
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 29 February 2024
Sec. Stroke
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1345914
This article is part of the Research TopicAdvances and controversies in ischemic stroke management: from prevention to diagnosis and acute treatmentView all 95 articles
Background and purpose: Significant differences in the outcomes observed in patients with acute ischemic stroke (AIS) have led to research investigations for identifying the predictors. In this retrospective study, we aimed to investigate the relationship of different clinical and imaging factors with the prognosis of AIS.
Materials and methods: All clinical and imaging metrics were compared between the good and poor prognosis groups according to the modified Rankin Scale (mRS) score at 90 days after discharge. Clinical factors included gender, age, NIHSS scores at admission, and other medical history risk factors. Imaging markers included the lesion’s size and location, diffusion, and perfusion metrics of infarction core and peripheral regions, and the state of collateral circulation. Spearman’s correlations were analyzed for age and imaging markers between the different groups. The Chi-square test and Cramer’s V coefficient analysis were performed for gender, collateral circulation status, NIHSS score, and other stroke risk factors.
Results: A total of 89 patients with AIS were divided into the good (mRS score ≤ 2) and poor prognosis groups (mRS score ≥ 3). There were differences in NIHSS score at the admission; relative MK (rMK), relative MD (rMD), relative CBF (rCBF) of the infarction core; relative mean transit time (rMTT), relative time to peak (rTTP), and relative CBF (rCBF) of peripheral regions; and collateral circulation status between the two groups (p < 0.05). Among them, the rMK of infarction lesions had the strongest correlation with the mRS score at 90 days after discharge (r = 0.545, p < 0.001).
Conclusion: Perfusion and diffusion metrics could reflect the microstructure and blood flow characteristics of the lesion, which were the key factors for the salvage ability and prognosis of the infarction tissue. The characteristics of the infarction core and peripheral regions have different effects on the outcomes. Diffusion of infarction core has strong relations with the prognosis, whereas the time metrics (MTT, TTP) were more important for peripheral regions. MK had a more significant association with prognosis than MD. These factors were the primary markers influencing the prognosis of cerebral infarction patients.
Acute ischemic stroke (AIS) is one of the common causes of death and disability worldwide (1). The recurrence rate is also high, which greatly reduces the patient’s quality of life. Many stroke survivors continue to experience dysfunction or long-term negative effects despite continuous rehabilitation (2). Therefore, stroke is a significant health problem and a financial burden on society (3). Prognostic prediction of AIS is essential to help patients and caregivers set appropriate clinical plans and monitor treatment during recovery and adaptation. In recent years, the mRS score has been used to evaluate neurological functional status after stroke (4), which can reflect characteristics of stroke recovery that are directly relevant to patients’ daily activities (5). Yet, it is challenging to predict neural function after an ischemic stroke. There are some potential indicators for long-term outcomes, such as age (6) and NIHSS score (4).
Magnetic resonance imaging (MRI) is commonly used for the diagnosis and evaluation of ischemic stroke, in which diffusion-weighted imaging (DWI) is particularly sensitive to reveal the range of cytotoxic edema (7, 8). Diffusion kurtosis imaging (DKI) complements the directionality of molecular diffusion (9). Both DWI and DKI could reveal the microstructure characteristics of the brain tissue (10). The lesion size obtained by DWI is an independent risk factor for the clinical outcome (11). Previous studies have shown that MD and MK closely relate to the necrotic regions at follow-up (12, 13). In addition, PWI provides the hemodynamic status of the infarct lesion and surrounding tissues. It can detect the severity and extent of the impaired perfusion for both the ischemic core and the surrounding brain regions (14, 15). Previous research has demonstrated a strong correlation between impaired brain perfusion and stroke recurrence and functional outcomes (16). MRA results can be used to evaluate the feeding artery of the lesion and can reflect the status of collateral circulation. Studies have found that the status of collateral circulation at the distal end of the lesion is significantly correlated with different prognoses (17).
This study aimed to investigate the correlation between the characteristics of an infarction, including the clinical and imaging features with its neurological outcome at the follow-up to explore the key factors in predicting the prognosis of an ischemic stroke.
AIS patients admitted to the Tianjin First Central Hospital were recruited for the study. All patients had an apparent onset of neurological impairment (communication dysfunction, unilateral face or limb numbness, etc.). Acute cerebral infarction was confirmed by the admission MRI scan, which included T1WI, T2WI, DWI, DKI, Magnetic Resonance Angiography (MRA), and perfusion-weighted imaging (PWI). The clinical history, including demographics, risk factors, neurological evaluation, and NIHSS score (18), was collected. The NIHSS score ranged from 0 to 42, where 0–1 was defined as normal or nearly normal; 2–4 was considered mild; 5–15 was considered moderate; 16–20 was considered moderate–severe and 21–42 was defined as a severe stroke (19). The exclusion criteria were small lesions (minimum diameter < 10 mm), bilateral lesions, intracranial hemorrhage or lesions, craniocerebral trauma or surgery history, and endovascular or intravenous thrombolytic therapy.
During the study period, 250 AIS patients were collected. Of them, 161 patients were excluded due to other intracranial space occupation or history of craniocerebral surgery, bilateral cerebral infarction (62 patients), and the maximum diameter of DWI high signal <10 mm. Finally, 89 patients were enrolled in the present study (Figure 1). After 3 months of admission, the mRS score was assessed and used as an indicator of the prognosis of AIS (20), with mRS ranging from 0 (asymptomatic) to 6 (death). For the patients with mRS Score between 0 and 2, it indicated that the patients could live independently, while the patients with mRS Score higher than 2 would need different degrees of help from others (21–23). Therefore, 89 patients were divided into two groups: the good prognosis group and the poor prognosis group. The good prognosis was defined as mRS score ≤ 2, and the poor prognosis was defined as mRS score ≥ 3 at 90 days after discharge. Their demographic characteristics are shown in Table 1.
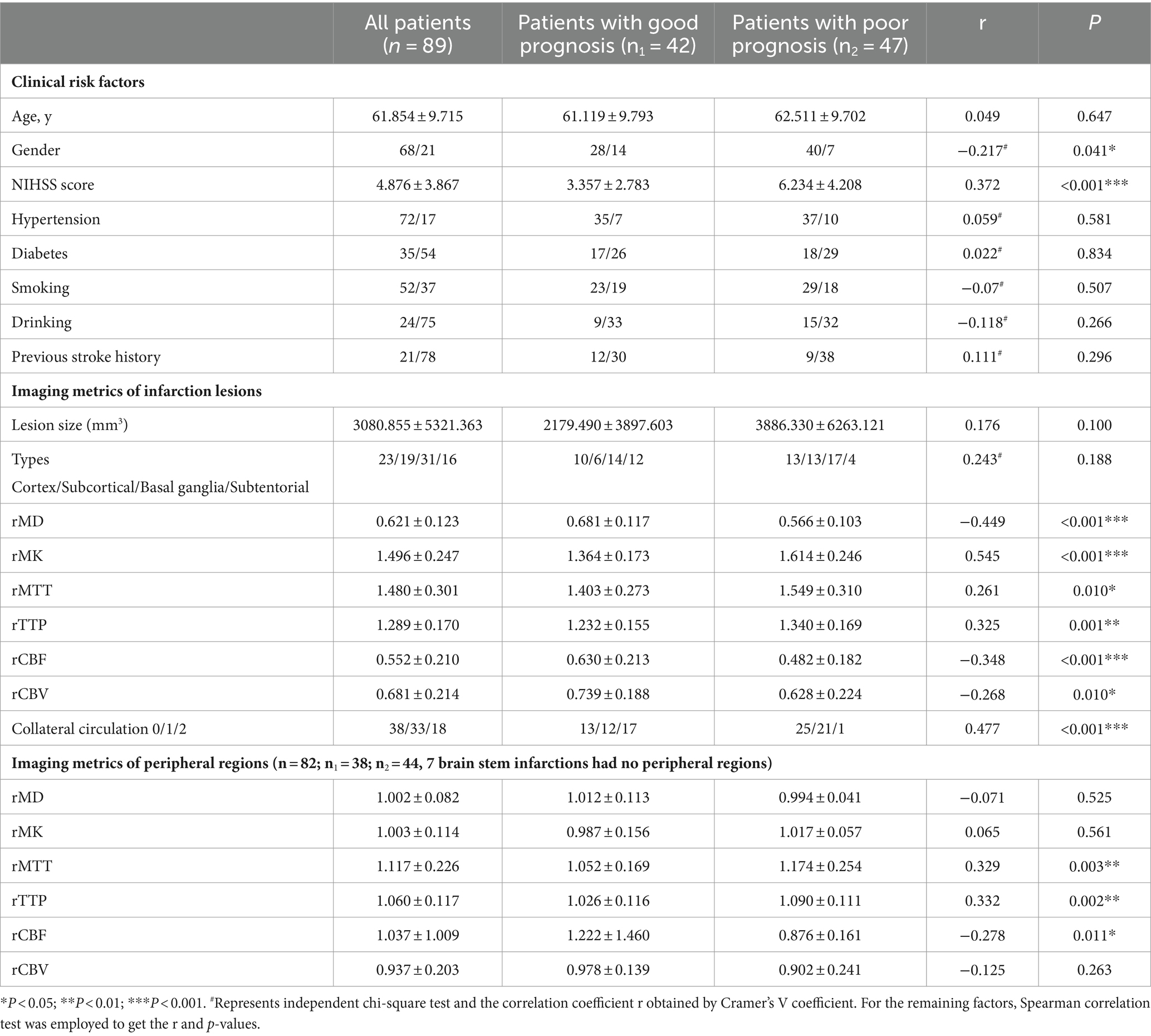
Table 1. The demographic, clinical and imaging features in different groups of patients (mean ± SD).
All MRI examinations (including T1WI, T2WI, DWI, DKI, MRA, and PWI) were performed using a 3 T Siemens Trio imager with a 32-channel head coil (Siemens Healthineers, Erlangen, Germany). The DWI was performed using a single-shot echo-planar sequence with repetition time (TR)/echo time (TE) = 5,000/93 ms, field of view (FOV) = 221 × 211 mm2, matrix = 183 × 384, axial slices (number/thickness/gap) = 20/5.0 mm/1.25 mm, and three diffusion directions with two b values (0, 1,000 s/mm)2; DKI was performed using a multi-band echo-planar sequence with TR/TE = 3,000/95 ms, FOV = 230 × 230 mm2, matrix = 183 × 384, axial slices (number/thickness/gap) = 20/5.0 mm/1.25 mm, and 20 diffusion directions with three b values (0, 1,000, 2,000 s/mm)2. PWI was collected after the injection of intravenous contrast agent, TR/TE = 1,480/32 ms, FOV = 230 × 230 mm2, axial slices (number/thickness/gap) = 20/5 mm/1.25 mm; MRA sequence was performed using TOF-3D technology, TR/TE = 27/3.59 ms, FOV = 230 × 230 mm2, slice thickness = 0.7 mm; total scan time for the imaging protocol was about 15 min.
The DKI data were converted to the NIfTI format and then processed for motion correction, eddy current correction, and Gaussian smoothing noise reduction (FMRIB Software Library, http://www.fmrib.ox.ac.uk/fsl/). The mean diffusivity (MD) and mean kurtosis (MK) maps were derived from diffusional kurtosis estimator software.1
The PWI data were post-processed by Siemens “Neuro Perfusion” software package to obtain the mean transit time (MTT) (s) and the time to peak (TTP) (s), cerebral blood flow (CBF) (ml/min/100 g), and cerebral blood volume (CBV) (ml/100 g).
The volume of the lesions was obtained by the area of all slices multiplied by the slice thickness. All other imaging metrics of infarction lesions and peripheral regions were measured at the maximum slice of the lesions.
The lesions were delineated in MRIcron software, and the ROI of the maximum slice was saved and copied to the DKI and PWI maps to obtain MD, MK, MTT, TTP, CBF, and CBV values. In addition to the acute infarct lesions, the ROI were also flipped to the opposite side to obtain the above metrics of the contra-lateral normal brain regions. The relative metrics of diffusion and perfusion were the ratio between the lesion’s values and the contra-lateral normal values, referred to as the rMTT, rTTP, rCBF, rCBV, rMD, and rMK of the infarction lesions.
Besides the characteristics of the infarction lesions, the relative metrics (rMTT, rTTP, rCBF, rCBV, rMD, and rMK) of the peripheral regions outside the infarction were also measured. The ROI of the peripheral region would be within the 2 cm outside the lesions and at the same blood supply region of the infarction.
The MRA vessels distal to the occlusion were evaluated as an indication of leptomeningeal collateral supply to the ischemic area. Compared to the contra-lateral normal cerebral hemisphere, a 3-point grading scale was used (0 = none/poor, 1 = fair, 2 = good/normal) (24).
Statistical analysis was performed using the Statistical Product and Service Solutions software (SPSS, Chicago, Ill). Medcalc and GraphPad Prism 82 software packages were used for graph rendering. All clinical and imaging metrics were compared between the good and poor prognosis groups. Student’s t-test or Mann–Whitney U-test was used for continuous variables with a normal or nonnormal distribution, and the Chi-square test was used for categorical variables. Continuous variables included age, lesion size, rMTT, rTTP, rCBF, rCBV, rMD, and rMK of infarction lesions and peripheral regions. Categorical variables included gender, history of hypertension, diabetes, smoking, drinking, previous stroke, NIHSS score, and grading of collateral circulation. Spearman’s correlations were determined between age, imaging markers, and different prognoses. The chi-square test and Cramer’s V coefficient were determined between gender, all risk factors, NIHSS score, grading of collateral circulation, and different prognoses. The ROC curve was used to compare the predictive efficacy of clinical and imaging metrics on the prognosis of the infarction, and the area under the curve (AUC) was calculated. p < 0.05 was considered a significant difference.
A total of 250 patients with AIS were collected, among which seven cases with surgery or intracranial lesions, 92 cases with small lesions, and 62 cases with bilateral lesions were excluded. Finally, 89 cases (68 males, 21 females; mean age 61.9 ± 9.7 years, age range 37–80 years) were included. A flow chart of the patient inclusion process in the study is depicted in Figure 1. The NIHSS score of all patients was assessed on admission (mean score 4.876 ± 3.867, score range 0–16). Meanwhile, 41 cases were diagnosed as mild stroke by the neurologist, 33 as moderate stroke, 2 as moderate to severe stroke, and the remaining 13 patients had no significant neurological impairment. The collateral circulation status evaluated by MRA was grade 0 in 38 cases, grade 1 in 33 cases, and grade 2 in 18 cases. At 90 days after discharge, 42 patients had a good prognosis and 47 patients had a poor prognosis. The demographic and clinical features of all patients in the two groups are shown in Table 1.
All 89 patients completed imaging examinations successfully. DWI images showed high signal in all cases, and most cases showed low signal lesions on MD images, high signal lesions on MK images, increased MTT, TTP, and decreased CBF. In some cases, MK and MD images showed heterogeneous signals, and CBF changes were not obvious. These patients without significant imaging changes (MK, MD, and CBF) had a better prognosis. Two typical cases are shown in Figure 2.
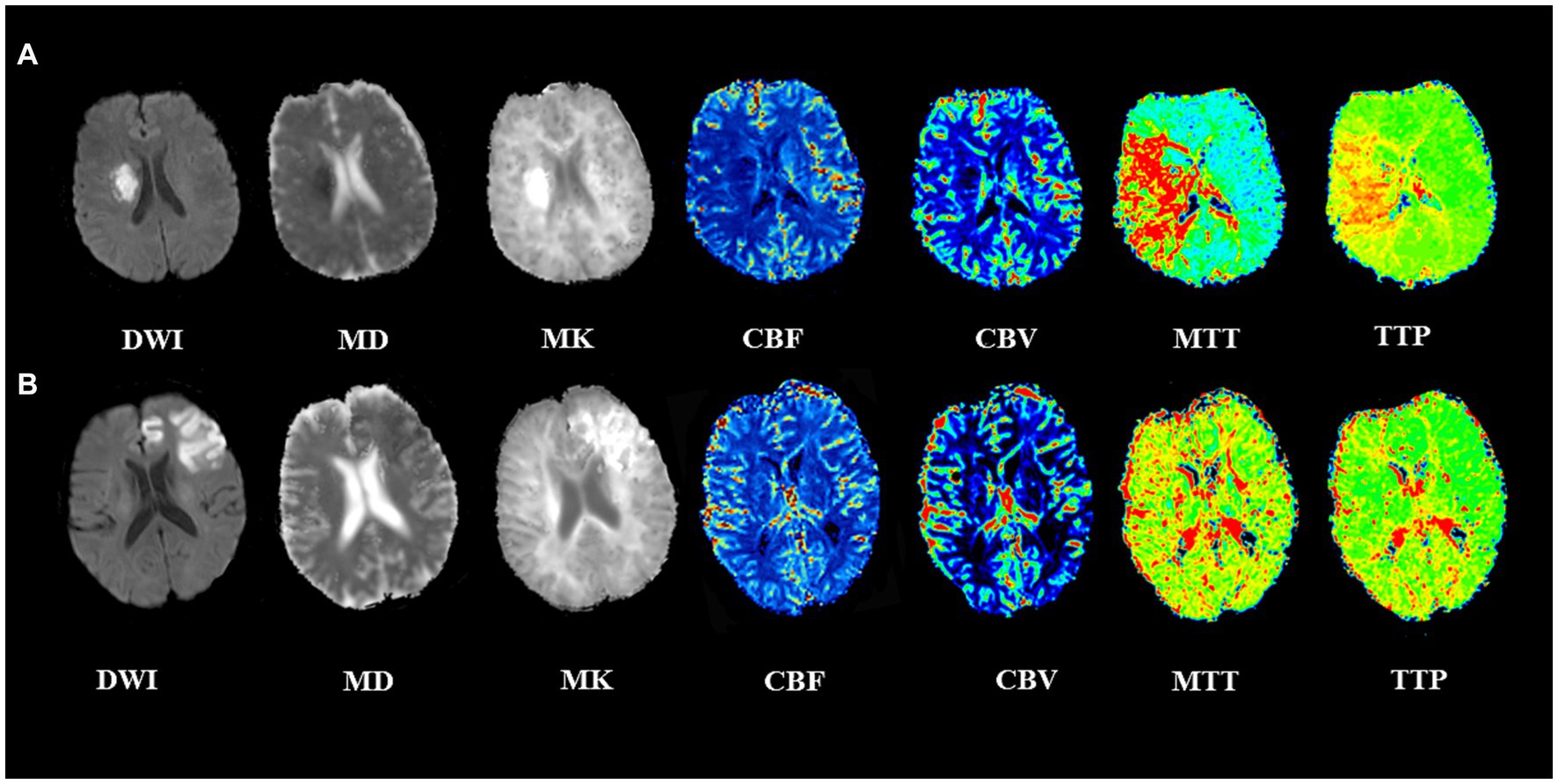
Figure 2. Two representative cases with acute ischemic stroke. (A) A 56-year-old man with left limb numbness for 3 days. The lesion adjacent to the right basal ganglia showed a low MD, high MK lesion, with a large area of increased MTT, TTP and decreased CBF. The admission NIHSS score was 3, 90-day mRS score was 4, indicating a poor prognosis. (B) A 62-year-old man with dysarthria for 2 h. The lesion at the left frontal cortex showed heterogeneous low MD, high MK, CBF, MTT, and TTP changes were not noticeable. The admission NIHSS score was 10, 90-day mRS score was 1, indicating a good prognosis.
Clinical information included age, gender, risk factors (hypertension, diabetes, previous stroke, smoking, and drinking history), and NIHSS score. As shown in Table 1, age and all history risk factors did not differ significantly with different prognoses. The prognosis of males was worse than that of females (p = 0.04). In addition, there was a significant positive correlation between NIHSS score and different prognoses (r = 0.384, p < 0.001). Despite having a weak correlation with prognosis, gender cannot be used as an independent indicator of prognosis, among all clinical features.
Infarction was categorized into cortical, subcortical, basal ganglia, and subtentorial based on the locations and blood supply. Other imaging features included lesion size, normalized diffusion (rMD and rMK), perfusion metrics (rMTT, rTTP, rCBF, and rCBV), and collateral circulation status. As shown in Table 1, lesion size and the types of cerebral infarction did not differ significantly with different prognoses. The other imaging metrics were statistically different between the two prognosis groups. rMK (r = 0.545, p < 0.001), rMD (r = −0.449, p < 0.001), and rCBF (r = −0.348, p < 0.001) were significantly correlated with functional prognosis, rMTT (r = 0.261, p = 0.01), rTTP (r = 0.325, p = 0.001), and rCBV (r = −0.268, p = 0.008) were correlated with functional prognosis.
In addition, different collateral circulation status also showed a significant correlation with prognosis (r = 0.477, p < 0.001).
As shown in Table 1, The diffusion characteristics (rMD and rMK) of the peripheral regions were not significant to the outcomes (Both p > 0.05). The rCBF of peripheral regions was mildly significant to the prognosis (r = −0.278, p = 0.011), but the time metrics (rMTT, rTTP) of peripheral regions showed more significant to the final prognosis (r = 0.329, p = 0.003 and r = 0.332, p = 0.002, respectively). The rCBV of peripheral regions showed no significance to the prognosis (r = −0.125, p = 0.263).
In all clinical and imaging metrics, the NIHSS score, rMD, rMK, rCBF of infarction lesions, and collateral circulation were most significantly correlated with the prognosis (p < 0.001). The ROC curves of these key markers in predicting the prognosis are presented in Figure 3. Areas under the curve of NIHSS score, rMD, rMK, rCBF, and collateral circulation were 0.701, 0.759, 0.815, 0.704, and 0.697, respectively.
AIS is one of the leading causes of disability and a significant cause of mortality among adults (25). The prevalence of AIS is high, and the annual incidence is steadily increasing, bringing a severe burden to patients and society. Therefore, early diagnosis, prognosis assessment, and treatment are essential.
Magnetic resonance imaging (MRI) is a commonly used diagnostic tool for stroke in the clinical setting (15). Imaging indicators are critical and can provide detailed information about the lesions. The prognosis of AIS is highly correlated with the imaging markers. The DWI sequence is widely used in the diagnosis of early cerebral infarction, and it is especially sensitive for detecting the location of cerebral infarction and the size of necrotic lesions in brain tissue (26). In addition to DWI, PWI can non-invasively and intuitively reflect the degree of cerebral blood flow perfusion attenuation. The changes in its perfusion indicators are consistent with the pathophysiological process of cerebral ischemia. PWI can detect a decrease in blood perfusion before DWI detects a cerebral infarction and can be used to assess perfusion improvement before and after cerebral ischemia treatment. Hence, PWI has clinical utility in the early detection and prognosis assessment of cerebral ischemic events (27).
DKI is a kurtosis imaging technology based on DWI and diffusion tensor imaging (DTI), and kurtosis is a statistical metric for quantifying the non-Gaussianity of a probability distribution (10). In normal tissues, the movement of water molecules usually deviates from Gaussian distribution due to the restrictions of the cell membrane and organelle membrane. Different physiological environments have different degrees of deviations from Gaussian distribution, and DKI technology can detect the deviation degree through high b values of diffusion gradients. A more significant deviation leads to a higher MK value. Therefore, the MK value can reflect the restricted degree of movement of water molecules in tissues, and thereby obtain information on subtle structural changes. Previous DKI studies on acute cerebral infarction have found higher MK and lower MD in infarction foci (28, 29). Although various parameters could be obtained from DKI, only MK and MD were mainly used in patients with AIS. Previous studies have shown that AK, RK, and other parameters are insufficient, and MK is the best evaluation parameter for ischemic lesions (30).
Various independent factors influence the prognosis of acute cerebral infarction. Numerous clinical metrics, including the patient’s age, onset time, symptoms, type and size of infarct lesions, concurrent diabetes and other disorders, as well as various treatment options, could affect the prognosis of patients (31, 32). The NIHSS score is frequently employed in the clinical setting to directly reflect patients’ neurological injury status. And the NIHSS score at admission was found to be correlated with the prognosis in this study; while other clinical information was not found to be significant when analyzed as independent variables. Zheng et al. (33) performed multiple evaluations of the NIHSS score after the onset of the disease and found a strong correlation between the NIHSS score at 7 days after the onset and the long-term prognosis of the patients. For imaging metrics, perfusion metrics that reflect the blood flow status of the infarct tissue are correlated with prognosis. In addition, other factors such as cell tolerance to ischemia and the presence or absence of penumbra may also affect the prognosis of patients. Due to the limitation of the examination technique, the mismatch between DWI and PWI cannot obtain the true penumbra, so the correlation between the penumbra condition around the lesion and different prognosis was not performed in this study. For the status of collateral circulation around the lesion, we found a significant correlation between it and prognosis, which is also consistent with some studies. The MK and MD metrics have a higher correlation with the prognosis and are apparent indicators of the degree of cell ischemia. They can indicate the swelling status of the cells in the infarct tissues after ischemia. The MK value has higher sensitivity and specificity than the MD value for predicting a patient’s prognosis. The inability of MD to fully capture structural complexity may be because it is influenced not only by tissue complexity but also by molecule concentration and other factors (9). Other studies also confirmed that MK has more specificity in determining tissue microstructural changes than MD (34).
Different cerebral infarction types and blood supply regions might have different sizes of infarction, as well as distinct imaging appearances and prognoses, which could be related to variations in local structure, blood supply, and peripheral circulation (35). However, our study did not find significant differences in prognosis among different types of cerebral infarctions, which may be due to the dependence of infarction types on other factors, particularly infarct size at admission.
This study had several limitations. First, the sample size was small. Due to the complexity of the clinical factors, the exclusion criteria were strict. For practical reasons, we could only include the lesions with diameter > 1 cm in the study, which might cause a bias in the results. Hence, large sample and multi-center studies are required. Second, the MRI examinations were performed on the second or third day of admission to avoid delays in emergency treatment. The imaging characteristics of the acute stage need to be further studied. Third, this study focused on the clinical history and key imaging indicators to evaluate the prognosis, but other factors, such as the laboratory tests, were not analyzed. Further studies are needed to confirm the results of this preliminary study.
Perfusion and diffusion metrics could reflect the microstructure and blood flow characteristics of the lesion, which were the key factors for the salvage ability and prognosis of the infarction tissue. The characteristics of the infarction core and peripheral regions have different effects on the outcomes. Diffusion of infarction core has strong relations with the prognosis, whereas the time metrics (MTT, TTP) were more important for peripheral regions. MK had a more significant association with prognosis than MD. The NIHSS score at admission could reflect the functional involvement and was correlated with patients’ outcomes. These factors were the primary clinical and imaging markers influencing the prognosis of cerebral infarction patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethics Committee of Tianjin First Central Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
CP: Conceptualization, Data curation, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. CH: Conceptualization, Data curation, Investigation, Methodology, Software, Writing – original draft. HL: Investigation, Software, Writing – original draft. XL: Funding acquisition, Writing – review & editing. WH: Conceptualization, Writing – review & editing. JL: Project administration, Supervision, Validation, Writing – review & editing. JY: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of Hainan Province (820RC783 and 2019RC388), the National Natural Science Foundation of Tianjin (21JCYBJC00840), and the Public Health Research Project of Hainan Provincial (22A200304).
The authors would like to thank all participants in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Herpich, F, and Rincon, F. Management of Acute Ischemic Stroke. Crit Care Med. (2020) 48:1654–63. doi: 10.1097/CCM.0000000000004597
2. Sennfält, S, Pihlsgård, M, Norrving, B, Ullberg, T, and Petersson, J. Ischemic stroke patients with prestroke dependency: characteristics and long-term prognosis. Acta Neurol Scand. (2021) 143:78–88. doi: 10.1111/ane.13328
3. Mozaffarian, D, Benjamin, EJ, Go, AS, Arnett, DK, Blaha, MJ, Cushman, M, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. (2016) 133:e38–e360. doi: 10.1161/CIR.0000000000000350
4. Ryu, W-S, Hong, K-S, Jeong, S-W, Park, JE, Kim, BJ, Kim, J-T, et al. Association of ischemic stroke onset time with presenting severity, acute progression, and long-term outcome: a cohort study. PLoS Med. (2022) 19:e1003910. doi: 10.1371/journal.pmed.1003910
5. Shuaib, A, Lees, KR, Lyden, P, Grotta, J, Davalos, A, Davis, SM, et al. NXY-059 for the treatment of acute ischemic stroke. N Engl J Med. (2007) 357:562–71. doi: 10.1056/NEJMoa070240
6. Ntaios, G, Lip, GYH, Vemmos, K, Koroboki, E, Manios, E, Vemmou, A, et al. Age- and sex-specific analysis of patients with embolic stroke of undetermined source. Neurology. (2017) 89:532–9. doi: 10.1212/WNL.0000000000004199
7. Chemmanam, T, Campbell, BCV, Christensen, S, Nagakane, Y, Desmond, PM, Bladin, CF, et al. Ischemic diffusion lesion reversal is uncommon and rarely alters perfusion-diffusion mismatch. Neurology. (2010) 75:1040–7. doi: 10.1212/WNL.0b013e3181f39ab6
8. Schaefer, PW, Grant, PE, and Gonzalez, RG. Diffusion-weighted MR imaging of the brain. Radiology. (2000) 217:331–45. doi: 10.1148/radiology.217.2.r00nv24331
9. Jensen, JH, Helpern, JA, Ramani, A, Lu, H, and Kaczynski, K. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. (2005) 53:1432–40. doi: 10.1002/mrm.20508
10. Jensen, JH, and Helpern, JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. (2010) 23:698–710. doi: 10.1002/nbm.1518
11. Thijs, VN, Lansberg, MG, Beaulieu, C, Marks, MP, Moseley, ME, and Albers, GW. Is early ischemic lesion volume on diffusion-weighted imaging an independent predictor of stroke outcome?: a multivariable analysis. Stroke. (2000) 31:2597–602. doi: 10.1161/01.STR.31.11.2597
12. Guo, Y, Zhang, Z, Zhang, G, Kong, L, Rao, H, Chen, W, et al. Evaluation of mean diffusion and kurtosis MRI mismatch in subacute ischemic stroke: comparison with NIHSS score. Brain Res. (2016) 1644:231–9. doi: 10.1016/j.brainres.2016.05.020
13. Zhu, L-H, Zhang, Z-P, Wang, F-N, Cheng, Q-H, and Guo, G. Diffusion kurtosis imaging of microstructural changes in brain tissue affected by acute ischemic stroke in different locations. Neural Regen Res. (2019) 14:272–9. doi: 10.4103/1673-5374.244791
14. Donnan, GA, Baron, J-C, Ma, H, and Davis, SM. Penumbral selection of patients for trials of acute stroke therapy. Lancet Neurol. (2009) 8:261–9. doi: 10.1016/S1474-4422(09)70041-9
15. Nael, K, and Kubal, W. Magnetic resonance imaging of acute stroke. Magn Reson Imaging Clin N Am. (2016) 24:293–304. doi: 10.1016/j.mric.2015.11.002
16. Straka, M, Albers, GW, and Bammer, R. Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging. (2010) 32:1024–37. doi: 10.1002/jmri.22338
17. Zhou, Y, Zhang, S, and Lou, M. Imaging markers in acute phase of stroke: implications for prognosis. Brain Hemorrhages. (2020) 1:19–23. doi: 10.1016/j.hest.2019.12.002
18. Appelros, P, Nydevik, I, and Viitanen, M. Poor outcome after first-ever stroke: predictors for death, dependency, and recurrent stroke within the first year. Stroke. (2003) 34:122–6. doi: 10.1161/01.STR.0000047852.05842.3C
19. Saber, H, and Saver, JL. Distributional validity and prognostic power of the National Institutes of Health stroke scale in US administrative claims data. JAMA Neurol. (2020) 77:606–12. doi: 10.1001/jamaneurol.2019.5061
20. Huybrechts, KF, Caro, JJ, Xenakis, JJ, and Vemmos, KN. The prognostic value of the modified Rankin scale score for long-term survival after first-ever stroke. Cerebrovasc Dis. (2008) 26:381–7. doi: 10.1159/000151678
21. Chen, Y, Nguyen, TN, Mofatteh, M, Abdalkader, M, Wellington, J, Yan, Z, et al. Association of Early Increase in body temperature with symptomatic intracranial hemorrhage and unfavorable outcome following endovascular therapy in patients with large vessel occlusion stroke. J Integr Neurosci. (2022) 21:156. doi: 10.31083/j.jin2106156
22. Sun, Y, Jou, E, Nguyen, TN, Mofatteh, M, Liang, Q, Abdalkader, M, et al. Predictors of futile recanalization after endovascular treatment in acute ischemic stroke: a multi-center study. Front Neurosci. (2023) 17:1279366. doi: 10.3389/fnins.2023.1279366
23. Tu, W-J, Zhao, S-J, Xu, D-J, and Chen, H. Serum 25-hydroxyvitamin D predicts the short-term outcomes of Chinese patients with acute ischaemic stroke. Clin Sci. (2014) 126:339–46. doi: 10.1042/CS20130284
24. Ernst, M, Forkert, ND, Brehmer, L, Thomalla, G, Siemonsen, S, Fiehler, J, et al. Prediction of infarction and reperfusion in stroke by flow- and volume-weighted collateral signal in MR angiography. AJNR Am J Neuroradiol. (2015) 36:275–82. doi: 10.3174/ajnr.A4145
25. Jadhav, AP, Desai, SM, Liebeskind, DS, and Wechsler, LR. Neuroimaging of acute stroke. Neurol Clin. (2020) 38:185–99. doi: 10.1016/j.ncl.2019.09.004
26. Keir, SL, and Wardlaw, JM. Systematic review of diffusion and perfusion imaging in acute ischemic stroke. Stroke. (2000) 31:2723–31. doi: 10.1161/01.STR.31.11.2723
27. Quarles, CC, Bell, LC, and Stokes, AM. Imaging vascular and hemodynamic features of the brain using dynamic susceptibility contrast and dynamic contrast enhanced MRI. NeuroImage. (2019) 187:32–55. doi: 10.1016/j.neuroimage.2018.04.069
28. Grinberg, F., Ciobanu, L., and Farrher, E., &. Shah, N. J. (2012). Diffusion kurtosis imaging and log-normal distribution function imaging enhance the visualisation of lesions in animal stroke models. NMR Biomed, 25, 1295–1304. doi: 10.1002/nbm.2802
29. Jensen, JH, Falangola, MF, Hu, C, Tabesh, A, Rapalino, O, Lo, C, et al. Preliminary observations of increased diffusional kurtosis in human brain following recent cerebral infarction. NMR Biomed. (2011) 24:452–7. doi: 10.1002/nbm.1610
30. Guo, Y-L, Li, S-J, Zhang, Z-P, Shen, Z-W, Zhang, G-S, Yan, G, et al. Parameters of diffusional kurtosis imaging for the diagnosis of acute cerebral infarction in different brain regions. Exp Ther Med. (2016) 12:933–8. doi: 10.3892/etm.2016.3390
31. Appelros, P, Stegmayr, B, and Terént, A. A review on sex differences in stroke treatment and outcome: sex differences in stroke treatment and outcome. Acta Neurol Scand. (2009) 121:359–69. doi: 10.1111/j.16000404.2009.01258.x
32. Leker, RR, Cohen, JE, Horev, A, Tanne, D, Orion, D, Raphaeli, G, et al. Impact of previous stroke on outcome after thrombectomy in patients with large vessel occlusion. Int J Stroke. (2019) 14:887–92. doi: 10.1177/1747493019841244
33. Lai, Y, Jou, E, Mofatteh, M, Nguyen, TN, Ho, JSY, Diana, F, et al. 7-day National Institutes of Health stroke scale as a surrogate marker predicting ischemic stroke patients’ outcome following endovascular therapy. Transl Neurosci. (2023) 14:20220307. doi: 10.1515/tnsci-2022-0307
34. Umesh Rudrapatna, S, Wieloch, T, Beirup, K, Ruscher, K, Mol, W, Yanev, P, et al. Can diffusion kurtosis imaging improve the sensitivity and specificity of detecting microstructural alterations in brain tissue chronically after experimental stroke? Comparisons with diffusion tensor imaging and histology. NeuroImage. (2014) 97:363–73. doi: 10.1016/j.neuroimage.2014.04.013
Keywords: acute ischemic stroke, NIHSS score, mRS score, diffusion, kurtosis, perfusion
Citation: Pei C, He C, Li H, Li X, Huang W, Liu J and Yin J (2024) Clinical and imaging markers for the prognosis of acute ischemic stroke. Front. Neurol. 15:1345914. doi: 10.3389/fneur.2024.1345914
Received: 28 November 2023; Accepted: 19 February 2024;
Published: 29 February 2024.
Edited by:
Matteo Foschi, Azienda Unità Sanitaria Locale (AUSL) della Romagna, ItalyReviewed by:
Xuxing Liao, Foshan Sanshui District People’s Hospital, ChinaCopyright © 2024 Pei, He, Li, Li, Huang, Liu and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Liu, Y2pyLmxpdWp1bkB2aXAuMTYzLmNvbQ==; Jianzhong Yin, amlhbnpob25nLnlpbkBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.