
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol., 31 January 2024
Sec. Stroke
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1346177
This article is part of the Research TopicAdvances and controversies in ischemic stroke management: from prevention to diagnosis and acute treatmentView all 95 articles
 Tsong-Hai Lee1
Tsong-Hai Lee1 Shinichiro Uchiyama2
Shinichiro Uchiyama2 Yohanna Kusuma3
Yohanna Kusuma3 Hou Chang Chiu4
Hou Chang Chiu4 Jose C. Navarro5
Jose C. Navarro5 Kay Sin Tan6
Kay Sin Tan6 Jeyaraj Pandian7
Jeyaraj Pandian7 Liang Guo8
Liang Guo8 Yoko Wong8†
Yoko Wong8† Narayanaswamy Venketasubramanian9*
Narayanaswamy Venketasubramanian9*  for the Asian Stroke Advisory Panel
for the Asian Stroke Advisory PanelBackground: Stroke burden is largely due to long-term impairments requiring prolonged care with loss of productivity. We aimed to identify and assess studies of different registered pharmacological therapies as treatments to improve post-stroke impairments and/or disabilities.
Methods: We performed a systematic-search-and-review of treatments that have been investigated as recovery-enhancing or recovery-promoting therapies in adult patients with stroke. The treatment must have received registration or market authorization in any country regardless of primary indication. Outcomes included in the review were neurological impairments and functional/disability assessments. “The best available studies” based on study design, study size, and/or date of publication were selected and graded for level of evidence (LOE) by consensus.
Results: Our systematic search yielded 7,801 citations, and we reviewed 665 full-text papers. Fifty-eight publications were selected as “the best studies” across 25 pharmacological classes: 31 on ischemic stroke, 21 on ischemic or hemorrhagic stroke, 4 on intracerebral hemorrhage, and 2 on subarachnoid hemorrhage (SAH). Twenty-six were systematic reviews/meta-analyses, 29 were randomized clinical trials (RCTs), and three were cohort studies. Only nimodipine for SAH had LOE A of benefit (systematic review and network meta-analysis). Many studies, some of which showed treatment effects, were assessed as LOE C-LD, mainly due to small sample sizes or poor quality. Seven interventions had LOE B-R (systematic review/meta-analysis or RCT) of treatment effects.
Conclusion: Only one commercially available treatment has LOE A for routine use in stroke. Further studies of putative neuroprotective drugs as adjunctive treatment to revascularization procedures and more confirmatory trials on recovery-promoting therapies will enhance the certainty of their benefit. The decision on their use must be guided by the clinical profile, neurological impairments, and target outcomes based on the available evidence.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=376973, PROSPERO, CRD42022376973.
Stroke is a major cause of death and disability with only a limited number of treatment options to improve functional outcomes or reduce death and disability after a stroke, including thrombolytic therapy, thrombectomy, early use of anti-platelets, decompression craniectomy for “malignant” infarcts, organized stroke care, and constraint-induced movement therapy (1). However, many patients do not receive time-sensitive acute stroke therapies for various reasons (2, 3). Alternative strategies using neuroprotectants have failed to live up to their earlier promise (4). Drug interventions that mediate recovery beyond the acute windows are, therefore, clinically important research targets.
As much as three-quarters of all stroke patients suffer impairments and disabilities, the most common of which are motor weakness (77.4%), urinary incontinence (48.2%), impaired consciousness (44.7%), dysphagia (44.7%), and impaired cognition (43.9%) (5). Transition from independence in activities of daily living to dependency between 3 and 12 months after a stroke may be observed in a high proportion of patients (6). At 5 years, functional and motor outcomes may deteriorate to the status at 2 months post-stroke (7). In a large multi-center clinical trial of stroke patients with one-third of participants coming from Asia, at a median follow-up of 4 years, 19–22% were disabled and 12–14% were dependent, requiring regular help with everyday activities (8).
Stroke burden is largely due to long-term impairments suffered after a stroke, requiring long-term care and loss of productivity (9–14). Improving the degree and chances of recovery will translate to an overall reduction in the burden and cost of stroke care. Apart from standard rehabilitation strategies, however, there is currently no common recommendation on pharmacological treatment for stroke recovery.
With the aging of the global population, the number of disabled stroke survivors is likely to rise. Clearly, treatments are needed to enhance recovery after stroke. Prematurely judging a treatment as ineffective may mean lost opportunities in moving stroke recovery research forward to benefit stroke sufferers. It is entirely possible that the apparent “lack” of the efficacy of neuro-recovery interventions thus far may not only be due to small sample sizes or varying severity of study subjects but also because of premature summative assessments and that following up at an extended time frame might show positive effects. Conversely, claiming a treatment as effective, when there is a lack of evidence, can be problematic as patients may be exposed unnecessarily to possible side effects or miss the opportunity of receiving a more appropriate treatment, in addition to incurring the costs of an ineffective intervention. A review of registered pharmacological therapies that have been investigated for improving post-stroke outcomes will help identify the types of available evidence, information on how research was conducted on them, key characteristics or factors related to treatment effects, and knowledge gaps in the pharmacological treatment of post-stroke patients that will be helpful in both clinical decision-making and planning future studies.
We, therefore, aimed to identify and assess studies of different registered pharmacological therapies investigated for improving post-stroke impairments and/or disabilities. The research questions we sought to answer are:
1. What is the best available evidence based on study design for different registered pharmacological therapies investigated for improving recovery after a stroke?
2. What stroke sub-populations and post-stroke outcomes are improved by these treatments, if any.
This systematic-search-and-review (15) was registered in the International Prospective Register of Systematic Reviews in 2022 (PROSPERO CRD42022376973).
Studies that have the following PICO study characteristics were included in the review:
Studies examining adult humans, aged 18 years or older, diagnosed with stroke were included. Studies addressing both adults and children were included if data provided for adults were reported separately.
Interventions were pharmacological therapies that have been investigated as recovery-enhancing or recovery-promoting treatments in patients who had suffered a stroke. These treatments must have received registration or market authorization in any country, either prescription or over-the-counter products, and may have primary indications for use in other medical conditions. Supplementary Table S1 lists the pharmacological classes and drugs investigated in the review.
Depending on the study type, a comparator (active or placebo) may have been included.
Stroke-related outcomes included overall function and motor recovery. Other clinical neurological domains were also considered. The following outcomes were excluded:
• Psychiatric—mood (e.g., depression, mania, anxiety, and apathy), sleep disorder, hypersexuality, emotionalism, delirium, etc.
• Cognitive—dementia, memory impairment, concentration, neglect, etc.
• Spasticity, contracture, sialorrhea, seizures, pain, and fatigue.
• Imaging and laboratory outcomes (e.g., lesion size, vasospasm, biomarkers, and transcranial magnetic stimulation parameters).
A literature search was carried out in PubMed, EMBASE, Scopus, the Cochrane Database of Systematic Reviews, the Cochrane Central Register of Controlled Trials (CENTRAL), and the Database of Abstracts of Reviews of Effects for published reports up to November 2022. The search was also supplemented by searching for trial protocols at https://www.clinicaltrials.gov and completing systematic reviews in PROSPERO. The search criteria included (i) both MeSH terms and free text related to “stroke” and “recovery”; (ii) each of the pharmacological classes/products listed, (iii) limited to the English language, and (iv) human subjects only. The search strategy for EMBASE is shown in Supplementary Table S2. The search syntax was adjusted accordingly in each search engine with the same criteria. To ensure literature saturation, the reference lists of included studies or relevant reviews were scanned.
Literature search results were uploaded to Covidence1 to facilitate collaboration among reviewers during the study selection process. The search results were grouped by pharmacological class. Duplicates were identified and removed automatically by Covidence and by manual checking. Titles and abstracts screening was conducted by at least one author, and only relevant studies were further retrieved and reviewed in the full text of the publication. Included studies were classified into one of the categories in decreasing level of evidence: systematic review and/or meta-analysis, randomized controlled trial (RCT), non-randomized controlled trial, cohort study, case–control study, case report, or opinion of expert(s).
The decision on selecting “the best available studies” based on study design was made by two reviewers. If multiple papers were identified under the same hierarchy, a decision was reached by consensus based on study size and date of publication for different stroke subtypes and/or outcomes. Any disagreement on study selection and data extraction was resolved by consulting a third author for arbitration.
Data extraction was performed using a pre-designed form for each included report. In cases of ambiguity of information, the study was elevated for adjudication by arbitrators.
Data extracted included patient demographics (age, gender, and country of origin), methodology (study design, sample size, and key stroke inclusion criteria), intervention details (dosage, frequency, duration of intervention, and type of control used), duration (stroke onset to study inclusion and follow-up period), and the reported outcomes (as dichotomous or continuous). If the outcome was reported as a composite measure, individual outcomes reported in the studies were extracted, if available. Whenever possible, we used results from an intention-to-treat analysis. For cross-over trials, we extracted data from the first period only to avoid any possible carry-over effects or the potential for stroke patients to recover spontaneously during cross-over.
The included studies were grouped by pharmacological class/product and stroke subtypes in the results, with short narratives highlighting the important points. The level of evidence was assessed according to the latest version of the American Stroke Association Level of Evidence (LOE) scheme (Table 1) (16). Individual studies were given only one level of evidence, but systematic reviews and meta-analyses may be given several levels of evidence for different analyses performed.
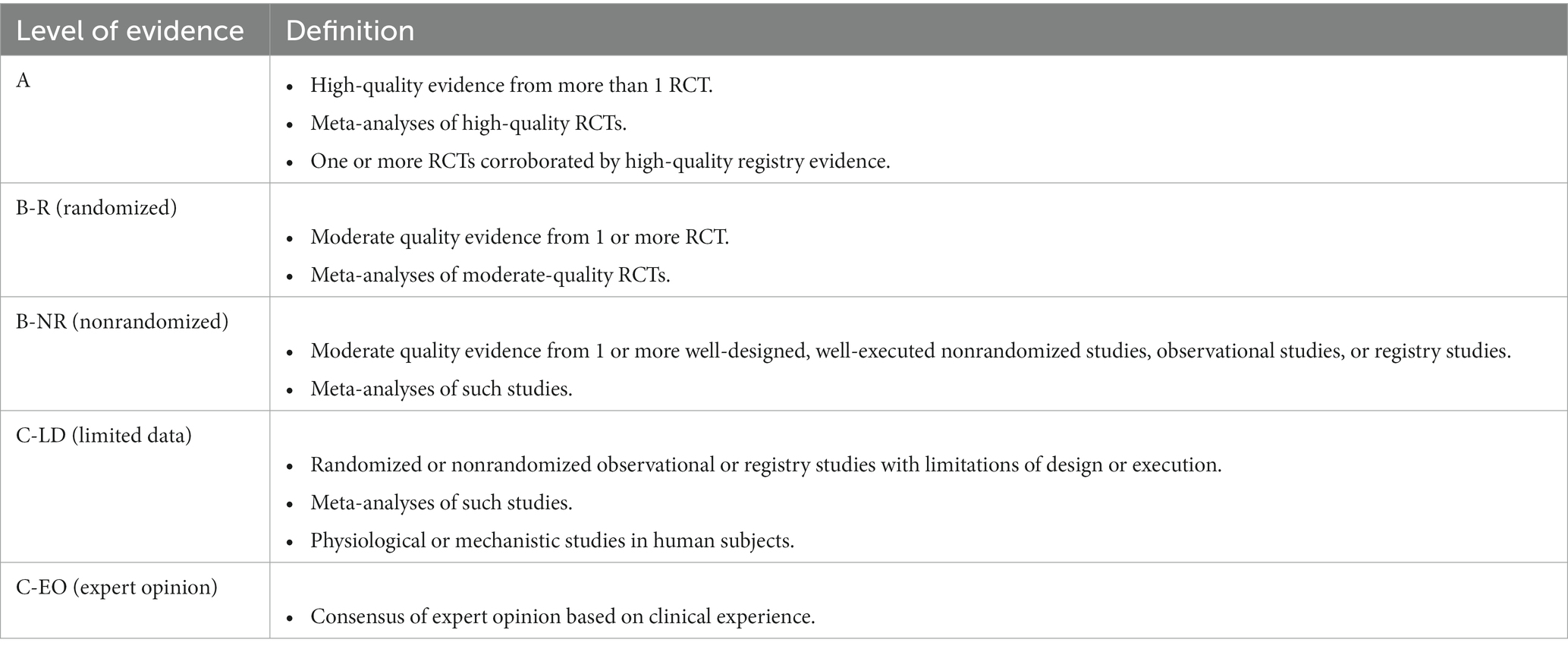
Table 1. American Stroke Association level of evidence scheme (16).
The systematic search yielded a total of 7,801 citations, of which 1,454 were duplicates. Of the remaining 6,347 papers screened by title and abstract, 5,680 did not meet one or more of the PICO criteria, while two papers could not be retrieved. After reviewing 665 full-text papers, a total of 58 publications were selected for inclusion as “the best” current studies across the different pharmacological classes (Figure 1).
Of the 58 publications included, 31 studies included patients with ischemic stroke, 21 on either “stroke” or “ischemic or hemorrhagic stroke,” four on intracerebral hemorrhage (ICH), and two on subarachnoid hemorrhage (SAH). Twenty-six were systematic reviews and/or meta-analyses, 29 were RCTs, and three were cohort studies. Among the included RCTs, the treatment window from stroke onset was up to 2 h (in one study), 5 h (one), 24 h (two), 48 h (four), 72 h (one), 96 h (one), 1 week (one), 2 weeks (one), 3 weeks (one), 1 month (three), 42 days (one), 3 months (one), 6 months (three), and > 6 months or “convalescing” or “chronic” (eight).
The characteristics of the 58 included studies are listed in Supplementary Table S3. The efficacy results of each study with their corresponding LOE (16) are summarized in Table 2, while the safety results are summarized in Table 3.

Table 2. Summary of efficacy results of included studies and assessed level of evidence according to the American Stroke Association scheme.
Eighty-five papers on SSRI in stroke were reviewed. The study selected was a systematic review and meta-analysis that included 13,029 patients from 38 fluoxetine, 13 paroxetine, eight sertraline, nine citalopram, five escitalopram, two citalopram or fluoxetine, and one sertraline or fluoxetine studies (17). Of the six studies at low risk of bias, all on fluoxetine, there was little to no difference in disability, independence, and motor deficit at the end of treatment between groups. When all studies, irrespective of the risk of bias, were included, SSRIs reduced disability scores but not the proportion of independent patients at the end of treatment, except for one study on citalopram (n = 642; RR 0.90, 95% CI 0.82–0.98; p = 0.01; LOE B-R).
Thirteen papers that investigated tricyclic antidepressants alone or together with other antidepressants in stroke were reviewed. A small RCT (n = 46) comparing maprotiline to placebo and fluoxetine in stroke patients who were unable to walk showed that maprotiline is no better than placebo and may hinder recovery in post-stroke patients undergoing rehabilitation (18). In another RCT (n = 83) that also compared nortriptyline to placebo and fluoxetine, treatment with anti-depressants, either fluoxetine or nortriptyline, improved modified Rankin Scale (mRS) scores compared to placebo independently of depression [mixed model time–treatment interaction t(105) = 2.91, p = 0.004; LOE C-LD] (19).
Fifty-one papers on botanicals were reviewed, of which nine were selected. In a small RCT, 100 patients with ischemic stroke within the prior 30 days were allocated to either di huang yin zi (DHYZ) or placebo for 12 weeks (20). Only 87 patients who completed the study were analyzed, which showed better Fugl-Meyer Assessment (FMA) scores in patients treated with DHYZ at 8 weeks (mean difference, MD, 7.0, 95% CI 1.6–12.4) and 12 weeks (MD 6.5, 95% CI 0.7–12.3) with higher Barthel Index (BI) scores on DHYZ at 12 weeks (MD 4.5, 95% CI 0.3–8.7) (LOE C-LD).
Two systematic reviews on ginkgo biloba, both on patients with ischemic stroke and published in 2020, were selected. One systematic review and meta-analysis included 13 studies (n = 1,466) (21). The pooled results suggest that ginkgo biloba was associated with improvement in neurological function on the National Institutes of Health Stroke Scale (NIHSS; MD −2.87, 95% CI −4.01 to −1.74; p < 0.00001), in activities of daily living (MD 9.52, 95% CI 4.66–14.38; p = 0.00001) and functional outcome (MD −0.50, 95% CI −0.63 to −0.37; p < 0.00001) at the end of the study. The second systematic review and meta-analysis included 15 studies (n = 1829) (22). Analyses showed that ginkgo biloba improved NIHSS (MD −1.39, 95% CI −2.15 to −0.62; p = 0.0004), Neurological Functional Deficit Scores (NFDS, RR 1.20, 95% CI 1.12–1.29; p < 0.00001), and activities of daily living (MD 5.72, 95% CI 3.11–8.33; p < 0.0001) compared with conventional therapy at different stages after an ischemic stroke. In both reviews, however, many of the studies were judged to be of poor quality and reliability due to the risk of bias (LOE C-LD).
Four studies were selected for MLC601/MLC901. An RCT (n = 150) included patients with ischemic stroke within 1 month of onset to either MLC601 or placebo for 3 months (23). Repeated measures analysis showed that motor recovery on FMA was higher in treated patients at 4 weeks (p < 0.001), 8 weeks (p = 0.001), and 12 weeks (p < 0.001) compared to control (LOE B-R). In a systematic review and meta-analysis of five RCTs (n = 1936), pooled analysis on functional recovery at the end of treatment (1 or 3 months) favored MLC601 (RR 1.64, 95% CI 1.05–2.57; p = 0.031; LOE B-R) (24). We prioritized the confidence interval in our interpretation of the results rather than the prediction interval calculated by the authors. A long-term follow-up study of patients with ischemic stroke (n = 880) showed that the odds ratio (OR) of functional independence was significantly increased at 6 months (1.49, 95% CI 1.11–2.01) and persisted up to 18 months (OR 1.36, 95% CI 1.01–1.83) on mRS and at 6 months (OR 1.55, 95% CI 1.14–2.10) on BI after treatment with MLC601 compared to placebo (LOE B-R) (25). One cohort study (n = 66) was the only paper available in patients with ICH (26). While patients treated with MLC601/MLC901 showed a sustained effect on neurological and functional recovery, this was not a controlled study.
Two studies were selected for Panax notoginseng, one each on ischemic stroke and ICH. The systematic review and meta-analysis on ischemic stroke included eight RCTs (n = 660) (27). However, seven of the eight studies were considered to be of poor quality. Pooled analysis (seven RCTs) indicated more improvement in neurological deficit with Panax notoginseng than control (RR 0.29, 95% CI 0.18–0.47, p = 0.00001) (LOE C-LD). Meta-analysis of two trials indicated a lower rate of death and dependency at 28 days (RR 0.63, 95% Cl 0.45–0.88; p = 0.0072), and one trial reported higher BI on Panax notoginseng (LOE C-LD). The systematic review and meta-analysis of intravenous Panax notoginseng in ICH patients included 20 studies (n = 1891), of which four were considered high-quality trials (28). Intravenous Panax notoginseng was associated with better “effectiveness rate” as defined in each study and often calculated as number of cases with desired grade of outcome out of total number in each group (OR 2.70; 95% CI 2.16–3.38; p < 0.00001), less neurological deficit (MD 4.36; 95% CI 3.07–5.65; p < 0.00001), and increased BI (MD 11.73; 95% CI 19.31–4.16; p = 0.002) (LOE B-R).
One hundred and seven papers on calcium antagonists were reviewed. Four papers were selected. A systematic review and meta-analysis included 34 RCTs (n = 7,731) on calcium antagonists in ischemic stroke, of which two studies included hemorrhagic stroke (n = 255) (29). The most studied calcium antagonists were nimodipine (26 RCTs) and flunarizine (3 RCTs). No effect on death or disability at the end of follow-up was shown by calcium antagonists as a group or by individual drugs. In a systematic review and network meta-analysis of prophylactic therapies after SAH, improvement in functional outcome at the end of follow-up was seen for nimodipine on Glasgow Outcome Scale (GOS, OR 1.46, 95% CI 1.07–1.99) and nicardipine on mRS (OR 8.80, 95% CI, 1.34–57.77), while magnesium did not reduce mortality or disability despite its effects on delayed cerebral ischemia and vasospasm (LOE A) (30).
A systematic review and meta-analysis of magnesium in ischemic or hemorrhagic stroke within 24 h of onset included seven RCTs (n = 4,347) (31). Compared with placebo, magnesium overall improved neither functional outcome (BI >60 or > 95) nor global outcome (mRS) at 90 days post-stroke. A subgroup meta-analysis of three RCTs that exclusively included only ischemic stroke patients (n = 164) resulted in lower mRS scores at 90 days post-stroke (weighted mean difference, WMD, −0.96, 95% CI −1.34 to −0.58; p < 0.00001), although this should be viewed with extreme caution given the limited number of patients (LOE C-LD). A recent sub-study of a large RCT that investigated the benefit of magnesium administration within 2 h of stroke symptom onset analyzed the subset of patients who suffered an ICH (n = 268) (32). In this sub-analysis, magnesium did not improve NIHSS or mRS at 90 days.
Thirty-six papers were reviewed, and two studies were selected. In a systematic review and meta-analysis of 10 RCTs (n = 4,543) on citicoline in patients with ischemic stroke, all were assessed as having a high risk of bias (33). Citicoline did not increase the proportion of patients with NIHSS ≤1 at 6 weeks (4 RCTs) or the proportion of patients with mRS <3 (four RCTs) compared with placebo. Four trials indicated that citicoline did not improve BI, while one study (n = 63) showed more patients on citicoline achieving BI scores ≥85 compared to control (RR 3.13, 95% CI 1.10–8.91; p = 0.03; LOE C-LD).
A placebo-controlled, blinded endpoint assessment RCT (n = 99) investigated the administration of citicoline immediately after recanalization therapy, either intravenous or endovascular, in patients with acute ischemic stroke (34). No differences between treatment groups were seen in neurological (NIHSS) or functional (mRS and BI) outcomes at 90 days.
Twenty-two papers were reviewed. Many of the studies were small and assessed cognitive outcomes at endpoints. A small RCT (n = 26) on donepezil in chronic post-stroke aphasia was selected (35). Donepezil given for 16 weeks was reported to improve Aphasia Quotient score on the Western Aphasia Battery (p = 0.037, Cohen’s d = 0.87) and Picture Naming on the Psycholinguistic Assessment of Language Processing in Aphasia (PALPA) (p = 0.025, Cohen’s d = 0.92), but not in other PALPA subtests, Communicative Activity Log, or the Stroke Aphasic Depression Questionnaire at endpoint (LOE C-LD).
A cohort study on pre-stroke usage of cholinesterase inhibitors (donepezil, galantamine, and rivastigmine) was selected (36). The study analyzed 805 patients with pre-stroke dementia within 7 days of an acute ischemic stroke and followed for 3 months. Patients were stratified according to pre-stroke usage of any cholinesterase inhibitor. Non-usage was associated with neurological deterioration by ≥2 points on NIHSS during hospitalization (OR 0.52, 95% CI 0.31–0.88; p = 0.01) and poor functional outcome at 3 months (mRS ≥3 OR 0.68, 95% CI 0.46–0.99; p = 0.048) after adjusting for potential confounding factor, as well as after propensity score matching (LOE B-NR).
Thirty papers were reviewed and two studies were selected. Although a systematic review of 11 trials (n = 329) on amphetamine was published in 2009 that concluded “no evidence exists at present to support the use of amphetamine after stroke,” and another one on CNS drugs in 2017 (37, 38), a more recently published RCT (n = 64) on amphetamine in patients with ischemic stroke and moderate-to-severe motor impairment was selected (39). Amphetamine or placebo was administered 1 h before physiotherapy every 4 days for six sessions. No overall treatment-associated differences in neurological, motor, walking, and functional scores were observed at the end of treatment or at 3 months.
A systematic review of modafinil (n = 138 in 12 studies) and amantadine (n = 128 in 10 studies) included studies with very varied stroke subtypes (ischemic, ICH, and SAH), some even including other neurological disorders (traumatic brain injury, dementia, etc.) (40). Forty different outcome measures with 141 domains were described across all studies. A positive response in at least one clinical effectiveness measure was reported in 83% of modafinil publications and 70% of amantadine publications. Quantitative analyses were not performed due to heterogeneity in the outcome measures.
Although an RCT (n = 21) on methylphenidate published in 2018 was available, it included only patients with post-stroke neglect (41). We, therefore, selected a study (n = 78) that compared methylphenidate or/and levodopa with a placebo in patients with a paretic arm and/or leg following a stroke that had occurred 15–180 days before (42). The study found that, compared with a placebo, treatment with methylphenidate, levodopa, or methylphenidate + levodopa combined with physiotherapy improved activities of daily living (ADL, p = 0.011) and NIHSS (p = 0.001) at 6 months but not motor recovery on FMA (LOE C-LD).
Of the 63 papers on colony-stimulating factors, four were selected. In a systematic review and meta-analysis of 11 studies (n = 1,275), 3 on EPO and 8 on G-CSF, in patients with ischemic or hemorrhagic stroke, EPO therapy was associated with an increase in death by the end of the trial (OR 1.98, 95% CI 1.17–3.33; p = 0.01) with no improvement on neurological impairment or functional outcome. G-CSF was associated with no significant reduction in early impairment and had no effect on functional outcome or death at the end of the trial (43). A more recent systematic review and meta-analysis of eight studies (n = 485), one on EPO and seven on G-CSF, also did not show improvement in NIHSS or BI at the end of the follow-up (44). A meta-analysis of G-CSF (n = 1,037 in 14 studies) in acute or subacute ischemic or hemorrhagic stroke concluded that while it was associated with a weakly significant improvement on BI (MD 8.65, 95% CI 0.98–16.32; p = 0.03), NIHSS was not improved at 3 months (LOE C-LD) (45).
A dose-escalation RCT (n = 96) of EPO in combination with human choriogonadotropin (hCG) in patients with supratentorial ischemic stroke was halted early (46). No significant difference in improvement on NIHSS was found between placebo and active treatment, whether analyzed together or separately, as well as on functional outcomes at 90 days.
Thirty-six papers on dopaminergics in stroke were reviewed. Although a meta-analysis (six RCTs, n = 795) of levodopa in stroke was available in 2020 (47), this was only published in abstract form and was performed in preparation for the Enhancement of Stroke REehabilitation with Levodopa (ESTREL) study. The meta-analysis showed a small non-significant trend for motor recovery in levodopa-treated stroke patients compared to control patients (Standardized Mean Difference, SMD, 0.15, 95% CI -−0.25 to 0.55). Heterogeneity between trials was considerable (I2 = 67%), and trials differed regarding phases (chronic or acute), dosage and duration of the study treatment, length of follow-up, and outcome measures.
The published results of the Dopamine Augmented Rehabilitation in Stroke (DARS) study, a randomized, double-blind, placebo-controlled trial in 593 patients who cannot walk independently within 5–42 days of stroke, were selected (48). In this study, levodopa did not improve walking after stroke, long-term disability, or functional outcome.
In a small RCT (n = 33), ropinirole did not improve gait velocity or motor recovery (49). Moreover, a cohort study suggests that the use of anti-Parkinson’s medications in patients after a stroke may be associated with longer rehabilitation length of stay and poorer functional status compared to those in the entire cohort (50).
No study on apomorphine met the criteria for review. The result of ESTREL is awaited.
Three papers were reviewed. One study (n = 57) that randomized ischemic stroke patients to either nicergoline or hydergine (no placebo arm) was analyzed by comparing outcomes before and after treatment in the same patient group rather than between groups (51). A small placebo-controlled RCT (n = 21) of oral hydergine for 12 weeks with a post-hoc crossover study (n = 15) in “convalescing geriatric” stroke patients was, therefore, selected (52). Analyses of both RCT and cross-over phases of this study showed no significant difference between hydergine and placebo on motor functions assessed that included muscle strength testing, hand grip, elbow flexion, walking, and sitting up.
Fourteen studies were reviewed. A systematic review published in 2018 that included four studies on clomethiazole and one study on diazepam (n = 3,838) was selected (53). Although there was an indication of potential benefit in the subgroup of patients with total anterior circulation stroke (RR 1.33, 95% CI 1.08–1.63; p = 0.01), no benefit was demonstrated overall on neurological impairments or disability for both ischemic and hemorrhagic stroke (LOE B-R).
No study on GABA antagonists, e.g., flumazenil, met the criteria for review and selection.
Eight papers were reviewed, of which three were selected. In the first systematic review and meta-analysis that included two RCTs (n = 119) of aminophylline in patients with ischemic stroke, there was no difference in early death and deterioration or death or disability at the end of the follow-up (54). A systematic review and meta-analysis of pentoxifylline and propentofylline, also in ischemic stroke, included five trials (n = 793) (55). No study on pentifylline was included. Death or disability was not reduced at the end of the follow-up (two trials). The data for neurological impairment and disability were not in a form suitable for analysis.
More recently, a small RCT (n = 64) investigated theophylline as an add-on treatment to thrombolytic therapy in acute ischemic stroke (56). While theophylline as an add-on to thrombolysis improved NIHSS score at 24 h more than thrombolysis alone (MD −3.6, 95% CI −7.1 to −0.1; p = 0.043; LOE C-LD), functional independence at 90 days was not different between treatment groups.
Three papers were reviewed, all relatively small RCTs, and two studies were selected. One RCT (n = 89) investigated the administration of moclobemide for 6 months in patients with post-stroke aphasia within 3 weeks of onset (57). Compared to placebo, treatment with moclobemide for 6 months did not enhance the regression of post-stroke aphasia at 6 and 12 months. Another RCT (n = 47) allocated patients within 2 weeks of stroke to either selegiline or placebo for 6 weeks in addition to standard rehabilitation (58). While cognitive functioning was improved in the selegiline-treated group, no significant difference in functional recovery on Functional Independence Measure (FIM) was observed at 2 and 6 weeks.
Four studies were reviewed, and one RCT on lithium (n = 66) was selected (59). There were overall no differences between lithium-treated and placebo-treated patients on improvements in the modified NIHSS and hand FMA scores at 30 days, although discrete differences on modified NIHSS (t-test p = 0.003) and hand FMA (t-test p = 0.003) in the cortical stroke subgroup (n = 27) were observed (LOE C-LD).
Seventy-two papers were reviewed and four papers on cerebrolysin were selected. In a recent systematic review and meta-analysis of seven RCTs (n = 1,601) that included patients within 48 h of ischemic stroke onset, cerebrolysin did not reduce all-cause mortality at the end of the follow-up, but may increase non-fatal serious adverse events (RR 2.15, 85% CI 1.01–4.55; p = 0.05) (60). There was not enough data to analyze death or dependency. In another earlier systematic review and meta-analysis of nine RCTs (n = 1879) that included patients within 72 h of ischemic stroke, more patients improved by ≥4 points on NIHSS at 21 or 30 days on cerebrolysin than placebo (OR 1.60, 95% CI 1.03–2.48; p = 0.035). In the meta-analysis of only more severe patients (NIHSS >12 at baseline) from three RCTs, mRS was improved at 90 days (MD 0.39, 95% CI 0.06–0.71; p = 0.02) (LOE B-R) (61). Similarly, cerebrolysin combined with rehabilitation in a small RCT (n = 66) of patients with subacute ischemic stroke improved FMA in the subgroup of patients with severe motor deficits at baseline (p < 0.05) but not in the overall study population (LOE C-LD) (62). A small RCT (n = 50) in patients with SAH showed cerebrolysin to be safe and well-tolerated but did not improve the global functional performance of patients even at 6 months (63).
Only one study met the PICO criteria for review. In a small (n = 20) randomized, double-blind, placebo-controlled trial, cycloserine given 1 h before motor training did not enhance motor learning or motor skill generalization in adults with weakness of upper and lower extremities from stroke (64).
Nine papers on NMDA antagonists in stroke were reviewed. In a pilot open-label RCT (n = 53) of patients with ischemic stroke within 24 h, memantine was associated with improvements in NIHSS during hospitalization (p < 0.0001) and BI at 3 months (p = 0.002) (LOE C-LD) (65). Patients with chronic post-stroke aphasia (n = 28) were randomized in an RCT to memantine or placebo alone for 16 weeks, after that combined with constraint-induced aphasia therapy (CIAT) for 2 weeks, then drug treatment alone for 2 weeks, 4 weeks of washout period, and followed by a 24-week open-label extension study of memantine (66). While memantine or CIAT alone improved aphasia severity, best outcomes on certain aphasia subdomains were achieved by memantine + CIAT at 16 weeks (p = 0.002) and 18 weeks (p = 0.0001), with the difference between treatment groups persisting on long-term follow-up (LOE C-LD).
No study on dextromethorphan was selected.
Eight studies were reviewed and two studies were selected. Atomoxetine, paired with 10 sessions of motor training, was investigated in a small pilot RCT (n = 12), which showed better recovery on upper limb FMA at the end of the treatment (MD 7.2, 95% CI 1.6–12.7; p = 0.016) but not at 1 month or on other motor assessment scales in patients with upper limb weakness ≥6 months after a stroke (LOE C-LD) (67). In another pilot randomized crossover study (n = 10), a single dose of reboxetine given before therapy in patients with “chronic” stroke increased tapping speed (ANOVA p = 0.048) and grip strength (ANOVA p = 0.003) in the paretic but not in the unaffected hand, with no further improvement noticed after physiotherapy alone (LOE C-LD) (68).
Eight studies of opioid antagonists in stroke were reviewed. A systematic review published in 2021 that included four studies on naloxone (n = 96) and three studies on nalmefene (n = 916) was selected (69). From this review, one small study (n = 44) on naloxone showed benefit on Neurological Status Score at 2 weeks (p < 0.01) and one study (n = 236) on nalmefene showed improvement on Glasgow Coma Scale (GCS) at 10 days (p < 0.05) and NIHSS at 20 days (p < 0.05) compared to controls (LOE C-LD). Meta-analysis was not performed because of the different parameters used in all studies.
Two papers were reviewed. A small placebo-controlled RCT (n = 74) of almitrine-raubasine in patients with non-acute ischemic stroke was selected (70). Almitrine-raubasine was associated with better BI scores at 1 month (t-test p = 0.001), 2 months (t-test p = 0.002), and 3 months (t-test p = 0.002), and mean improvement on NFDS (t-test p = 0.034) at 1 month (LOE C-LD).
Two papers, both on dalfampridine, were reviewed. The paper selected was an RCT (n = 377) that compared two doses of dalfampridine administered for 12 weeks to placebo in patients with walking deficits ≥6 months after an ischemic stroke (71). Dalfampridine, at either 7.5 or 10 mg dose, did not significantly increase walking performance at the end of treatment, although the study was terminated early before the full enrolment of 540 subjects due to an unblinded analysis that showed insufficient efficacy to support further recruitment.
Seventy-two papers, all on edaravone, were reviewed. In a systematic review and meta-analysis of 50,536 patients with ischemic stroke from 14 observational studies and five RCTs, edaravone treatment was overall associated with improved odds of excellent (mRS ≤1 OR 1.26, 95% CI 1.04–1.54; p = 0.02) and good (mRS ≤2 OR 1.31, 95% CI 1.03–1.67; p = 0.03) functional outcomes with lower mortality. However, study heterogeneity was high, and the effect was reduced to p > 0.05 when analysis was restricted to randomized trials only (72). A systematic review and meta-analysis of 17 RCTs on edaravone in patients with ischemic stroke treated with intravenous thrombolysis (n = 1877) showed that combined treatment with edaravone and alteplase reduced the NIHSS score at the end of treatment (MD 3.95, 95% CI 2.92–4.99; p < 0.00001), 7 days (MD 5.11, 95% CI 2.84–7.37; p < 0.00001), and 14 days (MD 3.11, 95% CI 2.23–3.99; p < 0.00001) compared with alteplase alone, with less occurrence of intracranial hemorrhage during hospitalization (LOE B-R) (73). A recent RCT (n = 1,194) compared edaravone alone with the combination of edaravone and dexborneol in patients with ischemic stroke within 48 h of onset. Improvement on NIHSS at 14 days (MD −0.40, 95% CI −0.72 to −0.08; p = 0.01) and mRS at 3 months (OR 1.42, 95% CI 1.12–1.81; p = 0.004) were better with combination treatment than edaravone alone (LOE B-R) (74).
A systematic review and meta-analysis of 38 RCTs on edaravone initiated within 7 days of ICH (n = 3,453) showed alleviation of neurological deficits (MD −5.44 95% CI −6.44 to −4.44; p < 0.00001), improved activities of daily living (MD 8.44 95% CI 7.65–9.23; p < 0.00001), and reduced hematoma volume with edaravone, although the included trials were of poor quality and high heterogeneity (LOE C-LD). No studies reported long-term functional outcomes (75).
Twenty-three papers were reviewed and two papers on piracetam were selected. In a systematic review and meta-analysis of three trials (n = 1,002) in acute ischemic stroke, most data came from one large trial and overall did not demonstrate the superiority of piracetam over control in improving functional outcome (BI) or reducing death or dependency at 3 months (76). Its role in post-stroke aphasia was investigated in a systematic review and meta-analysis of seven RCTs (n = 261), which showed that piracetam did not improve overall language performance but may benefit written language ability (SMD 0.35, 95% CI 0.04–0.66; p = 0.03), particularly more so during the first 12 weeks but not long term (LOE C-LD) (77).
Two papers were reviewed and selected. In a systematic review and meta-analysis of 26 RCTs on buflomedil in patients treated within the first few days of ischemic stroke (n = 2,756), the trials were generally of poor quality, and many were poorly reported (78). Only one trial (n = 200) reported long-term death and disability, with patients on buflomedil having a lower risk of death or disability than the control group at 3 months (RR 0.71, 95% CI 0.53–0.94; p = 0.02). Another trial (n = 85) reported less disability (MD 15.0, 95% CI 5.83–24.17, p = 0.0) while all 26 trials (n = 2,756) reported improvements in neurological deficits at the end of treatment on buflomedil, although evidence for any of these short-term outcomes was not considered robust (LOE C-LD).
A recent RCT (n = 937) compared cinepazide to placebo in patients with ischemic stroke within 48 h of onset (79). The study showed cinepazide injection to be safe and better than placebo in improving functional recovery (OR 0.607, 95% CI 0.460–0.801) and reducing disability (OR 0.719, 95% CI 0.542–0.956) at 3 months (LOE B-R).
We performed this systematic-search-and-review to identify the best available evidence of different registered pharmacological interventions for improving recovery after a stroke. Among the different pharmacological interventions reviewed, only one intervention, nimodipine in SAH, was shown to have level A evidence of treatment benefit based on a systematic review and network meta-analysis. Other treatments with LOE A studies did not demonstrate the benefit of intervention over control, namely SSRIs, calcium antagonists, and citicoline in ischemic stroke and magnesium, colony-stimulating factors, and GABA agonists in ischemic or hemorrhagic stroke.
Many of the reviewed selected papers were assessed as LOE C-LD, mostly due to small sample sizes or poor quality of studies, some of which showed treatment effects and required larger studies to provide better certainty of evidence. Of the studies assessed as LOE B-R, seven commercially available drugs showed treatment effects, although additional trials would further support their clinical use. While generally reported to be safe, it is important as well to be aware that certain treatments may have a detrimental effect on poststroke recovery (80). In our review, the risk may be increased for bone fracture on SSRI (17), non-fatal serious adverse events on cerebrolysin (60), and death at the end of the study on flunarizine and EPO (29, 43).
The studies of different interventions in our review included patients with a wide range of treatment time windows from stroke onset. As the underlying pathophysiological targets after an ischemic stroke differ between the time of injury and during repair (81–83), we may arbitrarily consider interventions given within 24 or 48 h as “neuroprotective,” of which re-establishment of blood flow and reperfusion to the injured brain tissue is currently the best strategy, and those administered beyond 48 h as “recovery-promoting” treatments. Recovery-promoting therapies should be viewed separately from those that enhance neuroprotection or reperfusion after a stroke since they have distinct therapeutic targets that are related to plasticity and growth after stroke with a therapeutic time window measured in days, weeks, or even months that may benefit a larger proportion of patients with stroke (84).
In our review, level B-R evidence of treatment effect at 3 months was available only for edaravone (particularly when combined with dexborneol) and cinepazide when administered within 48 h and clomethiazole (in total anterior circulation syndrome) within 12 h of acute ischemic stroke (53, 72, 74, 79). More recently, re-evaluating drugs as adjunctive therapies to revascularization have gained interest since many of these compounds were investigated when thrombolysis and endovascular thrombectomy were rarely available (85–88). Furthermore, despite the higher recanalization rate and efficacy of thrombectomy, approximately half of patients still had poor outcomes at 90 days (89). Our review identified four drugs recently tested in combination with revascularization attempts—citicoline, clomethiazole (included in the systematic review on GABA agonists), theophylline, and edaravone (34, 53, 56, 73). Some results are promising and such approach may be important to consider when designing future trials to re-assess supposed neuroprotective drugs, especially when taken in the context of learnings from recent studies of novel compounds given to patients receiving reperfusion therapies (90–92).
Among interventions administered beyond 48 h of stroke onset, level B-R evidence of therapeutic effect was available for SSRIs (at the end of treatment), MLC601/MLC901 (at 3 to 18 months), and cerebrolysin (at 1 or 3 months) in ischemic stroke, and Panax notoginseng (at 1 month) in ICH (17, 23–25, 28, 61). Our review also revealed investigations of other multi-modal approaches that included combination treatments, i.e., botanicals and EPO + hCG, and administering treatment together with or before planned rehabilitative training, particularly for potential recovery-promoting drugs. Combining different therapy principles is a logical step to further increase poststroke recovery, wherein a simplified theoretical scheme uses priming treatments synergistically with respective consolidation treatments (training) (93, 94). Further research on putative recovery-promoting treatments can benefit much from new approaches to patient selection, inclusion of more severe deficits, control interventions, appropriate outcome measures based on the intervention’s target and stage of stroke recovery, and longer duration of follow-up (95, 96).
Our systematic-search-and-review have several limitations. We did not include a standardized quality assessment tool in reviewing each of the papers as this is not required for a systematic-search-and-review study design especially aimed at exhaustive searches. Because of the broad range of treatments covered, we did not do any meta-analysis to obtain pooled results from different studies. This was, however, not the objective of the review and can be performed as a next step focusing on selected pharmacological classes or products. We included only papers published in the English language and may have excluded some well-conducted large studies published in other languages. One drug in particular, cortexin, had all studies only in non-English publications and was therefore not reviewed. As mentioned in the Methods section, we excluded studies that assessed psychiatric and cognitive outcomes, as well as spasticity, seizures, pain, and fatigue. We also excluded imaging and laboratory outcomes as surrogate markers since we were mainly interested in post-stroke clinical outcomes as hard endpoints. In addition, our review did not include studies on less usual causes of stroke, e.g., stroke in pregnancy and stroke in children. Finally, we focused mainly on treatments that clinicians would have ready access to rather than on investigational new drugs because we intended this review to guide clinical decision-making.
In conclusion, only one registered treatment has level A evidence for routine use in patients who suffer an acute stroke—nimodipine after SAH. There are, however, several commercially available treatments with level B evidence as either neuroprotective or recovery-promoting treatments. Further studies of putative neuroprotective drugs as adjunctive treatment to revascularization procedure, as well as more confirmatory studies on neuro-recovery treatments, will enhance the certainty of their benefit seen in clinical trials. As most molecular targets for therapy have biphasic roles in stroke pathophysiology during acute injury and in neurovascular remodeling in the recovery phase (82, 97), an intervention that failed as a neuroprotectant may not necessarily be of no benefit as a recovery-promoting treatment after a stroke. Even treatments with level C evidence may be candidates for larger studies, particularly those with signals on preclinical and clinical studies. Study designs must be based on the expected mechanism of action and stroke subtype and aimed at restoring the specific impairment at the optimal time window. Moreover, treatment for neuro-recovery may require a much longer duration than neuroprotective trials.
As the treatments we reviewed are registered products and may be available to clinicians and patients, the decision on their use must be guided by the clinical profile, neurological impairments, and outcomes they hope to improve based on the available evidence outlined.
The original contributions presented in the study are included in the article/Supplementary material; further inquiries can be directed to the corresponding author.
T-HL: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. SU: Formal analysis, Investigation, Methodology, Supervision, Validation, Writing – review & editing. YK: Formal analysis, Investigation, Validation, Writing – review & editing. HCC: Formal analysis, Investigation, Validation, Writing – review & editing. JCN: Formal analysis, Investigation, Validation, Writing – review & editing. KST: Formal analysis, Investigation, Validation, Writing – review & editing. JP: Formal analysis, Investigation, Validation, Writing – review & editing. LG: Data curation, Methodology, Project administration, Software, Supervision, Writing – review & editing. YW: Data curation, Methodology, Project administration, Software, Supervision, Writing – review & editing. NV: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors wish to sincerely thank Shi Luming of the Singapore Clinical Research Institute (Epidemiology) for her help in performing the search and Robert Gan of Moleac Singapore for his support in project management, coordination, retrieval of full-text publications, and logistics in the course of this endeavor. Both were not involved in the review, selection, analysis, and interpretation of the reports.
SU received funding for clinical trials related to edaravone, but was not involved in the assessment of edaravone for this paper. JCN received funding for clinical trials related to MLC601/MLC901. He was not involved in the assessment of MLC601/MLC901 for this paper. NV received funding for clinical trials related to MLC601/MLC901 that were paid to his institution. He was not involved in the assessment of MLC601/MLC901 for this paper.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1346177/full#supplementary-material
1. Powers, WJ, Rabinstein, AA, Ackerson, T, Adeoye, OM, Bambakidis, NC, Becker, K, et al. Guidelines for the early Management of Patients with acute ischemic Stroke: 2019 update to the 2018 guidelines for the early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
2. Mold, F, Wolfe, C, and McKevitt, C. Falling through the net of stroke care. Health Soc Care Community. (2006) 14:349–56. doi: 10.1111/j.1365-2524.2006.00630.x
3. Eissa, A, Krass, I, and Bajorek, BV. Optimizing the management of acute ischaemic stroke: a review of the utilization of intravenous recombinant tissue plasminogen activator (tPA). J Clin Pharm Ther. (2012) 37:620–9. doi: 10.1111/j.1365-2710.2012.01366.x
4. Ginsberg, MD. Current status of neuroprotection for cerebral ischemia: synoptic overview. Stroke. (2009) 40:S111–4. doi: 10.1161/STROKEAHA.108.528877
5. Lawrence, ES, Coshall, C, Dundas, R, Stewart, J, Rudd, AG, Howard, R, et al. Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke. (2001) 32:1279–84. doi: 10.1161/01.STR.32.6.1279
6. Ullberg, T, Zia, E, Petersson, J, and Norrving, B. Changes in functional outcome over the first year after stroke: an observational study from the Swedish stroke register. Stroke. (2015) 46:389–94. doi: 10.1161/STROKEAHA.114.006538
7. Meyer, S, Verheyden, G, Brinkmann, N, Dejaeger, E, De Weerdt, W, Feys, H, et al. Functional and motor outcome 5 years after stroke is equivalent to outcome at 2 months: follow-up of the collaborative evaluation of rehabilitation in stroke across Europe. Stroke. (2015) 46:1613–9. doi: 10.1161/STROKEAHA.115.009421
8. Fransen, M, Anderson, C, Chalmers, J, Chapman, N, Davis, S, MacMahon, S, et al. PROGRESS: effects of a perindopril-based blood pressure-lowering regimen on disability and dependency in 6,105 patients with cerebrovascular disease: a randomized controlled trial. Stroke. (2003) 34:2333–8. doi: 10.1161/01.STR.0000091397.81767.40
9. Katan, M, and Luft, A. Global burden of stroke. Semin Neurol. (2018) 38:208–11. doi: 10.1055/s-0038-1649503
10. Virani, SS, Alonso, A, Aparicio, HJ, Benjamin, EJ, Bittencourt, MS, Callaway, CW, et al. Heart Disease and Stroke Statistics-2021 Update: A Report from the American Heart Association. Circulation. (2021) 143:e254–743. doi: 10.1161/CIR.0000000000000950
11. Yousufuddin, M, Moriarty, JP, Lackore, KA, Zhu, Y, Peters, JL, Doyle, T, et al. Initial and subsequent 3-year cost after hospitalization for first acute ischemic stroke and intracerebral hemorrhage. J Neurol Sci. (2020) 419:117181. doi: 10.1016/j.jns.2020.117181
12. Gloede, TD, Halbach, SM, Thrift, AG, Dewey, HM, Pfaff, H, and Cadilhac, DA. Long-term costs of stroke using 10-year longitudinal data from the north East Melbourne Stroke incidence study. Stroke. (2014) 45:3389–94. doi: 10.1161/STROKEAHA.114.006200
13. Rajsic, S, Gothe, H, Borba, HH, Sroczynski, G, Vujicic, J, Toell, T, et al. Economic burden of stroke: a systematic review on post-stroke care. Eur J Health Econ. (2019) 20:107–34. doi: 10.1007/s10198-018-0984-0
14. Saka, O, McGuire, A, and Wolfe, C. Cost of stroke in the United Kingdom. Age Ageing. (2009) 38:27–32. doi: 10.1093/ageing/afn281
15. Grant, MJ, and Booth, A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Inf Libr J. (2009) 26:91–108. doi: 10.1111/j.1471-1842.2009.00848.x
16. Kleindorfer, DO, Towfighi, A, Chaturvedi, S, Cockroft, KM, Gutierrez, J, Lombardi-Hill, D, et al. 2021 guideline for the prevention of Stroke in patients with Stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. (2021) 52:e364–467. doi: 10.1161/STR.0000000000000375
17. Cochrane Stroke GroupLegg, LA, Rudberg, AS, Hua, X, Wu, S, Hackett, ML, et al. Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery. Cochrane Database Syst Rev. (2021) 2021:CD009286. doi: 10.1002/14651858.CD009286.pub4,
18. Dam, M, Tonin, P, De Boni, A, Pizzolato, G, Casson, S, Ermani, M, et al. Effects of fluoxetine and maprotiline on functional recovery in poststroke hemiplegic patients undergoing rehabilitation therapy. Stroke. (1996) 27:1211–4. doi: 10.1161/01.STR.27.7.1211
19. Mikami, K, Jorge, RE, Adams, HP, Davis, PH, Leira, EC, Jang, M, et al. Effect of antidepressants on the course of disability following stroke. Am J Geriatr Psychiatr. (2011) 19:1007–15. doi: 10.1097/JGP.0b013e31821181b0
20. Yu, M, Sun, ZJ, Li, LT, Ge, HY, Song, CQ, and Wang, AJ. The beneficial effects of the herbal medicine Di-huang-yin-zi (DHYZ) on patients with ischemic stroke: a randomized, placebo controlled clinical study. Complement Ther Med. (2015) 23:591–7. doi: 10.1016/j.ctim.2015.06.003
21. Chong, PZ, Ng, HY, Tai, JT, and Lee, SWH. Efficacy and safety of Ginkgo biloba in patients with acute ischemic Stroke: a systematic review and Meta-analysis. Am J Chin Med. (2020) 48:513–34. doi: 10.1142/S0192415X20500263
22. Ji, H, Zhou, X, Wei, W, Wu, W, and Yao, S. Ginkgol biloba extract as an adjunctive treatment for ischemic stroke: a systematic review and meta-analysis of randomized clinical trials. Medicine. (2020) 99:e18568. doi: 10.1097/MD.0000000000018568
23. Harandi, AA, Abolfazli, R, Hatemian, A, Ghragozlee, K, Ghaffar-Pour, M, Karimi, M, et al. Safety and efficacy of MLC601 in Iranian patients after Stroke: a double-blind, Placebo-Controlled Clinical Trial. Stroke Res Treat. (2011) 2011:721613. doi: 10.4061/2011/721613
24. González-Fraile, E, Martín-Carrasco, M, and Ballesteros, J. Efficacy of MLC601 on functional recovery after stroke: a systematic review and meta-analysis of randomized controlled trials. Brain Inj. (2016) 30:267–70. doi: 10.3109/02699052.2015.1118764
25. Venketasubramanian, N, Young, SH, Tay, SS, Umapathi, T, Lao, AY, Gan, HH, et al. Chen CL; CHIMES-E study investigators. CHInese medicine NeuroAiD efficacy on Stroke recovery—extension study (CHIMES-E): a multicenter study of Long-term efficacy. Cerebrovasc Dis. (2015) 39:309–18. doi: 10.1159/000382082
26. Kumar, R, Abu Bakar, A, Thanabalan, J, Paramasvaran, S, Toh, CJ, Jaffar, A, et al. Safety and use of MLC601/MLC901 (NeuroAiD™) in primary intracerebral hemorrhage: a cohort study from the NeuroAiD safe treatment registry. Brain Sci. (2020) 10:499. doi: 10.3390/brainsci10080499
27. Chen, X, Zhou, M, Li, Q, Yang, J, Zhang, Y, Zhang, D, et al. Sanchi for acute ischaemic stroke. Cochrane Database Syst Rev. (2008):CD006305. doi: 10.1002/14651858.CD006305.pub2
28. Xu, D, Huang, P, Yu, Z, Xing, DH, Ouyang, S, and Xing, G. Efficacy and safety of Panax notoginseng Saponin therapy for acute intracerebral hemorrhage, Meta-analysis, and Mini review of potential mechanisms of action. Front Neurol. (2015) 5:274. doi: 10.3389/fneur.2014.00274
29. Zhang, J, Liu, J, Li, D, Zhang, C, and Liu, M. Calcium antagonists for acute ischemic stroke. Cochrane Database Syst Rev. (2019) 2019:CD001928. doi: 10.1002/14651858.CD001928.pub3
30. Dayyani, M, Sadeghirad, B, Grotta, JC, Zabihyan, S, Ahmadvand, S, Wang, Y, et al. Prophylactic therapies for morbidity and mortality after aneurysmal subarachnoid hemorrhage: a systematic review and network Meta-analysis of randomized trials. Stroke. (2022) 53:1993–2005. doi: 10.1161/STROKEAHA.121.035699
31. Avgerinos, KI, Chatzisotiriou, A, Haidich, AB, Tsapas, A, and Lioutas, VA. Intravenous magnesium sulfate in acute Stroke. Stroke. (2019) 50:931–8. doi: 10.1161/STROKEAHA.118.021916
32. Naidech, AM, Shkirkova, K, Villablanca, JP, Sanossian, N, Liebeskind, DS, Sharma, L, et al. Magnesium sulfate and hematoma expansion: an ancillary analysis of the FAST-MAG randomized trial. Stroke. (2022) 53:1516–9. doi: 10.1161/STROKEAHA.121.037999
33. Martí-Carvajal, AJ, Valli, C, Martí-Amarista, CE, Solà, I, Martí-Fàbregas, J, and Bonfill, CX. Citicoline for treating people with acute ischemic stroke. Cochrane Database Syst Rev. (2020) 2020:CD013066. doi: 10.1002/14651858.CD013066.pub2
34. Agarwal, A, Vishnu, VY, Sharma, J, Bhatia, R, Garg, A, Dwivedi, S, et al. Citicoline in acute ischemic stroke: a randomized controlled trial. PLoS One. (2022) 17:e0269224. doi: 10.1371/journal.pone.0269224
35. Berthier, ML, Green, C, Higueras, C, Fernández, I, Hinojosa, J, and Martín, MC. A randomized, placebo-controlled study of donepezil in poststroke aphasia. Neurology. (2006) 67:1687–9. doi: 10.1212/01.wnl.0000242626.69666.e2
36. Wakisaka, Y, Matsuo, R, Nakamura, K, Ago, T, Kamouchi, M, and Kitazono, T. Pre-Stroke cholinesterase inhibitor treatment is beneficially associated with functional outcome in patients with acute ischemic Stroke and pre-Stroke dementia: the Fukuoka Stroke registry. Cerebrovasc Dis. (2021) 50:390–6. doi: 10.1159/000514368
37. Sprigg, N, and Bath, PMW. Speeding stroke recovery? A systematic review of amphetamine after stroke. J Neurol Sci. (2009) 285:3–9. doi: 10.1016/j.jns.2009.04.040
38. Yeo, S-H, Lim, Z-JI, Mao, J, and Yau, W-P. Effects of central nervous system drugs on recovery after Stroke: a systematic review and Meta-analysis of randomized controlled trials. Clin Drug Investig. (2017) 37:901–28. doi: 10.1007/s40261-017-0558-4
39. Goldstein, LB, Lennihan, L, Rabadi, MJ, Good, DC, Reding, MJ, Dromerick, AW, et al. Effect of Dextroamphetamine on Poststroke motor recovery: a randomized clinical trial. JAMA Neurol. (2018) 75:1494–501. doi: 10.1001/jamaneurol.2018.2338
40. Gagnon, DJ, Leclerc, AM, Riker, RR, Brown, CS, May, T, Nocella, K, et al. Amantadine and Modafinil as Neurostimulants during post-stroke care: a systematic review. Neurocrit Care. (2020) 33:283–97. doi: 10.1007/s12028-020-00977-5
41. Luauté, J, Villeneuve, L, Roux, A, Nash, S, Bar, J-Y, Chabanat, E, et al. Adding methylphenidate to prism-adaptation improves outcome in neglect patients. A randomized clinical trial. Cortex. (2018) 106:288–98. doi: 10.1016/j.cortex.2018.03.028
42. Lokk, J, Salman Roghani, R, and Delbari, A. Effect of methylphenidate and/or levodopa coupled with physiotherapy on functional and motor recovery after stroke--a randomized, double-blind, placebo-controlled trial. Acta Neurol Scand. (2011) 123:266–73. doi: 10.1111/j.1600-0404.2010.01395.x
43. Bath, PMW, Sprigg, N, and England, T. Colony stimulating factors (including erythropoietin, granulocyte colony stimulating factor and analogues) for stroke. Cochrane Database Syst Rev. (2013) 2013:CD005207. doi: 10.1002/14651858.CD005207.pub4
44. Chen, X, Sun, W, Zhong, P, and Wu, D. Colony-stimulating factors on mobilizing CD34+ cells and improving neurological functions in patients with stroke: a meta-analysis and a systematic review. Front Pharmacol. (2021) 12:704509. doi: 10.3389/fphar.2021.704509
45. Huang, X, Liu, Y, Bai, S, Peng, L, Zhang, B, and Lu, H. Granulocyte colony stimulating factor therapy for stroke: a pairwise meta-analysis of randomized controlled trial. PLoS One. (2017) 12:e0175774. doi: 10.1371/journal.pone.0175774
46. Cramer, SC, and Hill, MD. Human choriogonadotropin and epoetin alfa in acute ischemic stroke patients (REGENESIS-LED trial). Int J Stroke. (2014) 9:321–7. doi: 10.1111/ijs.12260
47. Engelter, ST, Altersberger, V, Meya, L, Wiesner, K, Wiegert, M, Held, J, et al. Enhancement of stroke rehabilitation with levodopa-the ESTREL-study. Neurologie und Rehabilitation. (2020) 25:S47.
48. Ford, GA, Bhakta, BB, Cozens, A, Hartley, S, Holloway, I, Meads, D, et al. Safety and efficacy of co-careldopa as an add-on therapy to occupational and physical therapy in patients after stroke (DARS): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. (2019) 18:530–8. doi: 10.1016/S1474-4422(19)30147-4
49. Cramer, SC, Dobkin, BH, Noser, EA, Rodriguez, RW, and Enney, LA. Randomized, placebo-controlled, double-blind study of ropinirole in chronic stroke. Stroke. (2009) 40:3034–8. doi: 10.1161/STROKEAHA.109.552075
50. Conroy, B, Zorowitz, R, Horn, SD, Ryser, DK, Teraoka, J, and Smout, RJ. An exploration of central nervous system medication use and outcomes in stroke rehabilitation. Arch Phys Med Rehabil. (2005) 86:S73–81. doi: 10.1016/j.apmr.2005.08.129
51. Elwan, O, Helmy, AA, Tamawy, ME, Naseer, MA, Banhawy, IE, Kader, AAA, et al. Ergoloids and ischaemic strokes; efficacy and mechanism of action. J Int Med Res. (1995) 23:154–66. doi: 10.1177/030006059502300302
52. Bochner, F, Eadie, MJ, and Tyrer, JH. Use of an ergot preparation (hydergine) in the convalescent phase of stroke. J Am Geriatr Soc. (1973) 21:10–7. doi: 10.1111/j.1532-5415.1973.tb00841.x
53. Liu, J, Zhang, J, and Wang, LN. Gamma aminobutyric acid (GABA) receptor agonists for acute stroke. Cochrane Database Syst Rev. (2018) 10:CD009622. doi: 10.1002/14651858.CD009622.pub5
54. Bath, PM. Theophylline, aminophylline, caffeine and analogues for acute ischaemic stroke. Cochrane Database Syst Rev. (2004) 2004:CD000211. doi: 10.1002/14651858.CD000211.pub2
55. Bath, PMW, and Bath-Hextall, FJ. Pentoxifylline, propentofylline and pentifylline for acute ischaemic stroke. Cochrane Database Syst Rev. (2004) 2004:CD000162. doi: 10.1002/14651858.CD000162.pub2
56. Modrau, B, Andersen, G, Mikkelsen, IK, Nielsen, A, Hansen, MB, Johansen, MB, et al. Theophylline as an add-on to thrombolytic therapy in acute ischemic Stroke: a randomized placebo-controlled trial. Stroke. (2020) 51:1983–90. doi: 10.1161/STROKEAHA.119.027446
57. Laska, AC, von Arbin, M, Kahan, T, Hellblom, A, and Murray, V. Long-term antidepressant treatment with moclobemide for aphasia in acute stroke patients: a randomised, double-blind, placebo-controlled study. Cerebrovasc Dis. (2005) 19:125–32. doi: 10.1159/000083256
58. Bartolo, M, Zucchella, C, Capone, A, Sandrini, G, and Pierelli, F. An explorative study regarding the effect of L-deprenyl on cognitive and functional recovery in patients after stroke. J Neurol Sci. (2015) 349:117–23. doi: 10.1016/j.jns.2014.12.039
59. Mohammadianinejad, SE, Majdinasab, N, Sajedi, SA, Abdollahi, F, Moqaddam, MM, and Sadr, F. The effect of lithium in post-stroke motor recovery: a double-blind, placebo-controlled, randomized clinical trial. Clin Neuropharmacol. (2014) 37:73–8. doi: 10.1097/WNF.0000000000000028
60. Ziganshina, LE, Abakumova, T, and Hoyle, CH. Cerebrolysin for acute ischaemic stroke. Cochrane Database Syst Rev. (2020) 7:CD007026. doi: 10.1002/14651858.CD007026.pub6
61. Bornstein, NM, Guekht, A, Vester, J, Heiss, W-D, Gusev, E, Hömberg, V, et al. Safety and efficacy of Cerebrolysin in early post-stroke recovery: a meta-analysis of nine randomized clinical trials. Neurol Sci. (2018) 39:629–40. doi: 10.1007/s10072-017-3214-0
62. Chang, WH, Park, CH, Kim, DY, Shin, YI, Ko, MH, Lee, A, et al. Cerebrolysin combined with rehabilitation promotes motor recovery in patients with severe motor impairment after stroke. BMC Neurol. (2016) 16:31. doi: 10.1186/s12883-016-0553-z
63. Woo, PYM, Ho, JWK, Ko, NMW, Li, RPT, Jian, L, Chu, ACH, et al. Randomized, placebo-controlled, double-blind, pilot trial to investigate safety and efficacy of Cerebrolysin in patients with aneurysmal subarachnoid hemorrhage. BMC Neurol. (2020) 20:401. doi: 10.1186/s12883-020-01908-9
64. Cherry, KM, Lenze, EJ, and Lang, CE. Combining d-cycloserine with motor training does not result in improved general motor learning in neurologically intact people or in people with stroke. J Neurophysiol. (2014) 111:2516–24. doi: 10.1152/jn.00882.2013
65. Beladi Moghadam, N, Pourheidar, E, Ahmadpour, F, Kafi, H, Salamzadeh, J, Nasiri, S, et al. The effects of memantine on the serum concentrations of matrix metalloproteinases and neurologic function of patients with ischemic stroke. J Clin Neurosci. (2021) 90:268–72. doi: 10.1016/j.jocn.2021.06.005
66. Berthier, ML, Green, C, Lara, JP, Higueras, C, Barbancho, MA, Dávila, G, et al. Memantine and constraint-induced aphasia therapy in chronic poststroke aphasia. Ann Neurol. (2009) 65:577–85. doi: 10.1002/ana.21597
67. Ward, A, Carrico, C, Powell, E, Westgate, PM, Nichols, L, Fleischer, A, et al. Safety and improvement of movement function after stroke with atomoxetine: a pilot randomized trial. Restor Neurol Neurosci. (2017) 35:1–10. doi: 10.3233/RNN-160673
68. Zittel, S, Weiller, C, and Liepert, J. Reboxetine improves motor function in chronic stroke: a pilot study. J Neurol. (2007) 254:197–201. doi: 10.1007/s00415-006-0326-5
69. Ortiz, JF, Cruz, C, Patel, A, Khurana, M, Eissa-Garcés, A, Alzamora, IM, et al. Opioid antagonist in the treatment of ischemic stroke. Brain Sci. (2021) 11:805. doi: 10.3390/brainsci11060805
70. Li, S, Long, J, Ma, Z, Xu, Z, Li, J, and Zhang, Z. Assessment of the therapeutic activity of a combination of almitrine and raubasine on functional rehabilitation following ischaemic stroke. Curr Med Res Opin. (2004) 20:409–15. doi: 10.1185/030079904125003080
71. Page, SJ, Kasner, SE, Bockbrader, M, Goldstein, M, Finklestein, SP, Ning, M, et al. A double-blind, randomized, controlled study of two dose strengths of dalfampridine extended release on walking deficits in ischemic stroke. Restor Neurol Neurosci. (2020) 38:301–9. doi: 10.3233/RNN-201009
72. Fidalgo, M, Ricardo Pires, J, Viseu, I, Magalhães, P, Gregório, H, Afreixo, V, et al. Edaravone for acute ischemic stroke—systematic review with meta-analysis. Clin Neurol Neurosurg. (2022) 219:107299. doi: 10.1016/j.clineuro.2022.107299
73. Hu, R, Guo, Y, Lin, Y, Tang, Y, Tang, Q, Wang, X, et al. Safety and efficacy of edaravone combined with alteplase for patients with acute ischemic stroke: a systematic review and meta-analysis. Pharmazie. (2021) 76:109–13. doi: 10.1691/ph.2021.0949
74. Xu, J, Wang, A, Meng, X, Yalkun, G, Xu, A, Gao, Z, et al. Edaravone Dexborneol versus Edaravone alone for the treatment of acute ischemic Stroke—a phase III, randomized, double-blind, Comparative Trial. Stroke. (2021) 52:772–80. doi: 10.1161/STROKEAHA.120.031197
75. Qin, M, Feng, L, Yang, C, Wei, D, Li, T, Jiang, P, et al. Edaravone use in acute intracerebral hemorrhage: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol. (2022) 13:935198. doi: 10.3389/fphar.2022.935198
76. Ricci, S, Celani, MG, Cantisani, TA, and Righetti, E. Piracetam for acute ischaemic stroke. Cochrane Database Syst Rev. (2012) 2012:CD000419. doi: 10.1002/14651858.CD000419.pub3
77. Zhang, J, Wei, R, Chen, Z, and Luo, B. Piracetam for aphasia in post-stroke patients: a systematic review and Meta-analysis of randomized controlled trials. CNS Drugs. (2016) 30:575–87. doi: 10.1007/s40263-016-0348-1
78. Wu, S, Zeng, Q, Liu, M, Yang, J, He, S, Lin, S, et al. Buflomedil for acute ischaemic stroke. Cochrane Database Syst Rev. (2015) 2015:CD009570. doi: 10.1002/14651858.CD009570.pub2
79. Ni, J, Chen, H, Chen, G, Ji, Y, Yi, F, Zhang, Z, et al. Efficacy and safety of cinepazide maleate injection in patients with acute ischemic stroke: a multicenter, randomized, double-blind, placebo-controlled trial. BMC Neurol. (2020) 20:282. doi: 10.1186/s12883-020-01844-8
80. Paolucci, S, and De Angelis, D. New developments on drug treatment rehabilitation. Clin Exp Hypertens. (2006) 28:345–8. doi: 10.1080/10641960600549454
81. Candelario-Jalil, E. Injury and repair mechanisms in ischemic stroke: considerations for the development of novel neurotherapeutics. Curr Opin Investig Drugs. (2009) 10:644–54.
82. Hurtado, O, Pradillo, JM, Alonso-Escolano, D, Lorenzo, P, Sobrino, T, Castillo, J, et al. Neurorepair versus neuroprotection in stroke. Cerebrovasc Dis. (2006) 21:54–63. doi: 10.1159/000091704
83. Dirnagl, U, Iadecola, C, and Moskowitz, MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. (1999) 22:391–7. doi: 10.1016/S0166-2236(99)01401-0
84. Cramer, SC. Drugs to enhance motor recovery after Stroke. Stroke. (2015) 46:2998–3005. doi: 10.1161/STROKEAHA.115.007433
85. Fisher, M, and Savitz, SI. Pharmacological brain cytoprotection in acute ischaemic stroke—renewed hope in the reperfusion era. Nat Rev Neurol. (2022) 18:193–202. doi: 10.1038/s41582-021-00605-6
86. Savitz, SI, Baron, JC, Fisher, M, and Consortium, SX. Stroke treatment academic industry roundtable x: brain cytoprotection therapies in the reperfusion era. Stroke. (2019) 50:1026–31. doi: 10.1161/STROKEAHA.118.023927
87. Mulder, IA, van Bavel, ET, de Vries, HE, and Coutinho, JM. Adjunctive cytoprotective therapies in acute ischemic stroke: a systematic review. Fluids Barriers CNS. (2021) 18:46. doi: 10.1186/s12987-021-00280-1
88. Vos, EM, Geraedts, VJ, van der Lugt, A, Dippel, DWJ, Wermer, MJH, Hofmeijer, J, et al. Systematic review—combining neuroprotection with reperfusion in acute ischemic Stroke. Front Neurol. (2022) 13:840892. doi: 10.3389/fneur.2022.840892
89. Goyal, M, Menon, BK, van Zwam, WH, Dippel, DW, Mitchell, PJ, Demchuk, AM, et al. HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
90. Hill, MD, Goyal, M, Menon, BK, Nogueira, RG, McTaggart, RA, Demchuk, AM, et al. ESCAPE-NA1 investigators. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): a multicentre, double-blind, randomised controlled trial. Lancet. (2020) 395:878–87. doi: 10.1016/S0140-6736(20)30258-0
91. Zhou, XF. ESCAPE-NA1 trial brings Hope of neuroprotective drugs for acute ischemic Stroke: highlights of the phase 3 clinical trial on Nerinetide. Neurosci Bull. (2021) 37:579–81. doi: 10.1007/s12264-020-00627-y
92. Chamorro, Á, Lo, EH, Renú, A, van Leyen, K, and Lyden, PD. The future of neuroprotection in stroke. J Neurol Neurosurg Psychiatry. (2021) 92:129–35. doi: 10.1136/jnnp-2020-324283
93. Sommer, CJ, and Schäbitz, WR. Fostering Poststroke recovery: towards combination treatments. Stroke. (2017) 48:1112–9. doi: 10.1161/STROKEAHA.116.013324
94. Sommer, CJ, and Schäbitz, WR. Principles and requirements for stroke recovery science. J Cereb Blood Flow Metab. (2021) 41:471–85. doi: 10.1177/0271678X20970048
95. Stinear, CM, Lang, CE, Zeiler, S, and Byblow, WD. Advances and challenges in stroke rehabilitation. Lancet Neurol. (2020) 19:348–60. doi: 10.1016/S1474-4422(19)30415-6
96. Cramer, SC, Koroshetz, WJ, and Finklestein, SP. The case for modality-specific outcome measures in clinical trials of stroke recovery-promoting agents. Stroke. (2007) 38:1393–5. doi: 10.1161/01.STR.0000260087.67462.80
Keywords: stroke, recovery, neuro-restoration, review, rehabilitation, evidence
Citation: Lee T-H, Uchiyama S, Kusuma Y, Chiu HC, Navarro JC, Tan KS, Pandian J, Guo L, Wong Y and Venketasubramanian N (2024) A systematic-search-and-review of registered pharmacological therapies investigated to improve neuro-recovery after a stroke. Front. Neurol. 15:1346177. doi: 10.3389/fneur.2024.1346177
Received: 15 December 2023; Accepted: 08 January 2024;
Published: 31 January 2024.
Edited by:
Raffaele Ornello, University of L'Aquila, ItalyReviewed by:
Guohui Jiang, Affiliated Hospital of North Sichuan Medical College, ChinaCopyright © 2024 Lee, Uchiyama, Kusuma, Chiu, Navarro, Tan, Pandian, Guo, Wong and Venketasubramanian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Narayanaswamy Venketasubramanian, cmFtYW5pX252QHJhZmZsZXNtZWRpY2FsLmNvbQ==
†ORCID: Yoko Wong orcid.org/0000-0002-1276-7060
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.