
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 01 May 2024
Sec. Stroke
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1384985
This article is part of the Research Topic Advances and controversies in ischemic stroke management: from prevention to diagnosis and acute treatment View all 95 articles
 Naveed Akhtar1†
Naveed Akhtar1† Mahesh Kate2†
Mahesh Kate2† Saadat Kamran1
Saadat Kamran1 Sujatha Joseph1
Sujatha Joseph1 Deborah Morgan1
Deborah Morgan1 Ryan Uy1
Ryan Uy1 Blessy Babu1
Blessy Babu1 Shobhna Shanti1
Shobhna Shanti1 Ashfaq Shuaib2*
Ashfaq Shuaib2*Objectives: Functional outcomes in patients with intracerebral hemorrhage (ICH) have not been well characterized in the Middle East and North Africa Region. We report the 30 and 90-day clinical outcomes in the native and expatriate of Qatar with ICH.
Methods: We evaluated the Glasgow Coma Scale (GCS), NIHSS, and imaging in the Qatar Stroke Registry (2013–22). The outcome measures were a modified Rankin Scale (mRS) at 90 days and mortality at 30 and 90 days. Unfavorable outcome was defined as mRS of 4–6. We performed non-parametric ROC analyses to measure the concordance index (C-index) to assess the goodness-of-fit of ICH score for predicting 30 day and 90-day mortality and functional outcome.
Results: 1,660 patients (median age of 49 (41.5–58) years; male 83.1%, expatriates 77.5%) with ICH, including supratentorial deep in 65.2%, cortical in 16.2%, infratentorial 16% and primary intraventricular in 2.5% were studied. The median baseline ICH volume was 7.5 (3.2–15.8) ml. An unfavorable outcome was seen in 673 (40.5%) patients at 90 days. The unfavorable 90-day outcome (mRS 4–6) was 49.2% in the native population vs. 44.4% in Africans, 39.0% in South Asian, 35.3% in Far Eastern, and 7.7% in Caucasians, p < 0.001. Mortality at 30 days and 90 days was 10.4 and 15.1%. Increasing age [OR (95% CI), 1.02 (1.00–1.03)], lower GCS [0.77 (0.73–0.80)], prior use of antiplatelet medications [1.82 (1.19–2.08)], higher ICH volume [1.03 (1.02–1.04)], and presence of any intraventricular hemorrhage [1.57(1.19–2.08)], were associated with unfavorable outcome.
Conclusion: In this relatively younger ICH cohort more than 75% were expatriates. The ICH volume, 90-day unfavorable outcome and mortality was lower in the expatriates compared to the local Arab population, likely related to the younger age and smaller size of the hemorrhages. Prognostic scoring systems may have to be modified in this population to avoid early withdrawal of care.
Despite improvements in medical and surgical therapies, acute intracerebral hemorrhage (ICH) carries a grave prognosis (1), with a 30-day mortality between 30–55% and fewer than 20% patients are functionally independent 6 months (2–5). Several tools are used to predict mortality and functional outcome following an ICH (6–11) with the ICH-score being one of the most common in clinical practice (6). A recent meta-analysis of 55 studies reported that ICH-score, while having a good discrimination with low scores, overestimated mortality with higher scores (12).
The clinical impact of ICH appears disproportionately high among lower-resource populations (13). In US-based studies, ICH incidence has been reported to be ≈1.6-fold greater among Black and Mexican (13). Internationally, ICH incidence is substantially higher in low-income countries and comprises ≈ 18% of all strokes (2–4). There are a few reports outlining prognosis of ICH from low resource countries (14–25). The number of patients in such series are small (19–22, 24), and the information collected retrospectively (18, 22, 23) making prediction modelling difficult to study. In a study from Netherlands, comparing the prognosis of ICH in ethnic populations, Surinamese men and women had higher rates if ICH and ischemic stroke compared to stroke patients from Morocco, Turkey, and the local Dutch population (26). In Scotland, the rates of ischemic stroke and ICH was higher in South Asian and Chinese compared to the White European origin subgroups (27).
In order to study admission trends, risk factors and prognosis in a large multiethnic population of patients from native (Arabs), and expatriates (South Asian, Southeast Asian), we investigated primary ICH admissions in the tertiary care centre in Qatar. We aimed to evaluate the outcome and prognosis in patients with ICH.
The Qatar Stroke Database was established in March 2013 at the Hamad General Hospital (HGH), Doha. All data on patients with suspected acute stroke, including transient ischemic attacks (TIAs), cerebral infarction, stroke mimics, ICH and cerebral venous thrombosis (CVT) are entered prospectively into the registry (28–32). Depending on the severity and location of the ICH, patients are admitted to the stroke ward, ICU or neurosurgery.
For inclusion in the study, the following criteria were use: A diagnosis of ICH that was proven by imaging and related to hypertension or amyloid angiopathy. Patients with primary ventricular ICH were included but hemorrhages related to AVMs or aneurysms were excluded. We also excluded ICH related to trauma, hemorrhagic transformation of an ischemic infarction and systemic coagulopathy. Patients with primary subdural hemorrhage and subarachnoid hemorrhage were also excluded.
Patient characteristics including age, sex, nationality, medical comorbidities, and prior medication were recorded. Ethnicity was defined based on social groups sharing cultural and language traits within the broad categories of Native Residents (Qatari and non-Qatari Arabs), South Asians (Indian, Pakistani, Bangladeshi, Sri Lankans, Nepalese and Myanmar) and the Southeast Asians (predominantly from Philippines). Data collected included pre-stroke modified Rankin scale (mRS), NIH Stroke Scale (NIHSS) score at admission, length of stay (LoS) in ED, LoS in the stroke ward (SW), general medical ward (GMW), neurosurgical ward or the ICH. The most recent population statistics from Qatar shows the local Qatari population to be 360,000 (11.6%). In the expatriates 2,760,000 (88.4%), South East Asians comprise the largest group comprising 1,680,000 (46.6%) followed by non-Qatari Arabs 535,000 (17.1%) and individuals from Philippines 236,000 (7.6%).
Patients or the public WERE NOT involved in the design, or conduct, or reporting, or dissemination plans of our research.
Patients’ plain head CT scans were analyzed to identify the following data: location (cortical, basal ganglia, thalamus, brainstem or cerebellum), ICH volume (cm3) measured using the method with the largest length in three dimensions divided by two (equation: ABC/2); presence or absence of intraventricular hemorrhage IVH. For the purpose of the present study, we combined the location of the hematoma in the basal ganglion and thalamus. Our main objective for the localization was to compare prognosis in patients with cortical, “deep” sub-cortical and brainstem hemorrhages. We did not use any cut-offs for the size of the hematoma in the study populations.
All patients had Glasgow Coma Scale (GCS) measured at admission and regularly during admission. The primary outcome measures were unfavorable mRS (4–6) at 90-day, 30-day and 90-day mortality. We also compared good outcome at 30-day and 90-days. Good outcome was defined as an mRS of 0–3. As a secondary objective, we also evaluated the favorable outcome as mRS of 0–2. This data is shown in Supplementary Table S1. The 30-day and 90-day data was collected by trained study coordinators by in person visits or via telephone assessments. The ICH-score was used to measure prognosis at admission. The ICH Score was the sum of individual points assigned as follows: GCS score 3 to 4 (=2 points), 5 to 12 (=1), 13 to 15 (=0); age 80-years yes (=1), no (=0); infratentorial origin yes (=1), no (=0); ICH volume > 30 cm3 (=1), <30 cm3 (=0); and intraventricular hemorrhage yes (=1), no (=0) (6).
We have previously shown that the percentage of patients with diabetes is high in the local and expatriate population in Qatar (28–32). An additional objective of our analysis was to evaluate the presence of diabetes on the prognosis in our ICH population.
Continuous variables (age, systolic BP, diastolic BP, NIHSS, GCS, BMI and ICH volume) are presented as median values with interquartile range. Categorical variables (sex, residential status, mode of arrival to the hospital, medical comorbidities, concomitant medications, hemorrhage location, ICH score, hospital admission status, and discharge disposition) are presented as counts and percentages. The study population was divided in two groups patients with favorable (mRS 0–3) and unfavorable (mRS 4–6) 90-day outcomes. Unadjusted univariable binary logistic regression analyses were performed to assess differences in continuous variables. The chi-square test was employed to compare categorical variables between groups. Variables with a p-value <0.05 were used for regression analyses. Multivariable binary logistic regression analyses were used to assess the effects of the individual predictors with respect to 30-day, 90-day mortality and 90-day functional outcome. We also performed non-parametric receiver operator curve (ROC) analyses to measure the area under the curve (AUC) for all the variables used in the multivariable logistic regression analysis. We performed non-parametric ROC analyses to measure the concordance index (C-index) to assess the goodness-of-fit of ICH score for predicting 30 day, 90-day mortality and functional outcome at 90 days. All statistical analysis was performed using STATA 18.0 BE (StatCorp LLC Texas, United States).
The study was approved by the Committee for Human Ethics Research, Academic Health Service at HMC (MRC-01-18-102).
1,660 patients with primary ICH were available for analysis. As shown in Figure 1, ICH represented 14.8% (1,674/11338) patients with final diagnosis of stroke. The median (IQR) age was 49 (41.5–58) years, men were (1,379, 83.1%) and expatriates were (1,286, 77.5%).
The details of the clinical features, risk factors, imaging, NIHSS and GCS at admission, ICH score, location and volume, admission location, and surgical intervention are summarized in Table 1. We compared the variables in two groups: favorable outcome (mRS 0–3) versus unfavorable outcome (mRS 4–6) at 90 days (Figure 2). The comparison of mRS 0–2 vs. 3–6 is shown in Supplementary Table S4. A favorable outcome was seen in 987 (59.5%). The better outcome in men compared to women may be related to the relatively young age of the male (48, 41–57 years) patients compared to female (53, 44–66 years, OR 0.96 95%CI 0.95–0.97). The older women reflect the higher percentage of native residents (125, 33.4%) compared to expatriate women (156, 12.1%, OR 3.6 95%CI 2.7–4.8) in the study population.
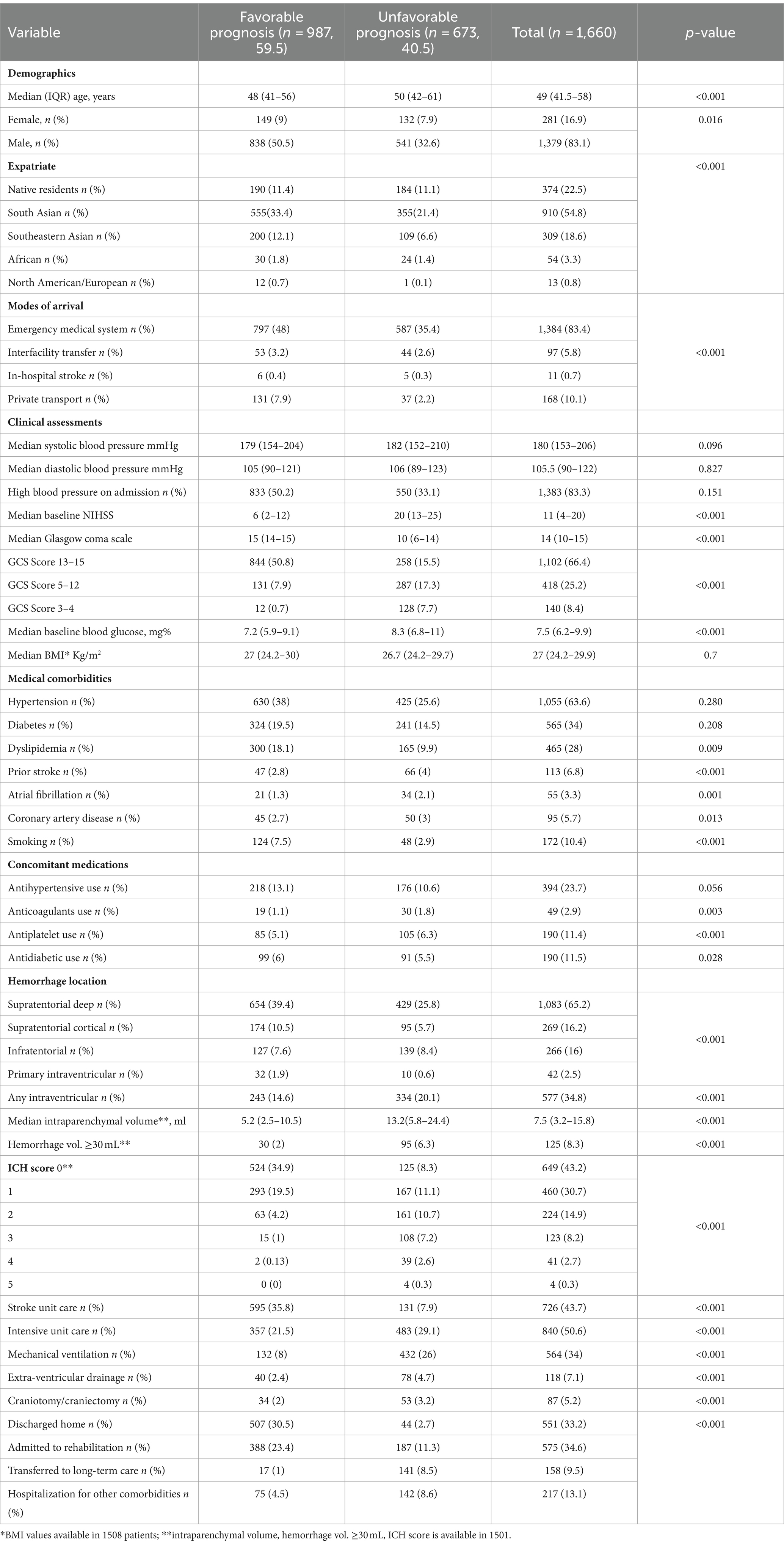
Table 1. Comparison of features related in ICH patients as defined by favorable (mRS 0–3) and unfavorable (mRS 4–6) outcome.

Figure 2. Prognosis at 90-days mentioned as Favorable (mRS 0-3) and Unfavorable (mRS 4-6) outcome- the unfavorable 90-day outcome was 49.2% in the native Arabs, 44.4% in Africans, 39.0% in South Asian, 35.3% in Far Eastern, and 7.7% in Caucasians, p< 0.001.
At 30 days 1,487 (89.6%) of patients were alive and 173 (10.4%) died. Most of the deaths were recorded during the hospitalization 156 (9.4%). The mortality at 90 days increased to 251 (15.1%), predominantly males (202/251, 80.5%).
Favorable outcome was seen in 59.5% of patients. Individuals with a favorable outcome were significantly younger, and had lower random glucose levels, lower NIHSS and higher GCS scores as shown in Table 1. Patients with favorable outcome were more likely to have smaller ICH volume on the initial imaging and supra-tentorial location. Active smoking and dyslipidemia, for unexplainable reasons, were more common in patients with a favorable outcome. The relationship of treatment including decompression surgery and hematoma evacuation is shown in Table 1.
The most common location for the ICH was sub-cortical in the basal ganglia or the thalamus (1,072/1660; 64.6%). ICH location was hemispheric in 280 (16.9%) patients, cerebellar in 152 (9.1%), brainstem in 114 (6.9%) patients, and primary intraventricular 42 (2.5%) patients. Most patients had small hemorrhages. The volume of the hemorrhage was less than 10 mL in patients 906 (60.4%) patients. The volume of the hematoma was between 10–19.9 mL in 311 (20.7%), 20–29.19 in 159 (10.6%) and more than 30 mL in 125 (8.3%) patients. The risk of early mortality and unfavorable outcome increased with increasing volume of the ICH. For each volume of the ICH, patients with infratentorial ICH were more likely to have a higher mortality at 30 and 90 days as shown in Figures 3A,B, 4. As is evident from Figure 4, the actual 30-day mortality in our patients was lower at all points of the ICH-score, when compared to the original publication (6). The younger age, smaller volume of ICHs and fewer infratemporal locations likely represent lower 30-day mortality in our study. There was a proportionate increase in unfavorable outcome with increasing ICH-score as shown in Figure 5.
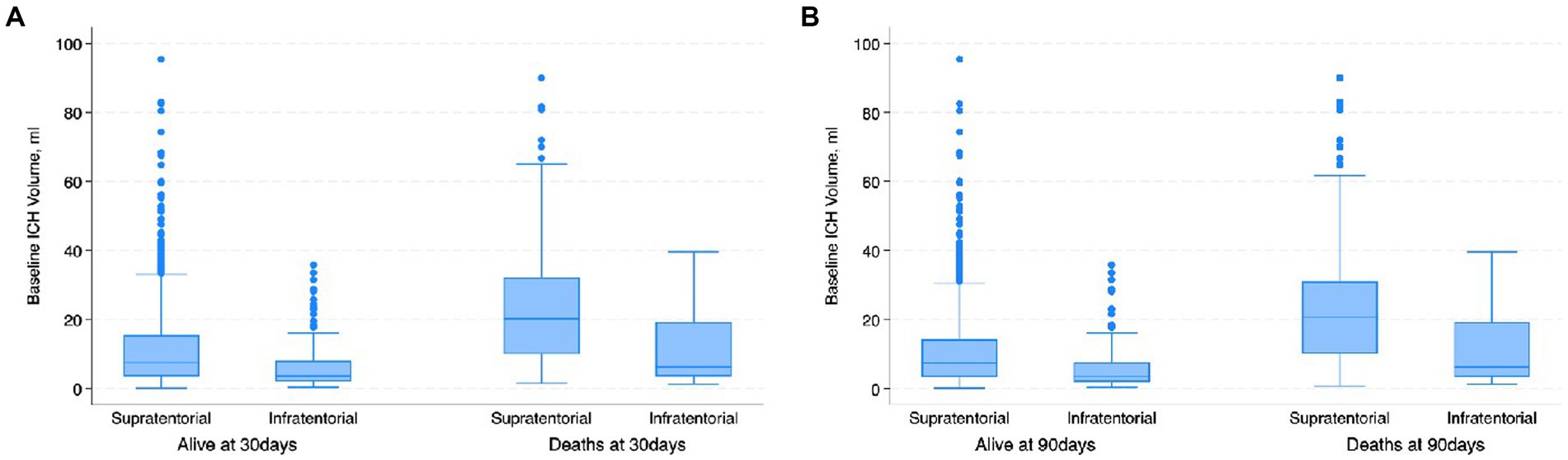
Figure 3. Boxplot. (A) Shows the distribution of the baseline ICH volume with respect to the location of the ICH and 30-day mortality. Patients with infratentorial locations had higher mortality at lower ICH volume; (B) shows the distribution of the baseline ICH volume with respect to the location of the ICH and 90-day mortality. Patients with infratentorial locations had higher mortality at lower ICH volume.
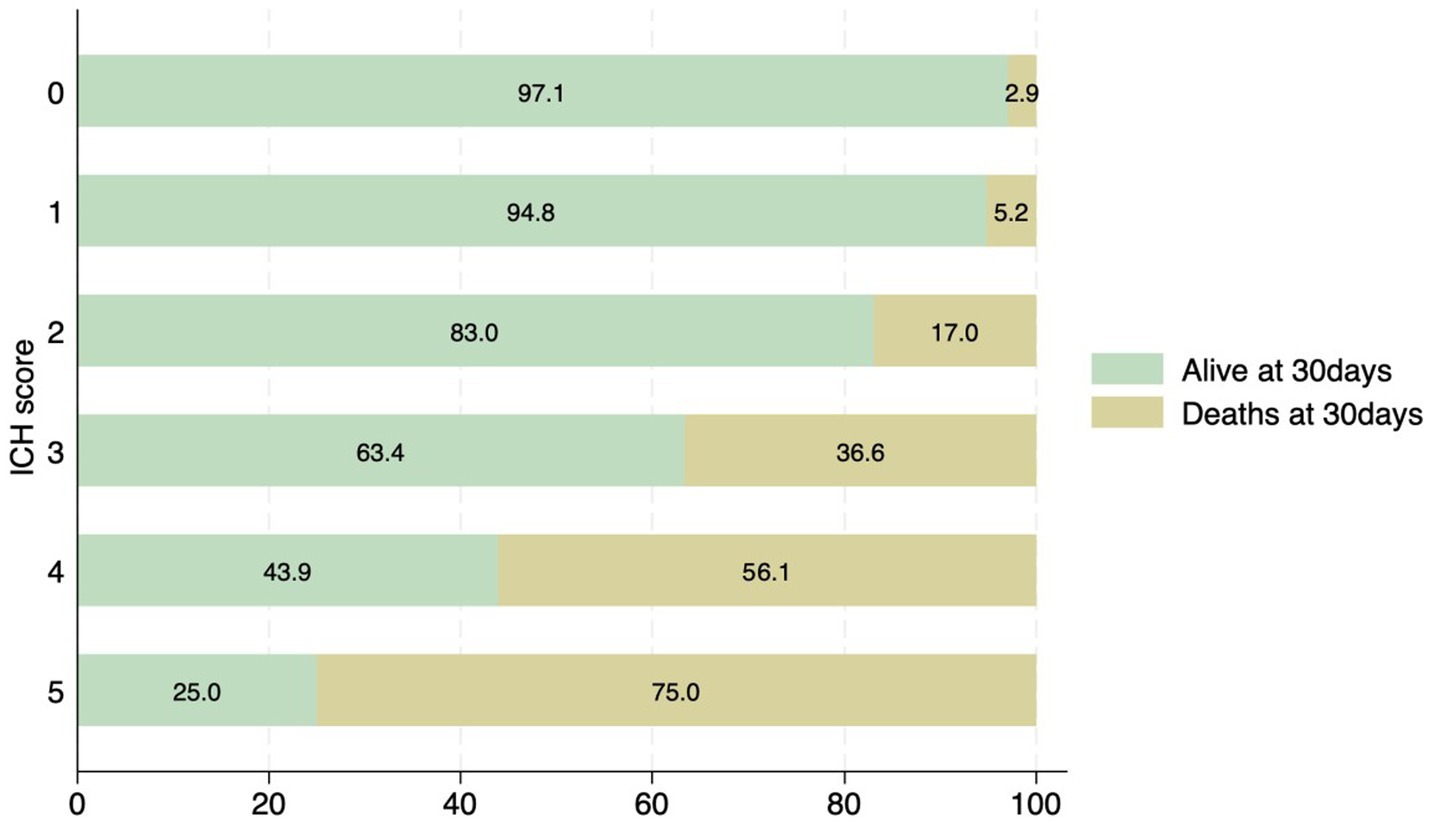
Figure 4. Distribution of patients with ICH score and associated mortality at 30 days. None of the patients had an ICH score of 6.
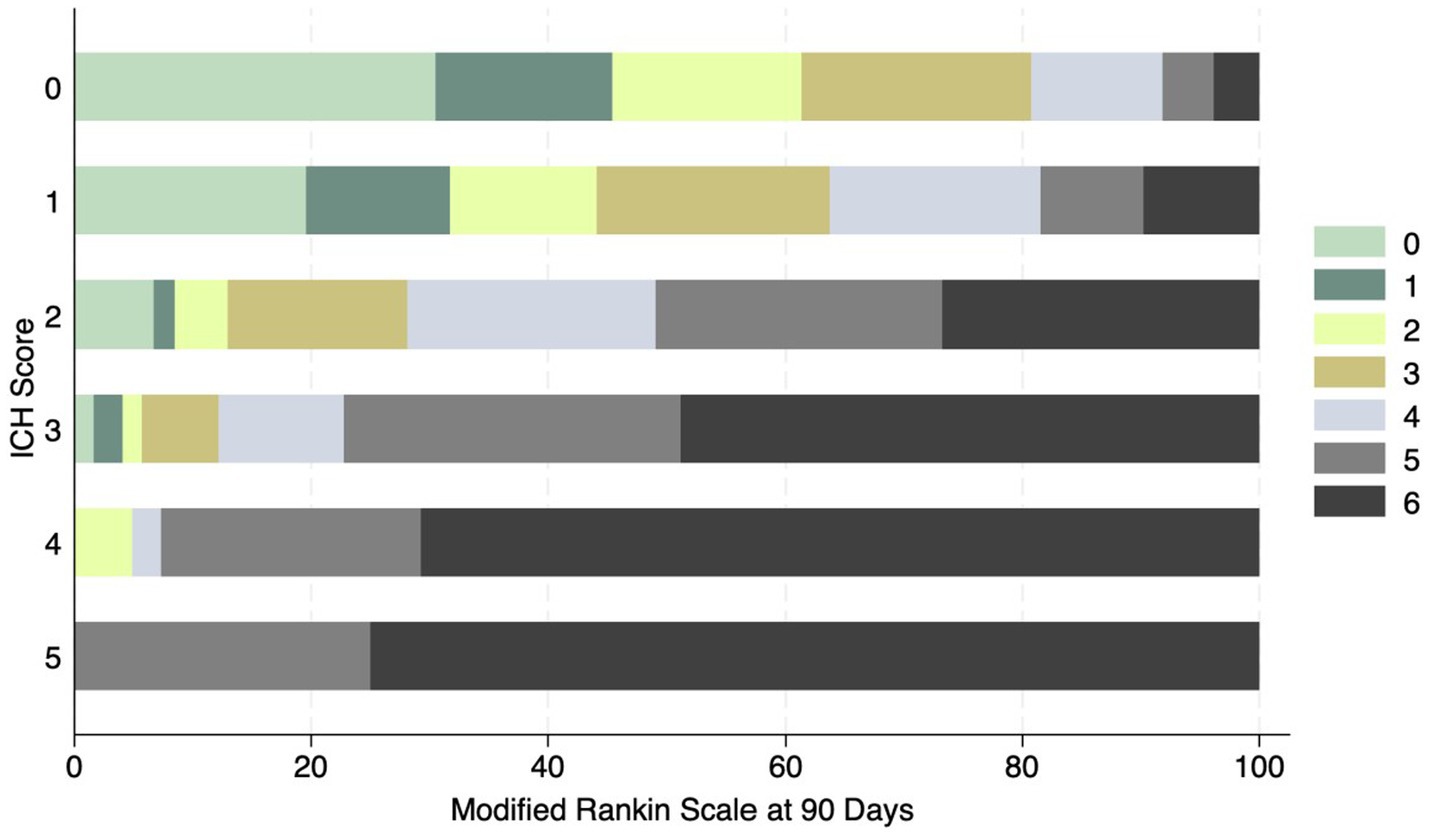
Figure 5. Distribution of mRS at 90 days and ICH score in ICH patients. No patient had a score of 6.
There were 49 (3%) patients on anticoagulation and 190 (11.4%) on antiplatelet medications at the time of the ICH (Supplementary Tables S1, S2). These patients were older (61 (55–74) vs. 48 (41–56), p < 0.001), and were more likely to have diabetes, CAD, atrial fibrillation and prior stroke. The severity of stroke as measured on the GCS and the NIHSS was not significantly different in patients with or without anticoagulants. ICH volume was also not significantly larger in patients on anticoagulation. Hemispheric location of the ICH was more common in patients taking anticoagulation (35.4% vs. 16.8%, p < 0.001). Despite the similar volume, and as shown in Supplementary Table S1, the 30-day (22.4% vs. 10.1%; p = 0.005) and 90-day (28.6% vs. 14.7%; p = 0.008) mortality was higher in the anticoagulated patients. The percentage of patients with a favorable outcome (mRS 0–3) at 90-days was also lower in patients on anticoagulation (38.8% vs. 61.2%. p < 0.003).
As shown in Supplementary Table S2, patients on antiplatelets were older (63.2 ± 12.1 vs. 49.3 ± 12.1, p = 0.001), and were more likely to have diabetes, CAD, atrial fibrillation and prior stroke. The severity of stroke was not significantly different in patients with or without antiplatelets. ICH volume was also similar in patients on antiplatelets. Hemispheric location of the ICH was more common in patients taking antiplatelets (25.1% vs. 16.3%, p < 0.001). Despite the similar volume of the ICH, and as shown in Supplementary Table S2, the 30-day (14.7% vs. 9.9%; p = 0.04) mortality was higher in patients with antiplatelets. The percentage of patients with a favorable outcome (mRS 0–3) at 90-days was also lower in patients on antiplatelet medications (44.7% vs. 55.3%. p < 0.001).
There were 564 (34%) patients with known diabetes at the time of the ICH. As Supplementary Table S3, these patients were older (56.6 ± 13.0 vs. 47.1 ± 12.4, p < 0.001), and were more likely to have prior stroke history, CAD and atrial fibrillation. The severity of stroke was higher in patients with diabetes. A GCS of 13–15 was seen in 34.4% of patients with diabetes and 47.8% of patients without diabetes (p < 0.001). Fewer patients with diabetes had an ICH volume < 10 mL (56.4 vs. 60.6, p < 0.001) and hemispheric location of the ICH was more common in patients with diabetes (19.8% vs. 16.6%, p = 0.02). The 30-day (13.2% vs. 8.8%; p < 0.001) and 90-day mortality (18.8% vs. 13.6%; p < 0.001) was significantly higher in the diabetic patients.
The multivariable analysis for the 30-day, 90-day mortality and 90-days unfavorable outcome are shown in Table 2. Baseline lower GCS, baseline ICH volume, and infratentorial location, presence of any intraventricular hemorrhage, no surgical intervention was all associated with a higher risk of 30-day and 90-day mortality and higher 90-day unfavorable outcome. The receiver operator curve analysis showed lower GCS score had the highest area-under the curve 0.80, 0.81 and 0.79 among all the variables to predict 30-day, 90-day mortality and 90-day unfavorable outcome (Table 2). Baseline ICH volume showed moderate concordance with AUC of 0.69, 0.71 and 0.71 to predict 30-day, 90-day mortality and 90-day unfavorable outcome. Other variables were fair.
Intracerebral hemorrhage comprised 14.8% of consecutive stroke patients admitted with acute stroke in Qatar. The patients were young, had male predominance and from multiple ethnicities, reflecting the population dynamics in Qatar where ~85% of the population is expatriate and mostly comprised of young male workers (28–32). The mortality at 30 days (10.4%) and 90 days (15.1%) was lower in our patients when compared to the previous reports of 30–55% in the literature (2–5). Smaller volume of the hematoma in most of our subjects [only 125 (8.3%) of patients with ICH of ≥30.0 mL/cm3], and smaller number of patients with low GCS of 3–4 [140 (8.4%)] likely contributed to the lower mortality. We are not sure of the reasons of the smaller size of the ICH in this population. Perhaps the younger age, especially in the patients from South East and Far East Asia may have been a factor for the smaller hemorrhages. We also noted a significantly lower mortality in our patients. It is possible that admission to a stroke unit or ICU in a majority of patients may have also contributed to the lower mortality. Maximally treated ICH patients have been shown to have lower mortality in a previous study (11). Increasing age, presence of vascular risk factors, especially diabetes, and the use of antithrombotic medications, especially anticoagulants, were most likely to associated with an unfavorable outcome.
Most studies on ICH focus on mortality, especially early deaths in the hospital and within the initial 30 days. Patients who survive the initial insult are however left with significant disability. The mRS is used globally to determine recovery following ischemic stroke and has been used in occasional ICH studies to evaluate recovery at 90 days to a year (21, 23, 33–35). An mRS of 0–3 (favorable outcome) at 90 days following the ICH was evident in 59.5% of patients in our study. In a smaller study of 243 patients, only 51% of patients reached an mRS of 0–2 at 3 months follow-up and 56% by one-year follow-up (34). The larger study with 3,255 patients from China also showed an mRS of 0–2% in 49% of patients at 90- days and 53% by one year (23). In the Swiss Stroke Registry, 2,650 patients with ICH were followed for functional recovery and 33.2% showed good functional recovery (mRS 0–2) at 3 months (35). In a study of 919 patients from the CLEAR-III and MISTIE-III trials, 11.5% died within 30 days and an mRS of 0–3 was seen in only 14.7% at 30 days (36). Finally, in the recent study from Montreal, similar to our study, favorable outcome (an mRS of 0–3 at 3 months) was evident in 50 percent of patients (33, 37). The mRS at 90-days may therefore be an important measure of recovery in addition to the mortality at 30-days following an ICH to determine prognosis.
There are some limitations to our research. Although the patients were all entered into the database prospectively, this is a retrospective analysis of the registry. Secondly, the analysis is from a single center in one country. The comparison of clinical and outcome data in a large number of patients from multiple ethnicities is, we believe, a major strength of the study. Thirdly, our follow-up is limited to the 90-day outcome as part of the registry data collection. As shown in the study by Hemphill from USA (6) and Wang et al. from China (23), recovery continues well beyond the initial 90 days, we do not have long-term outcome information from our research.
In summary, our study outlines the clinical and radiological features of ICH in a large multiethnic cohort of patients from a single center in Qatar. Our patients were younger and the hematomas were most frequently seen in the sub-cortical thalamic and basal ganglion region. The 30-day mortality was significantly better than what has been previously reported. The lower mortality may be related to the younger age at presentation, fewer patients with sub-tentorial hemorrhages and the small volume of the hematomas. Despite the lower mortality at 30 days, favorable outcome was evident in only 44.5% patients at 90 days.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Institutional Review Board of the Medical Research Centre at Hamad Medical Corporation (MRC-01-18-102). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
NA: Writing – original draft, Writing – review & editing. MK: Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. SK: Methodology, Writing – review & editing. SJ: Data curation, Writing – review & editing. DM: Data curation, Methodology, Writing – review & editing. RU: Data curation, Writing – review & editing. BB: Data curation, Methodology, Writing – review & editing. SS: Data curation, Methodology, Writing – review & editing. AS: Conceptualization, Methodology, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1384985/full#supplementary-material
1. Sheth, KN. Spontaneous intracerebral hemorrhage. N Eng J Med. (2023) 387:1589–96. doi: 10.1056/NEJMra2201449
2. Morotti, A, Boulouis, G, Dowlatshahi, D, Li, Q, Shamy, M, al-Shahi Salman, R, et al. Intracerebral hemorrhage expansion,: definitions, predictors, and prevention. Lancet. (2023) 22:159–71. doi: 10.1016/S1474-4422(22)00338-6
3. Cordonnier, C, Demchuk, A, Ziai, W, and Anderson, CS. Intracerebral haemorrhage: current approaches to acute management. Lancet. (2018) 392:1257–68. doi: 10.1016/S0140-6736(18)31878-6
4. Magid-Bernstein, J, Polster, RGS, Srinath, A, Romanos, S, and Awad, IA. Cerebral hemorrhage: pathophysiology, treatment and future directions. Circ Res. (2022) 130:1204–29. doi: 10.1161/CIRCRESAHA.121.319949
5. van Asch, CJJ, Luitse, MJ, Rinkel, GJE, van der Tweel, I, Algra, A, and Klijn, CJM. Incidence, case fatality, and functional outcome of intracerebral hemorrhage over time according to age, sex and ethnic origin: a systemic review and meta-analysis. Lancet Neurol. (2010) 9:167–76. doi: 10.1016/S1474-4422(09)70340-0
6. Hemphill, JC, Bonovich, DC, Bermertis, L, Manley, GT, and Johnston, SC. The ICH score. Stroke. (2001) 32:891–7. doi: 10.1161/01.STR.32.4.891
7. Tuhrim, S, Dambrosia, JM, Price, TR, Mohr, JP, Wolf, PA, Hier, DB, et al. Intracerebral hemorrhage external validation and extension of a model for prediction of 30-day survival. Ann Neurol. (1991) 29:658–63. doi: 10.1002/ana.410290614
8. Broderick, JP, Brott, TG, Duldner, JE, Tomsick, T, and Huster, G. Volume of intracerebral hemorrhage: a powerful and easily-to-use predictor of 30-day mortality. Stroke. (1993) 24:987–93. doi: 10.1161/01.STR.24.7.987
9. Lisk, DR, Pasteur, W, Rhoades, H, Putman, RD, and Grotta, JC. Early presentation of hemispheric intracerebral hemorrhage: prediction of outcome and guidelines for treatment alloca5tion. Neurology. (1994) 44:133–9. doi: 10.1212/WNL.44.1.133
10. Fahlström, A, Nittby Redebrandt, H, Zeberg, H, Bartek, J, Bartley, A, Tobieson, L, et al. A grading scale for surgically treated patients with spontaneous supratentorial intracerebral hemorrhage: the surgical Swedish ICH score. J Neurosurg. (2020) 133:800–7. doi: 10.3171/2019.5.JNS19622
11. Sembill, JA, Gerner, ST, Volbers, B, Bobinger, T, Lücking, H, Kloska, SP, et al. Severity assessment in maximally treated ICH patients. Neurology. (2017) 89:423–31. doi: 10.1212/WNL.0000000000004174
12. Gregório, T, Pipa, S, Cavaleiro, P, Atanásio, G, Albuquerque, I, Castro Chaves, P, et al. Original intracerebral hemorrhage score for the prediction of short-term mortality in cerebral hemorrhage: systemic review and meta-analysis. Crit Care Med. (2019) 47:857–64. doi: 10.1097/CCM.0000000000003744
13. Greenberg, SM, Ziai, WC, Cordonnier, C, Dowlatshahi, D, Francis, B, and Goldstein, JN. 2022 Guidelines for the management of patients with spontaneous intracerebral hemorrhage: a guideline from the American heart association/American Stroke Association. Stroke. (2022) 53:e282–361. doi: 10.1161/STR.0000000000000407
14. Fegin, VL, Lawes, CM, Bennett, DA, Baker-Collo, SL, and Parag, V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systemic review. Lancet Neurol. (2009) 8:355–69. doi: 10.1016/S1474-4422(09)70025-0
15. GBD Stroke Collaborators. Global, regional, and national stroke and its risk factors, 1990-2019: a systemic analysis for the global burden of disease study. Lancet Neurol. (2019) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
16. Saqqur, M, Salam, A, Akhtar, N, Ali, M, Joseph, S, Khan, A, et al. The incidence rate, mortality rate, and functional outcome of intracerebral hemorrhage according to age, sex, and ethic group in the state of Qatar. Int J Stroke. (2020) 15:NP3. doi: 10.1177/1747493019897856
17. Saqqur, M, Salam, A, and Akhtar, N. The prevalence, mortality rate and functional outcome if intracerebral hemorrhage according to age, sex, and ethnic group in the state of Qatar. Clin Neurol Neurosurg. (2020) 199:105255:106255. doi: 10.1016/j.clineuro.2020.106255
18. Wasay, M, Khatri, IA, Khealani, B, and Afaq, M. Temporal trends in risk factors and outcome of intracerebral hemorrhage over 18 years at a tertiary care hospital in Karachi, Pakistan. J Stroke CVD. (2012) 21:289–92. doi: 10.1016/j.jstrokecerebrovasdis.2010.09.001
19. Tshikwela, ML, and Longo-Mbenza, B. Spontaneous intracerebral hemorrhage: clinical and computed tomography findings in predicting in-hospital mortality in central Africans. J Nuerosci Rural Pract. (2012) 3:115–20. doi: 10.4103/0976-3147.98205
20. Albakr, A, Almatar, A, AlFajri, A, Zafar, A, Nazish, S, Shahid, R, et al. Important factors to expect the outcome after intracerebral hemorrhage. Neurologist. (2023) 28:310–5. doi: 10.1097/NRL.0000000000000491
21. Cheung, RT, and Zou, L-Y. Use of the original, modified, or new intracerebral hemorrhage score to predict mortality and morbidity after intracerebral hemorrhage. Stroke. (2003) 34:1717–22. doi: 10.1161/01.STR.0000078657.22835.B9
22. Buensuceso, AM. Predictors of mortality based on CT scan findings of patients admitted due to hypertensive intracerebral hemorrhage at the Philippine heart center. Phil Heart Center J. (2007) 13:155–60.
23. Wang, W, Lu, J, Wang, C, Wang, Y, Li, H, Zhao, X, et al. Prognostic value of ICH score and ICH-GS score in Chinese intracerebral hemorrhage patients: analysis from the China National Stroke Registry (CNSR). PLoS One. (2013) 8:e77429. doi: 10.1371/journal.pone.0077429
24. Narayan, SK, Sivaprasad, P, Sushma, S, Sahoo, RK, and Dutta, TK. Etiology, and outcome determinants of intracerebral hemorrhage in a south Indian population: a hospital-based study. Ann Indian Acad Neurol. (2012) 15:263–6. doi: 10.4103/0972-2327.104333
25. Qui, L, Upadhyaya, T, Qi See, ANA, Ng, YP, and King, NKK. Incidence of recurrent intracerebral hemorrhage in a multiethnic south Asian population. J Stroke CVD. (2016) 26:666–72. doi: 10.1016/j.jstrokecerebrovasdis.2016.10.044
26. Agyemang, C, van Oeffelen, AAM, Norredam, M, Kappelle, LJ, Klijn, CJM, Bots, ML, et al. Ethnic disparities in ischemic stroke, intracerebral hemorrhage and subarachnoid hemorrhage incidence in the Netherlands. Stroke. (2014) 45:3236–42. doi: 10.1161/STROKEAHA.114.006462
27. Bhopal, RS, Bansal, N, Fischbacher, CM, Brown, H, and Capewell, SScottish Health and Ethnic Linkage Study. Ethnic variations in the incidence and mortality of stroke in the Scottish health and ethnicity linkage study of 4.65 million people. Eur J Prev Cardiol. (2012) 19:1503–8. doi: 10.1177/1741826711423217
28. Akhtar, N, Kamran, S, Singh, R, Cameron, P, D’Souza, A, Imam, Y, et al. Beneficial effects of implementing stroke protocols require establishment of a geographically distinct unit. Stroke. (2015) 46:3494–501. doi: 10.1161/STROKEAHA.115.010552
29. Akhtar, N, Salam, A, Kamran, S, Bourke, P, Joseph, S, Santos, M, et al. Ethnic variation in acute cerebrovascular disease: analyses from the Qatar stroke registry. Eur Stroke J. (2016) 1:231–41. doi: 10.1177/2396987316663776
30. Akhtar, N, Kamran, S, Singh, R, Malik, RA, Deleu, D, Bourke, PJ, et al. The impact of diabetes on outcome after acute ischemic stroke: a prospective observational study. JSCVD. (2019) 28:619–26. doi: 10.1016/j.jstrokecerebrovasdis.2018.11.003
31. Wilkins, SS, Bourke, P, Salam, A, Akhtar, N, D'Souza, A, Kamran, S, et al. Functional stroke mimics: incidence and characteristics at a primary stroke center in the Middle East. Psychosom Med. (2018) 80:416–21. doi: 10.1097/PSY.0000000000000563
32. Wilkins, SS, Akhtar, N, Salam, A, Bourke, P, Joseph, S, Santos, M, et al. Acute post-stroke depression at a primary stroke center in the Middle East. PLoS. (2018) 13:e0208708. doi: 10.1371/journal.pone.0208708
33. Abulhasan, YB, Teitelbaum, J, Al-Ramadhani, K, Morrison, KT, and Mr, A. Functional outcomes and mortality in patients with intracerebral hemorrhage after intensive medical and surgical support. Neurology. (2023) 100:e1985–95. doi: 10.1212/WNL.0000000000207132
34. Hempill, JC, Farrant, M, and Neill, TA. Prospective validation of the ICH score for 12-month functional outcome. Neurology. (2009) 73:1088–94. doi: 10.1212/WNL.0b013e3181b8b332
35. Palm, E, Henschke, N, Wolf, J, Zimmer, K, Safer, A, Schröder, RJ, et al. Intracerebral hemorrhage in a population-based stroke registry (LuSSt): incidence, etiology, functional outcome and mortality. J Neurol. (2013) 260:2541–50. doi: 10.1007/s00415-013-7013-0
36. Shah, VA, Thompson, RE, Yenokyan, G, Acosta, JN, Avadhani, R, Dlugash, R, et al. One-year outcome trajectories and factors associated with functional recovery among survivors of intracerebral and intraventricular hemorrhage with initial severe disability. JAMA Neurol. (2022) 79:856–68. doi: 10.1001/jamaneurol.2022.1991
Keywords: hemorrhagic stroke, outcome, major adverse cardiovascular events, mortality, hematoma volume, ICH score
Citation: Akhtar N, Kate M, Kamran S, Joseph S, Morgan D, Uy R, Babu B, Shanti S and Shuaib A (2024) Short-term functional outcomes of patients with acute intracerebral hemorrhage in the native and expatriate population. Front. Neurol. 15:1384985. doi: 10.3389/fneur.2024.1384985
Received: 11 February 2024; Accepted: 19 April 2024;
Published: 01 May 2024.
Edited by:
Giovanni Merlino, Udine University Hospital, ItalyReviewed by:
Mohammad Wasay, Aga Khan University, PakistanCopyright © 2024 Akhtar, Kate, Kamran, Joseph, Morgan, Uy, Babu, Shanti and Shuaib. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ashfaq Shuaib, YXNoZmFxLnNodWFpYkB1YWxiZXJ0YS5jYQ==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.