
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 27 February 2024
Sec. Endovascular and Interventional Neurology
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1360161
Objectives: Restenosis is one of the important factors affecting the effectiveness of percutaneous transluminal angioplasty and stenting in the treatment of intracranial atherosclerotic stenosis. We aimed to clarify whether recruitable collateral flow could cause restenosis in patients treated with percutaneous transluminal angioplasty and stenting.
Material and methods: Our study retrospectively analyzed patients with symptomatic severe intracranial atherosclerotic stenosis (≥70%) who underwent percutaneous transluminal angioplasty and stenting. We enrolled 28 patients with restenosis and 71 patients without restenosis. We analyzed baseline data, perioperative events, and follow-up results of patients in the two groups. Binary logistic regression analysis was used to identify restenosis predictors.
Results: For preoperative stroke, the restenosis group had a greater likelihood of having a previous stroke (89.3%), which was less prevalent in the non-restenosis group (66.2%) (P = 0.020). The restenosis group had a higher rate of re-stroke (21.4 vs. 4.2%, P = 0.022). After binary logistic regression analysis, collateral circulation and residual stenosis were independent risk factors of restenosis, with overall risk (95% confidence intervals) of 5.034 (1.484–4.066, P < 0.001) and 1.064 (1.006–1.125, P = 0.030), respectively. Restenosis risk increased 1.456-fold for each collateral circulation grade increase. However, for each 1% increase in residual stenosis, restenosis risk increased by 5.9% (P = 0.03). The chance of restenosis is minimal when the residual stenosis rate after percutaneous transluminal angioplasty and stent implantation is 15.85%.
Conclusions: Good collateral circulation was significantly associated with restenosis in patients undergoing intracranial angioplasty, the residual stenosis rate tends to be 15.85% to reduce restenosis risk. Compared to patients with restenosis, those without restenosis have a low stroke risk during follow-up.
Intracranial atherosclerotic stenosis (ICAS) is the most common cause of ischemic stroke (1). Despite optimal drug therapy, patients with ICAS experience a high rate of re-stroke, disability, and death (2). Percutaneous transluminal angioplasty and stenting (PTAS) is a potential treatment that restores anterograde blood flow by remodeling the blood vessels (3). However, the safety and efficacy of PTAS have been widely questioned because of the high incidence of perioperative complications and recurrent stroke. The SAMMPRIS and VISIT trials showed greater rates of stroke and death within 30 days in patients treated with PTAS (4, 5). In the CASSISS trial, percutaneous intervention was found to be an effective treatment for symptomatic ICAS after applying strict inclusion criteria (6). A post-hoc analysis of SAMMPRIS found that patients with new infarcts had a 66.7% rate of restenosis during long-term follow-up (7). Studies have identified many factors influencing restenosis after PTAS for symptomatic ICAS, including age, diabetes, lesion length, and residual stenosis rate after treatment (8–10). As an alternative blood supply route, the brain's collateral circulation may provide blood flow when blood vessels are blocked. Collaterals can effectively reduce the loss of ischemic penumbra and extend the time window for acute stroke treatment (11). However, a post-hoc analysis based on the original warfarin-aspirin symptomatic intracranial disease (WASID) trial population of 569 patients found that good collateral circulation likely leads to recurrent stroke in patients with mild stenosis (12).
To date, no study has determined the effect of collateral circulation on restenosis after intracranial PTAS. Therefore, this study was conducted to investigate the possible role of different grades of collateral circulation on the risk of restenosis after intracranial PTAS.
We retrospectively analyzed the data of 99 patients treated with PTAS at the Affiliated Hospital of Qingdao University from 2019.1 to 2022.6. These patients met the following inclusion criteria: 1. symptomatic ICAS in patients undergoing PTAS, the symptoms include TIA or ischemic stroke; 2. the target vessels include the terminal internal carotid artery (C4–C7), the middle cerebral artery, the V4 segment of the vertebral artery and the basilar artery; 3. the rate of atherosclerotic stenosis was 70–99% as confirmed by DSA according to the WASID method (13); 4. willingness to be followed up, including undergoing a computed tomography angiography (CTA) or DSA; 5. more than 2 weeks from the onset of the ischemic event.
The exclusion criteria were: 1. another intracranial artery with stenosis >70%; patients with tandem lesions or stroke caused by perforator occlusion; 2. a non-atherosclerotic lesion, including cardioembolic stroke, moyamoya disease, vasculitis, dissection or tandem stenosis of extracranial and intracranial arteries; 3. concurrent intracranial pathology including tumors, aneurysms, or arteriovenous malformation (Table 1).
This retrospective study was conducted in accordance with the Helsinki Declaration (as revised in 2013) ethical standards and was approved by the ethics committee of the Affiliated Hospital of Qingdao University.
All patients received dual antiplatelet therapy (aspirin 100 mg and clopidogrel 75 mg daily) for >5 days before the procedure. During the first 3 months after the intervention, the patient continued dual antiplatelet therapy. Subsequently, long-term administration of either aspirin (100 mg/day) or clopidogrel (75 mg/day) was applied to the patients. Statins were also administered for at least 6 months after stenting.
All patients were administered general anesthesia. Usually, a 6-F guiding catheter was used for device delivery. A balloon (Boston Scientific, Natick, MA, USA) was placed in the stenotic segment for submaximal angioplasty. For initial treatment, primary balloon angioplasty alone is performed; stenting is considered in patients with insufficient dilation, marked acute dissection, or restenosis. The operators were instructed to choose whether to use stenting based on the morphological features of the lesion. The stent was placed at the lesion site after a submaximal angioplasty. After 15 min of observation, cerebral arteriography was repeated. The procedure was completed if the stent position was satisfactory and no residual stenosis >50% or acute thrombosis was observed.
Non-contrast head computed tomography (CT) was performed to exclude hemorrhage after the procedure. If patients were free of hemorrhage, GP IIb/IIIa receptor inhibitors were administered to those with a high risk of thrombosis. Blood pressure was maintained below 130/80 mmHg to prevent hyperperfusion syndrome.
Baseline demographics; vascular risk factors; and clinical, angiographic, and periprocedural data were collected. Vascular imaging studies, including CTA and DSA, were scheduled 12 months after the index procedure. Two experienced neurointerventionists interpreted the images. Collateral circulation was graded using the Astin/Sir Score (Figure 1). The primary endpoints were periprocedural complications (death, ischemic stroke, or hemorrhagic stroke), transient ischemic attacks, ischemic stroke, restenosis, and symptomatic restenosis. Symptomatic restenosis was defined as restenosis associated with TIA or stroke in the offending vessel territory. The WASID technique was used to calculate the percentage of patients with residual or recurrent stenosis. For patients evaluated using cerebral angiography, restenosis was defined as having lesions with >50% stenosis. The lesion locations were considered restenosis for patients evaluated using CTA if both the treated segment and the edge of the treated segment could not be displayed well or showed an apparent filling defect on CTA.
Statistical analysis was performed using SPSS version 26.0 for Windows (Armonk, NY: IBM Corp; 2019). Normality was assessed for continuous variables using the Shapiro–Wilk test. Continuous variables are expressed as the mean ± standard deviation (SD) or as the median with interquartile range and compared using Student's t-test or the Mann–Whitney U-test, as appropriate. Categorical variables were expressed as rates and compared with the chi-squared test or Fisher exact test; α = 0.05 was used as the test standard. Univariate analysis and the binary logistic regression model were performed on the data. The optimal residual stenosis rate was expressed using the receiver operating characteristic (ROC) curve.
Ninety-nine patients were enrolled, including 27 treated with balloon angioplasty and 72 treated with balloon dilatation plus stenting. We enrolled 28 patients with restenosis and 71 patients without restenosis. Hypertension was the most common risk factor in both groups (n = 4, 74.7%). Anterior circulation lesions were present in 53.5% of patients; no differences were found in age, sex, proportion of risk factors, and lesion location (P > 0.05). For preoperative stroke, the restenosis group had a greater likelihood of having a previous stroke (89.3%), which was less prevalent in the non-restenosis group (66.2%; P = 0.020) (Table 2).
During the operation, all stents were successfully delivered to the lesion; none failed to reach the location. The differences in the length and diameter of the used balloons and stents were not statistically significant between the two groups. Stenosis degrees after intervention [18.65 (10.17) vs. 14.30 (13.60), P = 0.04)] were significantly lower in the non-restenosis group than that in the restenosis group (Table 3).
In the restenosis and non-restenosis groups, the rates of any cause of safety endpoints within 30 days showed no statistical difference. In the restenosis group, one patient developed an asymptomatic intracerebral hemorrhage after surgery; the occurrence of restenosis was associated with stroke during follow-up [21.4% of restenosis patients (n = 6) vs. 4.2% of non-restenosis patients (n = 3); P = 0.022]. No significant differences were found in periprocedural complications, cerebral hemorrhage, death, and other characteristics (Table 4).
After binary logistic regression, we found that the Astin/Sir [odds ratio (OR) 5.034, 95% confidence interval (CI) 1.484–4.066; P < 0.001] and more severe residual stenosis (OR 1.064, 95% CI 1.006–1.125; P = 0.030) were independently associated with restenosis (Table 5). We constructed a binary logistic regression prediction model for restenosis after ICAS in the form of a nomogram, which was used to predict the effect of residual stenosis on restenosis. The risk of ISR was lowest when the residual stenosis rate was 15.85% after PTAS (sensitivity 71.4%; specificity 67.7%; Youden index 0.39), and the area under the curve (AUC) was 0.69 (95% CI: 0.58–0.79; P = 0.004) (Figure 2).
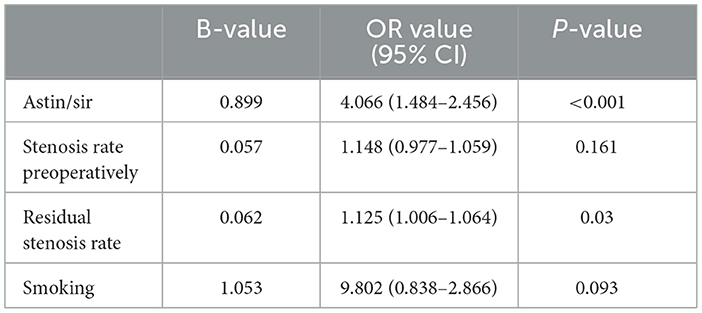
Table 5. Analysis of risk factors for restenosis after PTAS according to binary logistic regression analysis.
This study identified that poor collateral circulation could reduce the incidence of restenosis after PTAS for symptomatic ICAS. Compared to the no-restenosis group, the restenosis group had an increased risk of stroke recurrence during follow-up. Our analysis showed that reducing residual stenosis within a certain range could lower the restenosis rate.
A high restenosis rate is a frustrating outcome of PTAS (14). In a post-hoc SAMMPRIS analysis, 66.7% of cases with an ischemic event beyond 30 days were attributed to restenosis of the offending vessel during the follow-up. In patients with Wingspan stent implantation, the symptomatic restenosis rates at 1, 2, and 3 years were 9.6, 11.3, and 14%, respectively (7). In our study, 66.7% of the re-stroke patients had restenosis. Among patients with ipsilateral ischemic stroke, this rate was 71.4% (5/7). The specific influencing factors of restenosis remain inconclusive. Collateral circulation may be an important reason for this phenomenon.
Collateral circulation is closely related to stroke in patients with ICAS and has a protective effect on severe stenosis. People with good collateral circulation can tolerate ischemia longer and have a smaller core infarct volume than those with poor collateral circulation. The rate of progression from penumbra to infarction depends largely on the extent of collateral circulation and ultimately determines whether the patient has a fast or slow progression. Previous studies have shown that collateral circulation plays an important role in the occurrence of restenosis and strongly promotes the development of re-stroke (15, 16). Other studies found that collateral circulation might not predict the risk of restenosis (17). This phenomenon may be attributed to the differences in measurement and evaluation methods of collateral circulation.
In our study, people with good collateral circulation were more likely to develop restenosis after PTAS. The cause for this phenomenon may include the recruitment of collateral vessels. Collateral arteries in healthy tissues have little or no net collateral flow. In ischemic tissue, the pressure gradient exerted upon the collateral vasculature increases the blood flow of collateral circulation. Blood flow along collateral circulation comes from opposite directions. This “to and fro” flow increases when the vessel stenosis is relieved (18). This competitive flow may lower shear stress through these vessels dramatically (19). Local hemodynamic factors, low shear stress in particular, are known to critically affect the natural history of atherosclerosis. Increasing evidence now suggests that low shear stress may contribute to the development of restenosis (20). The proliferation of smooth muscle cells is one of the main reasons that promote the development of restenosis. Low shear stress promotes restenosis through interactions of shear sensing endothelial cells with smooth muscle cells (21, 22). Abundant collateral circulation may exacerbate this phenomenon. In addition, the proliferation of blood vessels significantly influences collateral circulation. A considerable amount of variability is evident in collateral circulation. The ideal configuration of the collaterals is reported in only a minority of cases. Mostly, the body requires arteriogenesis to relieve tissue ischemia. Unlike healthy collateral arteries, arterial neogenesis leads to significant tortuosity of collaterals, remodeling of the luminal diameter, and hemodynamic changes (23). This may further increase the risk of restenosis. Previous studies have found that the mechanisms of restenosis include smooth muscle cell proliferation, thrombus formation, intimal hyperplasia, and atherogenic plate formation (24). Changes in the flow shear stress and pressure gradient indicate a greater possibility of thrombus formation and lipid accumulation.
Submaximal angioplasty is an effective tool for the treatment of patients with ICAS. By marginally increasing the diameter of the affected blood vessel, blood flow is markedly increased, thus alleviating the patient's hypoperfusion-type symptoms. The specific degree of dilation of intracranial blood vessels has not yet been determined. Underexpansion appears to be an important mechanism of restenosis. However, excessive deployment of the balloon and stent can reduce the vessel curvature and change the hemodynamic pattern, inevitably leading to the destruction of endothelial cells and triggering neuroinflammation, ultimately causing restenosis (25, 26). Excessive dilatation also may lead to intimal damage and increase the risk of intracerebral hemorrhage. A post-hoc analysis revealed that the sensitivity and specificity curves identified different thresholds for the optimal stent expansion area after the intervention (27). Therefore, an appropriately sized balloon should be chosen to balance the risk of restenosis and perioperative complications. We found that the risk of ISR was lowest when the residual stenosis rate was 15.85% after PTAS. The sensitivity of the ROC curve was a little low, which may be related to the small sample size in the ISR group.
First, this study was a retrospective analysis. We enrolled patients who were willing to undergo follow-up imaging. Potential biases included patient selection and quantification of the degree of stenosis. Second, the small sample size collected in this study may cause statistical bias. In addition, because of the controversy regarding the conclusion of the PTAS end, we selected only patients who still had symptomatic ICAS despite receiving medical therapy.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (QYFY WZLL 28202). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and the institutional requirements. The study was conducted in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
YL: Conceptualization, Writing – original draft, Writing – review & editing. YS: Data curation, Methodology, Writing – review & editing. TL: Supervision, Validation, Writing – review & editing. PL: Conceptualization, Investigation, Writing – review & editing. GL: Data curation, Methodology, Writing – review & editing. YZ: Resources, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Qingdao Natural Science Foundation (23-2-1-186-zyyd-jch).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wang Y, Zhao X, Liu L, Soo YO, Pu Y, Pan Y, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke. (2014) 45:663–9. doi: 10.1161/STROKEAHA.113.003508
3. Zhou K, Cao Y, He XH, Qiu ZM, Liu S, Gong ZL, et al. A Comparison of safety and effectiveness between wingspan and neuroform stents in patients with middle cerebral artery stenosis. Front Neurol. (2021) 12:527541. doi: 10.3389/fneur.2021.527541
4. Derdeyn CP, Chimowitz MI, Lynn MJ, Fiorella D, Turan TN, Janis LS, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet. (2014) 383:333–41. doi: 10.1016/S0140-6736(13)62038-3
5. Zaidat OO, Fitzsimmons BF, Woodward BK, Wang Z, Killer-Oberpfalzer M, Wakhloo A, et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA. (2015) 313:1240–8. doi: 10.1001/jama.2015.1693
6. Gao P, Wang T, Wang D, Liebeskind DS, Shi H, Li T, et al. Effect of stenting plus medical therapy vs medical therapy alone on risk of stroke and death in patients with symptomatic intracranial stenosis: the CASSISS randomized clinical trial. JAMA. (2022) 328:534–42. doi: 10.1001/jama.2022.12000
7. Derdeyn CP, Fiorella D, Lynn MJ, Turan TN, Cotsonis GA, Lane BF, et al. Nonprocedural symptomatic infarction and in-stent restenosis after intracranial angioplasty and stenting in the SAMMPRIS trial (stenting and aggressive medical management for the prevention of recurrent stroke in intracranial stenosis). Stroke. (2017) 48:1501–6. doi: 10.1161/STROKEAHA.116.014537
8. Peng G, Zhang Y, Miao Z. Incidence and risk factors of in-stent restenosis for symptomatic intracranial atherosclerotic stenosis: a systematic review and meta-analysis. Am J Neuroradiol. (2020) 41:1447–52. doi: 10.3174/ajnr.A6689
9. Ueda T, Takaishi S, Yoshie T, Usuki N, Tatsuno K, Ohtsubo H, et al. Long-term outcome and factors associated with restenosis after combination therapy of balloon angioplasty and stenting for symptomatic intracranial stenosis. BMC Neurol. (2022) 22:477. doi: 10.1186/s12883-022-03009-1
10. Zhang X, Gong W, Meng Z, Li G, Liu P, Zhang Y, et al. A non-linear relationship between lesion length and risk of recurrent cerebral ischemia after stenting for symptomatic intracranial stenosis with hemodynamic impairment. Front Neurol. (2023) 14:1122708. doi: 10.3389/fneur.2023.1122708
11. Jung S, Gilgen M, Slotboom J, El-Koussy M, Zubler C, Kiefer C, et al. Factors that determine penumbral tissue loss in acute ischaemic stroke. Brain. (2013) 136(Pt 12):3554–60. doi: 10.1093/brain/awt246
12. Liebeskind DS, Cotsonis GA, Saver JL, Lynn MJ, Turan TN, Cloft HJ, et al. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol. (2011) 69:963–74. doi: 10.1002/ana.22354
13. Weinberger J. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. Curr Cardiol Rep. (2006) 8:7. doi: 10.1016/S0513-5117(08)70245-X
14. Gong W, Zhang X, Meng Z, Liu F, Li G, Xiao J, et al. Factors influencing the outcome of symptomatic intracranial artery stenosis with hemodynamic impairment after short and long-term stent placement. Front Neurol. (2022) 13:682694. doi: 10.3389/fneur.2022.682694
15. Jensen LO, Thayssen P, Lassen JF, Hansen HS, Kelbaek H, Junker A, et al. Recruitable collateral blood flow index predicts coronary instent restenosis after percutaneous coronary intervention. Eur Heart J. (2007) 28:1820–6. doi: 10.1093/eurheartj/ehm067
16. Nakae I, Fujita M, Fudo T, Iwase T, Tanaka T, Tamaki S, et al. Relation between preexistent coronary collateral circulation and the incidence of restenosis after successful primary coronary angioplasty for acute myocardial infarction. J Am Coll Cardiol. (1996) 27:1688–92. doi: 10.1016/0735-1097(96)00043-5
17. Perera D, Postema P, Rashid R, Patel S, Blows L, Marber M, et al. Does a well developed collateral circulation predispose to restenosis after percutaneous coronary intervention? An intravascular ultrasound study. Heart. (2006) 92:763–7. doi: 10.1136/hrt.2005.067322
18. Chalothorn D, Faber JE. Formation and maturation of the native cerebral collateral circulation. J Mol Cell Cardiol. (2010) 49:251–9. doi: 10.1016/j.yjmcc.2010.03.014
19. Nordgaard H, Swillens A, Nordhaug D, Kirkeby-Garstad I, Van Loo D, Vitale N, et al. Impact of competitive flow on wall shear stress in coronary surgery: computational fluid dynamics of a LIMA-LAD model. Cardiovasc Res. (2010) 88:512–9. doi: 10.1093/cvr/cvq210
20. Carlier SG, van Damme LC, Blommerde CP, Wentzel JJ, van Langehove G, Verheye S, et al. Augmentation of wall shear stress inhibits neointimal hyperplasia after stent implantation: inhibition through reduction of inflammation? Circulation. (2003) 107:2741–6. doi: 10.1161/01.CIR.0000066914.95878.6D
21. Virmani R, Farb A. Pathology of in-stent restenosis. Curr Opin Lipidol. (1999) 10:499–506. doi: 10.1097/00041433-199912000-00004
22. Balcells M, Martorell J, Olivé C, Santacana M, Chitalia V, Cardoso AA, et al. Smooth muscle cells orchestrate the endothelial cell response to flow and injury. Circulation. (2010) 121:2192–9. doi: 10.1161/CIRCULATIONAHA.109.877282
23. Faber JE, Chilian WM, Deindl E, van Royen N, Simons M. A brief etymology of the collateral circulation. Arterioscler Thromb Vasc Biol. (2014) 34:1854–9. doi: 10.1161/ATVBAHA.114.303929
24. Giustino G, Colombo A, Camaj A, Yasumura K, Mehran R, Stone GW, et al. Coronary in-stent restenosis: JACC state-of-the-art review. J Am Coll Cardiol. (2022) 80:348–72. doi: 10.1016/j.jacc.2022.05.017
25. Song X, Qiu H, Wang S, Cao Y, Zhao J. Hemodynamic and geometric risk factors for in-stent restenosis in patients with intracranial atherosclerotic stenosis. Oxid Med Cell Longev. (2022) 2022:6951302. doi: 10.1155/2022/6951302
26. Han Z, Li L, Zhao H, Wang R, Yan F, Tao Z, et al. MicroRNA-193a-5p rescues ischemic cerebral injury by restoring N2-like neutrophil subsets. Transl Stroke Res. (2023) 14:589–607. doi: 10.1007/s12975-022-01071-y
27. Sonoda S, Morino Y, Ako J, Terashima M, Hassan AH, Bonneau HN, et al. Impact of final stent dimensions on long-term results following sirolimus-eluting stent implantation: serial intravascular ultrasound analysis from the sirius trial. J Am Coll Cardiol. (2004) 43:1959–63. doi: 10.1016/j.jacc.2004.01.044
Keywords: restenosis, collateral circulation, intracranial, angioplasty, stroke
Citation: Li Y, Sun Y, Liu T, Liu P, Li G and Zhang Y (2024) Has collateral blood flow any effect on restenosis rate? Our experience. Front. Neurol. 15:1360161. doi: 10.3389/fneur.2024.1360161
Received: 22 December 2023; Accepted: 06 February 2024;
Published: 27 February 2024.
Edited by:
Yang-Ha Hwang, Kyungpook National University, Republic of KoreaReviewed by:
Natsuhi Sasaki, Fukui Red Cross Hospital, JapanCopyright © 2024 Li, Sun, Liu, Liu, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangwen Li, ZG9jdG9ybGlndWFuZ3dlbkAxNjMuY29t; Yong Zhang, YnJhdmV6aGFuZ0AxMjYuY29t
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.