- 1Center for Rehabilitation Medicine, Rehabilitation and Sports Medicine Research Institute of Zhejiang Province, Department of Rehabilitation Medicine, Zhejiang Provincial People's Hospital, Affiliated People's Hospital, Hangzhou Medical College, Hangzhou, Zhejiang, China
- 2School of Basic Medical Sciences, Zhejiang University School of Medicine, Hangzhou, China
Background: Intravaginal electrical stimulation (IVES) has been explored as a potential treatment for pelvic floor disorders (PFDs), although its efficacy remains a subject of debate. We aim to conducted a comprehensive meta-analysis of relevant trials.
Methods: This meta-analysis was performed under the PRISMA 2020 guideline. We meticulously searched for randomized controlled trial (RCT) studies in various databases, including PubMed, Cochrane Library, EMBASE, and ClinicalTrials.gov, spanning from inception to March 6, 2023. All studies included one treatment group of intravaginal electrical stimulation and the diseases spectrum of the studies involved different kinds of PFDs, including urinary incontinence, overactive bladder, etc. Risk of bias charts were used to assess the risk of bias in the studies and forest plots were used the demonstrate the overall effects.
Results: Our analysis encompassed a total of 13 RCT studies. In most of the assessed PFD cure outcomes, the results demonstrated positive effects of IVES therapy, as indicated by the following findings: daily voiding frequency (MD = −1.57, 95% CI = −3.08 to −0.06, I2 = 68%,), nocturia (MD = −1.07, 95% CI = −2.01 to −0.13, I2 = 71%), Pad test, and Urinary incontinence. Nevertheless, the data concerning the impact of IVES therapy on the quality of life of individuals with PFDs did not confirm these positive results.
Discussion: In light of the insufficiency in both the quality and quantity of the included studies, it is premature to draw a definitive conclusion regarding the efficacy of IVES therapy for treating PFDs. Nonetheless, our study does provide several pieces of evidence in support of the potential therapeutic effects of electrical stimulation therapy in this context. We recommend that further research in this area be conducted to provide more conclusive insights into the efficacy of IVES therapy for PFDs.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier: CRD42023442171.
1 Introduction
Pelvic floor disorder (PFD), also known as pelvic floor dysfunction, is characterized by a range of symptoms associated with the malfunction of the pelvic floor, including urinary incontinence (UI), pelvic organ prolapse (POP), and sexual dysfunction, etc. (1). Brief introduction of these subtypes are as follows.
Among UIs, there're SUI, UUI, and MUI (2). Stress urinary incontinence (SUI) characterizes the involuntary release of urine prompted by heightened abdominal pressure. Considering different treatment options for SUI, surgical interventions have evolved over time. In contrast, urge urinary incontinence (UUI) manifests as involuntary urine leakage accompanied by a strong sensation of urgency and an immediate need to urinate that cannot be delayed. Mixed urinary incontinence (MUI) encompasses a combination of SUI and UUI symptoms.
As for POP, it is a prevalent condition among women (3). Pelvic organ prolapse refers to the descent of one or more pelvic organs from their anatomical position, resulting in the formation of a bulge within the vaginal region, known as a prolapse. Normally, the pelvic organs are supported by the muscles and connective tissues of the pelvic floor, which ensure their proper placement and functioning. Its occurrence has been on the rise in tandem with the overall increase in life expectancy. A range of treatment options, both conservative and surgical in nature, are available to address this condition.
It is estimated that women have a 1 in 4 face a lifetime risk of experiencing PFD, while in many cultures these conditions may have a connection with stigmatization and women tend to suffer with the symptoms silently (4). However, previous researches (4) have pointed out that treatment of these symptoms can have positive effects on their quality of life and sexual satisfaction.
The risk factors of PFDs involved a large range of etiologic factors. Usual factors included increasing age, weight, parity, and a history of hysterectomy (5). Previous studies had proved that as women age the prevalence increases, with >40% of women older than 40 years old experiencing urinary incontinence (6). Therefore, it is recommended that annual screening for PFDs in women should be promoted regardless of whether risk factors were present.
According to previous guidelines (7), the spectrum of treatments for pelvic floor disorders spans from lifestyle and behavioral therapy to surgical interventions, pharmaceuticals, and the use of medical devices (8–10). Among the conservative treatment modalities, pelvic floor muscle training (PFMT) has been recommended as the first-line approach to manage PFD symptoms, particularly stress urinary incontinence (SUI) (11). However, the evidences for electrical stimulation (ES) of the pelvic floor muscles (PFMs) were variable and couldn't reach solid conclusions (11). Therefore, further comparison is needed to be done in this subject.
Pelvic floor muscle training (PFMT) is a structured exercise program which improves pelvic floor muscle strength, endurance, power, relaxation, or a combination of these. It has been reported that PFMT can prevent the symptoms of PFDs and is now considered to be applied before using other intravaginal devices (11). Meanwhile, previous study (12) had pointed out that PFMT combined with the additional treatment using PFMT devices, its effects can be maximized and improved.
Previous studies have proposed three theorized mechanisms for PFMT (13). The first and dominant mechanism is that PFMT exercises the levator ani muscle to increase the cross-sectional area of the key support muscle underlying the urethra (14). The PFMT programs based on this mechanism are called “Kegel's exercises” or pelvic floor muscle (PFM) exercises. The second mechanism targets the urethral striated muscle to maximize the awareness of the timing of the PFM (15) and the corresponding programs are called “the Knack,” “stress strategy,” and “perineal lock.” The third mechanism aims at the transversus abdominis (TrA) muscle to strengthen the core muscle, and the PFMT programs reliant on this theorized mechanism are typically referred to as core muscle training (16). However, it is unclear how much evidence is available to support these theorized mechanisms and further studies are needed to clarify this subject.
Intravaginal electrical stimulation (IVES) is a method of passive muscle activation through the direct stimulation of weakened muscles or nerve fibers. The mechanism of action of electrical stimulation is complicated. It consists of a direct action inducing pelvic floor striated muscle hypertrophy and activation of the detrusor inhibitory reflex arc.
Though IVES is recommended by some physicians (11), its actual efficacy remains controversial. Data from systematic review (17) indicated that there was insufficient evidence both in favor of and against the use of the IVES therapy for women with SUI, probably due to the variability in the interventions of the included trials and the inadequacy of trial data (18). As for surgical interventions, they were not recommended unless other treatments have proven ineffective (11).
Trials concerning IVES as therapies for women has proliferated in recent years, and as for provide guidance for clinical practice, there is a need for more comprehensive assessments of IVES, including a thorough exploration of side effects, comparisons with other therapeutic approaches, and evaluations of IVES as a monotherapy or in combination with other treatments. Moreover, heterogeneities are needed to be solved and explained between these trials. Hence, our study aimed to investigate on whether IVES can treat female PFD syndrome. Furthermore, we aim to assess the efficacy of intravaginal ES, whether administered in conjunction with other treatments or as a standalone therapy, in comparison to no intervention, sham ES, or any other conservative treatment.
2 Methods
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020 guidelines (19). We've registered this program in Prospero before the study (the registration ID was CRD42023442171). Meta-analysis is a secondary data analysis method that relies on existing academic literature, and it does not involve direct experimentation with human subjects. As such, no new ethical review or approval was required for this research.
2.1 Data sources and search strategy
We searched PubMed, Cochrane library, EMBASE, ClinicalTrials.gov from inception to March 6, 2023. Search terms included those related to electric stimulation, intravaginal, and their variants. The full search strategy is provided in Supplementary Table 1. We also extracted relevant articles that met the inclusion criteria for randomized controlled trials included in previous systematic reviews or meta-analyses.
2.2 Eligibility criteria
2.2.1 Types of studies
Only randomized controlled trials (RCTs) available as full-text articles were considered for inclusion.
2.2.2 Types of participants
Female patients diagnosed with pelvic floor dysfunction, including urinary incontinence, overactive bladder, inability to voluntarily contract the PFMs efficiently. However, due to the limitation of quantity of the studies involving both PFDs and IVES, the spectrum of PFDs only involves urinary incontinence (UI), detrusor instability, overactive bladder (OAB). Some subtypes of PFDs, such as pelvic organ prolapse (POP) cannot be fully explored due to insufficient data.
2.2.3 Types of interventions
The following comparisons were made: IVES as a monotherapy or in combination with other treatments vs. no active treatment, sham ES or other conservative treatments for pelvic floor dysfunction.
2.2.4 Types of outcomes
The primary outcomes were objective cure outcomes, defined as cure outcomes that were measured with objective measures and less susceptible to a variety of factors (including results of pad test, daily voiding frequency, PFM strength, etc.), and subjective cure outcomes, defined as cure outcomes that were measured with subjective measures like scales and more susceptible to a variety of factors, including results of International Consultation Incontinence Questionnaire-UI Short Form (ICIQ-SF, ICIQ-UI-SF), urgency. The secondary outcomes were outcomes concerning life quality, including results of incontinence quality of life scale (I-QoL), Spinal Cord Injury Quality-of-Life Measurement System (SCI-QoL), etc.
2.3 Data extraction
A 2-step data extraction process was conducted. During the first stage, according to the study titles and abstracts, two independent reviewers with no interests made decisions in a standardized data extraction form based on the eligibility criteria. During the second stage, studies that passed the previous stage were downloaded, and the full texts were reviewed. Any discrepancies or disagreements were resolved through discussion and consensus. The following information was extracted from each included study: first author, year of publication, country, population characteristic (age, number parity), intervention (type, frequency, pulse width, intensity, and duration), comparison, and outcomes.
2.4 Risk of bias assessment
Studies meeting the inclusion criteria were evaluated for methodological quality to assess the risk of bias employing the Cochrane Collaboration's risk of bias tool as described in the Cochrane Handbook for Systematic Reviews of Interventions; each quality item was graded into three levels: low risk, high risk, or unclear risk (20). The quality assessment covered the following domains: allocation concealment, bias in the allocation process, bias in the results (integrity and authenticity), bias in the measurement process, and selective outcome reporting. Independent assessment by two reviewers was performed in the study and disagreements in the process were solved by a third reviewer.
2.5 Data synthesis and analysis
Continuous outcomes were used for statistical efficacy analysis using Hedge's standardized mean difference (SMD) for pad test, PFM strength, quality of life and urinary incontinence episodes and mean difference (MD) for frequency, maximal cystometric capacity, nocturia, and urgency with 95% confidence intervals (CI) with the random effects model for pooling estimates for each analysis. Subgroup analysis between IVES monotherapy and IVES in combination with other traditional treatments were performed. Binary outcomes were analyzed using rate ratio (RR) with 95% CI with random effects model either, but no analysis for specific outcomes were included in the final article due to limited quantity of studies included. The significance of the pooled effects was evaluated by a Z-test, and a P-value of < 0.05 was considered significant. I2 statistic was used to examine overall heterogeneity between studies and values higher than 50% were defined to have high heterogeneity. All statistical analyses were performed using Review Manager 5.3 (Nordic Cochrane Center) and Stata software version 15.1 (StataCorp, USA).
3 Results
3.1 Study selection and characteristics of included studies
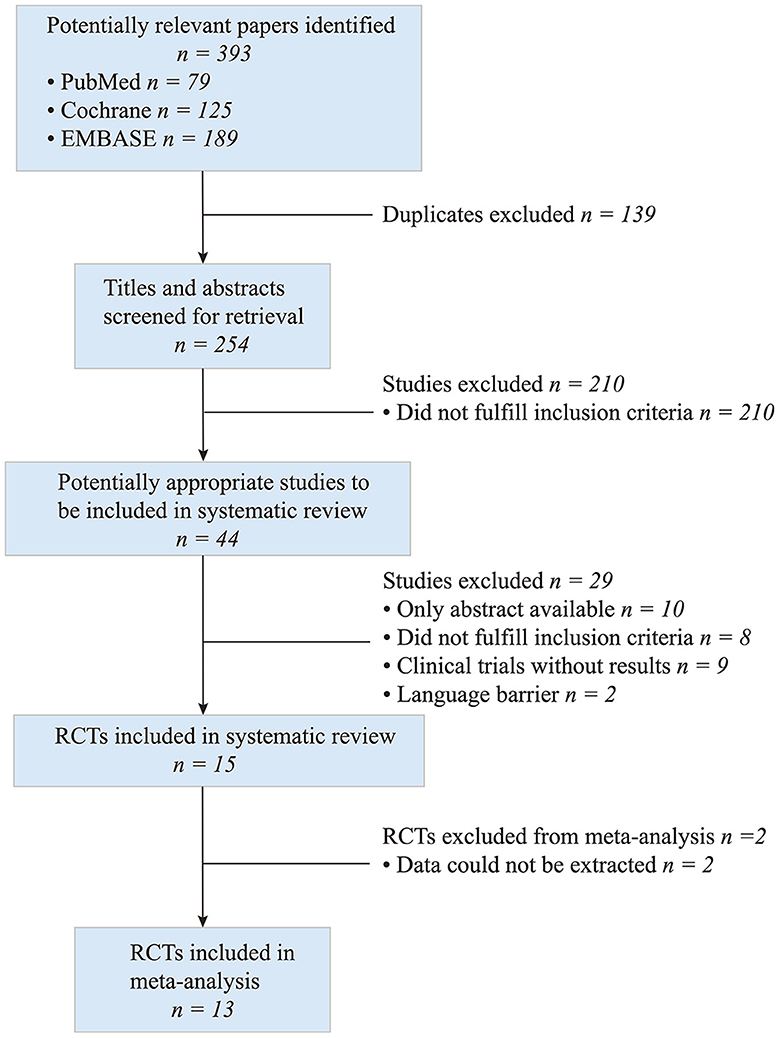
A total of 393 articles were identified by the electronic search. The titles, abstracts and full texts revealed that 13 met the inclusion criteria (Figure 1) and included 559 participants with pelvic floor muscle dysfunction.
The characteristics of included studies are presented in Table 1. Most of the studies included are single-centered and the studies come from the following countries: six in Brazil, two in Turkey, one in China, one in USA, one in Netherland, one in Denmark, and one in Poland. Target diseases were urinary incontinence (UI) (21–27), including stress urinary incontinence (SUI), mixed urinary incontinence (MUI), urge urinary incontinence (UUI), overactive bladder (OAB) (28–30) and other PFDs (31–33). Studies on nonspecific diseases concerning lower urinary tract were also included (23, 31, 33). As for intervention of the studies, nine studies involved IVES monotherapy (21, 22, 24–26, 28, 29, 32, 33) and the rest four studies involved other treatments in combination with IVES treatment, including surface EMG (sEMG) (27, 31), pelvic floor muscle training (PFMT) (23, 31), and bladder training (BT) (30).
Studies included were of high quality. There were low risk in randomization method, data integrity and selective reporting of all 13 studies (21–33), allocation concealment of six studies (21, 23, 24, 26, 30, 33), blind method for participants of 12 studies (21–23, 25–33), and blind method for outcome measurer of 10 studies (21–24, 27, 28, 30–33).
3.2 Results of individual studies and syntheses
3.2.1 Objective cure outcomes
Data from six studies suggested that intravaginal ES had a more preferable effect in improving daily voiding frequency than no active treatment or sham ES (MD = −1.57, 95% CI = −3.08 to −0.06, Figure 2A), but significant heterogeneity was found (I2 = 96%, P = 0.0008). Subgroup analysis of these studies regarding the comparison between IVES as a monotherapy and IVES in combination with other therapies (e.g., PFMT, EMG), the results confirmed that groups treated with IVES monotherapy showed significant improvements compared to groups combined with other therapies (Figure 2A).
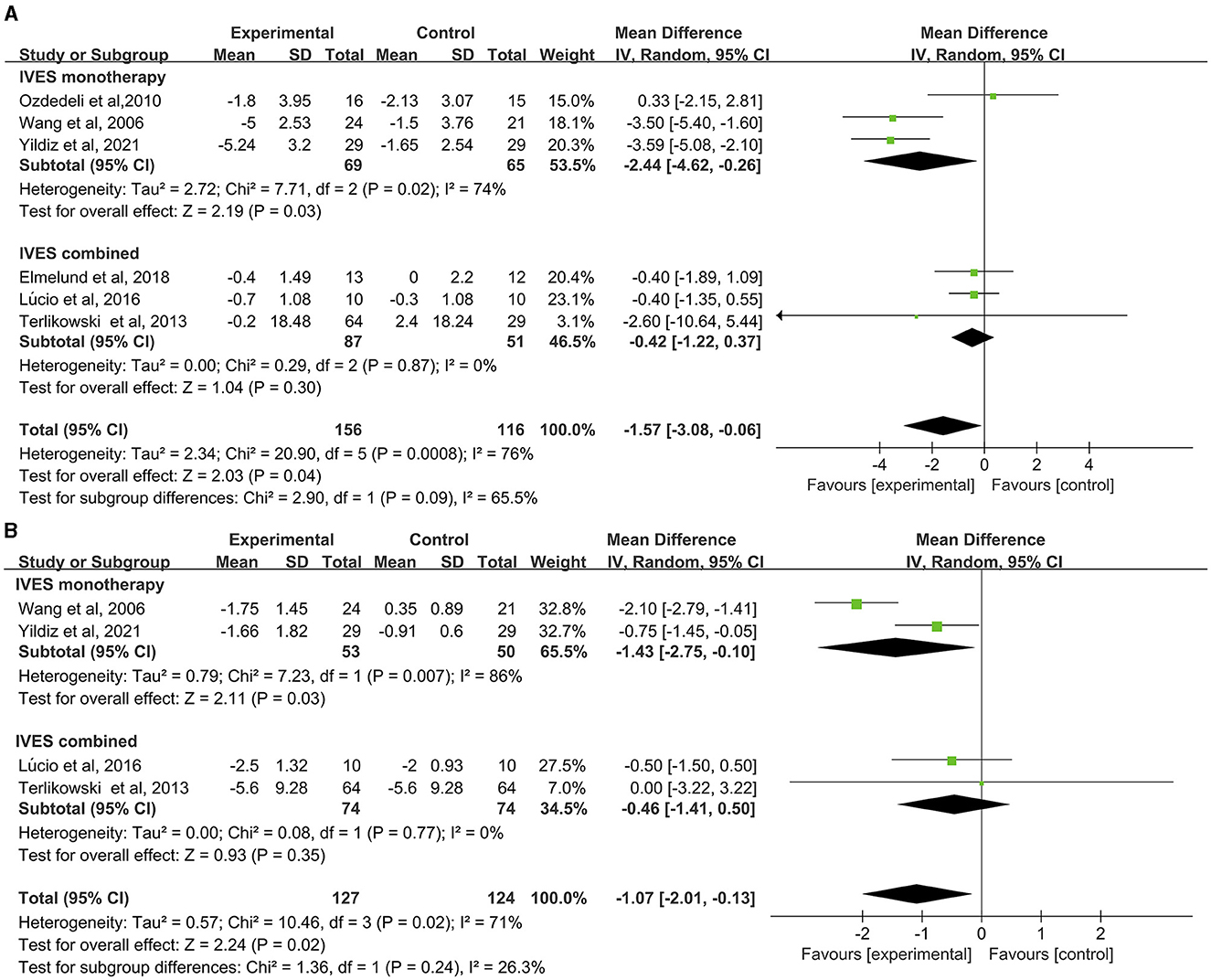
Figure 2. Forest plot of daily voiding frequency or nocturia outcome between the ES and other conservative treatment groups. (A) Daily voiding frequency; (B) nocturia.
Data from four studies showed that intravaginal ES is significantly effective in reducing nocturia than placebo or sham ES (MD = −1.07, 95% CI = −2.01 to −0.13, Figure 2B). Heterogeneity was also found in this analysis (I2 = 71%, P = 0.02). Subgroup analysis of the comparison between IVES as a monotherapy and IVES in combination with other therapies confirmed that IVES monotherapy contributed to a significant reduction in nocturia (Figure 2B), yet heterogeneity was still found in the comparison (I2 = 86%, P = 0.03).
Results of the pad test concerning seven studies was analyzed. Data suggested that IVES contributed to significant improvement in pad test results (MD = −0.51, 95% CI = −0.81 to −0.21, Figure 3A) as well as IVES monotherapy (MD = −0.54, 95% CI = −0.95 to −0.13) while no significant effect was found in IVES in combination with other therapies (MD = −0.43, 95% CI = −0.97 to 0.11) and no heterogeneity was found.
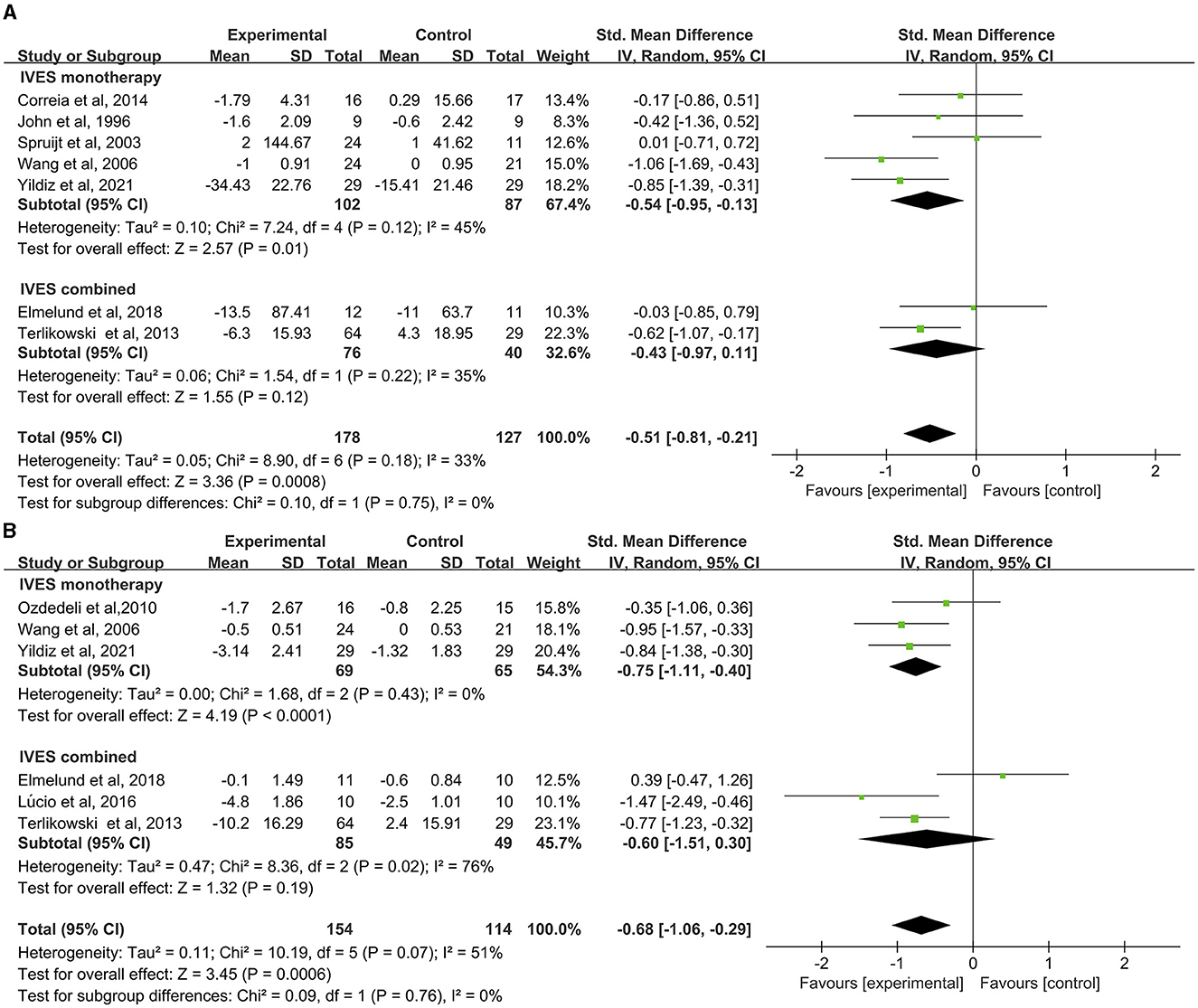
Figure 3. Forest plot of pad test or urinary incontinence result between the ES and other conservative treatment groups; (A) pad test. (B) Urinary incontinence.
Status of urinary incontinence based on patients' voiding diary (including frequency of urine loss, incontinence episodes) of six studies suggested significant improvement in urinary incontinence (Figure 3B), with minor heterogeneity found in the comparison (I2 = 51%, p = 0.07). Subgroup analysis found out that IVES as a monotherapy can contribute to improvement in urinary incontinence (Figure 3B) without heterogeneity found in this comparison.
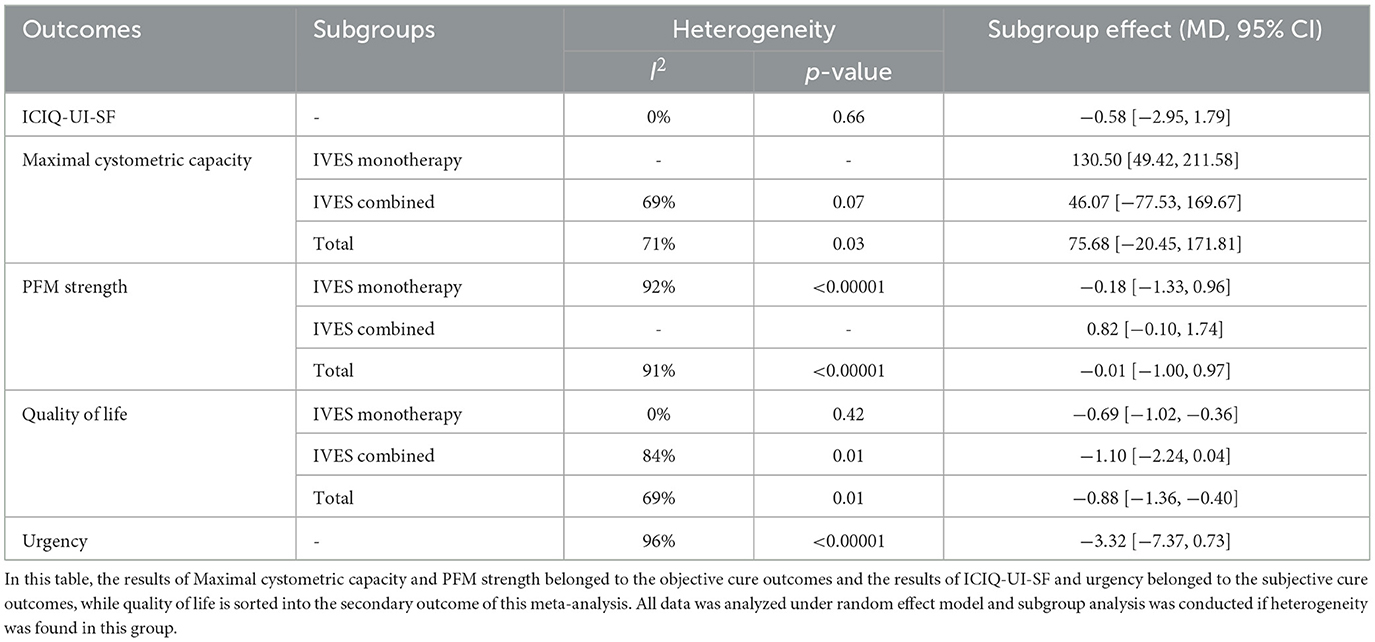
Data from three studies indicated that intravaginal ES is not significantly effective in improving maximal cystometric capacity than sham ES or trospium hydrochloride (Table 2). Heterogeneity was also found (I2 = 71%, p = 0.03). Subgroup analysis was not applied since only three studies was analyzed.
PFM strength (including objective and subjective PFM strength) of six studies was also compare between IVES group and control group. Data showed no significant improvement for IVES group (Table 2) with high heterogeneity (I2 = 91%, p < 0.00001). Meanwhile, subgroup analysis of IVES as monotherapy showed similar results and no significant difference was found.
3.2.2 Subjective cure outcomes
Data from two studies showed no significant improvement in scores of urinary incontinence scales (including ICIQ-UI-SF, ICIQ-SF) and no heterogeneity was found (Table 2). Self-reported urgency of three studies using subjective measurements (including VAS scale, 4-day voiding diary, etc.) indicated no significant difference between both groups (Table 2). No further subgroup analyses were performed as there was not enough studies in these outcomes.
3.2.3 Secondary outcome
Data from five studies suggested that IVES could lead to significant reduction in the impact of PFDs on quality of life (Table 2), while heterogeneity was detected (I2 = 69%, p = 0.01). Subgroup analysis showed that IVES monotherapy contributed to lesser PFDs impact on QoL level in women than sham ES or no active treatment with no heterogeneity, while IVES combined with other treatments (including PFMT, EMG) didn't show significant effect.
4 Discussion
The major goal of this meta-analysis is to analyze published trials concerning the effects of intravaginal electrical stimulation on women with Pelvic floor disorders, and determine whether IVES is an effective method to treat these clinical conditions. Pooled data from included trials have given several instructive conclusions as follows. The 11 studies involved in the analysis were of high quality.
Regarding most objective outcomes measuring the treatment of PFD symptoms (including daily voiding frequency, nocturia, pad test results, and UI status), pooled data from published clinical trials confirmed the positive effects of IVES therapy. As shown in subgroup analyses, it was confirmed that IVES monotherapy contributed to a more preferable effect than IVES combined with other treatments. However, intravaginal ES failed to yield statistically significant improvement in maximal cystometric capacity, PFM strength between the ES and other conservative treatment group. The small quantity of studies included may explain the negative result.
Regarding the two subjective outcomes included in this study, all kinds of analyses failed to yield any significant difference, whether for or against. The reason behind might be the insufficiency of the quantity and quality of evidence included as well as the lack of large-sample multi-centered random clinical trials. Additionally, the subjectivity of the measuring process of these outcomes may explain part of the results.
Concerning these outcomes, high-quality clinical trials had given similar results in support of ES therapy. Previous randomized controlled trials (34–36) have found significant improvement from baseline concerning objective outcome leakage episodes, pad testing, vaginal muscle strength, etc. Meanwhile, results of subjective outcomes including visual analog scores of urinary incontinence, King's Health Questionnaire score (34) also showed significant improvement from baseline, which made a supplement to our results.
Though presenting many evidence in support of intravaginal ES as a treatment for pelvic floor disorders, this meta-analysis didn't reach the conclusion that intravaginal ES therapy is better than other therapies, such as PFMT or drug intervention. Apart from the limited quantity of studies included that involved different kinds of other therapies, the failure of subgroup analysis of IVES combined with other therapies to show significant results also posed a question to whether IVES reigned over other therapies in treatment of PFM dysfunction. Opposite evidences were found in previous systematic reviews. Data from meta-analysis (37) suggested that it was too early to say whether ES is similar or superior to other active treatments like PFMT in effectiveness. Another explanation for this problem may stem from the fact that our search strategy is too broad. Pelvic floor dysfunction is a group of urinary dysfunctions caused by different pathophysiological mechanisms, and most of the participants in previous studies on this topic have been stress incontinence/mixed urinary incontinence. We believe that heterogeneity in the included study populations may be one of the reasons for the reduction in the significance of the results. Moreover, it is suggested (27, 37, 38) that intravaginal electrical stimulation may result in device-related adverse effect (including urinary tract infection, vaginal infection, etc.) and there was insufficient data to determine whose adverse effect tended to be larger. However, the studies analyzed in this systematic review included trials concerning ES with non-implanted devices other than IVES, which may affect the results. Nevertheless, the reasons of low significance of our results are worth exploring and future researches of these hypotheses mentioned above are needed.
As for the secondary outcome, we found improvement in quality of life (QoL) in both IVES and IVES monotherapy, which established a strong connection between quality of life in UI (or other PFM dysfunctions) women and IVES therapy for PFM dysfunction treatment.
Regarding this result, previous studies had given similar results. In the RCT conducted by Kargar Jahromi et al. (35), researchers observed a significant improvement from baseline in PFMT group for incontinence quality of life at 8.5 weeks, pointing out the connection between the alleviation of PFD symptoms like UI and quality of life. Meanwhile, data from Cavkaytar et al. (39) indicated a similar elevation in QoL level from baseline using different scales (including QoL form, Oxford scale, PGI-I) in group of home-based Kegel exercises. However, data from meta-analysis on IVES or ES therapy were rare in this subject.
The limitations of the present study should also be acknowledged. Firstly, the substantial level of heterogeneity suggests that the results obtained should be interpreted with caution. Secondly, due to the limitation in the relevant evidence, only a small number of studies were identified. Thirdly, the population of women with PFM dysfunction was not further subdivided into UI and OAB, etc. and other treatments combined with IVES (including PFMT, IVVS, EMG, etc.) were not further classified, which might weaken the accuracy of results. Fourthly, due to the current limited and inconsistent data, subgroup analysis concerning some outcomes (maximal cystometric capacity, urgency) and analysis to evaluate the effect of IVES combination therapy could not be performed. Fifthly, studies concerning IVES monotherapy were of small quantity, which left the conclusions of IVES monotherapy limited. Lastly, due to the limitation of the quantity of the studies involving both PFDs and IVES, some subtypes of PFDs, like POP and sexual dysfunction, cannot be fully explored in this article. As previous systematic review have pointed out that there was insufficient evidence for or against the use of intravaginal ES therapy for women with PFD symptoms like SUI, there's a lot of space remained to be explored in these results (17). Based on the current research status in this field, follow-up research can be carried out for some specific research questions, such as exploring the setting of IVES stimulation parameters, exploring the intervention of different disease subtypes in the disease spectrum of PFDs and exploring the mechanisms behind these physiotherapies, etc. Large-scale clinical trials and network meta-analyses are needed in these fields. These limitations mentioned above may weaken the significance of this study.
5 Conclusion
The conclusions should be drawn carefully because of the limited evidence quality and quantity. On the one hand, current evidences tended to support the cure effect of IVES monotherapy, while heterogeneity existed in some of the outcomes. On the other hand, there wasn't sufficient evidence for or against the use of intravaginal ES therapy combined with other therapies in women with SUI, partly due to the variability in the choice of other therapies to combine with IVES. Hence, there is a need for further high-quality randomized controlled studies on the effectiveness of intravaginal ES for UI.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
RC: Conceptualization, Data curation, Investigation, Methodology, Software, Writing – original draft. RW: Data curation, Investigation, Methodology, Writing – original draft. YY: Data curation, Methodology, Writing – original draft. KZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JL: Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Natural Science Foundation of Zhejiang Province, China (Grant No. LGF20H170012).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1378494/full#supplementary-material
References
1. MacLennan AH, Taylor AW, Wilson DH, Wilson D. The prevalence of pelvic floor disorders and their relationship to gender, age, parity and mode of delivery. BJOG. (2000) 107:1460–70. doi: 10.1111/j.1471-0528.2000.tb11669.x
2. Kobashi KC, Albo ME, Dmochowski RR, Ginsberg DA, Goldman HB, Gomelsky A, et al. Surgical treatment of female stress urinary incontinence: AUA/SUFU guideline. J Urol. (2017) 198:875–83. doi: 10.1016/j.juro.2017.06.061
3. Baeßler K, Aigmüller T, Albrich S, Anthuber C, Finas D, Fink T, et al. Diagnosis and therapy of female pelvic organ prolapse. Guideline of the DGGG, SGGG and OEGGG (S2e-Level, AWMF Registry Number 015/006, April 2016). Geburtshilfe Frauenheilkd. (2016) 76:1287–301. doi: 10.1055/s-0042-119648
4. Good MM, Solomon ER. Pelvic floor disorders. Obstet Gynecol Clin North Am. (2019) 46:527–40. doi: 10.1016/j.ogc.2019.04.010
5. Gill EJ, Hurt WG. Pathophysiology of pelvic organ prolapse. Obstet Gynecol Clin North Am. (1998) 25:757–69. doi: 10.1016/S0889-8545(05)70041-3
6. Wu JM, Vaughan CP, Goode PS, Redden DT, Burgio KL, Richter HE, et al. Prevalence and trends of symptomatic pelvic floor disorders in US women. Obstet Gynecol. (2014) 123:141–8. doi: 10.1097/AOG.0000000000000057
7. Okeahialam NA, Dworzynski K, Jacklin P, McClurg D. Prevention and non-surgical management of pelvic floor dysfunction: summary of NICE guidance. Br Med J. (2022) 2022:376. doi: 10.1136/bmj.n3049
8. Brown JS, Vittinghoff E, Wyman JF, Stone KL, Nevitt MC, Ensrud KE, et al. Urinary incontinence: does it increase risk for falls and fractures? Study of osteoporotic fractures research group. J Am Geriatr Soc. (2000) 48:721–5. doi: 10.1111/j.1532-5415.2000.tb04744.x
9. Huang AJ, Brown JS, Kanaya AM, Creasman JM, Ragins AI, Van Den Eeden SK, et al. Quality-of-life impact and treatment of urinary incontinence in ethnically diverse older women. Arch Intern Med. (2006) 166:2000–6. doi: 10.1001/archinte.166.18.2000
10. Tähtinen RM, Cartwright R, Tsui JF, Aaltonen RL, Aoki Y, Cárdenas JL, et al. Long-term impact of mode of delivery on stress urinary incontinence and urgency urinary incontinence: a systematic review and meta-analysis. Eur Urol. (2016) 70:148–58. doi: 10.1016/j.eururo.2016.01.037
11. Chêne G, Mansoor A, Jacquetin B, Mellier G, Douvier S, Sergent F, et al. Female urinary incontinence and intravaginal electrical stimulation: an observational prospective study. Eur J Obstet Gynecol Reprod Biol. (2013) 170:275–80. doi: 10.1016/j.ejogrb.2013.06.011
12. Arantes R, Viana R, Seabra E, Silva LF. What is there about endovaginal devices and their effects for pelvic floor muscle training? A systematic review. In: Martins Amaro A, et al. Proceedings of the 10th Congress of the Portuguese Society of Biomechanics. Cham: Springer (2023). p. 26.
13. Sheng Y, Carpenter JS, Ashton-Miller JA, Miller JM. Mechanisms of pelvic floor muscle training for managing urinary incontinence in women: a scoping review. BMC Womens Health. (2022) 22:161. doi: 10.1186/s12905-022-01742-w
14. Bø K. Pelvic floor muscle training is effective in treatment of female stress urinary incontinence, but how does it work? Int Urogynecol J Pelvic Floor Dysfunct. (2004) 15:76–84. doi: 10.1007/s00192-004-1125-0
15. Miller JM, Ashton-Miller JA, DeLancey JO. A pelvic muscle precontraction can reduce cough-related urine loss in selected women with mild SUI. J Am Geriatr Soc. (1998) 46:870–4. doi: 10.1111/j.1532-5415.1998.tb02721.x
16. Sapsford RR, Hodges PW, Richardson CA, Cooper DH, Markwell SJ, Jull GA. Co-activation of the abdominal and pelvic floor muscles during voluntary exercises. Neurourol Urodyn. (2001) 20:31–42. doi: 10.1002/1520-6777(2001)20:1<31::AID-NAU5>3.0.CO;2-P
17. Stania M, Niemiec B, Kamieniarz A, Chmielewska D. Intravaginal electrical stimulation as a monotherapy for female stress urinary incontinence: a systematic review and meta-analysis. Complement Ther Clin Pract. (2022) 49:101624. doi: 10.1016/j.ctcp.2022.101624
18. Vaughan CP, Markland AD. Urinary incontinence in women. Ann Intern Med. (2020) 172:ITC17–32. doi: 10.7326/AITC202002040
19. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. (2021) 134:178–89. doi: 10.1016/j.jclinepi.2021.03.001
20. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions 5.1. 0. Cochrane Collaboration (2011). p. 33–49. Available at: http://trainingcochraneorg/handbook (accessed November 12, 2018).
21. Amaro JL, Gameiro MO, Kawano PR, Padovani CR. Intravaginal electrical stimulation: a randomized, double-blind study on the treatment of mixed urinary incontinence. Acta Obstet Gynecol Scand. (2006) 85:619–22. doi: 10.1080/00016340500495058
22. Correia GN, Pereira VS, Hirakawa HS, Driusso P. Effects of surface and intravaginal electrical stimulation in the treatment of women with stress urinary incontinence: randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. (2014) 173:113–8. doi: 10.1016/j.ejogrb.2013.11.023
23. Elmelund M, Biering-Sørensen F, Due U, Klarskov N. The effect of pelvic floor muscle training and intravaginal electrical stimulation on urinary incontinence in women with incomplete spinal cord injury: an investigator-blinded parallel randomized clinical trial. Int Urogynecol J. (2018) 29:1597–606. doi: 10.1007/s00192-018-3630-6
24. Ignácio Antônio F, Bø K, Pena CC, Bueno SM, Mateus-Vasconcelos ECL, Fernandes ACNL, et al. Intravaginal electrical stimulation increases voluntarily pelvic floor muscle contractions in women who are unable to voluntarily contract their pelvic floor muscles: a randomised trial. J Physiother. (2022) 68:37–42. doi: 10.1016/j.jphys.2021.12.004
25. Smith JJ III. Intravaginal stimulation randomized trial. J Urol. (1996) 155:127–30. doi: 10.1016/S0022-5347(01)66567-4
26. Spruijt J, Vierhout M, Verstraeten R, Janssens J, Burger C. Vaginal electrical stimulation of the pelvic floor: a randomized feasibility study in urinary incontinent elderly women. Acta Obstet Gynecol Scand. (2003) 82:1043–8. doi: 10.1080/j.1600-0412.2003.00130.x
27. Terlikowski R, Dobrzycka B, Kinalski M, Kuryliszyn-Moskal A, Terlikowski SJ. Transvaginal electrical stimulation with surface-EMG biofeedback in managing stress urinary incontinence in women of premenopausal age: a double-blind, placebo-controlled, randomized clinical trial. Int Urogynecol J. (2013) 24:1631–8. doi: 10.1007/s00192-013-2071-5
28. Ozdedeli S, Karapolat H, Akkoc Y. Comparison of intravaginal electrical stimulation and trospium hydrochloride in women with overactive bladder syndrome: a randomized controlled study. Clin Rehabil. (2010) 24:342–51. doi: 10.1177/0269215509346092
29. Wang AC, Chih SY, Chen MC. Comparison of electric stimulation and oxybutynin chloride in management of overactive bladder with special reference to urinary urgency: a randomized placebo-controlled trial. Urology. (2006) 68:999–1004. doi: 10.1016/j.urology.2006.05.038
30. Yildiz N, Alkan H, Sarsan A. Efficacy of intravaginal electrical stimulation added to bladder training in women with idiopathic overactive bladder: a prospective randomized controlled trial. Int Braz J Urol. (2021) 47:1150–9. doi: 10.1590/s1677-5538.ibju.2021.0161
31. Lúcio A, D'ancona CA, Perissinotto MC, McLean L, Damasceno BP, de Moraes Lopes MH. Pelvic floor muscle training with and without electrical stimulation in the treatment of lower urinary tract symptoms in women with multiple sclerosis. J Wound Ostomy Continence Nurs. (2016) 43:414–9. doi: 10.1097/WON.0000000000000223
32. Mateus-Vasconcelos ECL, Brito LGO, Driusso P, Silva TD, Antônio FI, Ferreira CHJ. Effects of three interventions in facilitating voluntary pelvic floor muscle contraction in women: a randomized controlled trial. Braz J Phys Ther. (2018) 22:391–9. doi: 10.1016/j.bjpt.2017.12.006
33. Rodrigues MP, Barbosa LJF, Paiva LL, Mallmann S, Sanches PRS, Ferreira CF, et al. Effect of intravaginal vibratory versus electric stimulation on the pelvic floor muscles: a randomized clinical trial. Eur J Obstet Gynecol Reprod Biol X. (2019) 3:100022. doi: 10.1016/j.eurox.2019.100022
34. Gilling PJ, Wilson LC, Westenberg AM, McAllister WJ, Kennett KM, Frampton CM, et al. A double-blind randomized controlled trial of electromagnetic stimulation of the pelvic floor vs. sham therapy in the treatment of women with stress urinary incontinence. BJU Int. (2009) 103:1386–90. doi: 10.1111/j.1464-410X.2008.08329.x
35. Kargar Jahromi M, Talebizadeh M, Mirzaei M. The effect of pelvic muscle exercises on urinary incontinency and self-esteem of elderly females with stress urinary incontinency, 2013. Glob J Health Sci. (2014) 7:71–9. doi: 10.5539/gjhs.v7n2p71
36. Nissenkorn I, Shalev M, Radziszewski P, Dobronski P, Borkowski A, De Jong PR. Patient-adjusted intermittent electrostimulation for treating stress and urge urinary incontinence. BJU Int. (2004) 94:105–9. doi: 10.1111/j.1464-410x.2004.04856.x
37. Stewart F, Berghmans B, Bø K, Glazener CM. Electrical stimulation with non-implanted devices for stress urinary incontinence in women. Cochrane Database Syst Rev. (2017) 12:CD012390. doi: 10.1002/14651858.CD012390.pub2
38. Sand PK, Richardson DA, Staskin DR, Swift SE, Appell RA, Whitmore KE, et al. Pelvic floor electrical stimulation in the treatment of genuine stress incontinence: a multicenter, placebo-controlled trial. Am J Obstet Gynecol. (1995) 173:72–9. doi: 10.1016/0002-9378(95)90172-8
Keywords: intravaginal electrical stimulation, pelvic floor disorders, meta-analysis, systematic review, PRISMA
Citation: Chen R, Wang R, Yu Y, Zhao K and Li J (2024) Intravaginal electrical stimulation for the treatment of pelvic floor dysfunction: a systematic review and meta-analysis. Front. Neurol. 15:1378494. doi: 10.3389/fneur.2024.1378494
Received: 29 January 2024; Accepted: 24 July 2024;
Published: 13 August 2024.
Edited by:
Hari S. Sharma, Uppsala University, SwedenReviewed by:
Rui Viana, Fernando Pessoa Foundation, PortugalSeaab Imad Sahib, National Center for Toxicological Research (FDA), United States
Copyright © 2024 Chen, Wang, Yu, Zhao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juebao Li, bGlqdWViYW9AaG1jLmVkdS5jbg==; Kun Zhao, emhhb2t1bjA0MjhAemp1LmVkdS5jbg==
 Rongrong Chen
Rongrong Chen Rui Wang1
Rui Wang1 Kun Zhao
Kun Zhao