- 1Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria
- 2VASCage – Research Centre on Clinical Stroke Research, Innsbruck, Austria
Introduction: This study aimed to emphasize the importance of cranial nerve (CN) palsies in spontaneous cervical artery dissection (sCeAD).
Methods: A search term-based literature review was conducted on “cervical artery dissection” and “cranial nerve palsy.” English and German articles published until October 2023 were considered.
Results: Cranial nerve (CN) palsy in sCeAD is evident in approximately 10% of cases. In the literature, isolated palsies of CN II, III, VII, IX, X, and XII have been reported, while CN XI palsy only occurs in combination with other lower cranial nerve palsies. Dissection type and mural hematoma localization are specific to affected CN as CN palsies of II or III are solely evident in those with steno-occlusive vessel pathologies located at more proximal segments of ICA, while those with CN palsies of IX, X, XI, and XII occur in expansive sCeAD at more distal segments. This dichotomization emphasizes the hypothesis of a different pathomechanism in CN palsy associated with sCeAD, one being hypoperfusion or microembolism (CN II, III, and VII) and the other being a local mass effect on surrounding tissue (CN IX, X, XI, and XII). Clinically, the distinction between peripheral palsies and those caused by brainstem infarction is difficult. This differentiation is key, as, according to the reviewed cases, peripheral cranial nerve palsies in sCeAD patients mostly resolve completely over time, while those due to brainstem stroke do not, making cerebrovascular imaging appraisal essential.
Discussion: It is important to consider dissections as a potential cause of peripheral CN palsies and to be aware of the appropriate diagnostic pathways. This awareness can help clinicians make an early diagnosis, offering the opportunity for primary stroke prevention.
Introduction
Cervical artery dissection (CeAD) is defined by the evidence of a mural hematoma within the arterial wall of either the carotid or vertebral arteries and can occur spontaneously (sCeAD) or in timely association with trauma. In the general stroke population, a sCeAD is rare and attributes for 1–2% of all strokes. In young individuals (i.e., under the age of 50 years of age) however, sCeAD accounts for 10–25% of ischemic strokes, making it one of the primary causes in this age group (1–4). The clinical presentation of sCeAD varies considerably with local signs and symptoms (such as head/neck pain, Horner’s syndrome, cranial nerve palsies, and pulsatile tinnitus), typically preceding ischemic stroke. As early detection of local signs and symptoms due to sCeAD followed by early treatment offers the opportunity for primary stroke prevention, understanding the clinical spectrum of sCeAD is of utmost importance. Over the years, studies and narrative reviews have focused on the frequent local signs and symptoms such as head/neck pain and Horner’s syndrome, neglecting cranial nerve palsies attributable to sCeAD (5–7). Therefore, we aimed to put the current evidence into perspective and to give an overview of the pathomechanistic as well as clinical aspects of sCeAD-related CN palsies.
Methods
A search term-based literature review of PubMed was conducted to identify articles investigating cranial nerve palsies due to sCeAD. Search terms were “cervical artery dissection” AND “cranial nerve palsy.” Additionally, a search with the terms “cervical artery dissection” AND “insert cranial nerve” (e.g., “facial nerve”) was performed for all 12 cranial nerves. Titles and abstracts were screened, and the full texts of potentially relevant articles were obtained for review. The inclusion criteria comprised cranial nerve palsies due to spontaneous cervical artery dissection (sCeAD). Articles concerning cranial nerve palsies due to other causes such as traumatic cervical artery dissection, stroke, cancer, surgery, or local inflammation were excluded. A review of the literature was performed by the two main authors (BD and LMS). In total, 75 search results matched the inclusion criteria for this review; 22 were duplicates of different search terms. Finally, 53 publications were considered (Supplementary Table S1).
Epidemiology/pathophysiology
In line with the increasing availability of magnetic resonance imaging (MRI), absolute sCeAD diagnoses, especially in the vertebral arteries, have become more frequent (5, 8). The cause for sCeAD is essentially unknown. As environmental factors such as mild, non-penetrating head/neck trauma, or systemic infection are reported to be potential triggers for sCeAD, a multifactorial pathogenesis is likely (9–18). In addition, a subclinical connective tissue disorder has been proposed as a potential disease-promoting factor (19–28). Depending on the location of the pathognomonic vessel wall hematoma, two types of dissections can be differentiated: subintimal or subadventitial (29). It is assumed that subintimal dissections, which are present in over 80% of the cases, are associated with an intimal tear and subsequent anterograde blood flow from the vessel to the false lumen (inside-out theory) resulting in steno-occlusive vessel pathologies characterized by no significant diameter expansion, hence unlikely to affect nearby anatomical structures. A subadventitial wall hematoma originates from a rupture of the vasa vasorum and typically results in expansive vessel pathologies (outside-in theory) (schematic Figure 1) (4, 30–36).
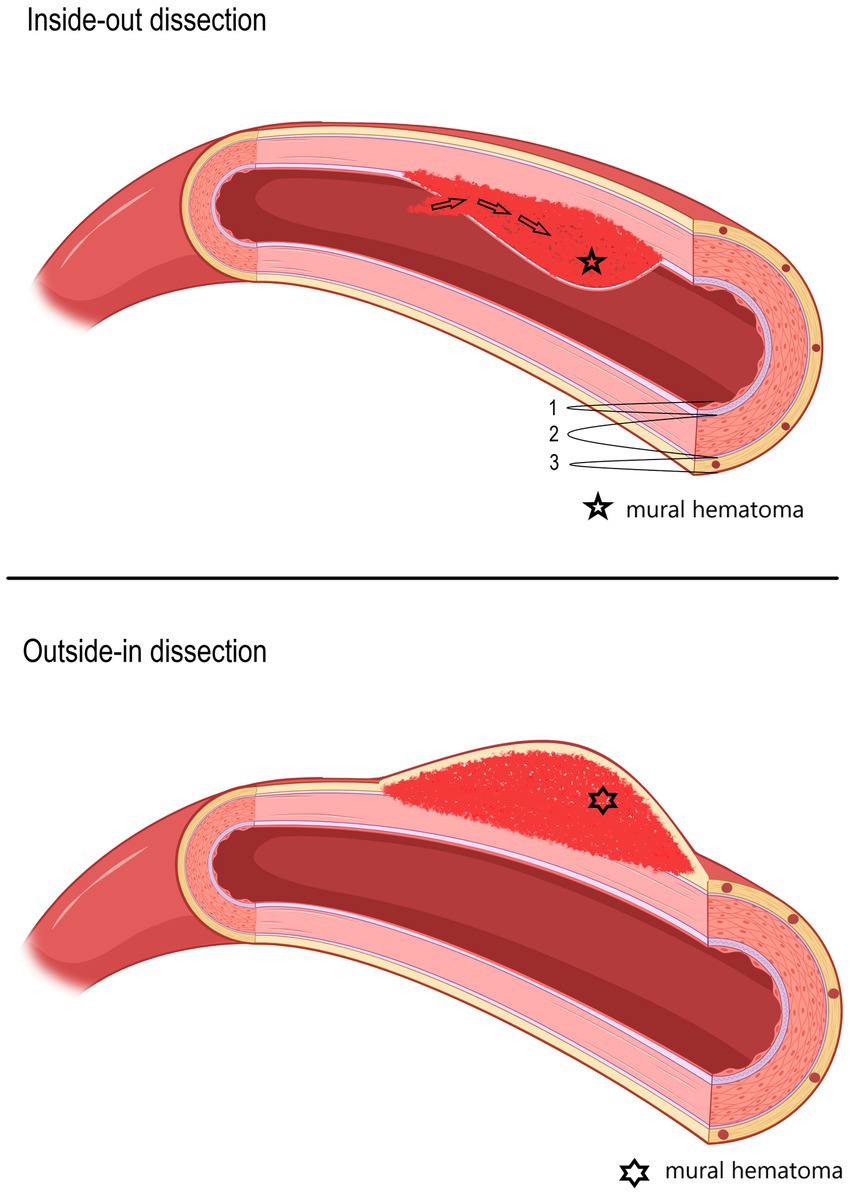
Figure 1. Schematic picture of two different hypothesized types of dissections (upper: inside-out; lower: outside-in), depending on the location of the mural hematoma. In the upper picture, an inside-out dissection is shown, and in the lower picture, an outside-in dissection is shown. 1—Tunica intima, 2—Tunica media, 3—Tunica externa (tunica adventitia) with vasa vasorum.
Clinical presentation—overall
The clinical presentation of patients with sCeAD ranges from asymptomatic to severe cerebral ischemic events. Hospital-based cohorts indicate a likelihood of sCeAD-related cerebral ischemia (TIA or stroke) ranging from 65 to 80%. These events usually exhibit an embolic pattern and, less frequently, occur in cases with high-grade stenosis or occlusion, including hemodynamic watershed infarcts (29, 30, 37–41). More than 80% of cases report at least one local symptom, with head/neck pain being by far the most frequent (5). The pain typically is characterized as pulling and/or dull, with mild-to-moderate progressive intensity. It often responds poorly to oral at-home analgesia and is specific to the ipsilateral side of the dissection (6). Another common local sign is the ipsilateral Horner’s syndrome, which is present in 28 to 58% of patients with internal carotid artery (ICA) dissection and can be the sole symptom in 10–12% of cases (1, 42–50). Tinnitus, often of pulsatile nature, is another possible local sign, occurring in 7–27% of cases and stems most likely from non-laminar flow in steno-occlusive sCeAD pathologies near the tympanic membrane (5, 43, 49, 51, 52).
sCeAD-related cranial nerve palsy
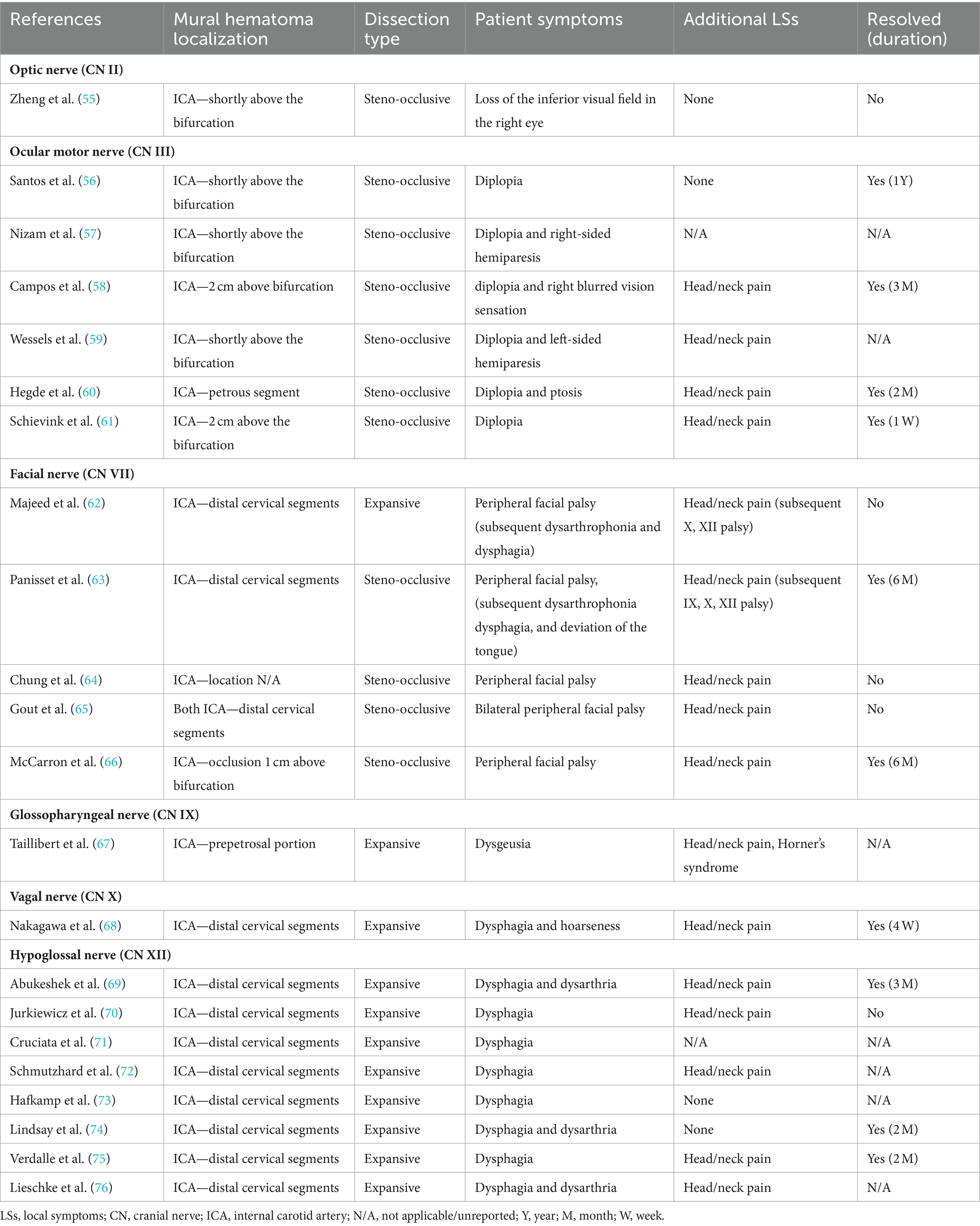
Cranial nerve palsies occur in 3–12% of all patients with sCeAD and can be the sole clinical sign in 0.5% (1–4, 53, 54). Table 1 presents the clinical characteristics of published case reports with isolated CN palsies due to sCeAD.
In summary, isolated CN II, III, VII, IX, X, and XII palsies due to sCeAD have been reported in the literature. Patients with CN II or III palsy exclusively had steno-occlusive sCeAD-related vessel pathologies, while those with isolated CN IX, X, or XII palsy primarily had expansive mural hematoma. Furthermore, the mural hematoma localization typically involved more proximal segments of the ICA in those with CN II or III palsy compared to others. Solely those with sCeAD-related VII palsies had different mural hematoma localization and dissection types. In total, 80% of cases where clinical data were available reported head/neck pain as an additional sCeAD-related local symptom.
CN palsies due to sCeAD can also be present as clinical syndromes, namely, Collet–Sicard, Villaret, or Tapia syndrome (1–5, 36, 43, 54, 63, 77). Table 2 holds clinical information on such syndromes previously described as attributable to sCeAD.
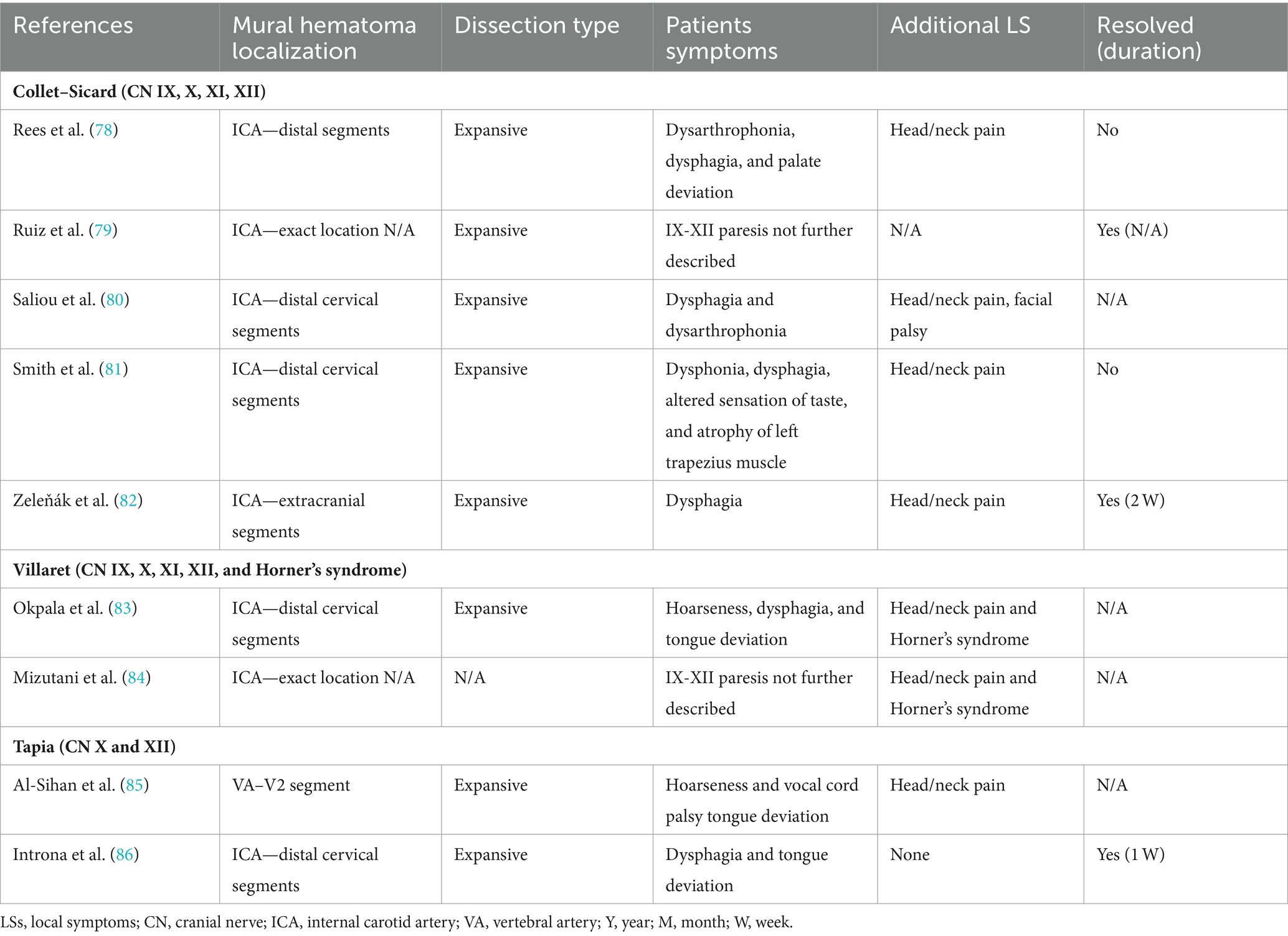
Table 2. Available data of articles reporting clinical syndromes of cranial nerve palsies due to sCeAD.
Clinical outcome was better in those with isolated CN palsies than those with clinical syndromes as 75% of patients with isolated palsies had complete resolution of symptoms compared to 60% of those with either Collet–Sicard, Villaret, or Tapia syndrome. However, the considerable amount of missing data on outcomes has to be mentioned.
Discussion
Hospital-based cohorts report that approximately three in four sCeAD cases suffer cerebral ischemia (1). However, local symptoms, such as head/neck pain, Horner’s syndrome, pulsatile tinnitus, and CN palsies, are the most frequent sCeAD-related symptoms and typically precede stroke (5, 47). Therefore, swift identification and management would enable primary stroke prevention. As previous studies and reviews have extensively covered more frequent local signs and symptoms in sCeAD, our review emphasizes that CN palsies are presentations that clinicians should not miss (5–7). In the literature, isolated palsies of CN II, III, VII, IX, X, and XII have been reported, while CN XI palsy only occurs in combination with other caudal CN palsies (Tables 1, 2). In view of the available literature, these palsies originate either from an expansive vessel wall hematoma causing a local mass effect on adjacent structures or as a consequence of peripheral nerve ischemia (i.e., microembolism or hypoperfusion of vasa nervorum) (36, 54, 62, 87). In cases where isolated CN palsies occur due to sCeAD, the available data depicted in Table 1 support such hypothetical pathomechanisms. CN IX, X, XI, and XII have a close anatomic vicinity to the ICA at the base of the skull and are therefore susceptible to mechanical stress (schematic Figure 2). As suggested by the published case reports in Table 1, patients who have isolated palsy of these CN also have a primarily expansive sCeAD-related vessel pathology, such as aneurysm formations (schematic Figure 1). On the other hand, those with isolated CN II or III palsy show steno-occlusive ICA pathologies due to sCeAD throughout. Therefore, the available literature supports the pathomechanism of microembolism or hypoperfusion of vasa nervorum in these cases. In addition to the solely mechanistic hypothesis of either local mass effect or hypoperfusion of vasa nervorum being causal to CN palsy, the localization of the sCeAD-related mural hematoma further supports this theory. Table 1 emphasizes that the mural hematoma in patients with CN II or III palsy involves more proximal parts of ICA, while in patients with CN IX, X, XI, or XII, the mural hematoma is primarily located at the base of the skull (schematic Figure 2). The only singular sCeAD-related CN palsy where different dissection types or mural hematoma localizations are reported is in CN VII palsy. In these cases, clinical presentation and patient history are crucial for accurate diagnosis and management.
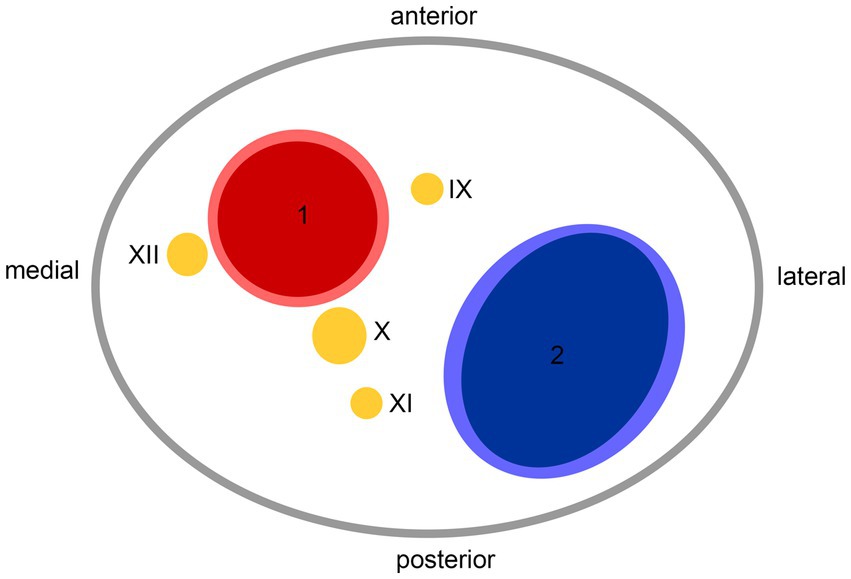
Figure 2. Anatomic scheme of axial view on left carotid sheath from caudal at the level of atlas; 1—internal carotid artery, 2—internal jugular vein, IX—glossopharyngeal nerve, X—vagus nerve, XI—accessory nerve, XII—hypoglossal nerve.
Careful clinical examination in individuals with CN palsy can reveal additional symptoms suggestive of sCeAD. In the reported cases, 80% had additional local symptoms (i.e., head/neck pain, pulsatile tinnitus, and Horner’s syndrome), with head/neck pain being the most frequent (16 of 20). Recently, a specific type of head/neck pain associated with sCeAD (acute onset, pulling pain with mild-to-moderate intensity, which continuously increases and does not respond to oral analgesia) has been reported (6). If available, imaging should be done using MRI as sCeAD with expansive vessel pathologies may be missed by ultrasound, especially if located at more distal ICA segments at the base of the skull (i.e., where CN are adjacent—schematic Figure 2) (88). Additionally, the most important differential diagnosis—brainstem ischemia-related CN palsies—could be revealed by MRI, as this distinction is sometimes difficult to identify clinically. A key difference is that peripheral CN palsies in sCeAD mostly occur in ICA dissections, while those caused by brainstem infarction relate to vertebral artery sCeAD (5). This emphasizes the necessity of a clear diagnosis, which, given the typical localizations of sCeAD primarily in the distal segments of ICA at the base of the skull and the vertebral artery (V3), should involve T1-weighted fat-saturated axial MRI imaging, if available. Here, surrounding the vessel lumen, either an isointense (first 5 days) or hyperintense crescent-shaped rim (>5 days after onset) can be found (89). However, a reported potential false-negative rate of MRI-based infra-tentorial ischemia detection of ~10% within the first 24 h after symptom onset has to be kept in mind (90). If MRI is not available, a combination of computed tomography angiography (CTA) and ultrasound can detect other, less specific signs of sCeAD, such as long tapered stenosis, false lumen and/or intima flap, and dissecting aneurysm (1, 91, 92). This is of clinical importance, especially in counseling patients, as a small hospital-based cohort analysis has shown that brainstem stroke-related CN palsies do not resolve over time, while peripheral CN do within a follow-up of 5 months (5). This was also true for the cases discussed within this review, as 72% of patients with available clinical follow-up information showed complete resolution of symptoms over time (Table 1). Even though observational studies have shown that planned stenting of sCeAD in the subacute setting is safe, we recommend a conservative approach in accordance to current treatment recommendations due to the benign prognosis of sCeAD-related peripheral CN palsies, which is in line with current guidelines (93).
Overall, CN palsy in sCeAD is evident in approximately 10% of cases. Although their prognosis is benign, it is important to consider sCeAD and the appropriate diagnostic pathways. This awareness can guide clinicians to make an early sCeAD diagnosis, offering the chance of primary stroke prevention.
Author contributions
BD: Writing – original draft, Writing – review & editing, Conceptualization, Methodology. MK: Conceptualization, Supervision, Writing – review & editing. SK: Supervision, Writing – review & editing. LM-S: Supervision, Writing – review & editing, Conceptualization, Methodology.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by VASCage—Research Centre on Clinical Stroke Research. VASCage is a COMET Centre within the Competence Centers for Excellent Technologies (COMET) program and funded by the Federal Ministry for Climate Action, Environment, Energy, Mobility, Innovation, and Technology, the Federal Ministry of Labour and Economy, and the federal states of Tyrol, Salzburg, and Vienna. COMET is managed by the Austrian Research Promotion Agency (Österreichische Forschungsförderungsgesellschaft). FFG Project number: 898252.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1364218/full#supplementary-material
References
1. Debette, S, and Leys, D. Cervical-artery dissections: predisposing factors, diagnosis, and outcome. Lancet Neurol. (2009) 8:668–78. doi: 10.1016/S1474-4422(09)70084-5
2. Robertson, JJ, and Koyfman, A. Cervical artery dissections: A review. J Emerg Med. (2016) 51:508–18. doi: 10.1016/j.jemermed.2015.10.044
3. Fusco, MR, and Harrigan, MR. Cerebrovascular dissections—A review part I: spontaneous dissections. Neurosurgery. (2011) 68:242–57. doi: 10.1227/NEU.0b013e3182012323
4. Tentschert, S, Kreuzer, S, Lalouschek, W, Lang, W, Nasel, CH, et al. Dissektion der Arteria carotis als Ursache ischämischer zerebrovaskulärer Ereignisse - Fallberichte und Diskussion. J Neurol Neurochir Psychiatr. (2003) 4:13–20.
5. Mayer, L, Boehme, C, Toell, T, Dejakum, B, Willeit, J, Schmidauer, C, et al. Local signs and symptoms in spontaneous cervical artery dissection: A single Centre cohort study. J Stroke. (2019) 21:112–5. doi: 10.5853/jos.2018.03055
6. Mayer-Suess, L, Frank, F, Töll, T, Boehme, C, Gizewski, ER, Ratzinger, G, et al. Head/neck pain characteristics after spontaneous cervical artery dissection in the acute phase and on a long-run. Cephalalgia. (2022) 42:872–8. doi: 10.1177/03331024221079298
7. Arnold, M, Baumgartner, RW, Stapf, C, Nedeltchev, K, Buffon, F, Benninger, D, et al. Ultrasound diagnosis of spontaneous carotid dissection with isolated Horner syndrome. Stroke. (2008) 39:82–6. doi: 10.1161/STROKEAHA.107.492652
8. Mayer-Suess, L, Geiger, M, Dejakum, B, Boehme, C, Domig, LM, Komarek, S, et al. Sex-differences in psychosocial sequelae after spontaneous cervical artery dissection. Sci Rep. (2022) 12:611. doi: 10.1038/s41598-021-04686-7
9. Dittrich, R, Rohsbach, D, Heidbreder, A, Heuschmann, P, Nassenstein, I, Bachmann, R, et al. Mild mechanical traumas are possible risk factors for cervical artery dissection. Cerebrovasc Dis. (2007) 23:275–81. doi: 10.1159/000098327
10. Caso, V, Paciaroni, M, and Bogousslavsky, J. Environmental factors and cervical artery dissection. Front Neurol Neurosci. (2005) 20:44–53. doi: 10.1159/000088134
11. Haldeman, S, Kohlbeck, FJ, and McGregor, M. Risk factors and precipitating neck movements causing vertebrobasilar artery dissection after cervical trauma and spinal manipulation. Spine. (1999) 24:785–94. doi: 10.1097/00007632-199904150-00010
12. Genius, J, Dong-Si, T, Grau, AP, and Lichy, C. Postacute C-reactive protein levels are elevated in cervical artery dissection. Stroke. (2005) 36:e42–4. doi: 10.1161/01.STR.0000158911.74006.d6
13. Grau, AJ, Buggle, F, Ziegler, C, Schwarz, W, Meuser, J, Tasman, AJ, et al. Association between acute cerebrovascular ischemia and chronic and recurrent infection. Stroke. (1997) 28:1724–9. doi: 10.1161/01.STR.28.9.1724
14. Guillon, B, Berthet, K, Benslamia, L, Bertrand, M, Bousser, MG, and Tzourio, C. Infection and the risk of spontaneous cervical artery dissection: a case-control study. Stroke. (2003) 34:e79–81. doi: 10.1161/01.STR.0000078309.56307.5C
15. Rubinstein, SM, Peerdeman, SM, van Tulder, MW, Riphagen, I, and Haldeman, S. A systematic review of the risk factors for cervical artery dissection. Stroke. (2005) 36:1575–80. doi: 10.1161/01.STR.0000169919.73219.30
16. Kumar, SD, Kumar, V, and Kaye, W. Bilateral internal carotid artery dissection from vomiting. Am J Emerg Med. (1998) 16:669–70. doi: 10.1016/S0735-6757(98)90172-3
17. Gutowski, NJ, Murphy, RP, and Beale, DJ. Unilateral upper cervical posterior spinal artery syndrome following sneezing. J Neurol Neurosurg Psychiatry. (1992) 55:841–3. doi: 10.1136/jnnp.55.9.841
18. Mokri, B, Sundt, TM, Wayne, OH, and Piepgras, DG. Spontaneous dissection of the cervical internal carotid artery. Ann Neurol. (1986) 19:126–38. doi: 10.1002/ana.410190204
19. Brandt, T, Orberk, E, Weber, R, Werner, I, Busse, O, Müller, BT, et al. Pathogenesis of cervical artery dissections: association with connective tissue abnormalities. Neurology. (2001) 57:24–30. doi: 10.1212/WNL.57.1.24
20. de Bray, JM, Marc, G, Pautot, V, Vielle, B, Pasco, A, Lhoste, P, et al. Fibromuscular dysplasia may herald symptomatic recurrence of cervical artery dissection. Cerebrovasc Dis. (2007) 23:448–52. doi: 10.1159/000101470
21. Tzourio, C, Cohen, A, Lamisse, N, Biousse, V, and Bousser, MG. Aortic root dilatation in patients with spontaneous cervical artery dissection. Circulation. (1997) 95:2351–3. doi: 10.1161/01.CIR.95.10.2351
22. Calvet, D, Boutouyrie, P, Touzé, E, Laloux, B, Mas, JL, and Laurent, S. Increased stiff ness of the carotid wall material in patients with spontaneous cervical artery dissection. Stroke. (2004) 35:2078–82. doi: 10.1161/01.STR.0000136721.95301.8d
23. Lucas, C, Lecroart, JL, Gautier, C, Leclerc, X, Dauzat, M, Leys, D, et al. Impairment of endothelial function in patients with spontaneous cervical artery dissection: evidence for a general arterial wall disease. Cerebrovasc Dis. (2004) 17:170–4. doi: 10.1159/000075787
24. Brandt, T, Hausser, I, Orberk, E, Grau, A, Hartschuh, W, Anton-Lamprecht, I, et al. Ultrastructural connective tissue abnormalities in patients with spontaneous cervicocerebral artery dissections. Ann Neurol. (1998) 44:281–5. doi: 10.1002/ana.410440224
25. Ulbricht, D, Diederich, NJ, Hermanns-Le, T, Metz, RJ, Macian, F, and Pierard, GE. Cervical artery dissection: an atypical presentation with Ehlers-Danlos-like collagen pathology? Neurology. (2004) 63:1708–10. doi: 10.1212/01.WNL.0000142970.09454.30
26. Uhlig, P, Bruckner, P, Dittrich, R, Ringelstein, EB, Kuhlenbaumer, G, and Hansen, U. Aberrations of dermal connective tissue in patients with cervical artery dissection (sCAD). J Neurol. (2008) 255:340–6. doi: 10.1007/s00415-008-0585-4
27. Mayer, L, Pechlaner, R, Barallobre-Barreiro, J, Boehme, C, Toell, T, Lynch, M, et al. Extracellular matrix protein signature of recurrent spontaneous cervical artery dissection. Neurology. (2020) 95:e2047–55. doi: 10.1212/WNL.0000000000010710
28. Popov, P, Chapot, R, Tanasković, S, Vekić, B, Sotirovic, V, Ilijevski, N, et al. Vocal cord paralysis as the first sign of spontaneous carotid dissection in a patient with extracranial internal carotid artery aneurysm. Vasc Endovasc Surg. (2016) 50:52–6. doi: 10.1177/1538574415627698
29. Mayer-Suess, L, Dejakum, B, Ratzinger, G, Gizewski, ER, Kiechl, S, and Knoflach, M. Clinical characteristics and outcome in expansive compared with steno-occlusive mural hematoma in spontaneous cervical artery dissection. Int J Stroke. (2023) 18:1186–92. doi: 10.1177/17474930231185032
30. Lucas, C, Moulin, T, Deplanque, D, Tatu, L, and Chavot, D. Stroke patterns of internal carotid artery dissection in 40 patients. Stroke. (1998) 29:2646–8. doi: 10.1161/01.STR.29.12.2646
31. Völker, W, Dittrich, R, Grewe, S, Nassenstein, I, Csiba, L, Herczeg, L, et al. The outer arterial wall layers are primarily affected in spontaneous cervical artery dissection. Neurology. (2011) 76:1463–71. doi: 10.1212/WNL.0b013e318217e71c
32. Morel, A, Naggara, O, Touzé, E, Raymond, J, Mas, JL, Meder, JF, et al. Mechanism of ischemic infarct in spontaneous cervical artery dissection. Stroke. (2012) 43:1354–61. doi: 10.1161/STROKEAHA.111.643338
33. Perry, BC, and Al-Ali, F. Spontaneous cervical artery dissection: the borgess classification. Front Neurol. (2013) 4:133. doi: 10.3389/fneur.2013.00133
34. Schievink, WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. (2001) 344:898–906. doi: 10.1056/NEJM200103223441206
35. Völker, W, Besselmann, M, Dittrich, R, Nabavi, D, Konrad, C, Dziewas, R, et al. Generalized arteriopathy in patients with cervical artery dissection. Neurology. (2005) 64:1508–13. doi: 10.1212/01.WNL.0000159739.24607.98
36. Sturzenegger, M, and Huber, P. Cranial nerve palsies in spontaneous carotid artery dissection. J Neurol Neurosurg Psychiatry. (1993) 56:1191–9. doi: 10.1136/jnnp.56.11.1191
37. Benninger, DH, Georgiadis, D, Kremer, C, Studer, A, Nedeltchev, K, and Baumgartner, RW. Mechanism of ischemic infarct in spontaneous carotid dissection. Stroke. (2004) 35:482–5. doi: 10.1161/01.STR.0000109766.27393.52
38. Binaghi, S, Saint-Maurice, JP, Laurian, C, and Houdart, E. Embolic stroke complicating cervical aneurysm. Cerebrovasc Dis. (2006) 22:196–8. doi: 10.1159/000093806
39. Sturzenegger, M. Spontaneous internal carotid artery dissection: early diagnosis and management in 44 patients. J Neurol. (1995) 242:231–8. doi: 10.1007/BF00919596
40. Peeters, A, Goffette, P, Dorban, S, Sindic, CJ, and Cosnard, G. An old dissecting aneurysm of the internal carotid artery presenting as acute stroke. Acta Neurol Belg. (2003) 103:179–82.
41. Lee, VH, Brown, RD, Mandrekar, JN, and Mokri, B. Incidence and outcome of cervical artery dissection: a population-based study. Neurology. (2006) 67:1809–12. doi: 10.1212/01.wnl.0000244486.30455.71
42. Dziewas, R, Konrad, C, Dräger, B, Evers, S, Besselmann, M, Lüdemann, P, et al. Cervical artery dissection: clinical features, riskfactors, therapy and outcome in 126 patients. J Neurol. (2003) 250:1179–84. doi: 10.1007/s00415-003-0174-5
43. Baumgartner, RW, Arnold, M, Baumgartner, I, Mosso, M, Gönner, F, Studer, A, et al. Carotid dissection with and without ischemic events: local symptoms and cerebral artery findings. Neurology. (2001) 57:827–32. doi: 10.1212/WNL.57.5.827
44. Béjot, Y, Aboa-Eboulé, C, Debette, S, Pezzini, A, Tatlisumak, T, Engelter, S, et al. Characteristics and outcomes of patients with multiple cervical artery dissection. Stroke. (2014) 45:37–41. doi: 10.1161/STROKEAHA.113.001654
45. Arnold, M, Cumurciuc, R, Stapf, C, Favrole, P, Berthet, K, and Bousser, MG. Pain as the only symptom of cervical artery dissection. J Neurol Neurosurg Psychiatry. (2006) 77:1021–4. doi: 10.1136/jnnp.2006.094359
46. Biedermann, B, Sojer, M, Stockner, H, Spiegel, M, and Schmidauer, C. Dissektionen der Arteria carotis interna und vertebralis: Ursachen, Symptome, Diagnostik und Therapie. J Neurol Neurochir Psychiatr. (2007) 8:7–18.
47. Biousse, V, D’Anglejan-Chatillon, J, Touboul, PJ, Amarenco, P, and Bousser, MG. Time course of symptoms in extracranial carotid artery dissections. A series of 80 patients. Stroke. (1995) 26:235–9. doi: 10.1161/01.STR.26.2.235
48. Bogousslavsky, J, Despland, PA, and Regli, F. Spontaneous carotid dissection with acute stroke. Arch Neurol. (1987) 44:137–40. doi: 10.1001/archneur.1987.00520140009010
49. Silbert, PL, Mokri, B, and Schievink, WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. (1995) 45:1517–22. doi: 10.1212/WNL.45.8.1517
50. Baumgartner, R, and Bogousslavsky, J. Clinical manifestations of carotid dissection. Front Neurol Neurosci. (2005) 20:70–6. doi: 10.1159/000088151
51. Fisher, CM, Ojemann, RG, and Roberson, GH. Spontaneous dissection of cervicocerebral. Can J Neurol Sci. (1978) 5:9–19. doi: 10.1017/S0317167100024690
52. Hart, RG, and Easton, JD. Dissections of cervical and cerebral arteries. Neurol Clin. (1983) 1:155–82. doi: 10.1016/S0733-8619(18)31177-0
53. Leys, D, Lucas, C, Gobert, M, Deklunder, G, and Pruvo, JP. Cervical artery dissections. Eur Neurol. (1997) 37:3–12. doi: 10.1159/000117396
54. Mokri, B, Silbert, PL, Schievink, WI, and Piepgras, DG. Cranial nerve palsy in spontaneous dissection of the extracranial internal carotid artery. Neurology. (1996) 46:356–9. doi: 10.1212/WNL.46.2.356
55. Zheng, X, Wang, Y, Chen, G, Ma, C, Yan, W, and Chen, M. Diagnosis of ischemic optic neuropathy caused by dissection of the internal carotid artery: A case report. Medicine (Baltimore). (2020) 99:e20034. doi: 10.1097/MD.0000000000020034
56. Santos, T, Morais, H, Oliveira, G, and Barros, P. Isolated oculomotor nerve palsy: a rare manifestation of internal carotid artery dissection. BMJ Case Rep. (2014) 2014:bcr2014205413. doi: 10.1136/bcr-2014-205413
57. Nizam, A, Yacoub, HA, and McKinney, JS. Internal carotid artery dissection heralded by an oculomotor nerve palsy: case report and literature review. Neurologist. (2011) 17:333–7. doi: 10.1097/NRL.0b013e318218030a
58. Campos, CR, Massaro, AR, and Scaff, M. Isolated oculomotor nerve palsy in spontaneous internal carotid artery dissection: case report. Arq Neuropsiquiatr. (2003) 61:668–70. doi: 10.1590/S0004-282X2003000400027
59. Wessels, T, Röttger, C, Kaps, M, Traupe, H, and Stolz, E. Upper cranial nerve palsy resulting from spontaneous carotid dissection. J Neurol. (2005) 252:453–6. doi: 10.1007/s00415-005-0673-7
60. Hegde, V, Coutinho, CM, and Mitchell, JD. Dissection of the intracranial internal carotid artery producing isolated oculomotor nerve palsy with sparing of pupil. Acta Neurol Scand. (2002) 105:330–2. doi: 10.1034/j.1600-0404.2002.1c259.x
61. Schievink, WI, Mokri, B, Garrity, JA, Nichols, DA, and Piepgras, DG. Ocular motor nerve palsies in spontaneous dissections of the cervical internal carotid artery. Neurology. (1993) 43:1938–41. doi: 10.1212/WNL.43.10.1938
62. Majeed, A, Ribeiro, NPL, Ali, A, Hijazi, M, and Farook, H. A rare presentation of spontaneous internal carotid artery dissection with Horner’s syndrome, VIIth, Xth and XIIth nerve palsies. Oxford Med Case Rep. (2016) 2016:omw078. doi: 10.1093/omcr/omw078
63. Panisset, M, and Eidelman, BH. Multiple cranial neuropathy as a feature of internal carotid artery dissection. Stroke. (1990) 21:141–7. doi: 10.1161/01.STR.21.1.141
64. Chung, SE, Yoon, TH, Lee, KM, Kim, HG, and Kim, BJ. A case report of multiple cervical artery dissection after peripheral type facial palsy and use of steroids. BMC Neurol. (2018) 18:74. doi: 10.1186/s12883-018-1080-x
65. Gout, O, Bonnaud, I, Weill, A, Moulignier, A, Quenet, JJ, Moret, J, et al. Facial diplegia complicating a bilateral internal carotid artery dissection. Stroke. (1999) 30:681–6. doi: 10.1161/01.STR.30.3.681
66. McCarron, MO, Metcalfe, RA, and Muir, KW. Facial nerve palsy secondary to internal carotid artery dissection. Eur J Neurol. (2000) 7:723–5. doi: 10.1046/j.1468-1331.2000.00159.x
67. Taillibert, S, Bazin, B, and Pierrot-Deseilligny, C. Dysgeusia resulting from internal carotid dissection. A limited glossopharyngeal nerve palsy. J Neurol Neurosurg Psychiatry. (1998) 64:691–2. doi: 10.1136/jnnp.64.5.691
68. Nakagawa, H, Kusuyama, T, and Ogawa, K. Isolated vagus nerve paralysis associated with internal carotid artery dissection. Auris Nasus Larynx. (2014) 41:118–20. doi: 10.1016/j.anl.2013.07.006
69. Abukeshek, T, Gbande, P, and Hamed, R. Hypoglossal nerve palsy due to internal carotid artery dissection with pseudoaneurysm formation: an unusual presentation. Acta Radiol Open. (2022) 11:20584601221111701. doi: 10.1177/20584601221111701
70. Jurkiewicz, MT, Stein, JM, Learned, KO, Nasrallah, IM, and Loevner, LA. Hypoglossal nerve palsy due to carotid artery dissection: an uncommon presentation of a common problem. Neuroradiol J. (2019) 32:123–6. doi: 10.1177/1971400918825485
71. Cruciata, G, Parikh, R, Pradhan, M, Shah, J, Greif, E, and Stein, EG. Internal carotid artery dissection and pseudoaneurysm formation with resultant ipsilateral hypoglossal nerve palsy. Radiol Case Rep. (2017) 12:371–5. doi: 10.1016/j.radcr.2017.01.016
72. Schmutzhard, J, Widmann, G, Abraham, I, Furtner, M, and Riechelmann, H. Headache and hypoglossal nerve palsy. HNO. (2011) 57:690–2. doi: 10.1007/s00106-009-1926-z
73. Hafkamp, HC, Van Der Goten, A, and Manni, JJ. Unilateral spontaneous dissection of the internal carotid artery presenting as hypoglossal nerve palsy. Eur Arch Otorrinolaringol. (2004) 261:405–8. doi: 10.1007/s00405-003-0696-6
74. Lindsay, FW, Mullin, D, and Keefe, MA. Subacute hypoglossal nerve paresis with internal carotid artery dissection. Laryngoscope. (2003) 113:1530–3. doi: 10.1097/00005537-200309000-00022
75. Verdalle, P, Herve, S, Kossowski, M, Felten, D, Courtois, A, Garcia, D, et al. Spontaneous dissection of the internal carotid artery in its extracranial portion, revealed by a hypoglossal paralysis: report of four cases. Ann Otol Rhinol Laryngol. (2001) 110:794–8. doi: 10.1177/000348940111000818
76. Lieschke, GJ, Davis, S, Tress, BM, and Ebeling, P. Spontaneous internal carotid artery dissection presenting as hypoglossal nerve palsy. Stroke. (1988) 19:1151–5. doi: 10.1161/01.STR.19.9.1151
77. Epstein, E, Khan, MA, Francis, D, Sada, P, and Thuse, M. Carotid artery dissection causing hypoglossal nerve palsy. BMJ Case Rep. (2012) 2012:bcr0120125636. doi: 10.1136/bcr.01.2012.5636
78. Rees, JH, Valentine, AR, and Llewelyn, JG. Spontaneous bilateral carotid and vertebral artery dissection presenting as a collet-Sicard syndrome. Br J Radiol. (1997) 70:856–8. doi: 10.1259/bjr.70.836.9486056
79. Ruiz, J, Varona, L, Martín-Gómez, JI, Pérez-Bas, M, Mateos, B, and Zarranz, JJ. Spontaneous internal carotid artery dissection as a cause of unilateral lower cranial nerve palsies. Neurologia. (1995) 10:391–3.
80. Saliou, V, Ben Salem, D, Ognard, J, Guellec, D, Marcorelles, P, Rouhart, F, et al. A collet-Sicard syndrome due to internal carotid artery dissection associated with cerebral amyloid angiopathy-related inflammation. SAGE Open Med Case Rep. (2018) 6:2050313X18777176. doi: 10.1177/2050313X18777176
81. Smith, R, Tassone, P, and Saada, J. Collet-Sicard syndrome as a result of unilateral carotid artery dissection. BMJ Case Rep. (2013) 2013:bcr2013200358. doi: 10.1136/bcr-2013-200358
82. Zeleňák, K, Zeleňáková, J, DeRiggo, J, Kurča, E, Kantorová, E, and Poláček, H. Treatment of cervical internal carotid artery spontaneous dissection with pseudoaneurysm and unilateral lower cranial nerves palsy by two silk flow diverters. Cardiovasc Intervent Radiol. (2013) 36:1147–50. doi: 10.1007/s00270-012-0472-3
83. Okpala, O, Von Essen, A, Cummins, G, and Manford, M. Teaching NeuroImages: internal carotid artery dissection presenting as Villaret syndrome. Neurology. (2018) 82:e110-1. doi: 10.1212/WNL.0000000000000266
84. Mizutani, S, Tsukuura, R, Matsumura, K, Watanabe, M, Hanakawa, I, and Kamata, T. Villaret’s syndrome caused by internal carotid artery dissection. Rinsho Shinkeigaku. (2011) 51:608–11. doi: 10.5692/clinicalneurol.51.608
85. Al-Sihan, M Jr, Schumacher, M, and Löhle, E. Tapia syndrome caused by a vertebral artery dissection. Ear Nose Throat J. (2011) 90:313–4. doi: 10.1177/014556131109000709
86. Introna, A, Chiumarulo, L, and Petruzzellis, M. Dissecating aneurysm of extracranial internal carotid artery presenting with Tapia syndrome in patient with essential thrombocythemia. Neurol Sci. (2017) 38:1893–5. doi: 10.1007/s10072-017-3017-3
87. Guidetti, D, Pisanello, A, Giovanardi, F, Morandi, C, Zuccoli, G, and Troiso, A. Spontaneous carotid dissection presenting lower cranial nerve palsies. J Neurol Sci. (2001) 184:203–7. doi: 10.1016/S0022-510X(01)00440-3
88. Benninger, DH, and Baumgartner, RW. Ultrasound diagnosis of cervical artery dissection. Front Neurol Neurosci. (2006) 21:70–84. doi: 10.1159/000092386
89. Provenzale, JM. MRI and MRA for evaluation of dissection of craniocerebral arteries: lessons from the medical literature. Emerg Radiol. (2009) 16:185–93. doi: 10.1007/s10140-008-0770-x
90. Kattah, JC, Talkad, AV, Wang, DZ, Hsieh, YH, and Newman-Toker, DE. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke. (2009) 40:3504–10. doi: 10.1161/STROKEAHA.109.551234
91. Leclerc, X, Godefroy, O, Salhi, A, Lucas, C, Leys, D, and Pruvo, JP. Helical CT for the diagnosis of extracranial internal carotid artery dissection. Stroke. (1996) 27:461–6. doi: 10.1161/01.STR.27.3.461
92. Benninger, DH, Georgiadis, D, Gandjour, J, and Baumgartner, RW. Accuracy of color duplex ultrasound diagnosis of spontaneous carotid dissection causing ischemia. Stroke. (2006) 37:377–81. doi: 10.1161/01.STR.0000198811.65068.16
93. Debette, S, Mazighi, M, Bijlenga, P, Pezzini, A, Koga, M, Bersano, A, et al. SO guideline for the management of extracranial and intracranial artery dissection. Eur Stroke J. (2021) 6:XXXIX-LXXXVIII. doi: 10.1177/23969873211046475
Glossary
Keywords: cranial nerve palsy, vertebral artery dissection, internal carotid artery dissection, cervical artery dissection, intramural hematoma
Citation: Dejakum B, Kiechl S, Knoflach M and Mayer-Suess L (2024) A narrative review on cervical artery dissection-related cranial nerve palsies. Front. Neurol. 15:1364218. doi: 10.3389/fneur.2024.1364218
Edited by:
Anna Bersano, IRCCS Carlo Besta Neurological Institute Foundation, ItalyReviewed by:
Andrew Southerland, University of Virginia Health System, United StatesPeggy Reiner, Assistance Publique Hopitaux De Paris, France
Copyright © 2024 Dejakum, Kiechl, Knoflach and Mayer-Suess. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lukas Mayer-Suess, bHVrYXMubWF5ZXJAaS1tZWQuYWMuYXQ=
 Benjamin Dejakum
Benjamin Dejakum Stefan Kiechl1,2
Stefan Kiechl1,2 Michael Knoflach
Michael Knoflach Lukas Mayer-Suess
Lukas Mayer-Suess