
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 15 March 2024
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1360741
This article is part of the Research Topic Clinical Therapy of Brain Tumors View all 17 articles
Background: This study aimed to investigate the effects of adjuvant beam radiation therapy (ABRT) on overall survival (OS) in patients with primary single intracranial atypical meningioma (AM), with a focus on age-related outcomes.
Methods: We conducted a retrospective study using data from SEER database. Our cohort consisted of patients diagnosed with a primary single intracranial AM tumor and had undergone surgery. The primary endpoint was OS. For survival analysis, univariable and multivariable Cox regression analysis were performed. A multivariable additive Cox model was used to assess the functional relationship between age and OS in patients with or without ABRT.
Results: Of the 2,759 patients included, 1,650 underwent gross total resection and 833 received ABRT. Multivariable Cox analysis indicated that ABRT did not significantly influence OS across the entire cohort. According to the multivariable generalized additive Cox model, the relative risk of all-cause mortality increased with advancing age in both ABRT-yes and ABRT-no group. ABRT-yes had a lower relative risk than ABRT-no when age ≤ 55 years old while a higher relative risk when age > 55 years old. Subsequent multivariable Cox analysis showed that ABRT was associated with a significant lower risk for all-cause mortality in patients with age ≤ 55 years old while a significant higher risk in patients with age > 55 years old.
Conclusion: Our study found that ABRT enhanced OS in younger primary single intracranial AM patients. But we also revealed a negative correlation between OS and ABRT in older patients.
Meningiomas are the most common primary tumors of the central nervous system, accounting for 40.0%, and are predominantly located in the cranial region (1). Atypical meningioma (AM) is the predominant subtype of WHO II meningiomas which accounts for 18.3% of total meningiomas (2). Surgery and adjuvant radiotherapy are two main treatment therapies for AM patients according to the 2016 and 2021 European Association of Neuro-Oncology (EANO) guidelines (3, 4). Surgery, aiming for Simpson I resection, stands as the primary treatment given that the extent of resection has been identified as an independent prognostic risk factor, supporting by abundant evidence (5–9). However, the efficacy of adjuvant beam radiation therapy (ABRT) for the treatment of AM patients remains an area of contention (4, 10). Conflicting results regarding ABRT’s effectiveness have been reported for AM patients. Some studies found ABRT was independently associated with worse overall survival (OS) and/or progression free survival (PFS) in AM patients (11–15), while some studies argued ABRT’s protective role against mortality and/or recurrence (9, 16–20). There are also studies that have found ABRT did not significantly influence the prognosis (21–25). The effect of ABRT in AM patients still needs to be elucidated.
Age is a recognized risk factor for the occurrence of meningiomas. Meningiomas mostly occur in old individuals and the incidence rate increase obviously with age, rarely occurring in children (1). Interestingly, the gender distribution of patients shifts with age, showing an initial rise in the female to male ratio that eventually declines in older populations (26). A previous study showed that tumor gene expression and chromosome abnormalities were associated with patient gender (27). Some studies also found that younger and older meningioma patients had different tumor pathology characteristics (28–32). Additionally, age could potentially influence the effect of radiation therapy. As some tumors’ clinical and biology behavior can change with age (33), and as aging process is associated with a decrease function of various organ systems, potentially heightening the risk of radiation-related side effects (34–37). The influence of age on the effect of radiation therapy with respect to prognosis has been discussed across various tumor types and many studies have elucidated that age might modulate the impact of radiation therapy, leading to prognosis variations among different age groups. Some studies reported that radiation therapy significantly decreased adverse outcomes in younger tumor patients, but increasing adverse outcomes in older patients (37–39). While some studies reported the opposed results (40–42). Considering impaired neurocognitive function and comorbidities might lead to radiation-induced toxicity, radiation therapy for old brain tumor patients is challenging (43).
The Surveillance, Epidemiology and End Results (SEER) database is a public database, which covers approximately 28% of population of United States. SEER database record demographic, oncology, treatment, and survival data. Surgery and radiation therapy information is released as part of the first course of treatment.1 And a lot of studies employed SEER data to discuss the efficacies of surgery type and adjuvant radiotherapy in a variety of tumor types (44–46). Some research studies have also assessed the role of ABRT in AM patients utilizing SEER or other database (6, 8, 25, 47). However, there are limited research studies which focus on the influence of age on the radiation therapy efficacy in AM patients who have undergone surgery. Hence, the aim of this study was to investigate impact of ABRT on OS in primary single intracranial AM patients with a focus on age, drawing upon a vast pool of carefully selected cases from SEER database.
The SEER database is publicly accessible, and we have obtained the access. Since all patients in this study were from this database, this study did not require the procedure, participate, or publish consent of patients, nor the approval of the ethics committee.
This research adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. Patients diagnosed with primary single intracranial AM who had undergone surgery were selected from a dataset containing 17 registries [Nov 2021 Sub (2000–2019)] in the SEER database. The inclusion criteria were: (1) Diagnosis of AM; (2) Primary site should be intracranial; and (3) Microscopic confirmation for each case. The exclusion criteria were: (1) History of tumors; (2) Clinical information was missing or unclear; (3) Surgery was not performed; (4) Chemotherapy was administered; (5) Age < 18 years old; and (6) Follow-up time ≤ 3 months. Details of the inclusion and exclusion criteria are presented in Supplementary Note 1. Meningioma sizes larger than 150 mm were rare and more likely to be coding errors, so the limit was set at 150 mm, in line with previous research (48). The research query can be found in Supplementary Note 2. The flow diagram is shown in Figure 1.
Demographic, oncological, treatment, and survival information were obtained for analysis. Demographic information included age, gender, race, and marital status. Oncological information such as year of diagnosis, tumor size, primary site, and laterality were recorded. Treatment information included surgery and ABRT (yes or no). Regarding surgery information, entries recorded as “55” and “30” under “Surg Prim Site (1998+)” were classified as gross total resection (GTR), while codes “20,” “21,” and “40” constituted subtotal resection (STR). Patients with “RX Summ--Surg/Rad Seq” recorded as “Radiation after surgery” and “Radiation recode” recorded as “Beam radiation” were identified as having undergone ABRT. The endpoint was OS, with a maximum follow-up period of 60 months.
The comparison of categorical variables between different groups was performed using Chi-square test or, when appropriate, Fisher’s exact test. The comparison of continuous variables was performed by Student’s t-test or Mann–Whitney U test where appropriate, as our previous study described (49). For OS analysis, Kaplan–Meier method was used to estimate OS rate. Univariable Cox regression analysis was performed and variables with p values less than 0.1 in univariable analysis were included in the multivariable Cox regression analysis. Hazard ratio (HR) and 95% confidence interval were calculated. A multivariable additive Cox proportional hazard model was used to assess the functional relationship between age and OS in patients with or without ABRT. The relative risk was calculated and then visualized with smooth spline curve. The abscissa value at the intersection point of the curves for the ABRT-yes group and the ABRT-no group was used as the cut-off value for age. The effect of ABRT was assessed using multivariable Cox regression analysis, and the interaction was inspected using likelihood ratio test in different age groups.
A p-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using R-4.2.0 (R Foundation for Statistical Computing, Vienna, Austria) and Empower Stats 4.1 (X&Y solutions, Inc. Boston, Massachusetts).
From the 4,138 patients who initially met the inclusion criteria (Figure 1), 1,379 were excluded, leaving 2,759 for study. All the patients were diagnosed by histology. The characteristics of the patients are shown in Table 1. The mean age was 58.5 ± 14.9 years old. Major patients were females (57.8%; female to male ratio = 1.37). Most patients were white race (73.5%). The mean tumor size was 49.0 ± 16.8 mm. Most tumor sites were identified as cerebral meninges, with only 0.7% being recorded as the brain. Cases of left laterality and right laterality were similar. And most patients (58.5%) were married. As for treatment, 1,650 (59.8%) patients underwent GTR and 1,109 (40.2%) patients underwent STR. Most patients (1926, 69.8%) did not received ABRT and 833 (30.2%) patients received it. There are some variables that are different between ABRT-yes and ABRT-no group, including age (p = 0.003), year of diagnosis (p < 0.001), tumor size (p = 0.001), laterality (p = 0.039), marital status (p = 0.040), and surgery type (p < 0.001). Patients received ABRT was younger and had a large tumor size. Notably, there was an observed increase in the ratio of ABRT-yes to ABRT-no trend over the years. Importantly, ABRT-yes group had a higher proportion (48.1% vs. 36.8%) of patients who received STR.
The maximum follow-up time was established at 60 months, during which 349 (12.7%) patients experienced all-cause mortality. The estimated 3-year OS rate was 90.3 ± 0.6%, the estimated 5-year OS rate was 82.8 ± 0.9%.
The univariable and multivariable Cox analysis results were shown in Table 2. Univariable Cox analysis showed that age (HR 1.06 [1.05, 1.07], p < 0.001), male gender (HR 1.26 [1.02, 1.56], p = 0.029), black race (HR 1.47 [1.12, 1.93], p = 0.005), tumor size (HR 1.01 [1.01, 1.02], p < 0.001), marital status (HR 3.76 [2.85, 4.97], p < 0.001 for ‘Widowed’; HR 1.83 [1.19, 2.80], p = 0.006 for ‘Other’), and STR (HR 1.41 [1.15, 1.75], p = 0.001) had a significant HR for OS. These variables and ABRT were enrolled in the multivariable analysis. Older age (HR 1.06 [1.05, 1.07], p < 0.001), male gender (HR 1.32 [1.05, 1.65], p = 0.016), black race (HR 1.47 [1.11, 1.95], p = 0.005), larger tumor size (1.01 [1.01, 1.02], p < 0.001), and STR (1.43 [1.16, 1.77], p < 0.001) were independent risk factors for OS. Interestingly, some marital status also had a significant impact for prognosis, such as widowed (2.08 [1.52, 2.84], p < 0.001) and single (1.78 [1.33, 2.39], p < 0.001). However, ABRT did not show a significant HR in the multivariable analysis in the overall population (1.21 [0.96, 1.54], p = 0.115).
Multivariable additive Cox proportional model was used to assess the functional relationship between age and the risk of all-cause mortality in patients with and without ABRT, respectively. The model was adjusted with variables which showed significant HR in the multivariable Cox analysis, including gender, race, marital status, tumor size, and surgery type. The HR was calculated at different ages and the results was shown in Figure 2. As we can see, the relative risk for OS increased with age in both ABRT-yes and ABRT-no group. But when age ≤ 55 years old, ABRT-yes had a lower relative risk for OS than ABRT-no. And when age > 55 years old, ABRT-yes was associated with a higher risk for OS.
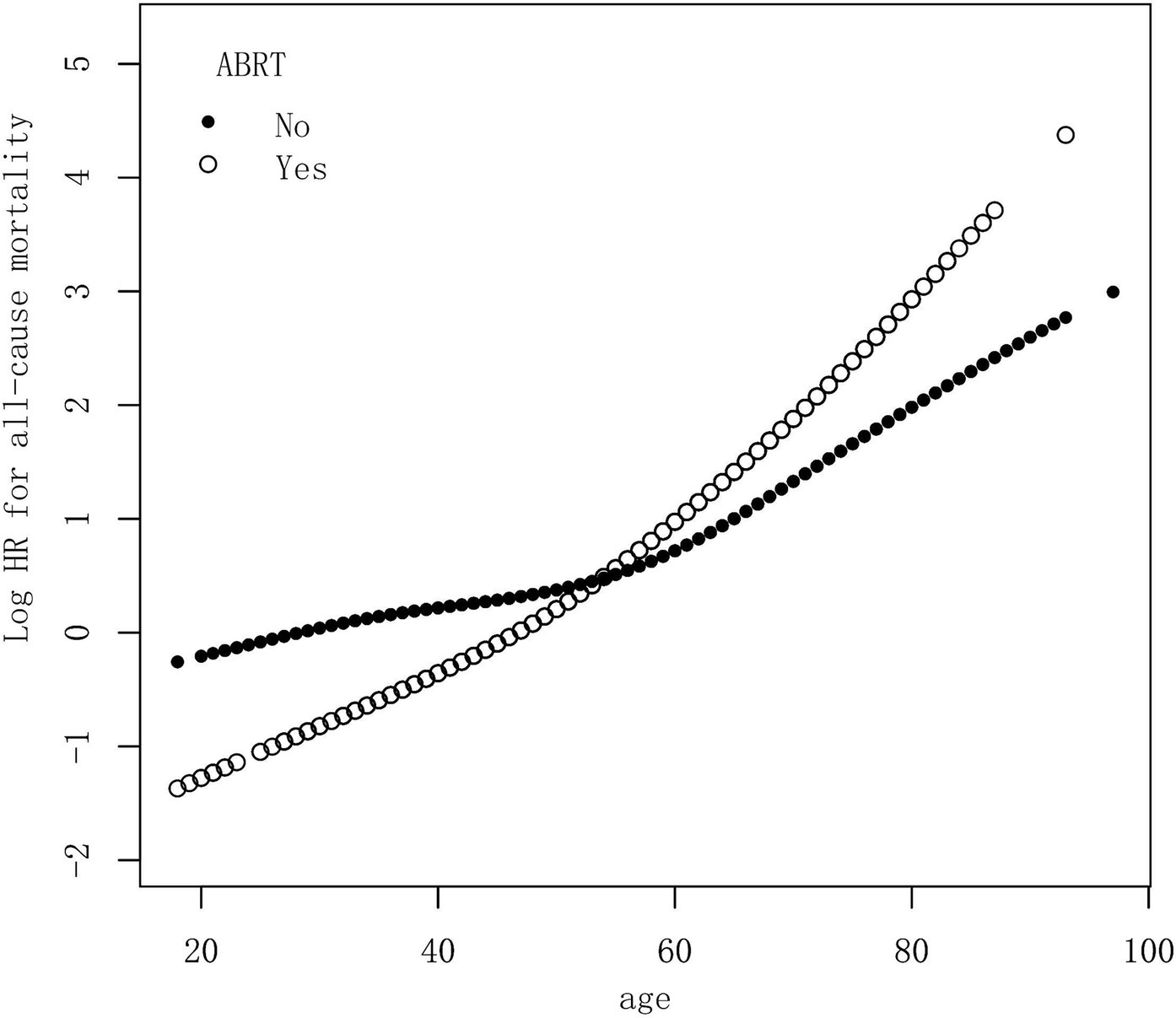
Figure 2. Multivariable additive Cox proportional hazard model demonstrated the relationship between age and the risk of all-cause mortality in patients with or without ABRT. The model was adjusted with variables, including gender, race, marital status, tumor size, and surgery type. HR, hazard ratio; ABRT, adjuvant beam radiation therapy.
The HR of ABRT for OS was calculated with different Cox model adjusted by different variables. As we can see in Table 3, the crude model was adjusted with no variable, the Adjust model I was adjusted with demographic variables, including gender, race, age, and marital status. The Adjust model II was adjusted with demographic, tumor, and treatment variables, including gender, race, age, marital status, tumor size, laterality, primary site, and surgery type. In the crude model, although the HR of ABRT in patients with age ≤ 55 years old was small (0.51 [0.26, 1.02]), the p value (0.058) did not meet the level of statistical significance. The HR of ABRT in patients with age > 55 years old was 1.20 [0.94, 1.53] (p = 0.152) in the crude model. And in Adjust model I, the HR of ABRT was 0.49 [0.25, 0.97] (p = 0.042) and 1.59 [1.23, 2.06] (p < 0.001) in patients with age ≤ 55 years old and > 55 years old, respectively. In Adjust model II, the HR of ABRT was 0.49 [0.25, 0.99] (p = 0.045) and 1.52 [1.17, 1.98] (p = 0.002) in patients with age ≤ 55 years old and > 55 years old, respectively. These results from multivariable adjusted models showed that ABRT associated with a lower risk of all-cause death in patients with age ≤ 55 years old while a higher risk of death in patients with age > 55 years old. And a significant interaction was observed between age group and ABRT across all three models (p = 0.015, 0.001, and 0.001 for Crude model, Adjust model I, and Adjust model II, respectively).
Our study aimed to investigate the effect of ABRT for OS in primary single intracranial AM patients, with a focus on the age. Our results demonstrated that in younger patients (age ≤ 55 years old) ABRT associated with a lower risk of all-cause mortality while in older patients (age > 55 years old) ABRT associated with a higher risk of death.
Given the small number of AM patients at individual centers, various studies have also used public database to investigate the efficacy of different treatment methods in AM patients. But the results of these studies are not consistent. In 2012, Stessin et al. (50) used SEER database to explore the effect of ABRT in nonbenign meningioma patients. They included both WHO II and III cases, enrolling and analyzing 657 patients together. After multivariable analysis, they found that ABRT did not appear as a significant prognostic factor. In 2015, Aizer et al. (6) also utilized the SEER database, enrolling 575 AM patients. The authors found ABRT did not affect OS. In 2018, Rydzewski et al. (5) enrolled 3,529 AM patients from the National Cancer Data Base and identified ABRT as a significant factor in enhancing OS in multivariable analysis. In 2019, Zeng et al. (8) also used SEER database and enrolled 1,014 AM patients, founding that ABRT did not significantly influence OS across the cohort.
When we used the SEER database, we enrolled a large number of AM patients (covering overing 2,700 cases), second only to Rydzewski et al.’s study (5), as far as we know. And we carefully selected patients and made the cohort homogeneous, consisting only of patients with primary single intracranial AM, and excluding patients with multiple meningiomas or spinal meningiomas. Given that AM is not a malignancy tumor and is associated with lower mortality compared to malignant tumors, such as glioblastoma (51), lung cancer (52), cervical cancer (53), and gastric cancer (54), the inclusion of these malignant tumors could introduce bias into the survival analysis of AM. So, patients with tumor history were also excluded. Additionally, to eliminate the impact of perioperative mortality, we included only patients with follow-up time exceeding 3 months. Strict inclusion and exclusion criteria can make our results more reliable.
The increased risk of all-cause mortality in older AM patients receiving ABRT can be attributed to two primary factors. Firstly, ABRT may not offer benefits to older AM patients, possibly due to heightened radiation toxicity in this age group. Studies have reported that elderly patients were more susceptible to brain atrophy and dementia induced by radiation than younger patients (55, 56). As a result, younger AM patients may benefit from ABRT with a good tolerance of radiation toxicity, while older patients may be less tolerable and thus experience worse outcomes from ABRT. Secondly, the increased mortality observed in older patients receiving ABRT could be due to the patients’ inherently worse oncological characteristics, such as a more aggressive pathological phenotype or larger postoperative residuals. This may suggest that treatment for tumors with ABRT in older patients was insufficient.
Despite observing an increased risk of all-cause mortality in older AM patients, we cannot conclusively state that ABRT will cause the deterioration of OS in older AM patients since the burden of proof needs to be high. The efficacy of ABRT in moderating AM prognosis remains to be validated through robust prospective research, such as the ROAM/EORTC-1308 trial (57).
Anyway, our observations underscore the need for refined treatment approaches for older AM patients. And it is gratifying to note that we observed ABRT noticeably improved OS in younger AM patients. This insight suggests that younger patients with AM might be more suitable candidates for ABRT, potentially guiding clinicians toward more aggressive treatment approaches in this demographic.
Future research should investigate the biological and pathological variances in AM tumors between younger and older patients. Biomarkers that can predict response to ABRT should also be identified. The 2021 EANO guidelines advocate for ABRT in WHO II meningioma patients, particularly patients without GTR. Our findings also revealed that patients receiving ABRT had a significantly higher proportion of STR (see Table 1). The impact of ABRT on patients with different extent of resection (GTR or STR) should also be explored in the future.
There are several limitations in this study. Firstly, the details of ABRT, including dose, time, fractionation, etc., are unavailable from SEER database. Secondly, the details of pathology information for tumors are also not available, such as Ki-67 index. Thirdly, we employed the additive Cox proportional hazard model to calculate the relative risk and visualized it using smooth spline curve, with the age cut-off value (55 years old) determined by the intersection point. Although the additive Cox proportional hazard model is commonly utilized to build smooth spline curves and identify cut-off values (58–64), several considerations must be considered. For instance, the inclusion of smoothing parameters might complicate the interpretation of the results and cause the overfitting. Moreover, the cut-off value was chose based on the statistical criteria alone, its medical and biological significance needs to be clarified. Lastly, PFS information and cause-specific death data are not available for AM patients in the SEER database. Nevertheless, our study’s results based on OS also warrant attention since OS is the gold standard primary endpoint in the tumor studies of clinical investigations (65, 66).
In conclusion, our study found that ABRT improved OS in younger primary single intracranial AM patients. We also revealed a negative correlation between OS and ABRT in older patients. This observation might stem from the long-term toxicity of radiation therapy for older patients. And it also might be attributed to the more invasive nature of tumors or larger postoperative residuals in this age group treated with ABRT, rendering the treatment insufficient. Our results call for a careful examination of both possibilities and further research is needed explore the optimal treatment strategies for AM patients, especially for elderly patients.
The data analyzed in this study was obtained from the National Institutes of Health (NIH), National Cancer Institute (NCI), Surveillance, Epidemiology, and End Results (SEER) database, the following licenses/restrictions apply: to request access to the Research Plus Data, users must login with an eRA Commons account that is associated with an institutional email address (.edu,.gov,.org, or work email address) for user authentication. Users with access only to the Research Data are not eligible to request specialized databases and cannot upgrade to Research Plus without an eRA Commons account or an HHS PIV card. Requests to access these datasets should be directed to SEER, https://seerdataaccess.cancer.gov/seer-data-access.
Ethical approval was not required for the studies involving humans because the SEER database is publicly accessible, and we have obtained the access. Since all patients in this study are from this database, this study does not require the approval of the ethics committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements because the patient data from SEER are strictly de-identified.
CL: Formal analysis, Methodology, Writing – original draft. JQ: Methodology, Writing – original draft. FX: Methodology, Writing – original draft. ZS: Methodology, Writing – review & editing. QL: Methodology, Writing – review & editing. YX: Conceptualization, Supervision, Writing – review & editing. XC: Conceptualization, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Program of Shanghai Municipal Commission of Health (No. 201940126), Program of Shanghai Tenth People’s Hospital (No. 2023YJXYSB008), and Program of Science and Technology Commission of Shanghai Municipality (No. 23141901100).
The authors wish to thank Jingfang Tang (Shanghai Putuo Center for Disease Control and Prevention) for her help with data analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1360741/full#supplementary-material
ABRT, adjuvant beam radiation therapy; AM, atypical meningioma; OS, overall survival; GTR, gross total resection; STR, subtotal resection; SEER, Surveillance, Epidemiology and End Results; HR, hazard ratio.
1. Ostrom, QT, Price, M, Neff, C, Cioffi, G, Waite, KA, Kruchko, C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2015-2019. Neuro Oncology. (2022) 24:v1–v95. doi: 10.1093/neuonc/noac202
2. Ren, L, Hua, L, Deng, J, Cheng, H, Wang, D, Chen, J, et al. Favorable long-term outcomes of chordoid meningioma compared with the other WHO grade 2 meningioma subtypes. Neurosurgery. (2022) 92:745–55. doi: 10.1227/neu.0000000000002272
3. Goldbrunner, R, Minniti, G, Preusser, M, Jenkinson, MD, Sallabanda, K, Houdart, E, et al. EANO guidelines for the diagnosis and treatment of meningiomas [review]. Lancet Oncol. (2016) 17:E383–91. doi: 10.1016/s1470-2045(16)30321-7
4. Goldbrunner, R, Stavrinou, P, Jenkinson, MD, Sahm, F, Mawrin, C, Weber, DC, et al. EANO guideline on the diagnosis and management of meningiomas. Neuro-Oncology. (2021) 23:1821–34. doi: 10.1093/neuonc/noab150
5. Rydzewski, NR, Lesniak, MS, Chandler, JP, Kalapurakal, JA, Pollom, E, Tate, MC, et al. Gross total resection and adjuvant radiotherapy most significant predictors of improved survival in patients with atypical meningioma. Cancer. (2018) 124:734–42. doi: 10.1002/cncr.31088
6. Aizer, AA, Bi, WL, Kandola, MS, Lee, EQ, Nayak, L, Rinne, ML, et al. Extent of resection and overall survival for patients with atypical and malignant meningioma. Cancer. (2015) 121:4376–81. doi: 10.1002/cncr.29639
7. Li, D, Jiang, P, Xu, S, Li, C, Xi, S, Zhang, J, et al. Survival impacts of extent of resection and adjuvant radiotherapy for the modern management of high-grade meningiomas. J Neuro Oncol. (2019) 145:125–34. doi: 10.1007/s11060-019-03278-w
8. Zeng, Q, Shi, F, and Guo, Z. Effectiveness of postoperative radiotherapy on atypical meningioma patients: a population-based study. Front Oncol. (2019) 9:34. doi: 10.3389/fonc.2019.00034
9. Li, H, Zhang, YS, Zhang, GB, Zhang, GJ, Wang, B, Li, D, et al. Treatment protocol, long-term follow-up, and predictors of mortality in 302 cases of atypical meningioma. World Neurosurg. (2019) 122:e1275–84. doi: 10.1016/j.wneu.2018.11.032
10. Preusser, M, Brastianos, PK, and Mawrin, C. Advances in meningioma genetics: novel therapeutic opportunities. Nat Rev Neurol. (2018) 14:106–15. doi: 10.1038/nrneurol.2017.168
11. Yoon, H, Mehta, MP, Perumal, K, Helenowski, IB, Chappell, RJ, Akture, E, et al. Atypical meningioma: randomized trials are required to resolve contradictory retrospective results regarding the role of adjuvant radiotherapy. J Cancer Res Ther. (2015) 11:59–66. doi: 10.4103/0973-1482.148708
12. Champeaux, C, and Dunn, L. World Health Organization grade II meningioma: a 10-year retrospective study for recurrence and prognostic factor assessment. World Neurosurg. (2016) 89:180–6. doi: 10.1016/j.wneu.2016.01.055
13. Champeaux, C, Wilson, E, Shieff, C, Khan, AA, and Thorne, L. WHO grade II meningioma: a retrospective study for outcome and prognostic factor assessment. J Neuro Oncol. (2016) 129:337–45. doi: 10.1007/s11060-016-2181-2
14. Champeaux, C, Houston, D, and Dunn, L. Atypical meningioma. A study on recurrence and disease-specific survival. Neurochirurgie. (2017) 63:273–81. doi: 10.1016/j.neuchi.2017.03.004
15. Garcia-Segura, ME, Erickson, AW, Jairath, R, Munoz, DG, and Das, S. Necrosis and brain invasion predict radio-resistance and tumor recurrence in atypical meningioma: a retrospective cohort study. Neurosurgery. (2020) 88:E42–8. doi: 10.1093/neuros/nyaa348
16. Dohm, A, McTyre, ER, Chan, MD, Fan, C, Isom, S, Bourland, JD, et al. Early or late radiotherapy following gross or subtotal resection for atypical meningiomas: clinical outcomes and local control. J Clin Neurosci. (2017) 46:90–8. doi: 10.1016/j.jocn.2017.08.023
17. Unterberger, A, Ng, E, Pradhan, A, Kondajji, A, Kulinich, D, Duong, C, et al. Adjuvant radiotherapy for atypical meningiomas is associated with improved progression free survival. J Neurol Sci. (2021) 428:117590. doi: 10.1016/j.jns.2021.117590
18. Hemmati, SM, Ghadjar, P, Grün, A, Badakhshi, H, Zschaeck, S, Senger, C, et al. Adjuvant radiotherapy improves progression-free survival in intracranial atypical meningioma. Radiat Oncol. (2019) 14:160. doi: 10.1186/s13014-019-1368-z
19. Chen, WC, Magill, ST, Wu, A, Vasudevan, HN, Morin, O, Aghi, MK, et al. Histopathological features predictive of local control of atypical meningioma after surgery and adjuvant radiotherapy. J Neurosurg. (2018) 130:1–8. doi: 10.3171/2017.9.Jns171609
20. Song, D, Xu, D, Han, H, Gao, Q, Zhang, M, Wang, F, et al. Postoperative adjuvant radiotherapy in atypical meningioma patients: a meta-analysis study. Front Oncol. (2021) 11:787962. doi: 10.3389/fonc.2021.787962
21. Rebchuk, AD, Alam, A, Hounjet, CD, Chaharyn, BM, Gooderham, PA, Yip, S, et al. Survival and recurrence outcomes following adjuvant radiotherapy for grade 2 intracranial meningiomas: 13-year experience in a tertiary-care center. World Neurosurg. (2022) 161:e748–56. doi: 10.1016/j.wneu.2022.02.088
22. Jenkinson, MD, Waqar, M, Farah, JO, Farrell, M, Barbagallo, GM, McManus, R, et al. Early adjuvant radiotherapy in the treatment of atypical meningioma. J Clin Neurosci. (2016) 28:87–92. doi: 10.1016/j.jocn.2015.09.021
23. Keric, N, Kalasauskas, D, Freyschlag, CF, Gempt, J, Misch, M, Poplawski, A, et al. Impact of postoperative radiotherapy on recurrence of primary intracranial atypical meningiomas. J Neuro Oncol. (2020) 146:347–55. doi: 10.1007/s11060-019-03382-x
24. Zhu, H, Bi, WL, Aizer, A, Hua, L, Tian, M, Den, J, et al. Efficacy of adjuvant radiotherapy for atypical and anaplastic meningioma. Cancer Med. (2019) 8:13–20. doi: 10.1002/cam4.1531
25. Zhang, GJ, Liu, XY, and You, C. Clinical factors and outcomes of atypical meningioma: a population-based study. Front Oncol. (2021) 11:676683. doi: 10.3389/fonc.2021.676683
26. Wiemels, J, Wrensch, M, and Claus, EB. Epidemiology and etiology of meningioma. J Neuro Oncol. (2010) 99:307–14. doi: 10.1007/s11060-010-0386-3
27. Tabernero, MD, Espinosa, AB, Maillo, A, Rebelo, O, Vera, JF, Sayagues, JM, et al. Patient gender is associated with distinct patterns of chromosomal abnormalities and sex chromosome linked gene-expression profiles in meningiomas. Oncologist. (2007) 12:1225–36. doi: 10.1634/theoncologist.12-10-1225
28. Maiuri, F, De Caro, ML, de Divitiis, O, Vergara, P, and Mariniello, G. Spinal meningiomas: age-related features. Clin Neurol Neurosurg. (2011) 113:34–8. doi: 10.1016/j.clineuro.2010.08.017
29. Sano, K, Wakai, S, Ochiai, C, and Takakura, K. Characteristics of intracranial meningiomas in childhood. Childs Brain. (1981) 8:98–106. doi: 10.1159/000119971
30. Zhao, X, Zhao, D, Wu, Y, Gao, W, Cui, H, Wang, Y, et al. Meningioma in the elderly: characteristics, prognostic factors, and surgical strategy. J Clin Neurosci. (2018) 56:143–9. doi: 10.1016/j.jocn.2018.06.011
31. Park, JS, Sade, B, Oya, S, Kim, CG, and Lee, JH. The influence of age on the histological grading of meningiomas. Neurosurg Rev. (2014) 37:425–9. doi: 10.1007/s10143-014-0537-7
32. Pant, I, Chaturvedi, S, Sarma, P, and Singh, G. Histopathological mapping of meningiomas: a 10-year retrospective analysis. Indian J Neurosurgery. (2021) 10:203–9. doi: 10.1055/s-0040-1718990
33. Khan, SA, Morris, M, Idrees, K, Gimbel, MI, Rosenberg, S, Zeng, Z, et al. Colorectal cancer in the very young: a comparative study of tumor markers, pathology and survival in early onset and adult onset patients. J Pediatr Surg. (2016) 51:1812–7. doi: 10.1016/j.jpedsurg.2016.07.015
34. Hoffe, S, and Balducci, L. Cancer and age: general considerations. Clin Geriatr Med. (2012) 28:1–18. doi: 10.1016/j.cger.2011.09.001
35. Hernández, L, Terradas, M, Camps, J, Martín, M, Tusell, L, and Genescà, A. Aging and radiation: bad companions. Aging Cell. (2015) 14:153–61. doi: 10.1111/acel.12306
36. Wang, K, and Tepper, JE. Radiation therapy-associated toxicity: Etiology, management, and prevention. CA Cancer J Clin. (2021) 71:437–54. doi: 10.3322/caac.21689
37. Zhang, T, Bi, N, Zhou, Z, Chen, D, Feng, Q, Liang, J, et al. The impact of age on the survival outcomes and risk of radiation pneumonitis in patients with unresectable locally advanced non-small cell lung cancer receiving chemoradiotherapy. J Thorac Dis. (2020) 12:4347–56. doi: 10.21037/jtd-20-2137
38. Yan, W, Christos, P, Nori, D, Chao, KS, and Ravi, A. Is there a cause-specific survival benefit of postmastectomy radiation therapy in women younger than age 50 with T3N0 invasive breast cancer? A SEER database analysis: outcomes by receptor status/race/age: analysis using the NCI surveillance, epidemiology, and end results (SEER) database. Am J Clin Oncol. (2013) 36:552–7. doi: 10.1097/COC.0b013e31825d529b
39. Valero, J, Peleteiro, P, Henríquez, I, Conde, A, Piquer, T, Lozano, A, et al. Age, gleason score, and PSA are important prognostic factors for survival in metastatic castration-resistant prostate cancer. Results of the uroncor group (uro-oncological tumors) of the Spanish Society of Radiation Oncology (SEOR). Clin Transl Oncol. (2020) 22:1378–89. doi: 10.1007/s12094-019-02274-w
40. Li, Y, Liu, H, Zhou, Y, Zhou, Z, Liu, W, Zhao, L, et al. The survival effect of radiotherapy on stage II/III rectal cancer in different age groups: formulating radiotherapy decision-making based on age. Front Oncol. (2021) 11:695640. doi: 10.3389/fonc.2021.783564
41. Xiao, G, Cao, Y, Qiu, X, Wang, W, and Wang, Y. Influence of gender and age on the survival of patients with nasopharyngeal carcinoma. BMC Cancer. (2013) 13:226. doi: 10.1186/1471-2407-13-226
42. He, MT, Lu, XX, and Gou, ZC. Effects of postmastectomy radiotherapy on survival in different age groups for patients with T3N0M0 breast cancer. Breast. (2021) 60:247–54. doi: 10.1016/j.breast.2021.11.006
43. Minniti, G, Filippi, AR, Osti, MF, and Ricardi, U. Radiation therapy for older patients with brain tumors. Radiat Oncol. (2017) 12:101. doi: 10.1186/s13014-017-0841-9
44. Lee, CM, Szabo, A, Shrieve, DC, Macdonald, OK, and Gaffney, DK. Frequency and effect of adjuvant radiation therapy among women with stage I endometrial adenocarcinoma. JAMA. (2006) 295:389–97. doi: 10.1001/jama.295.4.389
45. Fernandes, AT, Shinohara, ET, Guo, M, Mitra, N, Wilson, LD, Rengan, R, et al. The role of radiation therapy in malignant thymoma: a surveillance, epidemiology, and end results database analysis. J Thorac Oncol. (2010) 5:1454–60. doi: 10.1097/JTO.0b013e3181e8f345
46. Mell, LK, Carmona, R, Gulaya, S, Lu, T, Wu, J, Saenz, CC, et al. Cause-specific effects of radiotherapy and lymphadenectomy in stage I-II endometrial cancer: a population-based study. J Natl Cancer Inst. (2013) 105:1656–66. doi: 10.1093/jnci/djt279
47. Wang, C, Kaprealian, TB, Suh, JH, Kubicky, CD, Ciporen, JN, Chen, Y, et al. Overall survival benefit associated with adjuvant radiotherapy in WHO grade II meningioma. Neuro-Oncology. (2017) 19:1263–70. doi: 10.1093/neuonc/nox0007
48. Moreau, JT, Hankinson, TC, Baillet, S, and Dudley, RWR. Individual-patient prediction of meningioma malignancy and survival using the surveillance, epidemiology, and end results database. NPJ Digit Med. (2020) 3:12. doi: 10.1038/s41746-020-0219-5
49. Lu, GH, Gong, SG, Li, C, Zhao, QH, Jiang, R, Luo, CJ, et al. Prognostic value of gamma-glutamyltransferase in male patients with idiopathic pulmonary arterial hypertension. Front Cardiovasc Med. (2020) 7:580908. doi: 10.3389/fcvm.2020.580908
50. Stessin, AM, Schwartz, A, Judanin, G, Pannullo, SC, Boockvar, JA, Schwartz, TH, et al. Does adjuvant external-beam radiotherapy improve outcomes for nonbenign meningiomas? A surveillance, epidemiology, and end results (SEER)-based analysis. J Neurosurg. (2012) 117:669–75. doi: 10.3171/2012.7.JNS111439
51. Brown, NF, Ottaviani, D, Tazare, J, Gregson, J, Kitchen, N, Brandner, S, et al. Survival outcomes and prognostic factors in glioblastoma. Cancers. (2022) 14:3161. doi: 10.3390/cancers14133161
52. Ganti, AK, Klein, AB, Cotarla, I, Seal, B, and Chou, E. Update of incidence, prevalence, survival, and initial treatment in patients with non-small cell lung cancer in the US. JAMA Oncol. (2021) 7:1824–32. doi: 10.1001/jamaoncol.2021.4932
53. Cohen, CM, Wentzensen, N, Castle, PE, Schiffman, M, Zuna, R, Arend, RC, et al. Racial and ethnic disparities in cervical cancer incidence, survival, and mortality by histologic subtype. J Clin Oncol. (2023) 41:1059–68. doi: 10.1200/JCO.22.01424
54. Li, H, Zhang, H, Zhang, H, Wang, Y, Wang, X, and Hou, H. Survival of gastric cancer in China from 2000 to 2022: a nationwide systematic review of hospital-based studies. J Glob Health. (2022) 12:11014. doi: 10.7189/jogh.12.11014
55. Asai, A, Matsutani, M, Matsuda, T, Tanaka, Y, and Funada, N. Radiation-induced brain atrophy. Gan No Rinsho. (1989) 35:1325–9. jpn.
56. Stylopoulos, LA, George, AE, de Leon, MJ, Miller, JD, Foo, SH, Hiesiger, E, et al. Longitudinal CT study of parenchymal brain changes in glioma survivors. AJNR Am J Neuroradiol. (1988) 9:517–22.
57. Jenkinson, MD, Javadpour, M, Haylock, BJ, Young, B, Gillard, H, Vinten, J, et al. The ROAM/EORTC-1308 trial: radiation versus observation following surgical resection of atypical meningioma: study protocol for a randomised controlled trial. Trials. (2015) 16:519. doi: 10.1186/s13063-015-1040-3
58. Klein, JP, and Moeschberger, ML. Survival analysis: techniques for censored and truncated data. New York. 2nd: Springer (2003).
59. Perperoglou, A, Sauerbrei, W, Abrahamowicz, M, and Schmid, M. A review of spline function procedures in R. BMC Med Res Methodol. (2019) 19:46. doi: 10.1186/s12874-019-0666-3
60. Wood, SN, Pya, N, and Säfken, B. Smoothing parameter and model selection for general smooth models. J Am Stat Assoc. (2016) 111:1548–63. doi: 10.1080/01621459.2016.1180986
61. Ji, X, Wang, Y, Hu, Z, Ma, Y, Man, S, Li, K, et al. Effectiveness of subcutaneous tumor necrosis factor inhibitors in patients with ankylosing spondylitis: a real-world prospective observational cohort study in China. Front Pharmacol. (2019) 10:1476. doi: 10.3389/fphar.2019.01476
62. Wang, S, Zhou, Z, Fan, F, Qi, L, Jia, J, Sun, P, et al. Joint effect of non-invasive central systolic blood pressure and peripheral systolic blood pressure on incident hypertension in a Chinese community-based population. Sci Rep. (2018) 8:3229. doi: 10.1038/s41598-018-21023-7
63. Yu, Y, Li, M, Huang, X, Zhou, W, Wang, T, Zhu, L, et al. A U-shaped association between the LDL-cholesterol to HDL-cholesterol ratio and all-cause mortality in elderly hypertensive patients: a prospective cohort study. Lipids Health Dis. (2020) 19:238. doi: 10.1186/s12944-020-01413-5
64. Zhou, S, Jiang, W, Wang, H, Wei, N, and Yu, Q. Predictive value of pretreatment albumin-to-alkaline phosphatase ratio for overall survival for patients with advanced non-small cell lung cancer. Cancer Med. (2020) 9:6268–80. doi: 10.1002/cam4.3244
65. Driscoll, JJ, and Rixe, O. Overall survival: still the gold standard: why overall survival remains the definitive end point in cancer clinical trials. Cancer J. (2009) 15:401–5. doi: 10.1097/PPO.0b013e3181bdc2e0
Keywords: atypical meningioma, gross total resection, radiation therapy, overall survival, age
Citation: Li C, Qin J, Xue F, Shen Z, Lin Q, Xue Y and Chen X (2024) Rethinking the effects of adjuvant beam radiation therapy on overall survival in atypical meningioma patients: age considerations. Front. Neurol. 15:1360741. doi: 10.3389/fneur.2024.1360741
Received: 24 December 2023; Accepted: 04 March 2024;
Published: 15 March 2024.
Edited by:
Bo yuan Huang, Capital Medical University, ChinaReviewed by:
Sukwoo Hong, Toranomon Hospital, JapanCopyright © 2024 Li, Qin, Xue, Shen, Lin, Xue and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianzhen Chen, Y2hlbnhpYW56aGVueUAxMjYuY29t; Yajun Xue, eHVlX3lqQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.