
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol. , 23 May 2024
Sec. Pediatric Neurology
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1359955
Developmental Coordination Disorder (DCD) is a neurodevelopmental disorder characterized by deficits in motor skills, with gross and fine motor dysfunction being the main symptom. This condition greatly impairs children’s daily life, learning, and social interaction. Symptoms typically appear during preschool or school age, and if left untreated, they can persist into adulthood. Thus, early assessment and intervention are crucial to improve the prognosis. This study aims to review the existing literature on DCD, providing a comprehensive overview of the assessment for children with DCD in terms of body functions and structures, activities and participation, and environmental factors within the framework of the International Classification of Functioning, Disability, and Health - Children and Youth (ICF-CY). Additionally, specific rehabilitation interventions will be described, offering valuable insights for the clinical assessment and intervention of children with DCD.
Developmental Coordination Disorder (DCD) is a neurodevelopmental disorder that impairs a child’s ability to perform coordinated motor movements, leading to slow, clumsy, or inaccurate movements and difficulties in learning new movements (1). However, it is important to note that DCD is not caused by organic, intellectual, or psychological issues (2), but instead by irregularities in the brain areas responsible for processing motor information (3). The prevalence of DCD in children ranges from approximately 2 to 20%, with widely accepted international figures currently standing at 5–6% (4). DCD is characterized by deficiencies in both gross and fine motor skills. Children with DCD often exhibit clumsiness, lack of balance and coordination, and struggle with everyday tasks such as grasping objects, dressing, and writing. Additionally, DCD is frequently associated with other conditions such as developmental language disorders, attention deficit hyperactivity disorder, autism spectrum disorders, learning disabilities, and cognitive deficits (4–9).
Furthermore, limited mobility stemming from motor skill deficits can result in decreased opportunities for children with DCD to engage in sports or social activities. This, in turn, increases the risk of weight issues or obesity and may lead to social deficits like isolation, reduced participation in social activities, and difficulties in forming friendships (10–12). Moreover, individuals with DCD may also experience psychosocial challenges such as anxiety and depression (13, 14). These psychosocial problems can persist into adulthood and significantly impact adaptive behavior and mental health outcomes (15). Hence, early diagnosis and intervention are crucial in improving long-term prognosis for individuals with DCD.
The International Classification of Functioning, Disability and Health for Children and Youth (ICF-CY) is a comprehensive framework that categorizes human functioning and disability. It serves as a scientific tool to understand and explore health-related states, outcomes, and factors that influence them (16). The ICF-CY encompasses three main components: body functions and structures, activities and participation, and environmental factors (4, 16). Body functions and structures refer to the anatomical parts and physiological functions of the body systems. Activities pertain to the individual’s ability to perform tasks or actions. Participation relates to the individual’s engagement in various life situations. Environmental factors encompass the natural, social, and attitudinal aspects of people’s surroundings that influence and interact with the elements of body functions and structures, activities, and participation.
This review examines the assessment of children with DCD within the context of the ICF-CY framework. It provides an overview of how children’s functioning in terms of body functions and structures, activities and participation, and environmental factors is evaluated. Additionally, specific rehabilitative interventions are discussed to offer insights into the clinical assessment and treatment of children with DCD.
The assessment of DCD is underpinned by a variety of theoretical frameworks, including the following five (4, 17): Firstly, there’s the functional motor skill assessment, which relies on normative standards such as MABC-2, BOT-2, and other standardized tests designed to evaluate skill performance. Secondly, there’s assessment based on general ability, which concentrates on the child’s sensory-motor integration, encompassing factors like perceptual ability. Thirdly, there’s assessment rooted in neurodevelopmental theory. This approach examines the child’s neurological function and signs, including various hard and soft indicators. Fourthly, there’s the dynamical system-based assessment, which delves into biomechanical and kinematic analyses to understand motor function dynamics. Lastly, there’s the cognitive-neurological based assessment, which focuses on evaluating the brain’s functioning concerning motor skills. This review aims to provide a multilevel clinical assessment of children with DCD, drawing from the above assessment theories to comprehensively reflect motor development across different functional levels—behavioral, neurocognitive, and affective.
Clinical assessment and diagnosis of DCD involves various steps, including screening, history taking, clinical examination, and standardized diagnostic assessment (see Figure 1) (4). DCD is typically identified by parents or teachers when a child shows signs of delayed motor development, difficulties with coordination and daily activities. Screening questionnaires like the Developmental Coordination Disorder Parent Questionnaire (DCDQ) and Little DCDQ are commonly used to identify children with DCD based on parent reports, assessing motor control, fine and gross motor skills, and overall coordination. Once children are screened positive, a history and clinical examination are conducted to rule out other conditions that could be causing motor skill deficits. If no other condition is identified, further diagnostic assessments are carried out using standardized tests like the Movement Assessment Battery for Children, Second Edition (MABC-2), Bruininks - Oseretsky Test of Motor Proficiency, Second Edition (BOTMP-2), and Peabody Developmental Motor Scales, Second Edition (PDMS-2). The MABC-2 is the most widely used and well-studied test for diagnosing DCD, evaluating fine motor, gross motor, and balance skills. The BOTMP-2 assesses upper limb movement quality, coordination, balance, and bilateral coordination (4). On the other hand, the PDMS-2 assesses gross and fine motor development in young children and is commonly used for assessing motor function in various disease states (18). It is important to note that while the MABC-2 is considered the gold standard for assessing motor development, a single motor test may miss some cases of DCD. In such instances, the BOTMP-2 can be performed simultaneously to avoid missed diagnoses. These assessment tools are utilized by occupational therapists and physiotherapists to observe and evaluate a child’s performance on specific motor tasks. Table 1 summarizes the test category, applicable conditions, quality, strengths, limitations and the level of evidence for guideline (4) recommendations of commonly used assessment scales for DCD.

Figure 1. Clinical diagnosis of DCD. DCDQ, Developmental Coordination Disorder Parent Questionnaire; MABC-2, Movement Assessment Battery for Children, Second Edition; BOTMP-2, Bruininks - Oseretsky Test of Motor Proficiency, Second Edition; PDMS-2, Peabody Developmental Motor Scales, Second Edition; DCD, Developmental Coordination Disorder.
The assessment of children with DCD should not solely focus on standardized scales, but should take a holistic approach within the framework of the ICF in the field of rehabilitation medicine. In addition to evaluating impaired body functions and structures, it is crucial to assess restricted activities and participation, as well as consider environmental factors that may impact a child’ s functioning (see Figure 2).
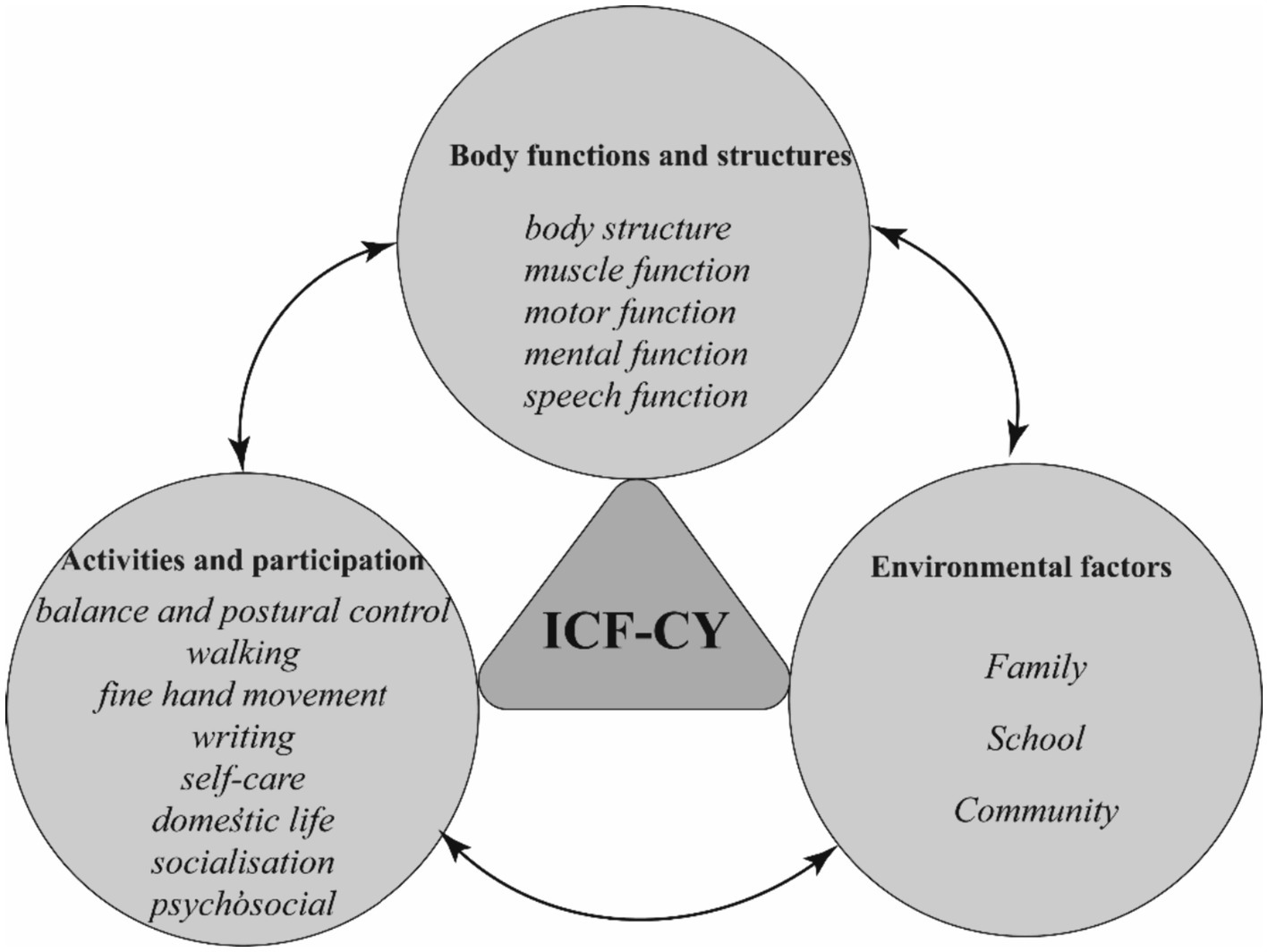
Figure 2. Rehabilitation assessment of children with DCD under the ICF framework. ICF-CY, International Classification of Functioning, Disability and Health for Children and Youth; DCD, Developmental Coordination Disorder.
The assessment of body functions and structures in children with DCD involves evaluating various aspects such as body structure, muscle function, motor function, mental function, and speech function (see Table 2).
Assessment of body structure and muscle function primarily revolves around evaluating the power system. An examination of the body structure looks for abnormal body posture, joint hypermobility, swelling or pain in the joints. Additionally, due to restricted activities and limited participation, children with DCD may encounter delays in skeletal development (19). Thus, it’s essential to screen them for skeletal maturity, considering their individual circumstances, including body mass index (BMI). Additional measurements and analyses, such as arm span and leg length, should be conducted if deemed necessary. Muscle function tests assess muscle tone and strength, which are often found to be low in children with DCD (20, 21). Motor function was assessed based on neurodevelopmental theories, including evaluations of motor reflex function, primary reflexes, and random motor control function derived from the dynamical system perspective. Children with DCD process motor reflexes differently than their peers, and reflexes like deep tendon reflexes, plantar reflexes, and adductor reflexes can be examined (22). Posture development, which relies on the integration of primary reflexes, is a prerequisite for movement (23). The Niklasson study (24) noted that unintegrated primary reflexes and vestibular dysfunction contribute to sensorimotor developmental delay and are important aspects for diagnosing DCD. Niklasson assessed primary reflexes and vestibular function by means of Retraining for Balance (RB), the RB-Physiological test detects abnormal primary reflexes, especially those related to the vestibular system, such as asymmetric tonic cervical reflexes, tonic labyrinth reflexes, symmetric tonic cervical reflexes, and Morrow’s reflexes (24), RB-Orientation and balance tests are used to detect vestibular function in children (25). However, international clinical guidelines (4) published by the European Academy of Childhood Disability (EACD) state that the role of mild cerebral developmental disorders and mild neurological dysfunction in the diagnosis of DCD is controversial. Therefore, the value of signs of abnormal motor development in the diagnosis of children with DCD needs to be verified by further studies. In terms of random motor control function, hand-eye coordination is usually poor in children with DCD, and tests such as the finger-nose test, finger-finger test, and alternating movements can be performed to assess coordination function (Figure 3).
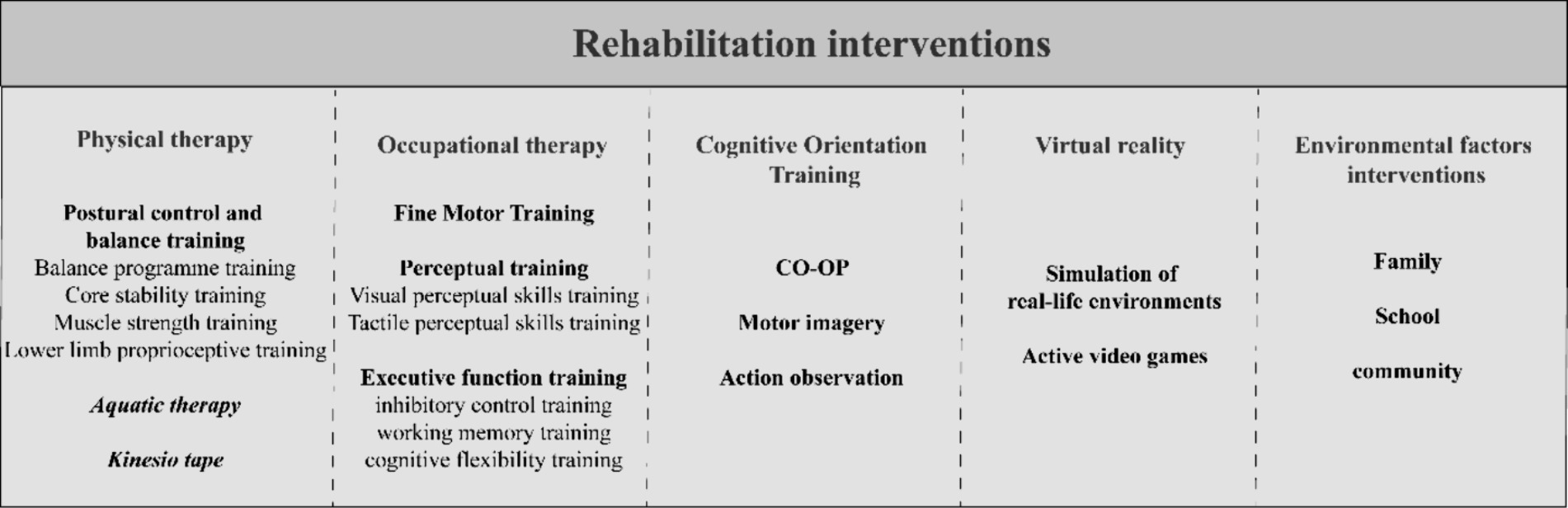
Figure 3. Clinical rehabilitation interventions for children with DCD. CO-OP, cognitive orientation to daily occupational performance; DCD, Developmental Coordination Disorder.
Assessment of mental functioning consists primarily of intelligence and advanced cognitive functions (executive functioning and motor planning) based on cognitive neurological assessment, and perceptual functioning based on general abilities. The Wechsler Intelligence Scale for Children is utilized to evaluate the children’s intellectual functioning (26). Poor executive functioning is also found in 40–60% of DCD cases (3, 27), including the three main areas of working memory (especially visuospatial working memory), inhibitory control and cognitive flexibility (27). Working memory can be assessed using the digit span test (28, 29), and visuospatial working memory can be assessed using the Groton Maze Learning Test (GMLT) and the block tapping test (30). Multiple test tasks such as the Go/NoGo test (31), Stroop test (32, 33), Tower of London test (31), etc. can be used to assess executive functions such as motor inhibition, cognitive inhibition, spatial problem solving and planning tasks. Moreover, children’s inhibitory control can be assessed by objectively recording reaction time and accuracy while individuals complete non-conflict and conflict tasks using the Flanker task (34). Longer reaction times and lower accuracy typically indicate poorer attentional inhibition. The Wisconsin Card Sorting Test (35) evaluates children’s cognitive flexibility and directly assesses their ability to think abstractly by reasoning analogically about sorting and matching cards. Recorded variables include categories completed, correct responses, total errors, perseverative errors, non-perseverative errors, and conceptual level responses. Motor planning was assessed by the “end-state-comfort” effect in the grip task, i.e., whether the selection of the object to be grasped was prioritized so that the movement could end comfortably (36). Assessment of perceptual function should focus on tactile perception and visual perception, previous studies have found that motor deficits in children with DCD are associated with tactile perceptual dysfunction, and that children with DCD have reduced tactile acuity compared to normal children (37, 38), a tactile discrimination task can be used to examine children’s tactile acuity. In terms of visual perception-related functioning, focusing on visuospatial perception as well as assessment of visuomotor skills, research studies have found that 5–47% of children with DCD are impaired on visual or visuospatial perception measures and 5–42% on visuomotor measures, visual perception plays a key role in the development of motor skills, and low functioning can lead to motor deficits (39). Therefore, identifying visual perception deficits is particularly important in assessing DCD. The standardized scale Test of Visual-Perceptual Skills-Fourth Edition (TVPS-4) provides a comprehensive test of visual-perceptual skills, The Visual Imagery Task and the Mental Rotation Task (40) assess visuospatial perception, involving tasks such as identifying three-dimensional shapes in various spatial orientations or angles through observation and imagination. Visual-motor skills can be assessed through the Visual-Motor Integration Test, hand-eye co-ordination (e.g., finger-nose test), replicated shapes, and three-dimensional block designs (26, 41). In addition, three-dimensional motion capture techniques are increasingly being used in the perceptual assessment of children with DCD, Wilmut (42) used digital calipers to detect DCD children’s judgement of the amount of space they could pass through a gap to assess the children’s perceptual bias and found that the children in the DCD group overestimated the amount of space required to pass through the gap (42).
Lastly, the development of motor skills can impact speech and language skills (43). Specific speech disorders, including phonological errors, syllable separation, rhythm abnormalities, and poor coordination of oral motor skills, are often observed in children with DCD (44). Assessments for speech include measures like maximal vocal duration, counting ability, oral rotation rate, and speech articulation. Additionally, it’s crucial to incorporate measures of verbal comprehension, utilizing specific tools like the verbal test, vocabulary test, reading fluency test, and reading comprehension test from the Wechsler Intelligence Scale, Fourth Edition (45). Other assessments to consider include words understood and words used in the MacArthur Communicative Development Inventory – words and gestures (MCDI) (46).
From a limitations perspective, the assessment of activities and participation of children with DCD includes a variety of areas such as balance and postural control, walking, fine hand movement, writing, self-care, domestic life, socialization, and psychosocial aspects (see Table 2). The assessment primarily relies on the dynamical system approach, supplemented by cognitive neurology.
Balance and postural control, which involve the ability to control the body in static and dynamic situations, can be assessed through both non-instrumented and instrumented tests (47). Non-instrumented tests mainly include functional balance tests such as the Berg Balance Scale, Timed Up and Go Test, Timed Up and Down Test, Limit of Stability and Functional Forward Extension Tests (48). However, these non-instrumented tests only provide a qualitative evaluation. On the other hand, instrumented tests, like the Virtual Reality Rehabilitation System (VRRS), can provide quantitative analysis of postural control. VRRS enables the collection of reliable balance data, such as swing area, swing speed, and number of oscillations, for assessing static balance and postural control in children with DCD (48, 49). Gait changes during walking are crucial in the assessment and early diagnosis of DCD. These changes are identified by analyzing the kinematics (movement analysis of body parts), kinetics (changes in the center of gravity, muscle dynamics, and joint dynamics), and plantar dynamics (changes in foot pressure) during walking in children. Tang (50) developed a framework using coronal 2D video recordings and posture estimation that allows distance-based features derived from conventional video recordings (e.g., mean distance from the left shoulder to the right hip or from the right shoulder to the left hip or 2D distance) to be utilized for the quantitative assessment of gait in children with DCD. Additionally, motion capture technology can measure, track, and record the movement patterns of objects in three-dimensional space, making it suitable for gait assessment in children with DCD (51). By attaching reflective markers to the major joints of the child, the 3D motion capture system can accurately evaluate gait data, including step length and gait speed, during walking. This provides an objective foundation for analyzing gait changes in children with DCD (52–54).
Standardized scales such as MABC-2, BOT-2, and PDMS-2 are commonly used for assessing fine hand activities, and they have demonstrated good reliability and validity. Additionally, precision instruments can provide quantitative assessment of upper limb and finger parameters during fine motor activities. Li proposed a fine motor assessment system that utilizes two cameras to record children’s performance in fine motor tasks. This system also incorporates automatic algorithms for task localization and single-task assessment, enabling automated scoring of fine motor skills and effective assessment for children with DCD (55). Three-dimensional motion capture can also be used to assess upper limb and finger movements during fine motor tasks in children with DCD. Using three-dimensional motion capture, Biancotto (56) discovered that children with DCD demonstrated a greater angle of spread between the thumb and forefinger during reaching and grasping tasks, compared to typically developing children. They also exhibited slower, more visually dependent, and more variable movements. Writing is a crucial everyday activity for children, and there are notable differences in movement trajectory and font size between children with DCD and typically developing children (4). A study showed that writing skills can accurately determine DCD in 94.9% of cases (57), Standardized scales such as the Handwriting Proficiency Screening Questionnaire and the Detailed Assessment of Speed of Handwriting Scale can be used to assess children’s writing skills (4). There are several tests available for assessing writing skills, which typically evaluate the efficiency of the handwriting process by counting the number of letters written within a designated time frame, and assess the quality of writing by evaluating handwriting legibility (58). Examination of children’s handwriting, including handwriting legibility and handwriting-generated movement. The legibility of handwriting can be assessed by means of the Handwriting Legibility Scale (59), and the handwriting-generated movement can be assessed by means of the Digital Writing Tablet and 3D Motion Technology (60, 61). Furthermore, digital writing tablets combined with 3D motion technology can analyze spatial and temporal data to evaluate handwriting accuracy and production movements (60), Additionally, 3D motion technology can monitor upper limb movement patterns during tasks such as copying and dictation, providing analysis on handwriting legibility, writing efficiency, and movement parameters related to writing performance (61). 3D Motion Capture holds extensive and versatile applications in the functional assessment of children with DCD. It enables precise measurement of perceptual sensations, dynamic capture of abnormal movement patterns during children’s walking for accurate assessment of gait parameters, and analysis and quantification of fine motor movements in hand grasping. This method offers a notably more precise and quantitative assessment compared to other tools.
Performance and engagement in activities of daily living (ADLs) are evaluated using the DCD Daily Scale, which encompasses motor performance (the degree to which the child completes the activity), daily participation (the extent to which the child engages in the activity), and ADL learning (the time taken by the child to learn the activity compared to their peers). This assessment provides insight into the children’s challenges in performing ADLs, learning, and participation, as well as the interplay between these areas (62, 63). Social skills are assessed using the Social Skills Improvement System Questionnaire (64), while psychosocial skills are evaluated through the Strengths and Difficulties Questionnaire (65, 66). The SDQ is a psychosocial assessment that measures the strengths and weaknesses of children and adolescents aged 4–16 years in domains such as emotional symptoms, behavioral problems, hyperactivity/inattention, peer relationship problems, and pro-social behavior (65). The Child Participation Scale (67) and the Canadian Occupational Performance Measure (68) are employed to assess children’s participation skills.
Environmental factors play a significant role in influencing the participation of children with DCD in activities, and parents often report facing more barriers and less support within their environment (4, 69–71). Assessing these factors involves evaluating the family, school, and community settings (see Table 2).
In the family environment, it is important to assess the personality traits of family members, living conditions, lifestyle, exercise spaces, and parental attitudes and behaviors. Understanding these factors provides insights into how the family environment may influence the child’s participation. In the school environment, the assessment should consider the availability of space and facilities, classroom activities, and interactions. Some indicators of the school’s psychological environment also warrant consideration, including measures of school adjustment, peer relationships, and teacher-student relationships. Evaluating these elements helps determine the level of support provided by the school and identifies potential barriers to participation. In the community environment, it is essential to assess outdoor sports spaces, the availability of playmates, and other factors that contribute to the child’s participation in community activities. Understanding the community environment provides insights into the opportunities available for the child to engage in various activities. Environmental factors can also be assessed using tools such as the Participation and Environment Measure-Children and Youth. This scale collects data on participation levels and the extent to which environmental factors either support or challenge children’s participation. It provides a comprehensive assessment of the home-school-community environments and their impact on children’s participation in activities. By evaluating environmental factors, professionals can gain a better understanding of the support and barriers that children with DCD may encounter in their daily lives. This information can guide interventions and strategies to enhance their participation in various activities (69).
By conducting a thorough assessment of the children’s body functions and structures, activities and participation, and environmental factors, a comprehensive understanding of their functional abilities can be obtained. This assessment provides a clear and detailed picture of the children’s overall functional status, enabling the development of personalized rehabilitation goals and targeted interventions.
Postural control plays a crucial role in the performance and development of gross motor skills (72). Postural control training for children with DCD includes many aspects of balance program training, core stability training, muscle strength training, and lower limb proprioceptive training. A study conducted by Zolghadr (73) implemented balance corrective exercise training for children with DCD. This training included tasks like side-to-side walking, reverse walking, standing with feet together, standing on one foot, postural correction, and planking. After 8 weeks of training, there was a significant improvement in the static and dynamic balance of the children with DCD.
Core stability training is particularly important as it helps prevent improper movement patterns by maintaining proper balance and posture during functional activities (74). Another study by Shahrbanian (75) focused on core stability training in children with DCD, which resulted in noticeable improvement in their reaction time, dynamic balance, and static balance. Balayi (74) conducted a combined core stability-hemisball training program to improve bilateral balance and coordination in children with DCD. The results showed that 8 weeks of this training significantly enhanced postural control, dynamic balance, and static balance.
In addition, muscle strength training is crucial in improving balance for children with DCD. Insufficient leg muscle strength and slow muscle force generation can limit their balance function, making it difficult for them to control their pelvis or execute other body movements in a timely manner. Therefore, muscle strength training is an essential component for improving balance (72). Fong (76) conducted resistance training on children with DCD, leading to improvements in muscle force and time to peak muscle power. This training effectively enhanced neuromuscular function, balance, and balance strategies in children with DCD.
Research suggests that balance dysfunction in children with DCD is related to impaired proprioception in the lower limbs (77, 78). Therefore, proprioceptive training can be utilized to improve balance and adjust postural control (79). Proprioceptive training includes repetitive somatosensory stimulation, somatosensory discrimination exercises, passive motor training, and active sensory-motor training with enhanced somatosensory feedback (80, 81), and individualized activities can be designed to target proprioception improvement based on each child’s skill level.
Furthermore, it has been proposed that light touch (LT) feedback provides additional information to the central nervous system about body sway and spatial orientation. This feedback triggers more stable postural control mechanisms to maintain body balance (82), Therapists can incorporate LT to promote immediate postural stabilization during balance training. Additionally, Chen (83) discovered that finger dipping can serve as an effective rehabilitation strategy to enhance sensitivity to light touch in children with DCD. This enhances the impact of LT on improving body sway in children with DCD.
The aquatic environment possesses unique properties such as buoyancy, turbulence, hydrostatic pressure, and resistance, which can effectively improve gross motor skills in children with DCD (84). The buoyancy of water reduces joint loading and enables anti-gravity movements, thereby enhancing trunk stability. The viscosity and resistance properties of water provide additional resistance, helping to build muscle strength (85). Additionally, aquatic therapy (AT) enhances tolerance to multi-sensory stimuli, promotes blood circulation through hydrostatic pressure and temperature stimulation, increases joint range of motion, and reduces muscle spasms (86). Furthermore, the water environment offers a safe setting for children to practice motor strategies and specific tasks. In water, children are more willing to attempt tasks and skills that they may avoid on land (87, 88). The Halliwick Method is a specific AT intervention designed for individuals with motor dysfunction. It is based on principles of hydrostatics, hydrodynamics, and body mechanics (89).The method consists of four phases: adaptation to the water, rotation, control of movement in the water and aquatic locomotion. It incorporates exercises focusing on breath control, floating unaided, balance, lateral, sagittal, and longitudinal control, rotational control, upward thrusting skills, and swimming strokes (86). During the learning process, children engage with each other, promoting social interaction and respiratory control. This interaction positively impacts joint mobility, stability, muscular strength and endurance, muscle tone, normalization of involuntary motor responses, control, gait, and psychological adaptation to water (86). In a study by Hillier (87), AT implemented for children with DCD effectively improved their gross motor skills. In clinical practice, training programs can be individualized to cater to the unique needs of each child, taking into account their learning styles, physical activity, skill levels, and psychological and emotional characteristics.
Kinesio tape (KT) is used to restore proper muscle function by stimulating the skin with the tape. When used as an aid during training, KT enhances muscle activation, postural control, and functional activity (90). In a study by Yam (90), KT was applied to the bilateral rectus femoris and gastrocnemius muscles in children with DCD. This resulted in improved dynamic balance performance, increased peak activation of the rectus femoris muscle, and prolonged time to peak muscle activation in specific extension directions. Another study by Li (91) investigated the effects of different KT patching methods in children with DCD, including no patching, gastrocnemius patching, tibialis anterior patching, and peroneus longus patching. The study found that muscle activity was significantly enhanced with the use of KT. Gastrocnemius patching improved balance during anterior–posterior swing, while tibialis anterior and gastrocnemius patching improved balance during medial-lateral swing. Additionally, KT facilitates improvement in proprioception. It provides mechanical pressure and stretch to the skin, stimulating skin mechanoreceptors and providing information about joint position and movement (91, 92).The repetitive stimulation enhances proprioceptive function in the body.
In occupational therapy (OT), the therapist assesses the child’s performance in activities of daily living, analyzes how the illness impacts occupational performance, and evaluates how the child’s environment influences their abilities (41). Based on this assessment, the therapist then selects suitable interventions for the child. In the case of children with DCD, OT primarily aims to improve fine motor skills, perceptual abilities, and executive functions.
Fine motor skills refer to the coordinated movements of the hand and the small muscle groups in the fingers and other parts of the hand (93). These skills involve various abilities, such as visual cues, tactile perception, and internal representation, and are crucial for children’s daily activities like picking up objects, grasping, manipulating, and tying shoelaces. The coordination of the small muscle groups in the hand serves as the foundation for precise movements. Improving hand strength and coordination can be achieved through exercises using grip strength devices, finger separation training, and two-hand coordination training. It is also important to pay attention to strengthening the proximal muscles, such as the shoulders and elbows, as they provide a stable base of support for distal control of the wrists, thumbs, and fingers, allowing the hand to be properly oriented in space and providing support and control of its movements (94).
Studies have shown that children with DCD often experience proprioceptive dysfunction in the shoulder, elbow, wrist, and hand joints, which contributes to their fine motor difficulties (95–97). Therefore, it is crucial to incorporate proprioceptive training for various upper limb joints. This can be achieved through stretching and weight-bearing exercises, such as static weight-bearing, upper limb balance exercises, dynamic stabilization exercises using a ball scapula, pinching activities, and active finger pinching tasks with or without elastic resistance (98–100). Additionally, elastic KT can be used to stimulate the local skin mechanoreceptors and proprioceptors in the surrounding tissues, thereby improving proprioception (92).
Visual and tactile perceptual skills training are crucial components of perceptual training for children with DCD. Visual feedback plays a significant role in the development of motor skills in children (101). The integration of visually perceived information into motor commands is essential for making adjustments to force output and correcting errors (102). Additionally, having good oculomotor control is important for accurately perceiving visual information (103). Eye movement control training incorporates various methods, such as visual training and quiet eye training (QET). Coetzee (104) implemented visual training in 32 children with DCD, combining perceptual and motor activities (balance, hand-eye coordination, bilateral integration, and vestibular integration) with visual exercises to enhance oculomotor control. After 18 weeks of treatment, significant improvements in oculomotor control skills, including visual tracking, fixation, alignment, and vergence, were observed in the children. QET involves maintaining fixation or tracking gaze on an object before initiating a critical action. It has been shown to effectively improve visuomotor control and attentional control (105). Norouzi (106) used QET with children with DCD to help them maintain visual attention and visuomotor coordination during coordination tasks, leading to enhanced bilateral arm coordination. Similarly, Rafique (103) implemented QET during a throwing and catching activity in children with DCD. They were instructed to fixate on the target position on the wall before throwing and then track the ball before catching it. This intervention effectively improved the visuomotor skills of the children, increased their concentration prior to throwing, and enhanced their anticipation and tracking ability. In addition to eye movement control training, engaging in sporting activities such as bowling, golf, cycling, football, skateboarding, and skiing have been found to improve physiological arousal, motor control skills, and expand visual attention in children with DCD (107).
Tactile perception also plays a crucial role in the daily activities of children with DCD, and improving tactile perception can enhance overall somatosensory functioning and fine motor control (108). Wang (38) implemented a tactile perception training program for children with DCD, where they matched objects based on six perceptual dimensions: texture, shape, hardness, size, weight, and contour. Following the training, significant improvements were observed in fine motor control tasks, fine motor integration, accuracy, manual dexterity, as well as visual perceptual functions such as visuospatial relations, visual memory, and visual sequential memory.
Children with DCD often experience deficits in three key executive functions: inhibitory control, working memory, and cognitive flexibility (86).Engaging in different ball sports and judo training can be beneficial for training these executive functions in children with DCD. In ball games, such as returning a randomly thrown ball from an opponent, individuals need to pay close attention to the situation, encode visual cues, and inhibit inappropriate actions or responses. This type of training can be effective in improving children’s attentional, reactive, and inhibitory controls (109). Tsai (109) conducted a study where children with DCD were trained in table tennis, the game requires quick planning based on actual visual information and executing appropriate actions or inhibitory responses. The findings revealed that 10 weeks of table tennis training led to significant improvements in inhibitory control, cognition, and motor skills in children with DCD. On the other hand, judo training involves practicing various falling, throwing, and grappling techniques in unpredictable environments, which demand timely visuospatial cues (such as joint positions and movement patterns) to anticipate and react to an opponent’s actions. This type of training enhances working memory capacity, particularly in visuospatial working memory (110). Additionally, judo training involves interference or response inhibition, as participants need to exercise self-control when facing an opponent during judo matches. Therefore, judo training can effectively improve children’s inhibitory control skills (111).
Numerous studies have highlighted DCD as a motor cognitive disorder (112, 113). It is well-established that children with DCD exhibit deficits in various aspects of movement anticipation, basic motor learning processes, and cognitive control, all of which significantly contribute to their difficulties in performing daily activities (3). Consequently, implementing cognitive-based strategies such as cognitive orientation to daily occupational performance (CO-OP), motor imagery and action observation (MI + AO) therapy can effectively enhance children’s memory, attention, and mental planning skills, thereby improving their overall occupational performance.
Cognitive orientation to daily occupational performance (CO-OP), as an approach, aims to enhance task-specific performance through the use of cognitively based strategies. This approach emphasizes gaining a comprehensive understanding of task requirements, acquiring problem-solving strategies, and identifying crucial aspects of performance. By focusing on cognitive aspects rather than direct barriers that restrict task performance, CO-OP facilitates the development of transferable strategies that can be applied to various tasks (114). CO-OP interventions offer children the opportunity to choose their objectives, which helps maintain their motivation and engagement throughout the therapy process. Additionally, conducting CO-OP sessions in small groups fosters a cooperative environment where children can act as both learners and mentors. During therapy, the therapist initially teaches the child a comprehensive cognitive strategy known as Goal-Plan-Do-Check. This strategy involves setting action goals, developing action plans to achieve those goals, executing the plans to complete the goals, and evaluating the results. Following this, the therapist guides the child through holistic strategies to identify specific strategies for solving the task at hand. These guiding techniques may include reinforcement, providing stimuli to provide feedback to the child, modeling or demonstrating the skill, and offering prompts to support the child’s discovery of successful strategies. Gradually, the prompts are reduced once the child has successfully completed the task with an improved plan (115, 116). In Thornton’s study (68), a group of children with DCD participated in a 10-week CO-OP intervention. The results showed that children in the CO-OP group demonstrated improvements in impairment, participation, and activity levels.
Similarly, Araújo’s study (116) investigated the effects of a 6-week CO-OP program involving both children with DCD and their parents. The study found that all the children showed improvements in their occupational performance on the goals they selected after receiving the CO-OP treatment. Furthermore, five of the children were able to apply the cognitive strategies they learned to tasks that were not specifically addressed in the treatment. CO-OP interventions have been proven effective in enhancing children’s task-specific performance by targeting cognitive strategies related to their chosen goals. Consequently, this approach leads to improvements in their overall activities and participation levels. Additionally, CO-OP facilitates the application of holistic or domain-specific strategies to other aspects of daily life as children’s cognitive strategies are enhanced.
MI therapy, which is a cognitive-based intervention, has also been shown to effectively improve motor planning in children with DCD (117, 118), MI involves mentally rehearsing or simulating movements from a first-person perspective, without physically executing the movements. Through MI, children can develop accurate internal models that aid in motor planning, activating the brain regions responsible for planning and executing motor skills (36, 117, 119, 120). Hoyroo (36) conducted a study to investigate the impact of MI on grip choice strategies in children with DCD. The participants, including both children with DCD and typically developing children, were engaged in a grip choice task with various color sequences. The MI group was instructed to mentally visualize the instructions for completing the movements before executing the task. The results indicated that children with DCD in the MI group demonstrated significantly improved motor planning abilities across all color sequences.
MI is frequently combined with AO in the treatment of motor planning difficulties (121). AO involves observing another individual performing a specific action to obtain precise visual and temporal motion cues. This visual information is then mapped onto a motion circuit and utilized to generate motion imagery that is closely related to the observed action (122). Both MI and AO activate brain regions that are involved in motor planning and execution, as they share neural networks associated with internal modeling processes (3, 121). Numerous studies have demonstrated that combining MI and AO can lead to improvements in motor skills and activities of daily living in children with DCD (123). In the Marshall study (124), children with DCD were divided into two groups: the MI + AO group (watching a video of the task while mentally imagining the sensations associated with performing the action) and the control group (watching a video unrelated to movement). During a visuomotor rotation task, the children in the MI + AO group demonstrated faster completion times, more goal-oriented eye movement patterns, and smoother movements. This study highlights the potential of combining MI and AO techniques in training for motion planning to enhance the development of internal models.
In recent years, the use of virtual reality (VR) has become increasingly prevalent in the rehabilitation of children with DCD, thanks to advancements in technology. VR offers a simulated experience of real-life environments, providing an enjoyable and accessible platform for children to enhance their confidence in their own abilities. This positive cycle of reinforcement helps to motivate children in overcoming challenges they face in their daily lives. Engel-Yeger’s study (125) illustrates how VR-based exercises and virtual games are used to provide enjoyable and successful experiences for children with DCD. These activities allow children to simulate the actions of their typically developing peers, ultimately leading to improvements in real-world activities and social participation skills.
Compared to traditional sedentary video games, virtual games are considered active video games that involve larger scale body movements in an interactive environment. An example of an active video game for VR systems is Wii Fit training. This game utilizes handheld controllers or balance board devices to track limb movements and translates this information into controlling avatars within a virtual gaming environment (107). In the Hashemi study (107), it was found that children with DCD who participated in 8 weeks of Wii Fit training experienced noteworthy enhancements in various aspects of visual perception, such as visual discrimination, visual memory, visual perception, visuospatial relations, visual form constancy, visual sequence memory, visual graphic ground, and visual closure. Improvements were also observed in executive function tasks, including planning, attention, simultaneous processing, and sequential processing. Furthermore, VR technology can be effectively utilized in AO and MI training. Through the use of VR, participants are able to perceive the virtual environment as if it were real and engage in interactive body movements to participate in the game or training session (126). VR offers a multitude of sensory feedback options, guiding the child’s attention towards external effects and motor outcomes. This in turn facilitates improved predictive motor control for children with DCD and enhances their ability to visualize and execute motor tasks (127).
Environmental factors are of utmost importance in the motor development of children with DCD. Creating an environment that is conducive to motor development allows children to consistently practice and enhance their motor skills. Therefore, interventions should not only focus on individual therapy but also include modifications to the child’s home, school, and community environments. These modifications aim to support the acquisition of motor skills and promote social participation among children with DCD. This entails providing suitable and sufficient space within the home, school, and community environments to accommodate various physical, cultural, and recreational activities. It is vital to encourage the active involvement of parents and peers in a wide range of activities that foster the motor development of children. Furthermore, the influence of electronic screens on children’s motor abilities cannot be disregarded as it becomes more prevalent. Research has shown that early exposure to electronic screens has a detrimental effect on children’s exercise duration, neuromusculoskeletal development, and motor skills (128–130). As a result, strict limitations should be implemented, ensuring that children’s exposure to electronic screens does not exceed 2 h per day.
Ecological intervention is a highly effective treatment for children with DCD. This approach not only focuses on restoring the individual’s motor function but also addresses environmental factors that contribute to DCD. These factors include the family, school, and community, all of which are considered essential components of the intervention (69). For example, home-based interventions during weekends can be implemented, involving parents actively in the treatment process. This can include improving parenting styles, providing encouragement and support to their children. In addition, peer-mediated physical activities can be introduced in school and community settings. These activities aim to promote the practice and acquisition of new motor skills in various activities, as well as enhance children’s social participation and ability to perform daily tasks. Ferguson conducted a nine-week health promotion program for children with and without DCD in a school setting, working to create child-friendly health conditions in the school and to influence the health-related knowledge and behaviors of children, educators, and parents, which resulted in improvements in the athletic performance of children with DCD as well as in their level of physical fitness (131).
Given the diverse impairments and limitations experienced by children with DCD, it is crucial to conduct a thorough assessment of their body functions and structures, activities and participation, and environmental factors. These assessments help to determine the extent and severity of the impairment of children’s body functions and structures, to identify the extent to which their activities and participation are limited, and to understand the environmental factors that hinder or promote them. Based on the assessment results, individualized rehabilitation goals can be established, and specific interventions can be formulated to address the unique needs of each child. Rehabilitation for children with DCD encompasses various interventions (Figure 3), such as physical therapy (PT), OT, cognitive orientation training, the use of emerging technologies like VR and the environmental factors. PT aims to reduce impairments, while OT focuses on enhancing limited activities and participation in daily life. However, it is important to note that improvements in impairments do not always lead to improvements in daily activities and participation (4, 132). Therefore, a multidisciplinary approach is often employed to address both impaired functioning and limited activities and participation in children with DCD.
In addition to other aspects, OT emphasizes family-centered therapy as a principle. It emphasizes the involvement of parents in their children’s treatment (132). Collaboratively, therapists and parents establish therapy goals, which can be related to education, self-care activities, or social interaction. Interventions are tailored to the specific abilities and needs of the child (41).Cognitive orientation training holds significant importance in the rehabilitation of children with DCD, as it is recognized as a motor cognitive disorder in numerous studies (112, 113). By acquiring problem-solving strategies at a cognitive level, children can enhance task-specific performance and improve their activities and participation. Moreover, the ability to generalize these strategies to other tasks is crucial (116). In this context, the use of emerging VR technologies offers enhanced and practical movement environments for the treatment of children with DCD, presenting a promising therapeutic approach. The treatment should also address the impact of environmental factors on the child’s functioning in society, their ability to perform actions or tasks, and their overall physical and mental well-being. The intervention of environmental factors encompasses all aspects of the family, school, and community environments. Throughout the treatment process, it is important for parents, teachers, and other individuals in the child’s surroundings to provide the necessary support and create a favorable external environment. By working together, a conducive environment can be established that facilitates the children’s acquisition of motor skills and enhances their social participation.
Regardless of the specific approach, intervention strategies for DCD can generally be classified into two main categories (133).The first category involves the application of activities aimed at addressing the underlying behavioral issues. This approach, known as the bottom–up process-oriented approach, focuses on reducing impairments and improving the child’s body functions and structures. The second category focuses on directly addressing the behavioral issues themselves. This approach, referred to as the top–down task-oriented approach, emphasizes changes at the level of activities and participation (For a categorization of the intervention strategies mentioned in this paper, see Table 3). In clinical practice, it is crucial to consider the generalizability of intervention effects to the different environments in which a child engages in daily activities and participates. Therefore, integrated interventions that focus on tasks are highly recommended for DCD. These interventions enable children to acquire problem-solving skills, enhance their motor planning and execution abilities, and apply the intervention’s effects to more complex everyday activities.
JG: Writing -original draft, Writing -review & editing. WS: Writing -original draft, Writing - review & editing. YZ: Writing -original draft, Writing -review & editing. DH: Writing -review & editing. JW: Writing -review & editing. AZ: Writing -review & editing. XK: Writing -review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The author disclosed receipt of the following financial support for the publication of this article: this work was supported by the Scientific Research Project of Traditional Chinese Medicine of Shanghai Hongkou District Health and Wellness Committee [grant number HKOGYOY-ZY-2023-24]; the Research Launch Project of The Fourth People’s Hospital Affiliated to Tongji University (grant number sykyqd02001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Smits-Engelsman, B, and Verbecque, E. Pediatric care for children with developmental coordination disorder, can we do better? Biom J. (2022) 45:250–64. doi: 10.1016/j.bj.2021.08.008
2. Cavalcante Neto, JL, Steenbergen, B, Zamunér, AR, and Tudella, E. Wii training versus non-Wii task-specific training on motor learning in children with developmental coordination disorder: a randomized controlled trial. Ann Phys Rehabil Med. (2021) 64:101390. doi: 10.1016/j.rehab.2020.03.013
3. Wilson, PH, Smits-Engelsman, B, Caeyenberghs, K, Steenbergen, B, Sugden, D, Clark, J, et al. Cognitive and neuroimaging findings in developmental coordination disorder: new insights from a systematic review of recent research. Dev Med Child Neurol. (2017) 59:1117–29. doi: 10.1111/dmcn.13530
4. Blank, R, Barnett, AL, Cairney, J, Green, D, Kirby, A, Polatajko, H, et al. International clinical practice recommendations on the definition, diagnosis, assessment, intervention, and psychosocial aspects of developmental coordination disorder. Dev Med Child Neurol. (2019) 61:242–85. doi: 10.1111/dmcn.14132
5. Green, D, and Payne, S. Understanding Organisational ability and self-regulation in children with developmental coordination disorder. Curr Dev Disord Rep. (2018) 5:34–42. doi: 10.1007/s40474-018-0129-2
6. Licari, MK, Reynolds, JE, Tidman, S, Ndiaye, S, Sekaran, SN, Reid, SL, et al. Visual tracking behaviour of two-handed catching in boys with developmental coordination disorder. Res Dev Disabil. (2018) 83:280–6. doi: 10.1016/j.ridd.2018.07.005
7. Archibald, LM, and Alloway, TP. Comparing language profiles: children with specific language impairment and developmental coordination disorder. Int J Lang Commun Disord. (2008) 43:165–80. doi: 10.1080/13682820701422809
8. Landgren, V, Fernell, E, Gillberg, C, Landgren, M, and Johnson, M. Attention-deficit/hyperactivity disorder with developmental coordination disorder: 24-year follow-up of a population-based sample. BMC Psychiatry. (2021) 21:161. doi: 10.1186/s12888-021-03154-w
9. Pieters, S, Roeyers, H, Rosseel, Y, van Waelvelde, H, and Desoete, A. Identifying subtypes among children with developmental coordination disorder and mathematical learning disabilities, using model-based clustering. J Learn Disabil. (2015) 48:83–95. doi: 10.1177/0022219413491288
10. Miller, HL, Sherrod, GM, Mauk, JE, Fears, NE, Hynan, LS, and Tamplain, PM. Shared features or co-occurrence? Evaluating symptoms of developmental coordination disorder in children and adolescents with autism Spectrum disorder. J Autism Dev Disord. (2021) 51:3443–55. doi: 10.1007/s10803-020-04766-z
11. Tal Saban, M, and Kirby, A. Empathy, social relationship and co-occurrence in young adults with DCD. Hum Mov Sci. (2019) 63:62–72. doi: 10.1016/j.humov.2018.11.005
12. Kilroy, E, Ring, P, Hossain, A, Nalbach, A, Butera, C, Harrison, L, et al. Motor performance, praxis, and social skills in autism spectrum disorder and developmental coordination disorder. Autism Res. (2022) 15:1649–64. doi: 10.1002/aur.2774
13. Draghi, TTG, Cavalcante Neto, JL, Rohr, LA, Jelsma, LD, and Tudella, E. Symptoms of anxiety and depression in children with developmental coordination disorder: a systematic review. J Pediatr. (2020) 96:8–19. doi: 10.1016/j.jped.2019.03.002
14. Draghi, TTG, Cavalcante Neto, JL, and Tudella, E. Symptoms of anxiety and depression in schoolchildren with and without developmental coordination disorder. J Health Psychol. (2021) 26:1519–27. doi: 10.1177/1359105319878253
15. Harrowell, I, Hollén, L, Lingam, R, and Emond, A. Mental health outcomes of developmental coordination disorder in late adolescence. Dev Med Child Neurol. (2017) 59:973–9. doi: 10.1111/dmcn.13469
16. Chien, CW, Rodger, S, Copley, J, and Skorka, K. Comparative content review of children's participation measures using the international classification of functioning, disability and health-children and youth. Arch Phys Med Rehabil. (2014) 95:141–52. doi: 10.1016/j.apmr.2013.06.027
17. Wilson, PH. Practitioner review: approaches to assessment and treatment of children with DCD: an evaluative review. J Child Psychol Psychiatry. (2005) 46:806–23. doi: 10.1111/j.1469-7610.2005.01409.x
18. Hua, J, Gu, G, Meng, W, and Wu, Z. Age band 1 of the movement assessment battery for children-second edition: exploring its usefulness in mainland China. Res Dev Disabil. (2013) 34:801–8. doi: 10.1016/j.ridd.2012.10.012
19. Tsang, WW, Guo, X, Fong, SS, Mak, KK, and Pang, MY. Activity participation intensity is associated with skeletal development in pre-pubertal children with developmental coordination disorder. Res Dev Disabil. (2012) 33:1898–904. doi: 10.1016/j.ridd.2012.05.015
20. Ip, A, Mickelson, ECR, and Zwicker, JG. Assessment, diagnosis, and management of developmental coordination disorder. Paediatr Child Health. (2021) 26:375–8. doi: 10.1093/pch/pxab047
21. Sueda, K, Hashimoto, R, and Ueda, T. Convergent validity of the developmental coordination disorder checklist using soft neurological signs. Brain and Development. (2022) 44:17–29. doi: 10.1016/j.braindev.2021.08.001
22. Raynor, AJ. Fractioned reflex and reaction time in children with developmental coordination disorder. Mot Control. (1998) 2:114–24. doi: 10.1123/mcj.2.2.114
23. Zafeiriou, DI. Primitive reflexes and postural reactions in the neurodevelopmental examination. Pediatr Neurol. (2004) 31:1–8. doi: 10.1016/j.pediatrneurol.2004.01.012
24. Niklasson, M, Rasmussen, P, Niklasson, I, and Norlander, T. Developmental coordination disorder: the importance of grounded assessments and interventions. Front Psychol. (2018) 9:2409. doi: 10.3389/fpsyg.2018.02409
25. Niklasson, M, Rasmussen, P, Niklasson, I, and Norlander, T. Adults with sensorimotor disorders: enhanced physiological and psychological development following specific sensorimotor training. Front Psychol. (2015) 6:480. doi: 10.3389/fpsyg.2015.00480
26. van Dyck, D, Baijot, S, Aeby, A, de Tiège, X, and Deconinck, N. Cognitive, perceptual, and motor profiles of school-aged children with developmental coordination disorder. Front Psychol. (2022) 13:860766. doi: 10.3389/fpsyg.2022.860766
27. Wilson, P, Ruddock, S, Rahimi-Golkhandan, S, Piek, J, Sugden, D, Green, D, et al. Cognitive and motor function in developmental coordination disorder. Dev Med Child Neurol. (2020) 62:1317–23. doi: 10.1111/dmcn.14646
28. Emrani, S, Lamar, M, Price, CC, Swenson, R, Libon, DJ, and Baliga, G. Neurocognitive operations underlying working memory abilities: an analysis of latency and time-based parameters. J Alzheimers Dis. (2023) 94:1535–47. doi: 10.3233/jad-230288
29. He, J, Yan, S, Song, Z, Lu, Q, Zhong, S, Lai, S, et al. Similarities and differences in working memory and neurometabolism of obsessive-compulsive disorder and major depressive disorder. J Affect Disord. (2022) 311:556–64. doi: 10.1016/j.jad.2022.05.069
30. Gonthier, C. Charting the diversity of strategic processes in visuospatial short-term memory. Perspect Psychol Sci. (2021) 16:294–318. doi: 10.1177/1745691620950697
31. Graziola, F, Garone, G, Grasso, M, Schirinzi, T, and Capuano, A. Working memory, attention and planning abilities in NKX2.1-related chorea. Parkinsonism Relat Disord. (2021) 88:24–7. doi: 10.1016/j.parkreldis.2021.05.021
32. Unterrainer, JM, Rahm, B, Loosli, SV, Rauh, R, Schumacher, LV, Biscaldi, M, et al. Psychometric analyses of the tower of London planning task reveal high reliability and feasibility in typically developing children and child patients with ASD and ADHD. Child Neuropsychol. (2020) 26:257–73. doi: 10.1080/09297049.2019.1642317
33. Kawabata, M, Lee, K, Choo, HC, and Burns, SF. Breakfast and exercise improve academic and cognitive performance in adolescents. Nutrients. (2021) 13:13. doi: 10.3390/nu13041278
34. Li, Y, Zhou, T, Lu, Y, Sang, M, Liu, J, He, X, et al. The association between the health-related physical fitness and inhibitory control in preschool children. BMC Pediatr. (2022) 22:106. doi: 10.1186/s12887-022-03163-y
35. Asgharian Asl, F, and Vaghef, L. The effectiveness of high-frequency left DLPFC-rTMS on depression, response inhibition, and cognitive flexibility in female subjects with major depressive disorder. J Psychiatr Res. (2022) 149:287–92. doi: 10.1016/j.jpsychires.2022.01.025
36. Bhoyroo, R, Hands, B, Wilmut, K, Hyde, C, and Wigley, A. Motor planning with and without motor imagery in children with developmental coordination disorder. Acta Psychol. (2019) 199:102902. doi: 10.1016/j.actpsy.2019.102902
37. Tseng, YT, Holst-Wolf, JM, Tsai, CL, Chen, FC, and Konczak, J. Haptic perception is altered in children with developmental coordination disorder. Neuropsychologia. (2019) 127:29–34. doi: 10.1016/j.neuropsychologia.2019.02.004
38. Wuang, YP, Huang, CL, and Wu, CS. Haptic perception training programs on fine motor control in adolescents with developmental coordination disorder: a preliminary study. J Clin Med. (2022) 11:11. doi: 10.3390/jcm11164755
39. Tsai, CL, Wilson, PH, and Wu, SK. Role of visual-perceptual skills (non-motor) in children with developmental coordination disorder. Hum Mov Sci. (2008) 27:649–64. doi: 10.1016/j.humov.2007.10.002
40. Milam, LA, Cohen, GL, Mueller, C, and Salles, A. Stereotype threat and working memory among surgical residents. Am J Surg. (2018) 216:824–9. doi: 10.1016/j.amjsurg.2018.07.064
41. Soref, B, Robinson, GL, and Bart, O. The effect of a short-term occupational therapy intervention on the participation and personal factors of preschoolers with developmental disabilities. Children (Basel). (2023) 10:10. doi: 10.3390/children10081401
42. Wilmut, K, Du, W, and Barnett, AL. Navigating through apertures: perceptual judgements and actions of children with developmental coordination disorder. Dev Sci. (2017) 20:20. doi: 10.1111/desc.12462
43. Varuzza, C, D’Aiello, B, Lazzaro, G, Quarin, F, de Rose, P, Bergonzini, P, et al. Gross, fine and visual-motor skills in children with language disorder, speech sound disorder and their combination. Brain Sci. (2023) 13:59. doi: 10.3390/brainsci13010059
44. Flapper, BC, and Schoemaker, MM. Developmental coordination disorder in children with specific language impairment: co-morbidity and impact on quality of life. Res Dev Disabil. (2013) 34:756–63. doi: 10.1016/j.ridd.2012.10.014
45. Wechsler, D. WISC-IV® Australian administration and scoring manual. Australia: Harcourt Assessment (2003).
46. Fletcher-Watson, S, Petrou, A, Scott-Barrett, J, Dicks, P, Graham, C, O’Hare, A, et al. A trial of an iPad™ intervention targeting social communication skills in children with autism. Autism. (2016) 20:771–82. doi: 10.1177/1362361315605624
47. Paillard, T, and Noé, F. Techniques and methods for testing the postural function in healthy and pathological subjects. Biomed Res Int. (2015) 2015:891390. doi: 10.1155/2015/891390
48. Martini, G, Beani, E, Filogna, S, Menici, V, Cioni, G, Battini, R, et al. New technological approach for the evaluation of postural control abilities in children with developmental coordination disorder. Children (Basel). (2022) 9:957. doi: 10.3390/children9070957
49. Beani, E, Filogna, S, Martini, G, Barzacchi, V, Ferrari, A, Guidi, E, et al. Application of virtual reality rehabilitation system for the assessment of postural control while standing in typical children and peers with neurodevelopmental disorders. Gait Posture. (2022) 92:364–70. doi: 10.1016/j.gaitpost.2021.12.008
50. Tang, W, van Ooijen, PMA, Sival, DA, and Maurits, NM. 2D gait skeleton data normalization for quantitative assessment of movement disorders from freehand single camera video recordings. Sensors (Basel). (2022) 22:22. doi: 10.3390/s22114245
51. Ozkaya, G, Jung, HR, Jeong, IS, Choi, MR, Shin, MY, Lin, X, et al. Three-dimensional motion capture data during repetitive overarm throwing practice. Sci Data. (2018) 5:180272. doi: 10.1038/sdata.2018.272
52. Jeon, S, Lee, KM, and Koo, S. Anomalous gait feature classification from 3-D motion capture data. IEEE J Biomed Health Inform. (2022) 26:696–703. doi: 10.1109/jbhi.2021.3101549
53. Parr, JVV, Foster, RJ, Wood, G, and Hollands, MA. Children with developmental coordination disorder exhibit greater stepping error despite similar gaze patterns and state anxiety levels to their typically developing peers. Front Hum Neurosci. (2020) 14:303. doi: 10.3389/fnhum.2020.00303
54. Parr, JVV, Foster, RJ, Wood, G, Thomas, NM, and Hollands, MA. Children with developmental coordination disorder show altered visuomotor control during stair negotiation associated with heightened state anxiety. Front Hum Neurosci. (2020) 14:589502. doi: 10.3389/fnhum.2020.589502
55. Li, R, Fu, H, Zheng, Y, Lo, WL, Yu, JJ, Sit, CHP, et al. Automated fine motor evaluation for developmental coordination disorder. IEEE Trans Neural Syst Rehabil Eng. (2019) 27:963–73. doi: 10.1109/tnsre.2019.2911303
56. Biancotto, M, Skabar, A, Bulgheroni, M, Carrozzi, M, and Zoia, S. Neuromotor deficits in developmental coordination disorder: evidence from a reach-to-grasp task. Res Dev Disabil. (2011) 32:1293–300. doi: 10.1016/j.ridd.2011.02.007
57. Rosenblum, S. Handwriting measures as reflectors of executive functions among adults with developmental coordination disorders (DCD). Front Psychol. (2013) 4:357. doi: 10.3389/fpsyg.2013.00357
58. Biotteau, M, Danna, J, Baudou, É, Puyjarinet, F, Velay, JL, Albaret, JM, et al. Developmental coordination disorder and dysgraphia: signs and symptoms, diagnosis, and rehabilitation. Neuropsychiatr Dis Treat. (2019) 15:1873–85. doi: 10.2147/ndt.S120514
59. Barnett, AL, Prunty, M, and Rosenblum, S. Development of the handwriting legibility scale (HLS): a preliminary examination of reliability and validity. Res Dev Disabil. (2018) 72:240–7. doi: 10.1016/j.ridd.2017.11.013
60. Prunty, M, and Barnett, AL. Accuracy and consistency of letter formation in children with developmental coordination disorder. J Learn Disabil. (2020) 53:120–30. doi: 10.1177/0022219419892851
61. Abu-Ata, A, Green, D, Sopher, R, Portnoy, S, and Ratzon, NZ. Upper limb kinematics of handwriting among children with and without developmental coordination disorder. Sensors (Basel). (2022) 22:22. doi: 10.3390/s22239224
62. van der Linde, BW, van Netten, JJ, Otten, B, Postema, K, Geuze, RH, and Schoemaker, MM. Activities of daily living in children with developmental coordination disorder: performance, learning, and participation. Phys Ther. (2015) 95:1496–506. doi: 10.2522/ptj.20140211
63. Delgado-Lobete, L, Montes-Montes, R, Pértega-Díaz, S, Santos-del-Riego, S, Hartman, E, and Schoemaker, MM. Motor performance and daily participation in children with and without probable developmental coordination disorder. Dev Med Child Neurol. (2022) 64:220–7. doi: 10.1111/dmcn.15036
64. Tal-Saban, M, Moshkovitz, M, Zaguri-Vittenberg, S, and Yochman, A. Social skills of kindergarten children with global developmental delay (GDD), with and without developmental coordination disorder (DCD). Res Dev Disabil. (2021) 119:104105. doi: 10.1016/j.ridd.2021.104105
65. Goodman, R. The strengths and difficulties questionnaire: a research note. J Child Psychol Psychiatry. (1997) 38:581–6. doi: 10.1111/j.1469-7610.1997.tb01545.x
66. Karras, HC, Morin, DN, Gill, K, Izadi-Najafabadi, S, and Zwicker, JG. Health-related quality of life of children with developmental coordination disorder. Res Dev Disabil. (2019) 84:85–95. doi: 10.1016/j.ridd.2018.05.012
67. Rosenberg, L, Jarus, T, and Bart, O. Development and initial validation of the children participation questionnaire (CPQ). Disabil Rehabil. (2010) 32:1633–44. doi: 10.3109/09638281003611086
68. Thornton, A, Licari, M, Reid, S, Armstrong, J, Fallows, R, and Elliott, C. Cognitive orientation to (daily) occupational performance intervention leads to improvements in impairments, activity and participation in children with developmental coordination disorder. Disabil Rehabil. (2016) 38:979–86. doi: 10.3109/09638288.2015.1070298
69. Izadi-Najafabadi, S, Ryan, N, Ghafooripoor, G, Gill, K, and Zwicker, JG. Participation of children with developmental coordination disorder. Res Dev Disabil. (2019) 84:75–84. doi: 10.1016/j.ridd.2018.05.011
70. Du, W, Ke, L, Wang, Y, Hua, J, Duan, W, and Barnett, AL. The prenatal, postnatal, neonatal, and family environmental risk factors for developmental coordination disorder: a study with a national representative sample. Res Dev Disabil. (2020) 104:103699. doi: 10.1016/j.ridd.2020.103699
71. Hua, J, Meng, W, Wu, Z, Zhang, L, Gu, G, and Zhu, L. Environmental factors associated with developmental coordination disorder in preschool children in urban area of Suzhou city. Zhonghua Er Ke Za Zhi. (2014) 52:590–5.
72. Fong, SSM, Ng, SSM, Guo, X, Wang, Y, Chung, RCK, Stat, G, et al. Deficits in lower limb muscle reflex contraction latency and peak force are associated with impairments in postural control and gross motor skills of children with developmental coordination disorder: a Cross-sectional study. Medicine (Baltimore). (2015) 94:e1785. doi: 10.1097/md.0000000000001785
73. Zolghadr, H, Sedaghati, P, and Daneshmandi, H. The effect of selected balance/corrective exercises on the balance performance of mentally-retarded students with developmental coordination disorder. USWR. (2019) 9:23–30. doi: 10.32598/PTJ.9.1.23
74. Balayi, E, Sedaghati, P, and Ahmadabadi, S. Effects of neuromuscular training on postural control of children with intellectual disability and developmental coordination disorders: neuromuscular training and postural control. BMC Musculoskelet Disord. (2022) 23:631. doi: 10.1186/s12891-022-05569-2
75. Shahrbanian, S, and Hashemi, A. The effects of Core stabilization training on balance and reaction time in children with developmental coordination disorder. JRSM. (2018) 8:83–91. doi: 10.29252/JRSM.8.16.83
76. Fong, SSM, Guo, X, Cheng, YTY, Liu, KPY, Tsang, WWN, Yam, TTT, et al. A novel balance training program for children with developmental coordination disorder: a randomized controlled trial. Medicine (Baltimore). (2016) 95:e3492. doi: 10.1097/md.0000000000003492
77. Chen, FC, Pan, CY, Chu, CH, Tsai, CL, and Tseng, YT. Joint position sense of lower extremities is impaired and correlated with balance function in children with developmental coordination disorder. J Rehabil Med. (2020) 52:jrm00088. doi: 10.2340/16501977-2720
78. Bartonek, Å, Eriksson, M, Ericson, A, Reimeringer, M, and Lidbeck, C. Evaluation of knee position sense in children with motor disabilities and children with typical development: a Cross-sectional study. Children (Basel). (2023) 10:10. doi: 10.3390/children10061056
79. Yoo, S, Park, SK, Yoon, S, Lim, HS, and Ryu, J. Comparison of proprioceptive training and muscular strength training to improve balance ability of taekwondo Poomsae athletes: a randomized controlled trials. J Sports Sci Med. (2018) 17:445–54.
80. Yeh, IL, Holst-Wolf, J, Elangovan, N, Cuppone, AV, Lakshminarayan, K, Cappello, L, et al. Effects of a robot-aided somatosensory training on proprioception and motor function in stroke survivors. J Neuroeng Rehabil. (2021) 18:77. doi: 10.1186/s12984-021-00871-x
81. Aman, JE, Elangovan, N, Yeh, IL, and Konczak, JÃ. The effectiveness of proprioceptive training for improving motor function: a systematic review. Front Hum Neurosci. (2014) 8:1075. doi: 10.3389/fnhum.2014.01075
82. Baldan, AM, Alouche, SR, Araujo, IM, and Freitas, SM. Effect of light touch on postural sway in individuals with balance problems: a systematic review. Gait Posture. (2014) 40:1–10. doi: 10.1016/j.gaitpost.2013.12.028
83. Chen, FC, Li, LL, Chu, CH, Pan, CY, and Tsai, CL. Finger soaking enhances effects of light touch on reducing body sway in children with developmental coordination disorder. J Rehabil Med. (2019) 51:217–24. doi: 10.2340/16501977-2524
84. Faíl, LB, Marinho, DA, Marques, EA, Costa, MJ, Santos, CC, Marques, MC, et al. Benefits of aquatic exercise in adults with and without chronic disease-a systematic review with meta-analysis. Scand J Med Sci Sports. (2022) 32:465–86. doi: 10.1111/sms.14112
85. Kargarfard, M, Shariat, A, Ingle, L, Cleland, JA, and Kargarfard, M. Randomized controlled trial to examine the impact of aquatic exercise training on functional capacity, balance, and perceptions of fatigue in female patients with multiple sclerosis. Arch Phys Med Rehabil. (2018) 99:234–41. doi: 10.1016/j.apmr.2017.06.015
86. Shariat, A, Najafabadi, MG, Dos Santos, IK, Anastasio, AT, Milajerdi, HR, Hassanzadeh, G, et al. The effectiveness of aquatic therapy on motor and social skill as well as executive function in children with neurodevelopmental disorder: a systematic review and meta-analysis. Arch Phys Med Rehabil. (2023) 105:1000–7. doi: 10.1016/j.apmr.2023.08.025
87. Hillier, S, McIntyre, A, and Plummer, L. Aquatic physical therapy for children with developmental coordination disorder: a pilot randomized controlled trial. Phys Occup Ther Pediatr. (2010) 30:111–24. doi: 10.3109/01942630903543575
88. McManus, BM, and Kotelchuck, M. The effect of aquatic therapy on functional mobility of infants and toddlers in early intervention. Pediatr Phys Ther. (2007) 19:275–82. doi: 10.1097/PEP.0b013e3181575190
89. Vodakova, E, Chatziioannou, D, Jesina, O, and Kudlacek, M. The effect of Halliwick method on aquatic skills of children with autism Spectrum disorder. Int J Environ Res Public Health. (2022) 19:19. doi: 10.3390/ijerph192316250
90. Yam, TTT, Or, PPL, Ma, AWW, Fong, SSM, and Wong, MS. Effect of Kinesio taping on Y-balance test performance and the associated leg muscle activation patterns in children with developmental coordination disorder: a randomized controlled trial. Gait Posture. (2019) 68:388–96. doi: 10.1016/j.gaitpost.2018.12.025
91. Li, LL, and Chen, FC. Effects of kinesio taping on static balance performance and muscle activity in children with developmental coordination disorder: a single-group pretest-posttest study. J Rehabil Med. (2023) 55:jrm13403. doi: 10.2340/jrm.v55.13403
92. Ager, AL, de Oliveira, FCL, Roy, JS, Borms, D, Deraedt, M, Huyge, M, et al. Effects of elastic kinesiology taping on shoulder proprioception: a systematic review. Braz J Phys Ther. (2023) 27:100514. doi: 10.1016/j.bjpt.2023.100514
93. Bondi, D, Robazza, C, Lange-Küttner, C, and Pietrangelo, T. Fine motor skills and motor control networking in developmental age. Am J Hum Biol. (2022) 34:e23758. doi: 10.1002/ajhb.23758
94. Steinhart, S, Weiss, PL, and Friedman, J. Proximal and distal movement patterns during a graphomotor task in typically developing children and children with handwriting problems. J Neuroeng Rehabil. (2021) 18:178. doi: 10.1186/s12984-021-00970-9
95. Tseng, YT, Tsai, CL, Chen, FC, and Konczak, J. Wrist position sense acuity and its relation to motor dysfunction in children with developmental coordination disorder. Neurosci Lett. (2018) 674:106–11. doi: 10.1016/j.neulet.2018.03.031
96. Tseng, YT, Tsai, CL, Chen, FC, and Konczak, J. Position sense dysfunction affects proximal and distal arm joints in children with developmental coordination disorder. J Mot Behav. (2019) 51:49–58. doi: 10.1080/00222895.2017.1415200
97. Tseng, YT, Lin, YH, Chen, YW, Tsai, CL, and Chen, FC. Impaired wrist position sense is linked to motor abnormalities in young adults with a probable developmental coordination disorder. Neurosci Lett. (2022) 772:136446. doi: 10.1016/j.neulet.2022.136446
98. Ager, AL, Borms, D, Bernaert, M, Brusselle, V, Claessens, M, Roy, JS, et al. Can a conservative rehabilitation strategy improve shoulder proprioception? A Systematic Review. J Sport Rehabil. (2020) 30:136–51. doi: 10.1123/jsr.2019-0400
99. Han, J, Waddington, G, Anson, J, and Adams, R. Does elastic resistance affect finger pinch discrimination? Hum Factors. (2013) 55:976–84. doi: 10.1177/0018720813481620
100. Li, L, Li, Y, Jia, P, Wang, S, Wang, W, and Liu, Y. Effect of pinch types on pinch force sense in healthy adults. Front Hum Neurosci. (2022) 16:990431. doi: 10.3389/fnhum.2022.990431
101. Pinero-Pinto, E, Romero-Galisteo, RP, Sánchez-González, MC, Escobio-Prieto, I, Luque-Moreno, C, and Palomo-Carrión, R. Motor skills and visual deficits in developmental coordination disorder: a narrative review. J Clin Med. (2022) 11:11. doi: 10.3390/jcm11247447
102. Mayhew, SD, Porcaro, C, Tecchio, F, and Bagshaw, AP. fMRI characterisation of widespread brain networks relevant for behavioural variability in fine hand motor control with and without visual feedback. NeuroImage. (2017) 148:330–42. doi: 10.1016/j.neuroimage.2017.01.017
103. Rafique, SA, and Northway, N. Relationship of ocular accommodation and motor skills performance in developmental coordination disorder. Hum Mov Sci. (2015) 42:1–14. doi: 10.1016/j.humov.2015.04.006
104. Coetzee, D, and Pienaar, AE. The effect of visual therapy on the ocular motor control of seven-to eight-year-old children with developmental coordination disorder (DCD). Res Dev Disabil. (2013) 34:4073–84. doi: 10.1016/j.ridd.2013.08.036
105. Vine, SJ, Moore, LJ, and Wilson, MR. Quiet eye training: the acquisition, refinement and resilient performance of targeting skills. Eur J Sport Sci. (2014) 14:S235–42. doi: 10.1080/17461391.2012.683815
106. Norouzi Seyed Hosseini, R, Norouzi, E, and Soleymani, M. Effects of quiet eye training on performance of bimanual coordination in children with DCD. Iran. J Child Neurol. (2021) 15:43–54. doi: 10.22037/ijcn.v15i4.18926
107. Hashemi, A, Khodaverdi, Z, and Zamani, MH. Effect of Wii fit training on visual perception and executive function in boys with developmental coordination disorders: a randomized controlled trial. Res Dev Disabil. (2022) 124:104196. doi: 10.1016/j.ridd.2022.104196
108. Kalagher, H, and Jones, SS. Developmental change in young children's use of haptic information in a visual task: the role of hand movements. J Exp Child Psychol. (2011) 108:293–307. doi: 10.1016/j.jecp.2010.09.004
109. Tsai, C-L. The effectiveness of exercise intervention on inhibitory control in children with developmental coordination disorder: using a visuospatial attention paradigm as a model. Res Dev Disabil. (2009) 30:1268–80. doi: 10.1016/j.ridd.2009.05.001
110. Ludyga, S, Mücke, M, Leuenberger, R, Bruggisser, F, Pühse, U, Gerber, M, et al. Behavioral and neurocognitive effects of judo training on working memory capacity in children with ADHD: a randomized controlled trial. Neuroimage Clin. (2022) 36:103156. doi: 10.1016/j.nicl.2022.103156
111. Ludyga, S, Tränkner, S, Gerber, M, and Pühse, U. Effects of judo on neurocognitive indices of response inhibition in preadolescent children: a randomized controlled trial. Med Sci Sports Exerc. (2021) 53:1648–55. doi: 10.1249/mss.0000000000002626
112. Caçola, P, Getchell, N, Srinivasan, D, Alexandrakis, G, and Liu, H. Cortical activity in fine-motor tasks in children with developmental coordination disorder: a preliminary fNIRS study. Int J Dev Neurosci. (2018) 65:83–90. doi: 10.1016/j.ijdevneu.2017.11.001
113. Zhao, W, Hui, M, Zhang, X, and Li, L. The relationship between motor coordination and imitation: an fNIRS study. Brain Sci. (2021) 11:11. doi: 10.3390/brainsci11081052
114. Yasunaga, M, Miyaguchi, H, Ishizuki, C, Kita, Y, and Nakai, A. Cognitive orientation to daily occupational performance: a randomized controlled trial examining intervention effects on children with developmental coordination disorder traits. Brain Sci. (2023) 13:13. doi: 10.3390/brainsci13050721
115. Mandich, A, and Polatajko, HJ. Enabling occupation in children: the cognitive orientation to daily occupational performance (CO-OP) approach. Otawa, ON: CAOT Publications ACE (2004).
116. Araújo, CRS, Cardoso, AA, and Magalhães, LC. Efficacy of the cognitive orientation to daily occupational performance with Brazilian children with developmental coordination disorder. Scand J Occup Ther. (2019) 26:46–54. doi: 10.1080/11038128.2017.1417476
117. Adams, ILJ, Lust, JM, Wilson, PH, and Steenbergen, B. Development of motor imagery and anticipatory action planning in children with developmental coordination disorder - a longitudinal approach. Hum Mov Sci. (2017) 55:296–306. doi: 10.1016/j.humov.2017.08.021
118. Adams, ILJ, Lust, JM, and Steenbergen, B. Development of motor imagery ability in children with developmental coordination disorder - a goal-directed pointing task. Br J Psychol. (2018) 109:187–203. doi: 10.1111/bjop.12274
119. Adams, IL, Lust, JM, Wilson, PH, and Steenbergen, B. Testing predictive control of movement in children with developmental coordination disorder using converging operations. Br J Psychol. (2017) 108:73–90. doi: 10.1111/bjop.12183
120. Hardwick, RM, Caspers, S, Eickhoff, SB, and Swinnen, SP. Neural correlates of action: comparing meta-analyses of imagery, observation, and execution. Neurosci Biobehav Rev. (2018) 94:31–44. doi: 10.1016/j.neubiorev.2018.08.003
121. Steenbergen, B, Krajenbrink, H, Lust, J, and Wilson, P. Motor imagery and action observation for predictive control in developmental coordination disorder. Dev Med Child Neurol. (2020) 62:1352–5. doi: 10.1111/dmcn.14612
122. Apšvalka, D, Cross, ES, and Ramsey, R. Observing action sequences elicits sequence-specific neural representations in Frontoparietal brain regions. J Neurosci. (2018) 38:10114–28. doi: 10.1523/jneurosci.1597-18.2018
123. Bek, J, Holmes, P, Webb, J, Craig, C, Franklin, Z, Sullivan, M, et al. Home-based training to increase functional Independence in Parkinson’s through action observation and imagery. Arch Phys Med Rehabil. (2018) 99:e65. doi: 10.1016/j.apmr.2018.07.225
124. Marshall, B, Wright, DJ, Holmes, PS, Williams, J, and Wood, G. Combined action observation and motor imagery facilitates visuomotor adaptation in children with developmental coordination disorder. Res Dev Disabil. (2020) 98:103570. doi: 10.1016/j.ridd.2019.103570
125. Engel-Yeger, B, Sido, R, Mimouni-Bloch, A, and Weiss, PL. Relationship between perceived competence and performance during real and virtual motor tasks by children with developmental coordination disorder. Disabil Rehabil Assist Technol. (2017) 12:752–7. doi: 10.1080/17483107.2016.1261305
126. Bailey, A, Barron, S, Curro, C, and Teague, E. Gaming the system strategies of camouflage In: A Ledeneva, editor. Global Encyclopaedia of informality, vol. 2. London, UK: UCL Press (2018). 181–342.
127. EbrahimiSani, S, Sohrabi, M, Taheri, H, Agdasi, MT, and Amiri, S. Effects of virtual reality training intervention on predictive motor control of children with DCD - a randomized controlled trial. Res Dev Disabil. (2020) 107:103768. doi: 10.1016/j.ridd.2020.103768
128. Chen, B, Bernard, JY, Padmapriya, N, Ning, Y, Cai, S, Lança, C, et al. Associations between early-life screen viewing and 24 hour movement behaviours: findings from a longitudinal birth cohort study. Lancet Child Adolesc Health. (2020) 4:201–9. doi: 10.1016/s2352-4642(19)30424-9
129. Howie, EK, Coenen, P, Campbell, AC, Ranelli, S, and Straker, LM. Head, trunk and arm posture amplitude and variation, muscle activity, sedentariness and physical activity of 3 to 5 year-old children during tablet computer use compared to television watching and toy play. Appl Ergon. (2017) 65:41–50. doi: 10.1016/j.apergo.2017.05.011
130. Hardy, LL, Ding, D, Peralta, LR, Mihrshahi, S, and Merom, D. Association between sitting, screen time, fitness domains, and fundamental motor skills in children aged 5-16 years: Cross-sectional population study. J Phys Act Health. (2018) 15:933–40. doi: 10.1123/jpah.2017-0620
131. Ferguson, GD, Naidoo, N, and Smits-Engelsman, BC. Health promotion in a low-income primary school: children with and without DCD benefit, but differently. Phys Occup Ther Pediatr. (2015) 35:147–62. doi: 10.3109/01942638.2015.1009230
132. Novak, I, and Honan, I. Effectiveness of paediatric occupational therapy for children with disabilities: a systematic review. Aust Occup Ther J. (2019) 66:258–73. doi: 10.1111/1440-1630.12573
133. Smits-Engelsman, BC, Blank, R, van der Kaay, AC, Mosterd-van der Meijs, R, Vlugt-van den Brand, E, Polatajko, HJ, et al. Efficacy of interventions to improve motor performance in children with developmental coordination disorder: a combined systematic review and meta-analysis. Dev Med Child Neurol. (2013) 55:229–37. doi: 10.1111/dmcn.12008
Keywords: developmental coordination disorder, rehabilitation, international classification of functioning, disability and health, child, physical and rehabilitation medicine
Citation: Gao J, Song W, Zhong Y, Huang D, Wang J, Zhang A and Ke X (2024) Children with developmental coordination disorders: a review of approaches to assessment and intervention. Front. Neurol. 15:1359955. doi: 10.3389/fneur.2024.1359955
Received: 22 December 2023; Accepted: 03 May 2024;
Published: 23 May 2024.
Edited by:
Giuseppina Sgandurra, Stella Maris Foundation (IRCCS), ItalyReviewed by:
José A. Barela, São Paulo State University, Campus Rio Claro, BrazilCopyright © 2024 Gao, Song, Zhong, Huang, Wang, Zhang and Ke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anren Zhang, YW5yZW4wMTI0QHRvbmdqaS5lZHUuY24=; Xiaohua Ke, a3hoMjJAdG9uZ2ppLmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.