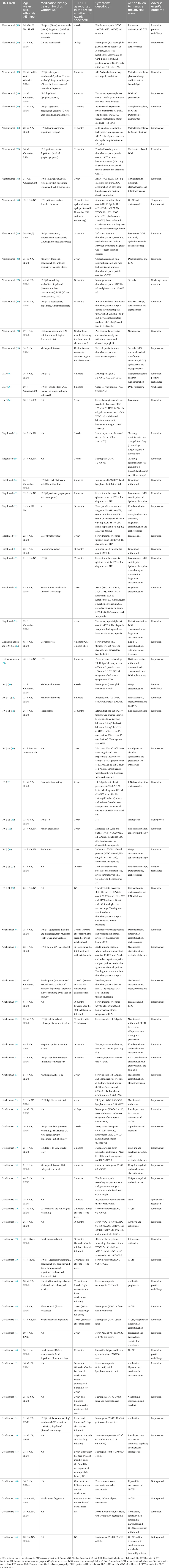
The main characteristics of included case reports and case series are reported in Tables 1, 2, which describe early-onset (events that occurred within 1 month after the first administration of the DMT) and late-onset (events that occurred after the first month of therapy) hematological disorders, respectively. Cases related to newborns are described in Table 3. An overview of the number of hematological disorders by DMTs is reported in Table 4. Lastly, the description of included case reports and series is presented hereafter by suspected drugs.
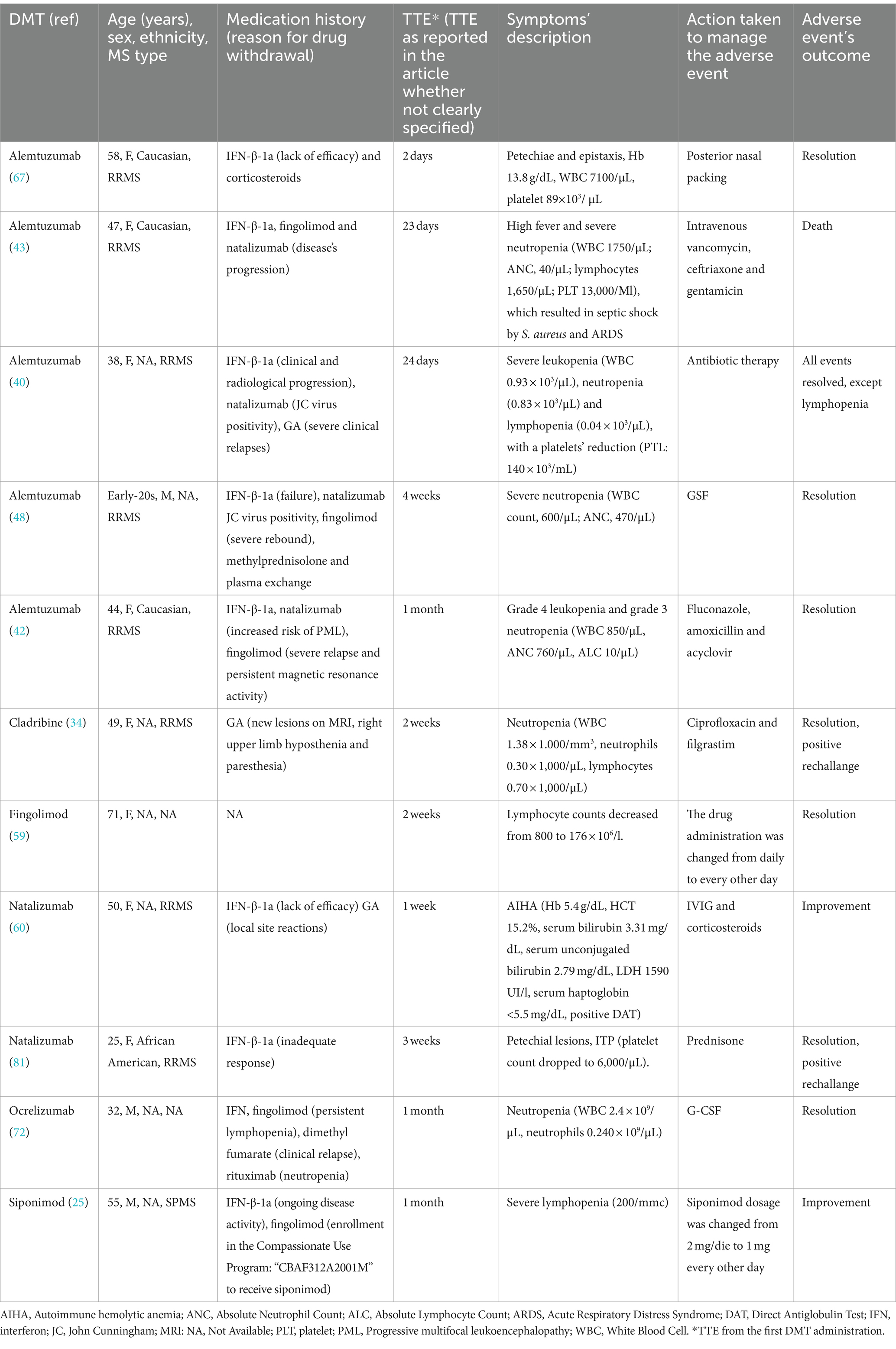
3.1 Alemtuzumab-induced hematological disorders
Sixteen case reports (21, 24, 27, 31, 33, 35, 40–45, 64, 67, 69, 74) and two case series (48, 77) described the occurrence of hematological disorders after alemtuzumab treatment. Of these studies, 5 concerned the occurrence of early-onset toxicities (40, 42, 43, 48, 67), including severe cases of leukopenia, neutropenia, lymphopenia and thrombocytopenia that occurred in four female and one male patients, aged <58 years, with a medication history that included at least IFN and/or natalizumab for all of them. Apart from one case that resulted in patient’s death (43), the remaining ones described the occurrence of hematological toxicities resulted in a full recovery. Alemtuzumab-induced late-onset hematological disorders were instead described in the remaining case reports and series (24, 27, 31, 33, 35, 41, 44, 45, 64, 69, 77). These events occurred in 8 male and 4 female patients [in one case (35) the sex was not reported]. The medication history included different therapies, mainly IFN, natalizumab and fingolimod for the majority of patients. The TTE ranged from 6 weeks to 45 months. ADRs reported were varied, with cases reporting AIHA, neutropenia, anemia and immune thrombocytopenia. In the majority of cases, the treatment with corticosteroids, intravenous immunoglobulins (IVIG) and transfusion led to improvements in bone marrow function. Neither of the reported cases had a fatal outcome.
Lastly, for two cases (21, 74) the time of hematological toxicity occurrence was unclear. Both cases concerned female patients aged <40 years, diagnosed with RRMS, who experienced cases of anemia and thrombocytopenia.
3.2 Natalizumab-induced hematological disorders
Nine studies described the occurrence of natalizumab-induced hematological disorders (37, 52–54, 60, 66, 73, 80, 81), of which 2 were case series (52, 60). Two cases of early onset hematological toxicity were found in the literature, of which one (60) concerning a 50-year-old female patient who experienced AIHA only 1 week after the first drug administration. The prompt treatment with IVIG and corticosteroids led to patient’s improvement after 2 months. The other case (81) concerned, instead, a 25-years-old African American woman who experienced ITP 3 weeks after the drug administration. Although the event resolved at the beginning, it re-occurred with the second infusion of the drug. The other cases were related to the occurrence of late-onset hematological toxicities (for which the TTE ranged from 12 weeks to 4 years), occurring in 5 female patients and 2 male patients aged <61 years. In one case (73) the patient’ sex was not specified. These cases were mainly related to the occurrence of severe anemia and thrombocytopenia (including autoimmune thrombocytopenia and thrombocytopenic purpura), and the majority with a progressive resolution after treatment with steroids and transfusion.
We also found 4 articles describing the occurrence of myelotoxicities in newborn from mothers who received natalizumab during pregnancy (29, 49, 50, 55). These cases were not included in the risk of bias assessment. Godano E et al. reported the case of a woman who had received natalizumab during pregnancy every 6 weeks. The baby was born at 37 + 5/7 weeks of gestation and blood tests showed the Hb was 8 g/dL and the hematocrit was 23.5%. The baby received therapy with erythropoietin (EPO) experiencing improvements of blood exams at 4 months of life (29). Another case was reported by Guilloton et al. who described the case of a 27-year-old woman who received the drug during pregnancy. The baby, was born 2 weeks before the term and blood exams performed 12 days after birth revealed a mild pancytopenia with leucopenia, thrombocytopenia and anemia. Blood exams normalized at 3 months of age (49). Ciron et al. reported the case of a 28-year-old woman who became pregnant while she was receiving natalizumab. The baby, was born at 40 weeks and 4 days of gestation. The newborn had a low platelet count at birth and experienced a improvement 10 days later (50). Lastly, Haghikia et al. described hematological abnormalities, including anemia and thrombocytopenia, among 10 infants whose mothers had received 1–9 natalizumab infusions during pregnancy (55). A detailed description of cases involving newborns is reported in Table 3.
3.3 Ocrelizumab-induced hematological disorders
Ten case reports (17, 23, 26, 28, 32, 37, 39, 70–72) and three case series (21, 65, 68) concerned the occurrence of hematological toxicities in patients receiving ocrelizumab. Apart from the case reported by Marrodan et al. (72) who described the occurrence of early onset neutropenia in a 32-year-old man, the remaining cases were related to late onset events with a TTE that ranged from 42 days to 5 years. Interferons and DMF mainly represented the medication history of these patients. Patients were aged <56 years and they presented multiple signs and symptoms that mainly included severe neutropenia. The antibiotic and antiviral prophylaxis together with G-CSF led to patients’ recovery in the majority of cases. For 4 cases described by Pang et al. (65) clinical and demographic data, including those related to the time to event, patients’ sex and medication history, were missing.
3.4 Interferon β-induced hematological disorders
Eight case reports (18, 58, 61, 63, 76, 77, 82, 83) and one case series (62) reporting the association IFN β-myeloid toxicity were found in the literature. Apart from one case (77) for which the time of toxicity occurrence was not inferable, all cases were related to late-onset events, occurring from 9 weeks to 10 years after the first drug administration. The majority of patients were female, younger than 52 years, with a medication history completely absent or mainly consisting in corticosteroids. The events mainly reported were neutropenia, anemia and thrombocytopenia. The treatment with conservative therapy and corticosteroids together with IFN β withdrawal led to full recovery in all patients.
3.5 Fingolimod-induced hematological disorders
Overall, five case reports (19, 51, 56, 57, 75) and 2 case series (47, 59) reported the occurrence of hematological toxicities following the treatment with fingolimod. Among cases reported by Tanaka et al. (59) there was one a case of lymphopenia that occurred 2 weeks after the beginning of fingolimod therapy in a 71-year-old woman that resolved after changing the frequency of the drug administration. The other cases reported in this case series were instead related to the occurrence of late-onset lymphopenia and neutropenia occurred after 5 weeks and 7 weeks, respectively, in 2 young women. For the remaining studies the TTE ranged from 2 months to 4 years. These cases mainly concerned cases of lymphopenia and AIHA. For the majority of cases fingolimod discontinuation and treatment with corticosteroids led to complete recovery of blood exams.
3.6 Dimethyl fumarate-induced hematological disorders
Three case reports (30, 38, 46) described the occurrence of late-onset hematological disorders (TTE ranged from 4 months to 3 years) in 3 women aged <50 years. In one case the medication history was not reported (38), while in the remaining cases patients had already received before IFN and glatiramer acetate. Anemia and lymphopenia were the events mainly reported. Only in one case (30) lymphopenia persisted for over 5 years despite treatment discontinuation. In their article, Zecca et al. (46) also reported that, after the review of medical records of their tertiary MS Centers (Lugano, Milan and Locarno), other 7 patients treated with DMF had discontinued because of ALC <0.5 × 109/L. These 7 cases were not considered for this review because of the lack of demographic, clinical and laboratory findings for the majority of them.
3.7 Other DMTs-induced hematological disorders
Finally, four case reports concerned the occurrence of hematological disorders following treatment with cladribine (34), siponimod (25), glatiramer acetate (79) and the combined therapy IFN/glatiramer acetate (22).
Maniscalco et al. reported a case of early non febrile neutropenia that occurred in 49-year-old RRMS female patient 2 weeks after the first cladribine cycle. The patient received ciprofloxacin for 5 days and filgrastim for 10 days with improvements in blood exams. After the second cladribine cycle, neutropenia occurred again, requesting a new cycle of ciprofloxacin and filgrastim with positive outcome (34).
Sparaco M et al. reported the case of a 55-year-old man who received siponimod in the context of the Compassionate Use Program: “CBAF312A2001M.” One month after the starting of the therapy, the patient developed severe lymphopenia (200/mmc). Siponimod dosage was changed from 2 to 1 mg/die with persistent lymphopenia (200/mmc) 1 month later, confirmed after another week. The drug was then administered at 1 mg every other day and 4 weeks later the lymphocyte count increased to 500/mmc (25).
Sagy I et al. described the occurrence of refractory symptomatic ITP in a 40-year-old woman 2 months after the initiation of therapy with glatiramer acetate. The condition was successfully managed by splenectomy (79).
The last case concerned a 65-year-old woman who developed mild to severe lymphopenia after having received glatiramer acetate and later IFN-β 1a. The patient started the treatment with glatiramer acetate in March 2007 and after 4 months her lymphocyte count decreased to 860 /μl. Four years later, IFN-β 1a was introduced and after 1 month the patient developed lymphopenia (22).
4 Discussion and conclusion
We carried out an extensive systematic review of studies published until January 5th 2024 on the Medline and Embase databases with the aim to provide an overview of case reports and series describing the occurrence of hematological disorders during the treatment with DMTs in patients with MS. We have reported data from 56 case reports and 11 case series concerning 84 adult patients who experienced myeloid toxicity during the treatment with a DMT and 13 newborns who developed this kind of toxicity due to a maternal exposure to a DMT. Out of 84 adults, 55 were female and 68 were younger than 50-year-old. When reported, RRSM was the most common form of MS. These demographic characteristics were expected considering that MS is a disease that worldwide affects more women than men (the prevalence ratio of MS of women to men is 2.3–3.5:1) and that the highest MS prevalence is in the age group 35–64 years (84–86).
Among myeloid toxicities, neutropenia was the most commonly reported, especially among patients treated with ocrelizumab. AIHA was frequently reported as well, mainly during the treatment with alemtuzumab. Overall, the DMTs most commonly reported as suspected in included case reports and series were alemtuzumab, natalizumab, ocrelizumab, IFN and fingolimod.
With regard to alemtuzumab, a risk of severe neutropenia and thrombocytopenia was already highlighted by the European Medicines Agency (EMA) at the end of a safety review carried out by the Pharmacovigilance Risk Assessment Committee at the request of the European Commission, under Article 20 of Regulation (EC) No 726/2004 (87, 88). In these communications, the EMA also reported that although rare, some ADRs, including thrombocytopenia, may occur 1 to 3 days of alemtuzumab infusion, while other events, mainly those autoimmune events, such as immune thrombocytopenic purpura, can occur within 48 months or longer after the last dose of alemtuzumab. With regard to the mechanisms underlying the occurrence of these events, thrombocytopenia might be the consequence both of a cytokine-released syndrome (in the presence of alemtuzumab, drug-dependent antibody binds to specific epitopes on platelet surface glycoproteins) or a complement-mediated lysis of circulating platelets (89). While neutropenia in addition, the drug is able to deplete lymphocytes, natural killer cells and monocytes by complement-mediated lysis of leukocytes expressing CD52 glycoprotein on their cell surfaces, leading to leukopenia and/or neutropenia (90, 91).
With regard to natalizumab, the majority of cases concerning adult patients were related to cases of anemia and thrombocytopenia. Data from a longitudinal study reported that the chronic treatment (18 months) was associated with significant modifications in complete blood cell count (increase in mean total white blood cell, lymphocyte, and eosinophil counts as a result of the inhibition in the transmigration of these cells into the central nervous systems that leads to their accumulation in peripheral blood) (92). Natalizumab blocks the alpha-4 subunit of the integrin molecules on leukocytes, leading to their extravasation into the CNS and intestinal tract (93). Contrary to what is observed with other DMTs, natalizumab is associated with an increase in CD4+, CD8+ T cells, CD19+ B cells, and NK cells in serum (94, 95). During natalizumab treatment, an increased release of CD34+ promotor cells from the bone marrow with a consequent increase of absolute lymphocyte counts in serum is observed. This increase tends to stabilize 3–6 months after starting treatment and lasts up to 6 months after discontinuation (96). On the other hand, the pathogenesis leading to thrombocytopenia is unclear, but overall authors of articles describing these cases mainly suggested a drug-induced immune-mediated mechanism, albeit in absence of antibodies against platelet. These effects, together with the fact that natalizumab induces a weakening of the immune systems and that it passes readily through the placenta during the third trimester of pregnancy, may explain the risk of hematological disorders such as anemia and thrombocytopenia in the newborns of mothers exposed to natalizumab. In addition, considering that the newborn’s immune system is under-developed at time of birth, the exposure to natalizumab may further impair its function and render the baby even more susceptible to infections (97, 98). However, notwithstanding a risk of hematological abnormalities in the newborn and spontaneous abortion at the same rate as that of the general population, the use of natalizumab is considered to be safe in pregnancy. The strict monitoring of patients is advisable to minimize the risk of such adverse outcomes.
Data from pivotal trials on ocrelizumab reported a decrease in neutrophil counts in 13–15% of patients with grade 4 neutropenia observed in up to 1% (99). Through the analysis of data reported in the FDA Adverse Event Reporting System (FAERS), Hammer H et al. aimed to identify risk factors of neutropenia in patients treated with ocrelizumab. They identified male sex, younger age and lower bodyweight as factors associated with ocrelizumab-related neutropenia (100). Studies involving rituximab and evaluating bone marrow functionality found that late-onset neutropenia was induced by the white cell line maturation arrest, which in turn is the result of excessive levels of B-cell activating factor (101, 102). As reported by Baird-Gunning (28), late-onset neutropenia can occur also several months after ocrelizumab infusion and it might be caused by alterations in growth factors that drive B-cell production inducing a significant reduction in neutrophils. Since this event is unpredictable and consequently not preventable, blood routine tests represent the main tool for risk mitigation.
Preclinical data on mechanisms underlying fingolimod-induced hematological abnormalities reported that fingolimod transiently increased platelets via S1pr1 activation on megakaryocytes (103), while further data reported that the drug is associated with lymphopenia due to an action on S1P receptor 1 by blocking the egress of lymphocytes from secondary lymphoid organs and preventing them from reaching inflamed tissues (104).
Lastly, few cases concerned the occurrence of hematological disorders in patients treated with IFNs. These DMTs are associated with a 20–30% drop of absolute lymphocyte count due to multiple mechanisms that include a reduction of dendritic cells and a down-regulation of the antigen presentation by antigen-presenting cells (APCs), a reduction in Th17 cells that in turn leads to a reduction of IL-17 release and induction of apoptosis of autoreactive T cells, a reduction of leukocyte migration via the blood–brain barrier into the CNS (105, 106).
Overall, the majority of cases referred to late-onset toxicities, that occurred more than 1 month after the beginning of the therapy with a DMT. Indeed, only 11 studies described the occurrence of early-onset hematological disorders, of which half of them were related to alemtuzumab. Generally, myeloid cytopenia, which include neutropenia, thrombocytopenia, and anemia, are the most common manifestations of drug-related myelotoxicity and the most common reasons for dose modifications or even therapy discontinuation (107). Late-onset neutropenia is confirmed when absolute neutrophil count (ANC) is <1.5 × 109/L and when it develops more than 4 weeks after last drug administration (108). Rarely neutropenia is severe and it can result in neutropenic fever and infection that require patient’s hospitalization, the need for broad-spectrum antibiotics, and the potential sequelae of bacteremia, up to be fatal (109).
AIHA is an acquired autoimmune disorder that develops when autoantibodies develop against self-antigens on the red blood cells, which leads to their destruction. The diagnosis is based on the presence of certain symptoms and laboratory findings including anemia, jaundice, splenomegaly, reticulocytosis, raised serum bilirubin, and a positive direct antiglobulin test (DAT), which detects the presence of antibodies or complement on the red blood cell (RBC) surface (110).
On the other hand, thrombocytopenia is defined in presence of platelet counts <100 × 109/L or > 50% drop in the platelet count from baseline. In severe cases, when the platelet count is <50 × 109/L, there is an increased risk of bleeding that can result in patient’s death. The mechanism underlying the occurrence of drug-induced thrombocytopenia can be either a decrease in platelet production (bone marrow toxicity) or an increased destruction (immune-mediated thrombocytopenia) (111). Many drugs seem to be related to the occurrence of this event, including carbamazepine, ceftriaxone, mirtazapine, oxaliplatin, penicillin, quinine, quinidine, rifampicin, NSAIDs, vancomycin and diuretics (112). Many case reports not included in this review described the occurrence of thrombotic microangiopathy during the treatment with IFN (113–116). Although these cases reported the occurrence of thrombocytopenia, they were not considered for inclusion in this systematic review considering that the reduction in platelets’ count is not the result of a myeloid toxicity rather than other conditions that are not related to suppression of myeloid function.
In conclusion, based on data summarized in this systematic review, the majority of DMTs currently used to treat MS seems to be associated with the occurrence of hematological disorders, even though to a different extent depending on the DMT. For this reason, blood tests need to be carried out before starting the treatment with a specific DMT; for example, due to the risk of ITP, complete blood counts with differential should be obtained prior to initiation of treatment with alemtuzumab and at monthly intervals thereafter until at least 48 months after the last infusion (117). Similarly, to due the risk of lymphopenia, before starting the therapy with dimethyl fumarate, a complete blood count, including lymphocytes, must be performed and repeated every 3 months. The treatment should not be initiated in patients with lymphocyte counts <0.5 × 109 /L. (118) These recommendations are also reported for fingolimod (119), natalizumab, when switching patients from another DMT (120) and ocrelizumab (due to the risk of occurrence of late neutropenia, measurement of blood neutrophils is recommended in patients with signs and symptoms of infection) (121). In general, apart from the regular monitoring of blood cells counts, in order to prevent potentially severe consequences of hematological disorders (infections and bleedings that might lead to patients’ death) and to improve their management, patients receiving DMTs should be educated about the signs and symptoms of myelotoxicity, such as unexplained fatigue, recurrent infections or bleeding (122–127). Indeed, although DMTs-induced hematological disorders seem to be, in the vast majority of cases, self-limiting and rarely associated with serious complications, their early recognition and management is essential for patients’ safety.
5 Limitations
This article has some limitations. First of all, the number of studies and included patients was modest, especially for some DMTs such as fingolimod, dimethyl fumarate, cladribine, siponimod and glatiramer acetate. Second, included cases were heterogeneous in terms of hematological disorders, which render the comparison across cases quite difficult. Third, incomplete data reporting was frequent across studies, including information on demographic characteristics (ethnicity, geographic region and education were lacking in the majority of studies), patients’ medical history (timelines of treatments was not frequently reported), and complete/consecutive inclusion of patients for case series. Lastly, apart from cases reporting a positive dechallenge (signs of myelotoxicity disappear after stopping the drug) and rechallenge (signs of myelotoxicity re-appear after introducing for the second time the drug) and for those cases without other clearly stated medical causes, it is not simple to establish a causal relationship for all cases included in this review. Based on these limitations, our results should be interpreted with caution and further ad hoc studies are strongly needed to better evaluate the myeloid toxicities of DMTs.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Author contributions
CS: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. VL: Investigation, Methodology, Writing – review & editing. OA: Data curation, Investigation, Writing – review & editing. DDGC: Investigation, Software, Writing – review & editing. MGS: Investigation, Writing – review & editing. VA: Conceptualization, Investigation, Writing – review & editing. LS: Data curation, Investigation, Writing – review & editing. GTM: Conceptualization, Data curation, Investigation, Writing – review & editing. AC: Conceptualization, Data curation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research has received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement n°115014, the resources of which are composed of financial contribution from the European Union’s Seventh Framework Program (FP7/2007–2013). More information is available at https://www.eu2p.org/about-eu2p/project (accessed on 8 November 2023).
Acknowledgments
The research leading to these results was performed in the framework of the European training program in Pharmacovigilance and Pharmacoepidemiology, Eu2P (for more information, visit www.eu2p.org, accessed on 15 June 2023).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1386527/full#supplementary-material
References
1. Auricchio, F, Scavone, C, Cimmaruta, D, Di Mauro, G, Capuano, A, Sportiello, L, et al. Drugs approved for the treatment of multiple sclerosis: review of their safety profile. Expert Opin Drug Saf. (2017) 16:1359–71. doi: 10.1080/14740338.2017.1388371
2. Klineova, S, and Lublin, FD. Clinical course of multiple sclerosis. Cold Spring Harb Perspect Med. (2018) 8:a028928. doi: 10.1101/cshperspect.a028928
3. Thompson, AJ, Banwell, BL, Barkhof, F, Carroll, WM, Coetzee, T, Comi, G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. (2018) 17:162–73. doi: 10.1016/S1474-4422(17)30470-2
4. Weiner, HL . A shift from adaptive to innate immunity: a potential mechanism of disease progression in multiple sclerosis. J Neurol. (2008) 255:3–11. doi: 10.1007/s00415-008-1002-8
5. Granziera, C, Derfuss, T, and Kappos, L. Time to change the current clinical classification of multiple sclerosis? JAMA Neurol. (2023) 80:128–30. doi: 10.1001/jamaneurol.2022.4156
6. Walton, C, King, R, Rechtman, L, Kaye, W, Leray, E, Marrie, RA, et al. Rising prevalence of multiple sclerosis worldwide: insights from the atlas of MS, third edition. Mult Scler. (2020) 26:1816–21. doi: 10.1177/1352458520970841
7. Tullman, MJ . Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. Am J Manag Care. (2013) 19:S15–20.
8. Maniscalco, G, Brescia Morra, V, Florio, C, Lus, G, Tedeschi, G, Cianfrani, M, et al. Preliminary results of the FASM study, an on-going Italian active pharmacovigilance project. Pharmaceuticals (Basel). (2020) 13:466. doi: 10.3390/ph13120466
9. Gajofatto, A, and Benedetti, MD. Treatment strategies for multiple sclerosis: when to start, when to change, when to stop? World J Clin Cases. (2015) 3:545–55. doi: 10.12998/wjcc.v3.i7.545
10. Alborghetti, M, Bellucci, G, Gentile, A, Calderoni, C, Nicoletti, F, Capra, R, et al. Drugs used in the treatment of multiple sclerosis during COVID-19 pandemic: a critical viewpoint. Curr Neuropharmacol. (2022) 20:107–25. doi: 10.2174/1570159X19666210330094017
11. Barbieri, MA, Sorbara, EE, Battaglia, A, Cicala, G, Rizzo, V, Spina, E, et al. Adverse drug reactions with drugs used in multiple sclerosis: an analysis from the Italian pharmacovigilance database. Front Pharmacol. (2022) 13:808370. doi: 10.3389/fphar.2022.808370
12. Carey, PJ . Drug-induced Myelosuppression. Drug Saf. (2003) 26:691–706. doi: 10.2165/00002018-200326100-00003
13. Schweitzer, F, Laurent, S, Fink, GR, Barnett, MH, Hartung, HP, and Warnke, C. Effects of disease-modifying therapy on peripheral leukocytes in patients with multiple sclerosis. J Neurol. (2021) 268:2379–89. doi: 10.1007/s00415-019-09690-6
14. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DGPRISMA Group. Preferred reporting items for systematic reviews and Meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
15. Cochrane Handbook for Systematic Reviews of Interventions . Version 6.4, (2023). Available at: https://training.cochrane.org/handbook/current (Accessed on March 20, 2024).
16. JBI . Critical Appraisal Tool. (2024). Available at: https://jbi.global/critical-appraisal-tools (Accessed on March 20th, 2024).
17. Alba Suárez, EM, Tallón Barranco, A, Puertas Muñoz, I, Chamorro Hernández, B, and Robles, MÁ. Non-late-onset neutropaenia following treatment of multiple sclerosis with ocrelizumab. Neurologia (Engl Ed). (2023) 38:463–6. doi: 10.1016/j.nrleng.2021.01.005
18. Cisarovsky, C, Théaudin, M, Bart, PA, Stalder, G, and Alberio, L. Severe late-onset drug-induced immune thrombocytopenia following IFN β-1a treatment: a case report of a 52-year-old woman with relapse-remitting multiple sclerosis. Case Rep Hematol. (2022) 2022:2767031. doi: 10.1155/2022/2767031
19. Najdaghi, S, Davani, DN, Ghajarzadeh, M, and Shaygannejad, V. Autoimmune hemolytic anemia after treatment with fingolimod in a patient with multiple sclerosis (MS): a case report and review of the literature. Autoimmun Rev. (2022) 21:103203. doi: 10.1016/j.autrev.2022.103203
20. Rossi, L, Dinoto, A, Bratina, A, Baldini, S, Pasquin, F, Bosco, A, et al. Neutropenia complicating anti-CD20 treatment in patients with multiple sclerosis: a retrospective case series and a systematic review of reported cases. Mult Scler Relat Disord. (2022) 68:104090. doi: 10.1016/j.msard.2022.104090
21. Chan, C, Beauchemin, P, Sayao, AL, and Carruthers, M. Autoimmune storm following alemtuzumab. BMJ Case Rep. (2022) 15:e248037. doi: 10.1136/bcr-2021-248037
22. Koseahmet, FB, Ozturk, M, and Celik, GGR. Lymphopenia and tuberculous lymphadenitis under immunomodulatory agents in a multiple sclerosis patient: follow-up of a challenging case. Ideggyogy Sz. (2022) 75:137–40. doi: 10.18071/isz.75.0137
23. Rauniyar, R, Rauniyar, R, Agrawal, A, Yadav, S, and Avula, S. Severe late-onset neutropenia induced by ocrelizumab in a multiple sclerosis patient: a case report. Clin Case Rep. (2022) 10:e05299. doi: 10.1002/ccr3.5299
24. Aitken, L, Patel, R, D'Rozario, J, and Choi, P. Alemtuzumab-induced red cell aplasia and other immune cytopenias: not so 'pure'. Immunotherapy. (2022) 14:95–9. doi: 10.2217/imt-2021-0163
25. Sparaco, M, Miele, G, and Bonavita, S. Severe lymphopenia switching from Fingolimod to Siponimod. Neurol Sci. (2021) 42:4837–8. doi: 10.1007/s10072-021-05546-y
26. Maniscalco, GT, Allegorico, L, Di Battista, ME, Annunziata, M, Salvatore, S, and Manzo, V. Late-onset neutropenia (LON) recur in a MS patient after the second cycle of ocrelizumab: a case report. Neurol Sci. (2021) 42:3933–5. doi: 10.1007/s10072-021-05379-9
27. Ganju, A, Stock, JC, and Jordan, K. Case report: delayed Alemtuzumab-induced concurrent neutropenia and thrombocytopenia in relapsing-remitting multiple sclerosis. J Pharm Pract. (2023) 36:168–72. doi: 10.1177/08971900211021235
28. Baird-Gunning, J, Yun, J, Stevenson, W, and Ng, K. Severe delayed-onset neutropenia induced by Ocrelizumab. Neurohospitalist. (2021) 11:59–61. doi: 10.1177/1941874420936438
29. Godano, E, Barra, F, Allodi, A, Ferraiolo, A, Laroni, A, Novi, G, et al. Erythropoietin therapy in a case of neonatal anemia after exposure to natalizumab throughout pregnancy. Ital J Pediatr. (2021) 47:69. doi: 10.1186/s13052-021-01025-4
30. Caldito, NG, O'Leary, S, and Stuve, O. Persistent severe lymphopenia 5 years after dimethyl fumarate discontinuation. Mult Scler. (2021) 27:1306–8. doi: 10.1177/1352458520988149
31. Erlich-Malona, N, Cahill, J, Chaudhry, S, Martin, J, and Rizvi, S. Cardiac sarcoidosis requiring ICD placement and immune thrombocytopenia following alemtuzumab treatment for multiple sclerosis. Mult Scler Relat Disord. (2021) 47:102599. doi: 10.1016/j.msard.2020.102599
32. Auer, M, Bsteh, G, Hegen, H, Wurth, S, Zinganell, A, Berger, T, et al. Late-onset neutropenia in a multiple sclerosis patient after first dose ocrelizumab switched from rituximab. Mult Scler Relat Disord. (2020) 43:102155. doi: 10.1016/j.msard.2020.102155
33. Alnahdi, MA, Aljarba, SI, and Al Malik, YM. Alemtuzumab-induced simultaneous onset of autoimmune haemolytic anaemia, alveolar haemorrhage, nephropathy, and stroke: a case report. Mult Scler Relat Disord. (2020) 41:102141. doi: 10.1016/j.msard.2020.102141
34. Maniscalco, GT, Annunziata, M, Ranieri, A, Alfieri, G, Renna, R, Iorio, WD, et al. Remission of early persistent cladribine-induced neutropenia after filgrastim therapy in a patient with relapsing-remitting multiple sclerosis. Mult Scler Relat Disord. (2020) 43:102151. doi: 10.1016/j.msard.2020.102151
35. Tzartos, JS, Valsami, S, Tzanetakos, D, Stergiou, C, Dandoulaki, M, Barbarousi, D, et al. Autoimmune hemolytic anemia, demyelinating relapse, and AQP1 antibodies after alemtuzumab infusion. Neurol Neuroimmunol Neuroinflamm. (2020) 7:e711. doi: 10.1212/NXI.0000000000000711
36. Zanetta, C, Robotti, M, Nozzolillo, A, Sangalli, F, Liberatore, G, Nobile-Orazio, E, et al. Late onset absolute neutropenia associated with ocrelizumab treatment in multiple sclerosis: a case report and review of the literature. J Neurol Sci. (2020) 409:116603. doi: 10.1016/j.jns.2019.116603
37. Rini, AM, Clerici, VT, Rinaldi, E, Modesto, M, Pastore, D, Confalonieri, PA, et al. Severe thrombocytopenia during Natalizumab therapy: a case report. J Neurol Sci. (2020) 409:116587. doi: 10.1016/j.jns.2019.116587
38. Quintanilla-Bordás, C, Castro-Izaguirre, E, Carcelén-Gadea, M, and Marín, M. The first reported case of drug-induced hemolytic anemia caused by dimethyl fumarate in a patient with multiple sclerosis. Transfusion. (2019) 59:1648–50. doi: 10.1111/trf.15151
39. Cohen, BA . Late-onset neutropenia following ocrelizumab therapy for multiple sclerosis. Neurology. (2019) 92:435–6. doi: 10.1212/WNL.0000000000006924
40. Maniscalco, GT, Cerillo, I, Servillo, G, Napolitano, M, Guarcello, G, Abate, V, et al. Early neutropenia with thrombocytopenia following alemtuzumab treatment for multiple sclerosis: case report and review of literature. Clin Neurol Neurosurg. (2018) 175:134–6. doi: 10.1016/j.clineuro.2018.11.002
41. Vakrakou, AG, Tzanetakos, D, Valsami, S, Grigoriou, E, Psarra, K, Tzartos, J, et al. A case of Alemtuzumab-induced neutropenia in multiple sclerosis in association with the expansion of large granular lymphocytes. BMC Neurol. (2018) 18:178. doi: 10.1186/s12883-018-1183-4
42. Galgani, S, Prosperini, L, Haggiag, S, Tortorella, C, and Gasperini, C. Early transient asymptomatic neutropenia associated with alemtuzumab treatment in multiple sclerosis: a case report. J Neurol. (2018) 265:2152–3. doi: 10.1007/s00415-018-8976-7
43. Yiannopoulou, KG, Papadimitriou, D, Anastasiou, AI, and Siakantaris, M. Neutropenia with fatal outcome in a multiple sclerosis patient 23 days after alemtuzumab infusion. Mult Scler Relat Disord. (2018) 23:15–6. doi: 10.1016/j.msard.2018.04.014
44. Di Ioia, M, Farina, D, Di Tommaso, V, Travaglini, D, Pietrolongo, E, Onofrj, M, et al. Simultaneous early-onset severe autoimmune hemolytic anemia and albuminuria during alemtuzumab treatment for multiple sclerosis. Mult Scler. (2018) 24:813–5. doi: 10.1177/1352458517743093
45. Meunier, B, Rico, A, Seguier, J, Boutiere, C, Ebbo, M, Harle, JR, et al. Life-threatening autoimmune warm hemolytic anemia following treatment for multiple sclerosis with alemtuzumab. Mult Scler. (2018) 24:811–3. doi: 10.1177/1352458517729766
46. Zecca, C, Antozzi, CG, Torri Clerici, V, Ferrazzini, M, Mantegazza, RE, Rossi, S, et al. Severe multiple sclerosis reactivation during prolonged lymphopenia after dimethyl fumarate discontinuation. Acta Neurol Scand. (2018) 137:623–5. doi: 10.1111/ane.12882
47. Yuen, HLA, Brown, S, Chan, N, and Grigoriadis, G. Immune thrombocytopenic purpura associated with fingolimod. BMJ Case Rep. (2017) 2017:bcr2017220590. doi: 10.1136/bcr-2017-220590
48. Gaitán, MI, Ysrraelit, MC, and Correale, J. Neutropenia in patients with multiple sclerosis treated with Alemtuzumab. JAMA Neurol. (2017) 74:1143–4. doi: 10.1001/jamaneurol.2017.1456
49. Guilloton, L, Pegat, A, Defrance, J, Quesnel, L, Barral, G, and Drouet, A. Neonatal pancytopenia in a child, born after maternal exposure to natalizumab throughout pregnancy. J Gynecol Obstet Hum Reprod. (2017) 46:301–2. doi: 10.1016/j.jogoh.2017.02.008
50. Ciron, J, Hautecoeur, P, Mathis, S, and Neau, JP. Natalizumab throughout pregnancy: risk of low platelet count in the newborn at delivery. Rev Neurol (Paris). (2016) 172:165–6. doi: 10.1016/j.neurol.2015.07.007
51. De Masi, R, Accoto, S, Orlando, S, De Blasi, V, Pasca, S, Scarpello, R, et al. Dramatic recovery of steroid-refractory relapsed multiple sclerosis following Fingolimod discontinuation using selective immune adsorption. BMC Neurol. (2015) 15:125. doi: 10.1186/s12883-015-0377-2
52. Monteleone, F, Buccisano, F, Boffa, L, Buttari, F, Di Veroli, A, Borriello, G, et al. Reversible hyporegenerative anemia during natalizumab treatment. Mult Scler. (2015) 21:257–8. doi: 10.1177/1352458514546516
53. Simone, AM, Ferraro, D, Vitetta, F, Marasca, R, Bonacorsi, G, Pinelli, G, et al. Severe anemia in a patient with multiple sclerosis treated with natalizumab. Neurology. (2014) 83:374–5. doi: 10.1212/WNL.0000000000000614
54. Cachia, D, Izzy, S, Berriosmorales, I, and Ionete, C. Drug-induced thrombocytopenia secondary to natalizumab treatment. BMJ Case Rep. (2014) 2014:bcr2013203313. doi: 10.1136/bcr-2013-203313
55. Haghikia, A, Langer-Gould, A, Rellensmann, G, Schneider, H, Tenenbaum, T, Elias-Hamp, B, et al. Natalizumab use during the third trimester of pregnancy. JAMA Neurol. (2014) 71:891–5. doi: 10.1001/jamaneurol.2014.209
56. La Mantia, L, Prone, V, Marazzi, MR, Erminio, C, and Protti, A. Multiple sclerosis rebound after fingolimod discontinuation for lymphopenia. Neurol Sci. (2014) 35:1485–6. doi: 10.1007/s10072-014-1800-y
57. Lysandropoulos, AP, and Benghiat, F. Severe auto-immune hemolytic anemia in a fingolimod-treated multiple sclerosis patient. Mult Scler. (2013) 19:1551–2. doi: 10.1177/1352458513493035
58. Münzel, EJ, Wimperis, JZ, and Williams, A. Relapsing-remitting multiple sclerosis and chronic idiopathic neutropenia: a challenging combination. BMJ Case Rep. (2013) 2013:bcr2012007936. doi: 10.1136/bcr-2012-007936
59. Tanaka, M, Park, K, and Tanaka, K. Reduced fingolimod dosage treatment for patients with multiple sclerosis and lymphopenia or neutropenia. Mult Scler. (2013) 19:1244–5. doi: 10.1177/1352458512472750
60. Midaglia, L, Rodriguez Ruiz, M, and Muñoz-García, D. Severe haematological complications during treatment with natalizumab. Mult Scler. (2012) 18:1644–6. doi: 10.1177/1352458512442262
61. Saeedi, M, Forughipour, M, Sasannezhad, P, and Shoeibi, A. Interferon-Beta-1b induced autoimmune hemolytic Anemia in a patient with MS: a case report. Iran Red Crescent Med J. (2011) 13:210–2.
62. Nabavi, SM, Hamzehloo, A, Shams, J, and Morsali, D. Reversible therapy-related dysplastic hematopoiesis following Beta interferon therapy in multiple sclerosis patients: report of 2 cases. Iran J Neurol. (2011) 10:32–4.
63. Aslam, AK, and Singh, T. Aplastic anemia associated with interferon beta-1a. Am J Ther. (2002) 9:522–3. doi: 10.1097/00045391-200211000-00011
64. Bourdin, V, Fossé, Q, Lambotte, O, Joly, B, Coppo, P, Anguel, N, et al. Alemtuzumab-induced immune-mediated thrombotic thrombocytopenic purpura: a newly described drug-related autoimmune disease. Br J Haematol. (2023) 204:1459–63. doi: 10.1111/bjh.19263
65. Pang, V, Seery, N, Wesselingh, R, Yeh, W, Zhong, M, Tan, T, et al. Neutropaenia complications from Ocrelizumab and rituximab treatment. Mult Scler Relat Disord. (2024) 81:105147. doi: 10.1016/j.msard.2023.105147
66. Azimi, A, Abna, Z, Ghadiri, F, and Mehrabi, F. Natalizumab-induced thrombocytopenia: a case report. Curr J Neurol. (2022) 21:64–5. doi: 10.18502/cjn.v21i1.9363
67. Mahmoudi, F, Emami, SA, Masaeli, F, and Rayatpisheh, N. Alemtuzumab-induced petechiae and epistaxis in a patient with relapsing-remitting multiple sclerosis: a case report. Clin Case Rep. (2023) 11:e8143. doi: 10.1002/ccr3.8143
68. Beigneux, Y, Louapre, C, Bihan, K, Roux, T, de Paz, R, Lubetzki, C, et al. Recurrence of severe symptomatic late-onset neutropenia on ocrelizumab. Mult Scler. (2024) 30:131–3. doi: 10.1177/13524585231206218
69. Kim, JH, Park, S, and Kim, W. Myelodysplastic syndrome in an alemtuzumab-treated multiple sclerosis patient. Neurol Sci. (2022) 43:5651–3. doi: 10.1007/s10072-022-06124-6
70. Lim, CSJ, Tye, JSN, Tan, K, and Yeo, T. Late-onset neutropenia following anti-CD20 therapies in multiple sclerosis and Neuromyelitis Optica Spectrum disorders: a report on two patients. Neuroimmunol Rep. (2022) 2:100136. doi: 10.1016/j.nerep.2022.100136
71. Kermode, WPH, Kavanagh, S, and Kermode, AG. Myeloid maturation arrest and severe late-onset neutropenia following ocrelizumab therapy in a patient with multiple sclerosis: a case report and review of the literature. Neuroimmunol Rep. (2021) 1:100012. doi: 10.1016/j.nerep.2021.100012
72. Marrodan, M, Laviano, J, Oneto, S, Reino, FM, Delorme, R, Fornillo, F, et al. Rituximab- and ocrelizumab-induced early- and late-onset neutropenia in a multiple sclerosis patient. Neurol Sci. (2021) 42:3893–5. doi: 10.1007/s10072-021-05357-1
73. Mansoor, S, Kelly, S, Burke, A, Adenan, MH, Joyce, E, Waters, A, et al. Natalizumab-induced hyporegenerative anaemia and leukopenia: a case report. Egypt J Neurol Psychiatry Neurosurg. (2020) 56:6. doi: 10.1186/s41983-019-0143-2
74. Love, B, and McCombe, JA. Anemia and sarcoidosis following treatment with alemtuzumab. Mult Scler Relat Disord. (2020) 46:102526. doi: 10.1016/j.msard.2020.102526
75. Mukharesh, L, and Rothstein, TL. Fingolimod-induced immune thrombocytopenic purpura (ITP). Clin Neurol Neurosurg. (2020) 197:106081. doi: 10.1016/j.clineuro.2020.106081
76. Shaygannejad, V, Fayyazi, E, Mirmosayyeb, O, and Toghianifar, N. Immune thrombocytopenia (ITP) in a patient with relapsing-remitting multiple sclerosis (RRMS) associated with b-interferon-1α treatment. Indian J Pharm Educ Res. (2018) 52:540–2. doi: 10.5530/ijper.52.3.62
77. Etemadifar, M, Sabeti, F, and Salari, M. Interferon beta-1b-induced thrombotic thrombocytopenic purpura-hemolytic uremic syndrome (TTP-HUS) in a patient treated for multiple sclerosis: a case report. Iran J Neurol. (2018) 17:91–4.
78. Obermann, M, Ruck, T, Pfeuffer, S, Baum, J, Wiendl, H, and Meuth, SG. Simultaneous early-onset immune thrombocytopenia and autoimmune thyroid disease following alemtuzumab treatment in relapsing-remitting multiple sclerosis. Mult Scler. (2016) 22:1235–41. doi: 10.1177/1352458516638558
79. Sagy, I, Shalev, L, Levi, I, Shleyfer, E, Valdman, S, and Barski, L. Glatiramer acetate-associated refractory immune thrombocytopenic Purpura. Eur J Case Rep Intern Med. (2016) 3:000399. doi: 10.12890/2016_000399
80. Seibert, JB, and Alvarez, E. Severe anemia in a patient with multiple sclerosis treated with natalizumab. Neurology. (2015) 84:861. doi: 10.1212/01.wnl.0000461938.49936.52
81. Stosic, M, De Jesus, P, McCarthy, J, Hutton, G, and Rivera, V. Immune thrombocytopenic purpura in a patient with multiple sclerosis treated with natalizumab. Neurology. (2011) 77:505–7. doi: 10.1212/WNL.0b013e318227b23f
82. Sahraian, MA, and Eshaghi, A. Concomitant multiple sclerosis and idiopathic thrombocytopenic purpura. Eur J Neurol. (2010) 17:e62–3. doi: 10.1111/j.1468-1331.2010.03098.x
83. Alanoglu, G, Kilbas, S, Arslan, C, Senol, A, and Kutluhan, S. Autoimmune hemolytic anemia during interferon-beta-I b treatment for multiple sclerosis. Mult Scler. (2007) 13:683–5. doi: 10.1177/1352458506071333
84. Ziello, A, Scavone, C, Di Battista, ME, Salvatore, S, Di Giulio Cesare, D, Moreggia, O, et al. Influenza vaccine hesitancy in patients with multiple sclerosis: a monocentric observational study. Brain Sci. (2021) 11:890. doi: 10.3390/brainsci11070890
85. Harbo, HF, Gold, R, and Tintoré, M. Sex and gender issues in multiple sclerosis. Ther Adv Neurol Disord. (2013) 6:237–48. doi: 10.1177/1756285613488434
86. Maniscalco, GT, Scavone, C, Mascolo, A, Manzo, V, Prestipino, E, Guglielmi, G, et al. The safety profile of COVID-19 vaccines in patients diagnosed with multiple sclerosis: a retrospective observational study. J Clin Med. (2022) 11:6855. doi: 10.3390/jcm11226855
87. European Medicines Agency . Use of multiple sclerosis medicine Lemtrada restricted while EMA review is ongoing. (2024). Available at: https://www.ema.europa.eu/en/documents/press-release/use-multiple-sclerosis-medicine-lemtrada-restricted-while-ema-review-ongoing_en.pdf (Accessed on February 14th, 2024).
88. European Medicines Agency . Measures to minimise risk of serious side effects of multiple sclerosis medicine Lemtrada. (2024). Available at: https://www.ema.europa.eu/en/documents/referral/lemtrada-article-20-procedure-measures-minimise-risk-serious-side-effects-multiple-sclerosis_en-0.pdf (Accessed on February 14th, 2024).
89. Puthenparampil, M, Rinaldi, F, Federle, L, Cazzola, C, Perini, P, and Gallo, P. Decreased platelet number in multiple sclerosis during alemtuzumab infusion: a common, transient and clinically silent phenomenon. Ther Adv Neurol Disord. (2017) 11:41056. doi: 10.1177/1756285617741056
90. Smith, A, Couvillion, R, Zhang, R, Killackey, M, Buell, J, Lee, B, et al. Incidence and management of leukopenia/neutropenia in 233 kidney transplant patients following single dose alemtuzumab induction. Transplant Proc. (2014) 46:3400–4. doi: 10.1016/j.transproceed.2014.07.070
91. LaMattina, JC, Mezrich, JD, Hofmann, RM, Foley, DP, D'Alessandro, AM, Sollinger, HW, et al. Alemtuzumab as compared to alternative contemporary induction regimens. Transpl Int. (2012) 25:518–26. doi: 10.1111/j.1432-2277.2012.01448.x
92. Bridel, C, Beauverd, Y, Samii, K, and Lalive, PH. Hematologic modifications in natalizumab-treated multiple sclerosis patients: an 18-month longitudinal study. Neurol Neuroimmunol Neuroinflamm. (2015) 2:e123. doi: 10.1212/NXI.0000000000000123
93. Fischer, S, Proschmann, U, Akgün, K, and Ziemssen, T. Lymphocyte counts and multiple sclerosis therapeutics: between mechanisms of action and treatment-limiting side effects. Cells. (2021) 10:3177. doi: 10.3390/cells10113177
94. Theien, BE, Vanderlugt, CL, Nickerson-Nutter, C, Cornebise, M, Scott, DM, Perper, SJ, et al. Differential effects of treatment with a small-molecule VLA-4 antagonist before and after onset of relapsing EAE. Blood. (2003) 102:4464–71. doi: 10.1182/blood-2003-03-0974
95. Vajkoczy, P, Laschinger, M, and Engelhardt, B. Alpha4-integrin-VCAM-1 binding mediates G protein-independent capture of encephalitogenic Tcell blasts to CNS white matter micro-vessels. J Clin Invest. (2001) 108:557–65. doi: 10.1172/JCI12440
96. Stüve, O, Cravens, PD, Frohman, EM, Phillips, JT, Remington, GM, von Geldern, G, et al. Immunologic, clinical, and radiologic status 14 months after cessation of natalizumab therapy. Neurology. (2009) 72:396–401. doi: 10.1212/01.wnl.0000327341.89587.76
97. Ramesh, V, Opara, CO, Khan, FY, Kabiraj, G, Kauser, H, Palakeel, JJ, et al. Adverse obstetric outcomes in pregnant women using Natalizumab for the treatment of multiple sclerosis: a systematic review. Cureus. (2022) 14:e29952. doi: 10.7759/cureus.29952
98. Airas, L . Exposure to natalizumab during pregnancy and lactation is safe - no. Mult Scler. (2020) 26:889–91. doi: 10.1177/1352458520917934
99. Gibiansky, E, Petry, C, Mercier, F, Günther, A, Herman, A, Kappos, L, et al. Ocrelizumab in relapsing and primary progressive multiple sclerosis: pharmacokinetic and pharmacodynamic analyses of OPERA I, OPERA II and ORATORIO. Br J Clin Pharmacol. (2021) 87:2511–20. doi: 10.1111/bcp.14658
100. Hammer, H, Kamber, N, Pistor, M, Diem, L, Friedli, C, Chan, A, et al. Ocrelizumab-related neutropenia: effects of age, sex and bodyweight using the FDA adverse event reporting system (FAERS). Mult Scler Relat Disord. (2022) 65:104015. doi: 10.1016/j.msard.2022.104015
101. Monaco, WE, Jones, JD, and Rigby, WF. Rituximab associated late-onset neutropenia-a rheumatology case series and review of the literature. Clin Rheumatol. (2016) 35:2457–62. doi: 10.1007/s10067-016-3313-y
102. Breuer, GS, Ehrenfeld, M, Rosner, I, Balbir-Gurman, A, Zisman, D, Oren, S, et al. Late-onset neutropenia following rituximab treatment for rheumatologic conditions. Clin Rheumatol. (2014) 33:1337–40. doi: 10.1007/s10067-014-2562-x
103. Zhang, L, Orban, M, Lorenz, M, Barocke, V, Braun, D, Urtz, N, et al. A novel role of sphingosine 1-phosphate receptor S1pr1 in mouse thrombopoiesis. J Exp Med. (2012) 209:2165–81. doi: 10.1084/jem.20121090
104. Mandala, S, Hajdu, R, Bergstrom, J, Quackenbush, E, Xie, J, Milligan, J, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. (2002) 296:346–9. doi: 10.1126/science.1070238
105. Dhib-Jalbut, S, and Marks, S. Interferon-beta mechanisms of action in multiple sclerosis. Neurology. (2010) 74:17–24. doi: 10.1212/WNL.0b013e3181c97d99
106. Haji, AM, Mofrad, MRK, and Schellekens, H. Interferon beta: from molecular level to therapeutic effects. Int Rev Cell Mol Biol. (2016) 326:343–72. doi: 10.1016/bs.ircmb.2016.06.001
108. Wolach, O, Bairey, O, and Lahav, M. Late-onset neutropenia after rituximab treatment: case series and comprehensive review of the literature. Medicine (Baltimore). (2010) 89:308–18. doi: 10.1097/MD.0b013e3181f2caef
109. Moore, DC . Drug-induced neutropenia: a focus on rituximab-induced late-onset neutropenia. P T. (2016) 41:765–8.
110. Kononowicz, JE, Farhan Ali, M, Palko, W, Pyper, S, and Agasthya, N. Rapidly progressing autoimmune hemolytic Anemia in a pediatric patient with COVID-19. Cureus. (2023) 15:e45633. doi: 10.7759/cureus.45633
111. van den Bemt, PM, Meyboom, RH, and Egberts, AC. Drug-induced immune thrombocytopenia. Drug Saf. (2004) 27:1243–52. doi: 10.2165/00002018-200427150-00007
112. Vayne, C, Guéry, EA, Rollin, J, Baglo, T, Petermann, R, and Gruel, Y. Pathophysiology and diagnosis of drug-induced immune thrombocytopenia. J Clin Med. (2020) 9:2212. doi: 10.3390/jcm9072212
113. Akita, S, Fujibayashi, K, Ueno, EI, Wakasa, M, Kawai, Y, and Kajinami, K. Thrombotic Microangiopathy after a 15-year treatment with interferon Beta-1b in a patient with multiple sclerosis: a case report and review of literature. Intern Med. (2023) 63:1113–7. doi: 10.2169/internalmedicine.1846-23
114. Mrabet, S, Dahmane, R, Raja, B, Fradi, A, Aicha, NB, Sahtout, W, et al. Thrombotic microangiopathy due to acquired complement factor I deficiency in a male receiving interferon-beta treatment for multiple sclerosis. Br J Clin Pharmacol. (2023) 89:1682–5. doi: 10.1111/bcp.15631
115. Taghavi, M, Stordeur, P, Collart, F, Dachy, B, Pozdzik, A, Mesquita, MDCF, et al. Interferon-β1a-induced thrombotic Microangiopathy: possible implication of the alternative pathway of the complement. Kidney Int Rep. (2022) 7:1917–21. doi: 10.1016/j.ekir.2022.05.002
116. Larochelle, C, Grand'maison, F, Bernier, GP, Latour, M, Cailhier, JF, and Prat, A. Thrombotic thrombocytopenic purpura-hemolytic uremic syndrome in relapsing-remitting multiple sclerosis patients on high-dose interferon β. Mult Scler. (2014) 20:1783–7. doi: 10.1177/1352458514523692
117. Lemtrada® . European Medicines Agency. (2024). Available at: https://www.ema.europa.eu/en/documents/product-information/lemtrada-epar-product-information_en.pdf (Accessed on May 14, 2024).
118. Tecfidera® . European Medicines Agency. (2024). Available at: https://www.ema.europa.eu/en/documents/product-information/tecfidera-epar-product-information_en.pdf (Accessed on May 14, 2024).
119. Gylenia® . European Medicines Agency. (2024). Available at: https://www.ema.europa.eu/en/documents/product-information/gilenya-epar-product-information_en.pdf (Accessed on May 14, 2024).
120. Tysabri . European Medicines Agency. (2024). Available at: https://www.ema.europa.eu/en/documents/product-information/tysabri-epar-product-information_en.pdf (Accessed on May 14, 2024).
121. Ocrevus® . European Medicines Agency. (2024). Available at: https://www.ema.europa.eu/en/documents/product-information/ocrevus-epar-product-information_en.pdf (Accessed on May 14, 2024).
122. Safety Monitoring for Multiple Sclerosis Patients on Disease Modifying Therapies . (n.d.). Available at: https://my.clevelandclinic.org/departments/neurological/depts/multiple-sclerosis/ms-approaches/safety-monitoring-multiple-sclerosis-patients-disease-modifying-therapies.
123. DMTs . Guidance on the Use and Monitoring of Disease Modifying Therapies (DMTs) for Multiple Sclerosis in Response to Risk of Coronavirus Pandemic. Available at: https://www.bsuh.nhs.uk/library/wp-content/uploads/sites/8/2020/04/Covid145_DMT-Monitoring-during-Pandemic.pdf.
124. Discontinuing Disease-Modifying Therapies in Multiple Sclerosis . (n.d.). Available at: https://practicalneurology.com/articles/2022-feb/discontinuing-disease-modifying-therapies-in-multiple-sclerosis.
125. DMTs . Guidance on the Use and Monitoring of Disease Modifying Therapies (DMTs) for Multiple Sclerosis in Response to Risk of Coronavirus Pandemic. (n.d.). Available at: https://www.bsuh.nhs.uk/library/wp-content/uploads/sites/8/2020/04/Covid145_DMT-Monitoring-during-Pandemic.pdf.
126. G-CSF . Guideline for the use of Granulocyte Colony Stimulating Factors (G-CSF) in Adult Haemato-Oncology Patients. (n.d.). Available at: https://archive.uhb.nhs.uk/Downloads/pdf/CancerPbGcsfAdultHaematoOncology.pdf.
Keywords: multiple scleorsis, DMT, hematological disorders, systematic reveiw, case reports, case series
Citation: Scavone C, Liguori V, Adungba OJ, Di Giulio Cesare D, Sullo MG, Andreone V, Sportiello L, Maniscalco GT and Capuano A (2024) Disease-modifying therapies and hematological disorders: a systematic review of case reports and case series. Front. Neurol. 15:1386527. doi: 10.3389/fneur.2024.1386527
Edited by:
Olwen C. Murphy, Johns Hopkins University, United StatesReviewed by:
Elda Ma Alba Suárez, Hospital Clínico San Carlos, SpainAlice Mariottini, University of Florence, Italy
Copyright © 2024 Scavone, Liguori, Adungba, Di Giulio Cesare, Sullo, Andreone, Sportiello, Maniscalco and Capuano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cristina Scavone, Y3Jpc3RpbmEuc2Nhdm9uZUB1bmljYW1wYW5pYS5pdA==
 Cristina Scavone
Cristina Scavone Valerio Liguori1,2
Valerio Liguori1,2 Olusola Jephthah Adungba
Olusola Jephthah Adungba Maria Giuseppa Sullo
Maria Giuseppa Sullo Liberata Sportiello
Liberata Sportiello Giorgia Teresa Maniscalco
Giorgia Teresa Maniscalco Annalisa Capuano
Annalisa Capuano