- 1Department of Orthopedics, The Second Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 2The First School of Clinical of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 3Department of Orthopedics, No. 903 Hospital of PLA Joint Logistic Support Force, Hangzhou, China
- 4Department of Orthopedics, The First Affiliated Hospital, Zhejiang University, Hangzhou, Zhejiang, China
- 5School of Biomedical Sciences, Faculty of Medicine, The Chinese University of Hong Kong, Shatin, Hong Kong SAR, China
- 6Department of Orthopedics, Third Xiangya Hospital, Central South University, Changsha, Hunan, China
- 7Zhejiang University School of Medicine, Hangzhou, Zhejiang, China
- 8Department of Neurology, Republican Research and Clinical Center of Neurology and Neurosurgery, Minsk, Belarus
- 9Department of Orthopedic Physical Therapy, Faculty of Physical Therapy, Nahda University in Beni Suef, Beni Suef, Egypt
Under standard conditions, nitrous oxide (N2O) manifests as a colorless, odorless gas with a mildly sweet taste. The compound finds applications in various fields, including its use as an aerosol propellants, an accelerant in motor racing, and an anesthetic in surgical procedures and dentistry. Unfortunately, the recreational misuse of N2O has become prevalent among young individuals due to its euphoric and hallucinogenic effects. Compounding this issue is the fact that nitrous oxide can be easily obtained from over-the-counter household items, facilitating its non-medical use. The global community has witnessed a surge in the recreational utilization of nitrous oxide gas in recent years. Despite the widespread non-medical abuse of N2O, there remains inadequate understanding of the potential adverse effects resulting from exposure to it. This paper provides an overview of management findings, laboratory and electrodiagnostic characteristics, as well as clinical presentations associated with neurological disorders induced by nitrous oxide usage.
Introduction
N2O is primarily utilized as an anesthetic agent. Distinguished from other inhalants, the inhalation of nitrous oxide induces a profound, transient, and pleasurable euphoria that is often described as mildly psychedelic and agreeable. It also subtly alters body image perception and can result in sensations of dissociation (1) along with its evanescent effects and rapid restoration of normal faculties, recreational users highly desire N2O for brief intoxication purposes, typically experiencing its effects within minutes. In recent years, the euphoric properties of N2O have led to widespread recreational use in the Western world (2). For instance, data collected from drug users across more than 30 countries through the 2019 Global Drug Survey (GDS) revealed that at least once in their lifetime, 90% of respondents had used N2O, positioning it as the tenth most popular substance in Western society after alcohol and tobacco.
The utilization patterns of N2O are similar across these nations with a particular prevalence of ‘whippets’—small canisters containing the gas. However, it is crucial to note that chronic exposure to elevated doses of N2O can result in significant neurological damage including cobalamin (vitamin B12) deficiency-induced neuropathy and even paralysis.
Therefore, it is imperative to understand and address the potential risks associated with prolonged inhalation of N₂O (3, 4). Additionally, the increasing incidence of individuals presenting at emergency departments with neurological impairments due to N₂O exposure highlights the concerning and serious nature of this trend (5).
Many of case reports have firmly established a clear correlation between the misuse of N₂O and a range of neurological and psychiatric disorders, including conditions such as subacute combined degeneration of the spinal cord, myelopathy, demyelinating polyneuropathy, peripheral neuropathy, and various mood and affective disturbances (6–8). Furthermore, fatalities resulting from N₂O inhalation have been documented (9). Currently, only a limited number of studies have focused on the peripheral neuropathy caused by abusive inhalation of nitrous oxide (10–12), highlighting a lack of awareness regarding the toxicity associated with N₂O abuse. Therefore, this study aims to comprehensively outline the clinical characteristics, mechanisms, and management strategies for N₂O-associated peripheral neuropathy.
The mechanisms of N2O neurotoxicity
The manifestation of peripheral neuropathy can occur through various mechanisms, including distal axonopathy, myelinopathy, and neuronopathy. Each mechanism involves distinct pathological processes that result in the degeneration or dysfunction of nerve fibers, thereby impairing their ability to effectively transmit signals (13–15).
To date, Vitamin B12, as called cobalamin, insufficiency has been extensively researched, while the exact mechanism of N2O remains unclear. Although it has been observed that individuals who persistently use N2O and experience neurological damage tend to have lower cobalamin levels, it is doubtful that a vitamin B12 shortage is the sole cause of such damage (11). Elevated serum levels of methylmalonate and homocysteine have proven to be more reliable biomarkers for brain injury following prolonged exposure to N2O (16), promoting further investigation into the specific metabolic pathways underlying the toxicity associated with N2O exposure.
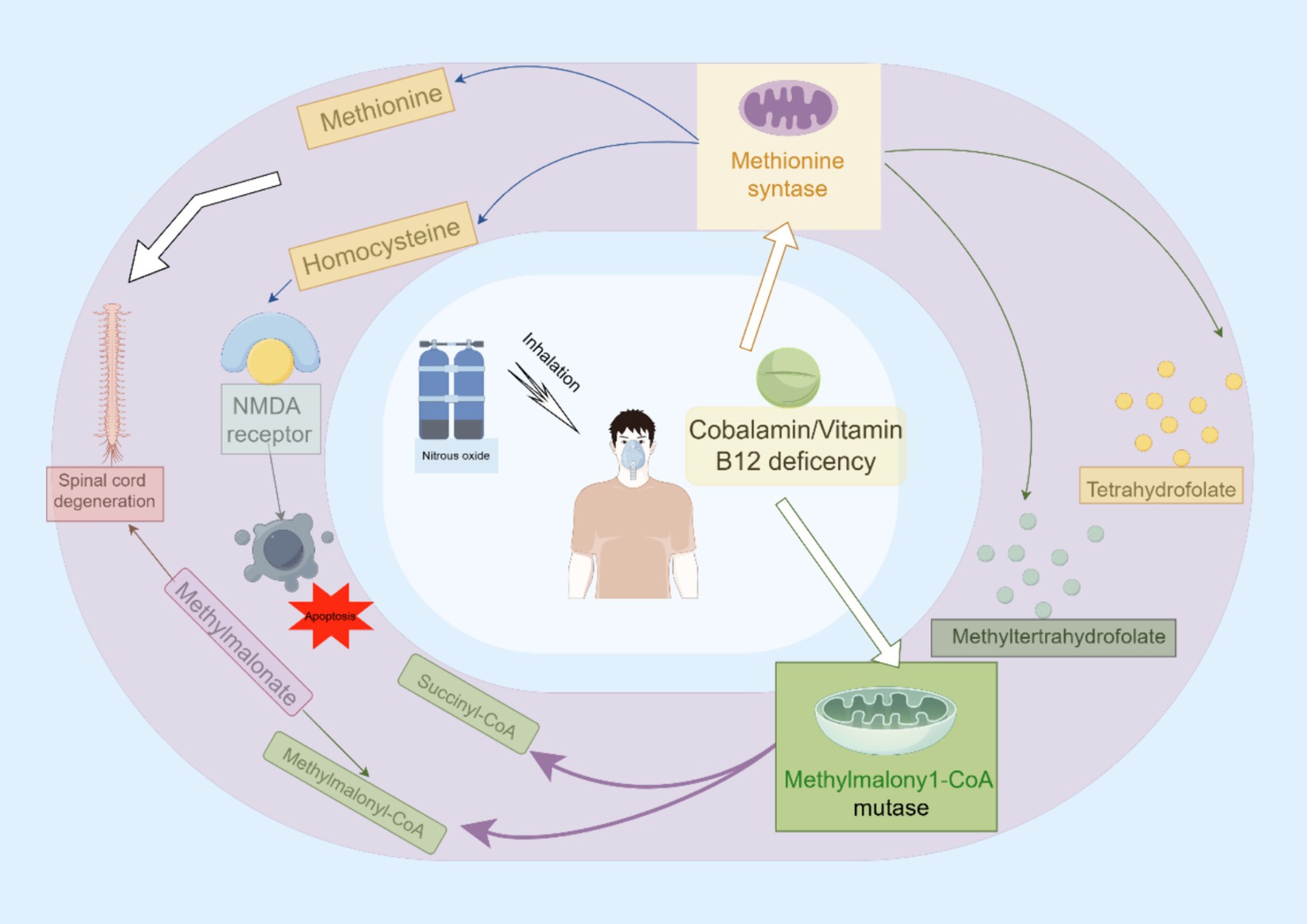
Cobalamin, also known as Vitamin B12, contains a cobalt ion at its core. Within the human body, it exists in two biologically active forms: methylcobalamin and adenosylcobalamin. Under specific conditions, N2O can induce neurotoxic effects primarily through various biochemical mechanisms. One significant pathway of neurotoxicity involves interference with Vitamin B12 metabolism. N2O oxidizes the cobalt ion from its functional +1 oxidation state to a non-functional +3 state (11). This oxidation renders cobalamin ineffective as a coenzyme for methionine synthase and methylmalonyl-CoA mutase (MMCoAM), thereby disrupting critical cellular processes such as DNA synthesis and energy production. The clinical implications of this disruption may include neurological dysfunction and hematological disorders due to impaired methionine synthesis and accumulation of homocysteine and methylmalonic acid (11). Furthermore, deficiency in MMCoAM enzymatic activity during lipid and carbohydrate biosynthesis leads to intracellular accumulation of methylmalonate acid (17, 18).
Another pathway involves methionine methyltransferase (MTR), a pivotal enzyme responsible for catalyzing the conversion of homocysteine and 5-methyltetrahydrofolate into tetrahydrofolate and methionine. Consequently, insufficient MTR enzymatic activity may result in an accumulation of homocysteine and 5-methyltetrahydrofolate, accompanied by reduced levels of methionine, tetrahydrofolate, and S-adenosylmethionine (19). Impairment in methionine and S-adenosylmethionine synthesis can disrupt the methylation process of myelin phospholipids, leading to various neurological consequences such as demyelination in the brain, spinal cord, and peripheral nervous system. Clinically, this disruption may manifest as megaloblastic anemia with potential progression to optic nerve atrophy (20, 21). Meanwhile, elevated levels of homocysteine can exert detrimental effects on physiological systems through distinct pathways: induction of oxidative stress resulting in reactive oxygen species (ROS) generation that triggers apoptotic cell death; activation of NMDA receptors (22). The activation of NMDA receptors has the potential to increase extracellular Ca2+ influx, cause mitochondrial Ca2+ overload and dysfunction along with ROS formation, potentially serving as the primary mechanism underlying homocysteine-mediated neurotoxicity (22).
Vitamin B12 depletion is not the only factor contributing to the neurotoxic effects observed after exposure to nitrous oxide (N2O); other substantial mechanisms are also involved. Neonatal cerebral structures are especially vulnerable to N2O-induced neurotoxicity, which occurs through antagonism of N-methyl-D-aspartate (NMDA) receptors (22). The activation dynamics of NMDA antagonists are widely recognized to produce divergent effects, ranging from neuroprotection to neurotoxicity (22). Short-term exposure to N2O can cause reversible vacuolization in neuronal cells, while prolonged exposure is associated with neuronal apoptosis. Importantly, vacuolization involves significant swelling of mitochondrial structures (23).
It has been suggested that a change in cerebral blood flow is one of the underlying mechanisms responsible for the neurotoxic ramifications of N2O, particularly in terms of cerebral damage (22). Furthermore, N2O alone can inhibit the biosynthesis of xanthine and various monoamines, such as norepinephrine, dopamine, and serotonin. This inhibition may lead to neurotoxic outcomes, subsequently triggering a cascade of events including cytokine disequilibrium, cerebral hypoxia, and acidosis (Figure 1) (24).
Clinical features
The symptoms of peripheral neuropathy vary depending on the location and type of nerve damage. Common manifestations include paresthesia in the hands and feet, muscle weakness or paralysis, impaired balance or coordination, as well as pain (25).
Abuse of N2O can lead to peripheral neuropathy, a condition marked by symptoms such as weakness, numbness, and unsteady gait (26). Notably, the weakness is more pronounced in the lower limbs compared to the upper limbs. Studies of nerve function have consistently demonstrated that this nerve damage involves both motor and sensory fibers, with a considerable loss of motor nerve axons in the lower extremities (26). Further investigations, including sural nerve biopsies, have confirmed that ongoing axonal degeneration is the primary pathological change in nerves affected by this condition (26, 27).
A retrospective study (28) spanning was conducted between 2018 and 2020, involving 76 patients diagnosed with neuropathy attributable to N2O misuse. The analysis of the collected data indicated that 36% of these patients exhibited an absence of response in nerve conduction assessments. Notably, the majority presented with reduced sensory and motor nerve conduction velocities, affecting 75 and 76% of the cohort, respectively. Additionally, diminished amplitudes in sensory nerve action potentials and compound muscle action potentials were observed in 57 and 59% of cases, respectively, along with prolonged distal motor latencies. The electrophysiological data (28) revealed diverse neuropathic presentations, with axonal neuropathy identified in 37 patients (49%), demyelinating peripheral neuropathy in 4 patients (5%), and mixed neuropathy in 12 patients (16%). The primary pathological features included predominant motor axonal damage in 67% of the upper and lower limb impairments, and sensory nerve demyelination accounting for 35% of the deficits. Furthermore, a subgroup analysis suggested a correlation between prolonged N2O exposure, extended illness duration, and the severity of motor axonal damage in the lower extremities.
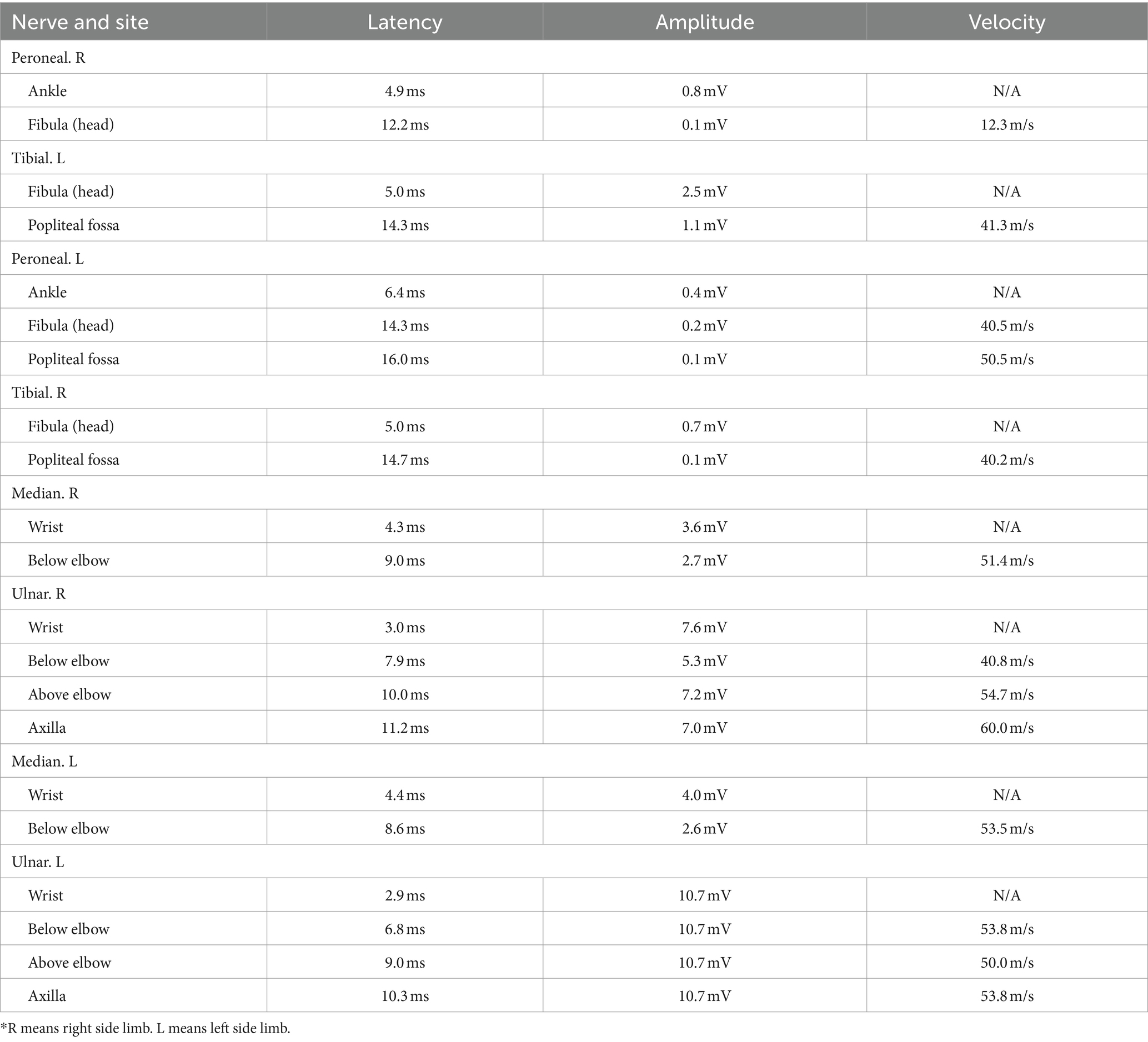
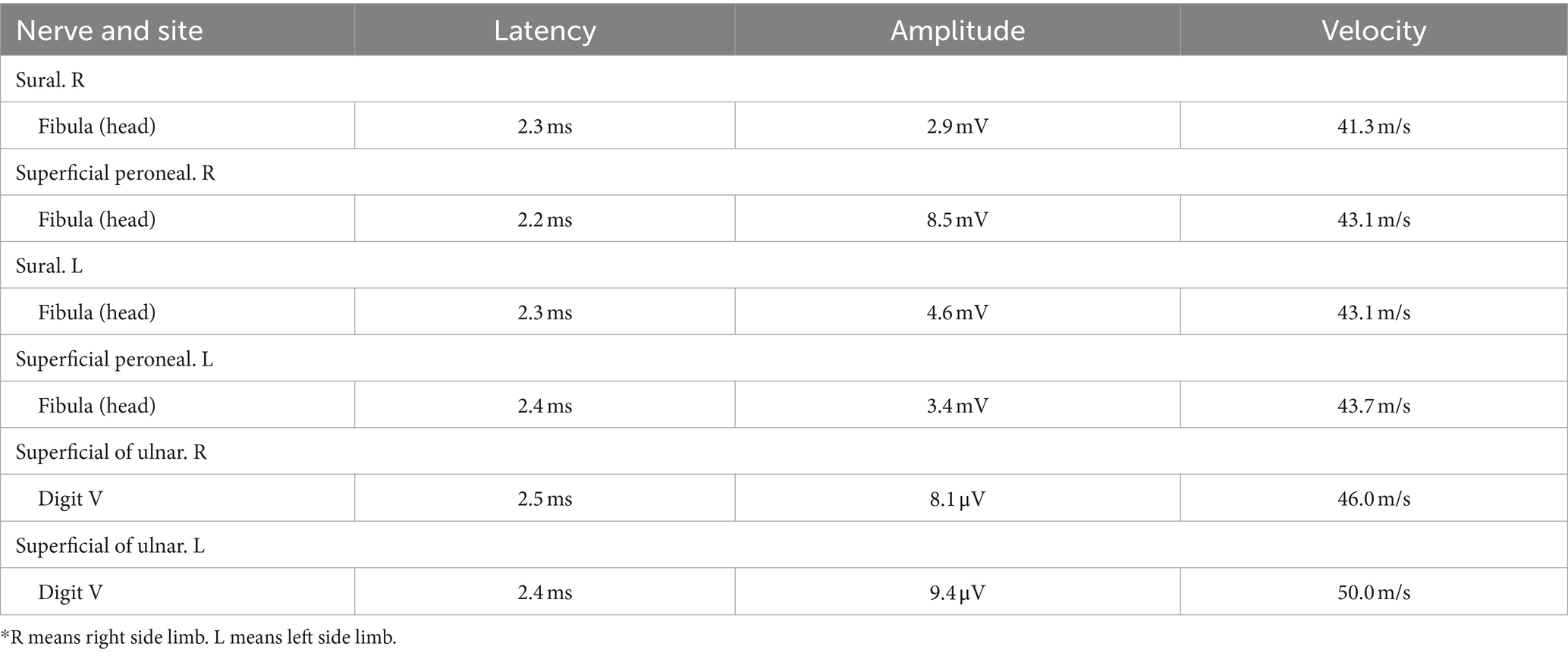
In our case series, the nerve conduction studies of a typical patient with peripheral neuropathy induced by N2O revealed complaints of fatigue and numbness in the bilateral lower limbs (Tables 1, 2) (1, 2).
Diagnosis
In clinical practice, several diagnostic tests are available to identify the underlying etiology and assess the extent of peripheral nerve damage. Commonly employed techniques include nerve conduction studies (NCS), electromyography (EMG), imaging modalities, and nerve biopsy (29). When assessing patients, especially younger individuals, who exhibit symptoms indicative of peripheral neuropathy or myelopathy, clinicians should contemplate the potential for N2O neurotoxicity. A detailed history of specific and prolonged N2O use and exposure is essential for diagnostic confirmation. However, it is important to note that some patients may not disclose their N2O usage during initial consultations, which can complicate the process of establishing a preliminary diagnosis. Moreover, Guillain-Barré syndrome (GBS) and N2O-related peripheral neuropathy share several similarities (11), necessitating additional biochemical testing and nerve conduction testing to be conducted (30).
Biochemical testing for functional vitamin B12 insufficiency, such as accessing homocysteine and methylmalonic acid levels, can be used to confirm the diagnosis in cases where there are consistent clinical symptoms and a history of significant N2O exposure (Table 1) (31). Furthermore, it is recommended to conduct nerve conduction studies in order to further characterize the involvement of the peripheral nervous system (Table 3) (32, 33).
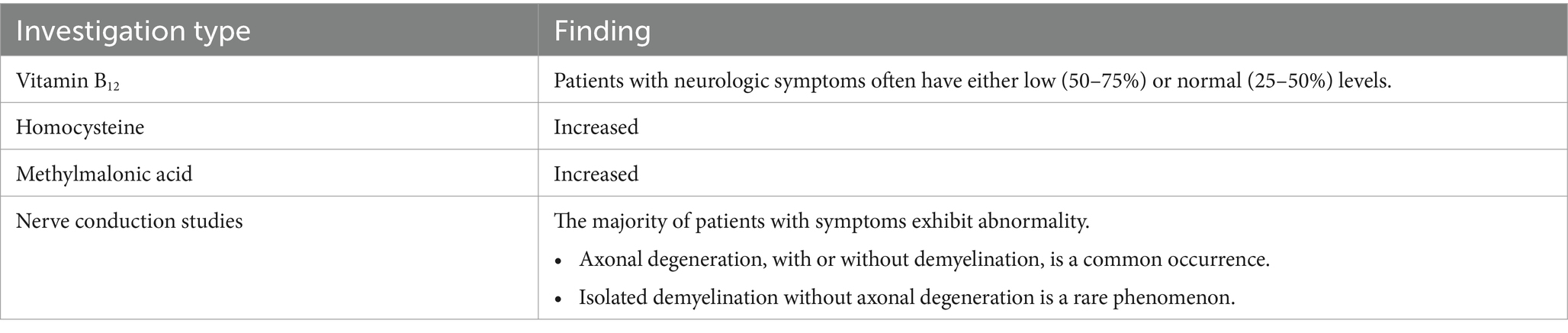
Table 3. Diagnostic examinations for individuals with suspected nitrous oxide poisoning (31).
A low concentration of vitamin B12 is observed in 54–72% of patients experiencing neurological issues due to N2O exposure (34, 35). This occurrence is more likely in individuals exhibiting symptoms after shorter doses, indicating increased susceptibility (35). Low concentrations of vitamin B12 in long-term users may be indicative of accelerated clearance (36, 37). This reduced enzymatic activity results in the accumulation of homocysteine and methylmalonic acid, with at least one of these being elevated in over 90% of patients (38). Consequently, these biomarkers are more sensitive indicators compared to vitamin B12 concentrations, as the latter can remain within the normal range in a significant proportion of users despite neurotoxicity. In an attempt to mitigate neurotoxicity and maintain normal levels of these biomarkers, some users supplement with additional vitamin B12. However, although this practice may potentially mislead clinicians, it does not offer complete protection against the neurological side effects associated with nitrous oxide usage (39, 40).
The majority of patients exhibiting symptoms demonstrate atypical results in nerve conduction studies (33–35). While a minority of these individuals exhibit signs of isolated demyelination, the predominant irregularity observed is axonal degeneration, which may occur with or without accompanying demyelination. It is noteworthy that individuals who regularly use N2O tend to experience more pronounced motor impairments compared to those with a deficiency of vitamin B12 not associated with nitrous oxide exposure (41).
In previous studies, MRI was used to diagnose lesions on the spinal cord and cerebral cortex (27). Based on our expertise, we recommend using ultrasonography to identify peripheral nerve impairments (42, 43). Echo enhancement around peripheral nerves can be observed with ultrasound (Figure 2).
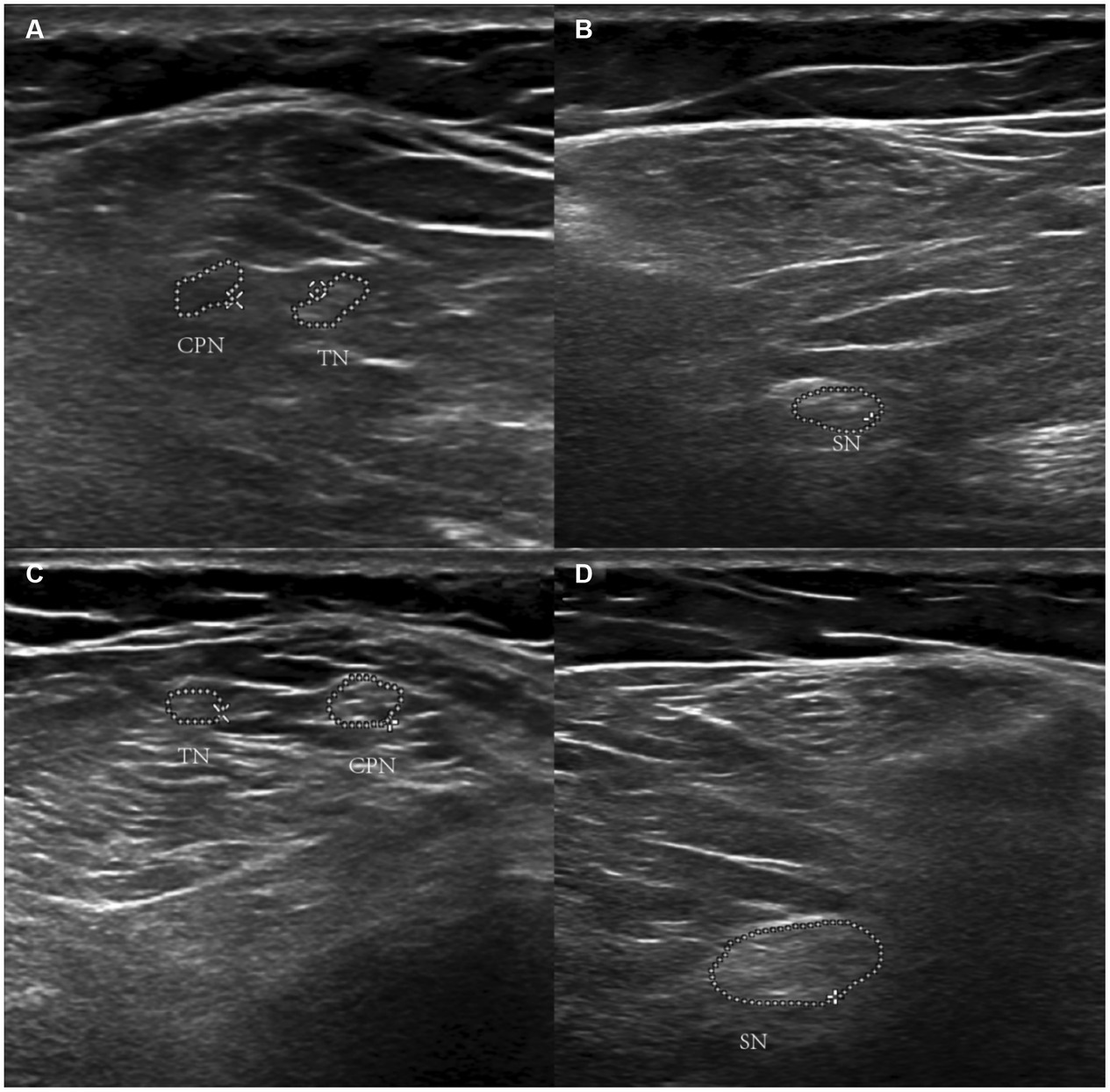
Figure 2. Ultrasonographic image of nitrous oxide-Induced peripheral neuropathy. Panels (A,B) show the echo enhancement around peripheral nerves in the left lower limb. Panels (C,D) are the right lower limb. *TN refers to the tibial nerve. CPN refers to common the peroneal nerve. SN refers to the sciatic nerve.
In conclusion, when patients with a history of N2O use present symptoms of peripheral neuropathy, physicians should consider the possibility of N2O-induced peripheral neuropathy. Further nerve conduction studies (NCS) can confirm the presence of peripheral nerve damage. Additionally, a concurrent decrease in Vitamin B12 levels can aid in diagnosing N2O-related peripheral neuropathy.
Treatment
Prior research has indicated that prolonged usage of N2O heightens the likelihood of neurological impairments, and discontinuing exposure to the toxin is the foremost crucial initial measure for treatment (11, 12, 30). Supplementation with vitamin B12 is advised, and in some cases, it may be combined with methionine, despite the limited evidence underpinning its efficacy (31). We propose an administration of 1,000 μg of vitamin B12, either subcutaneously or intramuscularly, on a daily basis for 1–2 weeks. Subsequently, either weekly injections administered by caregivers or daily oral doses of 2,000 μg should continue until symptomatic relief is achieved. This recommendation is based on the favorable safety profile of vitamin B12 (44, 45). Furthermore, we propose a secure and efficacious regimen of methionine supplementation, with an oral dosage of 1 g administered three times daily for a minimum of 4–6 weeks, or until significant symptomatic improvement is observed (46). Due to the potential for exacerbation of symptoms and prolonged recovery, initiating folate supplementation before the restoration of vitamin B12 levels is not recommended, as it is unlikely to benefit the patient (47, 48). In certain cases, integrating physical rehabilitation along with social and psychological support measures may be essential.
The beneficial effects of hyperbaric oxygen therapy (HBOT) in repairing peripheral nerve injuries have been well-documented in previous literature (49). A recent prospective study (50) assessed the efficacy of HBOT following primary nerve repair in patients with upper extremity nerve injuries. The study results shown that compared to the control group, the group treated with hyperbaric oxygen achieved a higher power score, exhibited a faster recovery rate, and demonstrated quicker impulse transmission. However, there is limited documentation regarding the use of HBOT for treating peripheral nerve injuries caused by N2O. In our experience, we are investigating the potential use of HBOT as an adjunctive treatment for patients with peripheral neuropathy induced by the abusive inhalation of N2O.
The prognosis for recovery varies among patients; however, the vast majority of patients (95–97%) exhibit some degree of improvement. It is important to note that despite months of therapeutic intervention, over one-third of hospitalized patients continue to manifest neurological symptoms (Figure 3 and Table 4) (34, 35).
Figure 3. A patient with peripheral neuropathy caused by excessive inhalation of nitrous oxide is undergoing treatment with hyperbaric oxygen therapy.
Conclusion
Nitrous oxide, is known for its cost-effectiveness and ease of procurement. It is widely utilized as a recreational substance, particularly among the adolescent population. Its consumption is a frequently overlooked as a potential cause of neurological disorders, primarily myelopathy, peripheral neuropathy, and encephalopathy, which may be accompanied by hematological abnormalities. Furthermore, it has the potential to induce functional vitamin B12 deficiency. Therefore, healthcare professionals are encouraged to consider and inquire about nitrous oxide use in patients presenting with unexplained clinical manifestations suggestive of vitamin B12 deficiency or other neurologic symptoms consistent with its usage.
In conclusion, a comprehensive understanding and recognition of the neurological implications associated with the utilization of N2O is imperative for healthcare professionals. By considering the potential involvement of N2O in patients presenting with inexplicable neurological symptoms or exhibiting signs of vitamin B12 deficiency, healthcare professionals can assume a pivotal role in early detection, diagnosis, and management of related conditions, thereby enhancing patient care and optimizing outcomes.
Author contributions
XZ: Writing – original draft, Writing - review & editing. FY: Writing – original draft, Writing – review & editing. WZ: Visualization, Writing – review & editing. YD: Investigation, Writing – review & editing. AA: Funding acquisition, Writing – review & editing. HZ: Methodology, Writing – review & editing. SE: Software, Writing – review & editing. VK: Supervision, Writing – review & editing. MA: Validation, Writing – review & editing. OA: Data curation, Formal analysis, Writing – review & editing. SA: Software, Writing – review & editing. HL: Conceptualization, Writing – review & editing. CW: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Beckman, NJ, Zacny, JP, and Walker, DJ. Within-subject comparison of the subjective and psychomotor effects of a gaseous anesthetic and two volatile anesthetics in healthy volunteers. Drug Alcohol Depend. (2006) 81:89–95. doi: 10.1016/j.drugalcdep.2005.06.002
2. van Amsterdam, JG, Nabben, T, and van den Brink, W. Increasing recreational nitrous oxide use: should we worry? A narrative review. J Psychopharmacol. (2022) 36:943–50. doi: 10.1177/02698811221082442
3. Oussalah, A, Julien, M, Levy, J, Hajjar, O, Franczak, C, Stephan, C, et al. Global burden related to nitrous oxide exposure in medical and recreational settings: a systematic review and individual patient data Meta-analysis. J Clin Med. (2019) 8:551. doi: 10.3390/jcm8040551
4. Vollhardt, R, Mazoyer, J, Bernardaud, L, Haddad, A, Jaubert, P, Coman, I, et al. Neurological consequences of recreational nitrous oxide abuse during SARS-CoV-2 pandemic. J Neurol. (2022) 269:1921–6. doi: 10.1007/s00415-021-10748-7
5. Lin, J-P, Gao, S-Y, and Lin, C-C. The clinical presentations of nitrous oxide users in an emergency department. Toxics. (2022) 10:112. doi: 10.3390/toxics10030112
6. Choi, C, Kim, T, Park, KD, Lim, OK, and Lee, JK. Subacute combined degeneration caused by nitrous oxide intoxication: a report of two cases. Ann Rehabil Med. (2019) 43:530–4. doi: 10.5535/arm.2019.43.4.530
7. Neveu, J, Perelman, S, Suisse, G, and Monpoux, F. Severe hyperhomocysteinemia and peripheral neuropathy as side effects of nitrous oxide in two patients with sickle cell disease. Arch Pediatr. (2019) 26:419–21. doi: 10.1016/j.arcped.2019.09.006
8. Edigin, E, Ajiboye, O, and Nathani, A. Nitrous oxide-induced B12 deficiency presenting with myeloneuropathy. Cureus. (2019) 11:e5331. doi: 10.7759/cureus.5331
9. Bäckström, B, Johansson, B, and Eriksson, A. Death from nitrous oxide. J Forensic Sci. (2015) 60:1662–5. doi: 10.1111/1556-4029.12879
10. Pichon, M, Majhadi, L, and Menn, A-M. Neurological manifestations induced by nitrous oxide abuse: a case series and review of literature. Neurologist. (2023) 29:113–9. doi: 10.1097/NRL.0000000000000531
11. Dong, M-X, Wang, Q, Xu, J-F, Hu, L, Yu, Y, and Li, T. Case report: recreational nitrous oxide abuse triggered peripheral neuropathy possibly through the immune-mediated pathogenesis. Front Neurol. (2022) 13:1033327. doi: 10.3389/fneur.2022.1033327
12. Richardson, PG. Peripheral neuropathy following nitrous oxide abuse. Emerg Medicine Australasia (2010). 22:88–90. doi: 10.1111/j.1742-6723.2009.01262.x
13. Stojkovic, T. Peripheral neuropathies: the rational diagnostic process. Rev Med Interne. (2006) 27:302–12. doi: 10.1016/j.revmed.2005.10.018
14. Bae, EH, Greenwald, MK, and Schwartz, AG. Chemotherapy-Induced Peripheral Neuropathy: Mechanisms and Therapeutic Avenues. Neurotherapeutics. (2021) 18:2384–2396. doi: 10.1007/s13311-021-01142-2
15. Escorcio Bezerra, ML, Pedroso, JL, Pinheiro, DS, Braga-Neto, P, Povoas Barsottini, OG, de Oliveira Braga, NI, et al. Pattern of peripheral nerve involvement in Machado-Joseph disease: neuronopathy or distal axonopathy? A clinical and neurophysiological evaluation. Eur Neurol. (2013) 69:129–33. doi: 10.1159/000345274
16. Kehoe, S, Hook, J, Nankivell, M, Jayson, GC, Kitchener, H, Lopes, T, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. (2015) 386:249–57. doi: 10.1016/S0140-6736(14)62223-6
17. Green, R, Allen, LH, Bjørke-Monsen, A-L, Brito, A, Guéant, J-L, Miller, JW, et al. Vitamin B12 deficiency. Nat Rev Dis Primers. (2017) 3:17040. doi: 10.1038/nrdp.2017.40
18. Green, R. Vitamin B12 deficiency from the perspective of a practicing hematologist. Blood. (2017) 129:2603–11. doi: 10.1182/blood-2016-10-569186
19. Ahn, SC, and Brown, AW. Cobalamin deficiency and subacute combined degeneration after nitrous oxide anesthesia: a case report. Arch Phys Med Rehabil. (2005) 86:150–3. doi: 10.1016/j.apmr.2004.01.019
20. Graber, JJ, Sherman, FT, Kaufmann, H, Kolodny, EH, and Sathe, S. Vitamin B12-responsive severe leukoencephalopathy and autonomic dysfunction in a patient with “normal” serum B12 levels. J Neurol Neurosurg Psychiatry. (2010) 81:1369–71. doi: 10.1136/jnnp.2009.178657
21. Green, R, and Kinsella, LJ. Current concepts in the diagnosis of cobalamin deficiency. Neurology. (1995) 45:1435–40. doi: 10.1212/WNL.45.8.1435
22. Savage, S, and Ma, D. The neurotoxicity of nitrous oxide: the facts and “putative” mechanisms. Brain Sci. (2014) 4:73–90. doi: 10.3390/brainsci4010073
23. Jevtovic-Todorovic, V, Beals, J, Benshoff, N, and Olney, JW. Prolonged exposure to inhalational anesthetic nitrous oxide kills neurons in adult rat brain. Neuroscience. (2003) 122:609–16. doi: 10.1016/j.neuroscience.2003.07.012
24. Scalabrino, G. Cobalamin (vitamin B12) in subacute combined degeneration and beyond: traditional interpretations and novel theories. Exp Neurol. (2005) 192:463–79. doi: 10.1016/j.expneurol.2004.12.020
25. Lehmann, HC, Wunderlich, G, Fink, GR, and Sommer, C. Diagnosis of peripheral neuropathy. Neurol Res Pract. (2020) 2:20. doi: 10.1186/s42466-020-00064-2
26. Wang, Q, Duan, X, Dong, M, Sun, S, Zhang, P, Liu, F, et al. Clinical feature and sural biopsy study in nitrous oxide-induced peripheral neuropathy. PLoS One. (2022) 17:e0274765. doi: 10.1371/journal.pone.0274765
27. Bao, L, Li, Q, Li, Q, Chen, H, Zhang, R, Shi, H, et al. Clinical, electrophysiological and radiological features of nitrous oxide-induced neurological disorders. NDT. (2020) 16:977–84. doi: 10.2147/NDT.S236939
28. Fang, X, Yu, M, Zheng, D, Gao, H, Li, W, and Ma, Y. Electrophysiologic characteristics of nitrous-oxide-associated peripheral neuropathy: a retrospective study of 76 patients. J Clin Neurol. (2023) 19:44–51. doi: 10.3988/jcn.2023.19.1.44
29. Pedersen, OB, Hvas, A-M, and Grove, EL. A 19-year-old man with a history of recreational inhalation of nitrous oxide with severe peripheral neuropathy and central pulmonary embolism. Am J Case Rep. (2021) 22:e931936. doi: 10.12659/AJCR.931936
30. Dong, Y, Alhaskawi, A, Zhou, H, Zou, X, Liu, Z, Ezzi, SHA, et al. Imaging diagnosis in peripheral nerve injury. Front Neurol. (2023) 14:1250808. doi: 10.3389/fneur.2023.1250808
31. Fortanier, E, Berling, E, Zanin, A, Guillou, AL, Micaleff, J, Nicolas, G, et al. How to distinguish Guillain‐Barré syndrome from nitrous oxide‐induced neuropathy: a 2‐year, multicentric, retrospective study. Eur J Neurol. (2023) 30:3296–306. doi: 10.1111/ene.15998
32. De Halleux, C, and Juurlink, DN. Diagnosis and management of toxicity associated with the recreational use of nitrous oxide. CMAJ. (2023) 195:E1075–81. doi: 10.1503/cmaj.230196
33. Patel, KK, Mejia Munne, JC, Gunness, VRN, Hersey, D, Alshafai, N, Sciubba, D, et al. Subacute combined degeneration of the spinal cord following nitrous oxide anesthesia: a systematic review of cases. Clin Neurol Neurosurg. (2018) 173:163–8. doi: 10.1016/j.clineuro.2018.08.016
34. Largeau, B, Karam, A, Potey, C, Caous, A, Tard, C, Carton, L, et al. Myeloneuropathy induced by recreational nitrous oxide use with variable exposure levels. Eur J Neurol. (2022) 29:2173–80. doi: 10.1111/ene.15370
35. Garakani, A, Jaffe, RJ, Savla, D, Welch, AK, Protin, CA, Bryson, EO, et al. Neurologic, psychiatric, and other medical manifestations of nitrous oxide abuse: a systematic review of the case literature: toxic effects of nitrous oxide abuse. Am J Addict. (2016) 25:358–69. doi: 10.1111/ajad.12372
36. Yu, M, Qiao, Y, Li, W, Fang, X, Gao, H, Zheng, D, et al. Analysis of clinical characteristics and prognostic factors in 110 patients with nitrous oxide abuse. Brain Behav. (2022) 12:e2533. doi: 10.1002/brb3.2533
37. Coimbra, CMF, Dias, SO, Regattieri, N, and Pires, FLG. Magnetic resonance findings in subacute combined degeneration. Arq Neuropsiquiatr. (2017) 75:488–8. doi: 10.1590/0004-282x20170068
38. Kondo, H, Osborne, ML, Kolhouse, JF, Binder, MJ, Podell, ER, Utley, CS, et al. Nitrous oxide has multiple deleterious effects on cobalamin metabolism and causes decreases in activities of both mammalian cobalamin-dependent enzymes in rats. J Clin Invest. (1981) 67:1270–83. doi: 10.1172/JCI110155
39. Metz, J. Cobalamin deficiency and the pathogenesis of nervous system disease. Annu Rev Nutr. (1992) 12:59–79. doi: 10.1146/annurev.nu.12.070192.000423
40. Wijesekera, NT, Davagnanam, I, and Miszkiel, K. Subacute combined cord degeneration: a rare complication of nitrous oxide misuse: a case report. Neuroradiol J. (2009) 22:194–7. doi: 10.1177/197140090902200210
41. Pugliese, RS, Slagle, EJ, Oettinger, GR, Neuburger, KJ, and Ambrose, TM. Subacute combined degeneration of the spinal cord in a patient abusing nitrous oxide and self-medicating with cyanocobalamin. Am J Health Syst Pharm. (2015) 72:952–7. doi: 10.2146/ajhp140583
42. Tani, J, Weng, H-Y, Chen, H-J, Chang, T-S, Sung, J-Y, and Lin, CS-Y. Elucidating unique axonal dysfunction between nitrous oxide abuse and vitamin B12 deficiency. Front Neurol. (2019) 10:704. doi: 10.3389/fneur.2019.00704
43. Dong, Y, and Lu, H. Editorial: surgical treatment of peripheral neuropathic pain, peripheral nerve tumors, and peripheral nerve injury. Front Neurol. (2023) 14:1266638. doi: 10.3389/fneur.2023.1266638
44. Kuzminski, AM, Del Giacco, EJ, Allen, RH, Stabler, SP, and Lindenbaum, J. Effective treatment of cobalamin deficiency with Oral cobalamin. Blood. (1998) 92:1191–8. doi: 10.1182/blood.V92.4.1191.416k15_1191_1198
45. Miller, JW. Proton pump inhibitors, H2-receptor antagonists, metformin, and vitamin B12 deficiency: clinical implications. Adv Nutr. (2018) 9:511S–8S. doi: 10.1093/advances/nmy023
46. Van Der Westhuyzen, J, Fernandes-Costa, F, and Metz, J. Cobalamin inactivation by nitrous oxide produces severe neurological impairment in fruit bats: protection by methionine and aggravation by folates. Life Sci. (1982) 31:2001–10. doi: 10.1016/0024-3205(82)90039-X
47. Marotta, DA, and Kesserwani, H. Nitrous oxide induced posterior cord myelopathy: beware of the methyl folate trap. Cureus. (2020) 12:e9319. doi: 10.7759/cureus.9319
48. Reynolds, EH. The risks of folic acid to the nervous system in vitamin B12 deficiency: rediscovered in the era of folic acid fortification policies. J Neurol Neurosurg Psychiatry. (2017) 88:1097–8. doi: 10.1136/jnnp-2017-316296
49. Leach, RM, Rees, PJ, and Wilmshurst, P. Hyperbaric oxygen therapy. BMJ. (1998) 317:1140–1143. doi: 10.1136/bmj.317.7166.1140
Keywords: nitrous oxide – N2O, peripheral neuropathy, abusive inhalation, neurological disorders, clinical characteristics
Citation: Zou X, Yi F, Zhou W, Dong Y, Alhaskawi A, Zhou H, Ezzi SHA, Kota VG, Abdulla MHAH, Alenikova O, Abdalbary SA, Lu H and Wang C (2024) Mechanisms and recent advances in the diagnosis and treatment of nitrous oxide-induced peripheral neuropathy: a narrative review. Front. Neurol. 15:1381938. doi: 10.3389/fneur.2024.1381938
Edited by:
Corrado Italo Angelini, University of Padua, ItalyReviewed by:
Mohamed Shatholy, Ain Shams University, EgyptTracy Peters, Fujian Medical University, China
Copyright © 2024 Zou, Yi, Zhou, Dong, Alhaskawi, Zhou, Ezzi, Kota, Abdulla, Alenikova, Abdalbary, Lu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changxin Wang, d2N4emNtdUAxNjMuY29t; Hui Lu, aHVpbHVAemp1LmVkdS5jbg==
 Xiaodi Zou
Xiaodi Zou Fangyu Yi
Fangyu Yi Weijie Zhou
Weijie Zhou Yanzhao Dong3
Yanzhao Dong3 Ahmad Alhaskawi
Ahmad Alhaskawi Sohaib Hasan Abdullah Ezzi
Sohaib Hasan Abdullah Ezzi Vishnu Goutham Kota
Vishnu Goutham Kota Mohamed Hasan Abdulla Hasan Abdulla
Mohamed Hasan Abdulla Hasan Abdulla Olga Alenikova
Olga Alenikova Sahar Ahmed Abdalbary
Sahar Ahmed Abdalbary Hui Lu
Hui Lu