
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Neurol. , 05 July 2024
Sec. Movement Disorders
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1382534
This article is part of the Research Topic An Interdisciplinary Approach Towards a Greater Understanding of Parkinson's Disease View all 6 articles
Primary familial brain calcification (PFBC), also known as Fahr’s disease, is a central nervous system calcium deposition disorder with symmetrical basal ganglia calcification. Most PFBC cases are caused by SLC20A2 gene variant. We report a Chinese female patient with PFBC and dopamine-responsive parkinsonism who had motor fluctuations and dyskinesia and recovered effectively after symptomatic medication adjustment. A novel heterozygous missense variant was found by whole-exome sequencing and proven harmful by family validation and genetic analysis. This example expands the phenotype of SLC20A2-associated PFBC patients and shows the clinical efficacy of dopaminergic replacement treatment.
Primary familial brain calcification (PFBC), originally known as Fahr’s disease, is a hereditary neurodegenerative disorder characterized by multiple symmetrical intracranial calcifications. The main clinical features include a combination of various movement disorders and neuropsychiatric symptoms (1). Over the past 12 years, seven genes associated with PFBC pathogenesis have been identified (2), including four dominant (SLC20A2, XPR1, PDGFB, and PDGFRB) and three recessive (MYORG, JAM2, and CMPK2) genes. Variants in SLC20A2 are the most common, with patients with PFBC with this allele having a higher probability of developing parkinsonism (1). However, unlike patients with Parkinson’s disease (PD), those with PFBC and a parkinsonism phenotype usually respond poorly to levodopa (3). Here, we report the case of a Chinese woman with PFBC due to a novel SLC20A2 variant, who presented with parkinsonism while responding well to dopaminergic therapy, exhibiting symptom fluctuations and levodopa-induced dyskinesia during progression.
The proband (patient I-2) was a 56-year-old Chinese woman with slowly deteriorating extremity tremors, rigidity, and bradykinesia for 4 years, and involuntary head, neck, perioral, and trunk movements for 3 years (see the timeline in Figure 1). The patient had no history of stroke, brain injury, dopamine-blocking medication, toxic agent exposure, or familial inheritance. In 2015, the patient initially presented with a right lower limb static tremor and rigidity, slowing daily activities. The symptoms were improved after Madopar (375 mg/d) monotherapy based on PD diagnosis. In 2019, the patient experienced tremors in the right upper and left limbs, rigidity, bradykinesia, and gait freezing. Medication onset was delayed, and the ON phase was shortened; Madopar dosage was later increased (750 mg/d). The drug improved parkinsonism; however, the patient experienced involuntary head and trunk swinging, mouth licking, and shoulder shrugging. Levodopa-induced dyskinesia disappeared during the OFF phase. The patient was referred to our hospital in 2022. The patient had diminished facial expressions and no eye movement abnormalities, dysarthria, or dysphagia during the ON phase of the physical assessment. The patient trunk slanted forward, arm swing was decreased, while beginning and turning were delayed. No dysmetria or dizziness was observed upon standing up. With increasing muscular tension, the right side became heavier than the left. The left Babinski’s sign was positive. The score on the Mini-Mental State Examination (MMSE) was 22/30 (Corrected), while the score on the Montreal Cognitive Assessment (MoCA) was 20/30 (Corrected). The patient’s (Unified Parkinson’s Disease Rating Scale) UPDRS score during the “OFF” phase was 126. After adjusting the anti-parkinsonian medication regimen, the patient’s “ON” phase UPDRS score was 49, indicating a 61.1% improvement. Laboratory tests revealed normal liver function, electrolyte (including calcium and phosphorus metabolism), metal trace elements such as copper and iron, thyroid hormones and antibodies, parathyroid hormone, tumor indicators, and vitamin metabolism. Brain computed tomography (CT) revealed multiple symmetrical calcifications (Figures 2A–C). Whole-exome sequencing (WES) identified a c.613G>C (p. Val205Leu) variant in SLC20A2. Sanger sequencing confirmed that the proband’s son (patient II-1) had the same variant (Figure 2E), exhibiting symmetrical calcifications on brain CT (Figure 2D) despite lacking symptoms. The proband was diagnosed with SLC20A2-associated PFBC. We lowered the dose of madopar (375 mg/d) and added stalevo (325 mg/d) and pramipexole (0.75 mg/d). No significant disease progression was documented in outpatient follow-ups. The patient’s UPDRS score was 124 during the “OFF” phase (after a 12-h medication withdrawal) and 52 during the “ON” phase. Furthermore, the proband’s son continues to show no symptoms.
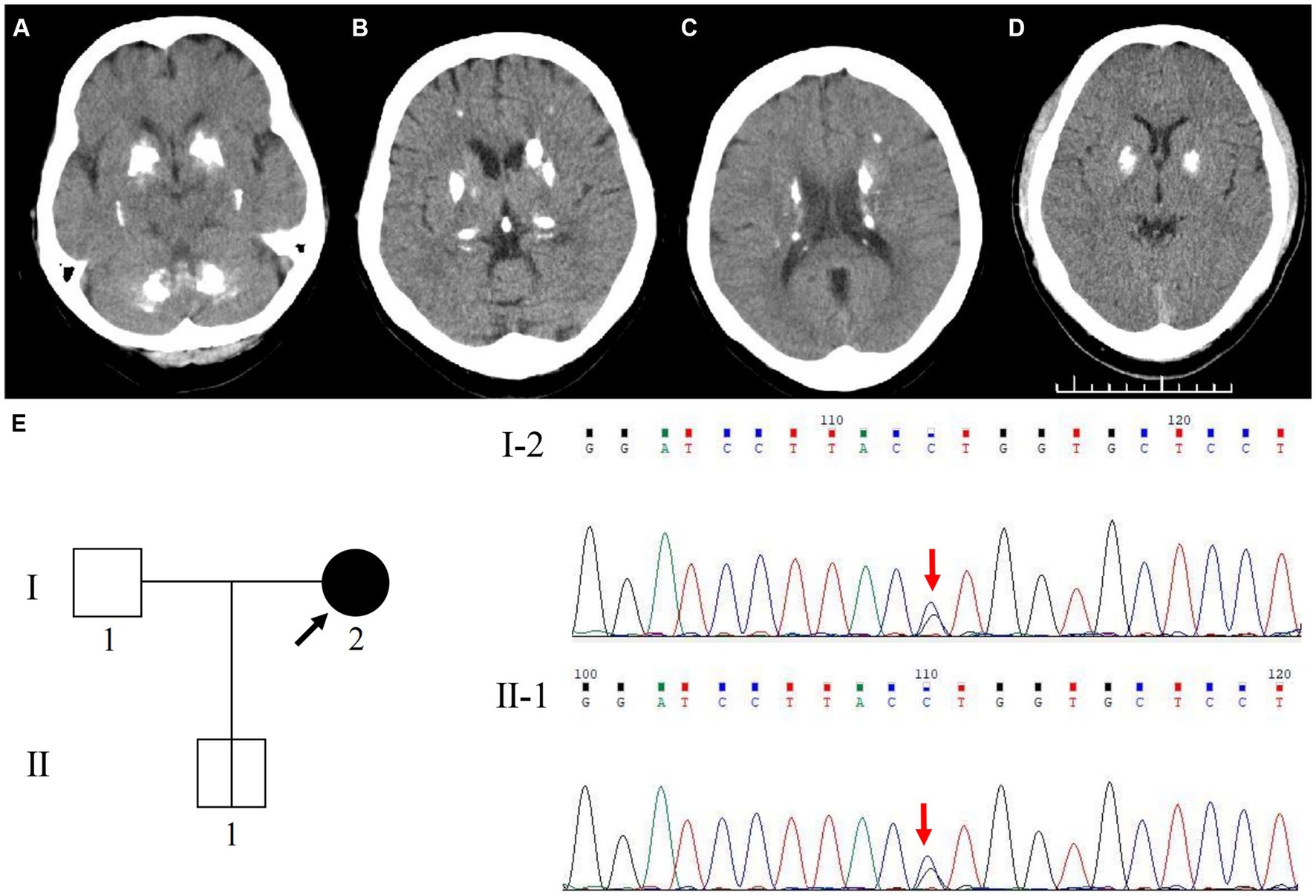
Figure 2. Cranial neuroimaging and Genetic analysis of SLC20A2 in the family. Axial cranial CT scan shows symmetric hyperdensity lesions in the bilateral basal ganglia, cerebellar dentate nuclei, thalamus, periventricular region, and subcortical white matter of the parietal lobe, with widened sulci and mild brain atrophy of the proband (A–C). Axial cranial CT scan shows symmetric hyperdensity lesions in the bilateral basal ganglia of the proband’s son (D). WES identified a novel variant site in the SLC20A2 gene, c.613G>C (p.Val205Leu). Genetic analysis revealed that the variant in the proband’s son (II-1) was inherited from his mother (I-2) (E).
PFBC is a rare hereditary calcium deposition disorder of the central nervous system that often manifests in individuals aged 40–50, with an estimated prevalence of 0.1% (2). PFBC is clinically heterogeneous. A study of 516 patients identified a wide range of neurological and psychiatric symptoms, from common motor symptoms such as parkinsonism, dystonia, and tremors to rarer symptoms such as ataxia, seizures, headache, vertigo, and urinary tract symptoms (1). Our patient presented with parkinsonism and cognitive impairment. Based on neuroimaging evidence of calcification, PFBC was suggested. Finally, using genetic testing, a novel SLC20A2 heterozygous missense variant was identified. This variant has not been reported in the gnomAD East Asian population database, ClinVar, or literature. The c.613G>C (p.Val205Leu) variant was predicted to be “Tolerated” by the SIFT software1 and “Benign” by the PolyPhen-2 software.2 Additionally, the proband’s son carried the same variant accompanied by brain calcification, suggesting a high concordance between the locus and clinical phenotype within the family, indicating it as a pathogenic variant. SLC20A2 pathogenic variants were the first to be identified (4) and are the most common among Chinese patients (5). Up to 60.3% of symptomatic patients carry SLC20A2 variants (1). SLC20A2 encodes the type III sodium phosphate transporter 2 protein (PiT2), which is specifically expressed in the choroid plexus of the brain. In vitro experiments have demonstrated that variants can directly disrupt cerebral phosphate homeostasis, increasing the uptake of phosphate in the extracellular matrix and cerebrospinal fluid, which is currently believed to be the primary cause of PFBC (6). Moreover, based on pathological studies and analyses of calcification composition, calcifications are primarily deposited around the outer membrane cells of cerebral microvessels, blood vessel walls, adjacent neurons, and glial cells. Hence, elevated phosphate levels leading to disturbances in the neurovascular unit (NVU) and induction of cell ossification are considered significant aspects of the complex mechanisms underlying ectopic central calcification in PFBC (6).
Brain calcifications in patients with PFBC are entirely penetrant and display similar radiological features (7). Various degrees of other typical calcified brain regions, including the bilateral basal ganglia, particularly the globus pallidus, as well as other areas such as the subcortical white matter, cerebellar dentate nucleus, thalamus, and cortex, have also been reported. However, although our patient exhibited cerebellar lesions, no clinical cerebellar signs were observed, consistent with research findings that no definite correlation between the severity and location of calcifications and clinical phenotype exists (7). This phenomenon may be related to the lower rate of clinical manifestations observed with SLC20A2 than with other PFBC causative genes (1). As the cooccurrence of SLC20A2-associated PFBC with dopamine-responsive parkinsonism is very unusual (3, 8–11), monitoring the dopamine responsiveness of the patient to assess the possible benefits of dopamine replacement therapy is essential. Notably, Yamada (10) and Manyam (11) discovered not only pathological calcification but also the presence of Lewy bodies in patients with dopamine-responsive PFBC. As PD is a prevalent neurodegenerative condition among older individuals, the possibility of comorbidity between PFBC and PD should not be overlooked. We were also intrigued by the unexpectedly favorable response to dopamine in this specific patient. Unfortunately, due to research facility limitations, we were unable to further explore the underlying neural mechanisms through measurements of striatal dopamine transporter uptake rate. This is a limitation of the paper.
PFBC is a slowly progressing neurodegenerative disease characterized by clinical heterogeneity and incomplete penetrance (6). Unexpected brain calcification seen on radiological imaging can be an initial diagnostic clue, even when clinical symptoms do not completely align (1). In the case report presented here, the proband’s son showed symmetrical basal ganglia calcification, but no clinical symptoms were observed during the two-year follow-up. However, it is speculated that as the proband’s son ages and intracranial calcification progresses, he may develop a phenotype similar to the proband in the future. Since there are currently no preventive or disease-modifying therapies for PFBC, it is important to enhance screening and follow-up for carriers within the family and provide genetic counseling.
In conclusion, this case confirms the clinical heterogeneity of PFBC associated with SLC20A2 variants and provided new clinical evidence for PFBC diagnosis. In the absence of effective disease-modifying treatments and calcium chelation medications, we achieved desirable therapeutic results using a customized pharmacological strategy, indicating that symptomatic treatment has positive clinical implications for patients with PFBC presenting with the parkinsonism phenotype.
The datasets presented in this article are not readily available because of ethical and privacy restrictions. Requests to access the datasets should be directed to the corresponding authors.
The studies involving humans were approved by the Medical Research Ethics Committee of the Affiliated Hospital of the Neurology Institute of Anhui University of Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
DS: Writing – original draft, Conceptualization, Data curation, Formal analysis. YW: Writing – original draft, Conceptualization, Data curation, Formal analysis. JW: Writing – original draft, Investigation, Methodology, Project administration. SW: Writing – original draft, Investigation, Methodology, Project administration. LZ: Writing – original draft, Investigation, Methodology, Project administration. KX: Writing – original draft, Investigation, Methodology, Project administration. YZ: Writing – review & editing, Supervision, Validation. XW: Writing – review & editing, Supervision, Validation.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors thank the patient and her family for placing their trust in us.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Balck, A, Schaake, S, Kuhnke, NS, Domingo, A, Madoev, H, Margolesky, J, et al. Genotype-phenotype relations in primary familial brain calcification: systematic MDSGene review. Mov Disord. (2021) 36:2468–80. doi: 10.1002/mds.28753
2. Carecchio, M, Mainardi, M, and Bonato, G. The clinical and genetic spectrum of primary familial brain calcification. J Neurol. (2023) 270:3270–7. doi: 10.1007/s00415-023-11650-0
3. Rohani, M, Poon, Y-Y, Naranian, T, and Fasano, A. SCL20A2 mutation mimicking fluctuating parkinson’s disease. Parkinsonism Relat Disord. (2017) 39:93–4. doi: 10.1016/j.parkreldis.2017.03.019
4. Wang, C, Li, Y, Shi, L, Ren, J, Patti, M, Wang, T, et al. Mutations in SLC20A2 link familial idiopathic basal ganglia calcification with phosphate homeostasis. Nat Genet. (2012) 44:254–6. doi: 10.1038/ng.1077
5. Ding, Y, and Dong, H-Q. A novel SLC20A2 mutation associated with familial idiopathic basal ganglia calcification and analysis of the genotype-phenotype association in Chinese patients. Chin Med J. (2018) 131:799–803. doi: 10.4103/0366-6999.228245
6. Xu, X, Sun, H, Luo, J, Cheng, X, Lv, W, Luo, W, et al. The pathology of primary familial brain calcification: implications for treatment. Neurosci Bull. (2023) 39:659–74. doi: 10.1007/s12264-022-00980-0
7. Tadic, V, Westenberger, A, Domingo, A, Klein, C, Kasten, M, and Kasten, M. Primary familial brain calcification with known gene mutations: a systematic review and challenges of phenotypic characterization. JAMA Neurol. (2015) 72:460–7. doi: 10.1001/jamaneurol.2014.3889
8. Ichikawa, Y, Tanaka, M, Kurita, E, Nakajima, M, Tanaka, M, Oishi, C, et al. Novel SLC20A2 variant in a Japanese patient with idiopathic basal ganglia calcification-1 (IBGC1) associated with dopa-responsive parkinsonism. Hum Genome Var. (2019) 6:44. doi: 10.1038/s41439-019-0073-7
9. Kumar, N, and Jog, M. Fahr’s disease presenting as late-onset levodopa-responsive parkinsonism. Can J Neurol Sci. (2017) 44:322–3. doi: 10.1017/cjn.2016.428
10. Yamada, M, Tanaka, M, Takagi, M, Kobayashi, S, Taguchi, Y, Takashima, S, et al. Evaluation of SLC20A2 mutations that cause idiopathic basal ganglia calcification in Japan. Neurology. (2014) 82:705–12. doi: 10.1212/WNL.0000000000000143
Keywords: primary familial brain calcification, Fahr’s disease, SLC20A2 gene, parkinsonism, motor fluctuation, levodopa-induced dyskinesia
Citation: Sun D, Wang Y, Wang J, Wang S, Zhu L, Xia K, Zhang Y and Wang X (2024) Primary familial brain calcification presenting with parkinsonism and motor complications caused by a novel SLC20A2 variant: a case report. Front. Neurol. 15:1382534. doi: 10.3389/fneur.2024.1382534
Received: 05 February 2024; Accepted: 27 June 2024;
Published: 05 July 2024.
Edited by:
Nardo Nardocci, IRCCS Carlo Besta Neurological Institute Foundation, ItalyReviewed by:
Barbara Garavaglia, IRCCS Carlo Besta Neurological Institute Foundation, ItalyCopyright © 2024 Sun, Wang, Wang, Wang, Zhu, Xia, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunyun Zhang, emhhbmd5eXNoeXlAc2h1dGNtLmVkdS5jbg==; Xun Wang, bmV1cm9kb2NAMTYzLmNvbQ==
†ORCID: Dandan Sun, orcid.org/0009-0000-4835-1756
Yu Wang, orcid.org/0000-0002-8098-2491
Jiawei Wang, orcid.org/0000-0001-7184-5922
Shijing Wang, orcid.org/0009-0009-2304-4405
Ling Zhu, orcid.org/0009-0003-7138-6561
Yunyun Zhang, orcid.org/0000-0003-0914-3406
Xun Wang, orcid.org/0000-0002-4637-7626
Kun Xia, orcid.org/0009-0007-6797-0115
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.