- 1Department of Neurology, University of Kentucky, Lexington, KY, United States
- 2Department of Neurology, HSHS Medical Group, O'Fallon, IL, United States
- 3Department of Neurology, Stanford Healthcare, Stanford, CA, United States
- 4Department of Neurology, Honor Health, Bob Bove Neurosciences Institute, Scottsdale, AZ, United States
Purpose: To describe the early experience of ravulizumab use in acetylcholine receptor antibody-positive generalized myasthenia gravis (AChR+ve gMG).
Methods: This multicenter retrospective study included AChR+ve gMG patients who were treated with ravulizumab and had both pre- and post-ravulizumab myasthenia gravis activities of daily living (MG-ADL) scores. Clinical information regarding MG history, concomitant treatment(s), MG-ADL, other MG-specific measures, and adverse events were recorded.
Results: A total of 18 patients with mean age of 61.83 (±16.08, n = 18) years were included in this cohort. In 10 complement inhibitor naive patients, a clinically meaningful reduction in mean Mg-ADL (baseline: 6.6 (±3.58) vs. 4.4 (±2.28), post ravulizumab) was seen. 6 out of 10 patients (60%) had clinically meaningful reduction post ravulizumab and two achieved minimum symptom expression (MSE). In 8 patients switched from eculizumab to ravulizumab, further reduction was noted in post ravulizumab mean MG-ADL (Baseline: 3.25 (±3.34) vs. 1.5 (±2.34) post ravulizumab). None of the patients who switched from eculizumab to ravulizumab experienced worsening symptoms. Eleven out of 14 (78.5%) patients on prednisone therapy were able to reduce their prednisone dose post-ravulizumab. None of the patients experienced any major side effects.
Conclusion: In our clinical practice, 60% of AChR+ve gMG complement inhibitor naive patients experienced a clinically meaningful improvement in MG-ADL scores with ravulizumab. Patients were safely switched from eculizumab to ravulizumab and had further improvement in their mean MG-ADL scores. Of those on prednisone therapy, the majority were able to reduce their prednisone dosage.
1 Introduction
The past 5 years have seen a significant increase in the number of FDA-approved therapies for the treatment of myasthenia gravis (MG). Since 2017, three complement inhibitors and two neonatal FC receptor (FcRn) antagonists have been approved for management of MG (1, 2). The first complement inhibitor to receive FDA approval for MG was eculizumab, a monoclonal antibody targeting C5 (3). In April 2022, a longer-acting form of C5 monoclonal antibody, ravulizumab was approved by the FDA for acetylcholine receptor antibody-positive generalized myasthenia gravis (AChR+ve gMG) patients (4). Although ravulizumab and eculizumab have similar mechanisms of action, ravulizumab has a longer half-life (51 vs. 14 days) and consequently has a lower infusion burden (5). Even though the mechanism of action of these two complement inhibitors is similar, clinical outcomes in the trial have subtle differences. The patient population in the eculizumab clinical trial had met the criteria for refractory AChR+ve MG, but this was not a requirement for the ravulizumab clinical trial. Additionally, unlike clinical trials for other hematological conditions such as paroxysmal nocturnal hemoglobinuria, patients with prior complement therapy were not included in the ravulizumab clinical trial (4–6). Therefore, the clinical effectiveness of switching from eculizumab to ravulizumab, as well as the effectiveness of ravulizumab in a diverse MG patient population is unclear even though recent publication highlights benefit in the long-term follow-up of phase 3 clinical trial patient population (7). To shed light on these questions, we report our clinical experience of ravulizumab from three large neuromuscular practices.
2 Materials and methods
2.1 Study design
Patients were identified through the neuromuscular practices of the investigators. Inclusion criteria were AChR+ve gMG, age ≥ 18 years, received at least one dose of ravulizumab, and had MG-ADL scores before and after ravulizumab treatment. We obtained patient information regarding MG-specific history, antibody status, history of thymoma, and thymectomy. MG-specific therapy at the time of ravulizumab initiation was obtained with special attention to any patients switching from eculizumab to ravulizumab or efgartigimod to ravulizumab. Patients who were not on complement inhibitor therapy prior to initiation of ravulizumab were considered complement inhibitor naive. Patients who were started on ravulizumab from May 2022 to May 2023 were included in this analysis.
2.2 Outcome measures
The main outcome measure for clinical effectiveness used in this analysis was MG-ADL, a primary outcome measure in most clinical trials (8). We assessed both clinically meaningful improvement in ADL and also a number of patients who achieved Minimum Symptom Expression (MSE, a MG-ADL score of 0 or 1). We also documented the changes in prednisone dose or other immunosuppressive therapies after starting ravulizumab. We reviewed any reported adverse events during the clinical evaluation. This study was approved by the Institutional Review Board at each institution.
3 Results
3.1 Baseline characteristics
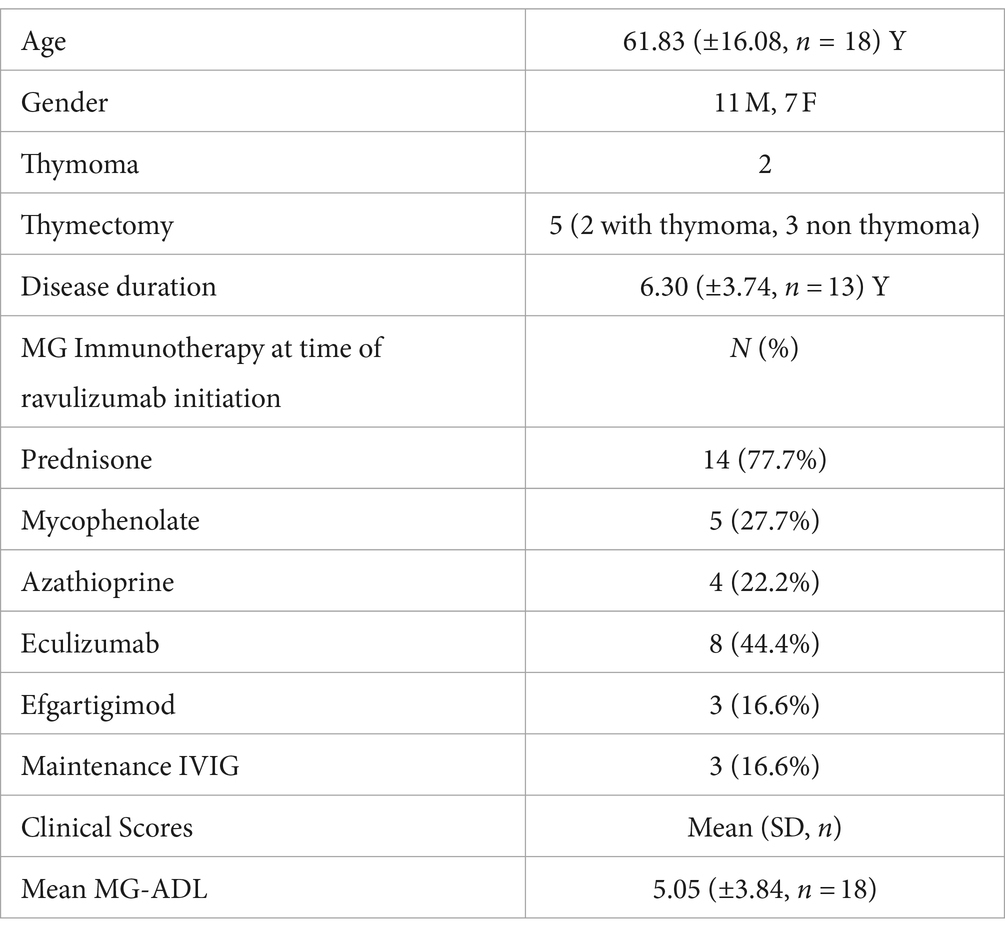
A total of 18 patients with a men age of 61.83 (±16.08, n = 18) years were included in this cohort. Among the 18, 11 were male and 7 were female. 16 patients were Caucasian and the remaining 2 were Hispanic.
10 out of 18 patients were complement inhibitor naive and were on corticosteroids and or corticosteroid sparing immunosuppressants prior to initiation of ravulizumab. Eight patients were transitioned from eculizumab to ravulizumab. The mean interval from MG diagnosis to ravulizumab initiation was 6.30 (±3.74, n = 13) years.
Seven patients had baseline cardiac comorbidities including hypertension, hyperlipidemia, atrial fibrillation, atrial flutter, and right bundle branch block. Four patients had diabetes, two patients had underlying pulmonary disease including chronic obstructive pulmonary disease (COPD) and obstructive sleep apnea (OSA) and one patient had breast cancer. Two patients with thymoma had undergone thymectomy prior to ravulizumab use. Three other patients without thymoma had prior thymectomies. The mean (SD) duration between thymectomy and initiation of ravulizumab was 4 (± 3.28; n = 5) years.
Prior to ravulizumab use, 14 patients were on prednisone with a mean dose of 19.21 (±15.72) mg/day. Nine patients were on steroid-sparing agents (5 on mycophenolate and 4 on azathioprine) and 3 patients were on maintenance intravenous immunoglobulins (IVIG). Additionally, 8 patients were on eculizumab and 3 were on efgartigimod at baseline and were then switched to ravulizumab. Baseline characteristics are shown in Table 1.
3.2 Clinical outcomes
3.2.1 MG-ADL score
3.2.1.1 Treatment response in complement inhibitor naive patients
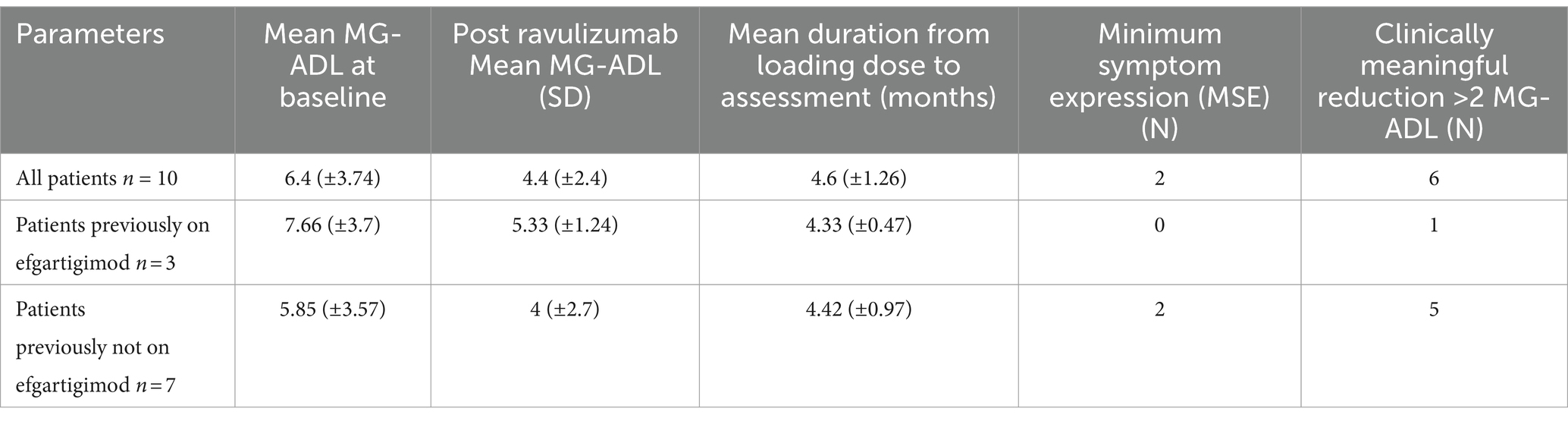
In 10 complement inhibitor naive patients, a clinically meaningful reduction in mean Mg-ADL (baseline: 6.4 (±3.74) vs. 4.4 (±2.4), post ravulizumab) was seen. 6 out of 10 (60%) patients noted a clinically meaningful reduction in MG-ADL score (>2 point change) and two (20%) patients achieved MSE. The mean time from loading dose to post ravulizumab MG-ADL assessment was 4.6 (±1.26) months. Disease characteristics pre and post ravulizumab for complement inhibitor naive patients (baseline MG-ADL >1) are shown in Table 2.
3.2.1.1.1 Patients switched from efgartigimod to ravulizumab
Three patients who were switched from efgaritigmod to ravulizumab noted a significant reduction in post ravulizumab mean MG-ADL (Baseline: 7.66 (± 3.7) vs. 5.33 (± 1.24) post ravulizumab). Figure 1 shows change in MG-ADL score, pre and post ravulizumab therapy for complement inhibitor naive patients.
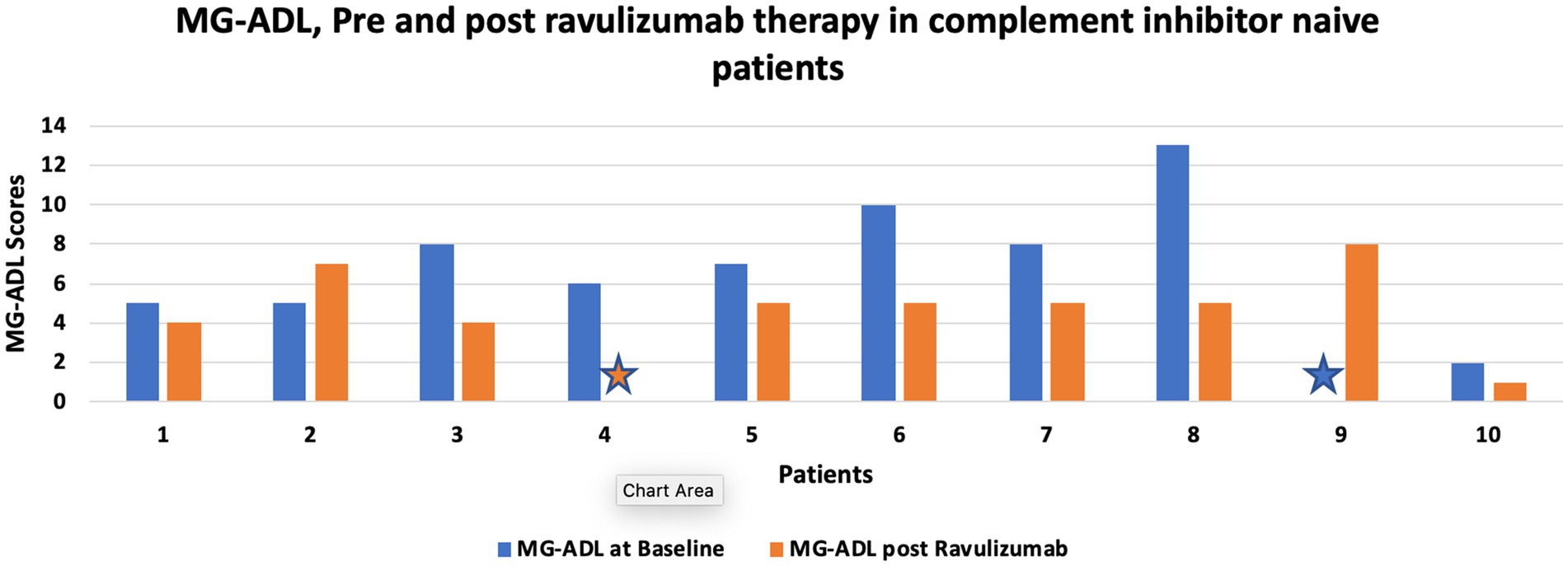
Figure 1. shows change in MG-ADL score, pre and post ravulizumab therapy for complement inhibitor naive patients. Star sign represents MG-ADL score of 0.
3.2.1.1.2 Reduction in prednisone dose
Eight out of 9, complement inhibitor naive patients were able to reduce their prednisone dosage, post ravulizumab therapy. The mean daily prednisone requirement decreased from baseline dose of 20.44 (± 11.69) mg daily to 15.55 (± 17.21) mg daily therapy over a mean duration of 4.7 (+ 1.20) months.
3.2.1.2 Treatment response in patients switched from eculizumab to ravulizumab
A total of 8 patients were switched from eculizumab to ravulizumab. Four patients with baseline MSE status maintained MSE status post ravulizumab. Out of the 4 patients without baseline MSE status, two patients achieved MSE status post ravulizumab therapy whereas the MG-ADL score remained unchanged for the remaining two patients. The mean time from loading dose to post ravulizumab MG-ADL assessment was 5 (±1.06) months.
Figure 2 shows change in MG-ADL score, pre and post ravulizumab therapy in patients who were switched from eculizumab to ravulizumab.
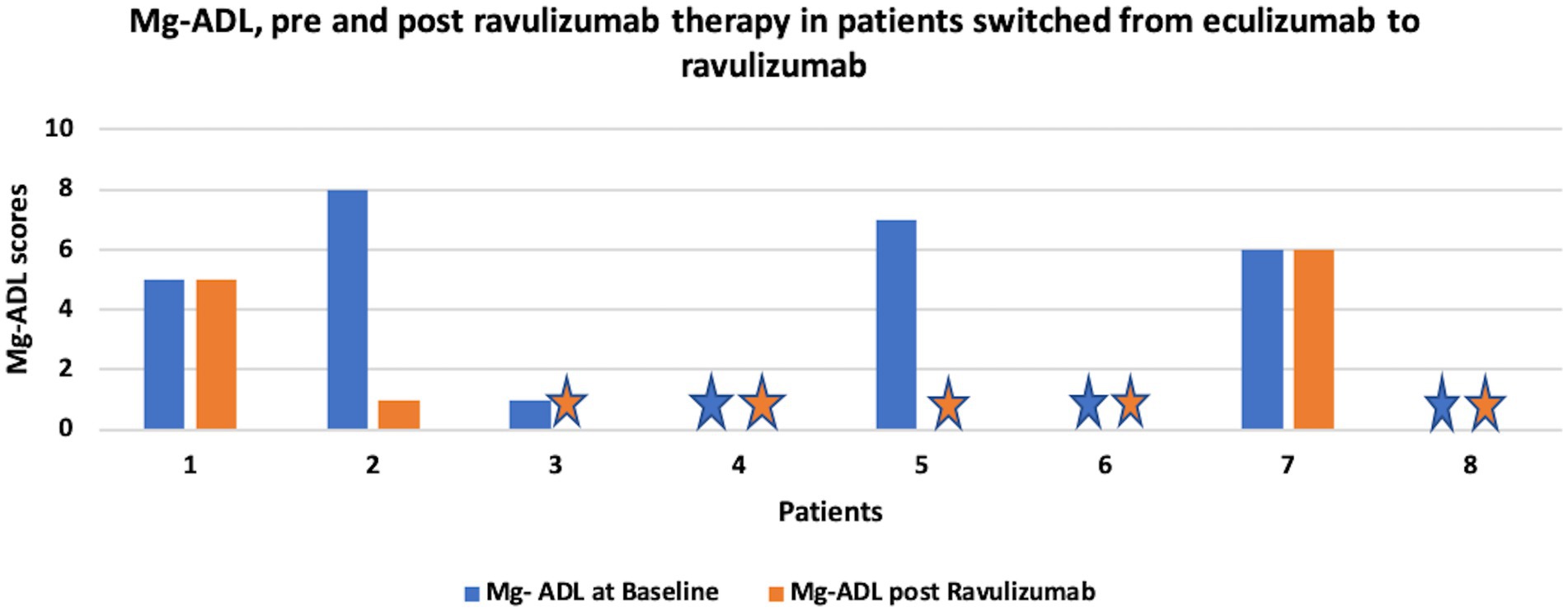
Figure 2. shows change in MG-ADL score, pre and post ravulizumab therapy in patients switched from eculizumab to ravulizumab. Star sign represents MG-ADL score of 0.
3.2.1.2.1 Reduction in prednisone dose
Three out of 5 patients switched from eculizumab to ravulizumab were able to reduce their prednisone dosage. The mean daily prednisone requirement decreased from baseline dose of 17 (±24.13) mg/day to 9 (±12.44) mg/day over a mean duration of 4.2 (± 1.09) months.
3.2.2 Other special interest populations
3.2.2.1 Patients previously on IVIG
Two out of three patients were receiving IVIG every 2 weeks prior to initiation of ravulizumab, one patient received IVIG as rescue therapy. One out of 3 patients, who were on IVIG therapy at baseline, noted a clinically meaningful reduction in MG-ADL score. MG-ADL score remained unchanged for one patient and worsened for the other patient.
3.2.2.2 Patients with prior thymoma
One of the two patients with a history of thymoma (s/p thymectomy) achieved MSE, while the other patient maintained MSE status after initiation of ravulizumab.
3.3 Side effects
4 patients experienced side effects, 3 had headaches and 1 had an upper respiratory tract infection and diarrhea. All side effects were mild, and patients were able to tolerate further infusions. None of the patients experienced any major side effects. The remaining 14 patients tolerated the infusions well without any documented side effects.
3.4 Discontinuation
Ravulizumab was discontinued for one, previously complement naive patient due to worsening MG-ADL. The time duration from loading dose of ravulizumab to discontinuation was 3 months.
4 Discussion
This retrospective case series highlights our experience with ravulizumab in AChR+ve generalized MG patients. In our cohort, more than 60% of complement inhibitor naïve patients had a clinically meaningful reduction in MG-ADL. Additionally, patients who were switched from eculizumab had a further reduction in MG-ADL. Finally, 78% of patients were able to reduce their prednisone dose after starting ravulizumab, revealing a steroid-sparing effect within a few months.
With dramatic interest in the development of novel therapies for MG, we expect increasing options in complement inhibition in patients with AChR+ve MG. Currently, two intravenous monoclonal antibody therapies, eculizumab, and ravulizumab with different half-lives are FDA approved. Another therapy, Zilucoplan, a peptide that binds to C5 and prevents the formation of a membrane attack complex, given daily by the subcutaneous route, received FDA approval in October 2023 (1). Additional oral and IV complement inhibitor therapies are in development (9).
With all these expensive therapeutic options, it is critical to identify the patients most likely to benefit from complement inhibition. Multiple case–control studies have evaluated markers of complement activation including CH50, byproducts of complement activation, but these markers do not correlate with the disease state or clinical responsiveness to complement inhibition. Cell-based analysis of antibodies from patients correlating AChR antibody titers and complement binding has revealed that not all patients with AChR+ve MG have significant complement binding seen only in 46.5% (72/155) serum samples revealing no complement activation (10). Additionally, complement binding did not always correlate with disease severity. More recent analysis suggests that the synergistic action of multiple AChR antibodies targeting different epitomes is critical for complement activation and disease severity (11). Nevertheless, these cell-based complement activation assays are not available for clinicians, therefore, no clear guidance is available for optimal patient selection for complement inhibitor therapy.
Our analysis included patients who were complement inhibitor therapy naïve and those who were already on eculizumab. 60% of complement inhibitor naive patients had a clinically meaningful improvement. Whereas none of the patients who were switched from eculizumab to ravulizumab had any worsening in MG-ADL and all patients maintained the clinical benefits. Interestingly, 2 symptomatic patients who switched from eculizumab to ravulizumab achieved MSE. Unlike clinical trials, where patients would qualify for complement therapy with active symptom burden (MG-ADL above 6), in practice, complement therapies are also used to reduce prednisone dose or simply immunosuppressive regimen.
Our analysis includes many patients who would not otherwise meet phase 3 clinical trial criteria such as patients who are on IVIG or those with a recent history of thymoma. In our study population, one of the two patients with a history of thymoma (s/p thymectomy) achieved MSE, while the other patient maintained MSE status after initiation of ravulizumab. This finding suggests that ravulizumab might be an effective option even in patients with thymoma, similar to publications showing the effectiveness of eculizumab in thymoma patients (12). Additionally, in our analysis, 3 patients were on IVIG. One of three patients on IVIG had clinical improvement in MG-ADL (improved from 7 to 0), this patient also had a history of thymoma. Another patient on IVIG had no change in ADL (5, before and after ravulizumab). The remaining one patient noted worsening of MG-ADL (worsened from 0 to 8). At baseline, this patient had MSE status on prednisone 5 mg daily, azathioprine 50 mg daily and IVIG therapy. He was transitioned to ravulizumab therapy due to the need for frequent IVIG infusions. For a brief period after initiation of ravulizumab, he was able to wean off both prednisone and azathioprine. Unfortunately, he had worsening symptoms, 4 months after loading dose, requiring initiation of prednisone 60 mg daily and azathioprine 50 mg daily. He continued ravulizumab therapy and his MG-ADL improved to 2, 6 months after loading dose of ravulizumab. In our analysis, there were no incidences of severe meningococcal or other severe bacterial infections. All patients were appropriately vaccinated before starting ravulizumab therapy. We did not find any other safety concerns.
Our retrospective analysis has a few limitations. We only obtained MG-ADL score as an outcome measure and there was variation in the timing of MG-ADL assessments among providers. The assessments were performed immediately before and whenever possible, post ravulizumab, and the exact date of clinical outcome was not pre-determined and so we cannot comment on the onset of benefit from ravulizumab. Side effects were only collected from notes and not from standardized case report forms and so are prone to recall bias and only major side effects were documented. Additionally, unlike clinical trials, we had patients who were not actively symptomatic, and therefore assessment of clinical benefit cannot be ascertained only from the MG-ADL scores.
5 Conclusion
In summary, this analysis provides evidence of the clinical effectiveness of ravulizumab in diverse clinical populations of AChR+ve MG patients. Further long-term analysis of these patients is needed to assess the persistence of the clinical benefit and need for chronic complement inhibition.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Stanford Institutional review board committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin because of the retrospective nature of the study.
Author contributions
NK: Writing – review & editing, Writing – original draft, Visualization, Validation, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. RG: Writing – review & editing, Visualization, Validation, Supervision, Methodology, Conceptualization. NG: Writing – review & editing, Validation, Supervision, Conceptualization. SuM: Writing – review & editing, Visualization, Validation, Supervision, Resources, Project administration, Data curation, Conceptualization. SrM: Writing – original draft, Methodology, Investigation, Writing – review & editing, Visualization, Validation, Supervision, Resources, Project administration, Data curation, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
RG served on the advisory board for Argenx, UCB, Janssen, Roche, and speakers bureau for Argenx and Alexion. NG has served on advisory board meetings for Argenx, Alexion, and UCB Pharma. SrM has served on advisory board meetings for Argenx, Alexion/Astrazaneca, ra/UCB, and Horizont Pharma. SuM speaker Bureau: Takeda, CSL Behring, Grifols, Catalyst, Alexion, Argenx. Consultant: UCB, Alexion, Argenx. Advisory Boards: Horizon, Argenx, Alexion, Grifols.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AChR+ve gMG, Acetylcholine receptor antibody-positive generalized myasthenia gravis; MG-ADL, Myasthenia gravis activities of daily living; MSE, minimum symptom expression; FcRn, neonatal FC receptor; COPD, chronic obstructive pulmonary disease; OSA, Obstructive sleep apnea; IVIG, Intravenous immunoglobulins.
References
1. Howard, JF Jr, Bresch, S, Genge, A, Hewamadduma, C, Hinton, J, Hussain, Y, et al. Safety and efficacy of zilucoplan in patients with generalised myasthenia gravis (RAISE): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Neurol. (2023) 22:395–406. doi: 10.1016/S1474-4422(23)00080-7
2. Bril, V, Drużdż, A, Grosskreutz, J, Habib, AA, Mantegazza, R, Sacconi, S, et al. Safety and efficacy of rozanolixizumab in patients with generalised myasthenia gravis (MycarinG): a randomised, double-blind, placebo-controlled, adaptive phase 3 study. Lancet Neurol. (2023) 22:383–94. doi: 10.1016/S1474-4422(23)00077-7
3. Howard, JF Jr, Utsugisawa, K, Benatar, M, Murai, H, Barohn, RJ, Illa, I, et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. (2017) 16:976–86. doi: 10.1016/S1474-4422(17)30369-1
4. Vu, T, Meisel, A, Mantegazza, R, Annane, D, Katsuno, M, Aguzzi, R, et al. Terminal complement inhibitor Ravulizumab in generalized myasthenia gravis. NEJM Evid. (2022) 1:EVIDoa2100066. doi: 10.1056/EVIDoa2100066
5. Vu, T, Ortiz, S, Katsuno, M, Annane, D, Mantegazza, R, Beasley, KN, et al. Ravulizumab pharmacokinetics and pharmacodynamics in patients with generalized myasthenia gravis. J Neurol. (2023) 270:3129–37. doi: 10.1007/s00415-023-11617-1
6. Kulasekararaj, AG, Hill, A, Rottinghaus, ST, Langemeijer, S, Wells, R, Gonzalez-Fernandez, FA, et al. Ravulizumab (ALXN1210) vs eculizumab in C5-inhibitor-experienced adult patients with PNH: the 302 study. Blood. (2019) 133:540–9. doi: 10.1182/blood-2018-09-876805
7. Meisel, A, Annane, D, Vu, T, Mantegazza, R, Katsuno, M, Aguzzi, R, et al. Long-term efficacy and safety of ravulizumab in adults with anti-acetylcholine receptor antibody-positive generalized myasthenia gravis: results from the phase 3 CHAMPION MG open-label extension. J Neurol. (2023) 270:3862–75. doi: 10.1007/s00415-023-11699-x. Epub 2023 Apr 27
8. Wolfe GI, Herbelin L, Nations SP, Foster B, Bryan WW. Barohn RJ myasthenia gravis activities of daily living profile. Neurology. (1999) 52:1487–9. doi: 10.1212/wnl.52.7.1487
9. U.S. National Library of Medicine. (2023). Study of ALXN2050 in adult participants with generalized myasthenia gravis. Available at: https://classic.clinicaltrials.gov/ct2/show/NCT05218096
10. Obaid, AH, Zografou, C, Vadysirisack, DD, Munro-Sheldon, B, Fichtner, ML, Roy, B, et al. Heterogeneity of acetylcholine receptor autoantibody-mediated complement activity in patients with myasthenia gravis. Neurol Neuroimmunol Neuroinflamm. (2022) 9:e1169. doi: 10.1212/NXI.0000000000001169
11. Rose, N, Holdermann, S, Callegari, I, Kim, H, Fruh, I, Kappos, L, et al. Receptor clustering and pathogenic complement activation in myasthenia gravis depend on synergy between antibodies with multiple subunit specificities. Acta Neuropathol. (2022) 144:1005–25. doi: 10.1007/s00401-022-02493-6
Keywords: acetylcholine receptor antibody positive, generalized myasthenia gravis, ravulizumab, MG-ADL, complement inhibition
Citation: Katyal N, Govindarajan R, Goyal N, Muley S and Muppidi S (2024) Ravulizumab use for acetylcholine receptor-positive generalized myasthenia gravis in clinical practice. Front. Neurol. 15:1378080. doi: 10.3389/fneur.2024.1378080
Edited by:
Paola Cavalcante, IRCCS Carlo Besta Neurological Institute Foundation, ItalyReviewed by:
Deepak Menon, National Institute of Mental Health and Neurosciences (NIMHANS), IndiaKimiaki Utsugisawa, Hanamaki General Hosipital, Japan
Copyright © 2024 Katyal, Govindarajan, Goyal, Muley and Muppidi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nakul Katyal, a2F0eWFsLm5ha3VsQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share last authorship
 Nakul Katyal
Nakul Katyal Raghav Govindarajan2
Raghav Govindarajan2 Neelam Goyal
Neelam Goyal Srikanth Muppidi
Srikanth Muppidi