- 1Department of Neurosurgery, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 2West China Centre of Excellence for Pancreatitis, Institute of Integrated Traditional Chinese and Western Medicine, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 3Department of Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, Sichuan, China
Background: Aneurysmal subarachnoid hemorrhage (aSAH) patients typically have poor prognoses. The anion gap (AG) has been proven to correlate with mortality in various critically ill patients. However, hypoalbuminemia can lead to underestimations of the true anion gap levels. This study was conducted to verify the prognostic value of single AG and albumin-corrected anion gap (ACAG) among aSAH patients.
Methods: Significant factors in the univariate logistic regression analysis were included in the multivariate logistic regression analysis to explore the risk factors for mortality in aSAH patients and to confirm the independent relationship between ACAG and mortality. The restricted cubic spline (RCS) was used to visually show the relationship between ACAG level and mortality risk of aSAH patients. The predictive model for mortality was developed by incorporating significant factors into the multivariate logistic regression analysis. The prognostic value of ACAG and the developed model was evaluated by calculating the area under the receiver operating characteristics curve (AUC).
Results: Among 710 aSAH patients, a 30-day mortality was observed in 20.3% of the cases. A positive relationship was demonstrated between the ACAG level and mortality in aSAH patients using the RCS curve. The multivariate logistic regression analysis helped discover that only six factors were finally and independently related to mortality of aSAH patients after adjusting for confounding effects, including the Hunt–Hess scale score (p = 0.006), surgical options (p < 0.001), white blood cell count (p < 0.001), serum chloride levels (p = 0.023), ACAG (p = 0.039), and delayed cerebral ischemia (p < 0.001). The AUC values for the AG, albumin, and ACAG in predicting mortality among aSAH patients were 0.606, 0.536, and 0.617, respectively. A logistic regression model, which includes the Hunt–Hess scale score, surgical options, white blood cell count, serum chloride levels, ACAG, and delayed cerebral ischemia, achieved an AUC of 0.911 for predicting mortality.
Conclusion: The ACAG is an effective prognostic marker for aSAH patients. A prognostic model incorporating ACAG could help clinicians evaluate the risk of poor outcomes among aSAH patients, thereby facilitating the development of personalized therapeutic strategies.
1 Introduction
With a reported annual incidence of 9.1 per 100,000 people in the world, aneurysmal subarachnoid hemorrhage (aSAH) is a type of hemorrhagic stroke with mortality ranging from 8.3% to 66.7% (1, 2). The high mortality of aSAH patients is attributable to both the initial severity of hemorrhage and the subsequent complications during hospitalizations (3). Acid-base disturbance and electrolyte disorder are prevalent among aSAH patients and correlate with the prognosis of aSAH patients (4–6). Evaluating the risk and severity of these disturbances is helpful for clinicians in the risk stratification of aSAH patients and for correcting these disturbances.
As an index for diagnosing and distinguishing metabolic acidosis, the anion gap (AG) is a comprehensive and readily available marker of acid-base balance calculated based on the following formula: AG = [Na+ (mmol/L) + K+ (mmol/L)] – [Cl−(mmol/L) + HCO (mmol/L)]. The AG has been considered a marker of tissue hypoperfusion and has been confirmed to be associated with mortality due to various diseases, including acute pancreatitis, acute myocardial infarction, congestive heart failure, and sepsis (7–11). However, AG levels can be underestimated with hypoalbuminemia. To address this, the albumin-corrected anion gap (ACAG) was developed to more accurately reflect true AG levels by accounting for the influence of albumin on measuring the true level of AG. Although some studies have explored the value of AG in risk stratification for stroke patients, including those diagnosed with ischemic stroke, intracerebral hemorrhage, or intracerebral infarction (12–14), only two studies have confirmed that AG is effective in the risk stratification of subarachnoid hemorrhage patients. These studies did not evaluate the accurate prognostic value of ACAG or establish a prognostic model incorporating it (15, 16). Therefore, we conducted this study to compare the different prognostic values between AG and ACAG and to develop a prognostic model for aSAH patients using ACAG.
2 Materials and methods
2.1 Patients
Patients confirmed with ruptured aneurysms and those receiving treatments in the West China Hospital, Sichuan University, between 1 January 2017 and 31 June 2019 were enrolled in this observational study. Some aSAH patients were excluded from this study to ensure the reliability of the conclusions: (1) patients transferred from other medical centers or admitted to our hospital 48 h after the onset of typical symptoms for aneurysm rupture (n = 8) and (2) patients without a history of albumin, AG, or other needed laboratory values (n = 27). A total of 710 aSAH patients were eventually enrolled in the study after screening (Figure 1). This observational study was conducted with the approval of the ethical review board of West China Hospital (2021–1684) and abided by the Declaration of Helsinki.
2.2 Data collection
Demographical information, including age, gender, history of smoking, history of alcoholism, and history of comorbidities such as diabetes mellitus and hypertension, was recorded. Initial blood pressure on admission and conventional clinical scores specified on aSAH patients, including the World Federation Neurosurgical Society (WFNS) score, the Hunt–Hess scale score, and the modified Fisher (mFisher) scale score, were collected. Information such as the location of the aneurysm, the presence of multiple aneurysms, and the occurrence of intraventricular hemorrhage was confirmed based on radiological findings. The values of white blood cell count, hemoglobin, albumin, serum creatinine, blood urea nitrogen, serum sodium, serum potassium, serum chloride, serum calcium, and serum AG were collected from laboratory records analyzing the first blood sample after admission (within 6 h after admission). Delayed cerebral ischemia was diagnosed based on the following process: the existence of a new focal functional defect (hemiplegia, aphasia, apraxia, hemianopsia, or neglect) or a decrease in the Glasgow Coma Scale (GCS) by at least two points, excluding other causes of secondary neurological deterioration such as fever, infectious complications, hydrocephalus, seizures, respiratory failure, or electrolyte disorders. The primary outcome of this study was 30-day mortality. The follow-up using telephone interviews lasted until 1 month after the aneurysm rupture.
2.3 Statistical analysis
Continuous variables were shown as mean ± standard deviation (normally distributed variables) and median (interquartile range) (non-normally distributed variables). Categorical variables were shown as counts (percentages). Patients were divided into two groups (survivors and non-survivors) based on their 30-day survival. Differences in the variables between these two groups were compared using χ2 tests or the Fisher test (categorical variables), the Independent Student's t-test (normally distributed variables), and the Mann—Whitney U-test (non-normally distributed variables). The restricted cubic spline (RCS) was used to visually demonstrate the relationship between the ACAG level and the mortality risk of aSAH patients. The multivariate logistic regression analysis included significant factors in the univariate logistic regression analysis to explore risk factors for mortality in aSAH patients and to confirm the independent relationship between ACAG and mortality. The predictive model for mortality was developed by incorporating significant factors into the multivariate logistic regression analysis. The prognostic value of ACAG and the developed model was evaluated by calculating the area under the receiver operating characteristics curve (AUC). The Z test was used to compare the AUC between the model and other scores.
A two-sided p < 0.05 was defined as statistically significant. Analyses were performed using SPSS 22.0 Windows software (SPSS, Inc., Chicago, IL) and R software (Version 3.6.1).
3 Results
3.1 Baseline comparison between survivors and non-survivors
A total of 144 aSAH patients experienced 30-day mortality, including 710 patients with a mortality rate of 20.3% (Table 1). Non-survivors had older age (p = 0.003), higher WFNS score (p < 0.001), higher Hunt-Hess score (p < 0.001), and higher mFisher score (p < 0.001) than survivors. Compared to the survivors, laboratory examinations showed that white blood cell count (p < 0.001), serum creatinine (p < 0.001), blood urea nitrogen (p = 0.027), serum sodium (p = 0.002), serum chloride (p < 0.001), AG (p < 0.001), and ACAG (p < 0.001) were all higher in the non-survivors. In contrast, the albumin level did not show statistical significance between the survivors and the non-survivors. Compared to the survivors, the non-survivors had a higher incidence of intraventricular hemorrhage (p = 0.007), delayed cerebral ischemia (p < 0.001), and a longer length of hospital stay (p < 0.001).
3.2 Association between ACAG and the mortality of aSAH patients
The positive relationship between the ACAG level and the mortality of aSAH patients is demonstrated using the unadjusted RCS curve shown in Figure 2A. The univariate logistic regression indicated that age (p = 0.001), WFNS score (p < 0.001), Hunt–Hess scale score (p < 0.001), mFisher scale score (p < 0.001), white blood cell count (p < 0.001), serum creatinine (p = 0.007), blood urea nitrogen (p = 0.046), serum sodium (p < 0.001), serum chloride (p < 0.001), ACAG (p < 0.001), intraventricular hemorrhage (p = 0.007), delayed cerebral ischemia (p < 0.001), surgical options (p < 0.001) were significantly correlated with the mortality of aSAH patients (Table 2). The multivariate logistic regression discovered that only six factors were finally and independently related to the mortality of aSAH patients after adjusting for confounding effects, including Hunt-Hess scale score (p = 0.006), surgical options (p < 0.001), white blood cells (p < 0.001), serum chloride (p = 0.023), ACAG (p = 0.039), and delayed cerebral ischemia (p < 0.001). After adjusting for the other five significant factors, the relationship between the ACAG level and the mortality of aSAH patients was still positive, as shown in Figure 2B.

Figure 2. (A) Unadjusted association between ACAG and the mortality of aSAH patients. (B) Adjusted association between ACAG and the mortality of aSAH patients.
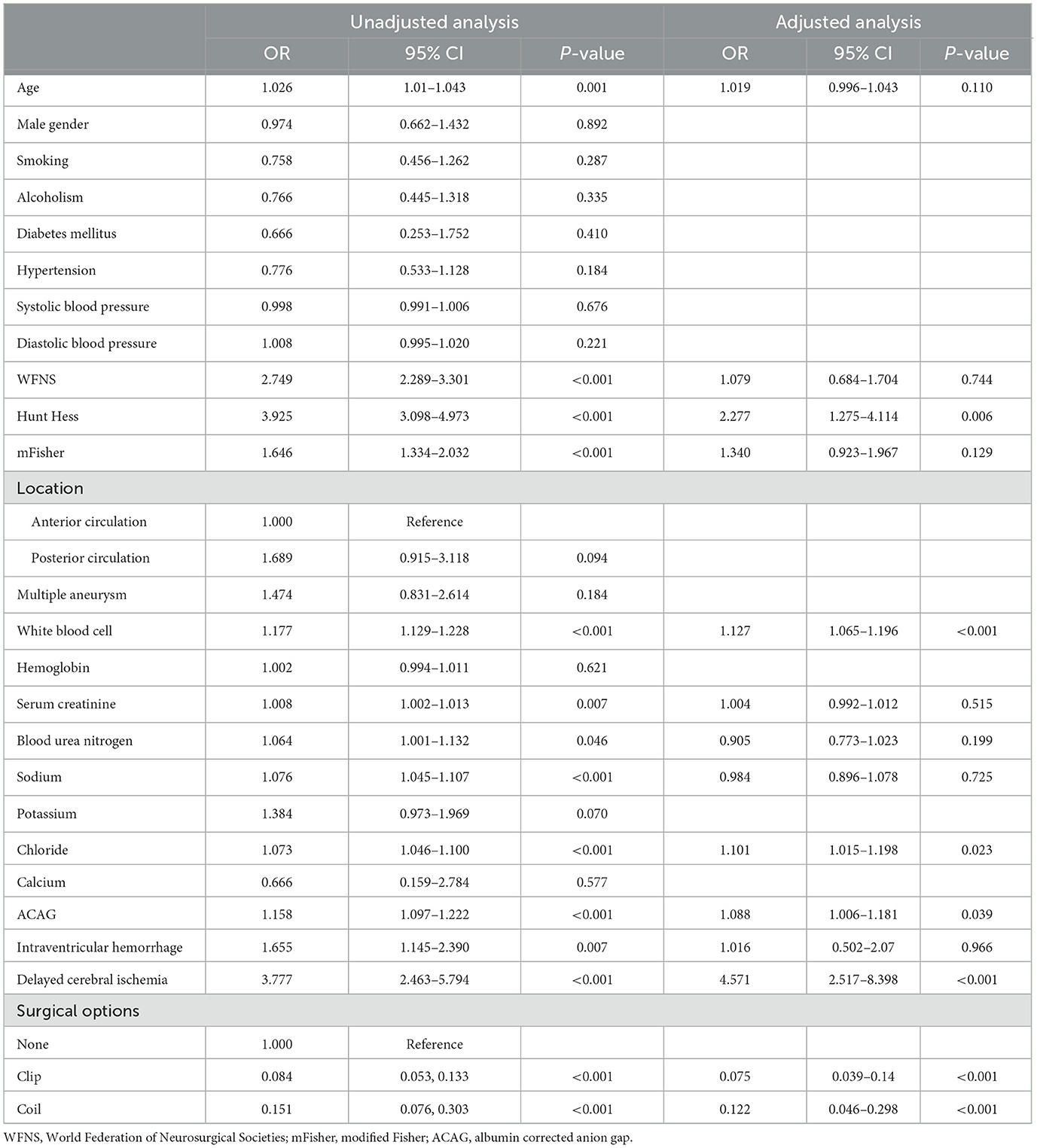
Table 2. Univariate and multivariate logistic regression analysis of risk factors for mortality in aSAH patients.
3.3 Prognostic value of ACGA in aSAH patients
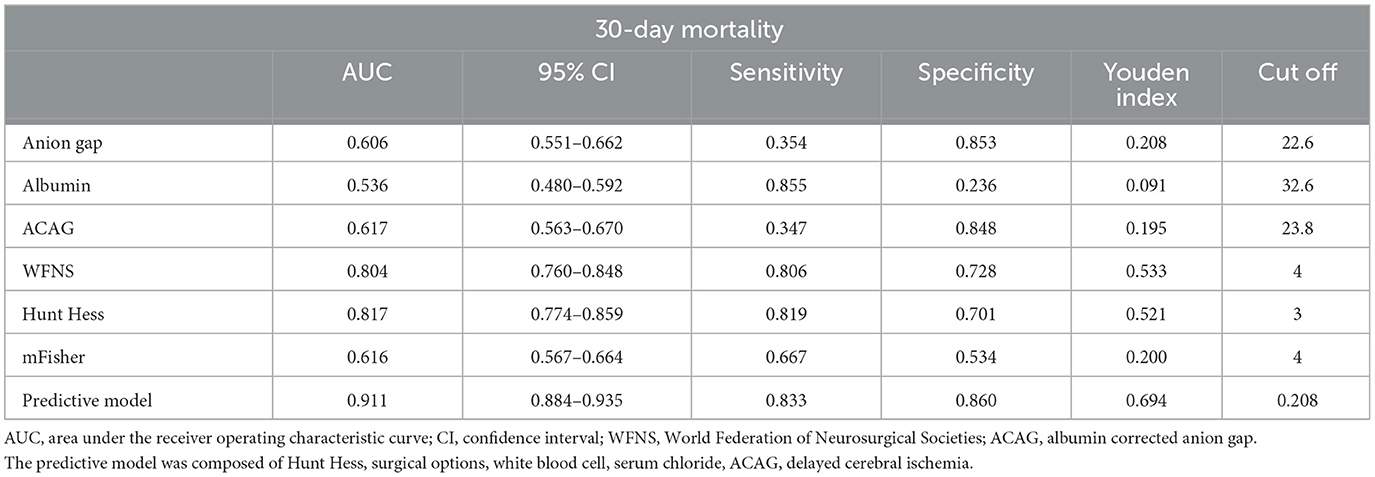
The AUC values for the AG, albumin, and ACAG in predicting the mortality of aSAH patients were 0.606, 0.536, and 0.617, respectively (Figure 3A; Table 3). The AUC values for conventional scoring systems, including the WFNS, Hunt–Hess, and mFisher scale scores, were 0.804, 0.817, and 0.616. A logistic regression model composed of the Hunt–Hess scale score, mFisher scale score, white blood cell count, serum chloride levels, ACAG, and delayed cerebral ischemia achieved an AUC of 0.911, which was significantly higher than that of the WFNS score (Z = 6.305, p < 0.001), the Hunt–Hess scale score (Z = 6.028, p < 0.001), the mFisher scale score (Z = 12.212, p < 0.001), and single ACAG (Z = 10.466, p < 0.001). The nomogram and calibration plot for this model are shown in Figures 3B, C.
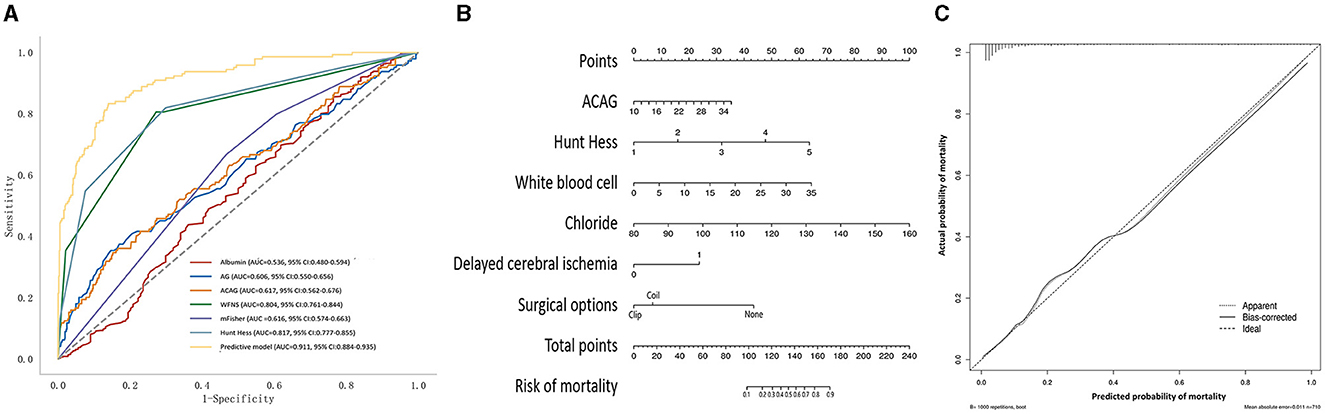
Figure 3. (A) The receiver operating characteristic curve of the prognostic model for the mortality of aSAH patients. (B) Nomogram of the prognostic model for the mortality of aSAH. (C) Calibration plot of the prognostic model for the mortality of aSAH.
4 Discussion
The prevalence of delayed cerebral ischemia and mortality was 16.5% and 20.3%, which was similar to the recently reported incidence (17–20). The high mortality rate makes risk evaluation crucial. As a readily available marker of acid-base balance, AG may be beneficial for the risk evaluation of prognoses among aSAH patients. The results of this study showed that, among aSAH patients, the non-survivors had higher levels of AG and ACAG than the survivors. The accuracy of ACAG in predicting the mortality of aSAH patients was higher than that of the original AG. As a marker identifying metabolic acidosis, the AG increased due to the overproduction of organic acids or the reduced renal excretion of anion (21). It is worth noting that aSAH leads to acidosis through multiple mechanisms, including hypoxia of cerebral tissue, accumulation of acidic metabolites, and other electrolyte disorders such as hypokalemia and hypocalcemia (4–6). Additionally, impaired renal function, which is commonly observed among aSAH patients, causes the accumulation of acidic metabolites (22–24). Therefore, the increased AG after aSAH may comprehensively reflect the abnormal metabolic state and renal dysfunction and thus contribute to the higher risk of mortality. Additionally, it could be deduced that elevated serum sodium levels cause an increase in AG based on the following formula: AG = [Na+ (mmol/L) + K+ (mmol/L)] – [Cl−(mmol/L) + HCO(25). The detrimental effect of hypernatremia on the prognosis of aSAH patients has been proven by several studies (6, 26–28). Finally, high serum AG levels have been found to be related to a high level of inflammatory biomarkers, including white blood cells and C-reactive protein (29). The inflammatory response after aSAH plays an important role in the pathophysiological process of brain injury and extracranial organ dysfunction (30–33).
Although many studies have explored the prognostic value of AG in various diseases, including acute pancreatitis, acute myocardial infarction, congestive heart failure, and sepsis, they have not compared the distinct impacts of single AG vs. ACAG (7–11). AG levels are often underestimated in cases of hypoalbuminemia, which commonly occurs during hospitalizations of aSAH patients (34). This underestimation may obscure the actual effect of acidosis on the prognosis of aSAH patients. Moreover, two recent studies that used AG for risk stratification in subarachnoid hemorrhage patients did not explore the supplementary effect of albumin correction or develop a prognostic model using ACAG (15, 16). The prognostic value of the ACAG was higher than that of the single AG in our study. When the measured AG is at the same level, a lower albumin level indicates a higher ACAG. This finding indicates that a high ACAG also partially reflects the effect of hypoalbuminemia on the prognosis of aSAH patients. Previous studies showed that hypoalbuminemia is associated with poor prognosis and complications after aSAH, including pneumonia, cerebral vasospasm, and delayed cerebral ischemia (35–38).
In addition to the AG, blood gas analysis could also be used to evaluate acid-base disturbances. The result of the blood gas analysis could be disturbed by compensatory respiratory alkalosis. Compared with the blood gas analysis needing arterial puncture, the AG is less expensive and more easily available in situations with limited resources (39). After being corrected using the albumin, the ACAG is useful for clinicians when evaluating the risk of poor prognosis among aSAH patients with relatively fewer resources.
Some limitations of this study should be noted. First, only the ACAG at admission was recorded, and any changes in ACAG were not tracked over time, so we could not evaluate the prognostic value of ACAG at different time points or ACAG changes among aSAH patients. Second, the record of blood lactate levels in our patient cohort was not complete, limiting our ability to compare the different values of lactate and ACAG. Third, only the survival outcome (30-day mortality) was analyzed; however, no other outcomes, including functional status and long-term cognitive status, were analyzed. The association between functional outcome and ACAG has been analyzed in our preliminary study, but we found that ACAG was not effective in predicting the functional outcome and thus did not include these results. Fourth, some confounders influencing the level of AG were not analyzed, including renal excretory function, the intravenous infusion volume, and the use of dehydration drugs. Fifth, some aneurysm-related morphological information, such as aneurysm size and aneurysm shape, was not collected. Our developed model may be improved after adding these variables. Finally, this study was conducted using the data from a single medical center in the southwestern region of China, highlighting the need for future studies in other regions and with larger sample sizes to validate our findings.
5 Conclusion
The ACAG is a prognostic marker for aSAH patients and could guide clinicians in correcting acid-base imbalances. A prognostic model incorporating ACAG could help clinicians evaluate the risk of poor outcomes among aSAH patients, thereby facilitating the development of personalized therapeutic strategies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of West China Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
RW: Conceptualization, Data curation, Formal analysis, Writing – original draft. JR: Data curation, Formal analysis, Writing – original draft. MH: Writing – review & editing. JX: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by 1.3.5 project for disciplines of excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University (2020HXFH036), Knowledge Innovation Program of the Chinese Academy of Sciences (JH2022007), General Program of the National Natural Science Foundation of China (82173175), and Sichuan Science and Technology Program (24QYCX0411 and 2024YFHZ0070).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. de Rooij NK, Linn FH, van der Plas JA, Algra A, Rinkel GJ. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatr. (2007) 78:1365–72. doi: 10.1136/jnnp.2007.117655
2. Nieuwkamp DJ, Setz LE, Algra A, Linn FH, de Rooij NK, et al. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol. (2009) 8:635–42. doi: 10.1016/S1474-4422(09)70126-7
3. Li R, Lin F, Chen Y, Lu J, Han H, Yan D, et al. In-hospital complication-related risk factors for discharge and 90-day outcomes in patients with aneurysmal subarachnoid hemorrhage after surgical clipping and endovascular coiling: a propensity score-matched analysis. J Neurosurg. (2021) 137:1–12. doi: 10.3171/2021.10.JNS211484
4. Alimohamadi M, Saghafinia M, Alikhani F, Danial Z, Shirani M, Amirjamshidi A, et al. Impact of electrolyte imbalances on the outcome of aneurysmal subarachnoid hemorrhage: a prospective study. Asian J Neurosurg. (2016) 11:29–33. doi: 10.4103/1793-5482.154978
5. Langer T, Zadek F, Carbonara M, Caccioppola A, Brusatori S, Zoerle T, et al. Cerebrospinal fluid and arterial acid-base equilibrium of spontaneously breathing patients with aneurismal subarachnoid hemorrhage. Neurocrit Care. (2022) 37:102–10. doi: 10.1007/s12028-022-01450-1
6. Qureshi AI, Suri MF, Sung GY, Straw RN, Yahia AM, Saad M, et al. Prognostic significance of hypernatremia and hyponatremia among patients with aneurysmal subarachnoid hemorrhage. Neurosurgery. (2002) 50:749–55. doi: 10.1097/00006123-200204000-00012
7. Mohr NM, Vakkalanka JP, Faine BA, Skow B, Harland KK, Dick-Perez R, et al. Serum anion gap predicts lactate poorly, but may be used to identify sepsis patients at risk for death: a cohort study. J Crit Care. (2018) 44:223–28. doi: 10.1016/j.jcrc.2017.10.043
8. Hu T, Zhang Z, Jiang Y. Albumin corrected anion gap for predicting in-hospital mortality among intensive care patients with sepsis: a retrospective propensity score matching analysis. Clin Chim Acta. (2021) 521:272–77. doi: 10.1016/j.cca.2021.07.021
9. Jian L, Zhang Z, Zhou Q, Duan X, Xu H, Ge L, et al. Association between albumin corrected anion gap and 30-day all-cause mortality of critically Ill patients with acute myocardial infarction: a retrospective analysis based on the mimic-IV database. BMC Cardiovasc Disord. (2023) 23:211. doi: 10.1186/s12872-023-03200-3
10. Lu J, Zhong L, Yuan M, Min J, Xu Y. Association between serum anion gap and all-cause mortality in patients with acute myocardial infarction: a retrospective study based on mimic-IV database. Heliyon. (2023) 9:e17397. doi: 10.1016/j.heliyon.2023.e17397
11. Li P, Shi L, Yan X, Wang L, Wan D, Zhang Z, et al. Albumin corrected anion gap and the risk of in-hospital mortality in patients with acute pancreatitis: a retrospective cohort study. J Inflamm Res. (2023) 16:2415–22. doi: 10.2147/JIR.S412860
12. Liu X, Feng Y, Zhu X, Shi Y, Lin M, Song X, et al. Serum anion gap at admission predicts all-cause mortality in critically ill patients with cerebral infarction: evidence from the mimic-III database. Biomarkers. (2020) 25:725–32. doi: 10.1080/1354750X.2020.1842497
13. Jhou HJ, Chen PH, Yang LY, Chang SH, Lee CH. Plasma anion gap and risk of in-hospital mortality in patients with acute ischemic stroke: analysis from the mimic-IV database. J Pers Med. (2021) 11:10004. doi: 10.3390/jpm11101004
14. Shen J, Li DL, Yang ZS, Zhang YZ. Anion gap predicts the long-term neurological and cognitive outcomes of spontaneous intracerebral hemorrhage. Eur Rev Med Pharmacol Sci. (2022) 26:3230–36. doi: 10.26355/eurrev_202205_28741
15. Ji L, Tong X, Wang K, Jiang Z, Liu A. Plasma anion gap and risk of in-hospital mortality in patients with spontaneous subarachnoid hemorrhage. Front Neurol. (2022) 13:1008030. doi: 10.3389/fneur.2022.1008030
16. Zhong C, Ye M, Hu L, Liu J. Association between high serum anion gap and all-cause mortality in non-traumatic subarachnoid hemorrhage: a retrospective analysis of the mimic-IV database. Front Neurol. (2022) 13:922099. doi: 10.3389/fneur.2022.922099
17. Labib H, Tjerkstra MA, Coert BA, Post R, Vandertop WP, Verbaan D, et al. Sodium and its impact on outcome after aneurysmal subarachnoid hemorrhage in patients with and without delayed cerebral ischemia. Crit Care Med. (2024) 52:752–63. doi: 10.1097/CCM.0000000000006182
18. Chikh K, Tonon D, Triglia T, Lagier D, Buisson A, Alessi MC, et al. Early metabolic disruption and predictive biomarkers of delayed-cerebral ischemia in aneurysmal subarachnoid hemorrhage. J Proteome Res. (2024) 23:316–28. doi: 10.1021/acs.jproteome.3c00575
19. Balança B, Bouchier B, Ritzenthaler T. The management of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Rev Neurol. (2022) 178:64–73. doi: 10.1016/j.neurol.2021.11.006
20. Han W, Yi HJ, Shin DS, Kim BT. Pan-immune-inflammation value predict delayed cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage. J Clin Neurosci. (2024) 121:47–52. doi: 10.1016/j.jocn.2024.02.003
21. Kraut JA, Madias NE. Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol. (2007) 2:162–74. doi: 10.2215/CJN.03020906
22. Guillemin L, Goronflot T, Desal H, Rozec B, Lakhal K. Early acute kidney injury in patients with non-traumatic subarachnoid hemorrhage who undergo catheter angiography: incidence, associated risk factors and impact on outcome. J Stroke Cerebrovasc Dis. (2022) 31:106488. doi: 10.1016/j.jstrokecerebrovasdis.2022.106488
23. Tujjar O, Belloni I, Hougardy JM, Scolletta S, Vincent JL, Creteur J, et al. acute kidney injury after subarachnoid hemorrhage. J Neurosurg Anesthesiol. (2017) 29:140–49. doi: 10.1097/ANA.0000000000000270
24. Eagles ME, Powell MF, Ayling OG, Tso MK, Macdonald RL. Acute kidney injury after aneurysmal subarachnoid hemorrhage and its effect on patient outcome: an exploratory analysis. J Neurosurg. (2019) 133:1–8. doi: 10.3171/2019.4.JNS19103
25. Miller PM, Dufour DR. Extreme hypernatremia with markedly increased anion gap. Clin Chem. (2015) 61:1422. doi: 10.1373/clinchem.2015.242313
26. Kumar AB, Shi Y, Shotwell MS, Richards J, Ehrenfeld JM. Hypernatremia is a significant risk factor for acute kidney injury after subarachnoid hemorrhage: a retrospective analysis. Neurocrit Care. (2015) 22:184–91. doi: 10.1007/s12028-014-0067-8
27. Jin D, Jin S, Liu B, Ding Y, Zhou F, Jin Y, et al. Association between serum sodium and in-hospital mortality among critically ill patients with spontaneous subarachnoid hemorrhage. Front Neurol. (2022) 13:1025808. doi: 10.3389/fneur.2022.1025808
28. Liu J, Li J, Zhang Q, Wang L, Wang Y, Zhang J, et al. Association between serum sodium levels within 24 h of admission and all-cause mortality in critically ill patients with non-traumatic subarachnoid hemorrhage: a retrospective analysis of the mimic-IV database. Front Neurol. (2023) 14:1234080. doi: 10.3389/fneur.2023.1234080
29. Farwell WR, Taylor EN. Serum anion gap, bicarbonate and biomarkers of inflammation in healthy individuals in a national survey. Cmaj. (2010) 182:137–41. doi: 10.1503/cmaj.090329
30. Wu F, Liu Z, Li G, Zhou L, Huang K, Wu Z, et al. Inflammation and oxidative stress: potential targets for improving prognosis after subarachnoid hemorrhage. Front Cell Neurosci. (2021) 15:739506. doi: 10.3389/fncel.2021.739506
31. Nie Z, Lin F, Li R, Chen X, Zhao Y, A. Pooled analysis of preoperative inflammatory biomarkers to predict 90-day outcomes in patients with an aneurysmal subarachnoid hemorrhage: a single-center retrospective study. Brain Sci 13. (2023). doi: 10.3390/brainsci13020257
32. Peng L, Li X, Li H, Zhong Y, Lian J, Gao H, et al. Relationship between peripheral blood inflammatory factors and prognosis of subarachnoid hemorrhage: a meta-analysis. Eur Neurol. (2023) 86:193–206. doi: 10.1159/000530208
33. Rostgaard N, Olsen MH, Capion T, MacAulay N, Juhler M. Inflammatory markers as predictors of shunt dependency and functional outcome in patients with aneurysmal subarachnoid hemorrhage. Biomedicines 11. (2023). doi: 10.3390/biomedicines11040997
34. Behrouz R, Godoy DA, Topel CH, Birnbaum LA, Caron JL, Grandhi R, et al. Early hypoalbuminemia is an independent predictor of mortality in aneurysmal subarachnoid hemorrhage. Neurocrit Care. (2016) 25:230–6. doi: 10.1007/s12028-016-0259-5
35. Shang F, Zhao H, Cheng W, Qi M, Wang N, Qu X, et al. Predictive value of the serum albumin level on admission in patients with spontaneous subarachnoid hemorrhage. Front Surg. (2021) 8:719226. doi: 10.3389/fsurg.2021.719226
36. Wang P, Zhang Y, Wang X, Peng L, Jia L, Li T, et al. Association between serum albumin and hospital-acquired infections after aneurysmal subarachnoid hemorrhage. Neurocrit Care. (2022) 37:424–34. doi: 10.1007/s12028-021-01421-y
37. Dicpinigaitis AJ, Galea VP, Sursal T, Al-Shammari H, Feldstein E, Ali S, et al. Low serum albumin as a risk factor for delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage: eicu collaborative research database analysis. J Neurosurg Sci. (2022). doi: 10.23736/S0390-5616.22.05604-1
38. Chen L, Jin Y, Wang L, Wei K, Li X, Jiang T, et al. Impact of human serum albumin level on symptomatic cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. Neurol Sci. (2024) 45:213–22. doi: 10.1007/s10072-023-07014-1
Keywords: serum anion gap, albumin, aneurysmal subarachnoid hemorrhage, prognosis, marker
Citation: Wang R, Rong J, Xu J and He M (2024) A prognostic model incorporating the albumin-corrected anion gap in patients with aneurysmal subarachnoid hemorrhage. Front. Neurol. 15:1361888. doi: 10.3389/fneur.2024.1361888
Received: 27 December 2023; Accepted: 24 May 2024;
Published: 19 June 2024.
Edited by:
Pradeep Kumar, All India Institute of Medical Sciences, IndiaReviewed by:
Mingchao Fan, The Affiliated Hospital of Qingdao University, ChinaYu Li, The First Affiliated Hospital of Kunming Medical University, China
Yunlin Chen, Second Affiliated Hospital of Chongqing Medical University, China
Copyright © 2024 Wang, Rong, Xu and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianguo Xu, eHVqZ0BzY3UuZWR1LmNu; Min He, aGVtaW4xOTkxMDMwNkB3Y2hzY3UuY24=
 Ruoran Wang
Ruoran Wang Juan Rong
Juan Rong Jianguo Xu
Jianguo Xu Min He
Min He