- 1Department of Radiotherapy, Medical University of Lublin, Lublin, Poland
- 2Brachytherapy Department, Saint John’s Cancer Center, Lublin, Poland
- 3Radiotherapy Department, Saint John’s Cancer Center, Lublin, Poland
- 4Department of Clinical Oncology and Chemotherapy, Medical University of Lublin, Lublin, Poland
- 5Hope Clinic Medical Center, Lublin, Poland
- 6Student Scientific Circle at the Department of Radiotherapy, Medical University of Lublin, Lublin, Poland
- 7Department of Medical Physics, Saint John’s Cancer Center, Lublin, Poland
- 8Department of Neurosurgery, Medical University of Lublin, Lublin, Poland
Introduction: Essential tremor (ET) is the most common movement disorder in adults, with an estimated incidence of up to 1% of the population and 5% of people older than 65 years of age. ET is manifested primarily by bilateral postural and kinetic tremor of the upper limbs with or without neurological symptoms and cognitive deficits. ET disrupts daily tasks and significantly lowers quality of life. Currently available medications alone are often insufficient to control severe symptoms. Several surgical treatment options are available, including stereotactic radiosurgery (SRS)—a minimally invasive treatment option aimed at relieving and controlling tremors.
Methods: We conducted a systematic review of the scientific literature on the use of SRS in the treatment of ET using PubMed, Scopus, Web of Science, Cochrane, ScienceDirect, and ClinicalTrials.gov registry and adhered to the PRISMA guidelines.
Results: The results obtained confirm the high efficacy and safety of the SRS procedure in treating drug-resistant intention tremor. The study results present high response rate reaching 80% and achievement of manual task improvement, lessening of the tremor and increase in the quality of life of the majority of the operated patients. The method also stands out for its favorable balance between efficiency and cost.
Disscusion: Stereotactic radiosurgery is a favourable, safe, efficient and cost-effective method in treatment of the essential tremor. Ongoing research is crucial to refine patient selection criteria for this procedure and further improve the effectiveness of the technique.
1 Introduction
Essential tremor (ET) is the most common movement disorder in adults, with an estimated incidence of up to 1% of the population and 5% of people older than 65 years of age (1). The Movement Disorder Society (MDS) defines ET as isolated tremor syndrome of bilateral upper limb action tremor for at least 3 years with or without tremor in other locations (e.g., head, voice, or lower limbs) and absence of other neurological signs, such as dystonia, ataxia, or parkinsonism (2). Familial, otherwise inherited, form of essential tremor constitutes of approximately 50% of cases. ET is manifested primarily by bilateral postural and kinetic tremor of the upper limbs with or without neurological symptoms, cognitive deficits, with some patients experiencing tremors of the head, neck or lower limbs, face, and vocal cords (3–6). Patients report difficulties with daily activities including eating, drinking, dressing, and writing. ET disrupts daily tasks and causes mental distress (1).
Patients diagnosed with ET usually initiate pharmacological therapy with primidone, propranolol, or topiramate. The safety profile, patients’ preferences, and confirmed efficacy are established as a first-line treatment in clinical practice. However, as the improvement rate of the pharmacotherapy is estimated for 50%, medications alone are often insufficient to control severe symptoms (7). To patients with severe spontaneous tremor, who do not achieve treatment response, several surgical options are available (8, 9).
Currently, there are four effective methods of surgical and radiotherapy treatment of patients with ET: deep brain stimulation (DBS), stereotactic radiosrugery (SRS), radiofrequency thalamotomy (RF), and focused ultrasound thalamotomy (FUS).
Stereotactic radiosurgery (SRS) is a minimally invasive treatment option aimed at relieving and controlling tremors. While the standard intervention remains deep brain stimulation, over the past 20 years, ventral intermediate thalamic nucleus (Vim), thalamotomy performed with SRS, has proven to be safe and effective (10). Radiosurgery becomes particularly important in patients with contraindications to surgery or who do not consent to surgical intervention (10). During SRS with GK Vim thalamotomy, patients are placed in a stereotactic frame under local anesthesia. Typically, target area is given a single central maximum dose of 130–152 Gy using a 4-mm collimator. Optimal planning minimizes the radiation exposure of the inner capsule. Modern SRS technique requires no drill holes or cranial electrode puncture and is a relatively non-invasive procedure (11).
2 Materials and methods
We conducted a systematic review according to the Population, Intervention, Control, Outcome, Study Design (PICOS) method, which is shown in Table 1. We followed the PRISMA 2020 (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement. We searched five databases which were PubMed, Scopus, Web of Science, Cochrane, ScienceDirect, and ClinicalTrials.gov registry. Additional evaluation was conducted via citation searching from selected articles. Two blinded authors independently performed searches using the keywords: (stereotactic radiosurgery or stereotactic radiotherapy or radiosurgery or SRS) AND essential tremor. We identified potential studies and exported them to a reference management program (Mendeley Desktop) for inclusion based on title and abstract and then the full article. The research involved an analysis of all studies published up to 30 November 2023. Although we considered ET cases only, in multiple studies, the only results available included groups of mixed tremor origin, such as Parkinson disease and multiple sclerosis tremor. These results were considered in our analysis and marked accordingly in the summary table below.
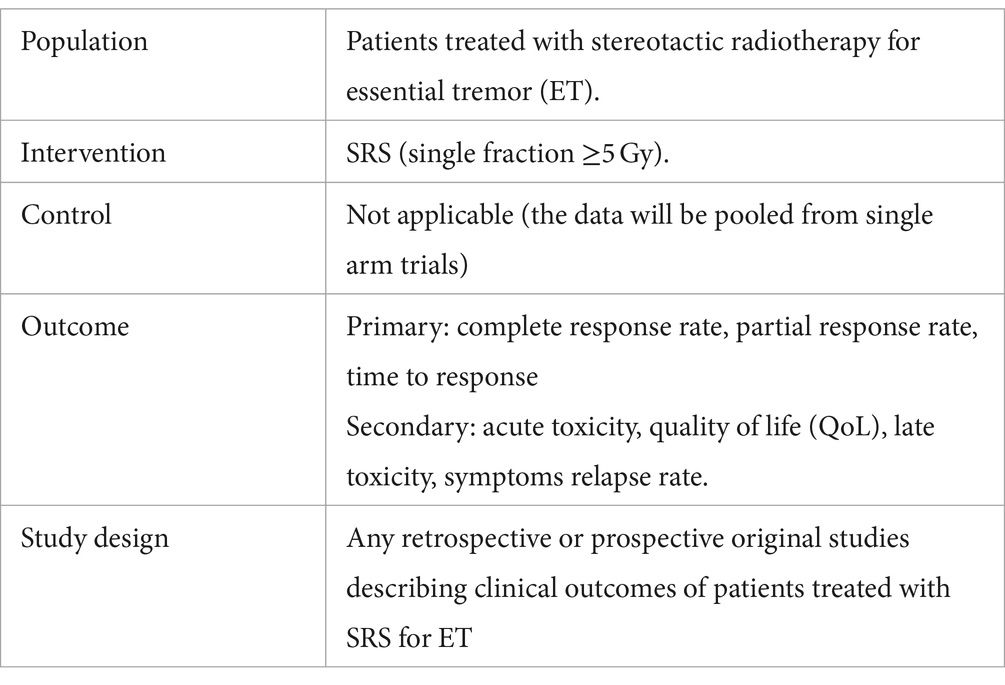
Table 1. Study design according to the population, intervention, control, outcome, and study design (PICOS) method.
2.1 Selection criteria
The inclusion criteria were as follows: (1) retrospective and prospective clinical trials with published results and (2) studies published in the English language.
Exclusion criteria were as follows: (1) lack of access to the full text of the manuscript, (2) studies without results and unclear results (3) case reports, (4) review studies, and (5) study protocols.
2.2 Data extraction
The extracted data consisted of the author, type of study, sample size, radiotherapy modality, target definition criteria, radiotherapy dose, dose constraints to organs at risk (OARs), time from SRS to response, complete response rates, partial response rate, early and late toxicity, and quality of life.
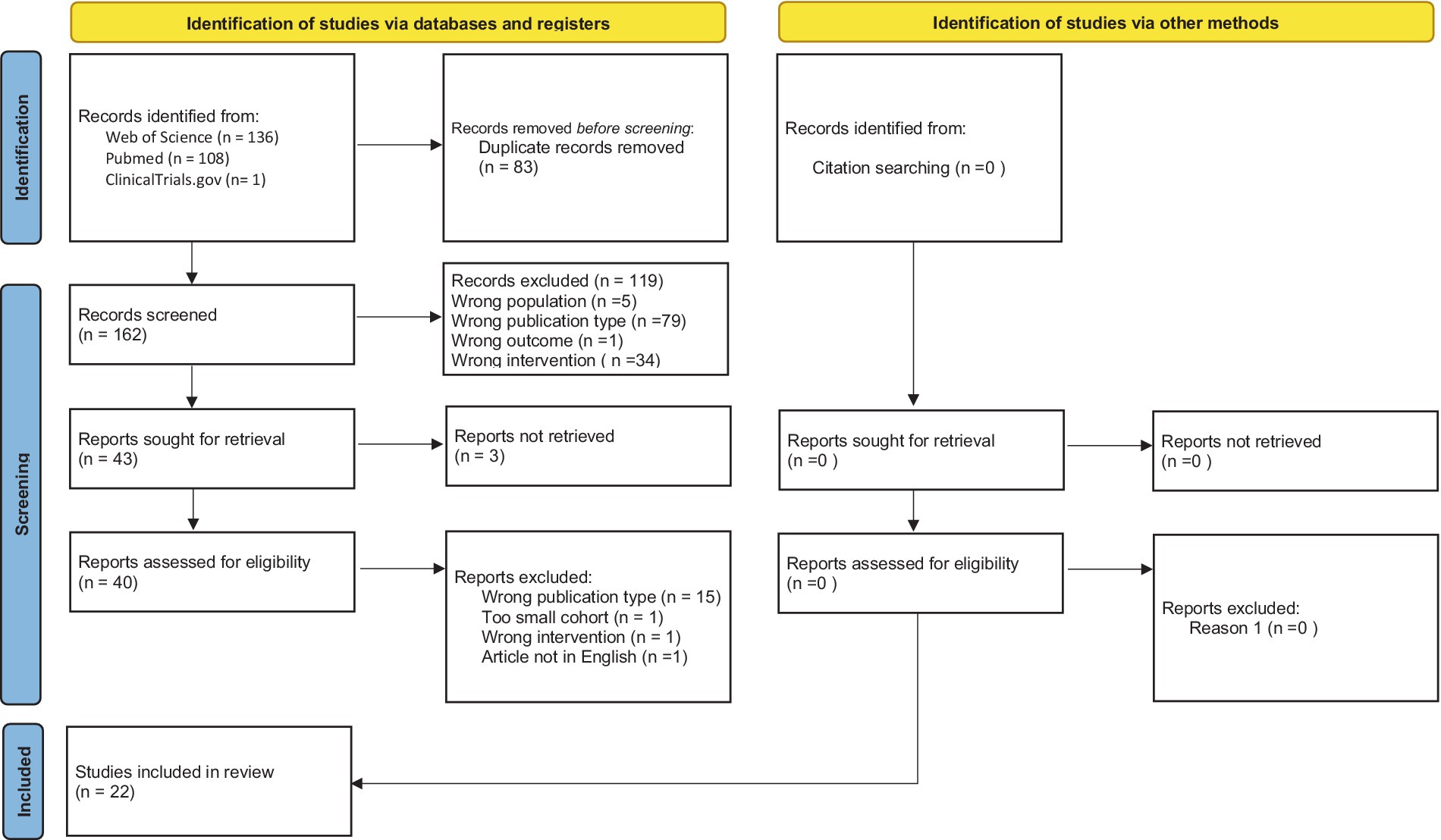
3 Results
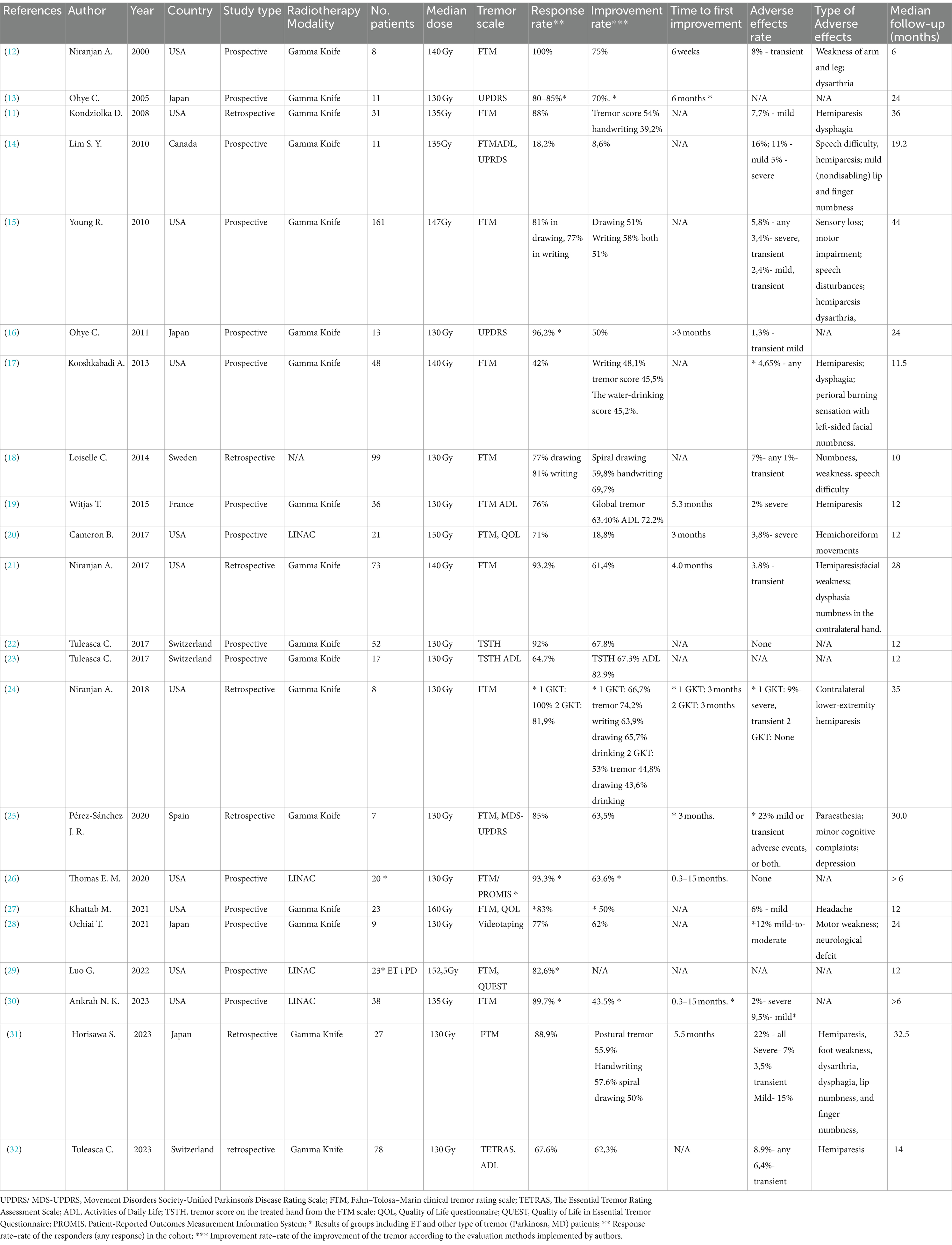
During searching databases and registry process, initially 245 studies were found (136—Web of Science, 108—PubMed, and 1—Clinicaltrials.gov). Before screening, we have deleted 83 duplicates. In the next step, we excluded titles and abstracts that did not follow the inclusion and exclusion criteria (119 articles). Full texts of qualified articles were analyzed, and selection was made after which 15 studies were excluded. Finally, 22 primary studies were included in systematic review. Figure 1 shows PRISMA 2020 flowchart with screening results. List and characteristics of included studies and their outcomes are shown in Table 2. In total, 15 included studies have prospective design and 7 studies are retrospective analyses.
3.1 Target definition
Anatomical visualization of the intermediate ventral nucleus (Vim) of the thalamus is not a trivial task. Therefore, for the targeting of the Vim, the indirect approach based on stereotactic measurements is widely used. Indirect targeting of the Vim for the procedure of radiosurgery is usually performed in several consecutive stages. The stereotactic coordinates for the target are set using the anterior commissure–posterior commissure (AC–PC) line as a reference of interest.
The authors of 22 publications that have been included in our analysis presented a (more or less detailed) description of target localizing for stereotactic radiosurgery. In most cases, the ventral intermediate nucleus target was localized using Guiot’s diagram: 25% of the AC–PC distance (plus 1-mm anterior to the PC), 2.5 mm above the AC–PC line, and 50% of the width of the third ventricle plus 11-mm lateral to the wall of the third ventricular wall (11, 12, 14, 24, 26). Chihiro et al. decided to mildly modify the target point taking into consideration that a more accurate localization of the thalamic nuclei can be specified using the ratio of the overall thalamic length instead of the conventional reference of posterior commissure. In this case, the target point was determined as 1-mm more medial and 1-mm more anterior, which should lead to more optimal target and, as a result, a better capsular and VO sparing (13).
3.2 Tremor evaluation criteria
Fahn–Tolosa–MarinClinical Rating Scale for Tremor (FTM) was the most common tool in the assessment of the severity of ET. This 0–4 point score system examines the tremor intensity and the disturbance of actions of writing, drawing spirals, and, optionally, drinking water or pouring liquids (12). The tremor score on the treated hand (TSTH) scale - a scoring system based on the FTM scale criteria - was used in two studies. Other scales used included Activities of Daily Living (ADL), Patient-Reported Outcome Measurement Information System (PROMIS), Essential Tremor Rating Assessment Scale (TETRAS), and Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS). Additionally, patients’ well-being was scored using Quality of Life in Essential Tremor Questionnaire (QUEST) and Quality of Life Questionnaire. Ochai replaced the rating scale system with videotaping of a tremor in the dominant upper limb with calculation of its frequency per 10 s [measured in decihertz (dHz)] as the assessment measure (17).
3.3 Radiation dose and modality
The majority of the presented studies performed either unilateral or bilateral SRS thalamotomy with Gamma Knife. In four trials, SRS was performed on LINAC (20, 26, 29, 30). One trial did not specify the modality used for SRS thalamotomy (18). Median dose used was 130 Gy in single fraction, although the dose range spread between 130 and 160 Gy. The study by Kondziolka and Lims relied on the 130–140 Gy dose range (11, 14), while 130–150 Gy dose was used in two other studies (12, 21). Other authors of included manuscripts used 140–160 Gy (20), 141–152 Gy (15), 145–160 Gy (29), 156–160 Gy (27), and 140 Gy (17).
In most cases, only singular, unilateral procedure is needed to achieve the therapeutic effect. Only two publications mention bilateral procedure as a part of the performed treatment (13, 15).
3.4 Response to SRS
The results in considered publications in a vast majority indicate significant improvement, estimating 50–60% in the postural tremor score, handwriting, drawing, and drinking. Mean improvement rate for tremor, writing, drawing, and drinking constitute 64, 58, 52, and 52%, respectively. The patients’ quality of life is increased in comparison to the pre-operation scores—57% for the ET group and 84.6% for the mixed group (25, 27). The results of the ET and Parkinson patients represent similar results (28).
Among patients with essential tremor, most studies report extremely high response rate, reaching excellent results. Median response rate (including sub-categories of writing and drawing for the same patients) constitutes of 81.8%, meanwhile median response constitutes 81%, which is considered as good is. The maximum rate of tremor reduction was depicted in the study by Niranjan et al., reaching 100% of complete tremor reduction (24). Only two authors report on the lack of response in patients, rating this subgroup as 11.1 and 15.1% (16, 31). The state of worsened intervention than before intervention was only mentioned in the study by Ohye et al. at a rate of 3.8% (16). However, the analyzed cohort was mixed, consisting mostly of patients with Parkinson disease who are more prone to developing more severe symptoms after surgery (16).
The 6-month duration was pinpointed by several authors, reaching between 25.2 and 59.3% of tremor score improvement (13, 15, 16, 27, 28). The median improvement rate at 6-month follow-up reached 49%.
During follow-up, no additional interventions were required in most of the cases due to achieved satisfactory tremor control. In the publications by Young, Loiselle, and Niranjan, secondary intervention was needed after tremor recurrence (15, 18, 24). In the study by Lim et al., two patients underwent open surgery due to treatment failure 31 and 24 months after the initial procedure (14). Young et al. reported that nine patients needed further treatment due to increase in tremor symptoms after SRS. Those patients underwent DBS or RFT procedures due to the recurrence of symptoms (15).
3.5 Time to response
Median onset of the tremor decrease falls within 3 months, spanning from 6 weeks to 6 months. The post-operative 6-month mark has been pinpointed by Khattab et al. as the period of the maximum benefit for the patient, although the tremor reduction was found to increase with time in other studies (16, 27, 28).
3.6 Follow-up
Follow-up periods varied depending on the study type, if it was either prospective, planned, clinical trial, or retrospective. The time frame varied between mean 6 months and mean 44 months, with individual scores reaching up to 152 months (21). The mean follow-up period of all publications is 14 months.
3.7 Adverse effects and safety profile
The rate of adverse effects (AEs) in publications varies between 0 and 23%, with median of 7.85%. Among all analyzed studies, out of 814 patients evaluated, only 58 experienced any adverse effects (7,12%). Most of the reported adverse effects are mild and transient. Severe AEs were described in two cases. They presented as complete steroid-irresponsive hemiparesis and chronic encapsulated expanding hematoma (CEEH), resulting in dysarthria, dysphagia, and hemibody numbness. Severe dysphagia in second patient lead to death because of aspiration pneumonia 60 months after GKT (24, 31). Limb weakness, numbness, mild and transient hemiparesis and speech disturbances including dysarthria belonged to the most frequent adverse effects reported in the post-SRS patients [12, 14, 15-, 22, 25, 29, 31–33]. Other reported ailment include headaches, depression, minor cognitive deficits, sensory loss, and hemichoreiform movements (20, 25, 27, 28). Tuleasca et al. reported a correlation between BED and the severity of adverse effects (32). Permanent adverse effects were found in 15 patients including contralateral weakness, numbness, and speech disturbances. In 6 of those cases, symptoms improved after 12 to18 months. Two cases were strictly sensory in nature and of no functional consequence. One was a cause of CEEH resulted in patient death (15, 18, 31). The detailed data about AE are presented in Table 2.
4 Discussion
4.1 Comparison of the effectiveness of SRS, DBS, and MRgFUS
DBS is currently the most commonly used surgical method for treating ET, yet its invasive nature remains a significant drawback. In contrast, FUS targeting the Vim offers similar efficacy without being invasive, which becomes particularly relevant, considering the risks of brain structure damage and complications associated with invasive procedures. While FUS, such as RF and GK thalamotomy, also creates permanent lesions, its non-invasive approach may be advantageous in managing bilateral symptoms, potentially reducing the risk of complications linked to invasive treatments. Since bilateral thalamotomy is generally avoided, direct comparisons of DBS with lesion treatment procedures are more relevant to unilateral treatments. These procedures appear to have similar improvement rates, but a formal comparison is needed. While patients often adapt to DBS therapy, there are reports of early relapses following FUS surgery. The benefits of SRS are not fully predictable, as many effects develop over months. Each procedure has its own advantages and limitations, which must be considered for optimal ET treatment results (33). These findings corroborate those of other studies (34, 35).
Despite comparisons between the efficacy and cost-effectiveness of SRS and FUS, it seems crucial to consider all the biological effects of each procedure deeply, especially in the context of combined treatment. Researchers suggest that these techniques are not necessarily competing. FUS, combined with radiotherapy, particularly in malignant disease treatment, is a common scenario. Here, FUS can initially remove the main tumor mass for immediate symptom relief, followed by irradiation of the surrounding BED to reduce local and regional recurrence. Conversely, initial irradiation can damage the ability of the cells to reproduce, followed by FUS to reduce the main tumor volume (36).
A study comparing the cost-effectiveness and efficiency of the different surgical methods of tremor treatment found SRS comparably cost-effective to MRgFUS and less expensive than DBS. However, if MRgFUS is less effective or more costly than estimated, SRS may be more cost-effective. This likelihood increases considering the longer duration and higher skill requirement for MRgFUS, potentially making SRS a more prevalent treatment for drug-resistant intractable tremor (37) (see Table 3).
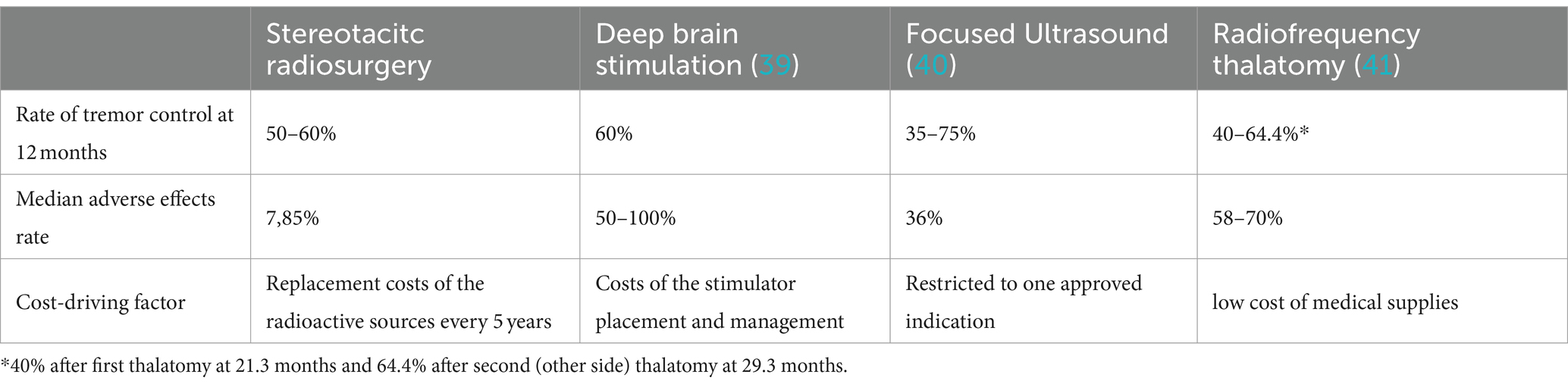
Table 3. Comparison of the efficacy, safety, and cost-effectiveness of the different thalamotomy methods.
SRS appears to be more advantageous than DBS procedure in the initial ET treatment. DBS seems to bear a great potential in case of unsuccessful SRS thalamotomy. DBS performed on the patients previously treated with radiosurgery indicate potential and effectiveness of such treatment modality after recurrence of symptoms. In the study by Lim et al., one patient with PD tremor remained unresponsive to the SRS and underwent DBS on the same side. During 4 months of follow-up, patient demonstrated 80% tremor reduction (14). Similarly Tuleasca et al. report a clinical case of a patient with ET treated initially with SRS and followed-up with bilateral DBS after the SRS failure. The initial response to SRS within 6 months was excellent, although patient further relapsed after 20 months. DBS procedure resulted in immediate and complete bilateral clinical alleviation lasting for 31 months. The clinical evidence and MRI examinations suggest the lasting effect of the SRS on the structure and functionality of the affected areas. The post-SRS reorganization initiates the potential for better DBS response (38).
4.2 Pathophysiology and response prediction
Tuleasca et al. have utilized the results from ET treatment with SRS to explore the pathophysiology of diseases in numerous publications. They propose that components of the visual system might significantly contribute to tremor onset (varying for the finger, hand, and head tremor) and its inhibition following interventions such as SRS-T (22, 23, 42–44). In 2017 study, Tuleasca indicated right visual association area as the tremor alleviation predictor with Brodmann area (BA) 18 as the only statistically significant cluster. High pretherapeutic gray matter density also correlated with better TSTH improvement (22).
The majority of the research do not associate any form of response pattern or radiological imagining results with the clinical outcome (13, 16, 19, 23, 26, 28, 31). Linear contrasting of the border between the thalamus and the internal capsule adjacent to the lesion site was found in patients with higher symptom improvement rate in comparison to the other individuals (28). Most hyperresponders are characterized by more pronounced lesions, often times accompanied by edema. Clinical outcome of this imagining is in most cases linked with severe adverse effects, including hemiparesis (11, 12, 14, 19, 21, 24, 28, 29, 31).
Luo et al. research found the radiation dose coverage to 0.1 cm3 of the autocontoured VIM structure as significantly higher in responders than nonresponders and a trendfor superior coverage of the autocontoured VIM at 20-Gy isodose level in the responders versus nonresponders (29). The center of the lesion was found 0.5 ± 0.1 mm laterally to the VIM in responders in comparison to 1.1 ± 0.5 mm in non-responders, associating patient’s response with the medial–lateral position (p < 0,019) (29). This finding suggests that the optimal isocenter position for clinical response is 0.7–0.9 mm lateral to the geometric center of the Vim with automatic contouring (29).
Diffusion tensor imaging fiber tracking constitutes a new potential treatment isocenter localization technique, enabling localization of the dentato-rubro-thalamic tract fibers, involved in the tremor etiopathology, which potentially intersects with the target. This solution is highly promising for developing patients-specific target network; however, it is greatly limited due to the equipment and software availability (29, 45).
4.3 BED in the SRS treatment
There is ongoing discussion among professionals about what dose should be administered to achieve a high clinical response rate and, at same time, low rates of radiation side effects. Modern research shows that there is a significant correlation involving dose-administered and BED (biologically effective dose) with rates of response and potential ARE. It was shown that the α/β ratio for normal brain is between 2 and 3 Gy (46, 47). Tuleasca et al. made an assumption that the α/β ratio for normal brain is 2.47 Gy. In their study, it was shown that with BED between 4,300 and 4,500 Gy2.47, optimal response rates limiting ARE can be achieved (32).
5 Conclusion
The results obtained confirm the high efficacy and safety of the SRS procedure in treating drug-resistant intention tremor. A significant proportion of patients experienced a clinically meaningful reduction in tremor, following SRS with the newly developed SRS GK technology. After SRS, a considerable number of patients showed improvements in activities such as tremor, writing, drawing, and drinking. Subsequent studies underscore the enhanced safety of SRS at certain therapeutic doses (up to 140 Gy), reflecting progress compared with earlier years when higher doses (160–180 Gy) were associated with more severe complications. The method also stands out for its favorable balance of efficiency and cost, making it a competitive alternative to MRgFUS and DBS. Ongoing research is crucial to refine patient selection criteria for this procedure and further improve the effectiveness of the technique.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. KS: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SS: Conceptualization, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. ARu: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. NK: Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. JK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft. ARo: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – review & editing. WK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – review & editing. MK: Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. IB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. SM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Louis, ED, and Ferreira, JJ. How common is the Most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. (2010) 25:534–41. doi: 10.1002/Mds.22838
2. Bhatia, KP, Bain, P, Bajaj, N, Elble, RJ, Hallett, M, Louis, ED, et al. Deuschl G; tremor task force of the International Parkinson and Movement Disorder Society. Consensus statement on the classification of tremors. From the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord. (2018) 33:75–87. doi: 10.1002/mds.27121
3. Chunling, W, and Zheng, X. Review on clinical update of essential tremor. Neurol Sci. (2016) 37:495–502. doi: 10.1007/S10072-015-2380-1
4. Chandran, V, and Pal, PK. Essential tremor: beyond the motor features. Parkinsonism Relat Disord. (2012) 18:407–13. doi: 10.1016/J.Parkreldis.2011.12.003
5. Jhunjhunwala, K, and Pal, PK. The non-motor features of essential tremor: a primary disease feature or just a secondary phenomenon? Tremor Other Hyperkinet Mov. (2014) 4:255. doi: 10.5334/Tohm.230
6. Louis, ED. Non-motor symptoms in essential tremor: a review of the current data and state of the field. Parkinsonism Relat Disord. (2016) 22:S115–8. doi: 10.1016/J.Parkreldis.2015.08.034
7. Hedera, P, Cibulčík, F, and Davis, TL. Pharmacotherapy of essential tremor. J Cent Nerv Syst Dis. (2013) 5:43–55. doi: 10.4137/JCNSD.S6561
8. Elble, RJ, Shih, L, and Cozzens, JW. Surgical treatments for essential tremor. Expert Rev Neurother. (2018) 18:303–21. doi: 10.1080/14737175.2018.1445526
9. Picillo, M, and Fasano, A. Recent advances in essential tremor: surgical treatment. Parkinsonism Relat Disord. (2016) 22:S171–5. doi: 10.1016/J.Parkreldis.2015.09.012
10. Elaimy, AL, Demakas, JJ, Arthurs, BJ, Cooke, BS, Fairbanks, RK, Lamoreaux, WT, et al. Gamma knife radiosurgery for essential tremor: a case report and review of the literature. World J Surg Oncol. (2010) 8:1–7. doi: 10.1186/1477-7819-8-20
11. Kondziolka, D, Ong, JG, Lee, JYK, Moore, RY, Flickinger, JC, and Lunsford, LD. Gamma knife Thalamotomy for essential tremor. J Neurosurg. (2008) 108:111–7. doi: 10.3171/Jns/2008/108/01/0111
12. Niranjan, A, Kondziolka, D, Baser, S, Heyman, R, and Lunsford, LD. Functional outcomes after gamma knife thalamotomy for essential tremor and MS-related tremor. Neurology. (2000) 55:443–6. doi: 10.1212/wnl.55.3.443
13. Ohye, C, Shibazaki, T, and Sato, S. Gamma knife thalamotomy for movement disorders: evaluation of the thalamic lesion and clinical results. J Neurosurg. (2005) 102:234–40. doi: 10.3171/jns.2005.102.s_supplement.0234
14. Lim, SY, Hodaie, M, Fallis, M, Poon, YY, Mazzella, F, and Moro, E. Gamma knife thalamotomy for disabling tremor: a blinded evaluation. Arch Neurol. (2010) 67:584–8. doi: 10.1001/archneurol.2010.69
15. Young, RF, Li, F, Vermeulen, S, and Meier, R. Gamma knife thalamotomy for treatment of essential tremor: long-term results. J Neurosurg. (2010) 112:1311–7. doi: 10.3171/2009.10.JNS09332
16. Ohye, C, Higuchi, Y, Shibazaki, T, Hashimoto, T, Koyama, T, Hirai, T, et al. Gamma knife thalamotomy for Parkinson disease and essential tremor: a prospective multicenter study. Neurosurgery. (2012) 70:526–35; discussion 535-6. doi: 10.1227/NEU.0b013e3182350893
17. Kooshkabadi, A, Lunsford, LD, Tonetti, D, Flickinger, JC, and Kondziolka, D. Gamma knife thalamotomy for tremor in the magnetic resonance imaging era. J Neurosurg. (2013) 118:713–8. doi: 10.3171/2013.1.JNS121111
18. Loiselle, C, Vermeulen, S, Meier, R, Li, F, and Young, R. Treatment of essential tremor with stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. (2014) 90:S167. doi: 10.1016/j.ijrobp.2014.05.668
19. Witjas, T, Carron, R, Krack, P, Eusebio, A, Vaugoyeau, M, Hariz, M, et al. A prospective single-blind study of gamma knife thalamotomy for tremor. Neurology. (2015) 85:1562–8. doi: 10.1212/wnl.0000000000002087
20. Cameron, BD, Wang, L, Cmelak, AJ, Luo, G, Yu, H, Hedera, P, et al. A prospective, observational trial of LINAC-based stereotactic radiosurgery for essential tremor and parkinsonian tremor. Int J Radiat Oncol Biol Phys. (2017) 99:S102. doi: 10.1016/j.ijrobp.2017.06.243
21. Niranjan, A, Ss, R, Kooshkabadi, A, Monaco, E 3rd, Jc, F, and Lunsford, L. Stereotactic radiosurgery for essential tremor: retrospective analysis of a 19-year experience. Mov Disord. (2017) 32:769–77. doi: 10.1002/Mds.26925
22. Tuleasca, C, Witjas, T, Van De Ville, D, Najdenovska, E, Verger, A, Girard, N, et al. Right Brodmann area 18 predicts tremor arrest after vim radiosurgery: a voxel-based morphometry study. Acta Neurochir. (2018) 160:603–9. doi: 10.1007/S00701-017-3391-X
23. Tuleasca, C, Najdenovska, E, Régis, J, Witjas, T, Girard, N, Champoudry, J, et al. Clinical response to Vim's thalamic stereotactic radiosurgery for essential tremor is associated with distinctive functional connectivity patterns. Acta Neurochir. (2018) 160:611–24. doi: 10.1007/S00701-017-3456-X
24. Niranjan, A, Ss, R, Ea, M, Jc, F, and Lunsford, L. Is staged bilateral thalamic radiosurgery An option for otherwise surgically ineligible patients with medically refractory bilateral tremor? J Neurosurg. (2018) 128:617–26. doi: 10.3171/2016.11.Jns162044
25. Pérez-Sánchez, JR, Martínez-Álvarez, R, Martínez Moreno, NE, Torres Diaz, C, Rey, G, Pareés, I, et al. Gamma knife® stereotactic radiosurgery as a treatment for essential and parkinsonian tremor: long-term experience. Neurologia. (2020) S0213-4853:30217–6. English, Spanish. doi: 10.1016/j.nrl.2020.05.014
26. Thomas, EM, Walker, H, Middlebrooks, EH, Fiveash, JB, Nicholas, A, Popple, RA, et al. Frameless Mlc-based Radiosurgical Thalamotomies on the modern linear accelerator platform—prospective phase I/ii clinical trial results. Int J Radiat Oncol Biol Phys. (2021) 111:S100. doi: 10.1016/j.ijrobp.2021.07.232
27. Khattab, M, Aj, C, Sherry, A, Luo, G, Wang, L, Yu, H, et al. Noninvasive Thalamotomy for refractory tremor by frameless radiosurgery. Int J Radiat Oncol Biol Phys. (2022) 112:121–30. doi: 10.1016/J.Ijrobp.2021.08.021
28. Ochiai, T. Gamma knife Thalamotomy for a medically refractory tremors: longitudinal evaluation of clinical effects and MRI response patterns. Acta Neurochir Suppl. (2021) 128:127–32. doi: 10.1007/978-3-030-69217-9_14
29. Luo, G, Cameron, BD, Wang, L, Yu, H, Neimat, JS, Hedera, P, et al. Targeting for stereotactic Radiosurgical Thalamotomy based on Tremortreatment response. J Neurosurg. (2021) 136:1387–94. doi: 10.3171/2021.7.Jns21160
30. Ankrah, NK, Thomas, E, Bredel, M, Middlebrooks, E, Walker, H, Fiveash, JB, et al. Frameless LINAC-based stereotactic radiosurgery is safe and effective for essential and parkinsonian tremor. Int J Radiat Oncol Biol Phys. (2023) 117:S173. doi: 10.1016/j.ijrobp.2023.06.640
31. Horisawa, S, Hayashi, M, Tamura, N, Kohara, K, Nonaka, T, Hanada, T, et al. Gamma knife Thalamotomy for essential tremor: a retrospective analysis. World Neurosurg. (2023) 175:e90–6. doi: 10.1016/j.wneu.2023.03.033
32. Tuleasca, C, Carey, G, Barriol, R, Touzet, G, Dubus, F, Luc, D, et al. Impact of biologically effective dose on tremor decrease after stereotactic radiosurgical thalamotomy for essential tremor: a retrospective longitudinal analysis. Neurosurg Rev. (2024) 47:73. doi: 10.1007/s10143-024-02296-1
33. Kl, W, Ren, Q, Chiu, S, Patel, B, Meng Fg, HW, and Aw, S. Deep brain stimulation and other surgical modalities for the management of essential tremor. Expert Rev Med Devices. (2020) 17:817–33. doi: 10.1080/17434440.2020.1806709
34. Rf, D, Dj, L, De Vloo, P, Fomenko, A, Hamani, C, Hodaie, M, et al. Outcomes from stereotactic surgery for essential tremor. J Neurol Neurosurg Psychiatry. (2019) 90:474–82. doi: 10.1136/Jnnp-2018-318240
35. Gr, G, Maugeri, R, Ge, U, Paolini, F, Bonosi, L, Meccio, F, et al. Dbs, Tcmrgfus, and gamma knife radiosurgery for the treatment of essential tremor: a Systematicreview on techniques, indications, and current applications. J Neurosurg Sci. (2022) 66:476–84. doi: 10.23736/S0390-5616.22.05524-2
36. Schlesinger, D, Lee, M, Ter Haar, G, Sela, B, Eames, M, Snell, J, et al. Aubry Jf.Equivalence of cell survival data for radiation dose and thermal dose in ablative treatments: Analysisapplied to essential tremor Thalamotomy by focused ultrasound and gamma knife. Int J Hyperth. (2017) 33:401–10. doi: 10.1080/02656736.2016.1278281
37. Vk, R, Jj, P, Ts, H, Ve, S, Kb, P, Wintermark, M, et al. Cost-effectiveness of focused ultrasound, radiosurgery, and Dbs for essential tremor. Mov Disord. (2017) 32:1165–73. doi: 10.1002/Mds.26997
38. Tuleasca, C, Pralong, E, Najdenovska, E, Cuadra, MB, Marques, JRF, Vingerhoets, F, et al. Deep brain stimulation after previous gamma knife thalamotomy of the vim for essential tremor is feasible! Clinical, electrophysiological, and radiological findings. Acta Neurochir. (2017) 159:1371–3. doi: 10.1007/s00701-017-3227-8
39. Koller, WC, Lyons, KE, Wilkinson, SB, Troster, AI, and Pahwa, R. Long-term safety and efficacy of unilateral deep brain stimulation of the thalamus in essential tremor. Mov Disord. (2001) 16:464–8. doi: 10.1002/mds.1089
40. Iorio-Morin, C, and Hodaie, M. Lozano AM adoption of focused ultrasound thalamotomy for essential tremor: why so much fuss about FUS? J Neurol Neurosurg Psychiatry. (2021) 92:549–54. doi: 10.1136/jnnp-2020-324061
41. Horisawa, S, Nonaka, T, Kohara, K, Mochizuki, T, and Kawamata, T. Takaomi Taira; bilateral radiofrequency ventral intermediate Thalamotomy for essential tremor. Stereotact Funct Neurosurg. (2023) 101:30–40. doi: 10.1159/000528825
42. Tuleasca, C, Régis, J, Najdenovska, E, Witjas, T, Girard, N, Champoudry, J, et al. Pretherapeutic functional imaging allows prediction of head tremor arrest after Thalamotomy for essential tremor: the role of altered interconnectivity between Thalamolimbic and supplementary motor circuits. World Neurosurg. (2018) 112:E479–88. doi: 10.1016/J.Wneu.2018.01.063
43. Tuleasca, C, Taw, B, Régis, J, Najdenovska, E, Witjas, T, Girard, N, et al. Normalization of aberrant Pretherapeutic dynamic functional connectivity of Extrastriate visual system in patients who underwent Thalamotomy with stereotactic radiosurgery for essential tremor: a resting-state functional Mri study. J Neurosurg. (2019) 132:1792–801. doi: 10.3171/2019.2.Jns183454
44. Tuleasca, C, Najdenovska, E, Régis, J, Witjas, T, Girard, N, Champoudry, J, et al. Pretherapeutic motor thalamus resting-state functional connectivity with visual areas predicts tremor arrest after Thalamotomy for essential tremor: tracing the Cerebello-Thalamo-Visuo-motor network. World Neurosurg. (2018) 117:E438–49. doi: 10.1016/J.Wneu.2018.06.049
45. Middlebrooks, E, Ra, P, Greco, E, Okromelidze, L, Hc, W, Da, L, et al. Connectomic basis for tremor control in stereotactic Radiosurgical Thalamotomy. Ajnr Am. J Neuroradiol. (2023) 44:157–64. doi: 10.3174/Ajnr.A7778
46. Hall, EJ, and Brenner, DJ. The radiobiology of radiosurgery: rationale for different treatment regimes for AVMs and malignancies. Int J Radiat Oncol Biol Phys. (1993) 25:381–5. doi: 10.1016/0360-3016(93)90367-5
Keywords: radiosurgery, tremor, essential tremor, tremor treatment, stereotactic radiotherapy
Citation: Bilski M, Szklener K, Szklener S, Rudzińska A, Kluz N, Klas J, Rodzajewska A, Kuryło W, Korga M, Baranowska I and Mańdziuk S (2024) Stereotactic radiosurgery in the treatment of essential tremor – a systematic review. Front. Neurol. 15:1370091. doi: 10.3389/fneur.2024.1370091
Edited by:
Alexandre Gironell, Universitat Autònoma de Barcelona, SpainReviewed by:
Angel Sesar, Complejo Hospitalario Universitario de Santiago, SpainConstantin Tuleasca, Centre Hospitalier Universitaire Vaudois (CHUV), Switzerland
Félix Javier Jiménez-Jiménez, Hospital Universitario del Sureste, Spain
Copyright © 2024 Bilski, Szklener, Szklener, Rudzińska, Kluz, Klas, Rodzajewska, Kuryło, Korga, Baranowska and Mańdziuk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Rudzińska, YW5uYS5ydWR6aW5za2EwNUBnbWFpbC5jb20=
 Mateusz Bilski
Mateusz Bilski Katarzyna Szklener
Katarzyna Szklener Sebastian Szklener5
Sebastian Szklener5 Anna Rudzińska
Anna Rudzińska Natalia Kluz
Natalia Kluz Izabela Baranowska
Izabela Baranowska