- 1Department of Neurology, Friedrich-Alexander-University Erlangen-Nürnberg (FAU), Erlangen, Germany
- 2Department of Neurosurgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 3Department of Neuroradiology, Friedrich-Alexander-University Erlangen-Nürnberg (FAU), Erlangen, Germany
Background and aims: General guideline recommendations in patients with intracerebral hemorrhage (ICH) include blood pressure-, temperature- and glucose management. The therapeutic effect of such a “care bundle” (blood pressure lowering, glycemic control, and treatment of pyrexia) on clinical outcomes becomes increasingly established. For the present study, we aimed to investigate associations of strict bundled care treatment (BCT) with clinical outcomes and characterize associations with key outcome effectors such as hematoma enlargement (HE) and peak perihemorrhagic edema (PHE).
Methods: We screened consecutive ICH patients (n = 1,322) from the prospective UKER-ICH cohort study. BCT was defined as achieving and maintaining therapeutic ranges for systolic blood pressure (110–160 mmHg), glucose (80–180 mg/dL), and body temperature (35.5–37.5°C) over the first 72 h. The primary outcome was the functional outcome at 12 months (modified Rankin Scale (mRS) 0–3). Secondary outcomes included mortality at 12 months, the occurrence of hematoma enlargement, and the development of peak perihemorrhagic edema. Confounding was addressed by a doubly robust methodology to calculate the absolute treatment effect (ATE) and by calculating e-values.
Results: A total of 681 patients remained for analysis, and 182 patients fulfilled all three BCT criteria and were compared to 499 controls. The ATE of BCT to achieve the primary outcome was 9.3%, 95% CI (1.7 to 16.9), p < 0.001; e-value: 3.1, CI (1.8). Mortality at 12 months was significantly reduced by BCT [ATE: −12.8%, 95% CI (−19.8 to −5.7), p < 0.001; e-value: 3.8, CI (2.2)], and no association was observed for HE or peak PHE. Significant drivers of BCT effect on the primary outcome were systolic blood pressure control (ATE: 15.9%) and maintenance of normothermia (ATE: 10.9%).
Conclusion: Strict adherence to this “care bundle” over the first 72 h during acute hospital care in patients with ICH was independently associated with improved functional long-term outcome, driven by systolic blood pressure control and maintenance of normothermia. Our findings strongly warrant prospective validation to determine the generalizability especially in Western countries.
Clinical trial registration:ClinicalTrials.gov, identifier [ID: NCT03183167].
Introduction
Intracerebral hemorrhage accounts for 11–22% of strokes and contributes to the burden of the disease with approximately 42% of the disability adjusted life-years due to the stroke (47 million life-years) (1, 2). Although the “one” breakthrough intervention improving functional outcome and mortality does not exist, a variety of treatment approaches possibly interacting with one another have been investigated (3). Baseline guideline-recommended interventions comprise an early and strict implementation of blood pressure, temperature, and glucose management (4). Over the last years, several large clinical trials and observational studies provided new evidence enhancing acute ICH care by investigating the potential benefit of single interventions (5–8). Nevertheless, the potential synergistic benefits of guideline-recommended treatments when combined remain elusive, especially in Western countries. Clustering interventions together as a care bundle lately revealed promising outcomes (9, 10). For example, Parry-Jones et al. focused on a “bundle” of treatments: reversal of coagulation status, referral to neurosurgery, blood pressure control, and admission to a neurological intensive care unit, and found a 6 to 12% absolute reduction in mortality (10). Most currently, the cluster-randomized INTERACT3 trial has been published demonstrating that implantation of a care bundle protocol in low- and middle-income countries without a previous standardized operating procedure for ICH patients resulted in improved functional outcome (11). This large trial (n = 7.036) included patients mainly from China and could document significant differences according to treatment allocation only for the parameter blood pressure, hence potentially driving the overall effect on the entire range of mRS estimates (common odds ratio 0.86; 95% CI: 0.76–0.97; p = 0·015).
The present study investigated whether the consistent and effective implementation of bundled care treatment (BCT) targets for systolic blood pressure, glucose levels, and temperature improved patient functional long-term outcomes after intracerebral hemorrhage. In addition, we aim to evaluate the key components of a care bundle and characterize associations with hematoma enlargement (HE) and peak perihemorrhagic edema (PHE).
Methods
Study participants and study design
We included patient data from the prospective single-center UKER-ICH registry [patients with spontaneous ICH; from 1 January 2006 until 31 December 2015 (NCT03183167)]. Detailed information and methods have been published previously (12–15). The study was approved by the local ethics committee and institutional review boards based on the central votes from Friedrich-Alexander-University Erlangen-Nuremberg, Germany (Re.No-4409 & 30_16B, 115_17B: “Retrospective analysis of patients with intracerebral haemorrhage,” approval date 20 June 2017) (12). Consent was obtained from patients or legal representatives. The procedures followed were in accordance with IRB ethical standards for human experimentation and the Helsinki Declaration of 1975. Patients with secondary ICH etiologies such as aneurysms, arteriovenous malformations, tumorous lesions, trauma, or coagulopathies other than oral anticoagulation were excluded (12–15). In addition, we excluded patients with symptom onset >8 h or withdrawal of therapy within 24 h according to previous studies (16) (Figure 1).
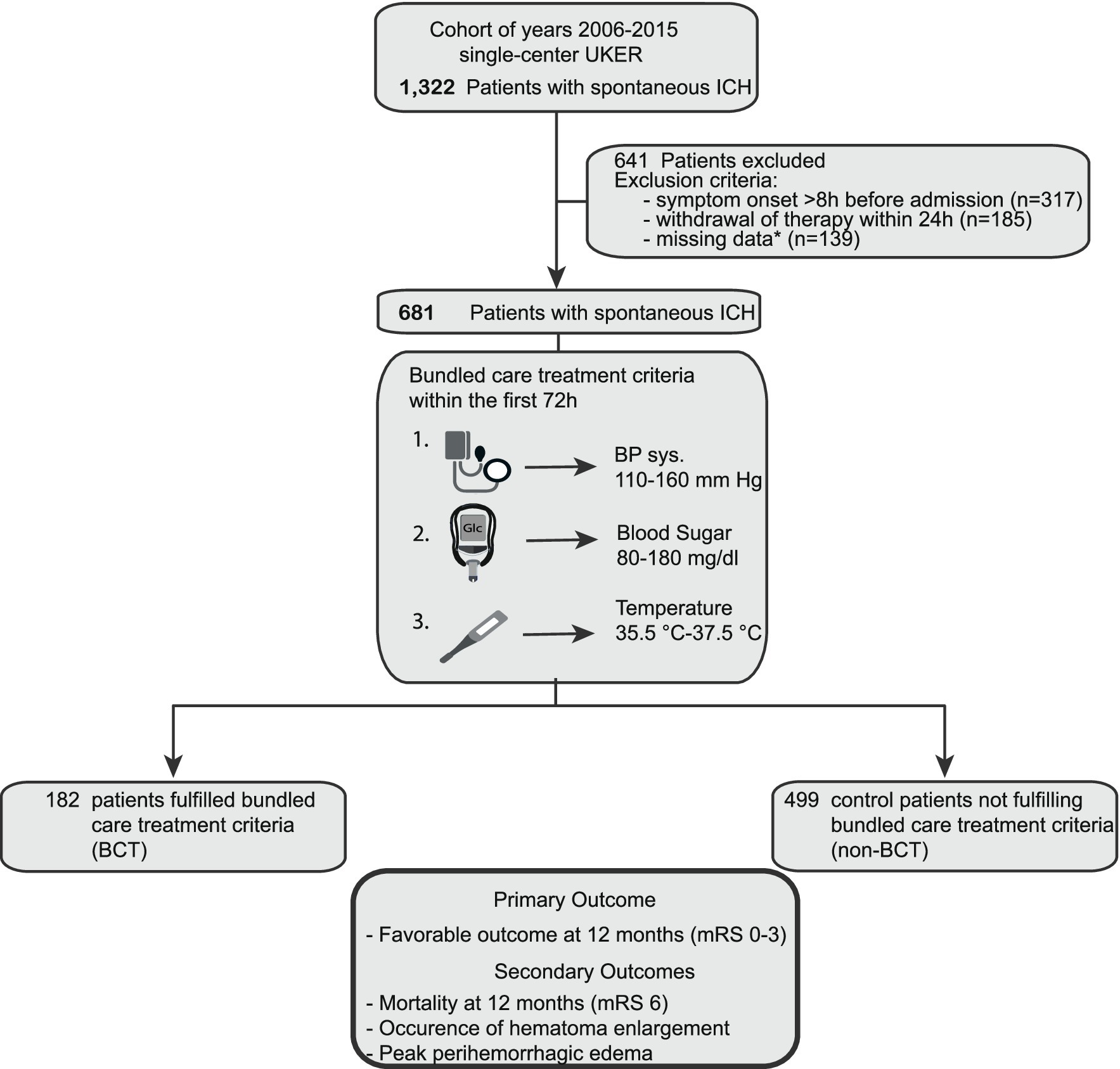
Figure 1. Flow chart of study participants. Overall, 1,322 patients with spontaneous ICH (years 2006–2015) were screened for analysis. 641 patients were excluded because of (i) symptom onset >8 h before admission (n = 317), (ii) withdrawal of therapy within the first 24 h (n = 185), and (iii) missing patient data (n = 139). 681 patients remained for primary and secondary outcome analyses. 182 fulfilled the bundle care treatment criteria, and 499 patients remained as controls. *Missing data consist of (i) more than 33.3% of maximum or minimum values of each BCT parameter over all 6-h intervals or (ii) follow-up imaging (dicom data for detailed analysis). BCT, bundled care treatment; ICH, intracerebral hemorrhage; UKER, Universitätsklinikum Erlangen Cohort of Patients With Spontaneous Intracerebral Hemorrhage.
Data acquisition
Data collection included baseline data on demographics (age and sex), prior comorbidities (hypertension, coronary artery disease, prior stroke, and abnormal kidney or liver function), prior medication (oral anticoagulation and antiplatelet medication), timing measures (time from symptom onset to first and second CT and time from symptom onset to treatment), and neurological status assessed using the National Institutes of Health Stroke Scale (NIHSS) upon hospital admission. In addition, the premorbid modified Rankin Scale was assessed (17). We evaluated hematoma characteristics (ICH location, intraventricular hemorrhage, and ICH volume) by neuroradiologists (S.H., S.L. and T.E.) blinded to clinical information. Assessment of mortality and functional outcome (modified Rankin Scale [mRS]) at 12 months was obtained by independent personnel, as previously reported (12). We recorded all available bundled care treatment (BCT) parameters, that is, measurement of systolic blood pressure, temperature, and blood sugar levels within the first 72 h.
Definition of BCT
Patients with ICH were categorized into two groups: one group with patients fulfilling the BCT criteria over the first 72 h (BCT) and the control group of patients not fulfilling the criteria (non-BCT). The BCT criteria consisted of three target parameters: systolic blood pressure, temperature, and glucose management. We defined the following target ranges according to standard operating procedures at our institution in place over the study period: (1) systolic blood pressure between 110 mmHg and 160 mmHg, (2) temperature between 35.5°C and 37.5°C, and (3) blood sugar levels between 80 mg/dL and 180 mg/dL. We evaluated all available measurements, divided these into 6-h intervals, and recorded maximum and minimum values over each period for each BCT parameter. Patients were categorized into the BCT group, if less than 33.3% of maximum or minimum values of each interval (i.e., >4/12) were outside of the predefined range over all 6-h intervals. We selected the systolic blood pressure range of <160 mmHg according to an internal standard operating procedure at our institution in place before the publication of the INTERACT-II trial data (7). Since 2013 this has been modified to a target systolic level of <140 mmHg7. Body core temperature was measured using the tympanic or bladder temperature, and the chosen range was considered normothermia (35.5°C and 37.5°C) according to previous studies (5, 18, 19). Regarding blood sugar levels, we selected a conventional range of 80–180 mg/dL according to current AHA guideline which recommends treating hypo- and hyperglycemia to prevent adverse events (6, 20, 21).
Primary and secondary outcomes
The primary outcome measure was the functional outcome at 12 months assessed by the ordinal modified Rankin Scale (mRS; 0: no deficit, through 5: severe disability and 6: death). Functional outcome was grouped into favorable (mRS 0–3) and unfavorable (mRS 4–6) outcomes, as previously described (8). Secondary outcomes compromised (i) mortality at 12 months, (ii) occurrence of hematoma enlargement defined as an ICH volume increase of more than 33% (relative) or 6 mL from initial to follow-up imaging, and (iii) peak perihemorrhagic edema (PHE). We assessed PHE on all available CT scans using a validated semi-automated threshold-based algorithm with a threshold range of 5–33 Hounsfield units (22). Peak PHE was defined as the maximum PHE volume measured during hospitalization and dichotomized according to the median split method (22).
Statistical analysis
Statistical analyses were performed using STATA (Version 14·2) and R x64 3.2.0.1 In general, confounders were considered relevant using a standardized mean difference larger than 20%. To address confounding, we used adjusted odds ratios (OR) and a doubly robust confounder-adjusted methodology to calculate adjusted absolute treatment effects, that is, augmented inverse probability weighting (AIPW) (23). These adjustments were applied in two ways: (A) identified confounders associated with an increased propensity for BCT and (B) validated confounders associated with primary and secondary endpoints; confounders were identified based on standardized mean differences (SMD). For exploratory analysis of the primary outcome, we used the aforementioned adjustment methodology. The categorization of non-dichotomous variables was split by the 50th percentile. Interactions of exploratory subgroup analyses were analyzed by the subgroup-defining variable (variable×intervention) and were considered significant for a p-value of <0.05. Sensitivity analyses compromised the evaluation of unmeasured confounding (e-values) (24). The average data missingness was approximately 7% per patient without difference between BCT and non-BCT patients. Missing functional outcome information was handled by multiple imputations by conditional specifications after assessment of missingness (25).
Results
Study population
We identified 681 patients from our prospective cohort study in spontaneous ICH (n = 1,322, Cohort of years 2006–2015 from UKER registry, Figure 1). A total of 182 patients (26.7%) fulfilled the strict BCT criteria and were compared to 499 controls (73.3%). We graphically displayed differences between these groups for all three BCT parameters over the first 72 h in Figure 2. Initial BCT measurements between groups were not significantly different (A, systolic blood pressure: 160 mmHg vs. 162 mmHg; B, temperature: 36.6°C vs. 36.7°C; blood sugar: 130 mg/dL vs. 132 mg/dL), and subsequent measurements over the 72-h time period significantly differed, that is, for systolic blood pressure in 92% (11/12, 6-h intervals), for temperature 75% (9/12, 6-h intervals), and for blood sugar levels 58% (7/12, 6-h intervals).
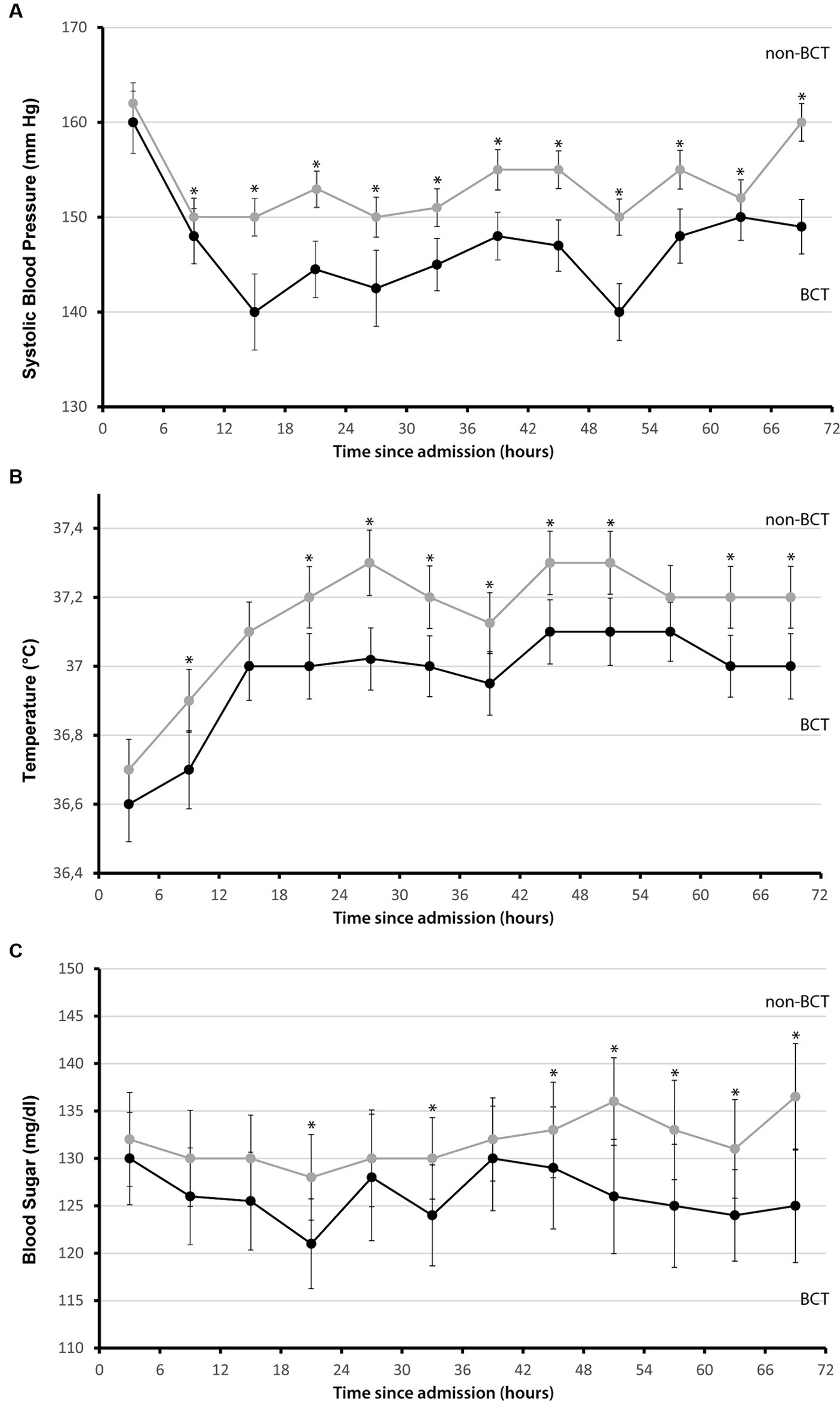
Figure 2. Systolic blood pressure, temperature, and glucose levels in 6-h intervals during the first 72 h mean values for (A) systolic blood pressure, mmHg, (B) temperature, °C, and (C) glucose levels, mg/dL measured in 6-h intervals since admission during the first 72 h separated for patients who fulfilled the bundle care treatment criteria (black) and for control patients (gray), maximum values were used. I bars indicated 95% confidence intervals. Asterisks mark significance *p ≤ 0.05.
Sensitivity analyses of confounders
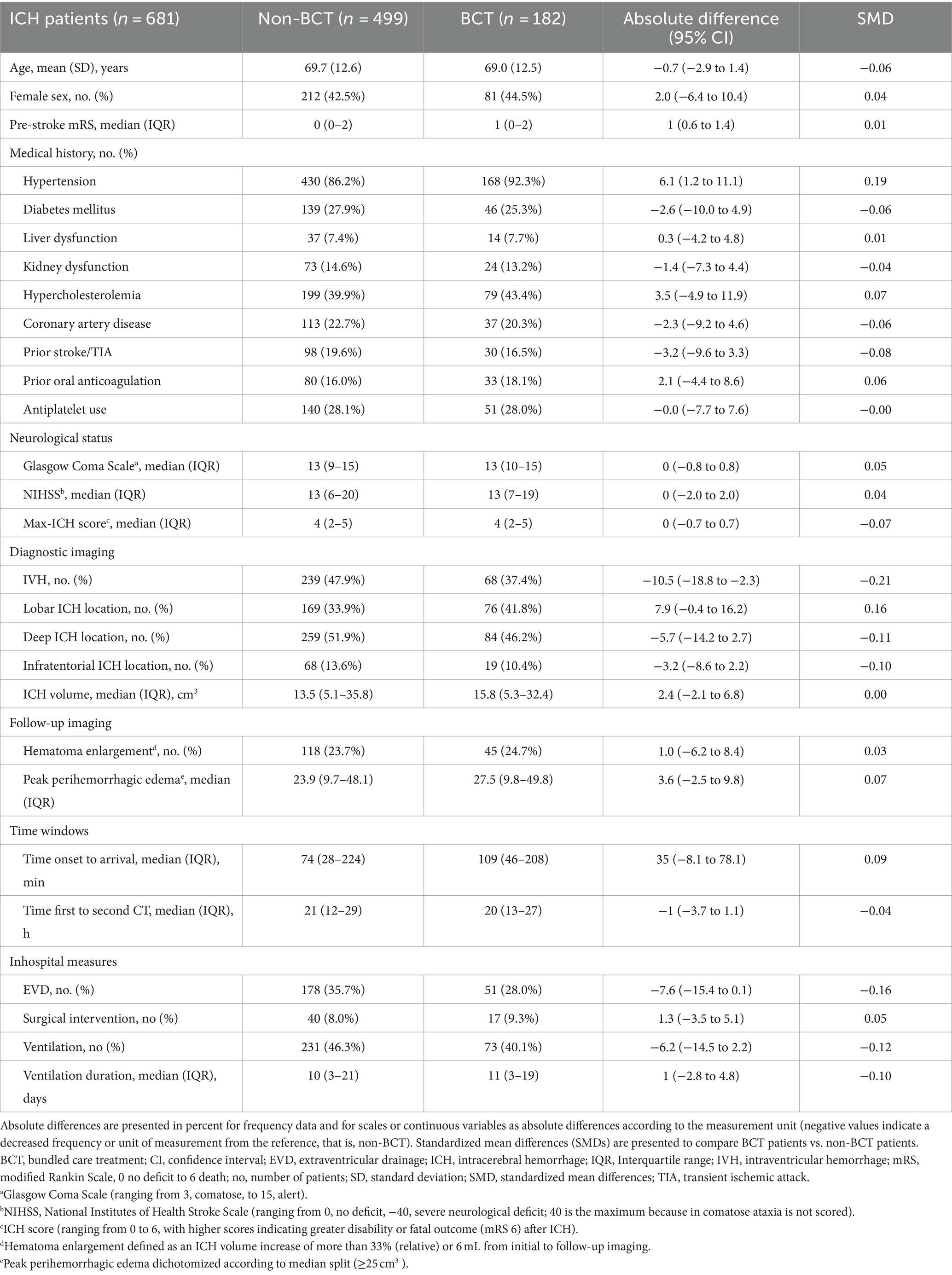
Baseline characteristics are shown in Table 1. We identified significant imbalances in BCT patients than in non-BCT patients, that is, more frequent prior diagnosis of hypertension [absolute difference (AD) 6.1, 95% CI (1.2 to 11.1) %; SMD 0.19], less frequent treatment with external ventricular drainage [AD −7.6, 95% CI (−15.4 to 0.1) %; SMD −0.16], more frequent lobar ICH location [AD 7.9, 95% CI (−0.4 to 16.2) %; SMD 0.16], and less frequent intraventricular hemorrhage [AD −10.5, 95% CI (−18.8 to −2.3) %; SMD −0.21]. For evaluation of confounding regarding the primary endpoint, sensitivity analysis was dichotomized according to functional outcome (mRS 0–3 at 12 months, Table 2). The results show that patients with mRS 0–3 at 12 months were younger [AD 7.1, 95% CI (5.2 to 8.9), years, %; SMD 0.58], had lower premRS [AD 1, 95% CI (0.8 to 1.2), SMD 0.64], had less comorbidity of prior stroke/TIA [AD 9.7, 95% CI (4.0 to 15.4), %; SMD 0.25], lower ICH volumes [AD 14.2, 95% CI (10.5 to 17.8), mL; SMD 0.67], lower NIHSS values [AD 9, 95% CI (7.6 to 10.4), SMD 0.84], more frequent rate of intraventricular hemorrhage [IVH, AD 23.9, 95% CI (16.7 to 31.2), %; SMD 0.50], and less frequent prior use of oral anticoagulation [AD 7.7 (2.2 to 13.2), %; SMD 0.21]. We accordingly performed sensitivity analyses for secondary outcomes: mortality at 12 months, occurence of hematoma enlargement and peak perihemorrhagic edema, dichotomized according to median split method (value 25 cm3), for details please see Supplementary Tables S1–S3.
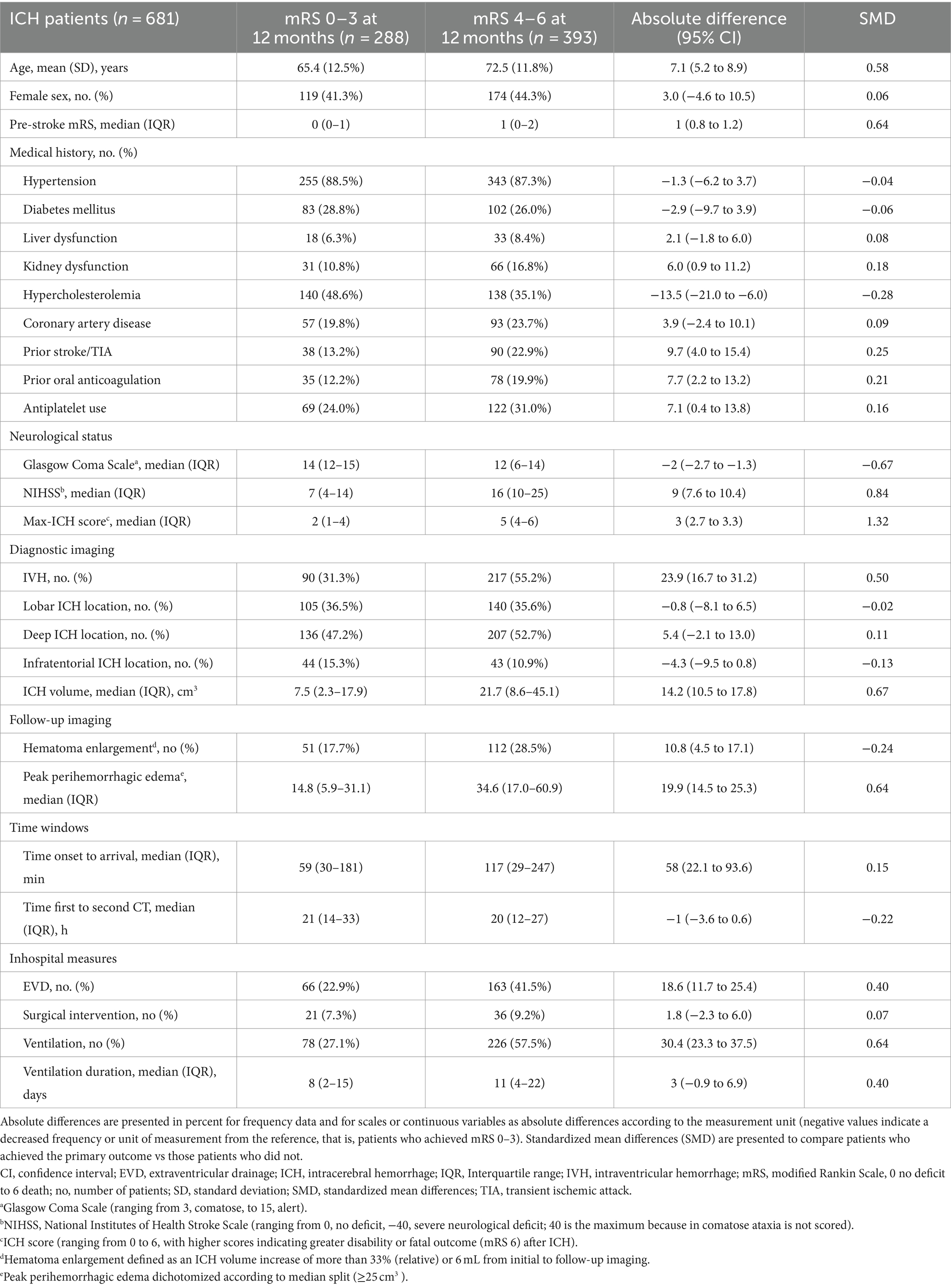
Table 2. Baseline characteristics: comparison of patients who achieved the primary outcome (mRS 0–3) at 12 months vs. those who did not.
Analyses of the primary and secondary outcomes
The adjusted absolute treatment effect (ATE) of BCT to achieve a favorable functional outcome at 12 months was 9.3%, 95% CI (1.7 to 16.9), p < 0·001 [adjusted OR, 1.86 (95% CI, 1.23–2.83), p < 0.005; (e-value, point estimate, 3.1, CI, 1.8)]. Among secondary outcomes, only mortality at 12 months was significantly reduced with an absolute treatment effect for BCT [ATE −12.8%, 95% CI (−19.8 to −5.7), p < 0.001; adjusted OR, 2.22 (95% CI, 1.40–3.53), p < 0.005; (e-value, point estimate, 3.8, CI, 2.2)]. There was no significant association of BCT regarding the occurrence of hematoma enlargement or peak perihemorrhagic edema [HE: ATE −0.1%, 95% CI (−6.8 to 7.8), p = 0.89; PHE: ATE −1.5%, 95% CI (−8.3 to 5.3, p = 0.65)] (Figure 3).
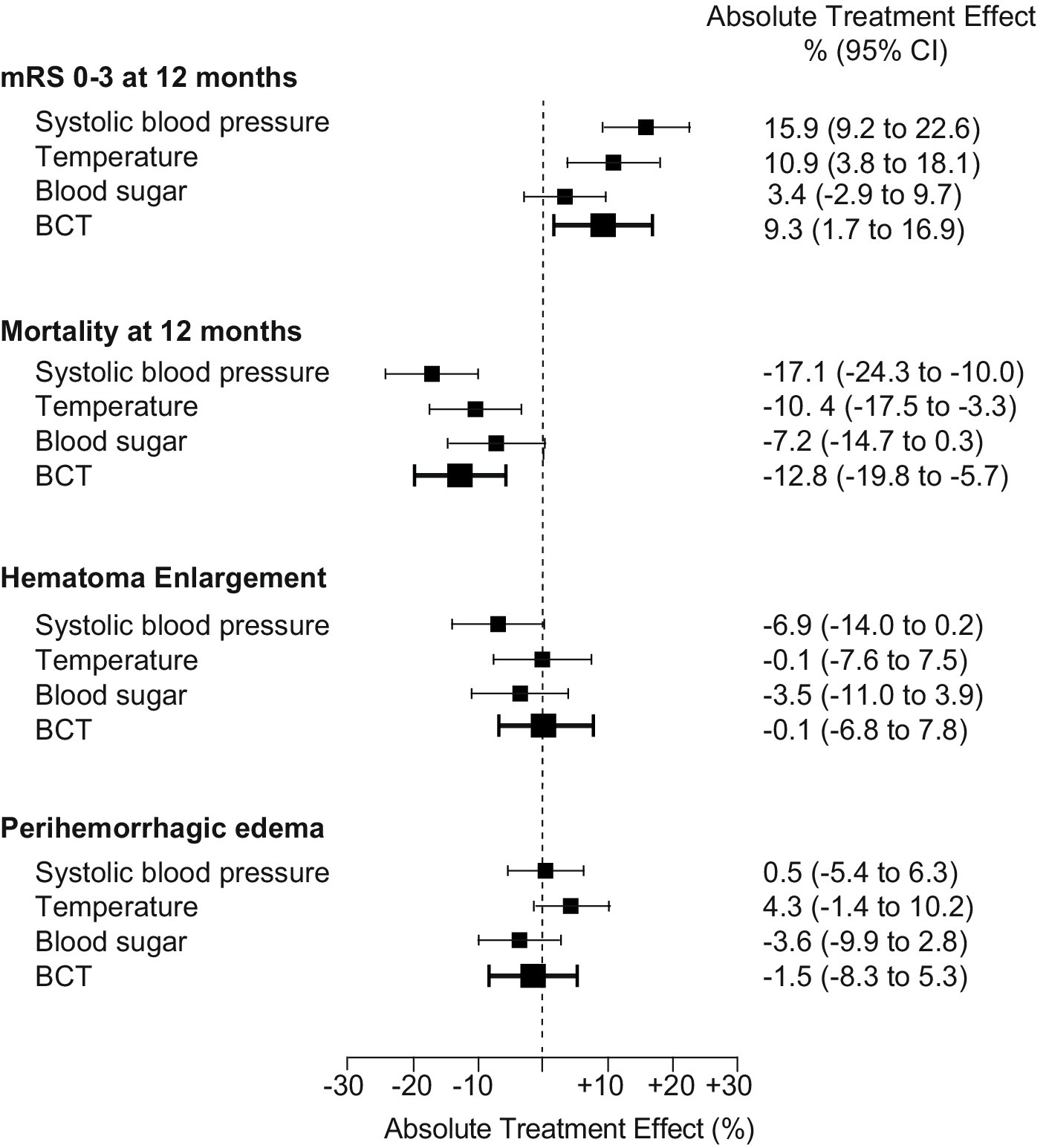
Figure 3. Associations of single BCT components to the absolute treatment effect. Results for the primary (modified Rankin Scale score 0–3 at 12 months) and secondary outcomes (mortality at 12 months), occurrence of hematoma enlargement, volume of peak perihemorrhagic edema, dichotomized according to median split (<25 cm3) are shown as adjusted absolute treatment effects separated for each target parameter: systolic blood pressure, temperature, and blood sugar, as well as BCT effect for categorized outcomes (bold), n = 681. Abbreviations/scores: BCT: bundled care treatment; hematoma enlargement was defined as an ICH volume increase of more than 33% (relative) or 6 mL from initial to follow-up imaging; peak perihemorrhagic edema was defined as maximum perihemorrhagic edema volume measured during hospitalization and dichotomized according to the median split method.
Exploratory subgroup analyses
For associations of BCT with the primary outcome according to predefined subgroups (Figure 4), significant ATE was found in younger patients aged 29–70 years, ATE 13.3%, 95% CI (2.8 to 23.8), in patients with higher NIHSS values, ATE 19.3%, 95% CI (8.8 to 29.7), in patients with larger ICH volumes (≥16.0 cm3), ATE 17.6%, 95% CI (7.1 to 28.2) and larger peak edema volumes (≥25.0 cm3), ATE 19.0%, 95% CI (8.1 to 30.0). In addition, significant ATE was found in patients with intraventricular hemorrhage, ATE 15.3%, 95% CI (4.1 to 26.5), and for patients without hematoma enlargement, ATE 16.1%, 95% CI (7.1 to 25.1). Significant interactions between treatment and subgroup categories were not detected (all p > 0.05).
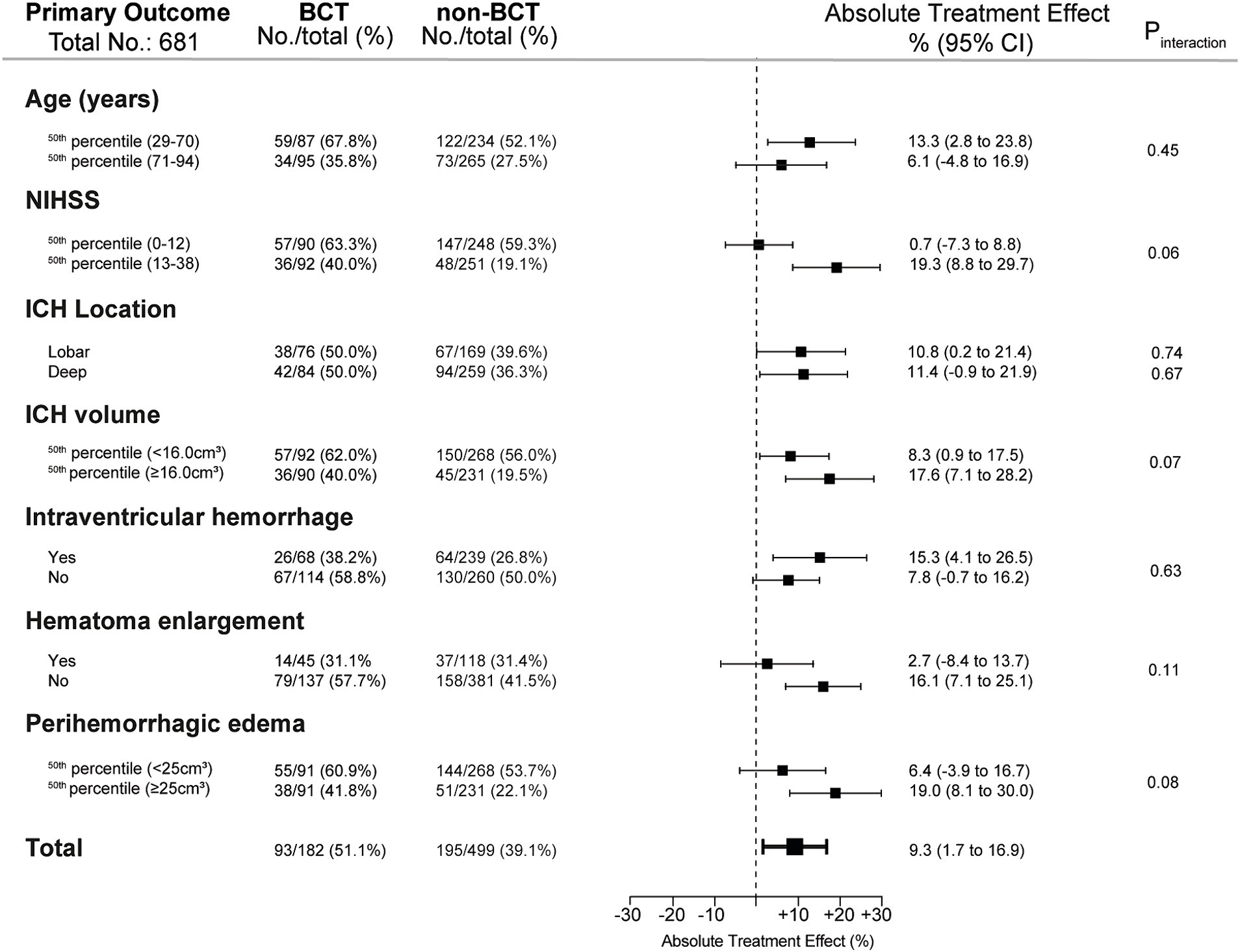
Figure 4. Exploratory subgroup analyses of the primary outcome. The results for the primary outcome (modified Rankin Scale score 0–3 at 12 months) are shown as crude frequency data and adjusted absolute treatment effects (n = 681). Interactions of exploratory subgroup analyses were analyzed by the subgroup-defining variable (variable×intervention) and were considered significant for p < 0.05. Abbreviations/scores: ICH: intracerebral hemorrhage; ICH volume was dichotomized according to median split (≥16.0 cm3). NIHSS, National Institutes of Health Stroke Scale. Hematoma enlargement defined as an ICH volume increase of more than 33% (relative) or 6 mL from initial to follow-up imaging; peak perihemorrhagic edema was defined as maximum perihemorrhagic edema volume measured during hospitalization and dichotomized according to the median split method (≥25.0cm3).
Contribution of the components of BCT to the overall treatment
To assess the contribution of each component of BCT to the overall effect, we investigated the treatment effect separated for each component, that is, glucose, blood sugar, and systolic blood pressure management (Figure 3). Therefore, ICH patients were categorized according to the above-mentioned target parameters (see also Methods). Accordingly, we addressed confounding by sensitivity analyses and doubly robust methodology as aforementioned. Among the three components, blood sugar management revealed no significant treatment effects for primary and secondary endpoints. Both systolic blood pressure and temperature management revealed a significant absolute treatment effect regarding the primary endpoint and mortality at 12 months [BP: mRS 0–3: ATE 15.9%, 95% CI (9.2 to 22.6), p < 0.001; mRS 6: ATE −17.1%, 95% CI (−24.3 to −10.0), p < 0.001; temperature: mRS 0–3: ATE 10.9%, 95% CI (3.8 to 18.1), p < 0.002; mRS 6: ATE −10.4%, 95% CI (−17.5 to −3.3), p < 0.001]. Regarding the occurrence of hematoma enlargement, only systolic blood pressure showed a trend toward a treatment effect [ATE −6.9%, 95% CI (−14.0 to 0.2), p = 0.06]. Neither overall BCT, nor the separated components revealed a significant treatment effect on peak PHE.
Discussion
As key findings, we observed that a “care bundle” consisting of strict control of systolic blood pressure lowering, glucose levels, and normothermia over 72 h was related to improved functional long-term outcomes and survival in patients with intracerebral hemorrhage. Specifically, BCT showed larger treatment effects in younger but more severely affected ICH patients. Furthermore, we could identify that especially systolic blood pressure control and maintenance of normothermia contributed to the effectiveness of BCT. The questions arise, what may be the underlying mechanisms for this treatment effect of BCT and what is the contribution of each component to this overall effect?
Within analysis of the single components of BCT, we confirmed that lowering blood pressure contributed to a reduction of hematoma enlargement which consequently translated to improved functional outcomes. These results are, with respect to previous studies, not surprising (12, 26–28). Guidelines recommend a careful titration of blood pressure lowering to ensure continuous smooth and sustained control, avoiding peaks and large variability in SBP based on INTERACT-2 and Antihypertensive Treatment of Acute Cerebral Hemorrhage 2 (ATACH-2) for early intensive BP lowering (7, 8). It is important to note that blood pressure reduction also carries potential risks as highlighted by the ATACH-2 trial (8). Nevertheless, early treatment of blood pressure may reduce the risk of hematoma enlargement and improve functional outcomes which is supported by our analyses.
More interestingly, also maintenance of normothermia contributed to a significant improvement in functional outcomes which is controversially debated (5, 19, 29–31). Possible detrimental mechanisms of fever are hypothesized to contribute to the development of peak PHE (18, 31–33). In addition, in patients who develop hyperthermia, underlying infections may be present, which can negatively impact their outcomes. Nevertheless, we did not observe a statistically significant association with maintenance of normothermia and larger peak PHE, but we observed a statistical trend, ATE: 4.3 (−1.4 to 10.2) deserving further more in-depth investigations. Theoretically, this potential effect may vary over time, may be associated with the interventional time window (0–72 h), and may differ according to the time course of peak PHE development (34). Importantly, early PHE within 72 h has been reported as a predictor of peak PHE volume (31).
Targeted glucose management as the third part of our treatment bundle did not reveal any significant treatment effect. It is recommended that either hyperglycemia or hypoglycemia should be treated to prevent adverse events that may worsen outcomes (20, 21, 35–37). Previous studies do not indicate that strict targeted glucose management leads to improved outcomes in patients with ICH, which is also not supported by our results. In general, episodes with severe hypo- or hyperglycemia should be avoided during intensive care treatment, and randomized controlled data provided that severe hypoglycemic episodes were independently associated with increased mortality, yet without causal inference (6). From our data, we did not observe more frequent severe hypoglycemia episodes (<60 mg/dL) within the measured 72-h interval between both groups [2/182 (1%) BCT vs. 5/499 (1%) non-BCT; data not shown]. However, the extent to which strict implementation of specific glucose target values in the treatment of intracerebral hemorrhage affects clinical outcomes is still controversial.
Consequently, lowering blood pressure and managing temperature are substantial components of BCT to improve outcomes in intracerebral hemorrhage. Even if glucose management seems to have an inferior role, it is essential to prevent hypo- or hyperglycemia events to prevent adverse events.
However, the recently published cluster-randomized INTERACT3 trial revealed that implementing a novel care bundle protocol (early intensive blood pressure lowering, management algorithms for hyperglycemia, pyrexia, and abnormal anticoagulation) at hospitals without a prior standardized operating procedure improves functional outcome (11). The trial was undertaken mainly in low- and middle-income countries (contributing >99% of patients), and the authors concluded that the incorporation of such a care bundle protocol resulted in improved functional outcomes and assumed that “the overall treatment effect seems to have been driven by intensive blood pressure lowering” (11). Importantly, the parameters of temperature and glucose management did not show significant inter-group differences, and therefore, the question of whether these parameters contribute to improved outcomes remains unanswered. Our study gives initial insights into the relevance of the individual bundle components and identifies blood pressure lowering and temperature management as drivers of this overall treatment effect. In addition, key outcome effectors such as hematoma enlargement and perihemorrhagic edema development including inflammatory processes seemed to be influenced and theoretically may be considered as mechanistically relevant to functional outcome. This assumption is supported by our observation that patients with more severe intracerebral hemorrhages (ICHs) and a higher incidence of intraventricular hemorrhage experienced a greater treatment effect.
Thereby, our study revealed that beneficial associations of BCT may exist when strictly controlled even in a hospital in Europe with existing SOPs executed at a dedicated neurological intensive care unit. INTERACT3 dominantly included patients with deep hypertensive ICH (88%), which was the effect-driving subgroup, as compared to data of this study with only 50% of patients with deep ICH. Hence, we provide data on the beneficial associations with strict BCT including all other ICH locations as non-trial selected real-world cohort.
Limitations of this study include low patient numbers in the BCT group, especially for exploratory analyses, and limitations inherent to the retrospective nature of this cohort study. In addition, data from this monocentric cohort may not be generalizable, and exact dosing and frequency of therapeutic medications were not part of this investigation. We cannot fully exclude that patients who stayed spontaneously within target ranges suffered less from major cerebrovascular and hemodynamic disruption as a consequence of the ICH. Therefore, we addressed confounding such as premorbid status, age, and ICH severity by robust statistical methodologies and sensitivity analyses. Furthermore, within the investigated time span (2005–2015), the guideline recommendation for systolic blood pressure lowering has changed following the publication of the INTERACT-II trial data (7). After 2013, this has been modified to a target systolic level of <140 mmHg at our institution. We did not observe bias over these differing treatment periods, that is, patients treated between 2013 and 2015 comprised 28% (n = 191/681) of the entire cohort, of which 25% (n = 46/182) were grouped into BCT compared to 29% (n = 145/499) grouped into non-BCT (data not shown). Potentially, we are missing stronger effects of more stringent blood pressure management due to the low proportion achieving less than 140 mmHg systolic according to current guideline recommendations. Bias due to confounding including also confounding by indication, residual bias, and unmeasured confounding cannot be fully ruled out but were addressed by robust statistical methodologies and sensitivity analyses.
Conclusion
Strict and consequent adherence to BCT consisting of systolic blood pressure lowering, treatment of pyrexia, and glucose management in patients with intracerebral hemorrhage was associated with improved functional outcomes at 12 months, specifically in younger patients with larger ICH volumes and larger edema volumes. Furthermore, we could identify that especially blood pressure lowering and treatment of pyrexia contributed to the beneficial associations of BCT whereas management of blood sugar seemed to have an inferior role. Future prospective studies are warranted to validate the effects of BCT and its components.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics statement
The studies involving humans were approved by local ethics committee and institutional review boards based on the central votes from Friedrich-Alexander-University Erlangen-Nuremberg, Germany (Re.No-4409 & 30_16B, 115_17B: “Retrospective analysis of patients with intracerebral haemorrhage,” approval date 20th June 2017). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AM: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing, Project administration, Validation. YS: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. VH: Data curation, Writing – review & editing. JS: Writing – review & editing. MS: Writing – review & editing. SH: Methodology, Writing – review & editing. SL: Methodology, Writing – review & editing. TE: Methodology, Writing – review & editing. BK: Writing – review & editing. BV: Methodology, Writing – review & editing. JK: Conceptualization, Project administration, Validation Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The present study was performed in (partial) fulfillment of the requirements for obtaining the degree “Dr. med.” (VH).
Conflict of interest
AM reports personal fees from Alexion Pharma Germany GmbH, outside the submitted work. BV reports personal fees from Pfizer AG/Bristol-Myers Squibb SA, personal fees from Bayer AG, grants from Institutional grant (Inselspital), personal fees from Ipsen Pharma, personal fees from CSL Behring, outside the submitted work. JK reports personal fees from Beohringer Ingelheim, personal fees from Biogen, personal fees from Boston Scientific, personal fees from Sanofi, personal fees from Bayer AG, personal fees from Alexion, outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1357815/full#supplementary-material
Footnotes
References
1. Haupenthal, D, Kuramatsu, JB, Volbers, B, Sembill, JA, Mrochen, A, Balk, S, et al. Disability-adjusted life-years associated with intracerebral hemorrhage and secondary injury. JAMA Netw Open. (2021) 4:e2115859. doi: 10.1001/jamanetworkopen.2021.15859
2. GBD 2019 Stroke Collaborators . Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/s1474-4422(21)00252-0
3. Sembill, JA, Huttner, HB, and Kuramatsu, JB. Impact of recent studies for the treatment of intracerebral hemorrhage. Curr Neurol Neurosci Rep. (2018) 18:71. doi: 10.1007/s11910-018-0872-0
4. Greenberg, SM, Ziai, WC, Cordonnier, C, Dowlatshahi, D, Francis, B, Goldstein, JN, et al. 2022 Guideline for the Management of Patients with Spontaneous Intracerebral Hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke. (2022) 53:e282–361. doi: 10.1161/STR.0000000000000407
5. Hervella, P, Rodríguez-Yáñez, M, Pumar, JM, Ávila-Gómez, P, da Silva-Candal, A, López-Loureiro, I, et al. Antihyperthermic treatment decreases perihematomal hypodensity. Neurology. (2020) 94:e1738–48. doi: 10.1212/wnl.0000000000009288
6. NICE-SUGAR Study InvestigatorsFinfer, S, Chittock, DR, Su, SY, Blair, D, Foster, D, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. (2009) 360:1283–97. doi: 10.1056/NEJMoa0810625
7. Anderson, CS, Heeley, E, Huang, Y, Wang, J, Stapf, C, Delcourt, C, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. (2013) 368:2355–65. doi: 10.1056/NEJMoa1214609
8. Qureshi, AI, Palesch, YY, Barsan, WG, Hanley, DF, Hsu, CY, Martin, RL, et al. Intensive blood-pressure lowering in patients with acute cerebral hemorrhage. N Engl J Med. (2016) 375:1033–43. doi: 10.1056/NEJMoa1603460
9. Middleton, S, McElduff, P, Ward, J, Grimshaw, JM, Dale, S, D'Este, C, et al. Implementation of evidence-based treatment protocols to manage fever, hyperglycaemia, and swallowing dysfunction in acute stroke (QASC): a cluster randomised controlled trial. Lancet. (2011) 378:1699–706. doi: 10.1016/s0140-6736(11)61485-2
10. Parry-Jones, AR, Sammut-Powell, C, Paroutoglou, K, Birleson, E, Rowland, J, Lee, S, et al. An intracerebral hemorrhage care bundle is associated with lower case fatality. Ann Neurol. (2019) 86:495–503. doi: 10.1002/ana.25546
11. Ma, L, Hu, X, Song, L, Chen, X, Ouyang, M, Billot, L, et al. The third intensive care bundle with blood pressure reduction in acute cerebral haemorrhage trial (INTERACT3): an international, stepped wedge cluster randomised controlled trial. Lancet. (2023) 402:27–40. doi: 10.1016/s0140-6736(23)00806-1
12. Kuramatsu, JB, Gerner, ST, Schellinger, PD, Glahn, J, Endres, M, Sobesky, J, et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA. (2015) 313:824–36. doi: 10.1001/jama.2015.0846
13. Gerner, ST, Kuramatsu, JB, Sembill, JA, Sprügel, MI, Endres, M, Haeusler, KG, et al. Association of prothrombin complex concentrate administration and hematoma enlargement in non-vitamin K antagonist oral anticoagulant-related intracerebral hemorrhage. Ann Neurol. (2018) 83:186–96. doi: 10.1002/ana.25134
14. Kuramatsu, JB, Sembill, JA, Gerner, ST, Sprügel, MI, Hagen, M, Roeder, SS, et al. Management of therapeutic anticoagulation in patients with intracerebral haemorrhage and mechanical heart valves. Eur Heart J. (2018) 39:1709–23. doi: 10.1093/eurheartj/ehy056
15. Sprügel, MI, Kuramatsu, JB, Gerner, ST, Sembill, JA, Beuscher, VD, Hagen, M, et al. Antiplatelet therapy in primary spontaneous and oral anticoagulation-associated intracerebral hemorrhage. Stroke. (2018) 49:2621–9. doi: 10.1161/strokeaha.118.021614
16. Sprigg, N, Flaherty, K, Appleton, JP, al-Shahi Salman, R, Bereczki, D, Beridze, M, et al. Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2): an international randomised, placebo-controlled, phase 3 superiority trial. Lancet. (2018) 391:2107–15. doi: 10.1016/s0140-6736(18)31033-x
17. Sprügel, MI, Sembill, JA, Kremer, S, Gerner, ST, Knott, M, Hock, S, et al. Evaluation of functional recovery following thrombectomy in patients with large vessel occlusion and Prestroke disability. JAMA Netw Open. (2022) 5:e2227139. doi: 10.1001/jamanetworkopen.2022.27139
18. Iglesias-Rey, R, Rodríguez-Yáñez, M, Arias, S, Santamaría, M, Rodríguez-Castro, E, López-Dequidt, I, et al. Inflammation, edema and poor outcome are associated with hyperthermia in hypertensive intracerebral hemorrhages. Eur J Neurol. (2018) 25:1161–8. doi: 10.1111/ene.13677
19. den Hertog, HM, van der Worp, HB, van Gemert, HM, Algra, A, Kappelle, LJ, van Gijn, J, et al. The paracetamol (acetaminophen) in stroke (PAIS) trial: a multicentre, randomised, placebo-controlled, phase III trial. Lancet Neurol. (2009) 8:434–40. doi: 10.1016/s1474-4422(09)70051-1
20. Béjot, Y, Aboa-Eboulé, C, Hervieu, M, Jacquin, A, Osseby, GV, Rouaud, O, et al. The deleterious effect of admission hyperglycemia on survival and functional outcome in patients with intracerebral hemorrhage. Stroke. (2012) 43:243–5. doi: 10.1161/strokeaha.111.632950
21. Wu, TY, Putaala, J, Sharma, G, Strbian, D, Tatlisumak, T, Davis, SM, et al. Persistent hyperglycemia is associated with increased mortality after intracerebral hemorrhage. J Am Heart Assoc. (2017) 6:6. doi: 10.1161/jaha.117.005760
22. Volbers, B, Staykov, D, Wagner, I, Dörfler, A, Saake, M, Schwab, S, et al. Semi-automatic volumetric assessment of perihemorrhagic edema with computed tomography. Eur J Neurol. (2011) 18:1323–8. doi: 10.1111/j.1468-1331.2011.03395.x
23. Kuramatsu, JB, Gerner, ST, Ziai, W, Bardutzky, J, Sembill, JA, Sprügel, MI, et al. Association of intraventricular fibrinolysis with clinical outcomes in intracerebral hemorrhage: an individual participant data meta-analysis. Stroke. (2022) 53:2876–86. doi: 10.1161/strokeaha.121.038455
24. Haneuse, S, VanderWeele, TJ, and Arterburn, D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA. (2019) 321:602–3. doi: 10.1001/jama.2018.21554
25. Chevret, S, Seaman, S, and Resche-Rigon, M. Multiple imputation: a mature approach to dealing with missing data. Intensive Care Med. (2015) 41:348–50. doi: 10.1007/s00134-014-3624-x
26. Moullaali, TJ, Wang, X, Martin, RH, Shipes, VB, Robinson, TG, Chalmers, J, et al. Blood pressure control and clinical outcomes in acute intracerebral haemorrhage: a preplanned pooled analysis of individual participant data. Lancet Neurol. (2019) 18:857–64. doi: 10.1016/s1474-4422(19)30196-6
27. Li, Q, Warren, AD, Qureshi, AI, Morotti, A, Falcone, GJ, Sheth, KN, et al. Ultra-early blood pressure reduction attenuates hematoma growth and improves outcome in intracerebral hemorrhage. Ann Neurol. (2020) 88:388–95. doi: 10.1002/ana.25793
28. Wang, X, Arima, H, Heeley, E, Delcourt, C, Huang, Y, Wang, J, et al. Magnitude of blood pressure reduction and clinical outcomes in acute intracerebral hemorrhage: intensive blood pressure reduction in acute cerebral hemorrhage trial study. Hypertension. (2015) 65:1026–32. doi: 10.1161/hypertensionaha.114.05044
29. Staykov, D, Schwab, S, Dörfler, A, and Kollmar, R. Hypothermia reduces perihemorrhagic edema after intracerebral hemorrhage: but does it influence functional outcome and mortality? Ther Hypothermia Temp Manag. (2011) 1:105–6. doi: 10.1089/ther.2011.0004
30. Broessner, G, Beer, R, Lackner, P, Helbok, R, Fischer, M, Pfausler, B, et al. Prophylactic, endovascularly based, long-term normothermia in ICU patients with severe cerebrovascular disease: bicenter prospective, randomized trial. Stroke. (2009) 40:e657–65. doi: 10.1161/strokeaha.109.557652
31. Volbers, B, Giede-Jeppe, A, Gerner, ST, Sembill, JA, Kuramatsu, JB, Lang, S, et al. Peak perihemorrhagic edema correlates with functional outcome in intracerebral hemorrhage. Neurology. (2018) 90:e1005–12. doi: 10.1212/wnl.0000000000005167
32. Honig, A, Michael, S, Eliahou, R, and Leker, RR. Central fever in patients with spontaneous intracerebral hemorrhage: predicting factors and impact on outcome. BMC Neurol. (2015) 15:6. doi: 10.1186/s12883-015-0258-8
33. Volbers, B, Fischer, U, and Huttner, HB. Inflammation, edema, hematoma and etiology – a rectangular relationship? Eur J Neurol. (2019) 26:e11. doi: 10.1111/ene.13829
34. Urday, S, Kimberly, WT, Beslow, LA, Vortmeyer, AO, Selim, MH, Rosand, J, et al. Targeting secondary injury in intracerebral haemorrhage--perihaematomal oedema. Nat Rev Neurol. (2015) 11:111–22. doi: 10.1038/nrneurol.2014.264
35. Lee, SH, Kim, BJ, Bae, HJ, Lee, JS, Lee, J, Park, BJ, et al. Effects of glucose level on early and long-term mortality after intracerebral haemorrhage: the acute brain bleeding analysis study. Diabetologia. (2010) 53:429–34. doi: 10.1007/s00125-009-1617-z
36. Oddo, M, Schmidt, JM, Carrera, E, Badjatia, N, Connolly, ES, Presciutti, M, et al. Impact of tight glycemic control on cerebral glucose metabolism after severe brain injury: a microdialysis study. Crit Care Med. (2008) 36:3233–8. doi: 10.1097/CCM.0b013e31818f4026
Keywords: ICH, bundle, treatment, PHE, HE
Citation: Mrochen A, Song Y, Harders V, Sembill JA, Sprügel MI, Hock S, Lang S, Engelhorn T, Kallmünzer B, Volbers B and Kuramatsu JB (2024) Influence of bundled care treatment on functional outcome in patients with intracerebral hemorrhage. Front. Neurol. 15:1357815. doi: 10.3389/fneur.2024.1357815
Edited by:
Jean-Claude Baron, University of Cambridge, United KingdomReviewed by:
Chuanyuan Tao, Sichuan University, ChinaFloris H. B. M. Schreuder, Radboud University Medical Centre, Netherlands
Copyright © 2024 Mrochen, Song, Harders, Sembill, Sprügel, Hock, Lang, Engelhorn, Kallmünzer, Volbers and Kuramatsu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joji B. Kuramatsu, am9qaS5rdXJhbWF0c3VAdWstZXJsYW5nZW4uZGU=
†These authors have contributed equally to this work
 Anne Mrochen
Anne Mrochen Yu Song
Yu Song Verena Harders
Verena Harders Jochen A. Sembill
Jochen A. Sembill Maximilian I. Sprügel1
Maximilian I. Sprügel1 Stefan Hock
Stefan Hock Bernd Kallmünzer
Bernd Kallmünzer Joji B. Kuramatsu
Joji B. Kuramatsu