
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Neurol., 09 May 2024
Sec. Experimental Therapeutics
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1372509
This article is part of the Research TopicComplementary and Alternative Therapy for Pain Disorders: From Bench to Clinical PracticeView all 18 articles
 Xiaoli Song1,2†
Xiaoli Song1,2† Qian Zhu1,2†
Qian Zhu1,2† Lanqian Su3†
Lanqian Su3† Lei Shi1,2
Lei Shi1,2 Hao Chi3
Hao Chi3 Yalan Yan3
Yalan Yan3 Mei Luo4
Mei Luo4 Xibin Xu4
Xibin Xu4 Baohong Liu5
Baohong Liu5 Zhengyang Liu6
Zhengyang Liu6 Jin Yang1,2*
Jin Yang1,2*Migraine is a prevalent and disabling neurovascular disorder, with women being more susceptible, characterized by unilateral throbbing headache, often accompanied by nausea and vomiting, and often associated with various comorbidities such as brain and cardiovascular diseases, which can have a serious impact on quality of life. Although nonsteroidal anti-inflammatory drugs (NSAIDs) are the main first-line medications for the treatment of pain, long-term use often leads to side effects and drug addiction, which emphasizes the need to investigate alternative pain management strategies with fewer adverse effects. Complementary and alternative medicine is a viable pain intervention often used in conjunction with traditional medications, including acupuncture, herbs, moxibustion, transcutaneous electrical stimulation, bio-supplements, and acupressure, which offer non-pharmacological alternatives that are now viable pain management options. This review focuses on the mechanistic doctrine of migraine generation and the role and potential mechanisms of Complementary and Alternative Therapies (CAT) in the treatment of migraine, summarizes the research evidences for CAT as an adjunct or alternative to conventional therapies for migraine, and focuses on the potential of novel migraine therapies (calcitonin gene-related peptide (CGRP) antagonists and pituitary adenylyl cyclase-activating peptide (PACAP) antagonists) with the aim of evaluating CAT therapies as adjunctive or alternative therapies to conventional migraine treatment, thereby providing a broader perspective on migraine management and the design of treatment programs for more effective pain management.
Migraine is a recurrent neurovascular disorder clinically characterized by unilateral throbbing moderate to severe headaches, often accompanied by other symptoms such as nausea and vomiting, and sensitivity to light and sound (1). According to epidemiologic studies, the incidence is 12–15% in the general population, and women are more commonly affected than men, especially in the most fertile age group, 25 to 55 years (2). Migraine, as the second most disabling neurological disorder, has co-morbid relationships with a variety of brain disorders (e.g., cerebral infarction, cerebral hemorrhage), cardiovascular disease, and epilepsy, and is a significant cause of disability (3). Migraine arises from a series of intracranial and extracranial changes due to neuronal dysfunction and carries the risk of changing from episodic migraine to chronic migraine, especially as the frequency of attacks increases and acute care medications are overused (4, 5).
Traditional treatments for migraine include a variety of acute care options (e.g., over-the-counter pain relievers (sometimes in combination with caffeine), nonsteroidal anti-inflammatory drugs, opioids) and migraine-specific medications (e.g., tretinoin and ergot) (6). Recent advances include the approval of CGRP antagonists for migraine prophylaxis in adults, such as erenumab, fremanezumab, and galcanezumab (7). While these therapeutic agents are effective in many individuals, they may not be appropriate for all patients, and some have contraindications or potential side effects (6). In addition, overuse of acute medications can lead to chronicity of migraine (8, 9).
Complementary and alternative therapies (CAT) are being explored as potential alternative treatments. They are becoming more widely recognized as a viable option for pain management because of their ability to relieve stressful effects, reduce recurrence and prevent chronic pain (10). CATs encompass a variety of forms including, but not limited to, transcutaneous electrical stimulation, herbs, acupuncture, acupressure, moxibustion, qigong, tai chi, yoga, and meditation (10, 11). These therapies include non-pharmacological options such as electrical nerve stimulation devices and magnetic stimulation devices that target various nerves such as the trigeminal, vagus and occipital nerves (12). Behavioral medicine techniques, such as biofeedback training and positive thinking, have also been used for some time to help manage migraines (13). These alternative therapies can provide more options for patients seeking relief from migraine symptoms, especially those who have not responded well to traditional therapies or are looking for non-pharmacological treatments. Research indicates that in the treatment of migraine, these alternative therapies demonstrate significant advantages that cannot be overlooked. For instance, acupuncture, a common form of CAT, not only matches the efficacy of mainstream pharmacological treatments but also offers a lower risk of side effects, providing patients with a safer and more appealing treatment option (14). Consequently, as our understanding of CAT deepens, these therapies not only offer a diverse array of treatment options for migraine sufferers but also drive innovation in chronic pain management, contributing to enhanced treatment outcomes and improved quality of life for patients.
Despite the fact that the pathogenesis of migraine is not clearly understood, there have been several theories that attempt to explain its cause, including vascular dysfunction, aseptic inflammatory response in the dura mater, and magnesium deficiency (15–17) (Figure 1A). Not only that, but there is evidence to support that migraine with aura is associated with cortical spreading depression (CSD), in which depolarizing waves generated by neurons and glial cell membranes in the cerebral cortex diffuse themselves along the cortex, leading to activation of trigeminal afferent pathways (18, 19). In particular, the caudal subnucleus of the spinal trigeminal nucleus (STN) sends out nociceptive-sensitive nerve fibers that transmit information about perceptual stimuli to the thalamus, leading to sensitization of tertiary neurons. During the diffusion of signals from the cerebral cortex, CSD may be associated with large potassium (K+) efflux, sodium (Na+) voltage-sensitive channel opening, and glutamate release (20).
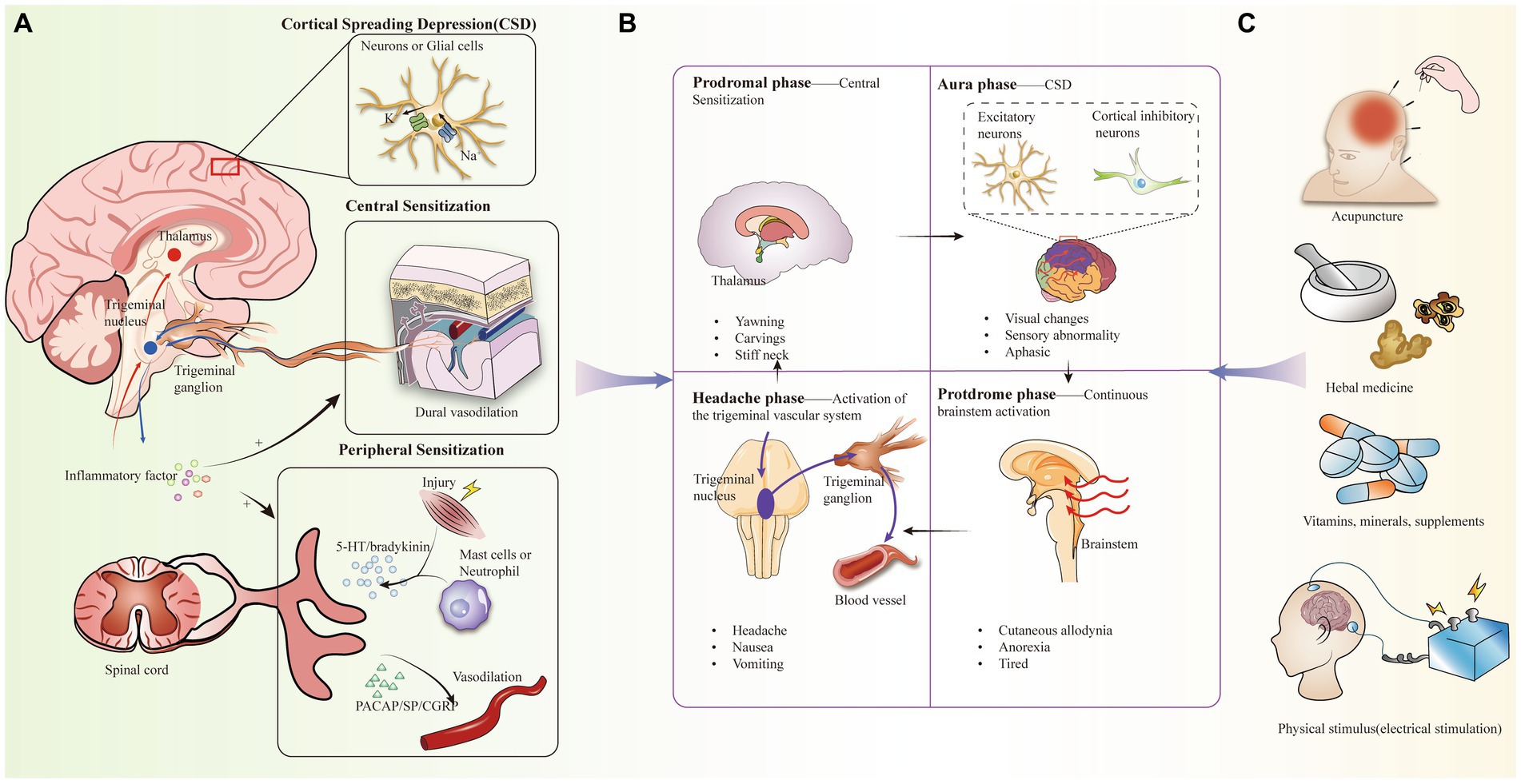
Figure 1. (A) Pathogenesis of migraine, including activation of the trigeminal vascular system, central and peripheral sensitization, cortical spreading inhibition, inflammation. (B) Clinical manifestations of the four periods of migraine and the corresponding mechanistic doctrines. (C) Complementary alternative therapies for migraine headaches.
In addition, most scholars now believe that activation of the trigeminal vascular system (TGVS) better explains the cause of migraine. Migraine attacks begin with triggers, especially migraine-inducing factors that alter central excitability, such as stress, sleep deprivation, fasting, and sound (21). Under these stimuli, the trigeminal nervous system is sensitized, which in turn induces the trigeminal ganglion (TG) to release a variety of neuropeptides, including CGRP, substance P (SP), and pituitary adenylate cyclase-activating polypeptide (PACAP) to participate in the neuroinflammatory response. At the same time, as TGVS is in a chronically activated state, it leads to a series of other changes, including mast cell degranulation and changes in meningeal vasodilatation. More interestingly, CSD can alter the permeability of the blood–brain barrier through activation and upregulation of matrix metalloproteinases (22, 23).
Considering that CGRP, as a key peptide, plays an important role in pain signaling, it has been demonstrated that CGRP release can be inhibited using herbs (24). The transient receptor potential ankyrin (TRPA) mediates CGRP release in neurogenic inflammation, and the study by Benemei et al. (25) demonstrates that Petasin inhibits CGRP signaling, achieving this through desensitization of TRPA. New studies have recently found that the PACAP pathway is independent of the CGRP pathway, and there are findings suggesting that frequent headache-induced reductions in PACAP and subsequent up-regulation of PACAP receptors play an important role in migraine progression (26–28). Therefore, PACAP antagonists may be a new therapeutic option for patients who are insensitive to CGRP antagonists (28). Perhaps, in the therapeutic regimen for migraine, CAT may play a role by inhibiting the PACAP signaling pathway. In addition, when treating chronic migraine and hyperparathyroidism (PTH), it may be more effective to consider combined inhibition of the CGRP and PACAP signaling pathways rather than inhibition of a single one of these signaling pathways (29).
Epidemiologic studies have found that 2.5–3% of patients with episodic migraine (EM) transition to chronic migraine (CM) in the second year (21, 30). The mechanism of its chronicity may be closely related to peripheral sensitization of primary afferent nerve fibers, secondary neurons in the STN, and central sensitization of higher neurons such as the thalamus (31, 32). In addition, the inflammatory response is closely related to peripheral sensitization and increased central sensitivity (33, 34). When activation of injury receptors occurs, primary afferent neurons, mast cells, and eosinophils in local tissues release a variety of chemicals, such as 5-hydroxytryptamine (5-HT) and bradykinin, which promotes neuroinflammation and modulates pain (35, 36). Among them, 5-HT 1F receptor agonists (e.g., Lasmiditan) are already in clinical trials (37). In addition, the introduction of CGRP as a target has been an important advance in migraine medication (38). CGRP levels increase when migraine attacks occur and decrease after treatment, thereby attenuating the vasodilating potency and central sensitizing effects of the pro-inflammatory neuropeptide CGRP, which has been confirmed in numerous studies to be a key neurotransmitter involved in migraine attacks (39, 40). A range of CGRP receptor antagonists and monoclonal antibodies to CGRP are currently in clinical trials, opening up new possibilities for migraine treatment drugs (41).
Migraine is categorized into a prodromal symptomatic phase, an aura phase, a headache phase, and a late headache phase, with headache phase symptoms manifesting as recurrent pain, nausea, and vomiting (32, 42–44). Currently, tretinoin, a 5-hydroxytryptamine 5-HT1B/1D receptor agonist, is the migraine-specific acute treatment of migraine during the headache phase, but it is not suitable for every patient, while the prophylactic effect is not good, and in recent years, the more promising alternative for acute-phase treatment has been complementary alternative therapies (CAT) (45) (Figure 1B). CAT has been used in the treatment of a wide range of pains, including mind–body interventional therapies (e.g., meditation), biologic based therapies (e.g., taking herbs and vitamins, dietary supplementation), physical therapy, and manual therapies including acupuncture (41, 46) (Figure 1C). Despite some methodological challenges, the effectiveness of these CAT modalities is supported by several studies (47) (Table 1).
Migraine may be associated with electrolyte disturbances, and magnesium deficiency may induce migraine by affecting cortical inhibition or leading to abnormalities in glutamatergic neurotransmission, which is seen as a potential mechanism for the magnesium-migraine association (53). Magnesium is involved in the regulation of the nervous system through multiple pathways, not only regulating vasodilatation by affecting mitochondrial metabolism, neurotransmitter release, and substance P release, but also attenuating neuroinflammation by inhibiting the nuclear factor κB pathway in pro-inflammatory cells (54, 55). Given the close link between inflammation and migraines, employing magnesium as a supplement emerges as a potent approach for the mitigation or prophylaxis of migraine episodes. In CAT, there have been several randomized clinical controlled trials supporting the use of magnesium as a supplement for the prevention of migraine attacks; however, most of the studies have been combination treatments in conjunction with other vitamins or bioorganic molecules (56). Using a randomized, multicenter, double-blind controlled trial, Gaul et al. demonstrated that treatment with supplements containing magnesium, riboflavin, and coenzyme Q10 reduces the frequency of migraine attacks, their clinical symptoms, and the burden of disease (56). Among these, riboflavin may protect nerves by reducing inflammation and anti-oxidative stress properties, suggesting its potential as a migraine preventive agent (57).
Herbal treatment has the advantages of holistic conditioning, multi-targeting, and long-lasting effects, which are conducive to individualized and fine-tuned treatment for migraine patients (58–60). In 22 years, Yang et al. (61) showed that in a rat model of nitroglycerin (NTG)-induced migraine, the Chinese herbal formula Xiongshao Zhitongfang (XZR) regulated NO, 5-HT, CGRP, and SP to normal levels, while inhibiting mast cell degranulation and the release of inflammatory factors, which resulted in attenuation of migraine symptoms. In addition, in a homozygous rat model, rhubarb extract from the traditional Chinese medicine Rheum palmatum also down-regulated the inflammatory response and alleviated migraine via the cGMP-PKG pathway (62). However, there is not much research available on how herbs can holistically condition the body and its long-term effects in treating migraines.
In addition to the commonly used pharmacological complementary alternative therapies, non-pharmacological treatments such as Remote Electrical Nerve Stimulation (REN), massage therapy, due to fewer adverse events, then it may be a more promising mode of treatment for migraine (63, 64). In randomized controlled trials, the frequency of migraine attacks was reduced in patients treated with rTMS therapy; also, rTMS was safer and more effective in treating chronic migraine (CM) when combined with amitriptyline (50, 65). The mechanisms involved may be related to the regulation of central and peripheral sensitization by rTMS (66).
From the perspective of qi and yin and yang concepts of Chinese medicine, acupuncture is based on meridians placing needles or pressing on specific locations on the patient’s skin to achieve therapeutic effects; from the perspective of physiology, the stimulation of high-threshold and tiny nerve fibers can transmit signals to specific brain regions mirrored by acupuncture points, leading to the release of endogenous opioids to achieve analgesic effects (67). Functional magnetic resonance imaging (MRI) data support that areas of brain activity in migraine patients who undergo acupuncture include the limbic system and the default mode network, as well as pain processing areas. The increased ALFF (Amplitude of Low-Frequency Fluctuation) values in these areas suggest that acupuncture may enhance spontaneous brain activity in patients with migraines (49). Acupuncture has been shown to be superior to sham surgery and placebo (68, 69). Meta-analyses have been performed to show that acupuncture reduces the frequency of migraine attacks more than pharmacologic prophylaxis and is less likely to result in withdrawals and reports of adverse effects due to adverse reactions (68).
Despite the large number of randomized controlled trials showing the benefits of complementary alternative therapies for migraine treatment, most studies have limitations, focusing mainly on methodological challenges (47). Common reasons for this include short follow-up time, small sample size, and patient loss, which leads to poor reproducibility and representativeness of the study results (70, 71). The limited availability of diagnostic criteria is also a challenge. Current diagnostic criteria do not adequately capture the heterogeneity of migraine, including underlying genetic and neurobiological factors. For example, a controlled trial of acupuncture treatment was unable to hypothesize a link between psychological and pain states because the Psychological Assessment Scale lacked an assessment of pain states (49). In addition, a recent meta-analysis showed a trend toward higher placebo responses in migraine prevention trials over the last 30 years (72). Another promblem is the impact of differences in patterns of regional culture. In Asian cultures, herbal treatments and tai chi are widely popular. However, these non-mainstream medical approaches may limit acceptance and application in non-Asian populations (73, 74).
Migraine is a mechanistically complex disorder caused primarily by neurovascular disorders, and its pathologic and physiologic processes are evolutionary and do not consist of a single mechanistic doctrine (75). Genetic and epigenetic susceptibility may also explain the development of migraine (76–78). Large genome-wide association studies have shown that genetics may contribute to altered brain morphology in individuals at high risk for migraine. Although genome-wide association studies have identified many susceptibility variants, including genetic factors shared with comorbidities, more in-depth studies exploring the overall susceptibility loci for migraine are needed to understand the cellular phenotypes resulting from migraine gene variants (26).
The design of future studies of complementary alternative treatments for migraine should ensure methodological rigor, reproducibility, and safety. 0Incorporation of CAT into migraine treatment should take into account the frequency of visits, the patient’s expectations of the treatment, and the psychological response to the treatment setting in order to avoid a high placebo response (26). Although most treatments are well tolerated with limited adverse effects, the possible risk of death due to carotid artery entrapment with high-speed chiropractic and hepatotoxicity of pyrrolizidine alkaloids in butterbur cannot be ignored (47, 79, 80). In addition, key areas of migraine research include further exploration of molecular markers and the use of imaging techniques to identify key mechanisms and triggers. In a longitudinal neuroimaging study, the duration of the aura phase of a spontaneous human migraine attack was found to be 48 h using MRI, and hypothalamic activation may serve as a potential marker for this staging (75, 81). In summary, the potential of CAT in migraine treatment is remarkable, offering a range of pharmacological and non-pharmacological options that can be tailored to the therapeutic needs of individual patients. While current research supports the efficacy of various CAT modalities, it is clear that more rigorous studies are needed to fully understand the mechanisms and optimize their integration into clinical practice.
XS: Conceptualization, Writing – original draft, Writing – review & editing. QZ: Data curation, Writing – original draft. LaS: Conceptualization, Writing – original draft. LeS: Writing – original draft. HC: Data curation, Writing – original draft. YY: Writing – original draft. ML: Methodology, Writing – original draft. XX: Writing – original draft. BL: Data curation, Writing – original draft. ZL: Data curation, Writing – original draft. JY: Data curation, Funding acquisition, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Tianjin Natural Science Foundation Project (21JCQNJC01160).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ferrari, MD, Goadsby, PJ, Burstein, R, Kurth, T, Ayata, C, Charles, A, et al. Migraine. Nat Rev Dis Primers. (2022) 8:2. doi: 10.1038/s41572-021-00328-4
2. Nappi, RE, Tiranini, L, Sacco, S, de Matteis, E, de Icco, R, Tassorelli, C, et al. Tassorelli C: role of estrogens in menstrual migraine. Cells. (2022) 11:11. doi: 10.3390/cells11081355
3. Robbins, MS . Diagnosis and Management of Headache: a review. JAMA. (2021) 325:1874–85. doi: 10.1001/jama.2021.1640
4. Katsarava, Z, Buse, DC, Manack, AN, and Lipton, RB. Defining the differences between episodic migraine and chronic migraine. Curr Pain Headache Rep. (2012) 16:86–92. doi: 10.1007/s11916-011-0233-z
5. Meng, ID, Dodick, D, Ossipov, MH, and Porreca, F. Pathophysiology of medication overuse headache: insights and hypotheses from preclinical studies. Cephalalgia. (2011) 31:851–60. doi: 10.1177/0333102411402367
6. Nichols, R, Doty, E, Sacco, S, Ruff, D, Pearlman, E, and Aurora, SK. Analysis of initial nonresponders to Galcanezumab in patients with episodic or chronic migraine: results from the EVOLVE-1, EVOLVE-2, and REGAIN randomized, double-blind, Placebo-Controlled Studies. Headache. (2019) 59:192–204. doi: 10.1111/head.13443
7. Hovaguimian, A, and Roth, J. Management of chronic migraine. BMJ. (2022) 379:e067670. doi: 10.1136/bmj-2021-067670
8. de Boer, I, Verhagen, IE, Souza, MNP, and Ashina, M. Place of next generation acute migraine specific treatments among triptans, non-responders and contraindications to triptans and possible combination therapies. Cephalalgia. (2023) 43:033310242211437. doi: 10.1177/03331024221143773
9. Hutchinson, S, Dodick, DW, Treppendahl, C, Bennett, NL, Yu, SY, Guo, H, et al. Ubrogepant for the acute treatment of migraine: pooled efficacy, safety, and tolerability from the ACHIEVE I and ACHIEVE II phase 3 randomized trials. Neurol Ther. (2021) 10:235–49. doi: 10.1007/s40120-021-00234-7
10. Côté, P, Yu, H, Shearer, HM, Randhawa, K, Wong, JJ, Mior, S, et al. Non-pharmacological management of persistent headaches associated with neck pain: a clinical practice guideline from the Ontario protocol for traffic injury management (OPTIMa) collaboration. Eur J Pain. (2019) 23:1051–70. doi: 10.1002/ejp.1374
11. Anheyer, D, Klose, P, Lauche, R, Saha, FJ, and Cramer, H. Yoga for treating headaches: a systematic review and Meta-analysis. J Gen Intern Med. (2020) 35:846–54. doi: 10.1007/s11606-019-05413-9
12. Gibson, W, Wand, BM, Meads, C, Catley, MJ, and O'Connell, NE. Transcutaneous electrical nerve stimulation (TENS) for chronic pain - an overview of Cochrane reviews. Cochrane Database Syst Rev. (2019) 4:Cd011890. doi: 10.1002/14651858.CD011890.pub3
13. Konstantinos, S, Vikelis, M, and Rapoport, A. Acute care treatment of migraine. J Neuroophthalmol. (2020) 40:472–84. doi: 10.1097/WNO.0000000000001053
14. Kim, CY, Hwang, EH, Heo, I, Park, SY, Shin, BC, and Hwang, MS. Efficacy of scalp acupuncture for migraine: a protocol for systematic review and meta-analysis. Medicine (Baltimore). (2022) 101:e30926. doi: 10.1097/MD.0000000000030926
15. Sacco, S, Ripa, P, Grassi, D, Pistoia, F, Ornello, R, Carolei, A, et al. Peripheral vascular dysfunction in migraine: a review. J Headache Pain. (2013) 14:80. doi: 10.1186/1129-2377-14-80
16. Vamos, E, Pardutz, A, Fejes, A, Tajti, J, Toldi, J, and Vecsei, L. Modulatory effects of probenecid on the nitroglycerin-induced changes in the rat caudal trigeminal nucleus. Eur J Pharmacol. (2009) 621:33–7. doi: 10.1016/j.ejphar.2009.08.034
17. Robblee, J . Breaking the cycle: unraveling the diagnostic, pathophysiological and treatment challenges of refractory migraine. Front Neurol. (2023) 14:1263535. doi: 10.3389/fneur.2023.1263535
18. Vitale, M, Tottene, A, Zarin Zadeh, M, Brennan, KC, and Pietrobon, D. Mechanisms of initiation of cortical spreading depression. J Headache Pain. (2023) 24:105. doi: 10.1186/s10194-023-01643-9
19. Melo-Carrillo, A, Strassman, AM, Schain, AJ, Adams, AM, Brin, MF, and Burstein, R. Combined onabotulinumtoxin a/atogepant treatment blocks activation/sensitization of high-threshold and wide-dynamic range neurons. Cephalalgia. (2021) 41:17–32. doi: 10.1177/0333102420970507
20. Kudo, C, Harriott, AM, Moskowitz, MA, Waeber, C, and Ayata, C. Estrogen modulation of cortical spreading depression. J Headache Pain. (2023) 24:62. doi: 10.1186/s10194-023-01598-x
21. Barbanti, P, Brighina, F, Egeo, G, Di Stefano, V, Silvestro, M, and Russo, A. Migraine as a cortical brain disorder. Headache. (2020) 60:2103–14. doi: 10.1111/head.13935
22. Costa, C, Tozzi, A, Rainero, I, Cupini, LM, Calabresi, P, Ayata, C, et al. Cortical spreading depression as a target for anti-migraine agents. J Headache Pain. (2013) 14:62. doi: 10.1186/1129-2377-14-62
23. Schain, AJ, Melo-Carrillo, A, Stratton, J, Strassman, AM, and Burstein, R. CSD-induced arterial dilatation and plasma protein extravasation are unaffected by Fremanezumab: implications for CGRP's role in migraine with Aura. J Neurosci. (2019) 39:6001–11. doi: 10.1523/JNEUROSCI.0232-19.2019
24. Borlak, J, Diener, HC, Kleeberg-Hartmann, J, Messlinger, K, and Silberstein, S. Petasites for migraine prevention: new data on mode of action, pharmacology and safety. A narrative review. Front Neurol. (2022) 13:864689. doi: 10.3389/fneur.2022.864689
25. Benemei, S, De Logu, F, Li Puma, S, Marone, IM, Coppi, E, Ugolini, F, et al. The anti-migraine component of butterbur extracts, isopetasin, desensitizes peptidergic nociceptors by acting on TRPA1 cation channel. Br J Pharmacol. (2017) 174:2897–911. doi: 10.1111/bph.13917
26. Grangeon, L, Lange, KS, Waliszewska-Prosół, M, Onan, D, Marschollek, K, Wiels, W, et al. Genetics of migraine: where are we now? J Headache Pain. (2023) 24:12. doi: 10.1186/s10194-023-01547-8
27. Guo, S, Jansen-Olesen, I, Olesen, J, and Christensen, SL. Role of PACAP in migraine: An alternative to CGRP? Neurobiol Dis. (2023) 176:105946. doi: 10.1016/j.nbd.2022.105946
28. Ernstsen, C, Christensen, SL, Rasmussen, RH, Nielsen, BS, Jansen-Olesen, I, Olesen, J, et al. The PACAP pathway is independent of CGRP in mouse models of migraine: possible new drug target? Brain. (2022) 145:2450–60. doi: 10.1093/brain/awac040
29. Guo, Z, Czerpaniak, K, Zhang, J, and Cao, YQ. Increase in trigeminal ganglion neurons that respond to both calcitonin gene-related peptide and pituitary adenylate cyclase-activating polypeptide in mouse models of chronic migraine and posttraumatic headache. Pain. (2021) 162:1483–99. doi: 10.1097/j.pain.0000000000002147
30. Spekker, E, Tanaka, M, Szabó, Á, and Vécsei, L. Neurogenic inflammation: the participant in migraine and recent advancements in translational research. Biomedicines. (2021) 10:76. doi: 10.3390/biomedicines10010076
31. Edvinsson, JCA, Viganò, A, Alekseeva, A, Alieva, E, Arruda, R, De Luca, C, et al. The fifth cranial nerve in headaches. J Headache Pain. (2020) 21:65. doi: 10.1186/s10194-020-01134-1
32. Andreou, AP, and Edvinsson, L. Mechanisms of migraine as a chronic evolutive condition. J Headache Pain. (2019) 20:117. doi: 10.1186/s10194-019-1066-0
33. Cruz-Almeida, Y, Aguirre, M, Sorenson, HL, Tighe, P, Wallet, SM, and Riley, JL 3rd. Age differences in cytokine expression under conditions of health using experimental pain models. Exp Gerontol. (2015) 72:150–6. doi: 10.1016/j.exger.2015.09.017
34. Wang, QY, Qu, YY, Feng, CW, Sun, WB, Wang, DL, Yang, TS, et al. Analgesic mechanism of acupuncture on neuropathic pain. Zhongguo Zhen Jiu. (2020) 40:907–12. doi: 10.13703/j.0255-2930.20190927-k0003
35. Liu, QQ, Yao, XX, Gao, SH, Li, R, Li, BJ, Yang, W, et al. Role of 5-HT receptors in neuropathic pain: potential therapeutic implications. Pharmacol Res. (2020) 159:104949. doi: 10.1016/j.phrs.2020.104949
36. Choi, SI, and Hwang, SW. Depolarizing effectors of bradykinin signaling in nociceptor excitation in pain perception. Biomol Ther (Seoul). (2018) 26:255–67. doi: 10.4062/biomolther.2017.127
37. Goadsby, PJ, Wietecha, LA, Dennehy, EB, Kuca, B, Case, MG, Aurora, SK, et al. Phase 3 randomized, placebo-controlled, double-blind study of lasmiditan for acute treatment of migraine. Brain. (2019) 142:1894–904. doi: 10.1093/brain/awz134
38. Dou, B, Li, Y, Ma, J, Xu, Z, Fan, W, Tian, L, et al. Role of Neuroimmune crosstalk in mediating the anti-inflammatory and analgesic effects of acupuncture on inflammatory pain. Front Neurosci. (2021) 15:695670. doi: 10.3389/fnins.2021.695670
39. Tzankova, V, Becker, WJ, and Chan, TLH. Diagnosis and acute management of migraine. CMAJ. (2023) 195:E153–e158. doi: 10.1503/cmaj.211969
40. Moye, LS, Siegersma, K, Dripps, I, Witkowski, W, Mangutov, E, Wang, D, et al. Delta opioid receptor regulation of calcitonin gene-related peptide dynamics in the trigeminal complex. Pain. (2021) 162:2297–308. doi: 10.1097/j.pain.0000000000002235
41. Russo, AF, and Hay, DL. CGRP physiology, pharmacology, and therapeutic targets: migraine and beyond. Physiol Rev. (2023) 103:1565–644. doi: 10.1152/physrev.00059.2021
43. Cuvellier, JC . Pediatric vs. adult Prodrome and postdrome: a window on migraine pathophysiology? Front Neurol. (2019) 10:199. doi: 10.3389/fneur.2019.00199
44. Brown, RB . Sodium chloride, migraine and salt withdrawal: controversy and insights. Med Sci (Basel). (2021) 9:67. doi: 10.3390/medsci9040067
45. Hautakangas, H, Winsvold, BS, Ruotsalainen, SE, Bjornsdottir, G, Harder, AVE, Kogelman, LJA, et al. Genome-wide analysis of 102, 084 migraine cases identifies 123 risk loci and subtype-specific risk alleles. Nat Genet. (2022) 54:152–60. doi: 10.1038/s41588-021-00990-0
46. Alipouri, M, Amiri, E, Hoseini, R, and Hezarkhani, LA. Effects of eight weeks of aerobic exercise and vitamin D supplementation on psychiatric comorbidities in men with migraine and vitamin D insufficiency: a randomized controlled clinical trial. J Affect Disord. (2023) 334:12–20. doi: 10.1016/j.jad.2023.04.108
47. Wells, RE, Beuthin, J, and Granetzke, L. Complementary and integrative medicine for episodic migraine: an update of evidence from the last 3 years. Curr Pain Headache Rep. (2019) 23:10. doi: 10.1007/s11916-019-0750-8
48. Xu, S, Yu, L, Luo, X, Wang, M, Chen, G, Zhang, Q, et al. Manual acupuncture versus sham acupuncture and usual care for prophylaxis of episodic migraine without aura: multicentre, randomised clinical trial. BMJ. (2020) 368:m697. doi: 10.1136/bmj.m697
49. Li, C, Li, X, He, K, Wu, Y, Xie, X, Yang, J, et al. Discovery of the mechanisms of acupuncture in the treatment of migraine based on functional magnetic resonance imaging and omics. Front Med. (2023) 17:993–1005. doi: 10.1007/s11684-023-0989-7
50. Leahu, P, Bange, M, Ciolac, D, Scheiter, S, Matei, A, Gonzalez-Escamilla, G, et al. Increased migraine-free intervals with multifocal repetitive transcranial magnetic stimulation. Brain Stimul. (2021) 14:1544–52. doi: 10.1016/j.brs.2021.10.383
51. Martins, LB, Rodrigues, A, Monteze, NM, Tibaes, JRB, Amaral, MHA, Gomez, RS, et al. Double-blind placebo-controlled randomized clinical trial of ginger (Zingiber officinale Rosc.) in the prophylactic treatment of migraine. Cephalalgia. (2020) 40:88–95. doi: 10.1177/0333102419869319
52. Slavin, M, Li, H, Khatri, M, and Frankenfeld, C. Dietary magnesium and migraine in adults: a cross-sectional analysis of the National Health and nutrition examination survey 2001-2004. Headache. (2021) 61:276–86. doi: 10.1111/head.14065
53. Domitrz, I, and Cegielska, J. Magnesium as an important factor in the pathogenesis and treatment of migraine-from theory to practice. Nutrients. (2022) 14:1089. doi: 10.3390/nu14051089
54. Khani, S, Hejazi, SA, Yaghoubi, M, and Sharifipour, E. Comparative study of magnesium, sodium valproate, and concurrent magnesium-sodium valproate therapy in the prevention of migraine headaches: a randomized controlled double-blind trial. J Headache Pain. (2021) 22:21. doi: 10.1186/s10194-021-01234-6
55. Su, N, Cai, P, Dou, Z, Yin, X, Xu, H, He, J, et al. Brain nuclei and neural circuits in neuropathic pain and brain modulation mechanisms of acupuncture: a review on animal-based experimental research. Front Neurosci. (2023) 17:1243231. doi: 10.3389/fnins.2023.1243231
56. Gaul, C, Diener, HC, and Danesch, U. Improvement of migraine symptoms with a proprietary supplement containing riboflavin, magnesium and Q10: a randomized, placebo-controlled, double-blind, multicenter trial. J Headache Pain. (2015) 16:516. doi: 10.1186/s10194-015-0516-6
57. Yamanaka, G, Suzuki, S, Morishita, N, Takeshita, M, Kanou, K, Takamatsu, T, et al. Experimental and clinical evidence of the effectiveness of riboflavin on migraines. Nutrients. (2021) 13:2612. doi: 10.3390/nu13082612
58. Huang, Y, Ni, N, Hong, Y, Lin, X, Feng, Y, and Shen, L. Progress in traditional Chinese medicine for the treatment of migraine. Am J Chin Med. (2020) 48:1731–48. doi: 10.1142/S0192415X2050086X
59. Shin, JM, Son, YJ, Ha, IJ, Erdenebileg, S, Jung, DS, Song, DG, et al. Artemisia argyi extract alleviates inflammation in a DSS-induced colitis mouse model and enhances immunomodulatory effects in lymphoid tissues. BMC Complement Med Ther. (2022) 22:64. doi: 10.1186/s12906-022-03536-x
60. Chen, S, Tang, Y, Gao, Y, Nie, K, Wang, H, Su, H, et al. Antidepressant potential of quercetin and its glycoside derivatives: a comprehensive review and update. Front Pharmacol. (2022) 13:865376. doi: 10.3389/fphar.2022.865376
61. Yang, S, Chen, C, Liu, X, Kang, Q, Ma, Q, Li, P, et al. Xiongshao Zhitong recipe attenuates nitroglycerin-induced migraine-like behaviors via the inhibition of inflammation mediated by nitric oxide synthase. Front Pharmacol. (2022) 13:920201. doi: 10.3389/fphar.2022.920201
62. Sun, S, Zheng, G, Zhou, D, Zhu, L, He, X, Zhang, C, et al. Emodin interferes with nitroglycerin-induced migraine in rats through CGMP-PKG pathway. Front Pharmacol. (2021) 12:758026. doi: 10.3389/fphar.2021.758026
63. Limakatso, K . Managing acute phantom limb pain with transcutaneous electrical nerve stimulation: a case report. J Med Case Rep. (2023) 17:209. doi: 10.1186/s13256-023-03915-z
64. Rinne, M, Garam, S, Häkkinen, A, Ylinen, J, Kukkonen-Harjula, K, and Nikander, R. Therapeutic exercise training to reduce chronic headache in working women: Design of a Randomized Controlled Trial. Phys Ther. (2016) 96:631–40. doi: 10.2522/ptj.20150267
65. Kalita, J, Kumar, S, Singh, VK, and Misra, UK. A randomized controlled trial of high rate rTMS versus rTMS and amitriptyline in chronic migraine. Pain Physician. (2021) 24:E733–e741. doi: 10.36076/ppj.2021.24.E733
66. Cosentino, G, Fierro, B, Vigneri, S, Talamanca, S, Paladino, P, Baschi, R, et al. Cyclical changes of cortical excitability and metaplasticity in migraine: evidence from a repetitive transcranial magnetic stimulation study. Pain. (2014) 155:1070–8. doi: 10.1016/j.pain.2014.02.024
67. Urits, I, Patel, M, Putz, ME, Monteferrante, NR, Nguyen, D, An, D, et al. Acupuncture and its role in the treatment of migraine headaches. Neurol Ther. (2020) 9:375–94. doi: 10.1007/s40120-020-00216-1
68. Linde, K, Allais, G, Brinkhaus, B, Fei, Y, Mehring, M, Vertosick, EA, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. (2016) 2016:Cd001218. doi: 10.1002/14651858.CD007587.pub2
69. Zhao, L, Chen, J, Li, Y, Sun, X, Chang, X, Zheng, H, et al. The long-term effect of acupuncture for migraine prophylaxis: a randomized clinical trial. JAMA Intern Med. (2017) 177:508–15. doi: 10.1001/jamainternmed.2016.9378
70. Toprak Celenay, S, Coban, O, Mete, O, and Karahan, N. An investigation of the effects of connective tissue massage in women with migraine: a controlled clinical trial. J Bodyw Mov Ther. (2023) 33:112–9. doi: 10.1016/j.jbmt.2022.09.008
71. Wojciech, K, Pawel, L, and Halina, RZ. Effects of feet reflexology versus segmental massage in reducing pain and its intensity, frequency and duration of the attacks in females with migraine: a pilot study. J Tradit Chin Med. (2017) 37:214–9. doi: 10.1016/S0254-6272(17)30047-X
72. Tepper, SJ, Cirillo, J, Kim, E, L'Italien, G, Tweedie, JM, Lodaya, K, et al. The temporal trend of placebo response in migraine prevention from 1990 to 2021: a systematic literature review and meta-analysis with regression. J Headache Pain. (2023) 24:54. doi: 10.1186/s10194-023-01587-0
73. Zheng, G, Xiong, Z, Zheng, X, Li, J, Duan, T, Qi, D, et al. Subjective perceived impact of tai chi training on physical and mental health among community older adults at risk for ischemic stroke: a qualitative study. BMC Complement Altern Med. (2017) 17:221. doi: 10.1186/s12906-017-1694-3
74. Millstine, D, and Chen, CY. Bauer B: complementary and integrative medicine in the management of headache. BMJ. (2017) 357:j1805. doi: 10.1136/bmj.j1805
75. Schulte, LH, Mehnert, J, and May, A. Longitudinal neuroimaging over 30 days: temporal characteristics of migraine. Ann Neurol. (2020) 87:646–51. doi: 10.1002/ana.25697
76. Mitchell, BL, Diaz-Torres, S, Bivol, S, Cuellar-Partida, G, Gerring, ZF, Martin, NG, et al. Elucidating the relationship between migraine risk and brain structure using genetic data. Brain. (2022) 145:3214–24. doi: 10.1093/brain/awac105
77. Russo, AF, Kuburas, A, Kaiser, EA, Raddant, AC, and Recober, A. A potential preclinical migraine model: CGRP-sensitized mice. Mol Cell Pharmacol. (2009) 1:264–70.
78. Papasavva, M, Vikelis, M, Siokas, V, Katsarou, MS, Dermitzakis, EV, Raptis, A, et al. Variability in oxidative stress-related genes (SOD2, CAT, GPX1, GSTP1, NOS3, NFE2L2, and UCP2) and susceptibility to migraine clinical phenotypes and features. Front Neurol. (2023) 13:1054333. doi: 10.3389/fneur.2022.1054333
79. von Gottberg, P, Hellstern, V, Wendl, C, Wolf, ME, Niehaus, L, Bäzner, H, et al. Combined anticoagulation and Antiaggregation in acute cervical artery dissection. J Clin Med. (2021) 10:4580. doi: 10.3390/jcm10194580
80. Kimel, K, Godlewska, S, Gleńsk, M, Gobis, K, Ośko, J, Grembecka, M, et al. LC-MS/MS evaluation of pyrrolizidine alkaloids profile in relation to safety of comfrey roots and leaves from polish sources. Molecules. (2023) 28:6171. doi: 10.3390/molecules28166171
81. Li, ML, Zhang, F, Chen, YY, Luo, HY, Quan, ZW, Wang, YF, et al. A state-of-the-art review of functional magnetic resonance imaging technique integrated with advanced statistical modeling and machine learning for primary headache diagnosis. Front Hum Neurosci. (2023) 17:1256415. doi: 10.3389/fnhum.2023.1256415
Keywords: migraine, pain management, alternative therapy, acupuncture, complementary therapy
Citation: Song X, Zhu Q, Su L, Shi L, Chi H, Yan Y, Luo M, Xu X, Liu B, Liu Z and Yang J (2024) New perspectives on migraine treatment: a review of the mechanisms and effects of complementary and alternative therapies. Front. Neurol. 15:1372509. doi: 10.3389/fneur.2024.1372509
Received: 18 January 2024; Accepted: 08 April 2024;
Published: 09 May 2024.
Edited by:
Shiyan Yan, Beijing University of Chinese Medicine, ChinaReviewed by:
Xuesong Wang, Hebei University of Chinese Medicine, ChinaCopyright © 2024 Song, Zhu, Su, Shi, Chi, Yan, Luo, Xu, Liu, Liu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin Yang, eWFuZ2ppbjk5MTIzQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.