
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
STUDY PROTOCOL article
Front. Neurol. , 05 July 2024
Sec. Neurotechnology
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1371284
This article is part of the Research Topic Photobiomodulation Therapy for Brain Disorders View all 5 articles
Introduction: Photobiomodulation (PBM) is a novel strategy for cognitive enhancement by improving brain metabolism and blood flow. It is potentially beneficial for patients with Alzheimer’s disease (AD). We present a study protocol for a randomised controlled trial designed to evaluate the efficacy and safety of PBM.
Method and analysis: This is a single-centre, parallel-group, randomised, sham-controlled study. We enroll patients with mild cognitive impairment or dementia due to AD and assigned them to receive either active or sham stimulation at home for 12 weeks, with three sessions per week (20 min each). The stimulation involves invisible near-infrared light delivered by five applicators (one in a nostril, one on the frontal scalp, and three on the occipital scalp). The primary outcome will be the mean change in the Alzheimer Disease Assessment Scale-cognition from baseline to Week 12. We will also measure cognitive function, activity of daily living, behavioral and psychological symptoms, and caregiver burden. We will collect data at clinics at baseline and Week 12 and remotely at home. We estimate a sample size of 30 (20 active and 10 sham) based on an expected mean difference of −6.9 and an SD of 4.8. We use linear models for the statistical analysis.
Ethics and dissemination: The National Center of Neurology and Psychiatry Clinical Research Review Board (CRB3200004) approved this study. The results of this study will be published in a scientific peer-reviewed journal. Trial registration details Japan Registry of Clinical Trials jRCTs032230339.
Dementia is a clinical syndrome characterized by impaired cognitive functions and reduced capacity to perform daily activities, as well as affecting the quality of life and social well-being of their caregivers. Prior to dementia, there is a stage of mild cognitive impairment, which is a condition of decreased cognitive performance that does not meet the criteria for dementia and preserves their ability for independent living (1). While some pharmacological treatments are available, such as cholinesterase inhibitors, memantine, and anti-amyloid antibodies, their efficacy and safety are still under debate. Anti-amyloid antibodies, in particular, have been recently approved in the United States and Japan, but their high cost, modest benefit, and potential risk of causing amyloid-related imaging abnormality, which can be fatal, raise questions about their clinical utility (2). Besides pharmacological interventions, several modifiable factors, such as physical activity, social engagement, and depression, have been identified as possible risk or protective factors for dementia (3).
Photobiomodulation (PBM) is a light therapy method that employs visible light (wavelengths around 400–700 nm) and near-infrared light (wavelengths around 810–1,100 nm). Its potential was first unveiled in 1968 when Mester et al. reported the positive effects of PBM on hair growth and wound healing in mice using a ruby laser (4). Since then, PBM has been investigated under various terms, including low-reactive level laser therapy (LLLT). More recently, it has been shown that light-emitting diodes (LEDs), which are more cost-effective than lasers, can also produce comparable results. Consequently, PBM encompasses interventions that utilize various types of light sources beyond lasers.
The mechanism underlying PBM’s effects varies depending on the wavelength of the light used. Notably, light in the red-near-infrared region offers superior tissue penetration and contributes to cell proliferation and growth promotion (5, 6), conversely, blue region light is expected to exhibit effects such as bactericidal effect and induction of cell apoptosis at high irradiation levels. In the case of low-level irradiation of red-near-infrared region light, it is believed that photons are absorbed by intracellular chromophores present in intracellular organelles (especially within mitochondria). Cytochrome C oxidase (CCO) is involved as a light receptor for this process (7), and the activation of CCO increases ATP production and activates cellular metabolic activity. It is also thought that the reactive oxygen species (ROS) and nitrogen oxide (NO) generated in this process promote gene transcription and cell repair. NO also has a role in regulating neurotransmitters in the central nervous system and vasodilation, which is thought to improve blood circulation. As a result, increased cerebral blood flow (8) and increased brain metabolism (9) have been observed with transcranial PBM.
The field of PBM has witnessed significant progress in improving brain stimulation parameters. Notably, the shift from continuous to pulsed stimulation has shown potential benefits, enhancing tissue absorption responses and possibly inducing specific brain wave rhythms, such as alpha waves at 10 Hz and gamma waves at 40 Hz (10). Furthermore, intranasal PBM may be related to direct or indirect stimulation of deeper brain regions (11), such as ventromedial prefrontal cortex and ventromedial orbitofrontal cortex (12). Coupled with the cost-effectiveness and miniaturization of LED devices, these improved parameters have heightened expectations for the effectiveness of PBM in neurological and psychiatric applications, especially in the context of cognitive enhancement. Consequently, a surge of clinical studies is expected to assess its impact in these domains. While the effectiveness of 40 Hz PBM on amyloid load in mice has yielded mixed results (13, 14), several clinical trials have shown potential in enhancing cognitive function in dementia patients.
For example, a sham-controlled clinical study by Chan et al. involved treating 18 patients with nine LEDs during a single stimulation session, which resulted in improved visual memory (15). Saltmarche et al. employed 5 patients, using pulsed 810 nm light at 10 Hz with an intensity of 14.2 mW/cm2 for sessions lasting 25 min, conducted once or twice weekly over 12 weeks, and observed improvements in Mini-Mental State Examination (MMSE (16)) and Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-Cog (17)) scores compared to baseline (18). Similarly, (19) Berman et al. recruited 11 patients and adopted pulsed 1,072 nm light at 10 Hz for 6 min daily over 28 consecutive days, noting improvements in quantitative electroencephalogram compared to a sham stimulation group (20). Nizamutdinov et al. administered continuous 1,060–1,080 nm light at an intensity of 23.1 mW/cm2 for sessions lasting 6 min, twice daily over 12 weeks to 60 patients, and reported improvements in MMSE and LMT-I scores compared to a sham stimulation group (21).
While the above paper used transcranial PBM, another clinical study has also used a nasal applicator to stimulate the deep brain trans nasally. Chao et al. utilized pulsed 810 nm light at 40 Hz with an intensity of 75 mW/cm2 for 20 min sessions thrice weekly over 12 weeks to 8 patients, reporting enhancements in ADAS-Cog and NPI scores compared to a sham stimulation group (19). This article also suggests increased cerebral perfusion and connectivity between the posterior cingulate cortex and lateral parietal nodes within the default-mode network in the PBM group. Our study try to extend this evidence with more number of participants in Japan to evaluate the utility of transcranial and intranasal PBM in early Alzheimer’s disease (AD) patients, who are expected to exhibit mild neurodegeneration due to dementia.
The main objective of this study is to assess the effectiveness and safety of 12 week photobiomodulation on cognition among mild cognitive impairment and mild dementia due to AD patients as assessed by ADAS-Cog.
This is a prospective, parallel, randomised, sham-controlled, superiority trial involving 30 participants with AD-related mild cognitive impairment or dementia, as per the DSM-5 criteria. The participants will be allocated to either an active or a sham group in a 2:1 ratio. The protocol adheres to the 2013 SPIRIT guidelines (22). The trial is registered with the Japan Registry of Clinical Trials (Trial ID: jRCTs032230339).
We will enroll outpatients with regular consultations from a single academic hospital in Tokyo, Japan: the National Center of Neurology and Psychiatry. Potential participants will be referred by their treating psychiatrists or neurologists and receive a brochure with brief information on the trial after their initial appointments. Participants who express interest will provide informed consent to the principal investigator or subinvestigators using the Informed Consent Form (Supplementary File S1). The study design, benefits and risks will be explained to the participants by the principal investigator and outcome assessors. Participants will undergo screening by a treating psychiatrist to verify their eligibility (shown below). The inclusion criteria are: (1) Age between 60 and 90 years at consent, (2) Prior visit to the study site, (3) Early AD diagnosis (mild AD or MCI due to AD) confirmed by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR) at the study site, (4) MMSE score between 15 and 30 points, (5) Availability of a study partner who cohabits or regularly (defined by 10 h or more per week) monitors the participant’s condition, (6) Ability to communicate and complete questionnaires via the internet or email, using a smartphone or personal computer (with or without study partner assistance), (7) Stable treatment for early AD (no change in drug or non-drug therapies for more than 4 weeks), (8) no major environmental changes expected within 12 weeks after the trial initiation, such as hospitalization, travel, relocation. The exclusion criteria are as follows; (1) people with cognitive impairment from causes other than AD (such as mental disorders, intellectual disabilities, etc.), (2) people who join a new clinical trial or study during this study period, (3) people with skin problems or foreign materials that interferes appropriate photobiomodulation, (4) people who are not suitable for this study according to the principal investigator or subinvestigators.
The study will employ a home-based PBM intervention using the Vielight Neuro Gamma 4 device (Vielight Inc., Tronto, Ontario, Canada), which delivers near-infrared emissions via five LED applicators (one intranasal, one frontal, and three occipital). The device will target the frontal, temporal, occipitoparietal, and naso-temporal regions (see Figure 1). Subjects will blindly receive either active or sham devices from the investigator at the time of study entry. At that time, the investigator will adjust the device to the appropriate angle and instruct the subject and study partner on the appropriate way of its use. The subject and study partner will be responsible for the daily removal and recharging of the device, and they will have free access to the device instructions and instructional videos created for the study. They will also report and communicate regularly with researchers to check for malfunctions and failure of the device.
The participants will be randomised into two groups: active or sham PBM. The active group will receive 40 Hz intermittent photoemission per session with 60 J/cm2 on the frontal region, 45 J/cm2 on the occipital region in total, and 15 J/cm2 intranasally. The sham group will receive a placebo device that mimics the appearance of the active device but does not emit any light for 20 min per session.
Regarding the dosage, it is thought that effective effects on cells emerge when a heat value of 0.1–6 J/cm2 is transferred as the effect of PBM on the skin surface,1 and considering the energy loss in the pathway, this product is designed to deliver 60 J/cm2 in the frontal region, 45 J/cm2 in the occipital region, and 15 J/cm2 in the nasal cavity in order to stimulate cranial nerves at a deeper level. Based on the output power of the product (100, 75, and 25 mW/cm2, respectively), the irradiation time is calculated to be 20 min (1,200 s).
Both devices will have a green light indicator and will turn off automatically after 20 min. The participants and their study partners are taught to do PBM at their home or wherever comfortable three times a week within the same range of a day (morning, afternoon, or evening). They will be unable to discern whether they are using the active or sham device. The assessors and participants will be blinded to the treatment allocation to enhance the study validity. At the end of Week 12, the participants will be asked to guess their treatment group to assess the blinding quality. The participants and their study partners will also monitor and report any adverse events to the researchers remotely, as well as submit periodic reports of efficacy, safety, and satisfaction. The study partners will keep track of the device usage frequency and occasionally contact the participants or their partners to encourage adherence to the study protocol.
Any changes in the concomitant therapy of the participants, such as starting, stopping, or adjusting the dose, should be documented, and reported to the researcher. The researcher will train the patient and study partner on operating the device at home and provide written instructions before the first use. The researcher will also verify that the patient and study partner can use the device correctly. A video tutorial is available for reference at any time, and any issues or questions regarding the device use are regularly monitored to enhance adherence. The participant will be withdrawn from the study if any of the following conditions apply, as determined by the principal investigator or subinvestigators when the participant revokes their consent or asks to stop participating in the research; the participant is affected by a disease outbreak or a pandemic; the device shows insufficient efficacy for the participant; the participant fails to attend the evaluation and cannot be reached; the principal investigator or subinvestigators judge that the participant’s mental and physical burden is too high to continue in this study; there is a serious breach or violation of the research protocol. The entire study may be terminated by the Independent Safety and Effectiveness Committee, which convenes when three or more serious adverse events related to the study device occur.
The main outcome of interest is mean change in ADAS-Cog scores from baseline to Week 12 between groups. ADAS-Cog is the de facto standard scale developed for assessing anti-dementia treatment. Other outcomes include global function, assessed by Clinical Dementia Rating scale; quality of life, evaluated by the DEMQOL (23) and DEMQOL-proxy (24); cognition, measured by the MMSE (with a score range of 15 to 30); global impression, rated by Participant’s Global Impression of Improvement (PGI-I) and Clinical Global Impression of Improvement (CGI-I); behavioral and psychological symptoms, monitored by Neuropsychiatric Inventory Questionnaire (NPI (25)); and caregivers’ burden, measured by Zarit’s Burden Interview (ZBI (26)). A specialized psychologist, who was blind to group assignment, scored all these outcomes except DEMQOL, DEMQOL-proxy, PGI-I, NPI and ZBI after a clinical interview. The psychologists conducted the assessments at baseline, Week 12, and the follow-up. The psychological evaluations at Week 12 and the follow-up had a 2 week window, respectively. Figure 2 summarizes the schedule for enrolment, intervention, and assessment. We will record any history of substance use disorder, dementia, psychiatric disorders, neurological disorders, brain injury and other conditions at baseline to exclude patients with clinically contraindicated conditions. Adverse events are any undesirable patient experience during PBM administration. We will monitor and record all adverse events throughout the study via self-report or clinical observations, regardless of causal relationship. We will follow up any untreatable adverse events after trial completion. The principal investigator will diagnose, treat, and explain any serious adverse events to the relevant patients. The subinvestigators will also report any information related to such adverse events to the principal investigator. The principal investigator is responsible for reporting any serious adverse events to the relevant authorities, including the clinical research review board, the Ministry of Health, Labour, and Welfare, and the pharmaceutical and medical device agencies.
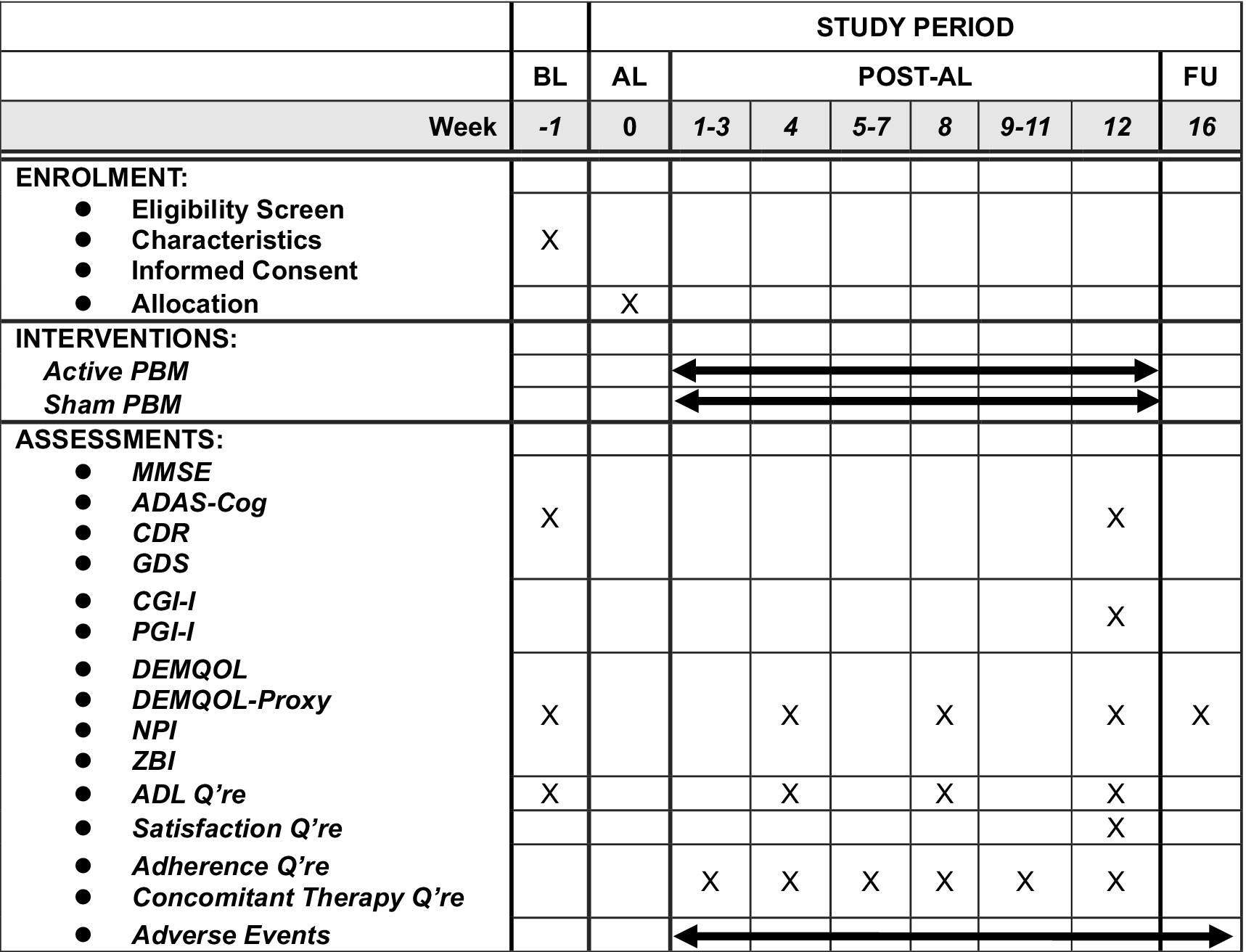
Figure 2. The schedule of this study. The time point of post-tDCS evaluation will be allowed within ±7 days and that of follow-up evaluation will be allowed within 14 days. ADAS-Cog, Alzheimer’s Disease Assessment Scale-Cognitive Subscale; ADL, activities of daily living; AL, allocation; BL, baseline; CDR, Clinical Dementia Rating; CGI-I, Clinical Global Impression-Improvement; FU, follow-up; GDS, geriatric depression scale; MMSE, Mini-Mental State Exam; NPI, Neuropsychiatric Inventory; PBM, photobiomodulation; Q’re, questionnaire; ZBI, Zarit Burden Interview.
Serious adverse events are any undesirable medical occurrences at any dose that: (1) Cause death. (2) Pose a threat to life. (3) Necessitate inpatient hospitalisation or prolong existing hospitalisation. (4) Lead to persistent or significant disability/incapacity. (5) Are congenital anomalies/birth defects. (6) Require interventions to prevent permanent impairment damage. The psychiatrists involved in the treatment will document the name, occurrence date, severity, grade, intervention implemented (if any), outcome and relationship to the study medication in an electronic clinical research form (eCRF). Any symptoms that were present at baseline and did not worsen during the study will not be considered as adverse events. Adverse events are classified into three categories in this study: Mild: asymptomatic or mild symptoms; clinical or diagnostic observations only; no intervention required; no impact on usual daily activities. Moderate: minimal, local or non-invasive intervention required; limiting age-appropriate instrumental activities of daily living (ADL); some impact on usual daily activities. Severe: medically significant, life-threatening or related to death; hospitalisation or the prolongation of hospitalisation required; disabling; limiting self-care in ADL; significant impact on usual daily activities.
We aim to enroll 30 participants. In Chao’s et al. report (19), the intervention group scored 32.3 ± 4.8 and the control group scored 39.2 ± 2.6 on ADAS-Cog, which is an efficacy evaluation item for cognitive function. In this trial, assuming a 1:2 allocation ratio of sham stimulation group to real stimulation group, an ADAS-Cog score of 39.2 for the sham stimulation group, an ADAS-Cog score of 32.3 for the real stimulation group, a standard deviation of 4.8, α = 0.05, and a power of 0.80, the required sample size was calculated to be 18 (6 for the sham stimulation group and 12 for the real stimulation group) using https://clincalc.com/stats/samplesize.aspx. Considering that this study includes patients with mild cognitive impairment, which is a preliminary stage of the previous study, and that the ADAS-Cog score may decrease overall, we multiplied the sample size by 1.5 to obtain 27, and further considered adherence and dropout cases to obtain 30.
We will use a computer-generated stratified sequence from the Mujinwari system2 to assign participants to the active or sham PBM group in a 2:1 ratio. Our custom electronic data capture (EDC) system, based on Google Workspace For Business (Google, United States), will collect all data. A cloud computer will keep group assignment and other personal identification data. It will ensure a balanced distribution of diagnosis types (e.g., major neurocognitive disorder or mild neurocognitive disorder). This randomisation method stratifies by diagnosis, and the independent personnel can access the allocation results and provide the appropriate device. The principal investigator and subinvestigators can access each participant’s allocation and disclose it to the participants and raters only after the complete study data is consolidated.
To evaluate the intervention, we will perform assessments by clinicians at baseline and Week 12 (Figure 2). Experienced psychologists unaware of the group allocations will conduct the baseline, Week 12 and follow-up evaluations. Participants and study partners will complete scheduled questionnaires and report adverse events between these visits. All data, except for the informed consent document, will be entered into the EDC anonymously and verified by independent data managers. Considering the device’s safety and the blindness of study personnel, we will not composite the specific data monitoring committee, but data monitors will oversee and review the trial progress. Participants can withdraw their consent or discontinue participating in the study at any time, but we will collect data as much as possible unless consent is withdrawn, or it is at least a serious deviation from the protocol. We will terminate the intervention if we observe any serious adverse events (SAEs). The following auditing standards for field work apply to this study based on the Clinical Research Act: audits will be conducted if any of the following criteria are met: (1) at least two SAEs that are causally related to the medical device are reported; (2) at least two serious protocol deviations are detected; (3) multicentre clinical trials are initiated; or (4) potential severe conflicts of interest that deviate from the prespecified plan for conflicts of interest are identified. We will report any necessary protocol amendments and their outcomes to both the clinical research review board and the Ministry of Health, Labour, and Welfare for registration on the Japan Registry of clinical trials website. After completing the trial, we will publish an original article to disseminate the data results.
We define two populations for our analyses. Full Analysis Set (FAS) will be those participants who are randomised and observed for efficacy assessment after taking active or sham devices home; Safety Analysis Set will be those who are randomised and observed for any safety assessment. Our primary analysis for efficacy will be FAS. Our primary analysis will be intention-to-treat and include all randomised patients in the active or sham group, and we will report their demographic characteristics. We will also perform per-protocol set analysis after excluding cases with protocol deviations as sensitivity analyses. To estimate the mean treatment effect, we will use a linear model for analysis of covariance (ANCOVA) to detect changes in ADAS-Cog, MMSE, CDR, PGI-I, CGI-I, DEMQOL, DEMQOL-Proxy, NPI, and ZBI as secondary outcomes at baseline and Week 12. The ANCOVA will incorporate the covariates of group, baseline score of each outcome and disease (mild cognitive impairment or dementia), which are the stratification factors of dynamic allocation. We will not plan any interim analyses. The primary statistical test will be a t-test for the difference of the adjusted means of ADAS-Cog between the groups at Week 12. We will also conduct t-tests for the follow-up period and/or the other outcomes. For missing data, we will impute the baseline score as a sensitivity analysis. We will use a Fisher’s exact test to assess the blinding integrity. Additionally, we will describe the demographic characteristics of the patients and compare them between the two groups using a two-sample t-test or Fisher’s exact test. We will also examine the correlations between the baseline characteristics, ADAS-Cog, MMSE, CDR, DEMQOL, DEMQOL-proxy, NPI-Q, and ZBI and the cognitive outcomes at Week 12 and follow-up. We will use SAS V.9.4 or later for the statistical analysis. The results will be significant at p < 0.05 and statistical tests will be conducted for two-tailed hypotheses.
Since dementia is a condition that involves insidious deterioration of cognitive and social functioning, the long-term effects of certain intervention should be clinically important. Thus, we should conduct a long-term trial with minimizing number of visits in hospitals to enhance adherence to the intervention. To this end, this device is suitable for home use, convenient and proper to wear or remove it. Besides applications at home needs fundamental safety of the device. This device has gained the status of General Wellness Device from the US Food and Drug Administration due to the safety and ability of home use. The remaining problem is the resource to conduct a huge clinical trial with long-term duration. Most other studies cannot overcome this and PBM studies have limited sample size generally. This study also fell to prey but the positive results of the properly conducted study hopefully evoke further and bigger clinical studies.
This study has several strengths, such as its rigorous sample size estimation for cognitive outcomes based on prior evidence, in contrast to many previous studies that did not perform adequate power calculations. Moreover, the device used in this study is unique in its ability to deliver near-infrared light through the nasal cavity. Given the long duration of 12 weeks for the participants and their caregivers, we implemented strategies to enhance adherence, such as minimizing the number of hospital visits and providing immediate contact with the researchers. The use of remote visits also improved the experience of the researchers. However, we also acknowledge some limitations of this study. First, the study is conducted at a single site, which may affect the generalizability of the findings. Second, the long-term effects of PBM on cognition cannot be evaluated. This is a common challenge in trials of anti-amyloid therapies, which require 78 weeks or more to show results and are still controversial regarding their sustained efficacy. Therefore, the optimal duration of PBM intervention remains an open question.
A potential limitation of this study is that it is conducted at a single centre and does not adequately assess the long-term outcomes of PBM.
We have submitted the protocol version 1.02 to an institutional review board for ethical approval by the National Center of Neurology and Psychiatry Clinical Research Review Board (CRB3200004), in accordance with the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects. The institutional clinical research review board will conduct initial and annual reviews of the protocol, starting from 1 September 2023. The principal investigator will provide safety and progress reports to the review board at least once a year, and within 3 months after the study termination or completion. These reports will contain the total number of patients enrolled, serious and non-serious adverse events that occurred, and summaries of the safety and monitoring board’s review. The clinical research review board and the Ministry of Health, Labour, and Welfare will receive prompt notification of any SAEs from the principal investigator. This study complies with the Clinical Trials Act and is registered with the Japan Registry of Clinical Trials. The study objectives will be explained to all study participants, who will provide informed consent before the principal investigator, the research coordinator or the research assistant enrolls them in the trial. Participation is voluntary and patients will undergo assessment after consenting. The clinical research review board will approve any protocol amendments that involve changes in the study protocol and/or the informed consent. Participants who need medical treatment will access it through their own medical insurance. Regarding data access and responsibility, the designated investigators (YY and TI) has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the analysis. Individual participant data generated in this study will be anonymised for transparency of trial results and adherence to ICMJE recommendations. They will be shared with researchers for secondary use of the data upon request per the data-sharing policy. The data curation will be completed by January 2026 and the results will be published by March 2026. The data will be retained for 5 years after the study completion.
YYo: Conceptualization, Writing – original draft, Writing – review & editing. TI: Supervision, Writing – original draft, Writing – review & editing. YYa: Supervision, Writing – original draft, Writing – review & editing. MM: Supervision, Writing – original draft, Writing – review & editing. AT: Supervision, Writing – original draft, Writing – review & editing. TN: Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (Grant-in-Aid for Young Scientists (Research Project Name: Photobiomodulation in Patients with Dementia and Mild Cognitive Impairment, Principal Investigator: YY, Grant Period: 2023–2025)) and did not receive any funding from specific companies.
We would like to express our gratitude to Dr. Mari Oba, who provided valuable statistical advice for this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1371284/full#supplementary-material
1. Duff, K. Mild cognitive impairment and dementia: definitions, diagnosis, and treatment. Arch Clin Neuropsychol. (2014) 29:691–2. doi: 10.1093/arclin/acu034
2. Mahase, E. Alzheimer’s disease: FDA approves lecanemab amid cost and safety concerns. BMJ. (2023) 380:e73. doi: 10.1136/bmj.p73
3. Livingston, G, Sommerlad, A, Orgeta, V, Costafreda, SG, Huntley, J, Ames, D, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. (2020) 396:413–46. doi: 10.1016/S0140-6736(20)30367-6
5. Stolik, S, Delgado, JA, Pérez, A, and Anasagasti, L. Measurement of the penetration depths of red and near infrared light in human ex vivo tissues. J Photochem Photobiol B. (2000) 57:90. doi: 10.1016/S1011-1344(00)00082-8
6. Migliario, M, Sabbatini, M, Mortellaro, C, and Renò, F. Near infrared low-level laser therapy and cell proliferation: the emerging role of redox sensitive signal transduction pathways. J Biophotonics. (2018) 11:e201800025. doi: 10.1002/jbio.201800025
7. Karu, TI. Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life. (2010) 62:607–10. doi: 10.1002/iub.359
8. Baik, JS, Lee, TY, Kim, NG, Pak, K, Ko, SH, Min, JH, et al. Effects of photobiomodulation on changes in cognitive function and regional cerebral blood flow in patients with mild cognitive impairment: a pilot uncontrolled trial. J Alzheimers Dis. (2021) 83:1513–9. doi: 10.3233/JAD-210386
9. Pruitt, T, Wang, X, Wu, A, Kallioniemi, E, Husain, MM, and Liu, H. Transcranial photobiomodulation (tPBM) with 1,064-nm laser to improve cerebral metabolism of the human brain in vivo. Lasers Surg Med. (2020) 52:807–13. doi: 10.1002/lsm.23232
10. Spera, V, Sitnikova, T, Ward, MJ, Farzam, P, Hughes, J, Gazecki, S, et al. Pilot study on dose-dependent effects of transcranial photobiomodulation on brain electrical oscillations: a potential therapeutic target in Alzheimer’s disease. J Alzheimers Dis. (2021) 83:1481–98. doi: 10.3233/JAD-210058
11. Zomorrodi, R, Loheswaran, G, Pushparaj, A, and Lim, L. Pulsed near infrared transcranial and intranasal photobiomodulation significantly modulates neural oscillations: a pilot exploratory study. Sci Rep. (2019) 9:6309. doi: 10.1038/s41598-019-42693-x
12. Salehpour, F, Gholipour-Khalili, S, Farajdokht, F, Kamari, F, Walski, T, Hamblin, MR, et al. Therapeutic potential of intranasal photobiomodulation therapy for neurological and neuropsychiatric disorders: a narrative review. Rev Neurosci. (2020) 31:269–86. doi: 10.1515/revneuro-2019-0063
13. Iaccarino, HF, Singer, AC, Martorell, AJ, Rudenko, A, Gao, F, Gillingham, TZ, et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature. (2016) 540:230–5. doi: 10.1038/nature20587
14. Soula, M, Martín-Ávila, A, Zhang, Y, Dhingra, A, Nitzan, N, Sadowski, MJ, et al. Forty-hertz light stimulation does not entrain native gamma oscillations in Alzheimer’s disease model mice. Nat Neurosci. (2023) 26:570–8. doi: 10.1038/s41593-023-01270-2
15. Chan, AS, Lee, TL, Hamblin, MR, and Cheung, MC. Photobiomodulation enhances memory processing in older adults with mild cognitive impairment: a functional near-infrared spectroscopy study. J Alzheimers Dis. (2021) 83:1471–80. doi: 10.3233/JAD-201600
16. Folstein, MF, Folstein, SE, and McHugh, PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. (1975) 12:189–98. doi: 10.1016/0022-3956(75)90026-6
17. Rosen, WG, Mohs, RC, and Davis, KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. (1984) 141:1356–64. doi: 10.1176/ajp.141.11.1356
18. Saltmarche, AE, Naeser, MA, Ho, KF, Hamblin, MR, and Lim, L. Significant improvement in cognition in mild to moderately severe dementia cases treated with transcranial plus intranasal photobiomodulation: case series report. Photomed Laser Surg. (2017) 35:432–41. doi: 10.1089/pho.2016.4227
19. Chao, LL. Effects of home photobiomodulation treatments on cognitive and behavioral function, cerebral perfusion, and resting-state functional connectivity in patients with dementia: a pilot trial. Photobiomodul Photomed Laser Surg. (2019) 37:133–41. doi: 10.1089/photob.2018.4555
20. Berman, MH, Halper, JP, Nichols, TW, Jarrett, H, Lundy, A, and Huang, JH. Photobiomodulation with near infrared light helmet in a pilot, placebo controlled clinical trial in dementia patients testing memory and cognition. J Neurol Neurosci. (2017) 8:176. doi: 10.21767/2171-6625.1000176
21. Nizamutdinov, D, Qi, X, Berman, MH, Dougal, G, Dayawansa, S, Wu, E, et al. Transcranial near infrared light stimulations improve cognition in patients with dementia. Aging Dis. (2021) 12:954–63. doi: 10.14336/AD.2021.0229
22. Chan, AW, Tetzlaff, JM, Altman, DG, Laupacis, A, Gøtzsche, PC, Krleža-Jerić, K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. (2013) 158:200–7. doi: 10.7326/0003-4819-158-3-201302050-00583
23. Gupta, SK, Viswanath, K, Thulasiraj, RD, Murthy, GV, Lamping, DL, Smith, SC, et al. The development of the Indian vision function questionnaire: field testing and psychometric evaluation. Br J Ophthalmol. (2005) 89:621–7. doi: 10.1136/bjo.2004.050732
24. Niikawa, H, Kawano, Y, Yamanaka, K, Okamura, T, Inagaki, H, Ito, K, et al. Reliability and validity of the Japanese version of a self-report (DEMQOL) and carer proxy (DEMQOL-PROXY) measure of health-related quality of life in people with dementia. Geriatr Gerontol Int. (2019) 19:487–91. doi: 10.1111/ggi.13646
25. Cummings, JL, Mega, M, Gray, K, Rosenberg-Thompson, S, Carusi, DA, and Gornbein, J. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology. (1994) 44:2308. doi: 10.1212/WNL.44.12.2308
Keywords: photobiomodulation, mild cognitive impairment, Alzheimer’s disease, decentralized trial, cognitive function
Citation: Yokoi Y, Inagawa T, Yamada Y, Matsui M, Tomizawa A and Noda T (2024) A randomized sham-controlled trial of transcranial and intranasal photobiomodulation in Japanese patients with mild cognitive impairment and mild dementia due to Alzheimer’s disease: a protocol. Front. Neurol. 15:1371284. doi: 10.3389/fneur.2024.1371284
Received: 16 January 2024; Accepted: 29 May 2024;
Published: 05 July 2024.
Edited by:
Luodan Yang, South China Normal University, ChinaReviewed by:
Denis E. Bragin, Lovelace Biomedical Research Institute, United StatesCopyright © 2024 Yokoi, Inagawa, Yamada, Matsui, Tomizawa and Noda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuma Yokoi, eS55b2tvaUBnbWFpbC5jb20=
†ORCID: Yuma Yokoi, https://orcid.org/0000-0002-4405-8322
Takuma Inagawa, https://orcid.org/0000-0001-6147-5008
Yuji Yamada, https://orcid.org/0000-0002-2169-1635
Takamasa Noda, https://orcid.org/0000-0003-0655-9350
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.