- 1Department of Neurosurgery, Clinical Hospital of Porto Alegre, Porto Alegre, Brazil
- 2Neuro-Oncology Post-Graduation, Sirio-Libanes Hospital, São Paulo, Brazil
- 3Department of Neurosurgery, Sirio-Libanes Hospital, São Paulo, Brazil
- 4Department of Neurosurgery, Cristo Redentor Hospital, Porto Alegre, Brazil
- 5Laboratory of Neuroscience, Sirio-Libanes Hospital, São Paulo, Brazil
Introduction: The Neurological Assessment for Neuro-Oncology (NANO) scale was elaborated to assess neurologic function in integration with radiological criteria to evaluate neuro-oncological patients in clinical setting and enable the standardization of neurological assessment in clinical trials. The objective of this study is the translation to Brazilian Portuguese and transcultural adaptation of NANO scale in patients with the diagnosis of glioblastoma, brain metastasis and low-grade glioma.
Methods: Patients with diagnosis of glioblastoma, brain metastasis, and low-grade glioma were prospectively evaluated between July 2019 and July 2021. The process of translating and cross-culturally adapting the NANO scale included: translation from English to Portuguese, synthesis and initial revision by an expert committee, back-translation from Portuguese to English, a second revision by the expert committee, and the application of the NANO scale. Regarding the reliability of the NANO scale, Cronbach’s alpha was employed to measure the internal consistency of all scale items and assess the impact of item deletion. Additionally, Spearman’s correlation test was used to evaluate the convergent validity between the NANO scale and Karnofsky Performance Scale (KPS).
Results: One hundred and seventy-four patients were evaluated. A statistically significant inverse relation (p < 0.001) between KPS and NANO scale was founded. The Cronbach’s alpha values founded for NANO scale were 0.803 for glioblastoma, 0.643 for brain metastasis, and 0.482 for low grade glioma.
Discussion: The NANO scale Brazilian Portuguese version proves to be reproducible and valid to evaluate neuro-oncological patients with glioblastoma and brain metastasis, presenting a strong correlation with KPS scale. Further studies are warranted to assess the validity and reliability of the scale in patients diagnosed with low-grade glioma.
Introduction
Central nervous system (CNS) tumors are responsible for 1.5% of all cancers and for 2.4% of all cancer deaths annually (1). In Brazil, the latest estimative predicted around 11,000 new cases of primary CNS tumors for 2020 (2). In the last years, a constant increase in studies evaluating new treatments in neuro-oncology, such as immunotherapy and target therapy, showed the necessity of a more precise response assessment in clinical trials and clinical practice. However, the best way to establish the evaluation criteria is not well defined in literature. While overall survival is considered an objective parameter and a gold standard in most studies, this outcome is limited to evaluate response assessment in diseases with prolonged course, as observed in low grade gliomas or meningiomas. Therefore, it is essential the implementation of response criteria that include both clinical and radiological findings, to promote a more complete evaluation (3, 4).
Even with the well stablished radiological response evaluation proposed by RANO group (4–7), the neurological outcome incorporated is simplified as “better,” “stable” or “worse.” The usual measurement of functional and clinical outcome in the oncological clinical practice and trials is by the Karnofsky Performance Status (KPS) (8) and Eastern Cooperative Oncology Group Performance Status (ECOG) (9). Despite the easy applicability in clinical trials and practice, the main limitation of these instruments in the neuro-oncologic patient is the inefficacy to translate the neurological status even with its direct influence in functional performance. Additionally, functional deterioration does not always signify disease progression. Prolonged use of corticosteroids, chemotherapy, immunotherapy, radiotherapy, infections, pre-existing comorbidities, and psychiatric conditions are examples of potential confounders in the functional evaluation of neuro-oncologic patients.
In 2017, a multidisciplinary international committee composed by neuro-oncology experts developed the Neurological Assessment in Neuro-Oncology (NANO) scale and evaluates neurological function in neuro-oncologic patients. The primary goal of NANO is to define clinical parameters objectively, to measure clinical response and disease progression related to tumoral activity. This scale was developed to be a simple, easy and fast-to-use tool that can be employed by any healthcare professional. To maintain its objectivity, the evaluation is based in direct observation rather relying on clinical history or reported symptoms (10).
Instruments that quantify and stratifies variables are essential to reproduce new data and aggregate more substantial information to evaluate outcomes and, finally, guide therapeutical decisions. Therefore, the translation and validation support the use of an instrument across different languages and cultures, standardizing the evaluation worldwide. The objective of this study is to translate and validate of the NANO scale to Brazilian Portuguese.
Methods
Patients diagnosed with brain tumors (presumably glioblastoma, low-grade glioma and brain metastasis) were prospectively evaluated between July 2019 and July 2021 at Sirio-Libanes Hospital, including both outpatient clinics and hospital admissions. Eligible patients were 18 years of or older with the previously mentioned CNS tumors, providing informed consent.
This study protocol was approved by the Institutional Review Board (ID 1294). Each patient underwent a neurological evaluation by a senior author’s staff neurosurgeon. The translation process of NANO scale included the following steps: translation from English to Portuguese, synthesis and initial revision by an expert committee composed by the senior authors (M.V.C.M, M.C.V., D.A.G., J.A.N.J., C.F., and G.F.G.), back-translation from Portuguese to English, a second revision by the expert committee, and the application of the NANO scale.
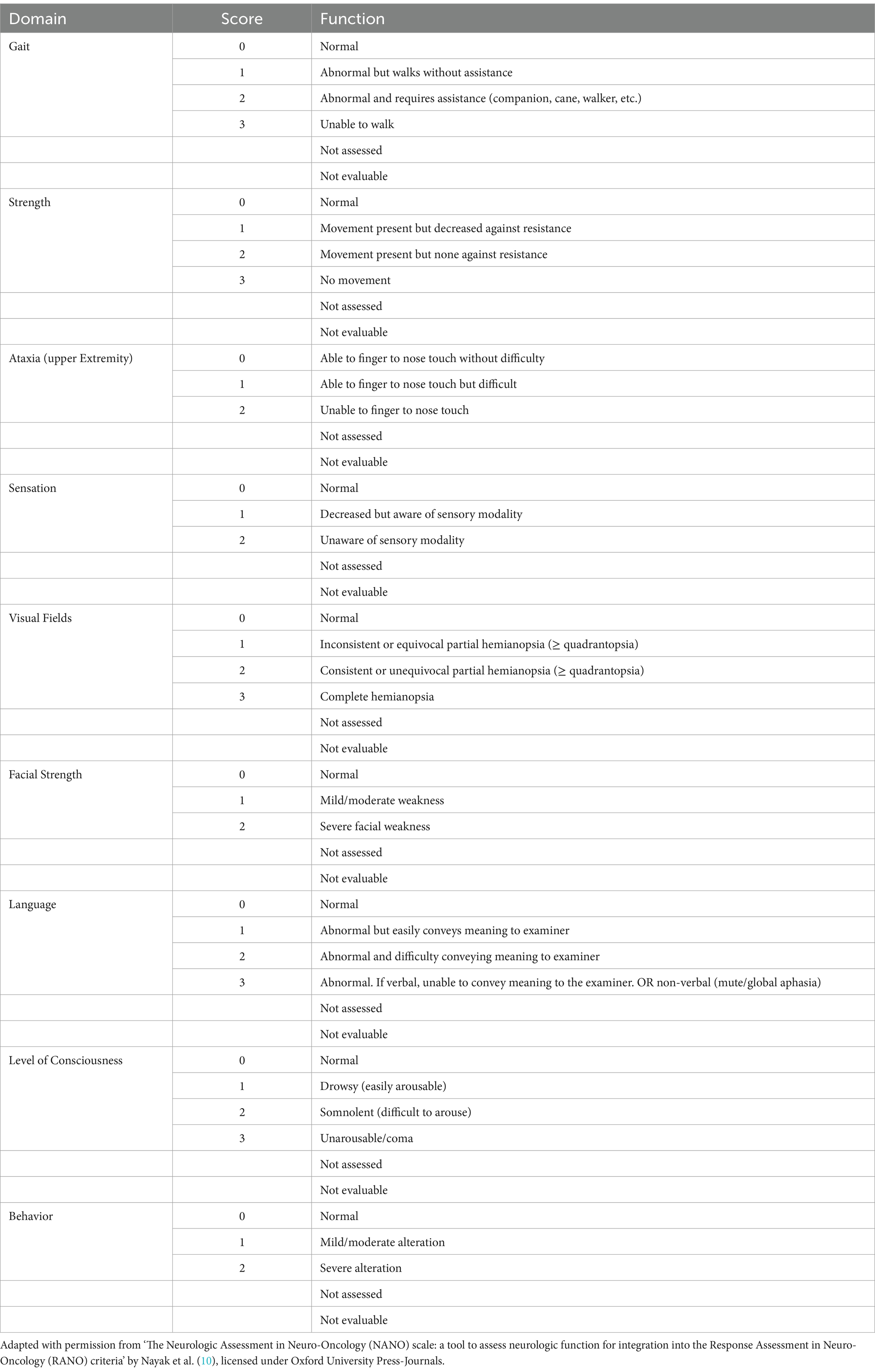
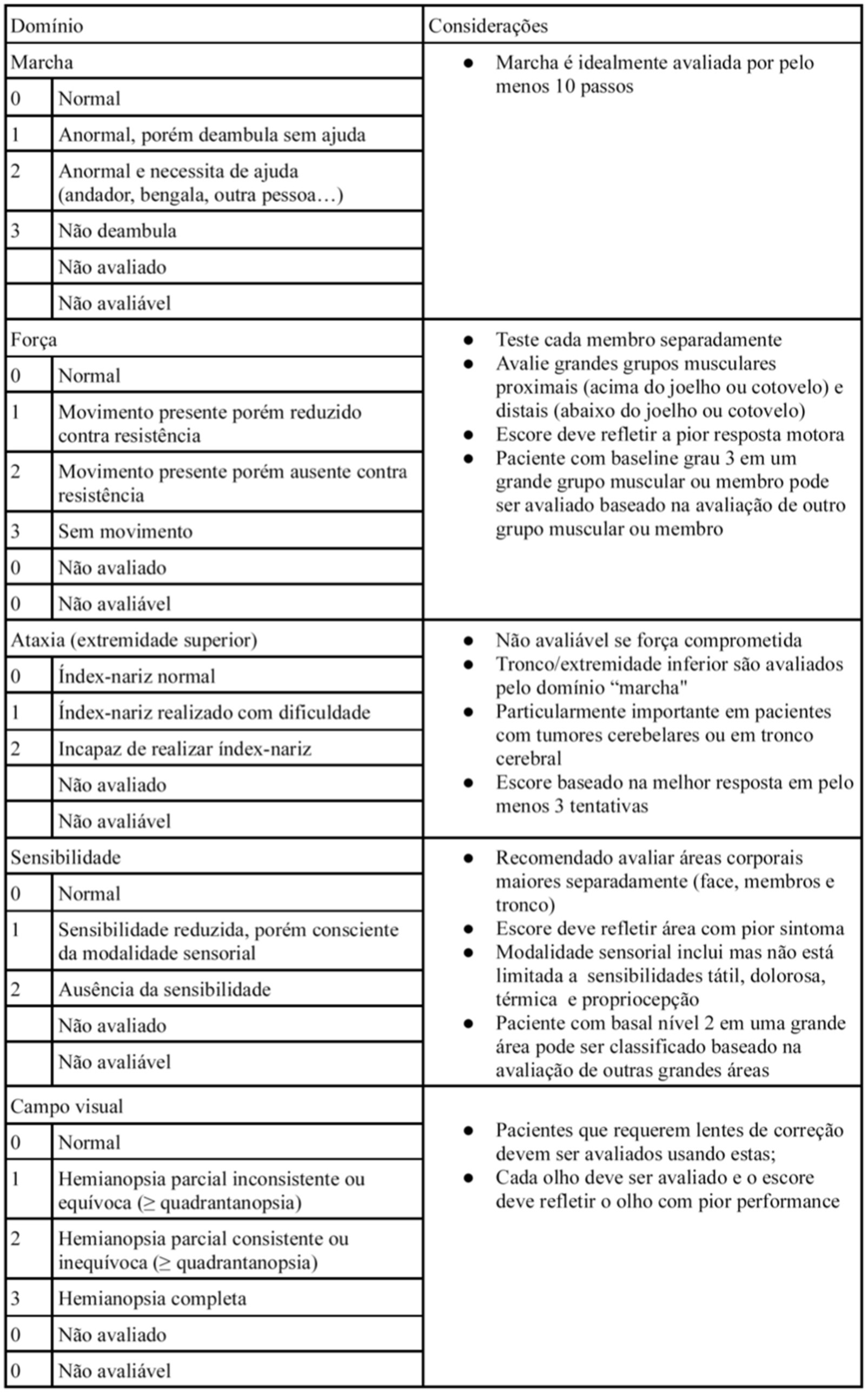
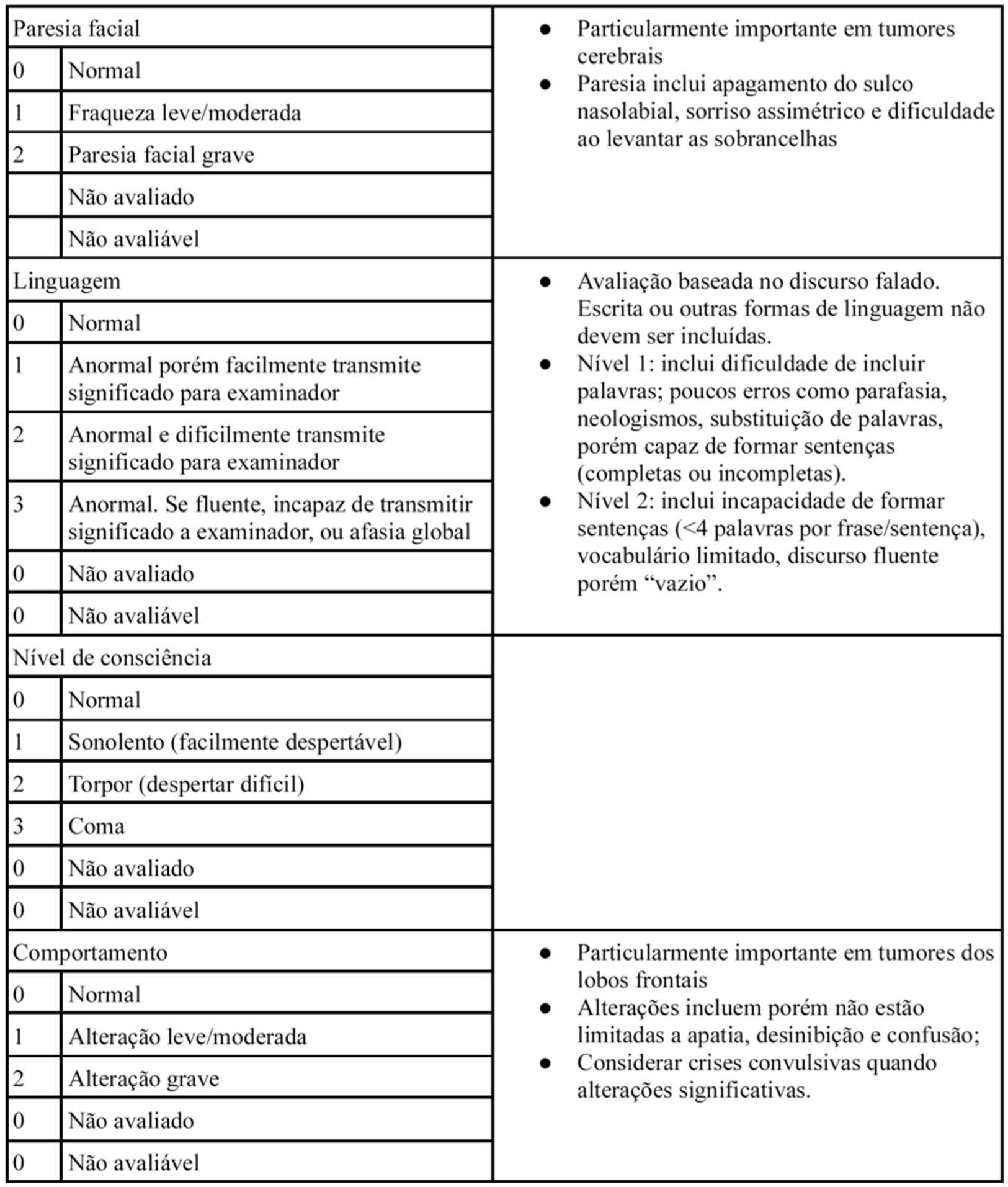
The NANO scale is an instrument that evaluates neurological function through nine domains: gait, strength, ataxia, sensibility, visual field, facial paralysis, language, level of consciousness and behavior. The scale domains were selected according to the most common neurological findings in patients with brain tumors. Each domain has three or four score levels, varying between 0 and 2 or 0 and 3. The zero score indicates normal function, and the highest level (2 or 3) refers to the worst deficit in that domain. Also, the options “not evaluated” and “not assessed” were added in each domain. “Not evaluated” is scored when any neurologic deficit is not attributed to tumoral activity, such as change in medications (corticosteroid therapy, sedatives, antiepileptic drugs), comorbid events (metabolic encephalopathy, post-ictal state, stroke), when the evaluation of one domain is affected by another domain (ataxia is not scored because of leg weakness, for example). “Not assessed” is scored when the examiner omits the evaluation of a domain (10). In Table 1, the NANO scale is described.
The descriptive analysis of the collected data included: age, gender, interview time (diagnosis or follow-up reviews), lesion location, treatment timing at the evaluation, KPS score, presence of seizures and corticosteroid use. The statistical analysis was conducted with SPSS® for Windows (SPSS Inc., Chicago, IL, USA). Categorical variables were presented as proportions and analyzed with Pearson’s Chi-square Test and Fisher’s Exact Test. The continuous variables were submitted to the Kolmogorov–Smirnov Test to verify the normal distribution. The comparison was conducted with Student T test (normally distributed data), Mann–Whitney U test and Kruskal-Wallis test (non-normally distributed data). The post-hoc Dunn test was performed when the null hypothesis was rejected in Kruskal-Wallis test. Concerning NANO scale confiability, Cronbach’s alpha was utilized to measure the internal consistency of all scale items and if item deleted. The correlation Spearman’s Test was used to evaluate the convergent validity between NANO scale and KPS.
Results
In regarding to the NANO scale translation to Brazilian Portuguese process, no inconsistency was identified in all stages, including translation, initial revision, back-translation and its final version (the Brazilian Portuguese version is described in Figure 1).
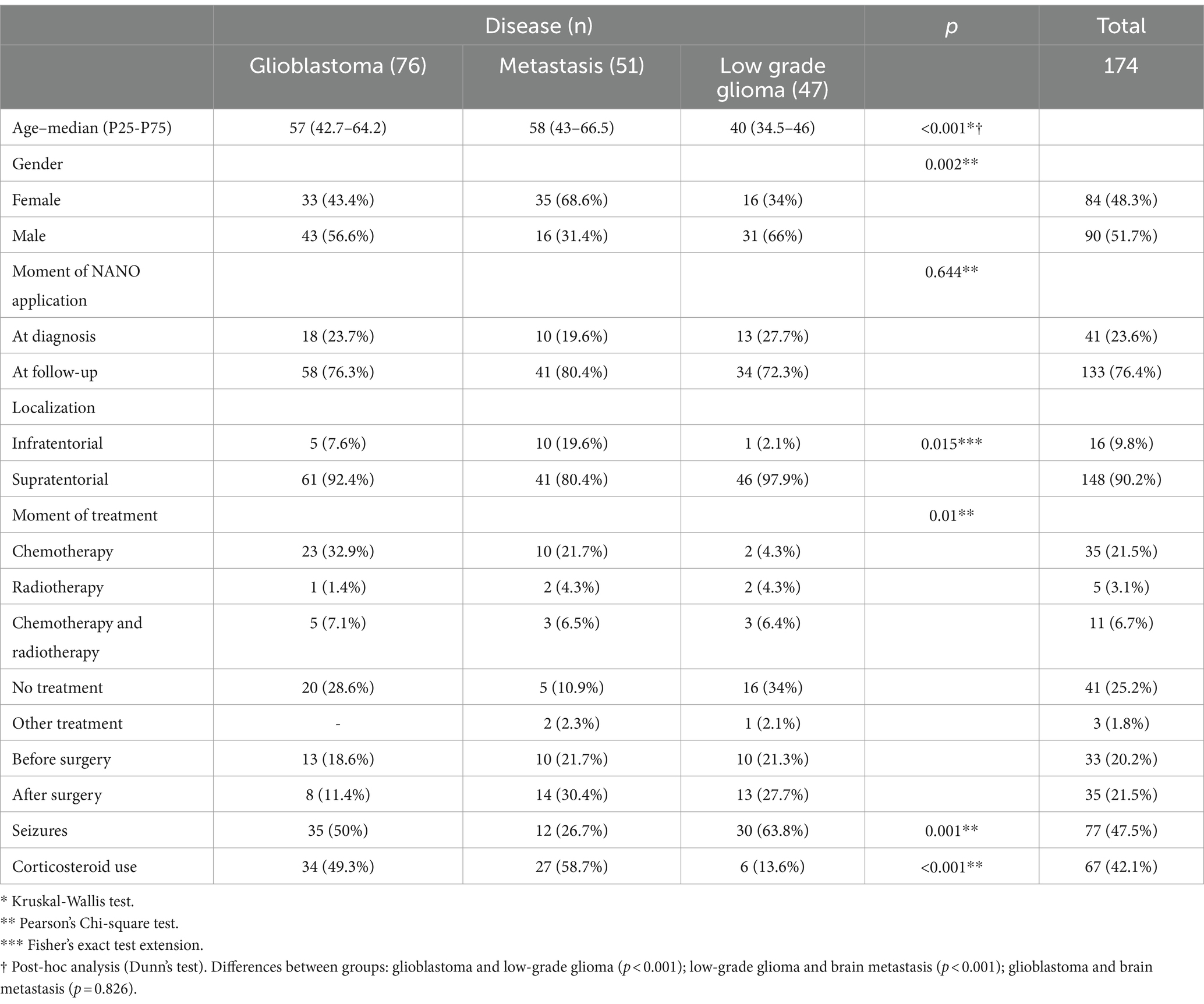
After the translation process, 174 patients were evaluated. The epidemiological and clinical characteristics are presented in Table 2. There are no difficulties in the understanding of scale items among examiners. The option “not assessed” was not scored in any patient evaluated.
Convergent validity
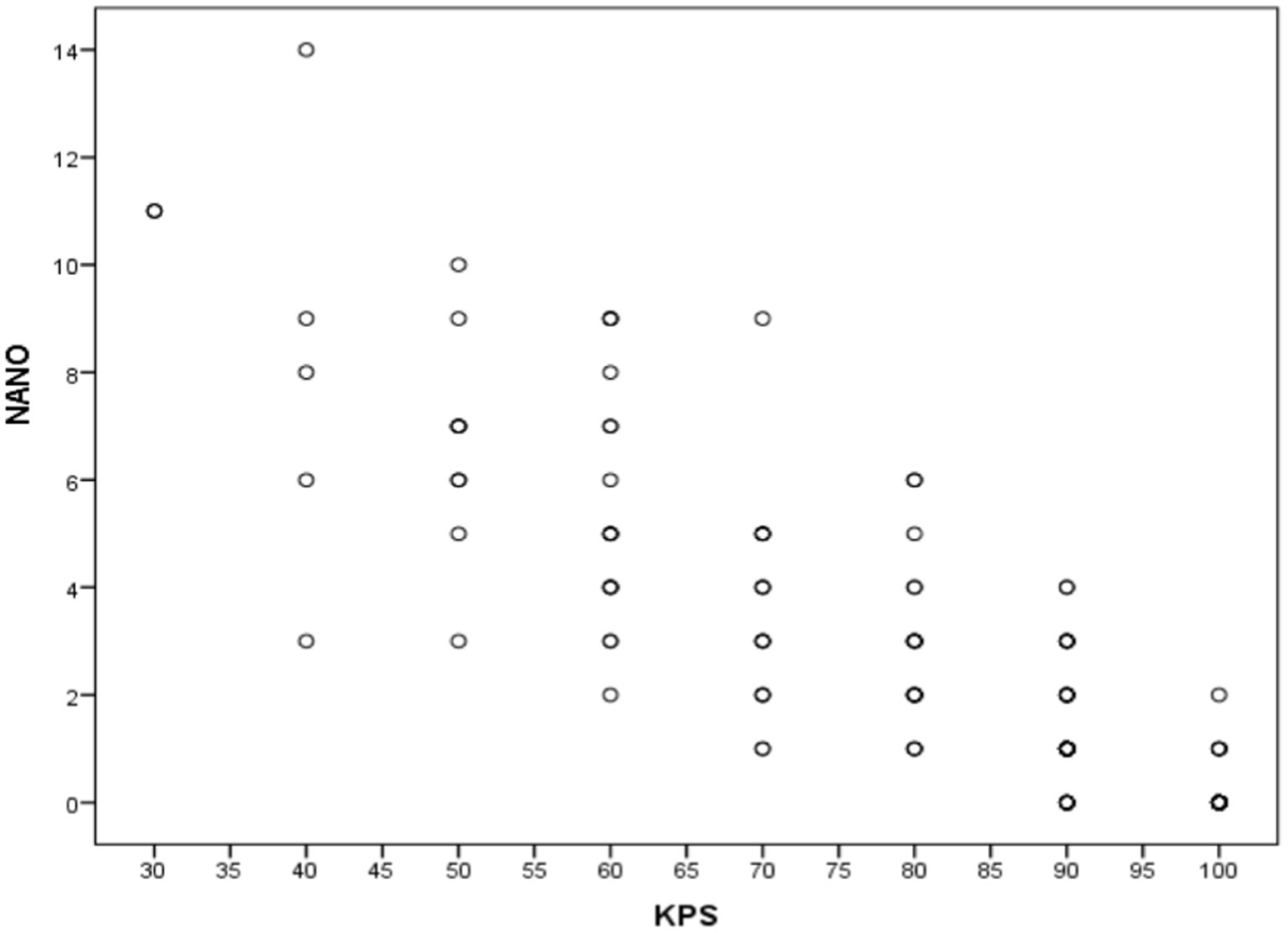
Figure 2 specifies the total Spearman correlation coefficients between NANO and KPS scale. The value encountered (−0.875) was statistically significant (p < 0.001). Figure 3 shows the amplitude of the Spearman correlation coefficients founded between NANO and KPS scale in each disease. A significant inverse correlation (p < 0.001) was observed in all diseases. The coefficients were: r = −0.886 in glioblastoma, r = −0.827 in brain metastasis and r = −0.872 in low grade glioma.

Figure 3. Spearman correlation coefficients between NANO and KPS scales in glioblastoma, brain metastasis and low-grade glioma groups.
Confiability
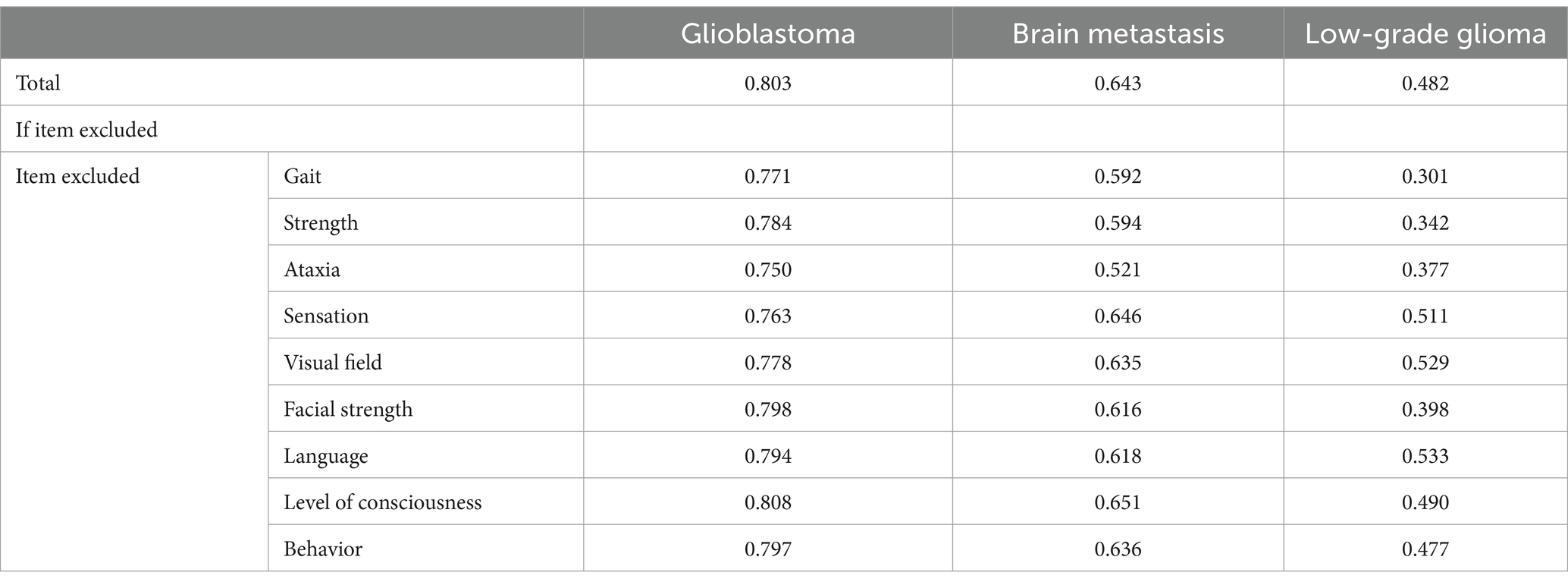
The Cronbach’s alpha values for NANO scale founded for each disease is specified as follows: 0.803 for glioblastoma, 0.643 for brain metastasis and 0.482 for low grade glioma. The total Cronbach’s alpha is 0.777. In Table 3, detailed information about Cronbach’s alpha when items are deleted is provided. Differences between patient groups were identified. When the items “gait,” “strength,” “ataxia,” “facial paralysis,” and “behavior” were excluded, the Cronbach’s alpha dropped below the total value, indicating that these items are essential for the scale’s reliability. In regarding to the exclusion of “consciousness” item, higher scores were observed in all groups, which infers the reduction of scale reliability with the presence of this domain. The “visual field” and “language” items showed lower scores when deleted in the glioblastoma and brain metastasis groups. Finally, the “sensibility” domain was important only in the glioblastoma group.
Discussion
This was the first study of translation and cross-cultural validation of NANO scale into Brazilian Portuguese. The primary objective was to translate and validate the NANO scale into Brazilian Portuguese with glioblastoma, brain metastasis and low-grade glioma patients. We chose these diseases due to the potential variability of identified symptoms and to assess whether there are specific particularities in each disease that could impact the NANO scale approach.
The radiological response criteria have already been validated, and the RANO criteria and its offspring (RANO-LGG, RANO-HGG, RANO-BM) (4–7, 11) are widely used in clinical trials. However, the clinical criteria also included in those instruments are too simplified and are commonly associated with the KPS and ECOG/WHO scores, which reflect only the general functional condition, without providing further details on the neurological status. Besides, radiological parameters not always mirror the neurological status. The greatest examples are the pseudoprogression and pseudo response phenomena (4, 6, 12). It is essential to perform a combined evaluation of radiological and more specific clinical criteria to determine treatment response. The NANO scale effectively addresses the limitations of other scores by characterizing the clinical status through nine domains that can be quantified and assessed longitudinally. The scale is suitable for use by all professionals involved in patient care.
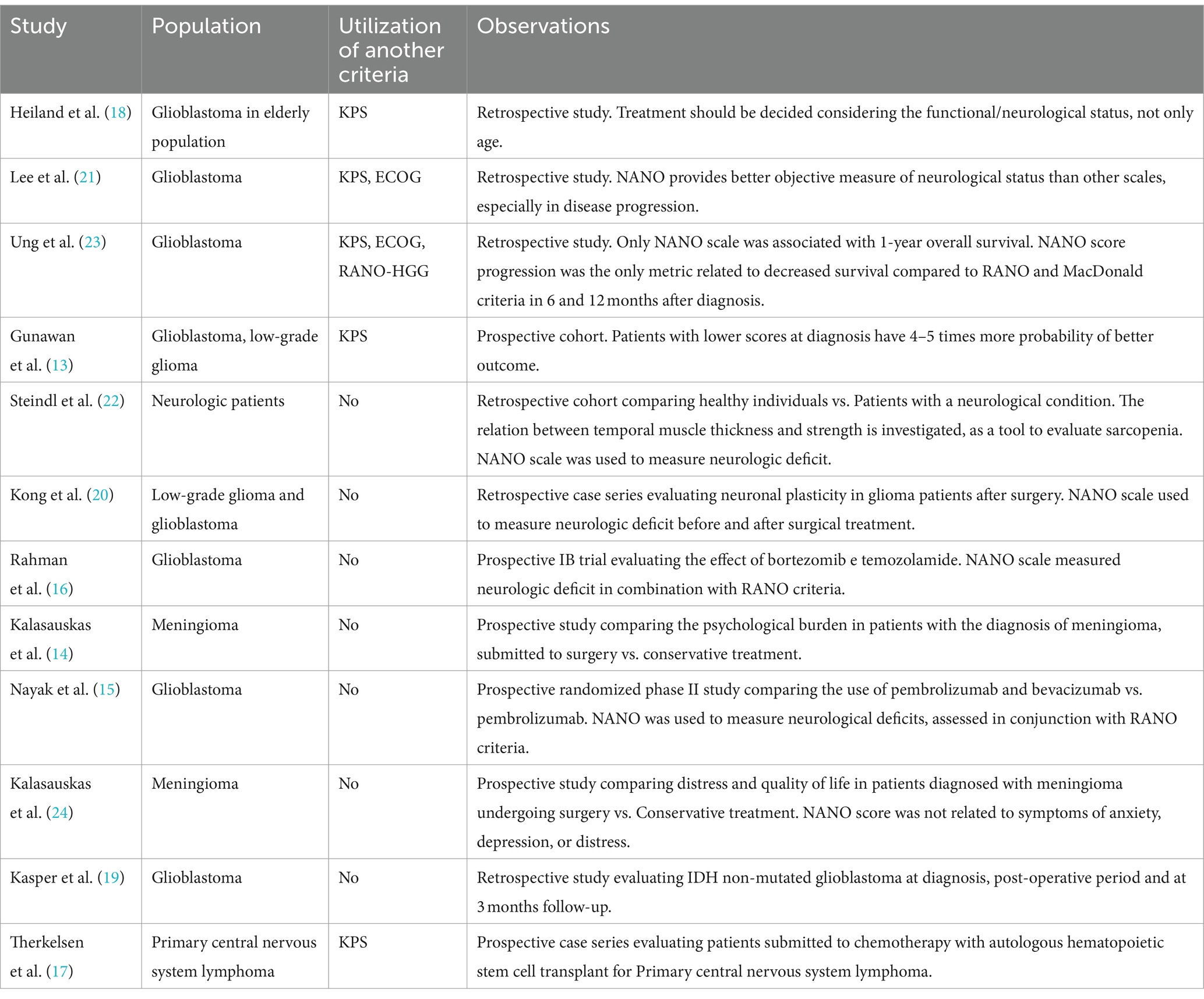
Although it is a novel instrument, the NANO scale has been utilized in various studies to date. These studies were heterogeneous, including patients with different diagnoses such as glioblastoma, low-grade glioma, meningioma, and neurological patients in general. The NANO scale has been employed in both prospective studies (13–17) and retrospective ones (18–23). Despite the need for NANO validation with retrospective data, there appears to be no significant loss of clinical information.
In Rahman et al. and Nayak et al., the NANO scale was used to measure neurological deficits in clinical trials in conjunction with the RANO criteria, following the approach proposed by the instrument’s authors (15, 16). Additionally, the NANO scale serves as an early predictor of disease progression in gliomas and a predictor of survival in glioblastoma when compared to other commonly used scales for assessing functional status. Good initial NANO and KPS scores predict 4–5 times better functional recovery 2 months after resection. Compared to KPS, the NANO scale exhibits a stronger correlation with better outcomes and is superior in predicting functional improvement (13). In a Korean study, 76 glioblastoma patients were retrospectively reviewed. In this study, the NANO scale demonstrated greater prognostic value at diagnosis and disease progression compared to the KPS and ECOG/WHO scales (21). In a retrospective study, a worse NANO score was the only metric associated with lower survival when compared to the RANO and MacDonald criteria at 6 and 12 months after treatment (23). As per Heiland et al.’s study, they assessed the functional response following surgical treatment in 342 elderly patients with glioblastoma. Patients with higher NANO scores were more likely to be submitted to biopsy than gross total resection (GTR: 2.57 vs. biopsy: 2.74; p < 0.05). In addition, lower NANO scores were significantly associated to longer survival (p = 0.001) (18). In Table 4, all the published studies that utilized the NANO scale are described.
As presented in the NANO validation publication (10), the option ‘not assessed’ was included in all domains. This option applies to situations in which the interviewer deliberately does not assign any scores to a particular domain, and subsequently, that domain should be excluded from further evaluations. However, during data collection, we observed that such circumstances did not arise. This observation suggests that the option ‘not assessed’ may be omitted in all domains in future studies. Few patients received ‘not evaluated’ scores, and as a result, it cannot be determined if this has a considerable influence on the final score. Additionally, this option was excluded in the evaluation between the groups.
Since this is the first instrument specifically designed to evaluate neurological status in neuro-oncological patients, there is no established “gold-standard.” Therefore, we used the convergent correlation with KPS score in the validation process, given its widespread application in neuro-oncology. A significant inverse correlation (p < 0.001) was found between the scales in all groups (glioblastoma: r = −0.886; metastasis: r = −0.827; low grade glioma: r = −0.872). Consequently, the higher the KPS score, the lower NANO score will be. To date, there are no published studies correlating the NANO with other instruments (13, 18, 21, 23).
Among the diverse ways to measure the reliability, we used the internal consistency, applying Cronbach’s Alpha as the statistical test. The internal consistency varied between groups: in the glioblastoma and metastasis patients, the Cronbach’s Alpha were acceptable (0.803 and 0.643, respectively). However, the Cronbach’s Alpha found in the low-grade glioma group was 0.482, which is an insufficient coefficient result. This lower value may be explained by the longer disease duration and milder signals and symptoms severity and variability compared to glioblastoma and brain metastasis, which can impair the scale’s evaluation in this specific group. It is possible that with a larger sample size, we may achieve better results in terms of internal consistency in this specific population.
In relation to Cronbach’s Alpha if an item is deleted, differences were observed between groups. This analysis allows us to assess the influence of each domain on the instrument’s internal consistency. If Cronbach’s Alpha decreases when an item is deleted, it indicates that this domain is important for the instrument’s reliability (25). As specified in Table 2, excluding “gait,” “strength,” “facial paralysis” and “behavior” domains decrease the Cronbach’s Alpha coefficient, indicating their key role in the instrument’s reliability. This finding may be explained by the higher objectivity and consistency in clinical evaluation, as well as their frequency, which allows for better internal consistency evaluation. Cronbach’s Alpha increases when this item is excluded, suggesting a reduction in reliability with the presence of this domain. However, this data may suggest the possibility of excluding this item to improve the scale’s performance. Nevertheless, since this is a novel instrument with recent applications, further studies are needed to confirm the significance of this item and the variability observed between groups, as shown in Table 3.
The NANO scale was developed to be applied alongside radiological criteria, providing a comprehensive evaluation and being useful for the follow-up of neuro-oncological patients, regardless of tumor histology. However, understanding the particularities found in each subpopulation is essential for refining the instrument and maximizing its utility in neuro-oncology. For instance, excluding the NANO validation publication (10), the instrument has not yet been applied to patients with cerebral metastasis. This measurement could be important since the assessment of clinical status significantly influences therapeutic decisions. For example, asymptomatic patients may undergo systemic treatments with good central nervous system penetrance, while those with focal symptoms could be treated with more localized therapies like surgery or stereotactic radiosurgery. Furthermore, with better quantification and monitoring of neurological symptoms, patients with brain metastasis have a greater chance of being included in clinical trials, a condition that is typically an exclusion criterion in studies (26).
The NANO scale is an instrument applied by healthcare professionals, with domains characterized by technical terms widely used in clinical practice, without expressions or terms that can lead to misunderstanding or the necessity to transcultural adaptation. Due to these factors, there were no disagreements in none of translation steps. The instrument’s application did not require prior training, and the provided instructions were sufficient for its execution. These characteristics encourage its use in clinical practice, promoting better communication among healthcare professionals.
Study limitations
The interobserver agreement could not be assessed due to an insufficient sample size required for this analysis. We plan to address this reliability analysis in future studies. The score ‘not evaluated’ was identified in a few cases; therefore, it was not possible to assess its influence on the validity and reliability of the NANO scale. The Cronbach’s alpha found in the low-grade glioma group was lower than recommended, and it was not possible to ensure the reliability of the NANO scale in this population. However, we intend to expand our sample size in future studies to provide better data for this specific population.
Conclusion
The NANO scale is a relatively new instrument for neuro-oncology evaluation. Consequently, the validation process is crucial to assess its ability to reliably reflect the patient’s neurological status. This instrument is not limited to one-time evaluations; rather, it can detect neurological changes throughout the clinical course of the disease. The Brazilian Portuguese version of the NANO scale proves to be a reproducible and valid tool for evaluating neuro-oncological patients, demonstrating a strong correlation with the KPS scale and adequate overall internal consistency. However, further studies are required to assess its reliability in patients with low-grade glioma, despite its good correlation with the KPS score.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Sirio-Libanes Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MV: Writing – review & editing, Writing – original draft, Visualization, Validation, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. DA: Writing – review & editing, Investigation, Data curation. PP: Writing – review & editing, Supervision. JN: Writing – review & editing, Validation, Methodology, Investigation, Data curation. CF: Writing – review & editing, Validation, Supervision, Methodology, Investigation, Formal analysis. GG: Writing – review & editing, Investigation, Data curation. AC: Writing – review & editing, Methodology. MM: Writing – review & editing, Writing – original draft, Supervision, Project administration, Investigation, Data curation, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors declare financial support was received for publication of this article by Sirio-Libanes Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rodrigues, DB, Lima, LO, Rodrigues Pereira, EL, Souza, UO, Fernandes de Oliveira, M, Lima, AM, et al. Epidemiologia das neoplasias intracranianas no Hospital do Servidor Público Estadual de São Paulo: 2010-2012. Arq Bras Neurocir. (2014) 33:06–12. doi: 10.1055/s-0038-1626192
2. BRASIL. [National Cancer Institute Jose Alencar Gomes da Silva (INCA)]. (2020). [Estimate/2020 – Incidence of Cancer in Brazil]. Rio de Janeiro: [National Cancer Institute Jose Alencar Gomes da Silva (INCA)]. Available at: https://www.inca.gov.br/sites/ufu.sti.inca.local/files//media/document//estimativa-2020-incidencia-de-cancer-no-brasil.pdf. (Accessed November 30, 2021).
3. Ballman, K, Buckner, JC, Brown, PD, Giannini, C, Flynn, PJ, LaPlant, BR, et al. The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro Oncol. (2007) 9:29–38. doi: 10.1215/15228517-2006-025
4. Eisele, SC, Wen, PY, and Lee, EQ. Assessment of brain tumor response: RANO and its offspring. Curr Treat Options Oncol. (2016) 17:35. doi: 10.1007/s11864-016-0413-5
5. Lin, NU, Lee, EQ, Aoyama, H, Barani, IJ, Barboriak, DP, Baumert, BG, et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. (2015) 16:e270–8. doi: 10.1016/S1470-2045(15)70057-4
6. Okada, H, Weller, M, Huang, R, Finocchiaro, G, Gilbert, MR, Wick, W, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. (2015) 16:e534–42. doi: 10.1016/S1470-2045(15)00088-1
7. Van Den Bent, M, Wefel, J, Schiff, D, Taphoorn, M, Jaeckle, K, Junck, L, et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. (2011) 12:583–93. doi: 10.1016/S1470-2045(11)70057-2
8. Karnofsky, DA, Burchenal, JH, Armistead, GC, Southam, CM, Bernstein, JL, Craver, LF, et al. Triethylene melamine in the treatment of neoplastic disease: a compound with nitrogen-mustard-like activity suitable for Oral and intravenous use. AMA Arch Intern Med. (1951) 87:477–516. doi: 10.1001/archinte.1951.03810040002001
9. Orr, ST, and Aisner, J. Performance status assessment among oncology patients: a review. Cancer Treat Rep. (1986) 70:1423–9.
10. Nayak, L, DeAngelis, LM, Brandes, AA, Peereboom, DM, Galanis, E, Lin, NU, et al. The neurologic assessment in neuro-oncology (NANO) scale: a tool to assess neurologic function for integration into the response assessment in neuro-oncology (RANO) criteria. Neuro Oncol. (2017) 19:625–35. doi: 10.1093/neuonc/nox029
11. Wen, PY, Norden, AD, Drappatz, J, and Quant, E. Response assessment challenges in clinical trials of gliomas. Curr Oncol Rep. (2010) 12:68–75. doi: 10.1007/s11912-009-0078-3
12. Pérez-Larraya, JG, Lahutte, M, Petrirena, G, Reyes-Botero, G, González-Aguilar, A, Houillier, C, et al. Response assessment in recurrent glioblastoma treated with irinotecan-bevacizumab: comparative analysis of the Macdonald, RECIST, RANO, and RECIST F criteria. Neuro Oncol. (2012) 14:667–73. doi: 10.1093/neuonc/nos070
13. Gunawan, PY, Islam, AA, July, J, Patellongi, I, Nasrum, M, and Aninditha, T. Karnofsky performance scale and neurological assessment of neuro-oncology scale as early predictor in glioma. Asian Pac J Cancer Prev. (2020) 21:3387–92. doi: 10.31557/APJCP.2020.21.11.3387
14. Kalasauskas, D, Keric, N, Ajaj, SA, Von Cube, L, Ringel, F, and Renovanz, M. Psychological burden in meningioma patients under a wait-and-watch strategy and after complete resection is high—results of a prospective single center study. Cancers. (2020) 12:3503. doi: 10.3390/cancers12123503
15. Nayak, L, Molinaro, AM, Peters, K, Clarke, JL, Jordan, JT, de Groot, J, et al. Randomized phase II and biomarker study of pembrolizumab plus bevacizumab versus pembrolizumab alone for patients with recurrent glioblastoma. Clin Cancer Res. (2021) 27:1048–57. doi: 10.1158/1078-0432.CCR-20-2500
16. Rahman, MA, Brekke, J, Arnesen, V, Hannisdal, MH, Navarro, AG, Waha, A, et al. Sequential bortezomib and temozolomide treatment promotes immunological responses in glioblastoma patients with positive clinical outcomes: a phase 1B study. Immun Inflammat Dis. (2020) 8:342–59. doi: 10.1002/iid3.315
17. Therkelsen, KE, Schaff, LR, Nandakumar, S, Omuro, AMP, DeAngelis, L, and Grommes, C. Long-term outcomes in primary CNS lymphoma after R-MVP and high-dose chemotherapy with autologous hematopoietic stem cell transplant. Neurology. (2023) 101:e710–6. doi: 10.1212/WNL.0000000000207490
18. Heiland, DH, Haaker, G, Watzlawick, R, Delev, D, Masalha, W, Franco, P, et al. One decade of glioblastoma multiforme surgery in 342 elderly patients: what have we learned? J Neuro Oncol. (2018) 140:385–91. doi: 10.1007/s11060-018-2964-8
19. Kasper, J, Wende, T, Fehrenbach, MK, Wilhelmy, F, Jähne, K, Frydrychowicz, C, et al. The prognostic value of NANO scale assessment in IDH-wild-type glioblastoma patients. Front Oncol. (2021) 11:790458. doi: 10.3389/fonc.2021.790458
20. Kong, NW, Gibb, WR, Badhe, S, Liu, BP, and Tate, MC. Plasticity of the primary motor cortex in patients with primary brain tumors. Neural Plast. (2020) 2020:1–9. doi: 10.1155/2020/3648517
21. Lee, J, Park, SH, and Kim, YZ. Prognostic evaluation of neurological assessment of the neuro-oncology scale in glioblastoma patients. Brain Tumor Res Treat. (2018) 6:22–30. doi: 10.14791/btrt.2018.6.e1
22. Steindl, A, Leitner, J, Schwarz, M, Nenning, KH, Asenbaum, U, Mayer, S, et al. Sarcopenia in neurological patients: standard values for temporal muscle thickness and muscle strength evaluation. J Clin Med. (2020) 9:1272. doi: 10.3390/jcm9051272
23. Ung, TH, Ney, DE, Damek, D, Rusthoven, CG, Youssef, AS, Lillehei, KO, et al. The neurologic assessment in neuro-oncology (NANO) scale as an assessment tool for survival in patients with primary glioblastoma. Neurosurgery. (2019) 84:687–95. doi: 10.1093/neuros/nyy098
24. Kalasauskas, D, Keric, N, Abu Ajaj, S, von Cube, L, Ringel, F, and Renovanz, M. Distress and quality of life do not change over time in patients with operated and conservatively managed intracranial meningioma. Acta Neurochir. (2021) 163:3417–24. doi: 10.1007/s00701-021-05004-w
25. Souza, AC, de Alexandre, NMC, Guirardello, E, de, B, Souza, AC, de Alexandre, NMC, et al. Psychometric properties in instruments evaluation of reliability and validity Epidemiologia e Serviços de Saúde. (2017) 26:649–659. doi: 10.5123/S1679-49742017000300022
Keywords: glioblastoma, brain metastasis, low-grade glioma, neuro-oncology, neurological performance, NANO scale
Citation: Velho MC, Andrade Gripp D, Pires de Aguiar PH, Nicacio JA Jr, Formentin C, Greggianin GF, Campos ACP and Maldaun MVC (2024) Translation and validation of the Neurological Assessment in Neuro-Oncology scale to Brazilian Portuguese. Front. Neurol. 15:1369625. doi: 10.3389/fneur.2024.1369625
Edited by:
Jakub Nalepa, Silesian University of Technology, PolandReviewed by:
Anna-Maria Barciszewska, Poznan University of Medical Sciences, PolandJoao Paulo Almeida, Mayo Clinic Florida, United States
Copyright © 2024 Velho, Andrade Gripp, Pires de Aguiar, Nicacio, Formentin, Greggianin, Campos and Maldaun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maíra Cristina Velho, mvelho@hcpa.edu.br
 Maíra Cristina Velho
Maíra Cristina Velho