
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 09 May 2024
Sec. Epilepsy
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1353366
Objectives: Seizures are common in children undergoing cardiopulmonary bypass (CPB). Cerebral oxygen saturation (ScO2) by near-infrared spectroscopy is routinely monitored in many centers, but the relations between the levels and changes of ScO2 and brain injuries remain incompletely understood. We aimed to analyze the postoperative profiles of ScO2 and cerebral blood flow velocity in different types of EEG seizures in relation to brain injuries on MRI.
Methods: We monitored continuous EEG and ScO2 in 337 children during the first 48 h after CPB, which were analyzed in 3 h periods. Cerebral blood flow peak systolic velocity (PSV) in the middle cerebral artery was measured daily by transcranial Doppler. Postoperative cerebral MRI was performed before hospital discharge.
Results: Based on the occurrence and spreading types of seizures, patients were divided into three groups as patients without seizures (Group N; n = 309), those with focal seizures (Group F; n = 13), or with secondarily generalized seizures (Group G; n = 15). There were no significant differences in the onset time and duration of seizures and incidence of status epilepticus between the two seizures groups (Ps ≥ 0.27). ScO2 increased significantly faster across Group N, Group G, and Group F during the 48 h (p < 0.0001) but its overall levels were not significantly different among the three groups (p = 0.30). PSV was significantly lower (p = 0.003) but increased significantly faster (p = 0.0003) across Group N, Group G, and Group F. Group F had the most severe brain injuries and the highest incidence of white matter injuries on MRI among the three groups (Ps ≤ 0.002).
Conclusion: Postoperative cerebral oxygenation showed distinct profiles in secondarily generalized and particularly focal types of EEG seizures in children after CPB. A state of ‘overshooting’ ScO2 with persistently low PSV was more frequently seen in those with focal seizures and more severe brain injury. Information from this study may have important clinical implications in detecting brain injuries when monitoring cerebral oxygenation in this vulnerable group of children after CPB.
Acquired brain injuries and neurodevelopmental impairment are common and potentially devastating comorbidities in children with congenital heart disease (CHD) undergoing CPB (1–3). Studies using continuous electroencephalographic (EEG) monitoring have reported that seizures occurred in 5–20% of patients during early postoperative period and was a marker for acute brain injury and associated with worse neurodevelopmental outcomes (1, 2, 4–6). According to the International League Against Epilepsy, seizures included focal and initially focal then secondarily generalized types depending on whether seizures widespread bilaterally or not (7). We and others have previously reported that among patients with seizures, the focal seizures occurred in 20–70% of patients and secondarily generalized type occurred in 15–70% (2, 4, 5). In addition to seizures, the EEG abnormal discharges also included some micro-scale patterns, such as spikes/sharp waves, which may occur when cerebral perfusion and oxygen supply are too limited to manifest seizures which demand much energy and oxygen supply (8–10). These micro-scale patterns have been related to neurologic risk conditions, e.g., neonatal asphyxia (11), but have not been reported in children after cardiac surgery.
The early postoperative period in children after CPB is characterized by the profound imbalance of systemic and cerebral oxygen transport with increased oxygen consumption and decreased oxygen delivery (12–14). It has been further reported that imbalanced systemic oxygen transport has important influences on early postoperative cerebral oxygen saturation (ScO2) (12). Cerebral metabolic rates and energy use may increase several times during seizures, thus worsening cerebral oxygenation status (8–10, 15). Nonetheless, the alteration of cerebral oxygenation and its relation with EEG abnormalities during the early post-CPB period remain largely unexplored.
Currently, near-infrared spectroscopy (NIRS), transcranial Doppler (TCD), EEG, and magnetic resonance imaging (MRI) are generally used to assess cerebral oxygenation and brain functional and anatomical injuries (1–6, 16–21). NIRS is routinely used in many centers to continuously monitor ScO2 which is the equilibrium of oxyhemoglobin and deoxyhemoglobin in a mixture of veins, arteries, and capillaries in the underlying tissue and reflects a regional state of oxygenation (16–20). Early postoperative low ScO2 has been reported to be associated with brain injury and neurodevelopmental impairment (18–20). But the clinical implications of its changes and the not uncommonly observed high levels of ScO2 during the early post-CPB period are incompletely understood. From ScO2 and systemic arterial oxygen saturation (SaO2), cerebral oxygen extraction ratio (CERO2) can be calculated, manifesting cerebral oxygen consumption relative to oxygen delivery. TCD measures cerebral blood flow velocity manifesting cerebral oxygen delivery (21).
Therefore, we aimed to investigate the profiles of cerebral oxygenation parameters in different types of EEG seizures and discharge abnormalities and to propose the potential pathophysiological mechanisms and clinical implications using these techniques.
After the institutional ethics approval (No. 46201) and informed consent obtained at the Guangzhou Women and Children’s Medical Center, a total of 337 patients were enrolled from January 2019 to December 2021 (Figure 1). The most complex CHD patient on the daily surgical list was screened to be approached for recruitment according to STS-EACTS (the Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery) Mortality Categories (4, 22). Patients with the recognizable syndrome of congenital anomalies, previous CPB, scalp vein puncture, postmenstrual age < 37 weeks, cerebral hemorrhage by Doppler ultrasound were excluded.
Patients were premedicated with 0.01 mg/kg penehyclidine hydrochloride. Anesthesia was induced with propofol (2–3 mg/kg), cis-atracurium (0.2–0.3 mg/kg), and sufentanil (0.5–1 μg/kg). It was maintained with sevoflurane (2–3%), dexmedetomidine (0.3–1 μg/kg/h). Standard cardiopulmonary bypass as described elsewhere (4). A bolus of heparin (500 units/kg) was administered to maintain activated clotting time of whole blood >480 s. Deep hypothermic circulatory arrest (DHCA) was performed without antegrade selective cerebral perfusion for the repair of aortic arch obstructive abnormalities.
Standard postoperative management was used as described elsewhere (4). Patients receive time-cycled pressure control/pressure support ventilation on arrival in the CICU, followed by non-invasive ventilation when appropriate. Sedation consisted of continuous intravenous infusion of sufentanil (0.2 μg/kg/min) and dexmedetomidine (2 μg/kg/min) and intermittent administration of midazolam (0.1 mg/kg). Inotropic and vasoactive drugs included dopamine, milrinone and epinephrine to maintain arterial blood pressure (systolic pressure 60–90 and 90–105 mmHg in neonates and children, respectively).
All patients had monitoring of heart rate, arterial blood pressure, rectal temperature, and SaO2.
Continuous Video-EEG was recorded using Nicolet monitor (CareFusion, Middleton, Wisconsin, United States). The recording of electrical activity was collected from scalp electrodes positioned in the FP1, C3, T3, O1, Cz, FP2, C4, T4, and O2 positions according to the international 10–20 system. Electrographic seizures was defined as epileptiform discharges averaging >2.5 Hz for ≥10 s, and status epilepticus as continuous seizures ≥10 min or for a total duration of ≥20% of any 60 min period of recording (23). The origin of seizures indicates the region of the initial seizure onset. Focal seizures was defined as localized and limited to one hemisphere (either a left-or right-sided lateralization) and secondarily generalized seizures was defined as initially focal then spreading to bilaterally diffused (7). Spikes/sharp waves were defined as high amplitude (≥2.5 times of background voltage) and short duration (<200 ms) (23). All EEGs were analyzed in 3 h periods by the qualified technicians (RYL and SYN) independently and finalized by SYN.
ScO2 was continuously measured using NIRS (INVOS 5100C, Medtronic & Covidien, Troy, MI, United States) (12). The sensors were placed on the children’s forehead below the hairline to the right and left of the midline and recorded every 3 h. Averaged bilateral ScO2 was used for Group N and Group G. In Group F, ipsilateral ScO2 to the onset of seizures and the other side of the brain were separately analyzed. CERO2 was calculated using the following equation: (SaO2 − ScO2)/SaO2.
PSV of the middle cerebral artery was measured with TCD with a 2 MHz pulse-wave ultrasound transducer, which was fixed above the zygomatic arch (Multi-Dop T; DWL Elektronische Systeme GmbH, Sipplingen, Germany) and interrogated the portion of the middle cerebral artery near its junction with the anterior cerebral artery (21). PSV was recorded on postoperative day 0, day 1, and day 2. Bilateral PSV was averaged for Group N and Group G. In Group F, we analyzed separately PSV ipsilateral to the onset of seizures and the other side of the brain.
MRI scans were performed on a 3 T Magentom Prisma scanner (Siemens, Munich, Germany) including standard T1, T2, diffusion-weighted imaging, and diffusion-tensor imaging at the median 9 (3–37) days after surgery. Brain injuries included white matter injury, stroke, and hemorrhage and were graded as mild, moderate, and severe using the standard method (24). All MRIs were evaluated by a pediatric neuroradiologist (MJZ).
Demographic data, STS-EACTS Mortality Categories (22), the duration of postoperative mechanical ventilation, CPB and aortic cross-clamp (ACC), Deep hypothermic circulatory arrest (DHCA), CICU, hospital stay, and death were collected (Table 1).
Data were described as median (range) or frequency (%) when appropriate. Comparisons of parameters across the three groups were made using the Kruskal–Wallis test for non-normal distribution variables and Chi-squared or Fish’s exact test for categorical variables when appropriate. Comparisons of seizures details between Group G and Group F were made using the Mann–Whitney U-test for non-normal distribution variables and the Chi-squared test for categorical variables. Mixed linear regression for repeated measures was used to analyze the profiles of variables. It was also used to compare the differences in levels and trends between groups with analysis of the effects of group interaction between time and group. The parameter estimates and probability values of the group effect (Pgroup) indicate the difference in the overall levels of each variable between the groups and the interaction of time and group (Pgroup × time) indicates the difference in trends of each variable between the groups. Logarithmic transformation was tested for time-related variables regarding the best fit of time. A p-value <0.05 was considered statistically significant (SAS 9.4, Cary, NC, the United States).
Postoperative EEG seizures occurred in 8.3% (28 out of 337) patients. There were 309 patients without seizures (Group N), 15 with secondarily generalized seizures (Group G), and 13 with focal seizures (Group F).
In Group G, the onset time of seizures ranged from 15–39 h (median 27) after surgery and lasted 1.6–646.8 min (median 84.9). Among them, 11 (73.3%) had status epilepticus. Seizure onset originated from occipital regions in 6 (40.0%), frontal in 2 (13.3%), and lateralized in 7 (46.7%). In Group F, the onset time of seizures ranged from 6 to 45 h (median 18) after surgery and lasted 3.6–772.9 min (median 106.3) and 10 (76.9%) had status epilepticus. Seizure onset originated from occipital regions in 8 (61.5%), central in 4 (30.8%), and frontal in 1 (7.7%). Seizures were lateralized in 1 (7.7%) and focal in 12 (92.3%). There were no significant differences in the onset time and duration of seizures and the incidence of status epilepticus (Ps ≥ 0.27) between the two seizures groups (Figure 2). The number of spikes/sharp waves was significantly larger (Pgroup < 0.0001) and decreased significantly faster (Pgroup*time = 0.02) across Group N, Group G, and Group F (Table 2, Figure 3 and Supplementary Table S1).
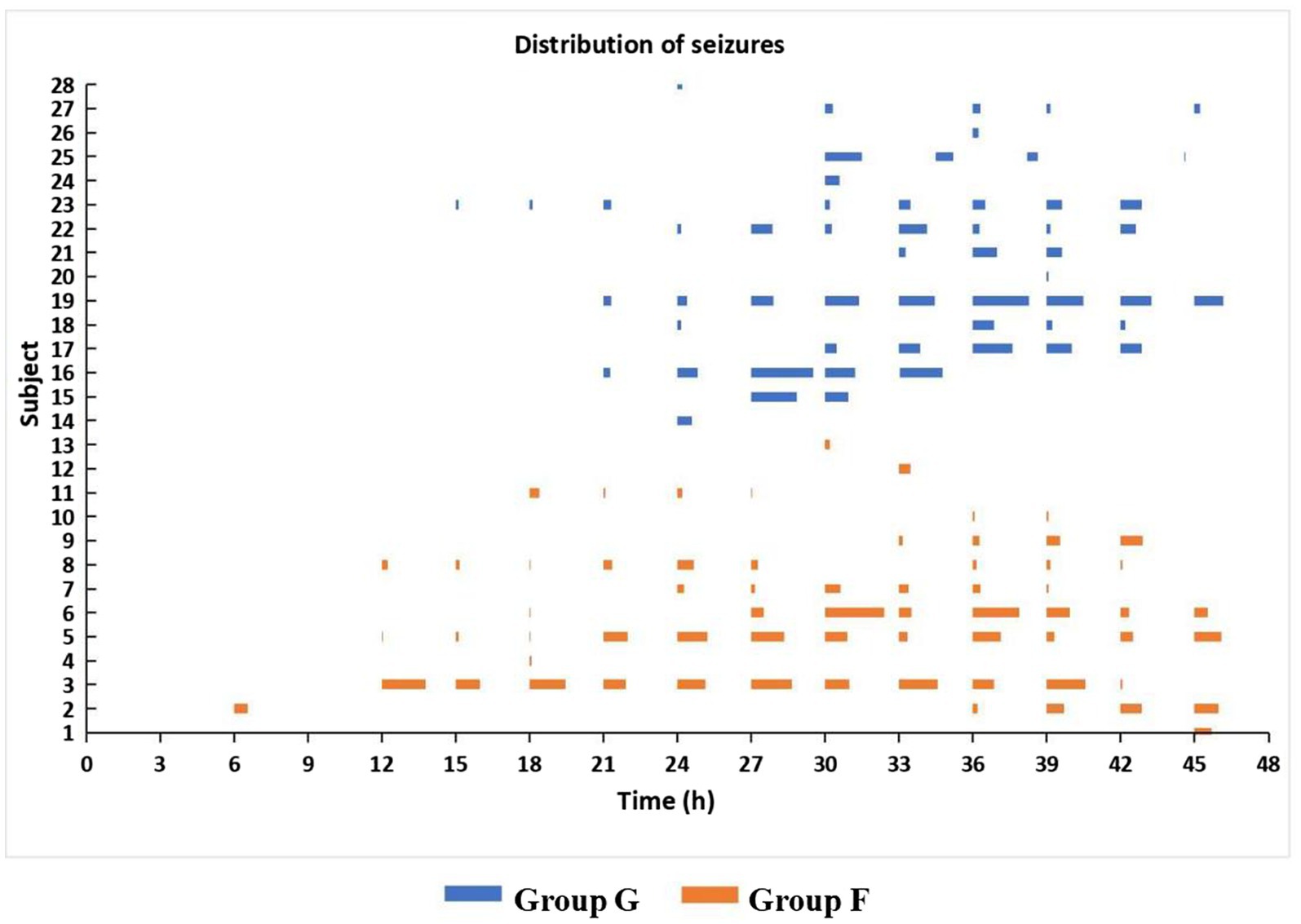
Figure 2. Time distribution of seizures in secondarily generalized seizure group (Group G) and focal seizure group (Group F) during the 48 h study period after CPB. Ps ≥ 0.27 for the onset time and duration of seizures and the incidence of status epilepticus.
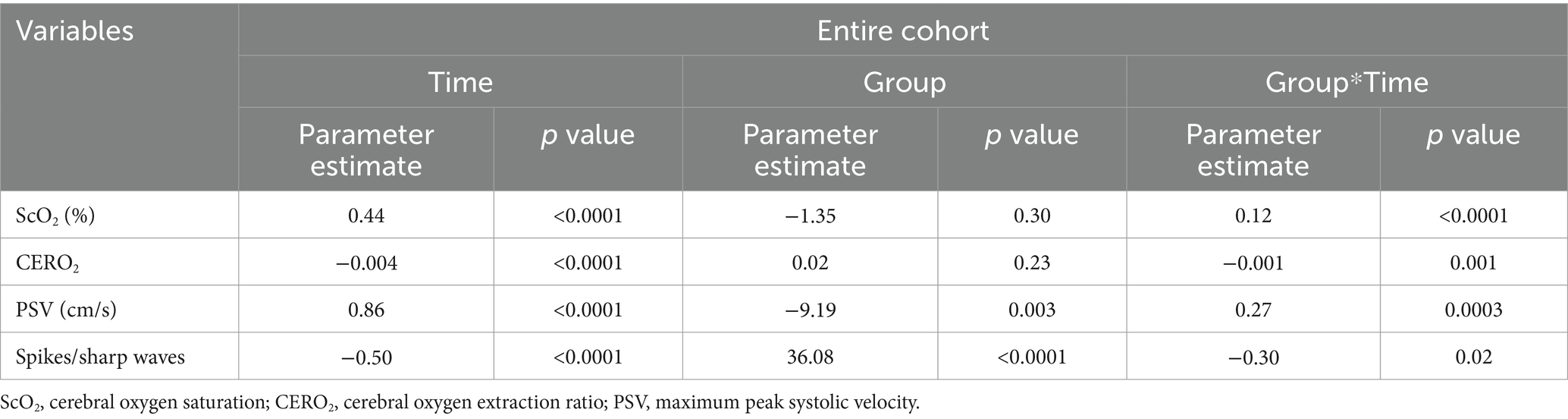
Table 2. Statistical results of the comparison of the changes of cerebral oxygenation parameters and spikes/sharp waves during the first 48 h after cardiac surgery among the three groups.
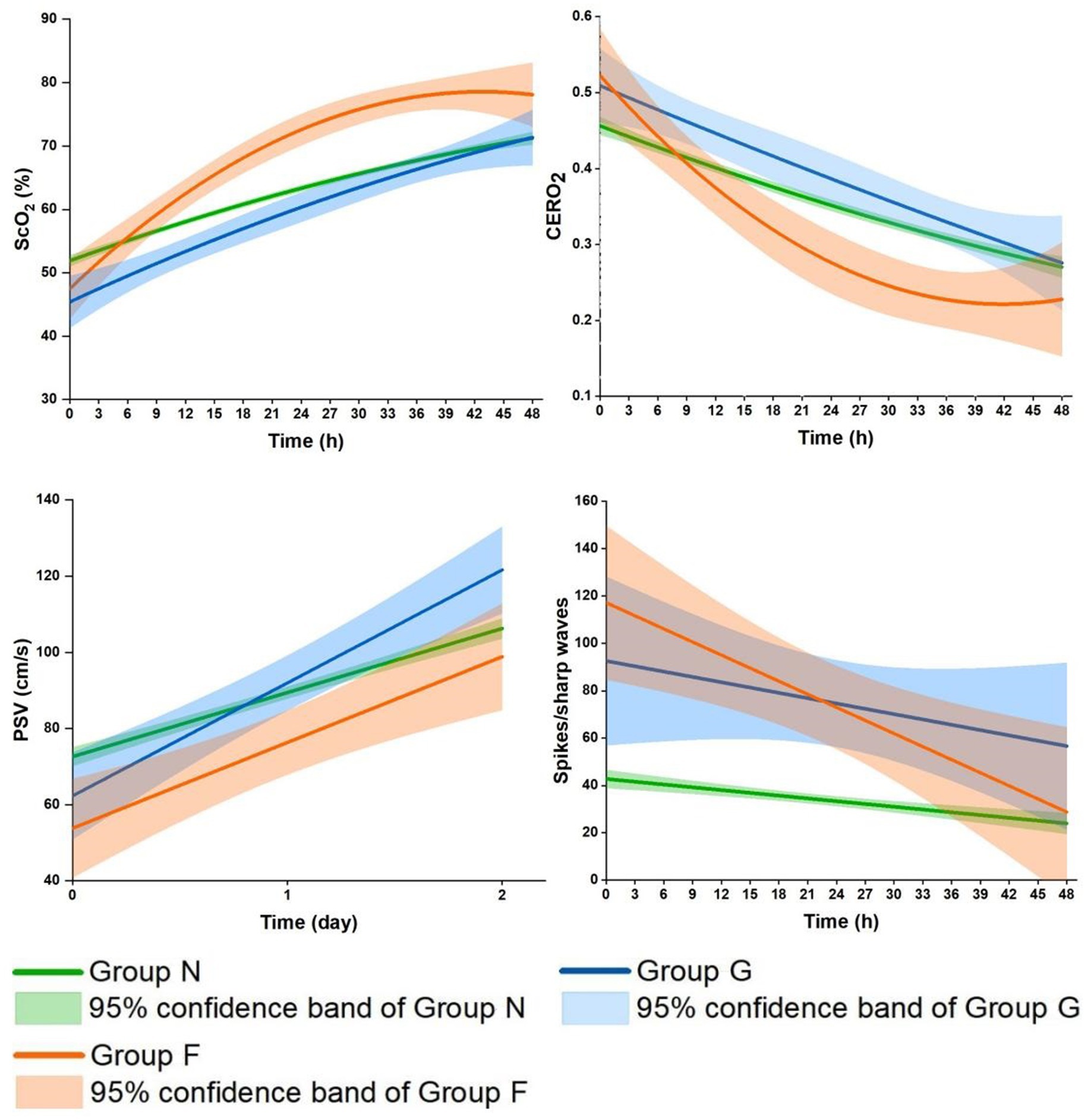
Figure 3. Comparisons of the profiles of the cerebral oxygenation parameters and spikes/sharp waves during the first 48 h after CPB among the three groups. Group G, secondarily generalized seizures group; Group F, focal seizures group; Group N, none-seizures group; ScO2, cerebral oxygen saturation; CERO2, cerebral oxygen extraction ratio; PSV, peak systolic velocity.
Across Group N, Group G, and Group F, patients’ age, weight, and body surface area (BSA) trended to be lower (Ps ≤ 0.10); STS-EACTS Mortality Categories was significantly higher and the durations of CPB and ACC were significantly longer and the use of DHCA was more frequent (Ps ≤ 0.001). There were no significant differences in the durations of DHCA, postoperative mechanical ventilation, CICU, and hospital stay (Ps ≥ 0.07) among the three groups. There were 8 deaths in Group N, and none in Group G and Group F (P > 0.99) (Table 1).
ScO2 significantly increased in the three groups during the 48 h (Supplementary Table S1). The increase was significantly faster across Group N, Group G and Group F (Ptime < 0.0001, Pgroup = 0.30, Pgroup*time < 0.0001). In more details, it linearly increased in Group N and Group G during this period (Ps < 0.0001); In Group G, it reached a level close to that in Group N by the end of 48 h; In Group F, it was significantly related to time after logarithmic transformation, with a faster increase followed by a slower increase (P < 0.0001), surpassing the level in Group N since 6–9 h after CPB. Similar but reciprocal profiles occurred in CERO2 (Ptime < 0.0001, Pgroup = 0.23, Pgroup*time = 0.001). The overall levels of PSV were significantly lower during the 48 h (Pgroup = 0.003), and increased more rapidly across Group N, Group G and Group F (Pgroup*time = 0.0003) (Table 2 and Figure 3). Further in Group F, there were no significant differences in ScO2, CERO2, and PSV between the two sides of the brain (Ps ≥ 0.17).
Postoperative MRI was undertaken in 65.3% (220 out of 337) of patients including 199 (64.4%) in Group N, 11 (73.3%) in Group G, and 10 (76.9%) in Group F (P = 0.52) (Table 3). There were no significant differences in the types (hemorrhage, white matter injury, and stroke) and degrees of brain injury between Group G and N. Compared to Group N and Group G, Group F had the most severe degree of brain injury (P = 0.002). All the patients in Group F had brain injury, being mild, moderate, and severe in 6, 1, and 3, respectively. Among the types of brain injury, the incidence of white matter injuries (50.0%) was highest in Group F (P = 0.0002). Another 4 (40%) patients had hemorrhage (subarachnoid in 1, subdural in 3), and 1 (10%) patient had stroke (Table 3).
The present study demonstrated distinct profiles of cerebral oxygenation parameters in Group G and particularly Group F compared to Group N, potentially attributable to the dynamic and complex relations between cerebral metabolism, oxygen consumption, and its delivery in the different types of seizures.
The early hours after CPB represent the most critical period of imbalanced cerebral oxygenation with decreased cerebral oxygen delivery and increased oxygen consumption, as indicated by the initially low ScO2 and PSV and high CERO2 in our cohort. In the two groups with seizures compared to Group N, the initial ScO2 and PSV were lower, and CERO2 higher, indicating a poorer balance of cerebral oxygenation (12, 16, 17, 25, 26). Clinically, patients with seizures were sicker, with significantly higher STS-EACTS Mortality Categories, longer time of CPB, ACC, and more frequent use of DHCA, which have been identified as risk factors for seizures in our previous study and others (1, 2, 4, 5).
Nonetheless, it should be noted that seizures did not occur during the most critical period until the median time of postoperative 27 and 18 h (P = 0.27) in Group G and Group F, respectively. This finding is consistent with other studies (2, 4, 6). Instead of seizures, the abnormal discharges were mostly manifested as micro-scale patterns, i.e., spikes/sharp waves in the two seizure groups, particularly in Group F. Speculatively, cerebral oxygenation may have too limited capacity to manifest seizures (9). A study using phosphorus-31 magnetic resonance spectroscopy in infants with seizures has revealed that high-energy phosphates decrease by 33% and mitochondrial oxidative phosphorylation increases by 45% during seizures, indicating a depleted cerebral energy state (8).
Later, seizures occurred and spikes/sharp waves decreased as cerebral oxygenation status recovered to a certain degree, as indicated by the increase in ScO2 and decrease in CERO2. In the two seizure groups, these changes were greater compared to Group N. This seems puzzling, as seizures induce an increase in cerebral oxygen consumption which would expectedly lead to poor cerebral oxygenation status (10, 15). Furthermore in Group G, ScO2 gradually and linearly increased, CERO2 decreased, and both became close to the levels in Group N by 48 h. In Group F, the increase in ScO2 and decrease in CERO2 were related to time after logarithmic transformation, with a faster increase/decrease followed by a slower increase/decrease. The changes of the two parameters were so much faster that they surpassed the levels in Group N since postoperative about 6–9 h coinciding with the onset of seizures.
The distinct profiles in ScO2 and CERO2 may indicate different underlying pathophysiological mechanisms between the two types of seizures. In Group G, the secondarily generalized seizures are resulted from hyperexcitability and hypersynchrony of multiple neurons all over the brain (27, 28). The potentially increased cerebral oxygen consumption may be overcompensated by the greater increase in oxygen delivery. This was supported by our data showing a greater increase in PSV on both hemispheres on the 1st and 2nd postoperative day. Nonetheless, ScO2 remained lower, and CERO2 higher, until the end of 48 h study period, indicating a generally worse cerebral oxygenation status in Group G compared to Group N. Sokol et al. reported the lower level of ScO2 during the ictal phase of secondarily generalized seizures compared to pre-ictal baseline in adults with medically refractory epilepsy (28). Despite all this, there was no significant difference in brain injuries on MRI between Group G and Group N. The most frequent brain injury was subdural hemorrhage (mild brain injuries) in both groups (n = 7 (63.6%) in Group G and n = 105 (52.8%) in Group N), and some patients did not show brain injuries on MRI (n = 4 (36.4%) and n = 87 (43.7%), respectively). Seizures are related to functional neurologic impairment and cause excitotoxic injury, which may not respond or lead to structural abnormalities observed on MRI (10).
Group F may represent a more complex scenario in terms of cerebral oxygenation alteration. The greatest increase in ScO2 and decrease in CERO2 occurred in the presence of persistently lower PSV. These data might suggest a greater reduction in cerebral oxygen consumption relative to the reduced oxygen delivery. Furthermore, there were the greatest amount of spikes/sharp waves during the 48 h in Group F, likely indicating overall worse EEG discharge abnormalities. The findings on MRI may provide more insights into the potential underlying mechanisms of this distinct cerebral oxygenation alteration. Group F had the most severe brain injury on MRI compared to Group G and Group N. In fact, all the patients with focal seizures had positive MRI findings, half of them had white matter injuries and the other half had hemorrhage or stroke. In consistency with our data, patients with focal seizures caused by febrile were more likely to have abnormal white matter signals and subcortical focal hyperintensity (29–31). The widespread seizure activity largely relies on the neuron networks of distinct neural circuits of transverse fibers in the white matter and spreads to more distant areas via the corpus callosum (32). Thus white matter injuries may disrupt that transmission pathway, which may help to explain our findings in patients with white matter injury in Group F. The other 40% of patients in Group F had hemorrhage (subarachnoid in 1, subdural in 3). In fact, the incidence of hemorrhage was not significantly different among the three groups. Patients with brain hemorrhage developed focal or secondarily generalized seizures, which may be determined by the different excitabilities across the neuron network in different patients (33). Furthermore, the network inhibition hypothesis suggests that seizure activity in one part of the brain may cause inhibition of other cortical regions (34). As such, the reasons for the findings in Group F might appear clear. The majority of focal seizures onset originated from the occipital region (61.5%) and central region (30.8%) thereby inhibiting the frontoparietal regions (35, 36). While focal seizures may increase oxygen consumption focally (28, 35, 37) we placed the NIRS sensors in the frontal regions and measured PSV in the middle cerebral artery where cerebral metabolism and oxygen consumption were inhibited. The inhibition of cerebral oxygen consumption was so much that the greatest increase in ScO2 and decrease in CERO2 occurred even when PSV remained consistently lowest in Group F compared to Group G and Group N. The inhibition appeared to have involved both hemispheres as there were no significant differences in ScO2, CERO2, and PSV on the two sides of the brain.
The main strength of this study was that we conducted a comprehensive and systematic investigation using NIRS, TCD, EEG, and MRI to evaluate cerebral oxygenation alteration in relation to brain injury. While ScO2 is widely used during and after CPB, there is limited knowledge about how to interpret the ScO2 values. Previous studies have mostly focused on reduced ScO2 (18–20). Our data provided information about the dynamic changes in ScO2 along with other cerebral oxygenation parameters in the complex relation to varied abnormal EEG discharges and brain injury on MRI. This information may be helpful to interpret the routinely monitored data at bedside. A state of ‘overshooting’ ScO2 with persistently low PSV was more frequently seen in those with focal seizures and more severe brain injury. More attention should be paid to the clinical management in such patients to protect their brain. This study has limitations. (1) This was a single-center study that recruited heterogeneous and relatively more complex CHD patients. Our data may not be applied to the overall patient populations in our center or other patient populations such as neonates with hypoplastic left heart syndrome and related anomalies who are known to have the most severe brain injuries (1, 38). (2) The STS-EACTS Mortality Categories was updated in 2021 (39) which was near the end of our study. We decided to keep using the earlier version throughout the study period in order to avoid certain bias that exists between the two versions. The information obtained in our study remained valid. (3) The mechanisms for the altered cerebral oxygenation in different groups were speculated based on previous animal and human experimental findings. (4) The effect of varied EEG discharge abnormalities and cerebral oxygenation status on long-term neurodevelopmental outcomes remains to be explored, which is being investigated in our center.
Distinct profiles of cerebral oxygenation parameters were found in patients with secondarily generalized or focal seizures in children after CPB compared to patients without seizures, which was potentially attributable to the dynamic and complex relations between cerebral oxygen consumption and its delivery in the different types of seizures. A state of ‘overshooting’ ScO2 with persistently low PSV was more frequently seen in those with focal seizures and more severe brain injury. More attention should be paid to the clinical management in such patients to protect their brain. Information from this study may have important clinical implications in detecting brain injuries when monitoring cerebral oxygenation in this vulnerable group of children after CPB.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Guangzhou Women and Children’s Medical Center. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
RL: Data curation, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. ND: Data curation, Methodology, Resources, Validation, Writing – review & editing. SN: Data curation, Resources, Validation, Writing – review & editing. MZ: Data curation, Resources, Validation, Writing – review & editing. JF: Data curation, Validation, Writing – review & editing. XC: Resources, Supervision, Validation, Visualization, Writing – review & editing. LM: Investigation, Resources, Supervision, Validation, Writing – review & editing. JL: Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Guangdong Provincial Key Laboratory of Research in Structural Birth Defect Disease and Guangzhou Women and Children’s Medical Center start-up fund to JL.
We thank all clinicians and nurses of the Heart Center, particularly those working at in the CICU for their support over the course of this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1353366/full#supplementary-material
1. Gaynor, JW, Jarvik, GP, Gerdes, M, Kim, DS, Rajagopalan, R, Bernbaum, J, et al. Postoperative electroencephalographic seizures are associated with deficits in executive function and social behaviors at 4 years of age following cardiac surgery in infancy. J Thorac Cardiovasc Surg. (2013) 146:132–9. doi: 10.1016/j.jtcvs.2013.04.002
2. Naim, MY, Gaynor, JW, Chen, J, Nicolson, SC, Fuller, S, Spray, TL, et al. Subclinical seizures identified by postoperative electroencephalographic monitoring are common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. (2015) 150:169–80. doi: 10.1016/j.jtcvs.2015.03.045
3. Beca, J, Gunn, JK, Coleman, L, Hope, A, Reed, PW, Hunt, RW, et al. New white matter brain injury after infant heart surgery is associated with diagnostic group and the use of circulatory arrest. Circulation. (2013) 127:971–9. doi: 10.1161/CIRCULATIONAHA.112.001089
4. Li, MY, Lou, XB, Cui, YQ, Lin, RY, Ning, SY, Li, LJ, et al. Assessment of postoperative risk factors for EEG abnormalities in routine clinical management after paediatric cardiopulmonary bypass. Interact Cardiovasc Thorac Surg. (2021) 33:301–8. doi: 10.1093/icvts/ivab081
5. Ghosh, S, Philip, J, Patel, N, Munoz-Pareja, J, Lopez-Colon, D, Bleiweis, M, et al. Risk factors for seizures and epilepsy in children with congenital heart disease. J Child Neurol. (2020) 35:442–7. doi: 10.1177/0883073820904912
6. Helmers, SL, Wypij, D, Constantinou, JE, Newburger, JW, Hickey, PR, Carrazana, EJ, et al. Perioperative electroencephalographic seizures in infants undergoing repair of complex congenital cardiac defects. Electroencephalogr Clin Neurophysiol. (1997) 102:27–36. doi: 10.1016/S0013-4694(96)95079-8
7. Fisher, RS, Cross, JH, French, JA, Higurashi, N, Hirsch, E, Jansen, FE, et al. Operational classification of seizure types by the international league against epilepsy: position paper of the ilae commission for classification and terminology. Epilepsia. (2017) 58:522–30. doi: 10.1111/epi.13670
8. Younkin, DP, Delivoria-Papadopoulos, M, Maris, J, Donlon, E, Clancy, R, and Chance, B. Cerebral metabolic effects of neonatal seizures measured with in vivo 31p nmr spectroscopy. Ann Neurol. (1986) 20:513–9. doi: 10.1002/ana.410200412
10. Rho, JM, and Boison, D. The metabolic basis of epilepsy. Nat Rev Neurol. (2022) 18:333–47. doi: 10.1038/s41582-022-00651-8
11. Biagioni, E, Boldrini, A, Bottone, U, Pieri, R, and Cioni, G. Prognostic value of abnormal eeg transients in preterm and full-term neonates. Electroencephalogr Clin Neurophysiol. (1996) 99:1–9. doi: 10.1016/0921-884X(96)95649-0
12. Li, J, Zhang, G, Holtby, H, Guerguerian, AM, Cai, S, Humpl, T, et al. The influence of systemic hemodynamics and oxygen transport on cerebral oxygen saturation in neonates after the Norwood procedure. J Thorac Cardiovasc Surg. (2008) 135:83–90.e2. doi: 10.1016/j.jtcvs.2007.07.036
13. Li, J, Schulze-Neick, I, Lincoln, C, Shore, D, Scallan, M, Bush, A, et al. Oxygen consumption after cardiopulmonary bypass surgery in children: determinants and implications. J Thorac Cardiovasc Surg. (2000) 119:525–33. doi: 10.1016/S0022-5223(00)70132-2
14. Hoffman, GM, Mussatto, KA, Brosig, CL, Ghanayem, NS, Musa, N, Fedderly, RT, et al. Systemic venous oxygen saturation after the Norwood procedure and childhood neurodevelopmental outcome. J Thorac Cardiovasc Surg. (2005) 130:1094–100. doi: 10.1016/j.jtcvs.2005.06.029
15. Sokoloff, MD, Plegue, MA, Chervin, RD, Barks, JD, and Shellhaas, RA. Phenobarbital and neonatal seizures affect cerebral oxygen metabolism: a near-infrared spectroscopy study. Pediatr Res. (2015) 78:91–6. doi: 10.1038/pr.2015.64
16. Claessens, NHP, Jansen, NJG, Breur, J, Algra, SO, Stegeman, R, Alderliesten, T, et al. Postoperative cerebral oxygenation was not associated with new brain injury in infants with congenital heart disease. J Thorac Cardiovasc Surg. (2019) 158:e861. doi: 10.1016/j.jtcvs.2019.02.106
17. Toet, MC, Flinterman, A, Laar, I, Vries, JW, Bennink, GB, Uiterwaal, CS, et al. Cerebral oxygen saturation and electrical brain activity before, during, and up to 36 hours after arterial switch procedure in neonates without pre-existing brain damage: its relationship to neurodevelopmental outcome. Exp Brain Res. (2005) 165:343–50. doi: 10.1007/s00221-005-2300-3
18. Aly, SA, Zurakowski, D, Glass, P, Skurow-Todd, K, Jonas, RA, and Donofrio, MT. Cerebral tissue oxygenation index and lactate at 24 hours postoperative predict survival and neurodevelopmental outcome after neonatal cardiac surgery. Congenit Heart Dis. (2017) 12:188–95. doi: 10.1111/chd.12426
19. Simons, J, Sood, ED, Derby, CD, and Pizarro, C. Predictive value of near-infrared spectroscopy on neurodevelopmental outcome after surgery for congenital heart disease in infancy. J Thorac Cardiovasc Surg. (2012) 143:118–25. doi: 10.1016/j.jtcvs.2011.09.007
20. Carra, G, Flechet, M, Jacobs, A, Verstraete, S, Vlasselaers, D, Desmet, L, et al. Postoperative cerebral oxygen saturation in children after congenital cardiac surgery and long-term total intelligence quotient: a prospective observational study. Crit Care Med. (2021) 49:967–76. doi: 10.1097/CCM.0000000000004852
21. Li, J, Zhang, G, Holtby, H, Bissonnette, B, Wang, G, Redington, AN, et al. Carbon dioxide—a complex gas in a complex circulation: its effects on systemic hemodynamics and oxygen transport, cerebral, and splanchnic circulation in neonates after the Norwood procedure. J Thorac Cardiovasc Surg. (2008) 136:1207–14. doi: 10.1016/j.jtcvs.2008.02.096
22. O’Brien, SM, Clarke, DR, Jacobs, JP, Jacobs, ML, Lacour-Gayet, FG, Pizarro, C, et al. An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg. (2009) 138:1139–53. doi: 10.1016/j.jtcvs.2009.03.071
23. Hirsch, LJ, Fong, MWK, Leitinger, M, LaRoche, SM, Beniczky, S, Abend, NS, et al. American clinical neurophysiology society’s standardized critical care eeg terminology: 2021 version. J Clin Neurophysiol. (2021) 38:1–29. doi: 10.1097/WNP.0000000000000806
24. Andropoulos, DB, Hunter, JV, Nelson, DP, Stayer, SA, Stark, AR, McKenzie, ED, et al. Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. J Thorac Cardiovasc Surg. (2010) 139:543–56. doi: 10.1016/j.jtcvs.2009.08.022
25. Greeley, WJ, Kern, FH, Ungerleider, RM, Boyd, JL, Quill, T, Smith, LR, et al. The effect of hypothermic cardiopulmonary bypass and total circulatory arrest on cerebral metabolism in neonates, infants, and children. J Thorac Cardiovasc Surg. (1991) 101:783–94. doi: 10.1016/S0022-5223(19)36647-4
26. Cheng, HH, Ferradal, SL, Vyas, R, Wigmore, D, McDavitt, E, Soul, JS, et al. Abnormalities in cerebral hemodynamics and changes with surgical intervention in neonates with congenital heart disease. J Thorac Cardiovasc Surg. (2020) 159:2012–21. doi: 10.1016/j.jtcvs.2019.08.045
27. Badawy, RA, Curatolo, JM, Newton, M, Berkovic, SF, and Macdonell, RA. Changes in cortical excitability differentiate generalized and focal epilepsy. Ann Neurol. (2007) 61:324–31. doi: 10.1002/ana.21087
28. Sokol, DK, Markand, ON, Daly, EC, Luerssen, TG, and Malkoff, MD. Near infrared spectroscopy (nirs) distinguishes seizure types. Seizure. (2000) 9:323–7. doi: 10.1053/seiz.2000.0406
29. Maytal, J, Krauss, JM, Novak, G, Nagelberg, J, and Patel, M. The role of brain computed tomography in evaluating children with new onset of seizures in the emergency department. Epilepsia. (2000) 41:950–4. doi: 10.1111/j.1528-1157.2000.tb00277.x
30. Van Landingham, KE, Heinz, ER, Cavazos, JE, and Lewis, DV. Magnetic resonance imaging evidence of hippocampal injury after prolonged focal febrile convulsions. Ann Neurol. (1998) 43:413–26. doi: 10.1002/ana.410430403
31. Hesdorffer, DC, Chan, S, Tian, H, Allen Hauser, W, Dayan, P, Leary, LD, et al. Are MRI-detected brain abnormalities associated with febrile seizure type? Epilepsia. (2008) 49:765–71. doi: 10.1111/j.1528-1167.2007.01459.x
32. Badawy, RA, Harvey, AS, and Macdonell, RA. Cortical hyperexcitability and epileptogenesis: understanding the mechanisms of epilepsy - part 2. J Clin Neurosci. (2009) 16:485–500. doi: 10.1016/j.jocn.2008.10.001
33. Lopes, MA, Junges, L, Woldman, W, Goodfellow, M, and Terry, JR. The role of excitability and network structure in the emergence of focal and generalized seizures. Front Neurol. (2020) 11:74. doi: 10.3389/fneur.2020.00074
34. Blumenfeld, H. What is a seizure network? Long-range network consequences of focal seizures. Adv Exp Med Biol. (2014) 813:63–70. doi: 10.1007/978-94-017-8914-1_5
35. Zhao, M, Nguyen, J, Ma, H, Nishimura, N, Schaffer, CB, and Schwartz, TH. Preictal and ictal neurovascular and metabolic coupling surrounding a seizure focus. J Neurosci. (2011) 31:13292–300. doi: 10.1523/JNEUROSCI.2597-11.2011
36. Schwartz, TH, and Bonhoeffer, T. In vivo optical mapping of epileptic foci and surround inhibition in ferret cerebral cortex. Nat Med. (2001) 7:1063–7. doi: 10.1038/nm0901-1063
37. Villringer, A, Planck, J, Stodieck, S, Botzel, K, Schleinkofer, L, and Dirnagl, U. Noninvasive assessment of cerebral hemodynamics and tissue oxygenation during activation of brain cell function in human adults using near infrared spectroscopy. Adv Exp Med Biol. (1994) 345:559–65. doi: 10.1007/978-1-4615-2468-7_74
38. Peyvandi, S, Kim, H, Lau, J, Barkovich, AJ, Campbell, A, Miller, S, et al. The association between cardiac physiology, acquired brain injury, and postnatal brain growth in critical congenital heart disease. J Thorac Cardiovasc Surg. (2018) 155:e293. doi: 10.1016/j.jtcvs.2017.08.019
Keywords: cardiopulmonary bypass, focal seizures, secondarily generalized seizures, cerebral oxygen saturation, brain injuries
Citation: Lin R, Du N, Ning S, Zhang M, Feng J, Chen X, Ma L and Li J (2024) Distinct profiles of cerebral oxygenation in focal vs. secondarily generalized EEG seizures in children undergoing cardiac surgery. Front. Neurol. 15:1353366. doi: 10.3389/fneur.2024.1353366
Received: 10 December 2023; Accepted: 25 April 2024;
Published: 09 May 2024.
Edited by:
Stefano Seri, Birmingham Women’s and Children’s Hospital, United KingdomReviewed by:
William Mcdevitt, Birmingham Women’s and Children’s Hospital, United KingdomCopyright © 2024 Lin, Du, Ning, Zhang, Feng, Chen, Ma and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia Li, amlhbGlfYmVpamluZ0AxMjYuY29t; Li Ma, bGltYTE2M0AxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.