
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol., 01 May 2024
Sec. Stroke
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1348695
Objective: To systematically evaluate the efficacy and safety of repetitive transcranial magnetic stimulation (rTMS) on language function in patients with non-fluent aphasia post-stroke.
Methods: We selected randomized clinical trials (RCT) that involved stroke patients with non-fluent aphasia, whose intervention was rTMS vs. no therapy or other therapy. Two researchers autonomously reviewed the literature based on the specified criteria for inclusion and exclusion and completed the process of data extraction, data verification, and quality evaluation. Meta-analysis was performed using RevMan 5.41 and Stata MP 172, while the assessment of risk of bias was carried out utilizing the Risk of Bias version 2 tool (RoB2)3.
Results: The meta-analysis involved 47 RCTs, encompassing 2,190 patients overall. The indexes indicated that rTMS has the potential to decrease the severity of non-fluent aphasia in stroke patients, including improvement of the capability of repetition, naming, and spontaneous language. The determination of BDNF in the serum of patients was also increased. In addition, rTMS reduced the likelihood of depression in stroke patients.
Conclusion: To summarize the relevant studies, rTMS has significant effects on improving the language abilities of stroke patients suffering from non-fluent aphasia, including the abilities of repetition, naming, and spontaneous language.
Stroke remains a primary cause of mortality and morbidity globally (1, 2). Approximately 38% of adult stroke victims are subsequently diagnosed with aphasia (3, 4), which further worsens the prognosis for these patients. The severity of aphasia is useful in predicting the functional autonomy of patients, as well as their short-term and long-term recovery outcomes following a stroke (5, 6). Patients suffering from post-stroke aphasia (PSA) exhibit significantly elevated mortality rates and poorer functional outcomes compared to those without the condition (7). Therefore, rehabilitation therapy after a stroke places a paramount emphasis on the restoration of language function.
Non-fluent aphasia (NFA), frequently observed in individuals recovering from stroke, results from damage to areas encompassing the left inferior frontal gyrus (IFG-L), namely Broca’s area, along with transcortical motor, global, and mixed transcortical aphasia (8, 9). Patients with NFA may exhibit notable impairments in language production, poor sentence repetition, poor verbal agility, and errors in sentence construction (9–11). Traditional speech and language therapies (SLTs) rarely lead to the complete restoration of linguistic functions. Therefore, it is clear that patients with NFA after stroke require more adjunctive and enhancing therapies (12).
Repetitive transcranial magnetic stimulation (rTMS) utilizes magnetic fields to elicit electrical currents within targeted areas of brain. This technique modulates cortical excitability in both the stimulated areas and distant regions by delivering consistent stimuli at extremely short intervals. This process helps restore inter-hemispheric balance and allows for precise control of stimulation parameters (frequency and location), significantly affecting the functional brain network (13). rTMS induces long-lasting neuroplastic changes and facilitates network-related brain reconstruction (14). Theta-burst stimulation (TBS), an advanced form of rTMS, is subdivided into intermittent theta-burst stimulation (iTBS) and continuous theta-burst stimulation (cTBS) (15). Different rTMS stimulation frequencies yield varied effects on cerebral cortex activity: high-frequency stimulation (≥5 Hz) and iTBS enhance local neuronal excitability, whereas low-frequency stimulation (≤1 Hz) and cTBS reduce it (16).
Martin et al. (17) first reported to support language recovery, yet conclusive findings are elusive, and influenced by various factors. An important consideration is whether SLT should be paired with rTMS. Several studies suggested that when employed as a standalone therapy, rTMS holds promise in producing language improvements in PSA (6, 18–20). On the contrary, the suggestion that rTMS could prime the brain for behavioral therapy, implying that it should be integrated with SLT, is met with challenges. Heterogeneity in SLT types and intensities among recent studies used alongside rTMS (21–24) contribute to the complexity of this argument. It is challenging to precisely define the individual contributions of rTMS and SLT and assess their collective impact on PSA rehabilitation that rTMS could offer a unique, complementary approach to treating aphasia. Current research into PSA rehabilitation has used rTMS to modulate interhemispheric interaction.
Numerous randomized controlled trials (RCTs) (25) suggest that rTMS may aid in the reconstruction and recovery of language abilities in individuals with PSA. Six systematic reviews (25–30) evaluated the impact of rTMS on PSA, with most reaching inconsistent conclusions or having loose exclusion criteria; the types of aphasia in patients were also ambiguously defined. Georgiou et al. (31) utilized the AMSTAR 2 tool to evaluate the quality of systematic reviews of RCTs focusing on the effectiveness of rTMS in aphasia rehabilitation following stroke before July 2017 and found that the quality of these studies was generally low. Another meta-analysis (32) identified the types of NFA in stroke patients, but the included literature was outdated. Consequently, we conducted a systematic review to furnish evidence-based information regarding the application of rTMS in treating NFA following a stroke. This involved analyzing a large number of studies and more relevant outcome indices, to identify new research directions.
This systematic review adheres to the guidelines set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (33). And the study protocol has been officially registered in PROSPERO (ID CRD42023434714).
Our PICO question was: in stroke patients with NFA, does rTMS, as compared to the absence of therapy or alternative treatments, reduce the severity of aphasia in patients, including naming, spontaneous language, and repetition abilities?
We searched nine commonly used electronic databases: PubMed, Cochrane Library, Embase, Web of Science, SinoMed, OVID, the China National Knowledge Infrastructure (CNKI), China Science and Technology Journal Database (VIP), and Wanfang Data for RCTs of rTMS for stroke patients with NFA. Furthermore, relevant systematic evaluations and reference lists of included studies were searched manually to ensure the comprehensiveness of included studies. Keywords were determined after preretrieval: repetitive transcranial magnetic stimulation, rTMS, TBS, stroke, cerebrovascular accident, aphasia, non-fluent aphasia, and post-stroke aphasia. The final literature search was conducted on January 8, 2024, using neither language nor publication date restrictions.
Inclusion criteria (1) study design: RCTs; (2) study population: individuals with NFA following a clinical diagnosis of stroke; (3) interventions: In addition to the interventions applied to the control group, the experimental group underwent rTMS. Alternatively, the experimental group received rTMS, whereas the control group received sham-rTMS. For studies encompassing more than two groups, the groups fulfilling the inclusion criteria were also included; (4) the outcome indicators ought to incorporate at least one metric of aphasia assessment, such as Western Aphasia Battery (WAB), Aphasia Battery of Chinese (ABC), China Rehabilitation Research Center Standard Aphasia Examination (CRRCAE), and Boston Naming Test (BNT).
Exclusion criteria: (1) duplicate studies or data cannot be extracted; (2) case studies, animal experimental studies, or reviews; (3) the unavailability of full text even after contacting the author via email.
Endnote X9 was utilized for document organization and deduplication. Two reviewers independently conducted literature screening, data extraction, and cross-verification according to predetermined inclusion and exclusion criteria. Any discrepancies between the reviewers were resolved by achieving a consensus with an unbiased third-party researcher. The process of extracting data entailed gathering details regarding the title, first author, publication year, the number of patients, diagnostic criteria, intervention and control protocols, rTMS parameters (e.g., stimulation site, frequency, intensity, and duration), outcome measures, any reported adverse events and follow-up duration.
Risk of bias assessment for the studies included was carried out utilizing the RoB2 tool (34). Two researchers evaluated the risk of bias independently, with any discrepancies being resolved by a third researcher.
Meta-analysis was conducted utilizing Revman 5.4 software. The mean differences (MD) along with the 95% confidence interval (CI) were employed for statistical analysis, and the standardized mean difference (SMD) was used when using different measurement methods or units. Statistically significant differences were indicated by p < 0.05, and the magnitude of heterogeneity was quantified using I2. I2 shows the proportion of heterogeneity in the total variation of effect size based on the Student–Newman–Keuls test, ranging from 0 to 100%. I2 ≤ 50% was deemed as an indication of low heterogeneity, employing the fixed-effects model for Meta-analysis; I2 > 50% was considered a clear indication of significant heterogeneity, utilizing the random-effects model.
The initial search returned 1,244 articles. Following the removal of duplicate articles, 607 studies were left, and 59 studies remained upon reviewing the titles and abstracts. Finally, 47 studies were included after a rigorous evaluation of the full-text articles (Supplementary Figure S1).
Out of the 48 studies (4, 23, 35–80), 37 studies (35–37, 39–49, 51, 52, 54–66, 68, 69, 72–74, 76, 78, 79) had reported diagnostic criteria for stroke and 35 (4, 35–39, 41–53, 55, 57–60, 62–65, 67–70, 74, 76, 77) for aphasia. In 43 studies (4, 23, 35–52, 54–65, 67–76, 78, 80), the age of the patient was reported as mean ± standard deviation, while two studies (53, 66) reported specific age information for each patient, and the age range was reported in two studies (77, 79). Except for two studies (50, 59) that failed to disclose the post-stroke time, the mean course of disease for patients in the other 44 studies (4, 23, 35–49, 51, 52, 54–58, 60–65, 67–80) ranged from 6.9 days to 4.46 years, and two studies (53, 66) reported specific course of disease for each patient. Of the total studies, 39 studies (4, 35–43, 45–48, 51–55, 58–70, 72–74, 77–80) specifically reported that the patients were right-handed, whereas the remaining 9 studies (23, 44, 49, 50, 56, 57, 71, 75, 76) failed to furnish this information. Duration of the intervention in 45 studies (4, 23, 35, 36, 38–40, 42–46, 48–80) spanned from 2 to 4 weeks, except for 2 studies (37, 41) with an intervention duration of 30 days and one (47) with 8 weeks. Regarding the content of the intervention, five studies (49, 50, 59, 71, 80) used rTMS or sTMS alone, and the remaining studies also had aphasia treatment components including SLT, acupuncture, and electroacupuncture (EA). Patients were followed up after treatment in 15 studies (4, 39, 40, 53, 57, 66, 67, 70–74, 77, 78, 80), ranging from 30 days to 12 months.
Adverse events were mentioned in 10 studies (4, 40, 42, 45, 51, 57, 60, 61, 73, 77). Six studies (4, 40, 42, 45, 60, 73) reported headache; three (40, 45, 51) reported dizziness; adverse effects of epilepsy seizures were seen in two studies (61, 77). One study (57) documented the occurrence of adverse events such as disorientation, injuries resulting from falls, and aspiration caused by dysphagia among both control and experimental participants, the ratio of aspiration was the highest in the experimental group (3 cases), and the control group (11 cases), which revealed that the combination of rTMS and speech training could reduce the complications and improve the therapeutic efficiency.
Regarding the NFA type, 16 studies (23, 37, 41, 42, 44, 45, 47–49, 54, 56, 57, 61, 74, 78, 79) included patients with Broca aphasia. Four studies (36, 53, 63, 64) included patients with global aphasia, and 5 studies (39, 43, 72, 73, 76) reported on the number of patients with different types of aphasia. The aphasia types of patients in the three studies (43, 72, 73) were global, Broca’s, and transcortical motor aphasia, and Wang’s (73) study included patients with mixed transcortical aphasia. In addition to the intervention received by the control group, the experimental group in one study (48) received high-frequency TMS (HF-rTMS) on the IFG-L. One study (67) performed bilateral cerebral pulse stimulation, with HF-rTMS (> 1 Hz) stimulating the left cerebral Broca area and low-frequency TMS (≤1 Hz) (LF-rTMS) stimulating the right Broca area; three studies (38, 46, 56) stimulated the right inferior frontal gyrus (IFG-R) with 0.5 Hz. Of the 40 studies with a frequency of 1 Hz, except for one study (53) in which the stimulation site was the right superior temporal gyrus, the stimulation site was the IFG-L. Three of these studies (32, 66, 80) further stimulated Brodmann 45 (pars triangularis) at the site of the IFG-R partition. Three studies (43, 58, 77) proceeded theta burst stimulation (TBS) on patients, one (77) conducted passive cTBS on the cerebellum in patients of the experimental group, and the other two (43, 58) conducted iTBS on the IFG-L. The critical characteristics of the included studies have been summarized in Table 1.
The outcomes of the risk-of-bias assessments conducted for each included study are shown in Supplementary Figures S2A,B.
Potential publication bias across the included studies was evaluated based on Egger’s tests in Stata MP 17, with a significance level set at p < 0.1 (Supplementary Table S1).
A total of 12 studies were excluded from this meta-analysis due to the following reasons. Specifically, the outcomes of two studies (71, 73) were shown as bar graphs, and two (66, 74) were presented as line charts rather than specific values. Individual outcome indicators in four studies (56, 58, 67, 74) could not be integrated with other studies. Zheng’s (77) date was unacquirable. Data in three studies (62–64) of Zhu was described by Median and quartile which could not be counted. Finally, a total of 35 studies were included.
Fifteen studies (23, 36–38, 41, 42, 44, 46, 51, 52, 57, 59, 61, 65, 75) involving 960 patients assessed aphasia quotient (AQ) in patients after rTMS stimulation. The random-effects model was utilized due to significant heterogeneity across the studies (I2 = 78%). Global language ability of the rTMS-treated group exhibited a substantial improvement in comparison to the control group [p < 0.00001] (Figure 1). Subgroup analysis was conducted according to the stroke stages of patients after removing a study (50) with unidentified stroke duration and another study (75) that patients were in the sequelae stage. The random-effects model was employed for the following studies: three studies (37, 41, 44) with patients in the acute phase [SMD = 1.41, 95% CI (0.91, 1.91), p < 0.00001, I2 = 78%] and 11 studies (23, 36, 38, 42, 46, 51, 52, 57, 59, 61, 65) with patients in the recovery stage [p < 0.00001, I2 = 77%] (Figure 2). Upon conducting sensitivity analysis, the removal of two studies (36, 57) from the subgroup of recovery, the following outcomes were observed: 9 studies (23, 38, 42, 46, 51, 52, 59, 61, 65) of recovery subgroup [p < 0.00001, I2 = 0%] (Figure 3). The findings suggest that rTMS has the potential to enhance the overall language abilities of stroke patients during both acute and recovery phases.
Twenty-two studies (23, 36–39, 41–44, 46, 48–52, 55, 59, 61, 65, 68, 69, 75) involving 1,228 patients assessed repetition ability in patients after rTMS treatment. The random-effects model was utilized due to significant heterogeneity across the studies (I2 = 85%). Repetition ability of rTMS-treated group exhibited a substantial improvement in comparison to the control group [p < 0.00001] (Figure 4). Subgroup analysis was conducted according to the stroke stages of patients after removing a study (50) with unidentified stroke duration. The random-effects model was employed for the following studies: four studies (37, 41, 44, 59) with patients in the acute stage [p < 0.00001, I2 = 84%]. The fixed-effects model was employed for the following studies: 14 studies (23, 36, 38, 39, 42, 43, 46, 48, 49, 51, 52, 55, 61, 65) with patients in the recovery stage [p < 0.00001, I2 = 41%] and three studies (68, 69, 75) with patients in the sequelae stage [p = 0.005, I2 = 0%] (Figure 5). These findings suggest that rTMS enhance repetition abilities in patients across various stages of stroke recovery. Notably, beyond immediate benefits, rTMS appears to exert medium- to long-term effects on language improvement. For individuals with NFA post-stroke, the positive effects of rTMS on speech enhancement persisted for up to 12 months (66).
Twenty-two (23, 36–38, 41–44, 46, 48–52, 55, 59–61, 65, 68, 69, 75) studies involving 1,229 patients evaluated naming ability in patients after rTMS. The random-effects model was utilized due to significant heterogeneity across the studies (I2 = 86%). Naming ability of the rTMS-treated group exhibited a substantial improvement in comparison to the control group [p < 0.00001] (Figure 6). Subgroup analysis was conducted according to the stroke stages of patients after removing a study (50) with unidentified stroke duration. The random-effects model was employed for the following studies: four studies (37, 41, 44, 59) with patients in the acute stage [p < 0.00001, I2 = 83%]. The fixed-effects model was employed for the following studies: 14 studies (23, 36, 38, 42, 43, 46, 48, 49, 51, 52, 55, 59, 61, 65) with patients in the recovery stage of [p < 0.00001, I2 = 1%] and three studies (68, 69, 75) with patients in the sequelae stage [p = 0.0003, I2 = 0%] (Figure 7). Naming is among the most challenging functions for stroke patients with NFA to regain. The restoration of naming capabilities necessitates the involvement of extensive neural networks, and rTMS has been shown to facilitate the recovery of these abilities across various stages of stroke, primarily by enhancing the connectivity among pertinent brain areas (65).
Seventeen studies (4, 23, 36–38, 41, 42, 44, 46, 48, 50–52, 55, 59, 61, 65) involving 1,046 patients assessed spontaneous language ability in patients after rTMS stimulation. The random-effects model was utilized due to significant heterogeneity across the studies (I2 = 67%). Spontaneous language ability of the rTMS-treated group exhibited a substantial improvement in comparison to the control group [p < 0.00001] (Figure 8). Subgroup analysis was conducted according to the stroke stages of patients after removing a study (50) with unidentified stroke duration and another study (75) that patients were in the sequelae stage. The random-effects model was employed for the following studies: four studies (37, 41, 44, 59) with patients in the acute stage [p < 0.00001, I2 = 64%]. The fixed-effects model was utilized for the following studies: 11 studies (23, 36, 38, 42, 46, 48, 51, 52, 55, 61, 65) with patients in the recovery stage [p < 0.00001, I2 = 0%] (Figure 9). These results suggested that rTMS ameliorated spontaneous language capability in patients in acute and recovery stages. Acupuncture therapy constitutes a significant component of complementary and alternative medicine, which has potential therapeutic effects in the treatment of PSA (81). The combined therapeutic application of acupuncture and rTMS on NFA has demonstrated a markedly superior efficacy compared to monotherapy. Specifically, the overall effectiveness of combining 1 Hz rTMS with acupuncture for NFA reached 96.66%, strongly associated with enhanced blood flow velocity and perfusion in the left middle cerebral artery (MCA) (44). Interaction between various acupuncture techniques and rTMS frequencies in producing distinct clinical outcomes warrants further investigation.
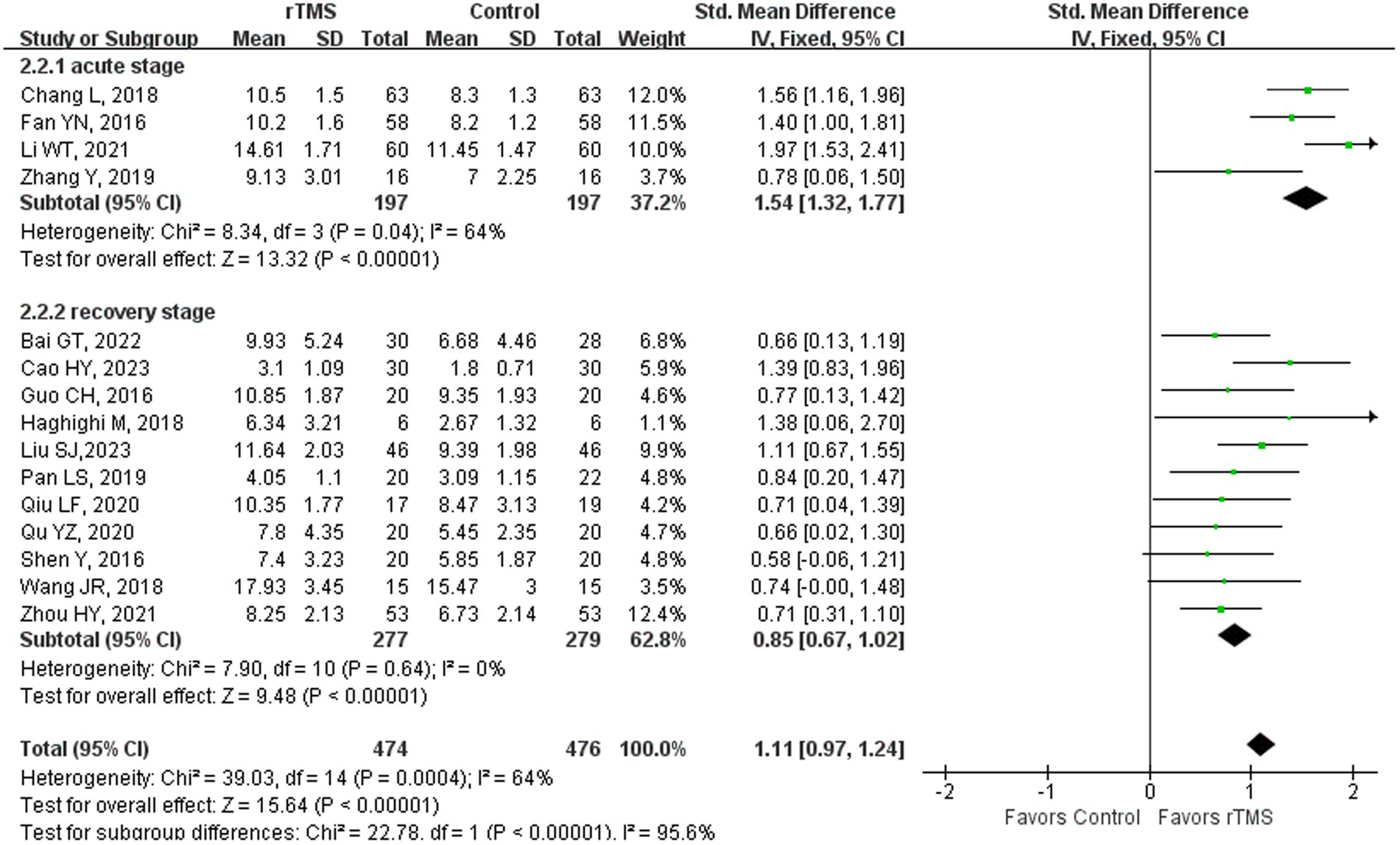
Figure 9. Spontaneous language capability after rTMS subgroup analysis according to different stroke stages.
Two studies (43, 65) involving 103 patients assessed the concentration of brain-derived neurotrophic factor (BDNF) within patients. The fixed-effects model was utilized due to low heterogeneity across the studies (I2 = 0%). The rTMS-treated group exhibited a substantial enhancement in serum BDNF concentration in comparison to the control group [p < 0.00001] (Figure 10). The findings indicated that rTMS has the potential to elevate the concentration of BDNF in the serum of stroke patients.
Two studies (48, 49) involving 84 patients used the Hamilton Depression Scale (HAMD) to evaluate the effects of rTMS on mood in stroke patients. The fixed-effects model was utilized due to low heterogeneity across the studies (I2 = 0%). The HAMD score of rTMS group was notably lower in comparison to the control group [p = 0.0005] (Figure 11). The findings indicated that rTMS had potential to ameliorate the depression of these patients.
Numerous research groups, including those led by Naeser, Hamilton, Heiss, and Thiel, have showcased the effectiveness of rTMS in the treatment of PSA through significant studies. Despite their contributions, these studies were excluded from the review for various reasons. Hamilton’s research indicated that rTMS offers sustained enhancements in picture naming and fluency in patients with NFA. However, limitations included the absence of a control group in one study (82) and crossover of sham group participants to actual rTMS treatment in another (71). Heiss and Thiel observed a delayed beneficial impact of LF-rTMS on the right pars triangularis (R IFG pr) in enhancing naming ability in subacute PSA patients, yet these individuals were not suffering from NFA (83–87). The Naeser team reported improvements in Phrase Length and picture naming on the BNT following the suppression of the posterior R IFG pr through the application of LF-rTMS in NFA patients. Nevertheless, the exclusion was due to the absence of control groups not comprised of stroke patients, rendering it impossible to extract and cross-reference assessment data (88–93).
The present study aimed to provide an updated overview of the current evidence about the efficacy of rTMS in treating NFA. Firstly, this review found that rTMS could improve NFA in stroke patients, evidenced by increased aphasia quotient (AQ) scores in the rTMS group. AQ is an indicator of aphasia severity and serves as a metric for assessing aphasia improvement (94). Subgroup analysis revealed that rTMS significantly enhanced repetition and naming abilities in stroke patients at various stages. Additionally, spontaneous language improvement was noted in both acute and recovery phase patients, although the effects in the sequelae stage require further investigation. In our pursuit to examine studies focusing on the impact of rTMS on language skills, we discovered that several of these studies provided valuable insights beyond our initial scope. Interestingly, this exploration led us to understand that depression alleviation and the increase of BDNF may also benefit from rTMS. Admittedly, these findings are only based on 2 studies (each), and further research is necessary.
Stroke damages brain regions responsible for language expression and auditory comprehension, leading to aphasia, which in turn worsens functional outcomes (95). Aphasia improvement is linked to the rebalancing of activity between the perilesional ipsilateral and contralateral hemispheres, making rTMS a promising method for promoting language recovery (5, 96). The meta-analysis primarily focused on single-site and LF-rTMS stimulation, uncovering a notable association between the activation level of the IFG-R and patients’ fluency (97). Lefaucheur et al. (16) proposed Level B evidence supporting the utilization of LF-rTMS on the IFG-R in patients suffering from NFA, especially when combined with SLT. Both HF-rTMS and LF-rTMS applied to one hemisphere, have demonstrated effectiveness in treating NFA (13). HF-rTMS enhances cerebral cortex excitability and revives bilateral cerebral hemisphere function by stimulating local neurons in the language center (98). However, HF-rTMS can cause intracranial hemorrhage and epilepsy, leading to its limited use in clinical and research settings. In one study (67), bilateral hemispheric stimulation (LF-rTMS applied to the right unaffected Broca’s area and HF-rTMS targeting the left affected Broca’s area) led to significant language function improvements, including repetition, naming, word comprehension, and fluency. These improvements were observed immediately after treatment and lasted for 2 months. Similarly, Vuksanović et al. (99) observed that the integration of cTBS applied to the right hemisphere with iTBS targeting the left, followed by 45 min of SLT, improved various language functions.
Aphasia, a neural network disorder, involves changes in the brain’s functional connections. The reinstatement of the language network structure and function is crucial for restoring language abilities in individuals with aphasia (100). Regional homogeneity (Reho) analysis revealed that in aphasia patients, the activation of the IFG-L and left cuneiform lobe was lower compared to normal subjects, and there was a reduction in the functional connection between the left medial temporal gyrus (MTG-L) and superior temporal gyrus (STG-L) (101). rTMS has been demonstrated to enhance the functional connections between the bilateral frontal lobes and the left temporal lobe (35). Lin et al. (69) investigated the relationship between functional connectivity in language-related regions and language performance. The LF-rTMS group demonstrated significant functional connectivity remodeling, which fostered positive changes in brain plasticity. This aligns with the theory of improving the “Inter-hemispheric competition pattern” (102). Therefore, imaging analysis holds a crucial role in comprehensively assessing disruption and remodeling of the language network following a stroke.
Regarding the risk of bias, Egger’s test results indicated that publication bias was not significant in the language domains such as repetition and overall language proficiency (p > 0.1). However, there was a significant presence of bias in naming and spontaneous speech categories (p < 0.1). Of the seven domains assessed by RevMan 5.4, the domain of random sequence generation exhibited the highest risk of bias.
The review also indicated that rTMS can enhance linguistic functions by increasing serum BDNF levels. BDNF, the most abundant neurotrophin in the cerebrum and predominantly found in the forebrain, is closely associated with cognitive and language functions. It plays a crucial role in facilitating the neuroplasticity process in PSA patients (65). LF-rTMS (43) and iTBS (65) have been shown to elevate BDNF levels in the peripheral serum of the rTMS group, reflecting changes in brain BDNF concentration. Post-stroke depression (PSD) affects 30–60% of stroke survivors (103), which also relates to the language performance of stroke patients (10). Recognized as an effective treatment for depression, rTMS is supported by level A evidence (16). The meta-analysis demonstrated that both LF-rTMS and HF-rTMS significantly reduced depression scores and improved mood in the meta-analysis. Our findings indicate that lower HAMD scores correlate with better linguistic function performance. In conclusion, notable improvements in mood or serum BDNF from rTMS in NFA patients positively influence linguistic functions.
Recognition of numerous constraints is essential in this systematic review. Firstly, the high proportion of Chinese literature may cause some publication bias. Secondly, clinical and methodological heterogeneity among the included literature may influence overall results, including the age of patients and varied rTMS treatment regimens. Thirdly, owing to restrictions on article length, only some meta-analysis findings were reported. In the future, we can discuss the therapeutic effects of different dosages of rTMS and different rTMS approaches, and design accurate rTMS parameters according to aphasia types, combined with functional imaging technology to further explore the related mechanisms.
Collectively, the reviewed literature provides compelling support for the utilization of rTMS as a viable non-pharmacological intervention aiding in the recovery of non-fluent aphasia post-stroke, including the ability of repetition, naming, and spontaneous language which may be accompanied by the improvement of serum BDNF and alleviation in depression in patients.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
JC: Writing – original draft. YJ: Methodology, Writing – review & editing, Formal analysis. TR: Data curation, Writing – review & editing, Software. YY: Supervision, Writing – review & editing. YL: Supervision, Writing – review & editing. YZ: Writing – review & editing. SY: Writing – review & editing, Funding acquisition, Visualization.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (Grant no. 82074513) and the Natural Science Foundation of Fujian Province of China (Grant no. 2021J01955).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1348695/full#supplementary-material
1. Greco, A, Occhipinti, G, Giacoppo, D, Agnello, F, Laudani, C, Spagnolo, M, et al. Antithrombotic therapy for primary and secondary prevention of ischemic stroke: JACC state-of-the-art review. J Am Coll Cardiol. (2023) 82:1538–57. doi: 10.1016/j.jacc.2023.07.025
2. Zhang, Y, He, X, Hu, S, Hu, S, He, F, Shen, Y, et al. Efficacy and safety of massage in the treatment of post-stroke insomnia: a protocol for systematic review and meta-analysis. Medicine. (2020) 99:e23598. doi: 10.1097/MD.0000000000023598
3. Vitti, E, and Hillis, AE. Treatment of post-stroke aphasia: a narrative review for stroke neurologists. Int J Stroke. (2021) 16:1002–8. doi: 10.1177/17474930211017807
4. Low, TA, Lindland, K, Kirton, A, Carlson, HL, Harris, AD, Goodyear, BG, et al. Repetitive transcranial magnetic stimulation (RTMS) combined with multi-modality aphasia therapy for chronic post-stroke non-fluent aphasia: a pilot randomized sham-controlled trial. Brain Lang. (2023) 236:105216. doi: 10.1016/j.bandl.2022.105216
5. Crosson, B, Rodriguez, AD, Copland, D, Fridriksson, J, Krishnamurthy, LC, Meinzer, M, et al. Neuroplasticity and aphasia treatments: new approaches for an old problem. J Neurol Neurosurg Psychiatry. (2019) 90:1147–55. doi: 10.1136/jnnp-2018-319649
6. Georgiou, AM, and Kambanaros, M. The effectiveness of transcranial magnetic stimulation (TMS) paradigms as treatment options for recovery of language deficits in chronic Poststroke aphasia. Behav Neurol. (2022) 2022:1–25. doi: 10.1155/2022/7274115
7. Poslawsky, IE, Schuurmans, MJ, Lindeman, E, and Hafsteinsdóttir, TB. A systematic review of nursing rehabilitation of stroke patients with aphasia. J Clin Nurs. (2010) 19:17–32. doi: 10.1111/j.1365-2702.2009.03023.x
8. Sinanović, O, Mrkonjić, Z, Zukić, S, Vidović, M, and Imamović, K. Post-stroke language disorders. Acta Clin Croat. (2011) 50:79–94.
9. Bonilha, L, Hillis, AE, Wilmskoetter, J, Hickok, G, Basilakos, A, Munsell, B, et al. Neural structures supporting spontaneous and assisted (entrained) speech fluency. Brain. (2019) 142:3951–62. doi: 10.1093/brain/awz309
10. Sheppard, SM, and Sebastian, R. Diagnosing and managing post-stroke aphasia. Expert Rev Neurother. (2021) 21:221–34. doi: 10.1080/14737175.2020.1855976
11. Haro-Martínez, A, Pérez-Araujo, CM, Sanchez-Caro, JM, Fuentes, B, and Díez-Tejedor, E. Melodic intonation therapy for post-stroke non-fluent aphasia: systematic review and Meta-analysis. Front Neurol. (2021) 12:700115. doi: 10.3389/fneur.2021.700115
12. Marchina, S, Norton, A, and Schlaug, G. Effects of melodic intonation therapy in patients with chronic nonfluent aphasia. Ann N Y Acad Sci. (2023) 1519:173–85. doi: 10.1111/nyas.14927
13. Starosta, M, Cichoń, N, Saluk-Bijak, J, and Miller, E. Benefits from repetitive transcranial magnetic stimulation in post-stroke rehabilitation. J Clin Med. (2022) 11:2149. doi: 10.3390/jcm11082149
14. Hara, T, Shanmugalingam, A, McIntyre, A, and Burhan, AM. The effect of non-invasive brain stimulation (nibs) on attention and memory function in stroke rehabilitation patients: a systematic review and Meta-analysis. Diagnostics. (2021) 11:227. doi: 10.3390/diagnostics11020227
15. Kesikburun, S . Non-invasive brain stimulation in rehabilitation. Turk J Phys Med Rehabil. (2022) 68:1–8. doi: 10.5606/tftrd.2022.10608
16. Lefaucheur, JP, Aleman, A, Baeken, C, Benninger, DH, Brunelin, J, di Lazzaro, V, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (RTMS): an update (2014-2018). Clin Neurophysiol. (2020) 131:474–528. doi: 10.1016/j.clinph.2019.11.002
17. Martin, PI, Naeser, MA, Theoret, H, Tormos, JM, Nicholas, M, Kurland, J, et al. Transcranial magnetic stimulation as a complementary treatment for aphasia. Semin Speech Lang. (2004) 25:181–91. doi: 10.1055/s-2004-825654
18. Chou, TY, Wang, JC, Lin, MY, and Tsai, PY. Low-frequency vs. Theta burst transcranial magnetic stimulation for the treatment of chronic non-fluent aphasia in stroke: a proof-of-concept study. Front Aging Neurosci. (2022) 13:800377. doi: 10.3389/fnagi.2021.800377
19. Fahmy, EM, and Elshebawy, HM. Effect of high frequency transcranial magnetic stimulation on recovery of chronic post-stroke aphasia. J Stroke Cerebrovasc Dis. (2021) 30:105855. doi: 10.1016/j.jstrokecerebrovasdis.2021.105855
20. Georgiou, A, Konstantinou, N, Phinikettos, I, and Kambanaros, M. Neuronavigated theta burst stimulation for chronic aphasia: two exploratory case studies. Clin Linguist Phon. (2019) 33:532–46. doi: 10.1080/02699206.2018.1562496
21. Ren, C, Zhang, G, Xu, X, Hao, J, Fang, H, Chen, P, et al. The effect of RTMS over the different targets on language recovery in stroke patients with global aphasia: a randomized sham-controlled study. Biomed Res Int. (2019) 2019:1–7. doi: 10.1155/2019/4589056
22. Hu, XY, Zhang, T, Rajah, GB, Stone, C, Liu, LX, He, JJ, et al. Effects of different frequencies of repetitive transcranial magnetic stimulation in stroke patients with non-fluent aphasia: a randomized, sham-controlled study. Neurol Res. (2018) 40:459–65. doi: 10.1080/01616412.2018.1453980
23. Haghighi, M, Mazdeh, M, Ranjbar, N, and Seifrabie, MA. Further evidence of the positive influence of repetitive transcranial magnetic stimulation on speech and language in patients with aphasia after stroke: results from a double-blind intervention with sham condition. Neuropsychobiology. (2017) 75:185–92. doi: 10.1159/000486144
24. Hebert, D, Lindsay, MP, McIntyre, A, Kirton, A, Rumney, PG, Bagg, S, et al. Canadian stroke best practice recommendations: stroke rehabilitation practice guidelines, update 2015. Int J Stroke. (2016) 11:459–84. doi: 10.1177/1747493016643553
25. Zhang, J, Zhong, D, Xiao, X, Yuan, L, Li, Y, Zheng, Y, et al. Effects of repetitive transcranial magnetic stimulation (rtms) on aphasia in stroke patients: a systematic review and meta-analysis. Clin Rehabil. (2021) 35:1103–16. doi: 10.1177/0269215521999554
26. Arheix-Parras, S, Barrios, C, Python, G, Cogné, M, Sibon, I, Engelhardt, M, et al. A systematic review of repetitive transcranial magnetic stimulation in aphasia rehabilitation: leads for future studies. Neurosci Biobehav Rev. (2021) 127:212–41. doi: 10.1016/j.neubiorev.2021.04.008
27. Gholami, M, Pourbaghi, N, and Taghvatalab, S. Evaluation of rtms in patients with poststroke aphasia: a systematic review and focused meta-analysis. Neurol Sci. (2022) 43:4685–94. doi: 10.1007/s10072-022-06092-x
28. Kielar, A, Patterson, D, and Chou, YH. Efficacy of repetitive transcranial magnetic stimulation in treating stroke aphasia: systematic review and meta-analysis. Clin Neurophysiol. (2022) 140:196–227. doi: 10.1016/j.clinph.2022.04.017
29. Li, T, Zeng, X, Lin, L, Xian, T, and Chen, Z. Effects of repetitive transcranial magnetic stimulation with different frequencies on post-stroke aphasia: a Prisma-compliant meta-analysis. Medicine. (2020) 99:e20439. doi: 10.1097/MD.0000000000020439
30. Wang, C, Nie, P, Wang, P, Wang, Y, Zang, Y, and Zhang, Y. The therapeutic effect of transcranial magnetic stimulation on post-stroke aphasia and the optimal treatment parameters: a meta-analysis. Arch Phys Med Rehabil. (2023). doi: 10.1016/j.apmr.2023.11.006
31. Georgiou, AM, Lada, E, and Kambanaros, M. Evaluating the quality of conduct of systematic reviews on the application of transcranial magnetic stimulation (TMS) for post-stroke aphasia rehabilitation. Aphasiology. (2019) 34:540–56. doi: 10.1080/02687038.2019.1632786
32. Zheng, Y, Liu, J, Wang, Y, Xing, J, Gu, Y, and Tang, J. Low-frequency repetitive transcranial magnetic stimulation for non-fluent aphasia after stroke: a systematic review. Chin J Rehabil. (2021) 36:365–71.
33. Page, MJ, McKenzie, J, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The Prisma 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
34. Sterne, JAC, Savović, J, Page, MJ, Elbers, RG, Blencowe, NS, Boutron, I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
35. Bai, G, Jiang, L, Sun, D, Meng, P, Yang, CH, Zhang, Z, et al. Based on the regional homogeneity method to explore the effect and mechanism of low-frequency repetitive transcranial magnetic stimulation on the auditory comprehension function of post- stroke patients with non-fluent aphasia. Chin J Rehabil Med. (2023) 38:606–12. doi: 10.3969/j.issn.1001-1242.2023.05.004
36. Cao, H, Zhang, W, Xu, T, and Cui, X. Effect of eye tracking training combined with repetitive transcranial magnetic stimulation on post-stroke complete aphasia. Chin J Rehabil. (2023) 38:552–5. doi: 10.3870/zgkf.2023.09.009
37. Chang, L . Clinical observation of low frequency repetitive transcranial magnetic stimulation on motor aphasia after acute cerebral infarction. Neural Injury Funct Reconstr. (2018) 13:53–4. doi: 10.16780/j.cnki.sjssgncj.2018.01.020
38. Shen, Y, Yin, Z, Zhou, Q, Cong, F, Yi, W, and Shan, CH. Low frequency, repetitive transcranial magnetic stimulation can alleviate non-fluent aphasia after stroke. Chin J Phys Med Rehabil. (2016) 3:170–4. doi: 10.3760/cma.j.issn.0254-1424.2016.03.003
39. Chen, Y, Gao, H, Wang, X, Zhou, Y, Ou, X, and Pan, C. Effect of low-frequency RTMS on rehabilitation of language and communication ability in patients with non-fluent aphasia after stroke. Chin J Gerontol. (2020) 40:709–12. doi: 10.3969/j.issn.1005-9202.2020.04.011
40. Shan, Y, Wang, L, Wang, J, Luo, Z, and Zeng, X. The effect of low frequency transcranial magnetic stimulation on aphasia after cerebral infarction. Chin J Phys Med Rehabil. (2012) 5:361–4. doi: 10.3760/cma.j.issn.0254-1424.2012.05.01
41. Fan, Y, and Zhao, J. Therapeutic effect of low-frequency repetitive transcranial magnetic stimulation on motor aphasia after acute cerebral infarction. Chin J Rehabil. (2016) 31:28–30. doi: 10.3870/zgkf.2016.01.008
42. Guo, C, Zhu, G, Deng, W, Ma, T, Du, A, and Zhao, Z. Effect of repetitive transcranial magnetic stimulation combined with memantine hydrochloride and speech therapy on the treatment of motor aphasia after cerebral infarction. China Med Herald. (2016) 13:83–6.
43. Jiang, X, Liu, Z, Su, Q, Zhao, Q, Xia, X, and Lu, F. Effect of intermittent theta burst transcranial magnetic stimulation on non-fluent aphasia after stroke. Chin J Rehabil Theory Pract. (2023) 29:839–43. doi: 10.3969/j.issn.1006.9771.2023.07.014
44. Li, W, Qin, H, Wei, X, and Zhao, P. Clinical observation on the treatment of post-stroke motor aphasia with consciousness-restoring resuscitation acupuncture combined with transcranial magnetic stimulation. J Pract Trad Chin Med. (2021) 37:858–60.
45. Li, Z, Zhao, Y, Ren, C, Cai, D, Wu, SH, and Fang, H. Mechanism in the treatment of subacute motor aphasia with low frequency repetitive transcranial magnetic stimulation by quantitative electroencephalography. Chin J Rehabil Med. (2018) 33:794–9. doi: 10.3969/j.issn.1001-1242.2018.07.008
46. Liu, S, Lv, M, Wang, Y, Liang, J, and Li, T. Contralateral inhibitory repetitive transcranial magnetic stimulation combined with donepezil in the treatment of post-stroke non-fluent aphasia. J Audiol Speech Pathol. (2023) 31:165–9. doi: 10.3969/j.issn.1006-7299.2023.02.016
47. Liu, S, and Hu, X. Clinical observation of acupuncture combined with repetitive transcranial magnetic stimulation in the treatment of motor aphasia after stroke. Clin J Trad Chin Med. (2022) 34:2146–50. doi: 10.16448/j.cjtcm.2022.1135
48. Pan, L, Ma, J, Peng, Y, Sun, J, and Zhu, B. Electro-acupuncture combined with high frequency repetitive transcranial magnetic stimulation in the treatment of motor aphasia after stroke. China J Chin Med. (2019) 34:1985–9. doi: 10.16368/j.issn.1674-8999.2019.09.463
49. Qiao, Y, Ma, J, Peng, Y, Sun, J, and Zhu, B. Clinical study of electro-acupuncture combined with Low- frequency rtms on motor aphasia after stroke. J Clin Acupunct Moxibust. (2019) 35:15–9. doi: 10.3969/j.issn.1005-0779.2019.10.005
50. Qin, Q . Efficacy of low-frequency repetitive transcranial magnetic stimulation combined with hyperbaric medicine aphasia after stroke. Med Diet Health. (2020) 18:53–4.
51. Qiu, L, Cai, Y, Jiang, Y, Lin, W, and Li, X. Effects of response elaboration training combined with RTMS in patients with non-fluent aphasia. Chin J Rehabil Med. (2020) 35:1192–7. doi: 10.3969/j.issn.1001-1242.2020.10.007
52. Qu, Y, Zhong, G, Lv, J, et al. Observation on the efficacy of low frequency repetitive transcranial magnetic stimulation in the treatment of non-fluent aphasia after subacute stroke. China Modern Doctor. (2020) 58:112–5.
53. Ren, C, Cai, D, Fang, H, Chen, P, Li, ZH, and Wu, SH. Effects of low frequency rtms over the right superior temporal gyrus on language function in subacute global poststroke aphasia patients. J Rehabil Med. (2018) 33:1055–9. doi: 10.3969/j.issn.1001-1242.2018.09.009
54. Wang, G, Liu, M, Sheng, Y, He, SH, Xie, J, and Long, J. Clinical study of RTMS combined with speech training in the treatment of aphasia after stroke. Shenzhen J Integr Trad Chin Western Med. (2021) 31:1–4. doi: 10.16458/j.cnki.1007-0893.2021.06.001
55. Wang, J, Li, J, Zhang, Y, Bai, G, Han, CH, Yang, CH, et al. Effect of low frequency repetitive transcranial magnetic stimulation on non-fluent aphasia after stroke. Chin J Rehabil Med. (2018) 33:1463–4. doi: 10.3969/j.issn.1001-1242.2018.12.018
56. Wu, G, Yang, H, and Pan, L. Effect and mechanism of repetitive transcranial magnetic stimulation on aphasia in patients with left hemisphere cerebral infarction. Shandong Med J. (2017) 57:65–7. doi: 10.3969/j.issn.1002-266X.2017.37.022
57. Xu, D, and Liu, H. Effect of repetitive transcranial magnetic stimulation combined with speech training on motor aphasia after stroke. Med Innov China. (2022) 19:147–50. doi: 10.3969/j.issn.1674-4985.2022.02.037
58. Zhang, D, Wang, J, Lu, J, Wang, P, Cheng, Y, Yuan, Y, et al. Efficacy comparison of noninvasive brain stimulation techniques on picture naming ability of non fluent aphasia patients after stroke with normal expression capacity. Chin J Cerebrovasc Dis. (2021) 18:84–90. doi: 10.3969/j.issn.1672-5921.2021.02.002
59. Zhang, Y, Yun, W, Zhang, M, Chen, Y, Cao, Y, and Zhou, X. The effects of low-frequency repetitive transcranial magnetic stimulation combined with hyperbaric oxygen in the treatment of non-fluent aphasia. Chin J Phys Med Rehabil. (2019) 7:512–6. doi: 10.3760/cma.j.issn.0254-1424.2019.07.008
60. Zheng, Y, Gu, Y, Yuan, L, Zhang, W, Shi, CH, and Tang, J. Attention should be paid to the effect of training combined with repetitive transcranial magnetic stimulation in the treatment of non-fluent aphasia after stroke. J Audiol Speech Pathol. (2022) 30:304–7. doi: 10.3969/j.issn.1006-7229.2022.03.015
61. Zhou, H, Yuan, L, Wen, Y, Jiang, W, Yang, L, Chen, H, et al. Effect of low-frequency repetitive transcranial magnetic stimulation combined with speech training on the rehabilitation of stroke aphasia. Neural Inj Funct Reconstr. (2021) 16:614–6. doi: 10.16780/j.cnki.sjssgncj.20201268
62. Zhu, H, Cheng, X, Liu, L, Tian, L, Rao, J, Liu, Y, et al. Clinical observation of inhibitory repetitive transcranial magnetic stimulation in stroke patients with non-fluent aphasia. Zhejiang Med J. (2023) 45:1140–1145+1151. doi: 10.12056/j.issn.1006-2785.2023.45.11.2022-232
63. Zhu, H, Zhang, X, Cheng, X, Rao, J, Zhang, Y, and Liu, L. Effect of inhibitory repetitive transcranial magnetic stimulation combined with mirror neuron training system for stroke patients with global aphasia. Chin J Rehabil. (2020) 35:563–7. doi: 10.3870/zgkf.2020.22.001
64. Zhu, H, Zhang, X, Cheng, X, Rao, J, Zhang, Y, and Liu, L. The effect of inhibitory repetitive transcranial magnetic stimulation combined with Mirror neuron training system on chronic global aphasia Poststroke. Chin J Stroke. (2021) 16:45–50. doi: 10.3969/j.issn.1673-5765.2021.01.008
65. Bai, G, Jiang, L, Huan, S, Meng, P, Wang, Y, Pan, X, et al. Study on Low-frequency repetitive transcranial magnetic stimulation improves speech function and mechanism in patients with non-fluent aphasia after stroke. Front Aging Neurosci. (2022) 14:883542. doi: 10.3389/fnagi.2022.883542
66. Barwood, CH, Murdoch, BE, Riek, S, O'Sullivan, JD, Wong, A, Lloyd, D, et al. Long term language recovery subsequent to low frequency rtms in chronic non-fluent aphasia. NeuroRehabilitation. (2013) 32:915–28. doi: 10.3233/NRE-130915
67. Khedr, EM, Abo el-Fetoh, N, Ali, AM, el-Hammady, DH, Khalifa, H, Atta, H, et al. Dual-hemisphere repetitive transcranial magnetic stimulation for rehabilitation of poststroke aphasia: a randomized, double-blind clinical trial. Neurorehabil Neural Repair. (2014) 28:740–50. doi: 10.1177/1545968314521009
68. Lin, BF, Hon, F, Lin, MY, Tsai, PY, and Lu, CF. Right arcuate fasciculus as outcome predictor after low-frequency repetitive transcranial magnetic stimulation in nonfluent aphasic stroke. Eur J Neurol. (2023) 30:2031–41. doi: 10.1111/ene.15808
69. Lin, BF, Yeh, SC, Kao, YJ, Lu, CF, and Tsai, PY. Functional remodeling associated with language recovery after repetitive transcranial magnetic stimulation in chronic aphasic stroke. Front Neurol. (2022) 13:809843. doi: 10.3389/fneur.2022.809843
70. Lopez-Romero, LA, Riano-Carreno, DM, Pachon-Poveda, MY, Mendoza-Sanchez, JA, Leon-Vargas, YK, Moreno-Pabon, A, et al. Efficacy and safety of transcranial magnetic stimulation in patients with non-fluent aphasia, following an ischaemic stroke. A controlled, randomised and double-blind clinical trial. Rev Neurol. (2019) 68:241–9. doi: 10.33588/rn.6806.2018300
71. Medina, J, Norise, C, Faseyitan, O, Coslett, HB, Turkeltaub, PE, and Hamilton, RH. Finding the right words: transcranial magnetic stimulation improves discourse productivity in non-fluent aphasia after stroke. Aphasiology. (2012) 26:1153–68. doi: 10.1080/02687038.2012.710316
72. Tsai, PY, Wang, CP, Ko, JS, Chung, YM, Chang, YW, and Wang, JX. The persistent and broadly modulating effect of inhibitory rtms in nonfluent aphasic patients: a sham-controlled, double-blind study. Neurorehabil Neural Repair. (2014) 28:779–87. doi: 10.1177/1545968314522710
73. Wang, CP, Hsieh, CY, Tsai, PY, Wang, CT, Lin, FG, and Chan, RC. Efficacy of synchronous verbal training during repetitive transcranial magnetic stimulation in patients with chronic aphasia. Stroke. (2014) 45:3656–62. doi: 10.1161/STROKEAHA.114.007058
74. YAŞA, İC, MAVİŞ, İ, ŞALÇİNİ, C, and MİDİ, İ. Comparing the efficiency of speech and language therapy and transcranial magnetic stimulation for treating Broca's aphasia. J Stroke Cerebrovasc Dis. (2023) 32:107108. doi: 10.1016/j.jstrokecerebrovasdis.2023.107108
75. Yoon, TH, Han, SJ, Yoon, TS, Kim, JS, and Yi, TI. Therapeutic effect of repetitive magnetic stimulation combined with speech and language therapy in post-stroke non-fluent aphasia. NeuroRehabilitation. (2015) 36:107–14. doi: 10.3233/NRE-141198
76. Yu, EH, Min, JH, Shin, YI, Ko, HY, and Ko, SH. Effect of repetitive transcranial magnetic stimulation on post-stroke non-fluent aphasia in relation with Broca's area. Brain Neurorehabil. (2021) 14:e15. doi: 10.12786/bn.2021.14.e15
77. Zheng, K, Chen, M, Shen, Y, Xu, X, Gao, F, Huang, G, et al. Cerebellar continuous Theta burst stimulation for aphasia rehabilitation: study protocol for a randomized controlled trial. Front Aging Neurosci. (2022) 14:909733. doi: 10.3389/fnagi.2022.909733
78. Chen, F, Wang, X, Zhan, C, Yang, L, Wang, Y, Sun, x, et al. Therapeutical effect of low-frequency repetitive transcranial magnetic stimulation on cerebral infarction aphasia and its possible mechanism. Chin J Cerebrovasc Dis. (2012) 6:246–51. doi: 10.3877/cma.j.issn.1673-9248.2012.05.002
79. Chen, F, Wang, X, Sun, X, Ke, SH, Wang, Y, Zhao, X, et al. Therapeutical effect of low-frequency repetitive transcranial magnetic stimulation on cerebral infarction aphasia and its effect on brain electrical activity. Chin J Cerebrovasc Dis. (2011) 5:96–101. doi: 10.3969/j.issn.1672-9248.2011.02.003
80. Barwood, CH, Murdoch, BE, Whelan, BM, Lloyd, D, Riek, S, O’ Sullivan, JD, et al. Improved language performance subsequent to low-frequency rtms in patients with chronic non-fluent aphasia post-stroke. Eur J Neurol. (2011) 18:935–43. doi: 10.1111/j.1468-1331.2010.03284.x
81. Sang, B, Deng, S, Zhai, J, Hao, T, Zhuo, B, Qin, C, et al. Does acupuncture therapy improve language function of patients with aphasia following ischemic stroke? A systematic review and meta-analysis. NeuroRehabilitation. (2022) 51:231–45. doi: 10.3233/NRE-220007
82. Harvey, DY, Podell, J, Turkeltaub, PE, Faseyitan, O, Coslett, HB, and Hamilton, RH. Functional reorganization of right prefrontal cortex underlies sustained naming improvements in chronic aphasia via repetitive transcranial magnetic stimulation. Cogn Behav Neurol. (2017) 30:133–44. doi: 10.1097/WNN.0000000000000141
83. Zumbansen, A, Black, SE, Chen, JL, J Edwards, D, Hartmann, A, Heiss, WD, et al. Non-invasive brain stimulation as add-on therapy for subacute post-stroke aphasia: a randomized trial (Northstar). Eur Stroke J. (2020) 5:402–13. doi: 10.1177/2396987320934935
84. Thiel, A, Black, SE, Rochon, EA, Lanthier, S, Hartmann, A, Chen, JL, et al. Non-invasive repeated therapeutic stimulation for aphasia recovery: a multilingual, multicenter aphasia trial. J Stroke Cerebrovasc Dis. (2015) 24:751–8. doi: 10.1016/j.jstrokecerebrovasdis.2014.10.021
85. Rubi-Fessen, I, Hartmann, A, Huber, W, Fimm, B, Rommel, T, Thiel, A, et al. Add-on effects of repetitive transcranial magnetic stimulation on subacute aphasia therapy: enhanced improvement of functional communication and basic linguistic skills. A randomized controlled study. Arch Phys Med Rehabil. (2015) 96:1935–1944.e2. doi: 10.1016/j.apmr.2015.06.017
86. Winhuisen, L, Thiel, A, Schumacher, B, Kessler, J, Rudolf, J, Haupt, WF, et al. Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: a combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke. (2005) 36:1759–63. doi: 10.1161/01.STR.0000174487.81126.ef
87. Weiduschat, N, Thiel, A, Rubi-Fessen, I, Hartmann, A, Kessler, J, Merl, P, et al. Effects of repetitive transcranial magnetic stimulation in aphasic stroke: a randomized controlled pilot study. Stroke. (2011) 42:409–15. doi: 10.1161/STROKEAHA.110.597864
88. Martin, PI, Treglia, E, Naeser, MA, Ho, MD, Baker, EH, Martin, EG, et al. Language improvements after TMS plus modified CILT: pilot, open-protocol study with two, chronic nonfluent aphasia cases. Restor Neurol Neurosci. (2014) 32:483–505. doi: 10.3233/RNN-130365
89. Garcia, G, Norise, C, Faseyitan, O, Naeser, MA, and Hamilton, RH. Utilizing repetitive transcranial magnetic stimulation to improve language function in stroke patients with chronic non-fluent aphasia. J Vis Exp. (2013) 77:e50228. doi: 10.3791/50228-v
90. Naeser, MA, Martin, PI, Theoret, H, Kobayashi, M, Fregni, F, Nicholas, M, et al. Tms suppression of right pars triangularis, but not pars opercularis, improves naming in aphasia. Brain Lang. (2011) 119:206–13. doi: 10.1016/j.bandl.2011.07.005
91. Naeser, MA, Martin, PI, Lundgren, K, Klein, R, Kaplan, J, Treglia, E, et al. Improved language in a chronic nonfluent aphasia patient after treatment with CPAP and TMS. Cogn Behav Neurol. (2010) 23:29–38. doi: 10.1097/WNN.0b013e3181bf2d20
92. Hamilton, RH, Sanders, L, Benson, J, Faseyitan, O, Norise, C, Naeser, M, et al. Stimulating conversation: enhancement of elicited propositional speech in a patient with chronic non-fluent aphasia following transcranial magnetic stimulation. Brain Lang. (2010) 113:45–50. doi: 10.1016/j.bandl.2010.01.001
93. Naeser, MA, Martin, PI, Nicholas, M, Baker, EH, Seekins, H, Helm-Estabrooks, N, et al. Improved naming after TMS treatments in a chronic, global aphasia patient – case report. Neurocase. (2005) 11:182–93. doi: 10.1080/13554790590944663
94. Ding, X, Zhang, S, Huang, W, Zhang, S, Zhang, L, Hu, J, et al. Comparative efficacy of non-invasive brain stimulation for post-stroke aphasia: a network meta-analysis and meta-regression of moderators. Neurosci Biobehav Rev. (2022) 140:104804. doi: 10.1016/j.neubiorev.2022.104804
95. Grönberg, A, Henriksson, I, Stenman, M, and Lindgren, AG. Incidence of aphasia in ischemic stroke. Neuroepidemiology. (2022) 56:174–82. doi: 10.1159/000524206
96. Bucur, M, and Papagno, C. Are transcranial brain stimulation effects long-lasting in post-stroke aphasia? A comparative systematic review and meta-analysis on naming performance. Neurosci Biobehav Rev. (2019) 102:264–89. doi: 10.1016/j.neubiorev.2019.04.019
97. Johnson, L, Yourganov, G, Basilakos, A, Newman-Norlund, RD, Thors, H, Keator, L, et al. Functional connectivity and speech entrainment speech entrainment improves connectivity between anterior and posterior cortical speech areas in non-fluent aphasia. Neurorehabil Neural Repair. (2022) 36:164–74. doi: 10.1177/15459683211064264
98. Chang, WK, Park, J, Lee, JY, Cho, S, Lee, J, Kim, WS, et al. Functional network changes after high-frequency RTMS over the Most activated speech-related area combined with speech therapy in chronic stroke with non-fluent aphasia. Front Neurol. (2022) 13:690048. doi: 10.3389/fneur.2022.690048
99. Vuksanović, J, Jelić, MB, Milanović, SD, Kačar, K, Konstantinović, L, and Filipović, SR. Improvement of language functions in a chronic non-fluent post-stroke aphasic patient following bilateral sequential theta burst magnetic stimulation. Neurocase. (2015) 21:244–50. doi: 10.1080/13554794.2014.890731
100. Iorga, M, Higgins, J, Caplan, D, Zinbarg, R, Kiran, S, Thompson, CK, et al. Predicting language recovery in post-stroke aphasia using behavior and functional MRI. Sci Rep. (2021) 11:8419. doi: 10.1038/s41598-021-88022-z
101. Li, H, Zhang, H, Xu, S, Wang, M, Zhang, J, Liu, J, et al. Altered spontaneous brain activity in Poststroke aphasia: a resting-state fmri study. Brain Sci. (2023) 13:300. doi: 10.3390/brainsci13020300
102. Carson, RG . Inter-hemispheric inhibition sculpts the output of neural circuits by co-opting the two cerebral hemispheres. J Physiol. (2020) 598:4781–802. doi: 10.1113/JP279793
Keywords: stroke, non-fluent aphasia, meta-analysis, repetitive transcranial magnetic stimulation, systematic review
Citation: Cheng J, Jiang Y, Rao T, Yang Y, Liu Y, Zhan Y and Yang S (2024) Repetitive transcranial magnetic stimulation for post-stroke non-fluent aphasia: a systematic review and meta-analysis of randomized controlled trials. Front. Neurol. 15:1348695. doi: 10.3389/fneur.2024.1348695
Received: 05 December 2023; Accepted: 16 April 2024;
Published: 01 May 2024.
Edited by:
Swathi Kiran, Boston University, United StatesReviewed by:
Xun Luo, Kerry Rehabilitation Medicine Research Institute, ChinaCopyright © 2024 Cheng, Jiang, Rao, Yang, Liu, Zhan and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shanli Yang, NDk2ODg0MDBAcXEuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.