
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol. , 18 April 2024
Sec. Multiple Sclerosis and Neuroimmunology
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1380654
 Anas Elgenidy1,2
Anas Elgenidy1,2 Nagham Nader Abdelhalim1
Nagham Nader Abdelhalim1 Mohammed Al-mahdi Al-kurdi3*
Mohammed Al-mahdi Al-kurdi3* Lobna A. Mohamed4
Lobna A. Mohamed4 Mohamed M. Ghoneim5
Mohamed M. Ghoneim5 Ahmed Wagdy Fathy6
Ahmed Wagdy Fathy6 Hazem Khaled Hassaan7
Hazem Khaled Hassaan7 Ahmed Anan8
Ahmed Anan8 Omar Alomari9
Omar Alomari9Background: Recent years have seen the emergence of disease-modifying therapies in multiple sclerosis (MS), such as anti-cluster of differentiation 20 (anti-CD20) monoclonal antibodies, aiming to modulate the immune response and effectively manage MS. However, the relationship between anti-CD20 treatments and immunoglobulin G (IgG) levels, particularly the development of hypogammaglobulinemia and subsequent infection risks, remains a subject of scientific interest and variability. We aimed to investigate the intricate connection between anti-CD20 MS treatments, changes in IgG levels, and the associated risk of hypogammaglobulinemia and subsequent infections.
Method: PubMed, Scopus, Embase, Cochrane, and Web of Science databases have been searched for relevant studies. The “R” software utilized to analyze the occurrence of hypogammaglobulinemia, infections and mean differences in IgG levels pre- and post-treatment. The subgrouping analyses were done based on drug type and treatment duration. The assessment of heterogeneity utilized the I2 and chi-squared tests, applying the random effect model.
Results: Thirty-nine articles fulfilled our inclusion criteria and were included in our review which included a total of 20,501 MS patients. The overall prevalence rate of hypogammaglobulinemia was found to be 11% (95% CI: 0.08 to 0.15). Subgroup analysis based on drug type revealed varying prevalence rates, with rituximab showing the highest at 18%. Subgroup analysis based on drug usage duration revealed that the highest proportion of hypogammaglobulinemia occurred in individuals taking the drugs for 1 year or less (19%). The prevalence of infections in MS patients with a focus on different infection types stratified by the MS drug used revealed that pulmonary infections were the most prevalent (9%) followed by urinary tract infections (6%), gastrointestinal infections (2%), and skin and mucous membrane infections (2%). Additionally, a significant decrease in mean IgG levels after treatment compared to before treatment, with a mean difference of 0.57 (95% CI: 0.22 to 0.93).
Conclusion: This study provides a comprehensive analysis of the impact of anti-CD20 drugs on serum IgG levels in MS patients, exploring the prevalence of hypogammaglobulinemia, based on different drug types, treatment durations, and infection patterns. The identified rates and patterns offer a foundation for clinicians to consider in their risk-benefit.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=518239, CRD42024518239.
Multiple sclerosis (MS) is a complex autoimmune disorder affecting the central nervous system, characterized by demyelination and nerve function impairment (1). MS predominantly affects young adults, typically beginning between 20 to 40 years of age, and is a significant contributor to neurological disability in this age group (2). The disease presents with diverse clinical manifestations and has a multifactorial etiology involving genetic and environmental factors. The main aspect of MS pathology involves the formation of demyelinating lesions, which are predominantly found in the white and grey matter of the brain, along with the spinal cord (1). The immune system and inflammation play crucial roles in the neurodegenerative process of MS (3). From a clinical perspective, MS can exhibit two primary trajectories: relapsing or progressive (4). The most frequent presentation involves relapsing-remitting MS (RRMS), characterized by discrete episodes of neurological dysfunction, followed by partial, complete, or no recovery. However, it is important to acknowledge the growing body of evidence suggesting the presence of progression independent of relapse activity (3, 4). With time, the frequency of RRMS relapses generally diminishes. However, a gradual decline often emerges, leading to a continuous worsening of symptoms, a phase known as secondary progressive MS (SPMS) (1). Diagnosis is based on clinical manifestations, radiological findings (notably MRI T2 lesions), and laboratory evidence (including cerebrospinal fluid-specific oligoclonal bands). These constituent elements collectively adhere to the guidelines outlined in the 2017 McDonald criteria (5).
In recent years, various therapeutic interventions, including disease-modifying therapies (DMTs), have been developed to manage MS effectively. DMTs, such as anti-cluster of differentiation 20 (anti-CD20) monoclonal antibodies, aim to modulate the immune response and ameliorate the disease course. These treatments have revolutionized MS management by targeting B cells, which play a pivotal role in the pathogenesis of MS (3).
The intriguing relationship between anti-CD20 treatments, including rituximab, ocrelizumab, ofatumumab, ublituximab, and immunoglobulin G (IgG) levels has garnered scientific interest. However, its noteworthy to mention that these treatments can lead to low levels of immunoglobulin, known as hypogammaglobulinemia (6). The impact on IgG levels and the subsequent risk of infections varies among individuals and types of MS. Different anti-CD20 therapies may also have variable impacts on IgG levels. While advancements in anti-CD20 treatments have greatly benefited patients, there are inconsistencies and heterogeneity in the available data regarding the connection between these treatments, IgG levels, and the risk of infections. Currently, there is a lack of standardized data to guide physicians in adjusting treatment dosages based on IgG levels. Addressing these knowledge gaps is crucial for enhancing the quality of life for individuals living with MS. However, results from studies in this area have differed.
This review aims to investigate the relationship between anti-CD20 MS treatments, IgG levels, and the risk of hypogammaglobulinemia and infections. By doing so, it aims to inform discussions on anti-CD20 MS treatment strategies and improve treatment decisions to prevent severe infections in patients.
The study was designed and reported with adherence to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines (7). We submitted the research protocol for this systematic review to the International Prospective Registry of Systematic Reviews (PROSPERO) database1 and assigned the PROSPERO ID: CRD42024518239. All working group members agreed on the study protocol before beginning the literature search.
We conducted a comprehensive search across five electronic databases (Medline via PubMed, Scopus, Embase, Cochrane, and Web of Science) from their inception up to Jan 25, 2024, to identify relevant studies. We used the Medical Subject Headings (Mesh) database to retrieve the synonyms of our search strategy, and the terms were combined using “OR” and “AND” Boolean operators, following the Cochrane Handbook for Systematic Reviews (Chapter 4.4.4) (8). The search strategy utilized were as follows: (“Immunoglobulin OR globulin OR Antibody OR Ig OR AB AND (G OR GAMMA OR γ) OR Hypogammaglobulinemia OR Agammaglobulinemia”) AND ((Multiple OR disseminated OR “Acute Fulminating”) AND (sclerosis OR “encephalomyelitis disseminata” OR “ADEM”)) OR MS AND (“Rituximab OR Mabthera OR Anti-CD20 OR Rituxan OR Ocrelizumab OR Ublituximab OR Ofatumumab OR Riabni OR Ruxience OR Truxima OR GP2013 OR CD20 OR IDEC-C2B8 OR “IDEC C2B8”). The detailed search terms and results for each database are provided in Supplementary Table S1.
Following the removal of duplicates by the endnote software, two authors screened the retrieved studies independently based on our predefined eligibility criteria using titles and abstracts. Subsequently, the list of included studies was subjected to further scrutiny by two authors. Studies deemed relevant and any conflicts were subjected to full-text screening. Additionally, we conducted a manual search by reviewing the references of the articles included, and literature reviews for possible relevant studies.
We included studies that met the following criteria: (1) all studies regardless of language or study type, and (2) studies that examined the effects of anti-CD20 monoclonal antibody treatments on serum IgG levels among diverse subtypes of MS patients. We excluded the following: (1) reviews, editorial correspondence, book chapters, animal and laboratory experiments, and in vitro inquiries, and (2) studies that did not explicitly mention serum IgG levels as a quantifiable factor. This meticulous approach ensures that the included studies meet high standards of quality and relevance.
Two authors independently extracted the data from the studies included entering the collected information into a pre-piloted Excel spreadsheet. To guarantee data accuracy and consistency, another author meticulously reviewed the completed extraction sheet, reconciled any discrepancies, and validated the precision of the data.
The data extraction encompassed several key elements, starting with study characteristics such as the first author’s name, publication year, study design, geographical location, and duration of the disease under investigation. Population characteristics, including sample size, gender distribution, age, duration of anti-CD20 treatment, type of MS, and interventions administered, were also recorded.
Furthermore, when applicable data on population characteristics were gathered based on after and before anti-CD20 administration, Expanded Disability Status Scale (EDSS) scores at the start and at the end of treatment, and different phases of the condition (PPMS, RRMS, SPMS). For the quantitative analysis, the same authors independently extracted information; the serum IgG levels in patients with MS, which was also extracted for different types of MS when applicable, and for different subtypes of anti-CD20 drugs data necessary to analyze the correlation of anti-CD20 treatment with serum IgG levels, and crucial variables for conducting the necessary statistical analysis. They also extracted data for different types of infections such as GIT, skin, pulmonary, and urinary. The data of interest were collected in the form of event, total, mean, and standard deviation. Data reported using different formats were converted into mean and standard deviation values using the website developed by McGrath et al. (9) which is an online tool used to facilitate the estimation of sample mean and standard deviation, ensuring consistency in the data presentation and analysis.
Our meta-analysis was conducted using the meta-package of the R software (R version 4.1.0) (10), This package provided a systematic approach to extracting and analyzing data from the selected studies. We performed an initial analysis to calculate the percentage of occurrence of hypogammaglobulinemia among MS patients undergoing treatment with anti-CD20 drugs. Additionally, a subgroup analysis was performed based on drug type and treatment duration to identify specific effects. We also calculated the mean difference in IgG levels by comparing baseline (pre-treatment) and post-treatment levels among different drug types. Furthermore, we calculated the infections rate during treatment, including pulmonary, urinary tract, gastrointestinal, and skin/mucous membrane infections. The proportion of patients developing specific infections for each drug was calculated, providing insights into infection patterns associated with anti-CD20 therapies.
We assessed the heterogeneity using the I2 and chi-squared tests and applied the random effect model. Heterogeneity was considered substantial when I2 was more than 50% at a p-value <0.05. Mean differences were reported with 95% confidence intervals for continuous data. Publication bias was assessed visually using a funnel plot when enough studies were included in the analysis (n ≥ 10). For all the outcomes, we conducted leave-one-out meta-analyses, in which each of the meta-analyses was repeated by removing a single study, one at a time, to demonstrate how each study influences the total estimate.
Two authors independently assessed the quality of the included studies and resolved any disagreements through discussion with a third author. The updated Cochrane risk of bias tool for randomized trials (ROB 2.0) was used to evaluate the risk of bias in the RCT studies (11). The risk of bias table covered biases related to randomization, deviations from expected interventions, missing data, outcome measurement, and selection of reported results. Each trial was categorized as high risk, some concerns, or low risk based on the assessment. In addition, we used the NIH tool to assess the quality of cohort and cross-sectional studies, which consisted of 14 questions related to the research methodology (12). Each question is answered with yes, no, or unclear. The risk bias of the included case-control studies was determined using the Newcastle–Ottawa Scale (NOS), a star system consisting of nine questions, with a point awarded for each answer marked with an asterisk (13).
Following full-text screening, we identified 39 articles that met our inclusion criteria and were included in our review. These articles included six randomized clinical trials, 29 observational cohort, one was cross-sectional study, and three case control studies (Figure 1) (14–52).
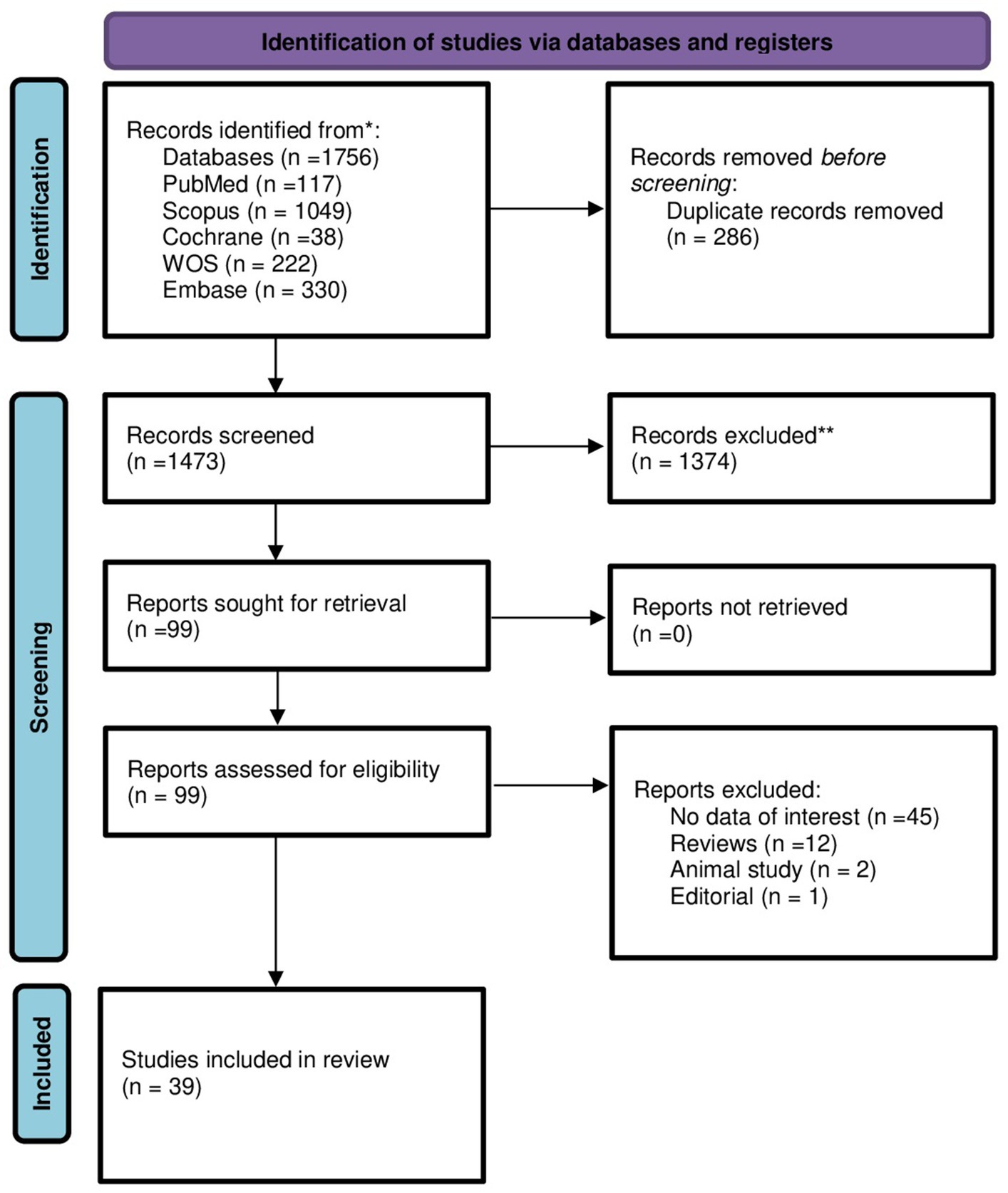
Figure 1. PRISMA flow diagram for new systematic reviews showing the selecting process of the articles which included searches of databases and registers only.
The relevant studies encompassed a total of 20,501 MS patients. Geographically. These studies were conducted in various countries, including France, Italy, Sweden, the USA, Qatar, Croatia, Switzerland, Norway, Denmark, Canada, Australia, Spain, Sweden, Greece, and the United Kingdom, providing a global perspective on MS treatments. The interventions investigated involve a range of anti-CD20 drugs, including therapies like rituximab, ocrelizumab, ofatumumab, and ublituximab. The patient populations studied in these diverse MS investigations represented a wide range of ages, typically spanning from the late 20s or early 30s to beyond 70 years. Sex distribution across the studies varies, with the percentage of female participants typically ranging from approximately 45 to 75%, indicating a higher representation of females across the studies. The studies also included different MS subtypes, such as Relapsing-Remitting MS (RRMS), Secondary Progressive MS (SPMS), Primary Progressive MS (PPMS), and Progressive-Relapsing MS (PRMS), ensuring a comprehensive representation of the disease spectrum. The duration of the disease, measured in years since onset or diagnosis, covers a broad spectrum, ranging from a few years to several decades. EDSS scores, indicating disability levels, vary across the studies, generally spanning from low scores indicative of mild disability to higher scores associated with more severe disability. In some studies, the specific type of immunosuppressive therapy used among the previously mentioned three drugs was not specified, so we referred to them collectively as “Anti CD20 drugs” in our analysis. A summary of the baseline characteristics of the included studies is presented in Table 1. To simplify the results of our subgroup analysis, we have provided a table summarizing the different correlations explored between the aforementioned factors (Table 2).
We identified 28 studies involving 12,012 patients that reported on the prevalence rate of hypogammaglobulinemia in patients of MS. The prevalence rate in the overall analysis was 11% (95% CI: 0.08 to 0.15), with substantial heterogeneity [I2 > 91% (p < 0.01)]. Examining subgroups based on drug type indicated that rituximab exhibited the highest prevalence at 18%, followed by ocrelizumab at 11%, non-specified anti-CD20 at 10%, and ofatumumab at 2%. The statistical test for subgroup differences yielded significance, indicating that the prevalence of hypogammaglobulinemia varies across drug types (rituxumab, ocrelizumab, ofatumumab, anti-CD20 (NS)) subgroups (p interaction <0.01) (Figure 2). The hypogammaglobulinemia thresholds determined by the included articles are listed in Table 3.
We identified 20 studies involving 9,405 patients revealed that the duration of drug intake was a significant factor in hypogammaglobulinemia development. The prevalence rate of hypogammaglobulinemia in patients who take these drugs was 11% (95% CI: 0.07 to 0.16), with substantial heterogeneity [I2 > 96% (p < 0.01)] (Figure 3). Upon conducting subgroup analysis based on drug usage duration, it was observed that the highest proportion of hypogammaglobulinemia development occurred in individuals taking these drugs for 1 year or less (19%), followed by 4 years (13%), 2 years (12%), and 3 years (7%). There was no statistically significant difference between the six subgroups regarding duration of treatment with the specified disease modifying therapy (1 year or less, about 2 years, about 3 years, about 4 years, about 5 years or about 8 years) denoting that the proportion of hypogammaglobulinemia development does not vary majorly across different groups of drug intake duration (p interaction = 0.06).
In this analysis, our aim was to identify prevalent infection types that occurred during the patients’ treatment. The analysis was stratified for each infection type based on the MS drug used during the study.
Fourteen studies involving 6,598 patients indicated the prevalence rate of pulmonary infections in MS patients. These infections ranged from upper respiratory tract issues like rhinitis and sinusitis to lower tract infections, including pneumonia requiring hospitalization. The prevalence rate in the overall analysis was 9% (95% CI: 0.04 to 0.20), with substantial heterogeneity [I2 > 98% (p < 0.01)] (Figure 4). Examining subgroups based on drug type indicated that patients who used ocrelizumab exhibited the highest prevalence of pulmonary infections at 26%, followed by rituxumab at 6%, non-specified anti-CD20 at 3%, and ofatumumab at 1%. The statistical test for subgroup differences yielded significance, indicating that the prevalence of pulmonary infections varies across drug types (rituxumab, ocrelizumab, ofatumumab), subgroups (p interaction <0.01).
Thirteen studies involving 4,484 patients indicated the prevalence rate of urinary tract infections (UTI) in MS patients. These infections ranged from upper to lower tract infections. The prevalence rate in the overall analysis was 6% (95% CI: 0.03 to 0.11), with substantial heterogeneity [I2 > 95% (p < 0.01)] (Figure 5). Examining subgroups based on drug type indicated that patients use ocrelizumab exhibited the highest prevalence of urinary infections at 13%, followed by rituxumab at 3%, non-specified anti-CD20 at 3%, and ofatumumab at 2%. The statistical test for subgroup differences did not yield significance, indicating that the prevalence of urinary infections does not vary across drug types (rituxumab, ocrelizumab, ofatumumab), subgroups (p interaction <0.01).
Nine studies involving 2,664 patients indicated the prevalence rate of gastrointestinal (GIT) infections in MS patients. The prevalence rate in the overall analysis was 2% (95% CI: 0.01 to 0.04), with substantial heterogeneity [I2 > 79% (p < 0.01)] (Figure 6). Examining subgroups based on drug type indicated that patients use ocrelizumab exhibited the highest prevalence of gastrointestinal infections at 4%, followed by ofatumumab at 3%, rituxumab at 1%, and non-specified anti-CD20 at 1%. The statistical test for subgroup differences did not yield significance, indicating that the prevalence of gastrointestinal infections does not vary across drug types (rituxumab, ocrelizumab, ofatumumab), subgroups (p interaction = 0.12).
Five studies involving 1,058 patients indicated the prevalence rate of skin and mucous membrane infections in MS patients. The prevalence rate in the overall analysis was 2% (95% CI: 0.00 to 0.09), with substantial heterogeneity [I2 > 90% (p < 0.01)] (Figure 7). Examining subgroups based on drug type indicated that patients use ocrelizumab exhibited the highest prevalence of gastrointestinal infections at 6%, followed by rituxumab at 0%. The statistical test for subgroup differences yielded significance, indicating that the prevalence of skin and mucous membrane infections varies across drug types (rituxumab, ocrelizumab), subgroups (p interaction <0.01).
Nine studies involving 4,405 patients indicated the prevalence rate of herpes virus infections in MS patients. The prevalence rate in the overall analysis was 1% (95% CI: 0.00 to 0.03), with substantial heterogeneity [I2 > 90% (p < 0.01)] (Figure 8). Examining subgroups based on drug type indicated that patients use ocrelizumab exhibited the highest prevalence of gastrointestinal infections at 4%, followed by rituxumab at 1%; ofatumumab at 0%. The statistical test for subgroup differences yielded significance, indicating that the prevalence of skin and mucous membrane infections varies across drug types (rituxumab, ocrelizumab and ofatumumab), subgroups (p interaction <0.01).
Our analysis aimed to investigate the relationship between the MS drugs and IgG levels drop by comparing the mean IgG before and after drug intake taking into account the drug type and duration of the drug intake.
Ten studies involving 3,589 MS patients demonstrated significantly lower IgG level in post treatment group compared to pre-treatment. The mean difference was 0.57 (95% CI: 0.22 to 0.93), with substantial heterogeneity (I2 > 80%) (Figure 9). However, when subgrouping by different drugs only Ofatumumab showed statistically significant IgG levels drop of −0.06 mean difference (−0.25; 013, I2 = 82%, p = 0.02) while ocrelizumab, rituximab and anti-CD20 all showed no statistical significance (p = 0.10, 0.26, 0.56 respectively). Subgroups of different drugs [rituxumab, ocrelizumab, ofatumumab, anti-CD20 (NS)], Figure 9 shows statistical significance in mean differences of serum IgG levels decrease (p interaction = <0.01). The statistical test for subgroup differences yielded significance, indicating that the mean differences of serum IgG levels vary across drug types [rituxumab, ocrelizumab, ofatumumab, anti-CD20 (NS)], subgroups (p interaction <0.01).
Regarding the duration of drug intake, the overall showed a mean difference of 0.57 in IgG level between pre-drug and post-drug intake (0.17; 0.93, I2 = 79%, p < 0.01) (Figure 10). Subgroup analysis based on drug usage duration revealed that drug intake for one year or less showed no significant change when comparing pre to post mean IgG level (p = 0.43). Subgroups of treatment duration of about 3 years also show no statistically significant mean difference of IgG level. There were not enough studies available for drug intake durations of 2 years, 1.5 years or 4 years to apply the random effects model on the data. Subgroups of different treatment durations (1 year or less, 1.5 years, about 2 years, about 3 years, about 4 years) Figure 10 showed no statistical significance in mean differences of serum IgG levels decrease (p interaction = 0.33).
The assessment of potential publication bias through funnel plots yielded consistently revealed no evidence of publication bias or asymmetry. Overall, funnel plot assessments consistently revealed no evidence of publication bias or asymmetry. In the subgroup analysis based on drug type and IgG levels in serum, the linear regression Egger’s test yielded no detectable funnel plot asymmetry (p = 0.5908). Similarly, the investigation into treatment duration and its relationship with hypogammaglobulinemia showed no funnel plot asymmetry, as indicated by the Egger’s test result of p = 0.9035. Furthermore, in the analysis of mean differences in IgG levels pre-drug and post-drug intake, the Egger’s test for the overall results once again demonstrated no funnel plot asymmetry (p = 0.9035).
We conducted leave-one-out analyses to evaluate the impact of individual studies on the overall findings within each subgroup analysis. Across all subgroup analyses based on drug type, IgG levels in serum, treatment duration, and infection type, no single study significantly influenced the results. This suggests the robustness and stability of the reported data.
We utilized various tools to assess the quality of the 36 studies included in our analysis. We used the ROB2 tool for six RCTs; three of them were rated as good quality (24, 26, 51), and the other three studies were of low quality (23, 36, 46) due to the limited available data in the studies. We used the NIH tool for 30 Cohort and cross-sectional studies, 19 of them were of good quality (14, 15, 17–22, 25, 27, 28, 32, 35, 37, 40, 41, 47, 50, 52), 11 studies were of fair quality (16, 29, 30, 38, 39, 42–45, 48, 49). Three studies were assessed using the Newcastle-Ottawa Scale, with two scoring nine stars (31, 33), and one scoring eight stars (34). We also encountered challenges with incomplete data in some conference abstracts, leading to a fair quality rating for many of the assessments. Further details can be found in the Supplementary material containing the tables of quality assessment.
To our knowledge this is the first large population-based study investigating the intricate connection between anti-CD20 MS treatments, changes in IgG levels, and the associated risk of hypogammaglobulinemia and subsequent infections. In our comprehensive analysis, we examined 36 studies aiming to elucidate the link between the immunosuppressive impacts of various anti-CD20 monoclonal antibody drugs used in MS and the occurrence of hypogammaglobulinemia, along with the resulting infections. The overall analysis indicates a significant prevalence of hypogammaglobulinemia (11%) among MS patients take anti-CD20 drugs. Examining subgroups based on drug type indicated that rituximab exhibited the highest prevalence at 18%, followed by ocrelizumab at 11%, non-specified anti-CD20 at 10%, and ofatumumab at 2%. Chronic usage of anti-CD20 therapies, such as rituximab and ocrelizumab, has been associated with a decline in immunoglobulin levels, particularly IgG and IgM. The decrease in IgG levels may persist even after the discontinuation of therapy. Also, anti-CD20 therapies, particularly ofatumumab and ocrelizumab, are effective in treating MS but pose a higher risk of infections.
Various disease-modifying therapies for MS differ in mechanisms, efficacy, and safety profiles, with a focus on targeting the immune response (53). Anti-CD20 therapies such as rituximab, ocrelizumab, ofatumumab, and ublituximab have proven effective and well-tolerated in MS patients through clinical trials, thereby expanding treatment options (54). Specifically, the anti-CD20 monoclonal antibodies ofatumumab and ocrelizumab, approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), demonstrate efficacy in relapsing multiple sclerosis (RMS) by delaying disease progression, reducing relapses, and limiting new lesion formations on brain scans (55, 56). Even though B-cell depleting (anti-CD20) therapy is effective in treating MS patients (32, 57), it comes with the highest infection risk among MS disease-modifying therapies (58).
As a complication of anti-CD20 therapy, a common occurrence is a decline in immunoglobulin levels. In the phase 3 trials for rituximab and ocrelizumab, there was an observed increase in the percentage of patients with serum IgG and IgM levels below the normal range, however, ofatumumab and ublituximab resulted in a rise in the proportion of patients displaying IgM levels below the lower limit of normal (LLN), while not impacting IgG levels (32, 53–59).
Hypogammaglobulinemia can manifest during prolonged anti-CD20 therapy (60, 61). One hypothesis suggests that despite anti-CD20 treatment not directly affecting IgG and IgM-producing plasma cells, it delays the regeneration of B-cells (54). Reports indicate the time required for B-cell regeneration post-treatment: 24 weeks for ofatumumab, 72 weeks (ranging 27–175) for ocrelizumab, and 70 weeks (ranging 0.1–75) for ublituximab (56). Even after 48 weeks from rituximab discontinuation, B-cell levels were merely 30.7% of baseline values (57). Another explanation involves certain B-cell subsets experiencing impaired reconstitution; for instance, post-rituximab therapy, regenerated B cells mainly comprise naïve B cells with fewer differentiated memory B cells (40, 62). These regenerated naive B cells display reduced ability to become plasma cells, leading to decreased IgG and IgA production while still capable of producing IgM (61). This suggests that the impact of anti-CD20 therapies on immunoglobulin levels might involve complex interactions between different types of B cells, affecting humoral immunity (54, 63, 64).
Our overall analysis shows a statistically significant decrease in IgG levels from pre- to post-drug intake and a significant hypogammaglobulinemia development rate among anti-CD20 drugs users. Ofatumumab showed a significant decrease in IgG levels, while ocrelizumab, and rituximab, did not show statistical significance. Saidha et al. (65) conducted a systematic review of clinical trials and real-world evidence (RWE) studies to investigate alterations in immunoglobulin levels in individuals diagnosed with RMS who underwent treatment with either ocrelizumab or ofatumumab, as well as to understand the correlation between changes in Ig levels and the occurrence of infections. The obtained results emphasized that the most frequently documented outcome was the variation in IgG levels. Among the ocrelizumab trial groups, four trial populations observed a decline in IgG levels over 24 to 336 weeks follow-up period. Conversely, in the five ofatumumab trial groups monitored for 104 to 168 weeks, a temporary drop in IgG levels occurred at week 48, but no sustained decrease was noted thereafter. In both ocrelizumab and ofatumumab trials, IgM displayed a declining trend over time. Additionally, a reduction in mean IgA levels was observed in the ocrelizumab treatment group throughout the 336-week follow-up period.
Our analysis also revealed that the duration of drug intake significantly influenced hypogammaglobulinemia development. However, no statistically significant difference was observed in the subgroup analysis based on different treatment durations, indicating that reduced IgG levels within 1 year did not necessarily lead to increased hypogammaglobulinemia with prolonged treatment. In a recent retrospective analysis of a considerable group of individuals with MS who were administered rituximab or ocrelizumab, 3.7% exhibited a decline in their IgG levels, dropping below 5 g/L on average after an exposure period of approximately 29.7 months (44).
Several studies in the literature have sought to investigate the potential association between low IgG levels and demographic characteristics of patients. In a study by Mears et al. (40) involving 184 patients treated with rituximab and ocrelizumab, 22 patients experienced hypogammaglobulinemia. Those with hypogammaglobulinemia were more likely to be aged ≥50 years and exhibited lower initial IgG levels. In another prospective observational study encompassing all patients with MS after undergoing a median of 5 (1–6) cycles of rituximab (32). The research revealed that age was linked to an increased risk of reduced IgG levels below 6 g/L, while there was no significant association with sex or a history of immunosuppressive treatment.
Evidence from certain clinical trials and observational studies has suggested an relationship between Ig antibody levels and infection rates, as well as infection severity in patients with MS (17, 65). Having an improved understanding of such risks is particularly relevant within the context of B cell-depleting therapeutic strategies because one of the main functions of B cells is antibody production. In a broader sense, people with MS taking B cell-depleting therapies that may interfere with the generation and/or release of Ig antibodies in response to infectious exposures may accordingly have a greater risk for serious infections (65). Our study reports an overall infection rate of 11% among MS patients receiving anti-CD20 drugs with ocrelizumab shows the highest prevalence of pulmonary infections (24%), urinary tract infections (10%), gastrointestinal infections (4%), and skin and mucous membrane infections (6%). This infection rate represents the proportion of MS patients who developed hypogammaglobulinemia out of the total number of patients on anti-CD20 drugs.
For rituximab, multiple retrospective studies have indicated that within the MS patient cohort receiving rituximab, a subset displaying reduced levels of IgG experienced escalated rates of severe infections necessitating hospitalization, prolonged antibiotic therapies, or intravenous antibiotic interventions when compared to counterparts with higher IgG levels (44). Another three observational studies encompassing a range of 59 to 1,000 patients, undergoing up to 3.5 years of rituximab treatment, established an augmented infection risk associated with IgG deficiency in MS patients, whereas IgM deficiency did not exhibit a similar association (17, 32, 33). Notably, one study highlighted those patients manifesting diminished IgG levels encountered elevated rates of severe infections even before their IgG levels declined, attributed to factors such as advanced age, extended disease duration, and diminished CD19 count (expressed in pro-B cells, B cells, and short-lived plasma cells) (63). These findings emphasize the intricate interplay of various factors contributing to infection risk beyond solely IgG levels.
Screening for hypogammaglobulinemia is vital as it helps identify patients at risk of severe infections (66). Therefore, initiation of anti-CD20 therapy demands careful monitoring due to the increased potency of infections. However, the medical literature lacks a universally agreed upon threshold for defining hypogammaglobulinemia in MS studies (67). Various definitions of the lower limit of normal (LLN) for immunoglobulins (IgG and IgM) have been utilized across different clinical investigations. The LLN for these immunoglobulins can differ depending on factors such as patient age and the specific clinical laboratory used (67). The range of studies included in our analysis provides a diverse spectrum of reported values for defining hypogammaglobulinemia in MS. Across the studies, the reported cutoff values vary considerably, with the lowest being 5 mg/dL and the highest reaching 7 mg/dL. Considering this broad range, it is evident that there is substantial variability in the literature regarding the threshold for defining hypogammaglobulinemia. To suggest the most suitable cutoff value, a balance between inclusivity and clinical relevance must be struck. Based on the distribution of reported values, a cutoff between 6 and 7 mg/dL emerges as a pragmatic choice, capturing a significant portion of the reported data while maintaining clinical significance. Therefore, a cutoff value of approximately 6–7 mg/dL may offer a reasonable compromise in defining hypogammaglobulinemia in MS studies.
Finally, anti-CD20 therapies have shown a link to higher chances of severe COVID-19 infection and hospitalization, as indicated by real-world data. While there was speculation regarding hypogammaglobulinemia contributing to this heightened risk, a retrospective study involving 758 patients found no significant association between IgG levels below 700 mg/dL and COVID-19 (68). Additionally, in individuals with MS, factors such as advanced age, Black ethnicity, lack of ambulatory ability, existing health conditions, and the use of glucocorticoids have been linked to an increased likelihood of COVID-19 hospitalization or death (53).
This study has some limitations to be addressed. Notably, challenges arose from incomplete data within certain conference abstracts, prompting a fair quality rating for several assessments. Moreover, the study acknowledges the presence of moderate to high heterogeneity in some analyses, may be attributable to differences in populations, ethnicity, and study designs. Mitigation strategies, such as random-effects models and subgroup analyses, were employed, with leave-one-out analyses conducted to validate the precision of estimates across various subanalysis groups. The intricate nature of confounding factors, particularly those associated with infections, poses complexities to the analysis, acknowledging that the considerable challenge in drawing definitive conclusions due to the high degree of heterogeneity. Additionally, the apparent differing rate of hypogammaglobulinemia may be influenced by patient-specific factors that could have varied between the groups receiving different medications, such as disease severity, duration, and individual immune response variations. Further investigation into these potential confounders is warranted to better elucidate the relationship between anti-CD20 MS treatments, IgG levels, and the risk of hypogammaglobulinemia. Also, the study underscores a limitation concerning the scarcity of studies, notably for specific drugs like ofatumumab. The limited data for certain interventions necessitates caution in drawing definitive conclusions, and future research endeavors addressing these gaps could significantly enhance the depth of understanding in this domain.
In conclusion, the study reveals a noteworthy prevalence rate of hypogammaglobulinemia development among MS patients take anti-CD20 therapies, with rituximab demonstrating the highest prevalence of low IgG concentration when compared to other MS drugs. This study contributes valuable insights into the immunosuppressive effects, infection risks, and implications of anti-CD20 therapies in MS treatment. The identified relationships and patterns offer a foundation for clinicians to consider in their risk-benefit assessments and underscore the importance of ongoing monitoring and research to optimize therapeutic strategies and patient outcomes in the context of MS treatment.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
AE: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. NA: Writing – review & editing, Writing – original draft, Methodology, Investigation, Data curation. MA-k: Writing – original draft, Visualization, Resources. LM: Writing – original draft, Resources, Methodology, Investigation. MG: Writing – original draft, Resources, Project administration, Methodology. AF: Writing – original draft, Investigation. HH: Writing – original draft, Resources, Project administration, Investigation. AA: Writing – original draft, Resources, Methodology, Investigation. OA: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1380654/full#supplementary-material
1. Filippi, M, Bar-Or, A, Piehl, F, Preziosa, P, Solari, A, Vukusic, S, et al. Multiple sclerosis. Nat Rev Dis Primers. (2018) 4:43. doi: 10.1038/s41572-018-0041-4
2. Yeshokumar, AK, Narula, S, and Banwell, B. Pediatric multiple sclerosis. Curr Opin Neurol. (2017) 30:216–21. doi: 10.1097/WCO.0000000000000452
3. Sharrad, D, Chugh, P, Slee, M, and Bacchi, S. Defining progression independent of relapse activity (PIRA) in adult patients with relapsing multiple sclerosis: a systematic review☆. Mult Scler Relat Disord. (2023) 78:104899. doi: 10.1016/j.msard.2023.104899
4. Müller, J, Cagol, A, Lorscheider, J, Tsagkas, C, Benkert, P, Yaldizli, Ö, et al. Harmonizing definitions for progression independent of relapse activity in multiple sclerosis: a systematic review. JAMA Neurol. (2023) 80:1232–45. doi: 10.1001/jamaneurol.2023.3331
5. McGinley, MP, Goldschmidt, CH, and Rae-Grant, AD. Diagnosis and treatment of multiple sclerosis. JAMA. (2021) 325:765. doi: 10.1001/jama.2020.26858
6. Smith, JB, Hellwig, K, Fink, K, Lyell, DJ, Piehl, F, and Langer-Gould, A. Rituximab, MS, and pregnancy. Neurol Neuroimmunol Neuroinflamm. (2020) 7:e734. doi: 10.1212/NXI.0000000000000734
7. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. (2021) 18:e1003583. doi: 10.1371/journal.pmed.1003583
8. Higgins, JPT, Thomas, J, Chandler, J, Cumpston, M, Li, T, Page, MJ, et al. Cochrane handbook for systematic reviews of interventions. Hoboken, NJ: John Wiley & Sons (2020) Available at: www.training.cochrane.org/handbook.
9. McGrath, S, Zhao, X, Steele, R, Thombs, BD, Benedetti, A, Levis, B, et al. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. (2020) 29:2520–37. doi: 10.1177/0962280219889080
10. Balduzzi, S, Rücker, G, and Schwarzer, G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. (2019) 22:153–60. doi: 10.1136/ebmental-2019-300117
11. Sterne, JAC, Savović, J, Page, MJ, Elbers, RG, Blencowe, NS, Boutron, I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
12. National Heart, Lung and Blood Institute . (2019). Study quality assessment tools. Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
13. Lo, CK-L, Mertz, D, and Loeb, M. Newcastle–Ottawa scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. (2014) 14:45. doi: 10.1186/1471-2288-14-45
14. Bauthman, MS . Effectiveness of anti-cluster of differentiation 20 as a disease-modifying therapy in multiple sclerosis across its different phenotypes at the University Hospital of Caen. Cureus. (2022) 14:e22120. doi: 10.7759/cureus.22120
15. Capasso, N, Palladino, R, Cerbone, V, Spiezia, AL, Covelli, B, Fiore, A, et al. Ocrelizumab effect on humoral and cellular immunity in multiple sclerosis and its clinical correlates: a 3-year observational study. J Neurol. (2023) 270:272–82. doi: 10.1007/s00415-022-11350-1
16. Chang, L, Jehli, L, Simkover, R, and Cooper, J. ECTRIMS 2019—poster session 3. Mult Scler J Exp Transl Clin. (2019) 25:581–805. doi: 10.1177/1352458519868081
17. Disanto, G, Ripellino, P, Riccitelli, GC, Sacco, R, Scotti, B, Fucili, A, et al. De-escalating rituximab dose results in stability of clinical, radiological, and serum neurofilament levels in multiple sclerosis. Mult Scler J. (2021) 27:1230–9. doi: 10.1177/1352458520952036
18. Evertsson, B, Hoyt, T, Christensen, A, Nimer, FAL, Foley, J, and Piehl, F. A comparative study of tolerability and effects on immunoglobulin levels and CD19 cell counts with ocrelizumab vs. low dose of rituximab in multiple sclerosis. Mult Scler J Exp Transl Clin. (2020) 6:205521732096450. doi: 10.1177/2055217320964505
19. Garcia-Cañibano, B, Ouanes, S, Ganesan, GS, Yousuf, W, Humos, B, Baig, T, et al. Real-world experience of ocrelizumab in multiple sclerosis in an Arab population. J Drug Assess. (2021) 10:106–13. doi: 10.1080/21556660.2021.1989193
20. Graves, J, Vinayagasundaram, U, Mowry, EM, Matthews, IR, Marino, JA, Cheng, J, et al. Effects of rituximab on lymphocytes in multiple sclerosis and neuromyelitis optica. Mult Scler Relat Disord. (2014) 3:244–52. doi: 10.1016/j.msard.2013.10.003
21. Habek, M, Piskač, D, Gabelić, T, Barun, B, Adamec, I, and Krbot, SM. Hypogammaglobulinemia, infections and COVID-19 in people with multiple sclerosis treated with ocrelizumab. Mult Scler Relat Disord. (2022) 62:103798. doi: 10.1016/j.msard.2022.103798
22. Hallberg, S, Boremalm, M, Evertsson, B, Lillvall, E, Johansson, F, Lycke, J, et al. ECTRIMS 2019—oral presentations. Mult Scler J Exp Transl Clin. (2019) 25:3–130. doi: 10.1177/1352458519868070
23. Hauser, SL, Cross, AH, Winthrop, K, Wiendl, H, Nicholas, J, Meuth, SG, et al. Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years. Mult Scler J. (2022) 28:1576–90. doi: 10.1177/13524585221079731
24. Hawker, K, O’Connor, P, Freedman, MS, Calabresi, PA, Antel, J, Simon, J, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. (2009) 66:460–71. doi: 10.1002/ana.21867
25. Iliopoulou, VA, Starvaggi Cucuzza, C, Gorczyca, A, Manouchehrinia, A, Piehl, F, and Fink, K. ECTRIMS 2021—ePoster. Mult Scler J Exp Transl Clin. (2021) 27:134–740. doi: 10.1177/13524585211044667
26. Kappos, L, Li, D, Calabresi, PA, O’Connor, P, Bar-Or, A, Barkhof, F, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet. (2011) 378:1779–87. doi: 10.1016/S0140-6736(11)61649-8
27. Karlowicz, JR, Klakegg, M, Aarseth, JH, Bø, L, Myhr, KM, Torgauten, HM, et al. Predictors of hospitalization due to infection in rituximab-treated MS patients. Mult Scler Relat Disord. (2023) 71:104556. doi: 10.1016/j.msard.2023.104556
28. Khatri, B, Van Zealand, P, Tarima, S, Schutten, S, Baker, A, Sershon, L, et al. Hypogammaglobulinemia and infection rates in ocrelizumab treated multiple sclerosis patients over 3 years: a real-world single center study. ECTRIMS 2021—ePoster. Mult Scler J. (2021) 27:134–740. doi: 10.1177/13524585211044667
29. Mehta, N, Balasa, A, Shukla, N, Fisher, K, and Lotze, T. Effect of rituximab on immunoglobulin levels in pediatric multiple sclerosis patients. Neurology. (2021) 96:4398. doi: 10.1212/wnl.96.15_supplement.4398
30. Nobile, S, and Beauchemin, P. Ocrelizumab and hypogammaglobulinemia: a real-world retrospective MS cohort study. ECTRIMS 2022—poster. Mult Scler J. (2022) 28:130–691. doi: 10.1177/13524585221123687
31. Oksbjerg, NR, Nielsen, SD, Blinkenberg, M, Magyari, M, and Sellebjerg, F. Anti-CD20 antibody therapy and risk of infection in patients with demyelinating diseases. Mult Scler Relat Disord. (2021) 52:102988. doi: 10.1016/j.msard.2021.102988
32. Perriguey, M, Maarouf, A, Stellmann, JP, Rico, A, Boutiere, C, Demortiere, S, et al. Hypogammaglobulinemia and infections in patients with multiple sclerosis treated with rituximab. Neurol Neuroimmunol Neuroinflamm. (2022) 9:e1115. doi: 10.1212/NXI.0000000000001115
33. Peters, J, and Longbrake, EE. Infection risk in a real-world cohort of patients treated with long-term B-cell depletion for autoimmune neurologic disease. Mult Scler Relat Disord. (2022) 68:104400. doi: 10.1016/j.msard.2022.104400
34. Rempe, T, Elfasi, A, Rodriguez, E, Vasquez, M, Graves, J, and Kinkel, R. Ocrelizumab extended interval dosing compared to standard dosing—a safe alternative with significantly decreased immunoglobulin M deficiency rates. ECTRIMS 2022 LB poster. Mult Scler J. (2022) 28:956–83. doi: 10.1177/13524585221126909
35. Seery, N, Sharmin, S, Li, V, Nguyen, AL, Meaton, C, Atvars, R, et al. Predicting infection risk in multiple sclerosis patients treated with ocrelizumab: a retrospective cohort study. CNS Drugs. (2021) 35:907–18. doi: 10.1007/s40263-021-00810-3
36. de Seze, J, Bar-Or, JCA, Cross, AH, Kappos, L, Selmaj, K, Wiendl, H, et al. Effect of ofatumumab on serum immunoglobulin levels and infection risk in relapsing multiple sclerosis patients from the phase 3 ASCLEPIOS I and II trials. Neurology. (2021) 96:1300. doi: 10.1212/WNL.96.15_supplement.1300
37. Torgauten, HM, Myhr, KM, Wergeland, S, Bø, L, Aarseth, JH, and Torkildsen, Ø. Safety and efficacy of rituximab as first- and second line treatment in multiple sclerosis—a cohort study. Mult Scler J Exp Transl Clin. (2021) 7:205521732097304. doi: 10.1177/2055217320973049
38. Tran, V, Miller, PA, Jarrar, R, Olson, J, Miller, T, and Miravalle, A. Real world efficacy and safety profile of ocrelizumab therapy in patients with multiple sclerosis. ECTRIMS 2019—poster session 3. Mult Scler J. (2019) 25:581–805. doi: 10.1177/1352458519868081
39. Tsao, L, Otani, IM, and Bove, R. Hypogammaglobulinemia in multiple sclerosis patients receiving disease-modifying immunomodulatory agents. J Allergy Clin Immunol. (2019) 143:AB16. doi: 10.1016/j.jaci.2018.12.051
40. Mears, V, Jakubecz, C, Seeco, C, Woodson, S, Serra, A, and Abboud, H. Predictors of hypogammaglobulinemia and serious infections among patients receiving ocrelizumab or rituximab for treatment of MS and NMOSD. J Neuroimmunol. (2023) 377:578066. doi: 10.1016/j.jneuroim.2023.578066
41. Derfuss, T, Bermel, R, Lin, C, Hauser, S, Kappos, L, Vollmer, T, et al. Risk factors for serious infections in patients with MS receiving long-term ocrelizumab treatment: multivariate analyses. ePosters. Eur J Neurol. (2022) 29:374–837.
42. Vollmer, B, Declusin, A, Nair, K, Sillau, S, Corboy, J, Vollmer, T, et al. Ocrelizumab real-world safety and effectiveness in the two years of treatment in multiple sclerosis. MSVirtual 2020—poster abstracts. Mult Scler J. (2020) 26:118–659. doi: 10.1212/WNL.96.15_supplement.4796
43. Vollmer, B, Vollmer, T, Corboy, J, and Alvarez, E. Risk factors for developing lymphopenia and hypogammaglobulinemia in anti-CD20 treated patients with multiple sclerosis. Neurology. (2020) 94:5218. doi: 10.1212/WNL.94.15_supplement.5218
44. Vollmer, BL, Wallach, AI, Corboy, JR, Dubovskaya, K, Alvarez, E, and Kister, I. Serious safety events in rituximab-treated multiple sclerosis and related disorders. Ann Clin Transl Neurol. (2020) 7:1477–87. doi: 10.1002/acn3.51136
45. Vollmer, B, Ijadi, N, Declusin, A, Nair, K, Sillau, S, Corboy, J, et al. Two-year real-world experience with ocrelizumab in the treatment of patients with multiple sclerosis. ECTRIMS 2021—ePoster. Mult Scler J. (2021) 27:134–740. doi: 10.1177/13524585211044667
46. Wiendl, H, De Seze, J, Bar-Or, A, Correale, J, Cross, AH, Kappos, L, et al. Serum immunoglobulin levels and infection risk in the phase 3 trials of ofatumumab in relapsing multiple sclerosis. MSVirtual 2020—poster abstracts. Mult Scler J. (2020) 26:118–659.
47. Zoehner, G, Miclea, A, Salmen, A, Kamber, N, Diem, L, Friedli, C, et al. Reduced serum immunoglobulin G concentrations in multiple sclerosis: prevalence and association with disease-modifying therapy and disease course. Ther Adv Neurol Disord. (2019) 12:175628641987834. doi: 10.1177/1756286419878340
48. Xavier, B, Lafaurie, M, Treiner, E, Walter, O, Pugnet, G, Martin-Blondel, G, et al. POS1178 prescribing rituximab in patients with auto-immune diseases and acquired hypogammaglobulinemia: description of the risk of severe infection in 121 patients before the SARS-CoV2 era. Ann Rheum Dis. (2022) 81:917.1–917.91917. doi: 10.1136/annrheumdis-2022-eular.1194
49. Wiendl, H, De Seze, J, Bar-Or, A, Correale, J, Cross, AH, Kappos, L, et al. Effect of ofatumumab on serum immunoglobulin levels and infection risk in patients with relapsing multiple sclerosis over 3.5 years, poster presented at ECTRIMS 2021 congress. (2021) 27:134–740. doi: 10.1177/13524585211044667
50. Salzer, J, Svenningsson, R, Alping, P, Novakova, L, Björck, A, Fink, K, et al. Rituximab in multiple sclerosis: a retrospective observational study on safety and efficacy. Neurology. (2016) 87:2074–81. doi: 10.1212/WNL.0000000000003331
51. Montalban, X, Hauser, SL, Kappos, L, Arnold, DL, Bar-Or, A, Comi, G, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. (2017) 376:209–20. doi: 10.1056/NEJMoa1606468
52. López Ruiz, R, Eichau, S, Guerra Hiraldo, JD, Dotor García-Soto, J, Ruiz De Arcos, M, and Ruiz-Peña,. Real world data on the use of ocrelizumab. Incidence of lymphopenia, B-cell and immunoglobulins evolution, ECTRIMS 2021—ePoster. Mult Scler J. (2021) 27:134–740. doi: 10.1177/13524585211044667
53. Klein, A, Flaskamp, M, Berthele, A, Held, F, Muratovic, H, and Hemmer, B. The impact of disease-modifying therapies on immunoglobulin blood levels in patients with multiple sclerosis: a retrospective cross-sectional study. Ther Adv Neurol Disord. (2023) 16:175628642311626. doi: 10.1177/17562864231162661
54. Alvarez, E, Longbrake, EE, Rammohan, KW, Stankiewicz, J, and Hersh, CM. Secondary hypogammaglobulinemia in patients with multiple sclerosis on anti-CD20 therapy: pathogenesis, risk of infection, and disease management. Mult Scler Relat Disord. (2023) 79:105009. doi: 10.1016/j.msard.2023.105009
55. Hauser, SL, and Cree, BAC. Treatment of multiple sclerosis: a review. Am J Med. (2020) 133:1380–1390.e2. doi: 10.1016/j.amjmed.2020.05.049
56. Gassman, AL, Nguyen, CP, and Joffe, HV. FDA regulation of prescription drugs. N Engl J Med. (2017) 376:674–82. doi: 10.1056/NEJMra1602972
57. Hauser, SL, Waubant, E, Arnold, DL, Vollmer, T, Antel, J, Fox, RJ, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. (2008) 358:676–88. doi: 10.1056/NEJMoa0706383
58. Luna, G, Alping, P, Burman, J, Fink, K, Fogdell-Hahn, A, Gunnarsson, M, et al. Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. JAMA Neurol. (2020) 77:184–91. doi: 10.1001/jamaneurol.2019.3365
59. Steinman, L, Fox, E, Hartung, HP, Alvarez, E, Qian, P, Wray, S, et al. Ublituximab versus teriflunomide in relapsing multiple sclerosis. N Engl J Med. (2022) 387:704–14. doi: 10.1056/NEJMoa2201904
60. De La Torre, I, Leandro, MJ, Valor, L, Becerra, E, Edwards, JCW, and Cambridge, G. Total serum immunoglobulin levels in patients with RA after multiple B-cell depletion cycles based on rituximab: relationship with B-cell kinetics. Rheumatology. (2012) 51:833–40. doi: 10.1093/rheumatology/ker417
61. Nishio, M, Fujimoto, K, Yamamoto, S, Endo, T, Sakai, T, Obara, M, et al. Delayed redistribution of CD27, CD40 and CD80 positive B cells and the impaired in vitro immunoglobulin production in patients with non-Hodgkin lymphoma after rituximab treatment as an adjuvant to autologous stem cell transplantation. Br J Haematol. (2007) 137:349–54. doi: 10.1111/j.1365-2141.2007.06584.x
62. Colucci, M, Carsetti, R, Serafinelli, J, Rocca, S, Massella, L, Gargiulo, A, et al. Prolonged impairment of immunological memory after anti-CD20 treatment in pediatric idiopathic nephrotic syndrome. Front Immunol. (2019) 10:1653. doi: 10.3389/fimmu.2019.01653
63. Margoni, M, Preziosa, P, Filippi, M, and Rocca, MA. Anti-CD20 therapies for multiple sclerosis: current status and future perspectives. J Neurol. (2022) 269:1316–34. doi: 10.1007/s00415-021-10744-x
64. Tellier, J, and Nutt, SL. Plasma cells: the programming of an antibody-secreting machine. Eur J Immunol. (2019) 49:30–7. doi: 10.1002/eji.201847517
65. Saidha, S, Bell, J, Harold, S, Belisario, JM, Hawe, E, Shao, Q, et al. Systematic literature review of immunoglobulin trends for anti-CD20 monoclonal antibodies in multiple sclerosis. Neurol Sci. (2023) 44:1515–32. doi: 10.1007/s10072-022-06582-y
66. Barmettler, S, Ong, MS, Farmer, JR, Choi, H, and Walter, J. Association of immunoglobulin levels, infectious risk, and mortality with rituximab and hypogammaglobulinemia. JAMA Netw Open. (2018) 1:e184169. doi: 10.1001/jamanetworkopen.2018.4169
67. Otani, IM, Lehman, HK, Jongco, AM, Tsao, LR, Azar, AE, Tarrant, TK, et al. Practical guidance for the diagnosis and management of secondary hypogammaglobulinemia: a work group report of the AAAAI primary immunodeficiency and altered immune response committees. J Allergy Clin Immunol. (2022) 149:1525–60. doi: 10.1016/j.jaci.2022.01.025.35176351
Keywords: anti-CD20, meta-analysis, multiple sclerosis, hypogammaglobulinemia, infections
Citation: Elgenidy A, Abdelhalim NN, Al-kurdi MA-m, Mohamed LA, Ghoneim MM, Fathy AW, Hassaan HK, Anan A and Alomari O (2024) Hypogammaglobulinemia and infections in patients with multiple sclerosis treated with anti-CD20 treatments: a systematic review and meta-analysis of 19,139 multiple sclerosis patients. Front. Neurol. 15:1380654. doi: 10.3389/fneur.2024.1380654
Received: 01 February 2024; Accepted: 03 April 2024;
Published: 18 April 2024.
Edited by:
Mark Slee, Flinders University, AustraliaReviewed by:
Stephen Bacchi, Royal Adelaide Hospital, AustraliaCopyright © 2024 Elgenidy, Abdelhalim, Al-kurdi, Mohamed, Ghoneim, Fathy, Hassaan, Anan and Alomari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammed Al-mahdi Al-kurdi, bW9oYW1tZWRtYWhkaWt1cmRpQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.