
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol., 03 April 2024
Sec. Neuromuscular Disorders and Peripheral Neuropathies
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1372861
 Yuhui Qin1,2†
Yuhui Qin1,2† Siyuan Chen1,2†
Siyuan Chen1,2† Qian Gui3†
Qian Gui3† Teng Zhang4
Teng Zhang4 Yanan Li1
Yanan Li1 Zhijuan Du1,2
Zhijuan Du1,2 Yahui Lv1,2
Yahui Lv1,2 Xiangyu Du1,2
Xiangyu Du1,2 Yi Hu1*
Yi Hu1* Zhefeng Liu1*
Zhefeng Liu1*Background: Immune checkpoint inhibitors (ICI)-induced myasthenia gravis (MG) is an uncommon but potentially fatal neurotoxicity. We aim to help physicians familiarize themselves with the clinical characteristics of ICI-induced MG, facilitating early diagnosis and prompt intervention.
Methods: We searched the Chinese People’s Liberation Army General Hospital medical record system from January 2017 to August 2023 for patients diagnosed with ICI-induced MG. We systematically reviewed the literature until August 2023 to identify all similar patients. We collected clinical information on these patients.
Results: 110 patients were identified, 9 from our institution and 101 from case reports. In our institution, Median age was 66 years (range: 49–79 years). 6 were males. The most common was lung cancer (n = 4). All patients had no previous history of MG and received PD-1 or PD-L1 inhibitors. The median time from ICI initiation to first MG symptoms was 4 weeks (range: 2–15 weeks). ICIs were discontinued in all patients. Most patients initially received high-dose corticosteroids, and their symptoms improved. Some patients are discharged with corticosteroids maintenance therapy. In addition, 55 patients (50%) with concomitant myositis and/or myocarditis and MG-induced mortality were more common in the myositis and/or myocarditis group (10.9% vs. 34.5%, p = 0.016). Overlap of myositis with MG (OR = 3.148, p = 0.009) and anti-AChR antibody positivity (OR = 3.364, p = 0.005) were both significantly associated with poor outcomes.
Conclusion: Our study reveals the prognosis of ICI-induced MG and suggests that myositis and/or myocarditis are severe comorbidities of ICI-induced MG, emphasizing the importance of early diagnosis and clinical intervention.
Immune checkpoint inhibitors (ICI) mainly include anti-programmed cell death protein 1 (PD-1) antibodies (e.g., nivolumab, pembrolizumab, tislelizumab, etc.), anti-programmed Cell Death Protein-Ligand 1 (PD-L1) antibodies (e.g., atezolizumab, durvalumab, etc.), and anti-cytotoxic T Lymphocyte-Antigen 4 (CTLA-4) antibodies (e.g., ipilimumab, etc.). Typically, tumors directly or indirectly reduce the intensity and extent of the immune response through immune checkpoints to maintain the self-tolerance of tumor cells in their surrounding normal tissues and to evade immune detection. Specifically, targeted binding of anti-PD1, anti-PDL1, and anti-CTLA4 antibodies enhances the anti-tumor immune response and accelerates host-mediated destruction of malignant cells by promoting immune surveillance (1). ICI have been widely used to treat non-small cell lung cancer (NSCLC), metastatic melanoma, renal cell carcinoma, and other tumors (2, 3). Nonetheless, the incidence of adverse drug reactions is on the rise, especially the non-specific characteristics of neurological Immune-induced adverse events (NirAEs), which are challenging to recognize and treat (4).
Immune-induced adverse events (IrAEs) have the potential to affect any organ system. However, the gastrointestinal tract, endocrine glands, skin, liver, and lungs are the most frequently involved, with a lower incidence of NirAEs. However, it is associated with higher mortality (5). The most commonly reported symptom of NirAEs is headache. They may also involve the peripheral and central nervous system (6). Myasthenia gravis (MG) is an autoimmune disorder impacting the neuromuscular junction, commonly identified by symptoms like ptosis and diplopia. In severe cases, it may involve the respiratory and masticatory muscles, leading to dyspnea and dysphagia. Acetylcholine receptor antibodies (AChR-Abs) and anti-muscle specific kinase antibodies (anti-MuSK Abs) are highly diagnosis-specific, with detection rates of around 85 and 10%, respectively (7, 8). ICI-induced MG is more difficult to diagnose and is often combined with myositis and/or myocarditis, with a rapid progression of the disease, often leading to patient death (9). Early recognition and effective clinical management are crucial. We reviewed our institution’s database and searched the literature for relevant case reports to summarize the prognostic and clinical characteristics of 110 patients with MG in the context of receiving ICI.
Patients diagnosed with ICI-induced MG at PLAGH between January 2017 and August 2023 constituted the study cohort. We conducted a comprehensive search on PubMed and Embase for case reports, series, and observational studies documenting cancer and MG patients undergoing ICI until August 2023 without imposing language or research design limitations. The search strategy and terms can be found in Supplementary File 1. Diagnostic criteria for ICI-induced MG are described in Supplementary File 2. Figure 1 illustrates the flow chart for screening case reports. Additionally, the quality appraisal of the reported cases from the literature is detailed in Supplementary Table 3. Then, the complete texts of the chosen articles were examined. We manually looked through the references of the included articles. Each patient had a comprehensive clinical profile.
We retrieved patient demographic and baseline characteristic data from PLAGH and literature-identified patients. Data from our institution and case reports were divided into two groups: MG alone and MG concomitant myositis and/or myocarditis. The two groups’ clinical and diagnostic characterization, management, and outcomes were evaluated and compared.
The study underwent statistical analysis using SPSS 26 and GraphPad Prism 8.0.2. For categorical data assessment, frequencies and percentages were employed, while medians and ranges described continuous data. The significance of categorical variables was compared between the two groups using the χ2 test. Continuous variables were assessed using the Mann–Whitney U test. Univariate binary logistic regression models were employed to calculate odds ratios (ORs) for the association between specific clinical or demographic variables and the risk of adverse events in MG patients. Additionally, a multivariate binary logistic regression model was utilized to examine the components significantly linked to negative results. All tests were two-sided, and statistical differences were deemed significant if p < 0.05.
Out of 961 unique articles identified in the literature, 96 publications, detailing 101 patients, met the inclusion criteria, with an additional 9 patients identified from PLAGH. Consequently, our final analysis encompassed a total of 110 patients; Median age was 72 years (range: 30–90 years), 67 were males. The most common type of cancer was melanoma (n = 34), followed by lung cancer (n = 31). 12 patients had a previous history of MG. Most patients received PD-1 inhibitors. 55 patients were diagnosed with MG combined with myositis and/or myocarditis. Among them, myositis was also diagnosed in 20 and myocarditis in 25; 10 had the triad of MG/myositis/myocarditis. For patients with the triad of MG/myositis/myocarditis, all patients had a rapid onset of illness after receiving the first or second cycle of ICI therapy. Almost all patients received steroids, and the remaining common treatments included intravenous immunoglobulin (IVIG; n = 9), acetylcholinesterase inhibitors (n = 5), and mechanical ventilation (n = 5). Eventually, 7 patients reported death.
9 patients from PLAGH were diagnosed with ICI-induced MG. The clinical data of the patients were summarized in Table 1. Their median age was 66 years (range: 49–79 years). 6 patients were male. The most common type of cancer was lung cancer (n = 4), followed by esophageal cancer (n = 2). None of the 9 patients had a previous history of MG, and all received PD-1 or PD-L1 inhibitors. 4 patients were treated with ICI combined with targeted therapy, three with ICI combined with chemotherapy, one with ICI combined with HDAC inhibitor, and one with ICI alone therapy. The PDL1-expression level on tumor cells was available for 5 patients: < 1% for 3%, 1%–49% for 1, and >50% for 1. Of these patients, 5 patients developed symptoms immediately after their first or second ICI. The median time from ICI initiation to first MG symptoms was 4 weeks (range: 2–15 weeks) (Figure 2).
The most common symptoms at first presentation were ptosis (n = 5) and dysphagia (n = 5), and the rest of the frequent symptoms included dyspnea and limb weakness and excluded exacerbation of symptoms due to progression of the primary tumor. Myocarditis was also diagnosed in 6 patients, myositis was diagnosed in 3 patients, and liver injury in 2 patients. 4 patients were found to have positive anti-AChR antibodies. 7 patients were detected with elevated creatine phosphokinase (CPK; median 2,208 IU/L, range: 380–9,994 IU/L). 2 patients had electromyography showing myogenic injury, and one patient had a muscle biopsy showing disseminated myofibrillar necrosis with type II myofibrillar atrophy. Computed tomography or magnetic resonance imaging was performed in 7 patients to rule out brain metastases or acute intracranial events. 3 patients had abnormal electrocardiograms on admission, demonstrating third-degree atrioventricular and right bundle branch blocks.
ICIs were stopped for all patients. They all received corticosteroids, 5 with acetylcholinesterase inhibitors, 6 with immunoglobulins, and the rest of the treatments included rituximab and mycophenolate mofetil (MMF). Overall, MG symptoms improved in 8 and worsened in 1 patient. 3 patients reported death, and the cause of death was systemic organ failure, all due to the tumor. Six patients are currently alive.
We grouped a total of 110 patients who were hospitalized and reported in the literature. The control group was patients with MG alone (without comorbid myositis and/or myocarditis), and the experimental group was patients with MG comorbid with myositis and/or myocarditis. We compared the clinical characteristics and prognosis of the two groups as shown in Table 2. Our results showed no statistically significant differences between the two groups in terms of age, gender, previous MG, and type of ICI. The specific information is shown in Table 2 and Supplementary Tables 4, 5. Secondly, regarding the clinical features at the onset, the myositis and/or myocarditis group developed MG-induced symptoms earlier after ICI treatment than patients with MG alone (p < 0.001). Anti-AchR antibody positivity was more common in the myositis and/or myocarditis group (p = 0.029). Regarding treatment, there were no statistically significant differences between the two groups in the use of corticosteroids, intravenous immunoglobulin, and plasma exchange. A relatively higher proportion of patients with MG alone were treated with anticholinesterase inhibitors (72.7% vs. 40.0%, p = 0.001). In addition, because patients with myositis and/or myocarditis were more severely ill and more likely to have myasthenia gravis-associated respiratory failure, more patients received mechanical ventilation compared to patients with MG alone (16.4% vs. 41.8%, p = 0.006). Finally, in terms of prognosis, symptoms being more challenging to treat in the myositis and/or myocarditis group, MG-induced mortality was higher in the myositis and/or myocarditis group (10.9% vs. 34.5%). In contrast, MG alone responded better to treatment, with more deaths due to cancer progression (12.7% vs. 5.5%). Our study suggests that myositis and/or myocarditis are common comorbidities of ICI-induced MG, which severely affects the prognosis of patients.
The results of univariate binary logistic regression analysis of the association between clinical characteristics and ICI-induced MG prognosis are shown in Table 3. Shorter cycles of ICI treatment (OR = 4.929, p = 0.003) and earlier onset of symptoms (OR = 2.501, p = 0.023) were negatively associated with ICI-induced MG adverse outcomes. The overlap of myositis with MG (OR = 3.148, p = 0.009) and Anti-AChR antibody positivity (OR = 3.364, p = 0.005) was significantly associated with poor ICI-induced MG outcomes. However, we did not find a correlation between the degree of CPK elevation and adverse disease outcomes. We incorporated variables such as cancer type, type of ICI, treatment cycle, onset, Anti-AChR antibody status, combined with myositis, and treatments into the multivariate binary logistic regression model. The results of the multivariate analysis indicated a negative association between the combined with myositis and Anti-AChR antibody positivity with outcomes in ICI-induced MG.
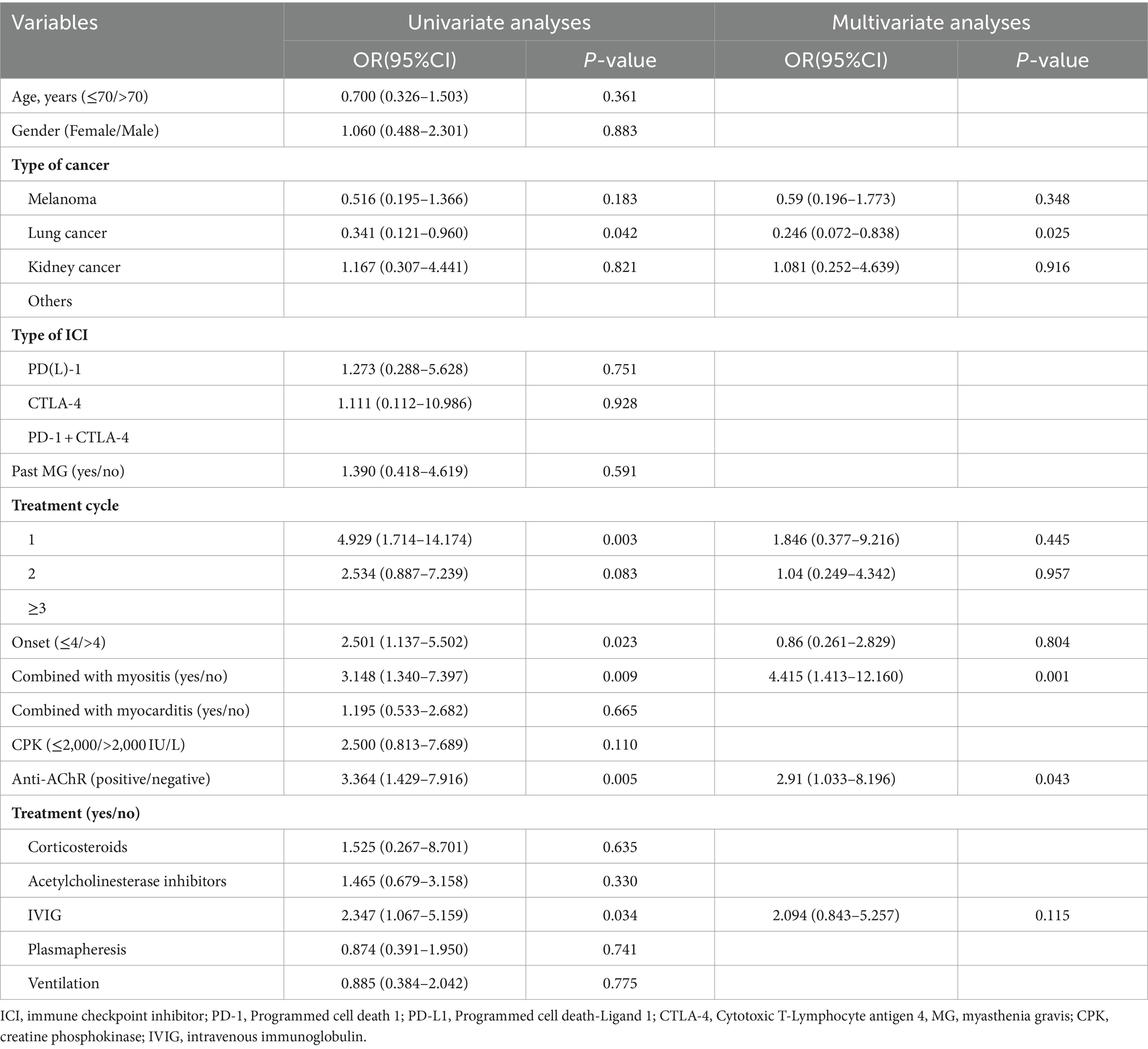
Table 3. Univariate and multivariate analyses for factors affecting the prognosis of patients with ICI-related MG (n = 110).
There is insufficient literature to provide evidence suggesting the risk of ICI re-initiation after NirAEs. Our review of case reports identified nine patients who were retreated with the same or a different ICI after initial MG remission due to a lack of effective alternative therapy to manage their advanced malignancies (melanoma [n = 6], lung cancer [n = 1], ovarian cancer [n = 1], and uterine carcinosarcoma [n = 1]). 6 patients received the same initial medication (anti-PD1), and the 2 patients were switched from pembrolizumab to nivolumab. Another patient was switched from nivolumab combined with ipilimumab to nivolumab monotherapy. After the first MG remission, all patients continued prophylaxis with corticosteroids, pyridostigmine, and IVIG. Recurrence of MG was seen in only 1 patient, and the irAE was seen in 2 cases (including 1 thyroiditis and 1 hepatitis). 7 patients ultimately had partial or complete tumor responses, and one patient died due to rapid tumor progression. Efficacy evaluation was not reported in another case.
We searched medical records and reviewed the literature for ICI-induced MG. NirAEs are less common, with incidence rates of only 1%–5% (10), with an incidence of 0.2% for irAE-MG. Compared with other irAEs, the mortality rates are high (11). According to meta-analyses of clinical trials, the incidence of any grade of irAE was 66 and 72% for PD-1/PD-L1 inhibitors and CTLA-4 inhibitors, respectively. The incidence of severe irAE was 14 and 24% for PD-1/PD-L1 inhibitors and CTLA-4 inhibitors, respectively (12, 13), and 0.3%–1.3% were lethal (14).
There were no differences in the clinical features and prognosis of ICI-induced MG between patients with previous MG episodes and those experiencing new-onset disease. The actual incidence of MG episodes following ICI in patients with a previous MG diagnosis remains unknown, primarily due to the absence of pertinent cohort studies. In addition, conventional studies often exclude individuals with a history of autoimmunity, posing challenges in evaluating the impact of ICI in substantial subject cohorts. The precise mechanism through which ICI induces MG remains currently unknown. It remains questionable whether patients with new-onset MG have subclinical autoimmunity that manifests only after exposure to ICI. Studies have demonstrated that CTLA-4 knockout mice can spontaneously develop MG (15). Furthermore, specific CTLA-4 genetic variants predispose individuals to MG, particularly in Caucasian and East Asian populations (16, 17). Overexpression of PD-1 is linked to favorable outcomes in autoimmune diseases, as it facilitates CD8 T cell depletion; however, PD-1 inhibitors may worsen symptoms in individuals with pre-existing MG (18). Currently, additional studies are required to validate these findings.
A review of 110 cases supports that ICI-induced MG is a life-threatening irAE that rapidly deteriorates shortly after ICI initiation. 32 patients presented with dyspnea requiring mechanical ventilation. In addition, we found that ICI-induced MG was often concurrent with myocarditis and/or myositis. 110 patients were grouped into a control group without other irAEs and an experimental group with other irAEs, such as myocarditis and myositis. The results showed a higher mortality in the MG combined myositis/myocarditis group. Our study supports that ICI-mediated MG and its overlapping syndrome occur early after the initiation of therapy and are associated with significant mortality.
ICI-induced MG have a 57%–83% positive rate of anti-AchR antibodies, in addition to the common neostigmine test and ice test, and some patients may be combined with hyper CPK and even positivity of myositis-associated antibodies, which often suggests that simultaneous combination of ICI-induced myositis may be possible. In our study, 70 patients were positive for anti-AchR antibodies, in addition to Anti-AchR, Anti-Striated muscle antibodies (antititin, anti-heart muscle, and anti-skeletal muscle autoantibodies) have been identified in both ICI-induced MG and myositis (19, 20), While myositis-specific autoantibodies tend to be negative (21–23). Müller-Jensen et al. studies have demonstrated that the presence of ICI-induced neuromuscular disease in cancer patients with 80 and 88% sensitivity and specificity, respectively, for the detection of these autoantibodies. Neuromuscular autoantibodies may serve as viable markers for the diagnosis and potential prediction of life-threatening ICI-induced neuromuscular diseases (24).
7 patients were treated with PD-1 combination with CTLA-4 inhibitor, six of whom had comorbid myositis and/or myocarditis, suggesting that the combination of the two drugs may be associated with a higher incidence of irAE. The reported incidence and distribution of irAE may vary by drug type, PD-1, PD-L1, CTLA-4, or combination. The incidence of serious irAE is as high as 27% with anti-CTLA4 compared to 16% with anti-PD1 and may increase to 55% when both therapies are used concurrently (25). Meanwhile, the incidence and severity of irAE are higher when CTLA-4 inhibitors are used alone or in combination with PD-1 or PD-L1 drugs, such as ipilimumab and nivolumab, regardless of the treatment of the primary tumor (26–28). A meta-analysis showed that the risk of irAE was elevated in solid tumors when ICI was added to chemotherapy, regardless of the drug (and tumor type) used (29).
Myocarditis and NirAEs are life-threatening initial irAEs, and clinicians should be more cautious in evaluating ICI rechallenge in these patients. Therefore, more evidence is needed to assess the safety and efficacy of rechallenge. Pembrolizumab and nivolumab were the most frequently utilized ICI in various studies, serving as both primary and secondary therapies. Moreover, these agents share similar three-dimensional structures and effector mechanisms; nevertheless, pembrolizumab exhibits a higher affinity for recombinant human PD-1 compared to nivolumab (30). Kan et al. demonstrated an enhanced response in four melanoma patients treated initially with nivolumab and subsequently with pembrolizumab (31). In a cohort encompassing diverse cancer types, predominantly melanoma and lung cancer, Simonaggio et al. reported a modest improvement in overall response rate with anti-PD-1 or anti-PD-L1 inhibitors. This finding suggests that, within the context of successful and well-tolerated primary treatment, which may support re-treatment with the same drug or drug group, the initial response to ICI treatment could serve as a crucial predictor of re-challenge efficacy (32, 33). Currently, prior or combined radiotherapy, chemotherapy, or targeted therapy is regarded as a promising strategy to enhance the effectiveness of both primary and secondary immunotherapy. Research by Niki et al., Watanabe et al., and Xu et al. has indicated that patients who exhibited a positive response to a second ICI received intermittent treatment with radiotherapy, chemotherapy, or targeted therapy, particularly in patients with NSCLC (34–36).
9 patients in MG symptomatic remission could be rechallenged with ICI without compromising ICI efficacy at a reduced dose of steroids, prophylaxis with pyridostigmine, and/or IVIG after carefully evaluating available treatment options. There needs to be adequate literature on the impact of rechallenge on survival, irAE recurrence, and the incidence of new irAE. Despite the risk of recurrent irAE, initiating ICI after discontinuation for prior MG may be partially safe with careful clinical monitoring and aggressive prevention. An investigation on the safety of rechallenge revealed that the recurrence rate of irAE varied based on the organ involved in the initial irAE. It was lower in patients re-initiated with the same ICI drug or ICI combination compared to those re-initiated with a different ICI drug or ICI combination (p = 0.02). The median duration of ICI discontinuation to re-initiation and the severity of the initial irAE were not predictive of recurrent irAE after ICI re-initiation (37). Previous literature shows that periodic irAE is not as severe as the first one and that patients continue to respond to medication after the episode (38).
As shown in the current study, ICI-induced MG is often combined with myositis and/or myocarditis and is significantly associated with poor prognosis (39). We observed that patients with combined myositis and/or myocarditis had a significantly shorter time to disease onset after the first or second dose of ICI treatment than patients with MG alone. This trend toward early onset of disease may indicate that flares and/or progression in the myositis and/or myocarditis group were more rapid in patients with poorer outcomes. Although ICI toxicity may occur at any time during treatment, Our findings align with prior studies indicating that fatal ICI toxicity often manifests early, potentially within 4 weeks of treatment initiation or shortly after the initial dose (40). Other studies have confirmed troponin as a possible predictor and have used elevated creatinine and decreased urea and hemoglobin as early biomarkers in dying patients (41).
ICI-related MG hardly resolves spontaneously and requires immediate hospitalization once detected. In terms of treatment, patients require immediate discontinuation of ICI. The current study found that this group of patients responds poorly to acetylcholinesterase inhibitor therapy unless symptoms are nonprogressive and mild, but high-dose methylprednisolone shock combined with IVIG or plasma exchange has been recommended as an important therapeutic measure in studies of ICI-related MG treatment. It can be used to alleviate myasthenia gravis crisis. In addition, a combination of immunomodulators such as MMF, rituximab, and infliximab may be considered in addition to the above treatment options. Patients with overlap syndrome have a potentially fatal risk of rapid progression to serious adverse events, and the main interventions currently available for this group of patients include recognition of the triad, airway support, administration of high-dose methylprednisolone, and ensuring early involvement of the multidisciplinary team.
The fact that ICI-induced MG is a relatively uncommon complication limits the study. There are no established diagnostic standards for diagnosing MG, myositis, and myocarditis. In particular, MG can be diagnosed in some patients who are negative for AChR antibodies. The results of our study will help clinicians familiarize themselves with the clinical features of ICI-induced MG. They will help them to make an early diagnosis of the disease and intervene promptly. In addition, our findings offer a safety signal for patients with no available alternatives in advanced stages that may help clinicians balance each patient’s advantages and disadvantages.
Although ICI-induced MG is rare, it often involves the respiratory muscles, leading to dyspnea and high mortality. Moreover, most patients remain symptomatic after treatment, seriously affecting their quality of life. In addition, half of the patients with ICI-induced MG often have a combination of myositis and/or myocarditis, which contributes to the rapid deterioration of the patient’s disease. Therefore, it is essential to recognize this possible complication as early as possible in patients treated with ICI, and the necessity of a multidisciplinary strategy and multimodal active treatment should also be recognized.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
YQ: Conceptualization, Data curation, Formal analysis, Methodology, Software, Supervision, Writing – original draft, Writing – review & editing. SC: Conceptualization, Data curation, Formal analysis, Methodology, Software, Supervision, Writing – original draft, Writing – review & editing. QG: Conceptualization, Data curation, Formal analysis, Methodology, Software, Supervision, Writing – original draft, Writing – review & editing. TZ: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. YLi: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. ZD: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. YLv: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. XD: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. YH: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing. ZL: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1372861/full#supplementary-material
1. Tang, J, Shalabi, A, and Hubbard-Lucey, VM. Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol. (2018) 29:84–91. doi: 10.1093/annonc/mdx755
2. Haslam, A, and Prasad, V. Estimation of the percentage of US patients with Cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. (2019) 2:e192535. doi: 10.1001/jamanetworkopen.2019.2535
3. Eggermont, AMM, Blank, CU, Mandala, M, Long, GV, Atkinson, V, Dalle, S, et al. Adjuvant Pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. (2018) 378:1789–801. doi: 10.1056/NEJMoa1802357
4. Vogrig, A, Muñiz-Castrillo, S, Farina, A, Honnorat, J, and Joubert, B. How to diagnose and manage neurological toxicities of immune checkpoint inhibitors: an update. J Neurol. (2022) 269:1701–14. doi: 10.1007/s00415-021-10870-6
5. Larkin, J, Chmielowski, B, Lao, CD, Hodi, FS, Sharfman, W, Weber, J, et al. Neurologic serious adverse events associated with Nivolumab plus Ipilimumab or Nivolumab alone in advanced melanoma, including a case series of encephalitis. Oncologist. (2017) 22:709–18. doi: 10.1634/theoncologist.2016-0487
6. Cuzzubbo, S, Javeri, F, Tissier, M, Roumi, A, Barlog, C, Doridam, J, et al. Neurological adverse events associated with immune checkpoint inhibitors: review of the literature. Eur J Cancer. (2017) 73:1–8. doi: 10.1016/j.ejca.2016.12.001
7. Hoch, W, McConville, J, Helms, S, Newsom-Davis, J, Melms, A, and Vincent, A. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med. (2001) 7:365–8. doi: 10.1038/85520
8. Lindstrom, JM, Seybold, ME, Lennon, VA, Whittingham, S, and Duane, DD. Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology. (1976) 26:1054–9. doi: 10.1212/WNL.26.11.1054
9. Safa, H, Johnson, DH, Trinh, VA, Rodgers, TE, Lin, H, Suarez-Almazor, ME, et al. Immune checkpoint inhibitor related myasthenia gravis: single center experience and systematic review of the literature. J Immunother Cancer. (2019) 7:319. doi: 10.1186/s40425-019-0774-y
10. Wang, Y, Zhou, S, Yang, F, Qi, X, Wang, X, Guan, X, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and Meta-analysis. JAMA Oncol. (2019) 5:1008–19. doi: 10.1001/jamaoncol.2019.0393
11. Larkin, J, Chiarion-Sileni, V, Gonzalez, R, Grob, JJ, Cowey, CL, Lao, CD, et al. Combined Nivolumab and Ipilimumab or monotherapy in untreated melanoma. N Engl J Med. (2015) 373:23–34. doi: 10.1056/NEJMoa1504030
12. Martins, F, Sofiya, L, Sykiotis, GP, Lamine, F, Maillard, M, Fraga, M, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. (2019) 16:563–80. doi: 10.1038/s41571-019-0218-0
13. Marini, A, Bernardini, A, Gigli, GL, Valente, M, Muñiz-Castrillo, S, Honnorat, J, et al. Neurologic adverse events of immune checkpoint inhibitors: a systematic review. Neurology. (2021) 96:754–66. doi: 10.1212/WNL.0000000000011795
14. Wang, DY, Salem, JE, Cohen, JV, Chandra, S, Menzer, C, Ye, F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and Meta-analysis. JAMA Oncol. (2018) 4:1721–8. doi: 10.1001/jamaoncol.2018.3923
15. Waterhouse, P, Penninger, JM, Timms, E, Wakeham, A, Shahinian, A, Lee, KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. (1995) 270:985–8. doi: 10.1126/science.270.5238.985
16. Chuang, WY, Ströbel, P, Gold, R, Nix, W, Schalke, B, Kiefer, R, et al. A CTLA4high genotype is associated with myasthenia gravis in thymoma patients. Ann Neurol. (2005) 58:644–8. doi: 10.1002/ana.20577
17. Sun, L, Meng, Y, Xie, Y, Zhang, H, Zhang, Z, Wang, X, et al. CTLA4 variants and haplotype contribute genetic susceptibility to myasthenia gravis in northern Chinese population. PLoS One. (2014) 9:e101986. doi: 10.1371/journal.pone.0101986
18. McKinney, EF, Lee, JC, Jayne, DR, Lyons, PA, and Smith, KG. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature. (2015) 523:612–6. doi: 10.1038/nature14468
19. Sechi, E, Markovic, SN, McKeon, A, Dubey, D, Liewluck, T, Lennon, VA, et al. Neurologic autoimmunity and immune checkpoint inhibitors: autoantibody profiles and outcomes. Neurology. (2020) 95:e2442–52. doi: 10.1212/WNL.0000000000010632
20. Mammen, AL, Rajan, A, Pak, K, Lehky, T, Casciola-Rosen, L, Donahue, RN, et al. Pre-existing antiacetylcholine receptor autoantibodies and B cell lymphopaenia are associated with the development of myositis in patients with thymoma treated with avelumab, an immune checkpoint inhibitor targeting programmed death-ligand 1. Ann Rheum Dis. (2019) 78:150–2. doi: 10.1136/annrheumdis-2018-213777
21. Touat, M, Maisonobe, T, Knauss, S, Ben Hadj Salem, O, Hervier, B, Auré, K, et al. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology. (2018) 91:e985–94. doi: 10.1212/WNL.0000000000006124
22. Müller-Jensen, L, Knauss, S, Ginesta Roque, L, Schinke, C, Maierhof, SK, Bartels, F, et al. Autoantibody profiles in patients with immune checkpoint inhibitor-induced neurological immune related adverse events. Front Immunol. (2023) 14:1108116. doi: 10.3389/fimmu.2023.1108116
23. Shelly, S, Triplett, JD, Pinto, MV, Milone, M, Diehn, FE, Zekeridou, A, et al. Immune checkpoint inhibitor-associated myopathy: a clinicoseropathologically distinct myopathy. Brain Commun. (2020) 2:181. doi: 10.1093/braincomms/fcaa181
24. Kartolo, A, Sattar, J, Sahai, V, Baetz, T, and Lakoff, JM. Predictors of immunotherapy-induced immune-related adverse events. Curr Oncol. (2018) 25:e403–10. doi: 10.3747/co.25.4047
25. Johansen, A, Christensen, SJ, Scheie, D, Højgaard, JLS, and Kondziella, D. Neuromuscular adverse events associated with anti-PD-1 monoclonal antibodies: systematic review. Neurology. (2019) 92:663–74. doi: 10.1212/WNL.0000000000007235
26. Wongvibulsin, S, Pahalyants, V, Kalinich, M, Murphy, W, Yu, KH, Wang, F, et al. Epidemiology and risk factors for the development of cutaneous toxicities in patients treated with immune-checkpoint inhibitors: a United States population-level analysis. J Am Acad Dermatol. (2022) 86:563–72. doi: 10.1016/j.jaad.2021.03.094
27. Molina, GE, Zubiri, L, Cohen, JV, Durbin, SM, Petrillo, L, Allen, IM, et al. Temporal trends and outcomes among patients admitted for immune-related adverse events: a single-center retrospective cohort study from 2011 to 2018. Oncologist. (2021) 26:514–22. doi: 10.1002/onco.13740
28. Biewenga, M, van der Kooij, MK, Wouters, M, Aarts, MJB, van den Berkmortel, F, de Groot, JWB, et al. Checkpoint inhibitor induced hepatitis and the relation with liver metastasis and outcome in advanced melanoma patients. Hepatol Int. (2021) 15:510–9. doi: 10.1007/s12072-021-10151-4
29. Fujiwara, Y, Horita, N, Namkoong, H, and Galsky, MD. The effect of adding immune checkpoint inhibitors on the risk of pneumonitis for solid tumours: a meta-analysis of phase III randomised controlled trials. Eur J Cancer. (2021) 150:168–78. doi: 10.1016/j.ejca.2021.03.012
30. Kan, T, Takahagi, S, Kawai, M, Matsubara, D, Tanaka, A, and Hide, M. Rechallenge of programmed cell death 1 inhibitor after an interval with dacarbazine treatment may be effective for advanced malignant melanoma. J Dermatol. (2020) 47:907–10. doi: 10.1111/1346-8138.15408
31. Chiarion-Sileni, V, Pigozzo, J, Ascierto, PA, Simeone, E, Maio, M, Calabrò, L, et al. Ipilimumab retreatment in patients with pretreated advanced melanoma: the expanded access programme in Italy. Br J Cancer. (2014) 110:1721–6. doi: 10.1038/bjc.2014.126
32. Robert, C, Schadendorf, D, Messina, M, Hodi, FS, and O'Day, S. Efficacy and safety of retreatment with ipilimumab in patients with pretreated advanced melanoma who progressed after initially achieving disease control. Clin Cancer Res. (2013) 19:2232–9. doi: 10.1158/1078-0432.CCR-12-3080
33. Simonaggio, A, Michot, JM, Voisin, AL, Le Pavec, J, Collins, M, Lallart, A, et al. Evaluation of Readministration of immune checkpoint inhibitors after immune-related adverse events in patients with Cancer. JAMA Oncol. (2019) 5:1310–7. doi: 10.1001/jamaoncol.2019.1022
34. Niki, M, Nakaya, A, Kurata, T, Yoshioka, H, Kaneda, T, Kibata, K, et al. Immune checkpoint inhibitor re-challenge in patients with advanced non-small cell lung cancer. Oncotarget. (2018) 9:32298–304. doi: 10.18632/oncotarget.25949
35. Watanabe, H, Kubo, T, Ninomiya, K, Kudo, K, Minami, D, Murakami, E, et al. The effect and safety of immune checkpoint inhibitor rechallenge in non-small cell lung cancer. Jpn J Clin Oncol. (2019) 49:762–5. doi: 10.1093/jjco/hyz066
36. Xu, Z, Hao, X, Yang, K, Wang, Q, Wang, J, Lin, L, et al. Immune checkpoint inhibitor rechallenge in advanced or metastatic non-small cell lung cancer: a retrospective cohort study. J Cancer Res Clin Oncol. (2022) 148:3081–9. doi: 10.1007/s00432-021-03901-2
37. Allouchery, M, Lombard, T, Martin, M, Rouby, F, Sassier, M, Bertin, C, et al. Safety of immune checkpoint inhibitor rechallenge after discontinuation for grade ≥2 immune-related adverse events in patients with cancer. J Immunother Cancer. (2020) 8:e001622. doi: 10.1136/jitc-2020-001622
38. Chennamadhavuni, A, Abushahin, L, Jin, N, Presley, CJ, and Manne, A. Risk factors and biomarkers for immune-related adverse events: a practical guide to identifying high-risk patients and Rechallenging immune checkpoint inhibitors. Front Immunol. (2022) 13:779691. doi: 10.3389/fimmu.2022.779691
39. Brahmer, JR, Lacchetti, C, and Thompson, JA. Management of Immune-Related Adverse Events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline summary. J Oncol Pract. (2018) 14:247–9. doi: 10.1200/JOP.18.00005
40. Moslehi, JJ, Salem, JE, Sosman, JA, Lebrun-Vignes, B, and Johnson, DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. (2018) 391:933. doi: 10.1016/S0140-6736(18)30533-6
Keywords: myasthenia gravis, immune checkpoint inhibitors, immunotherapy, immune-related adverse events, neurotoxicity
Citation: Qin Y, Chen S, Gui Q, Zhang T, Li Y, Du Z, Lv Y, Du X, Hu Y and Liu Z (2024) Prognosis of immune checkpoint inhibitor-induced myasthenia gravis: a single center experience and systematic review. Front. Neurol. 15:1372861. doi: 10.3389/fneur.2024.1372861
Received: 18 January 2024; Accepted: 07 March 2024;
Published: 03 April 2024.
Edited by:
Jens Schmidt, Immanuel Klinik Rüdersdorf, GermanyReviewed by:
Wladimir Bocca Vieira De Rezende Pinto, Federal University of São Paulo, BrazilCopyright © 2024 Qin, Chen, Gui, Zhang, Li, Du, Lv, Du, Hu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Hu, aHV5aTMwMXpseGJAc2luYS5jb20=; Zhefeng Liu, bHpmMTIyMEBzaW5hLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.