- 1Department of Rehabilitation, School of Nursing, Jilin University, Changchun, China
- 2Department of radiology, First Hospital of jilin University, Changchun, China
- 3Department of Rehabilitation Medicine, First Hospital of Jilin University, Changchun, China
Objectives: In recent years, there has been an increase in the number of randomized clinical trials of BTX-A combined with ESWT for the treatment of post-stroke spasticity. This has made it possible to observe the benefits of combination therapy in clinical practice. Therefore, this paper reviews the effectiveness of BTX-A in combination with ESWT for the treatment of post-stroke spasticity.
Methods: By October 2023, a systematic review was conducted in the databases PubMed, Cochrane, Embase, Medline, Web of Science, China National Knowledge Infrastructure, Wan Fang Database, China Biology Medicine disc and China Science and Technology Journal Database were systematically searched. We included randomized controlled trials that reported outcome metrics such as MAS, FMA, and MBI score. Studies were excluded if MAS was not reported. The quality of the included studies was assessed by the Cochrane Collaboration’s tool for assessing risk of bias, and the AMSTAR quality rating scale was selected for self-assessment.
Results: A total of 70 articles were included in the initial search, and six were ultimately included. The results of the included studies showed that the combination therapy was effective in reducing MAS scores and improving FMA and MBI scores in patients with spasticity compared to the control group. Combination therapy has also been shown to improve joint mobility and reduce pain in spastic limbs.
Conclusion: Cumulative evidence from clinical randomized controlled trial studies suggests that the combination therapy is effective in reducing lower limb spasticity and improving mobility after stroke. However, more clinical trials are still needed to corroborate the evidence regarding the efficacy of BTX-A combined with shockwave therapy.
Systematic Review Registration: The system review can be searched in the PROSPERO database (CRD42023476654).
1 Introduction
The incidence of stroke is increasing (1, 2), and most patients are left with limb hemiparesis disorders, of which muscle spasms are more common (3, 4). Spasticity is a movement disorder characterized by a velocity-dependent increase in muscle tone due to a hyperactive detachment reflex, often accompanied by a hyperactive tendon reflex, and is a form of Upper motor neuron syndrome (UMN) (5). The spasticity that develops after stroke can limit the use of the affected limb, causing pain, contractures or falls and impairing gait (6). However, long-term hypertonia and spasticity can lead to permanent joint contracture deformity, hindering the functional rehabilitation of paralyzed limbs, and is one of the major causes of disability (7, 8). Post-stroke dyskinesia has a significant impact on motor function, balance, gait and ability to perform activities of daily living, and usually, patients can improve with intensive exercise training (9). However, the first issue to deal with in achieving this is the effect of spasticity (muscle stiffness) on muscle tone and flexibility (10). Spasticity can interfere with or stop exercise prescription (11). In stroke patients, the burden of spasticity on patients, caregivers and society is enormous. Healthcare costs associated with stroke patients with spasticity are four times higher compared to those without spasticity (12).
Traditional treatments for spasticity include Botulinum Toxin A (BTX-A) (13, 14), phenol (15), baclofen (16, 17), surgical correction (18), Extracorporeal Shockwave Therapy (ESWT) (19), and rehabilitation (20–23). Botulinum toxin A (BTX-A) is a neurotoxin whose primary site of action is the nerve endings and cranial nuclei. It reduces muscle hyperactivity by inhibiting the release of acetylcholine at the neuromuscular junction (it cleaves the 25 kDa synaptosome-associated protein, which is required for vesicle docking and therefore for neurotransmitter release) (24). Thus, BTX-A injections can reduce spasticity by temporarily paralyzing muscle activity and reducing muscle hypertonia (25, 26). At present, BTX-A is a first-line agent for the treatment of focal and multifocal spasticity (27). Much of the literature has demonstrated that BTX-A injections can treat muscle imbalances, decrease muscle tone, and improve muscle function (27). However, studies have also proposed that BTX-A and placebo have similar effects in reducing spasticity (28). And the maximum efficacy of BTX-A in PSS was seen at about 3–4 weeks, and the clinically significant effect of BTX-A was maintained for about 4–8 weeks, after which time the effect gradually declined (14, 29). This finding suggests that BTX-A may need to be supplemented with a multidisciplinary team (MDT) approach together as part of a rehabilitation program to promote sustained clinical outcomes (30).
Extracorporeal shock wave therapy (ESWT) is a non-invasive, relatively inexpensive treatment that is widely used with post-stroke spasticity. ESWT are widely used in the treatment of kidney stones, urethral stones, urinary stones, and biliary stones by releasing energy into the tissues (31). The mechanism of its application to reduce spasticity is still uncertain. Some researchers have proposed the hypothesis that the high energy delivered by ESWT affects the mechanical properties of the muscle and disrupts the functional link between actin and myosin, therefore relieving muscle spasm (32). Another hypothesis on the mechanism of action of ESWT suggests that ESWT promotes the production of nitric oxide (NO), which is involved in the formation of neuromuscular junctions, neurotransmission, memory formation and synaptic plasticity. In addition, it increases blood supply to tissues and activates growth factors in spastic muscles through angiogenesis (33). Kenmoku T et al. found through animal experiments that low-frequency ESWT inhibits acetylcholine binding to receptors and reduces excitability at the neuromuscular junction, thereby relieving muscle spasm (34). Several studies have reported that ESWT improves post-stroke spasticity and enhances motor performance, and has a promising application (35, 36). Therefore, the addition of ESWT in combination with BTX-A can be considered in order to obtain a sustained therapeutic effect in a timely manner. Although ESWT has been demonstrated as a new technology for PSS treatment in several studies, there are fewer studies on the use of BTX-A in combination with ESWT for the relief of PSS.
Several clinical trials have reported in recent years on the therapeutic efficacy of the combination of BTX-A and ESWT in the treatment of poststroke spasticity. A systematic review of existing clinical randomized controlled trial studies may allow a more precise assessment of their effectiveness. If effectiveness is confirmed, it would help to promote the combined use of BTX-A and ESWT. Therefore, the aim of our study was to systematically evaluate the effect of combined BTX-A and ESWT therapy on poststroke spasticity compared with conventional rehabilitation with or without BTX-A, or other interventions.
2 Methods
2.1 Retrieval strategy
The protocol was registered in the PROSPERO database (CRD42023476654) and all search results were evaluated according to the PRISMA statement. Extensive searches were performed on databases such as PubMed, Cochrane, Embase, Medline, Web of Science, China National Knowledge Infrastructure, Wan Fang Database, China Biology Medicine disc (CBM), and China Science and Technology Journal Database. The search was conducted from the time of the library’s creation until 20 October 2023 to identify potential studies exploring the effects of ESWT combined with botulinum toxin injections for the treatment of post-stroke spasticity. Chinese search terms were “stroke,” “spasticity,” “hypertonia,” “shock wave therapy,” “extracorporeal shock wave,” “botulinum toxin,” “botulinum toxin,” and “botulinum toxin type A,” etc. English search terms were “Stroke,” “Brain Infarction,” “Muscle Spasticity,” “Spasm,” “Botulinum Toxins, Type A,” “Botulinum Toxins,” “Extracorporeal Shockwave Therapy,” etc. The specific search formula is in Supplementary Table 1.
2.2 Study eligibility
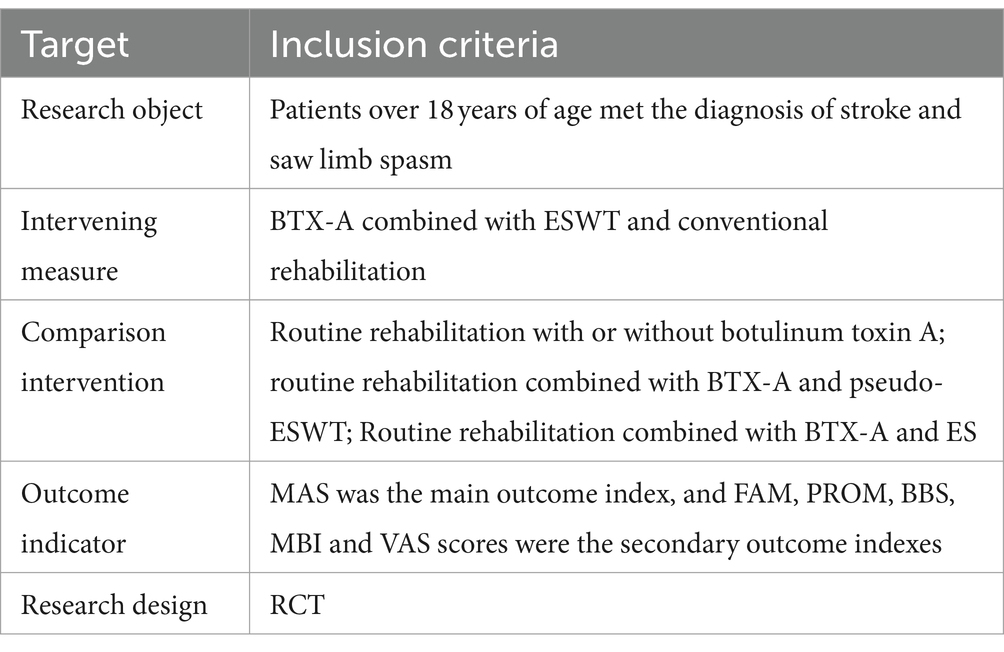
We included clinical randomized controlled trials involving patients aged 18 years or older with poststroke spasticity to analyze the efficacy of BTX-A injections combined with shockwave therapy in the treatment of spasticity. Specifically speaking, our intervention criteria for inclusion in the literature needed to include both BTX-A and ESWT. On this basis, there are studies that have also combined conventional rehabilitation at the same time, which are also included in our inclusion. Screening by title, abstract browsing, and full-text reading were used to determine whether articles were included. Criteria for inclusion were developed according to the PICOS principles of Population, Intervention, Comparator, Outcomes and Study designs and are summarized in Table 1. Literature, Meta-analyses, reviews, conferences, case reports, animal experiments and non-randomized controlled trials that did not meet the inclusion criteria were excluded.
2.3 Study selection and data abstraction
Two researchers independently assessed eligibility for inclusion, and disagreements were resolved through a third researcher. Titles and abstracts were screened to identify relevant studies, and then the full text was carefully evaluated. References cited in selected articles were also examined to identify potentially relevant studies. The Modified Ashworth Rating Criteria Score (MAS) was extracted as the primary outcome. Secondary outcomes included simplified Fugl-Meyer scale (FAM) scores, modified Barthel Index (MBI) scores, visual analog scale (VAS), daily spasticity frequency (SFS), passive joint mobility (PROM), and Berg Balance Scale (BBS) scores. Changes in these scores represent certain functional changes.
2.4 Quality assessment
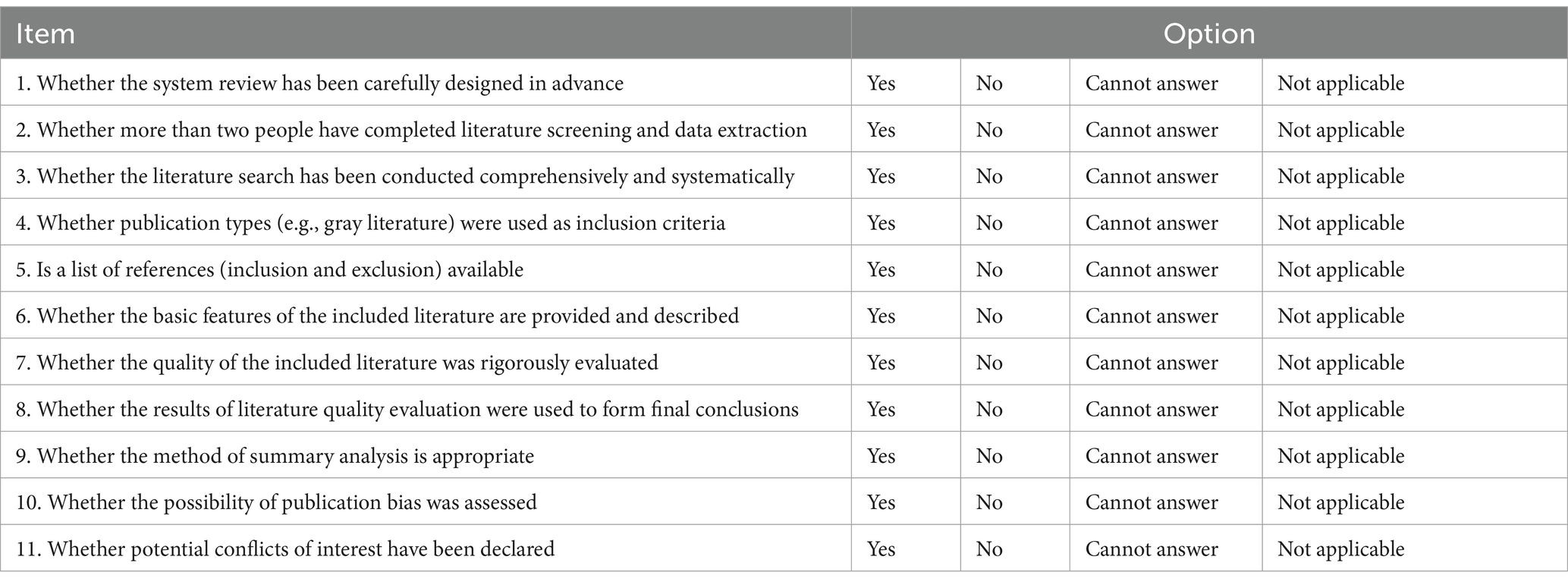
The quality of included studies was assessed by the Cochrane Collaboration’s tool for assessing risk of bias, which is recommended for use in systematic reviews of interventions in the Cochrane Handbook version 5.1.0. We assessed seven areas of bias, including selection bias, implementation bias, detection bias, attribution bias, reporting bias, and other sources of bias. Judgments were expressed as “high risk,” “low risk,” or “unclear risk,” and quality assessment data were generated by RevMan version 5.4. The AMSTAR quality assessment form was also used for self-assessment.
3 Results
3.1 Search results
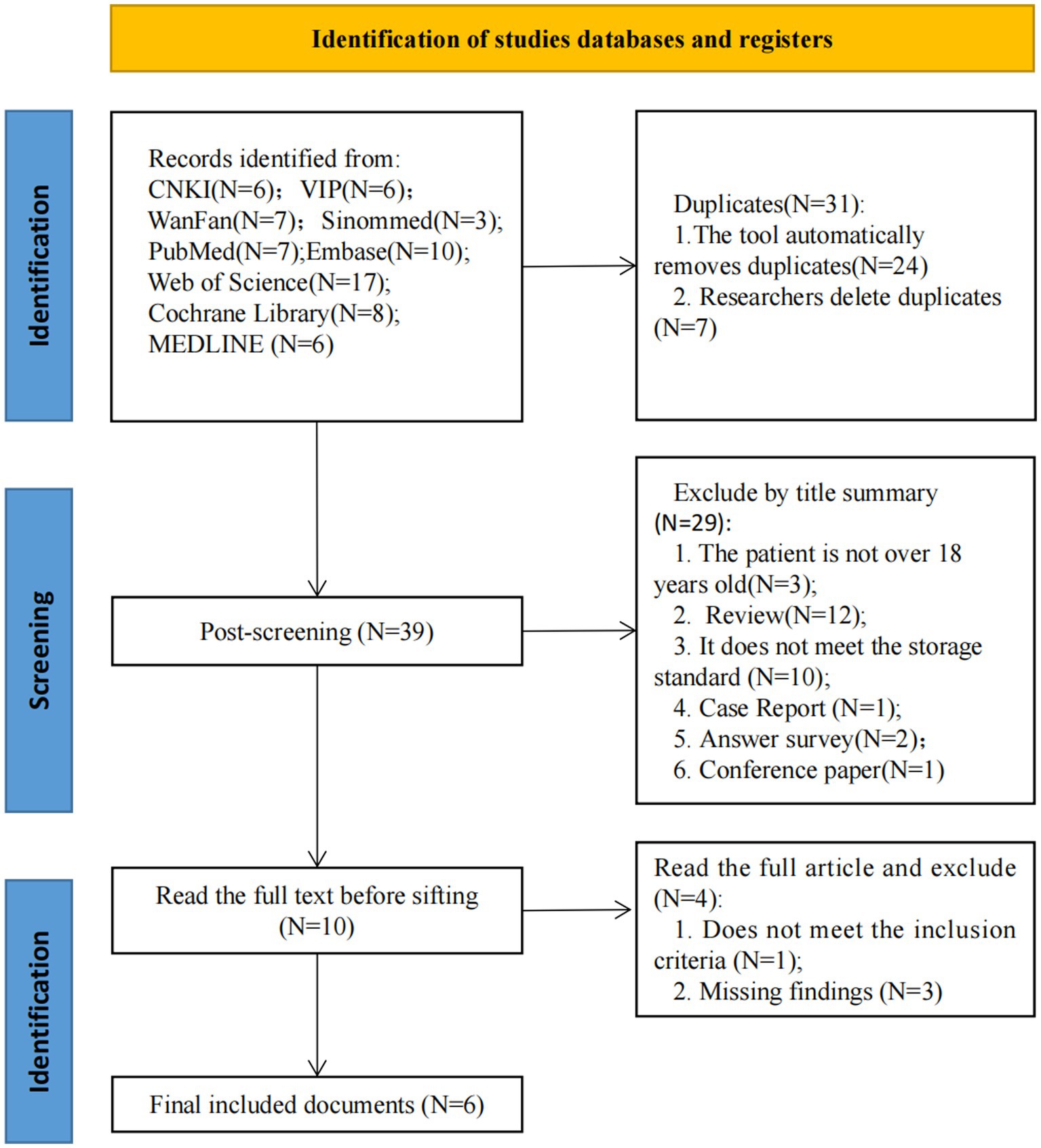
The initial search produced 70 articles, and 39 records were screened after removing duplicates. 29 records were excluded after assessing titles and abstracts of potentially relevant studies. The full text of 10 articles was then scrutinized, and 4 articles were excluded because they did not meet the inclusion criteria or had missing study results (Figure 1). Finally, 6 RCTs were included in this systematic review (37–42).
3.2 Characteristics of included systematic review
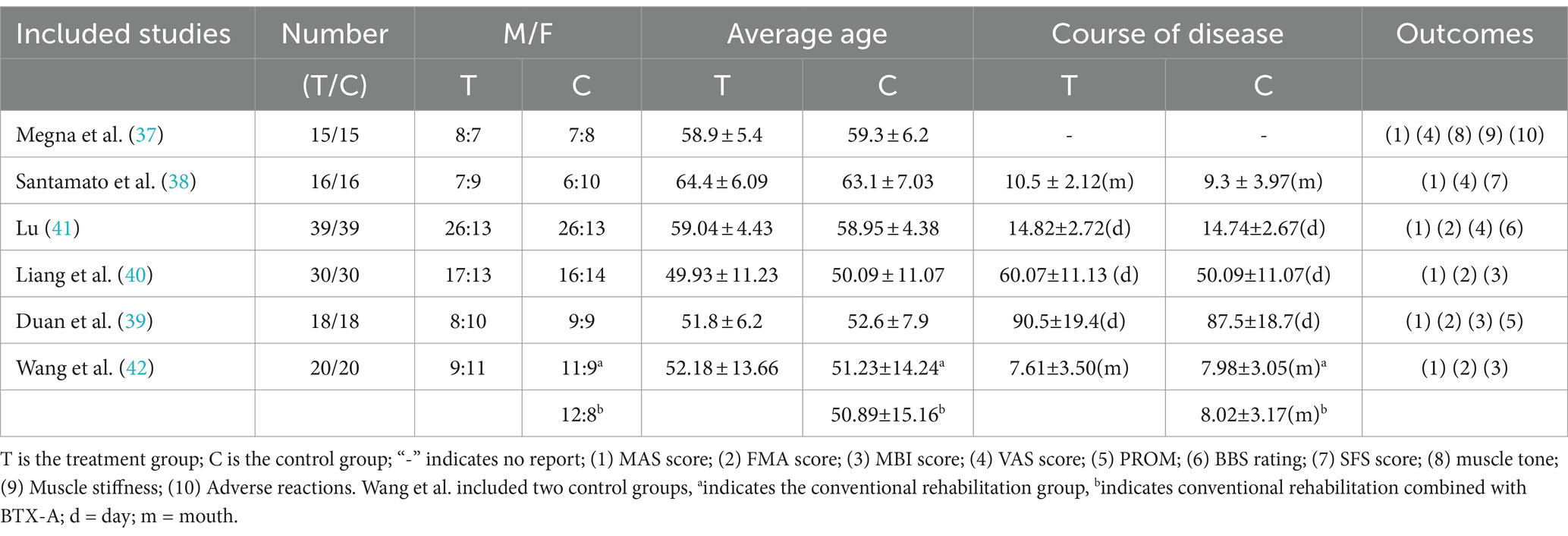
The included studies were published from 2013 to the present and contained both English (N = 2) (37, 38) and Chinese (N = 4) (39–42) literature. Five of them were prospective randomized controlled studies and one was a retrospective randomized controlled study. The characteristics of these studies are displayed in Table 2. Participants’ baseline functional levels are shown in Supplementary Table 2. The sample sizes ranged from 30 to 78. All experimental groups received local injection of botulinum toxin combined with shockwave therapy, while the control group received different treatments, including conventional rehabilitation (N = 3) (40–42), conventional rehabilitation combined with botulinum toxin type A (N = 2) (37, 42), conventional rehabilitation combined with botulinum toxin injections and pseudo-shockwave therapy (N = 1) (39), and botulinum toxin injections combined with electrical stimulation (N = 1) (38). The study by Hong Wang et al. (42) included two control groups, conventional rehabilitation combined with or without botulinum toxin. Inclusion and exclusion criteria for each article are recorded in Supplementary Table 3.
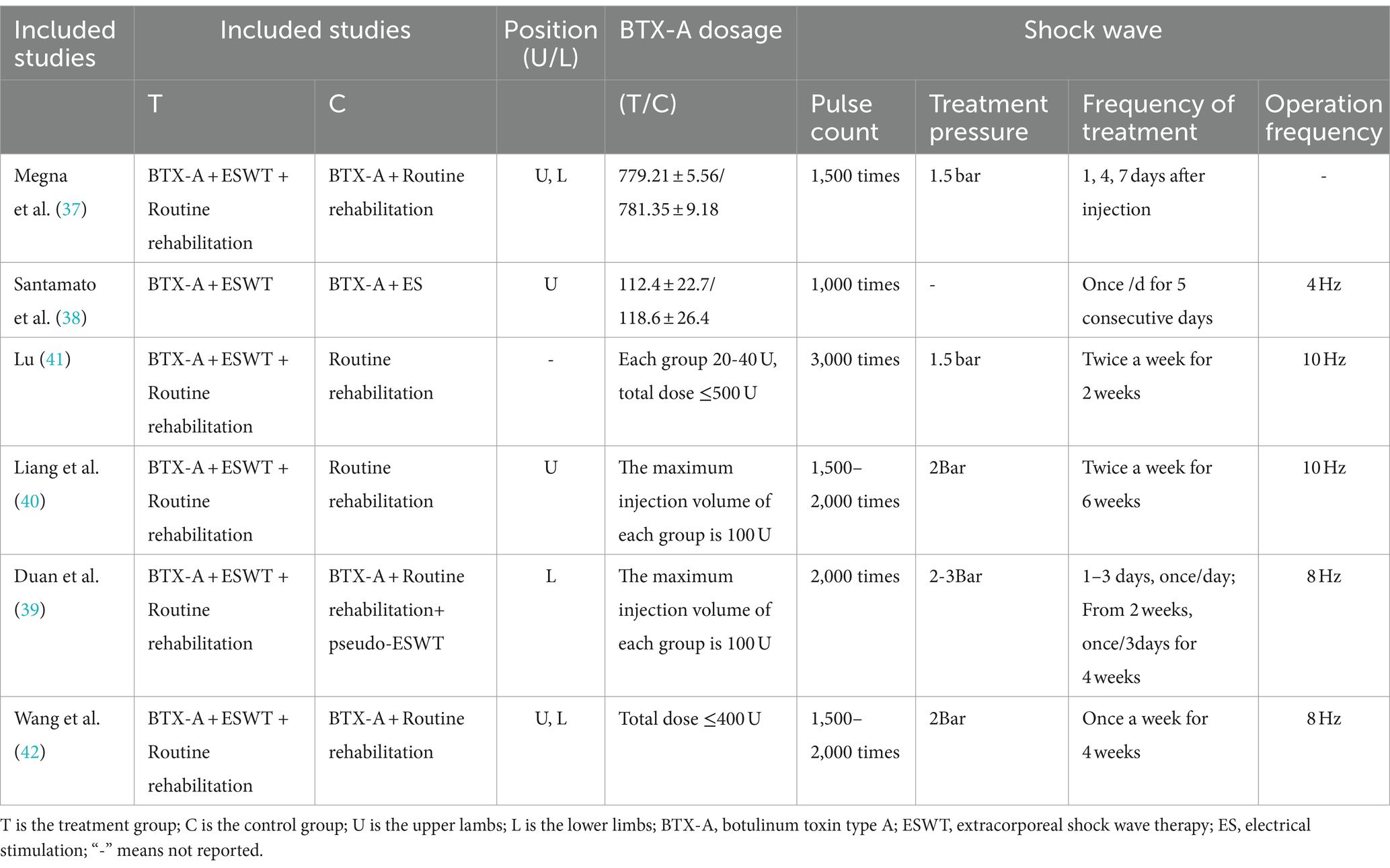
The specific treatment parameters for botulinum toxin injections and shockwave therapy for each of the included studies are shown in Table 3. The dose of botulinum toxin used varied from study to study, with the injected dose ranging from 20 to 120 units per muscle group in most studies, and the total dose not exceeding 500 U. In fact, one study had an injection dose of 779.21 ± 5.56/781.35 ± 9.18 U in the control and experimental groups (37). The therapeutic parameters of shock waves used varied from study to study (number of pulses 1,000–3,000, therapeutic pressure 1.5–3 bar, frequency 4–10 Hz). In addition, one study did not mention the frequency of operation of shock wave therapy (37).
In the six included articles, the treatment sites for BTX-A injections and shockwaves were as follows: gastrocnemius (37, 39, 42), soleus (39, 42), tibialis posterior (42), bunion (42), phalanges (42), forearm superficial finger flexors (38), biceps brachii (BB) (37), and superficial finger flexors (SFD) (37). In addition, two studies did not report specific treatment sites (40, 41).
3.3 Quality assessment and bias analysis
The quality of the included studies was evaluated using the Cochrance Risk of Bias Assessment Tool and the AMSTAR Quality Evaluation Form recommended by the Cochrance Collaborative Group.
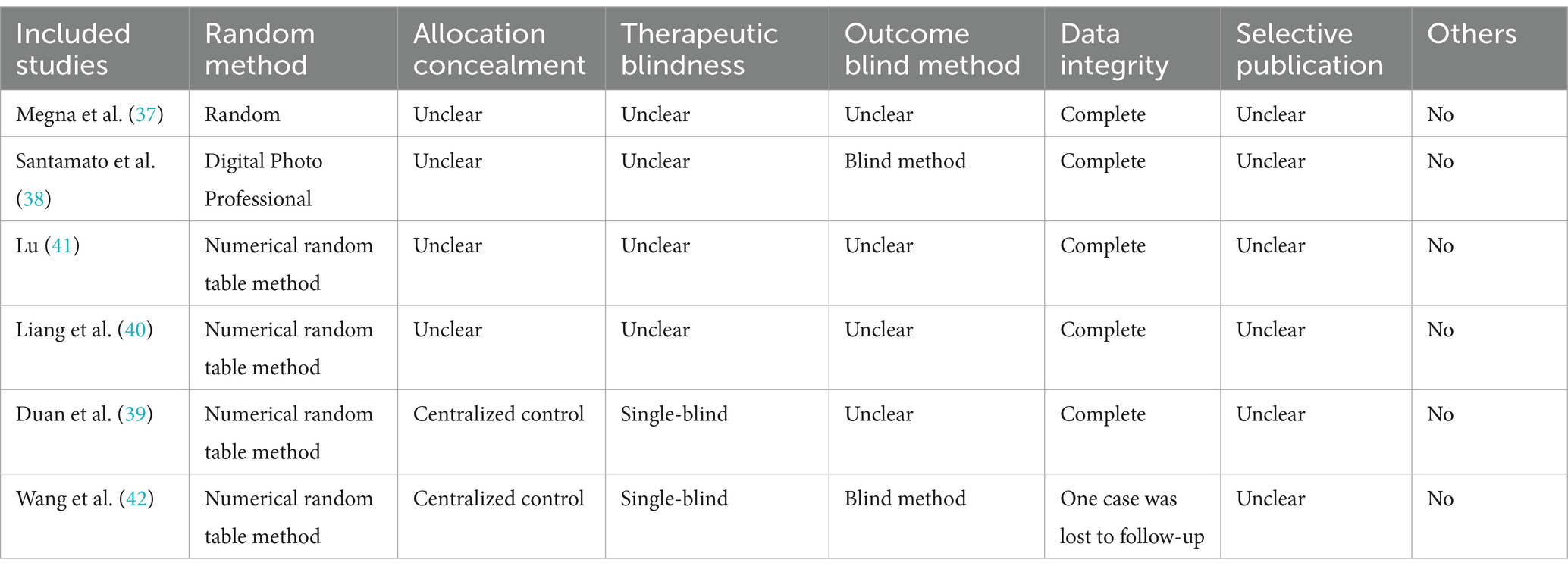
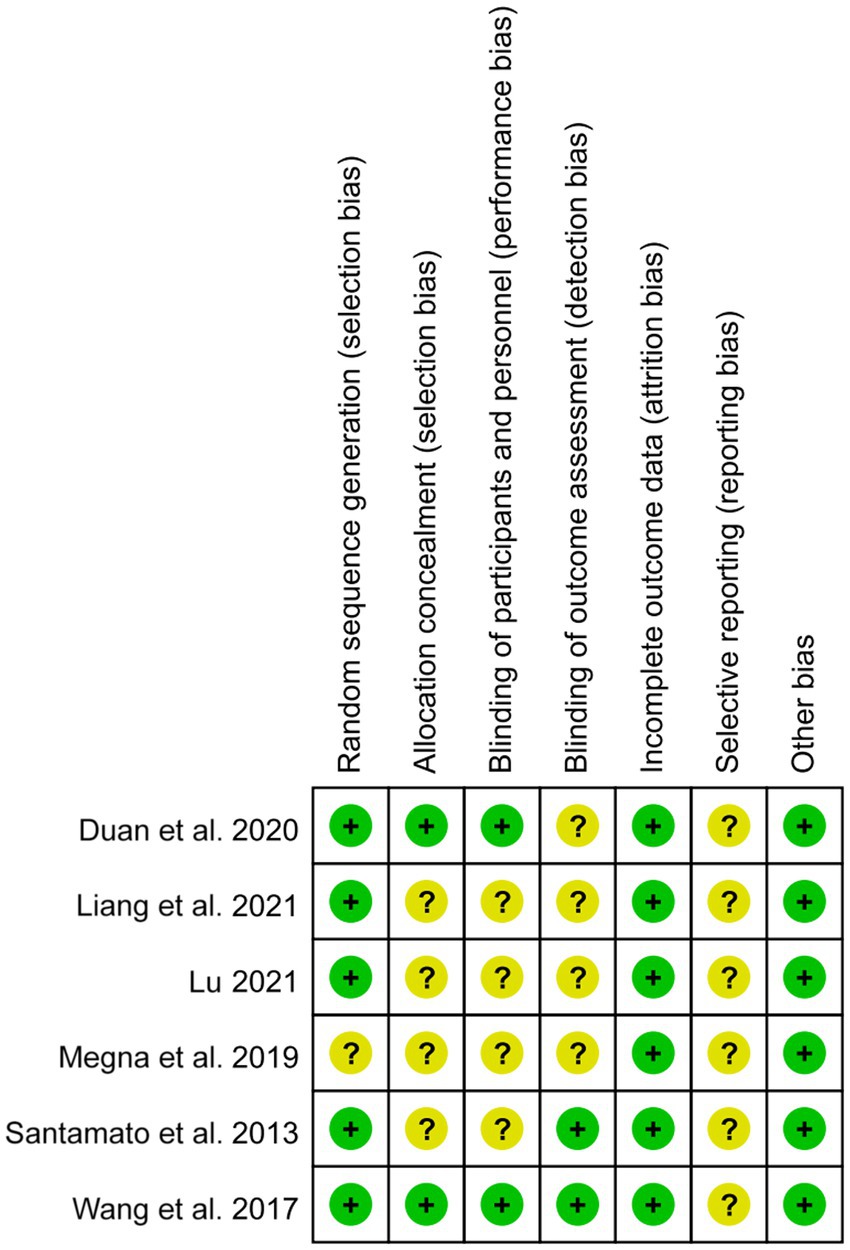
The risk of Cochrance bias assessment is shown in Table 4: (1) Random sequence generation: 4 papers used random number table method for random grouping and were judged to be low risk (39–42), 1 paper used software for randomization and was judged to be low risk (38), and the remaining 1 paper only mentioned the word “random” without describing the method of randomization, so it was not possible to judge the risk status (2, 37). (2) Concealment of the allocation plan: 2 articles concealed the allocation plan for patients and were judged to be low risk (39, 42), and 4 included articles did not describe the concealment of the allocation plan (37, 38, 40, 41). (3) Double-blinding of investigators and subjects: 2 were single-blinded but would not affect outcome indicators and were judged to be low risk (39, 42). The remaining 4 papers did not mention blinding and did not have enough information to judge whether they were high or low risk (37, 38, 40, 41). (4) Blinded evaluation of study outcomes: 2 literature assessors were not aware of the subgroups and judged them to be low risk (38, 42), and the remaining 4 were not described and did not have enough information to judge whether they were high or low risk (37, 39–41). (5) Completeness of outcome data: 5 included studies had complete outcome data and were judged to be low risk (37–41), and 1 had an explanation of the lost visit data and could be judged to be low risk (42). (6) Selective publication: There was not enough information to make a judgment of high or low risk. (7) Bias from other sources: None of the studies introduced bias from other sources and were judged to be at low risk (Figures 2, 3).

Figure 3. Bias risk graph. The authors’ judgment of each item at risk of bias was expressed as a percentage across all included studies.
The quality evaluation is strictly in accordance with the requirements of the AMSTAR quality evaluation form for systematic evaluation, strict quality control, eligible, the results are shown in Table 5.
3.4 Outcome
3.4.1 Spasticity
Modified Ashworth Scale (MAS) is a commonly used scale for clinical evaluation of patients’ muscle tone and assessing the degree of muscle spasticity (43). The MAS classifies the degree of spasticity into grades 0-IV: (1) Grade 0: normal muscle tone; (2) Grade I: a slight increase in muscle tone, with minimal resistance at the end of the joint movement; (3) Grade I+: a slight increase in muscle tone, with minimal resistance in the posterior 50% of the joint range of motion; (4) Grade II: an increase in muscle tone in the majority of the joint range of motion, but still able to carry out passive activities; (5) Grade III: a significant increase in muscle tone, with difficulty in passive activities; (6) Grade IV: stiffness occurs and inactivity is not possible (44). This measure is reliable by assessing the degree of resistance and the point of resistance during manual stretching of the muscle (45). The outcome indicators varied among the studies. However, the included studies all used the MAS to subjectively report treatment outcomes. The results showed that on the basis of conventional rehabilitation and BTX-A, the efficacy of supplemental ESWT therapy for spasticity relief was better than the control group, and the differences were statistically significant (p < 0.05) (37–42).
In addition, Megna et al. performed an objective assessment of muscle tone by MyotonPro®. One month after the treatment, muscle tone was significantly reduced (pre = 29.11 ± 1.43, 1 M = 20.01 ± 1.54) and was significantly different from the control group (pre = 28.34 ± 1.23; 1 M = 22.20 ± 1.56). The study also evaluated muscle hardness. Compared with the control group (pre = 312.89 ± 4.25, 1 M = 212.34 ± 1.34), ESWT supplementation treatment (pre = 311.65 ± 2.43, 1 M = 204.81 ± 2.21) reduced muscle hardness more effectively (p < 0.05) (37).
One study evaluated the daily spasm frequency scale (SFS). SFS is generally recorded by the patients themselves as the number of spasms occurring per hour or per day (46). Santamato et al. categorized 32 patients into either a shockwave group or an electrical stimulation (ES) group. Both groups received conventional rehabilitation and injections of BTX-A. The results showed that the combination of BTX-A and f-ESWT had a better effect on the reduction of spasticity grade (p < 0.05) (38).
3.4.2 Athletic ability
The simplified Fugl-Meyer assessment (FMA) has an excellent retest reliability and is internationally recognized (47). The FMA is based on Twitchell and Brunnstrom’s observations of the sequential recovery of motor function, assessing reflex activity of the upper and lower extremities as well as synergistic and dissociative movements (48). It is the most widely used quantitative measure of motor recovery after stroke (49). Four studies have reported the results of FMA scores, which showed that BTX-A injection followed by combined ESWT therapy improved patients’ upper or lower extremity motor function (p < 0.05) (39–42).
The results of the study by Duan et al. showed that the MAS score and PROM of the observation group were significantly better than those of the control group after 2 and 4 weeks of treatment (p < 0.05). This suggests that BTX-A combined with low-frequency ESWT can more effectively alleviate the spasticity state of the calf triceps muscle after stroke and improve the joint mobility. The FMA and MBI scores of the observation group were also significantly better than those of the control group after 4 weeks of treatment (p < 0.05). This suggests that compared with local injection of BTX-A alone, BTX-A combined with ESWT treatment did not significantly improve the motor function and ADL ability of the affected lower limb in the short term (2 weeks). Rather, it was only after a longer period of regular rehabilitation training (4 weeks) that the observation group significantly outperformed the control group in terms of motor function and ADL ability. This also suggests that the purpose of BTX-A injection and low-frequency ESWT is not only to alleviate muscle spasm, but also to promote the recovery of limb motor function through active exercise based on the reduction of muscle tone (39).
One study evaluated balance function. The Berg balance scale (BBS) has a total of 14 items, each with five levels ranging from 0 to 4 out of a possible 56 points. The total score ultimately reflects the overall balance ability of the subject. The higher the score, the better the balance (50). Lu recruited 78 patients for a 2-week treatment and assessed the patients’ balance ability before (32.23 ± 3.62) and 2 weeks after (42.65 ± 4.50) the treatment. The results suggested that the combination therapy was able to improve the balance of the patients (p < 0.05) (41).
A study evaluated passive range of motion (PROM). Duan et al. performed botulinum toxin injections in patients with post-stroke triceps spasticity of the calf and gave shockwave therapy 1–3 days post-injection, once/day, and from week 2 onwards, once/3 days for 4 weeks. The passive knee mobility of the patients was assessed before (25.58 ± 4.52), 2 weeks (44.37 ± 8.02) and 4 weeks (56.92 ± 8.79) after the treatment. The results indicated that the combination therapy was able to improve the contracture status of the joint (p < 0.05). Moreover, shockwave therapy was able to improve the passive joint mobility of patients in this study compared to the pseudo-ESWT group (pre = 27.02 ± 4.39, 2 W = 36.74 ± 6.75, 4 W = 45.48 ± 7.86; p < 0.05) (39).
3.4.3 Activity of daily living
The modified Barthel index (MBI) is a commonly used scale to assess the ability to perform activities of daily living (ADL; including eating, grooming, bathing, toileting, dressing, diaphragm control, postural transfer, and walking up and down stairs). 0–100 points are scored, with higher scores indicating better ADL ability of patients (51). Three studies reported the results of Modified Barthel Index (MBI) scores, which suggested that the combined management of conventional rehabilitation, BTX-A and ESWT therapies could improve patients’ self-care ability (p < 0.05) (39, 40, 42).
3.4.4 Pain
The Visual Analog Scale (VAS) is one of the most commonly used unidimensional measures of pain intensity and is characterized by accuracy, simplicity, and sensitivity (52). Three studies have assessed patients’ self-perceived pain before and after treatment by VAS scores, demonstrating that combination therapy can reduce patients’ pain (37, 38, 41).
3.4.5 Adverse event
The combined management of conventional rehabilitation, BTX-A and ESWT therapy may have a favorable safety profile, with only one study reporting adverse events. One case (3.3%) in the experimental group reported localized muscle weakness with a duration of 7 days. The control group reported one case (3.3%) of localized muscle weakness and one case (3.3%) of transient generalized weakness (37).
3.4.6 Follow-up outcome
Three studies reported follow-up results. Megna et al. performed shockwave therapy on days 1, 4, and 7 after BTX-A injection and followed up. In this study, during follow-up, an increase in MAS scores was found in patients at 2 months (2.4 ± 0.6) and 3 months (3.2 ± 0.2) compared to 1 month post-treatment (1.5 ± 0.25), and the same trend was observed for muscle tone (1 M =20.01 ± 1.54,2 M =24.87 ± 1.78, 3 M =28.99 ± 1.11), muscle hardness (1 M = 204.81 ± 2.21, 2 M = 240.31 ± 2.31, 3 M = 309.12 ± 3.89), and VAS scores (1 M = 5.0 ± 0.6, 2 M = 6.10 ± 0.9, 3 M = 8.8 ± 0.5) (37). The frequency of shockwave therapy used by Santamato et al. was 1x/d for 5d after BTX-A injection. Spasticity grade increased at 30d (1.75 ± 0.45) and 90d (1.58 ± 0.52) follow-up compared to 15d post-treatment (1.37 ± 0.5) (38). The study by Wang et al. provided similar conclusions, with an increase in spasticity (1 M = 1.45 ± 0.16, 4 M = 2.14 ± 0.19) and a decrease in motor function (1 M = 28.56 ± 1.56, 4 M = 19.21 ± 3.09) and ADL (1 M = 58.29 ± 5.32, 4 M = 49.83 ± 5.41) found in the patients during follow-up (42). This may suggest that we need to consider the appropriate frequency of shockwave therapy as well as the treatment period for long-term spasticity management.
4 Discussion
Post-stroke spasticity (PSS) is defined as involuntary muscle activity, which can be caused by intermittent or persistent motor-sensory control deficits. PSS is secondary to damage to the upper motor elements and is a manifestation of the gradual recovery of the function of the body’s pyramidal tracts (53). However, PSS can lead to contractual deformities accompanied by pain and limited joint movement, limiting patients’ daily activities and reducing their quality of life (54). For focal spasticity, BTX injections are considered the most effective and safest treatment strategy (55). Although highly utilized, the efficacy of BTX-A may decrease after the end of the application cycle, and compensation by increasing the dose has limited effects in terms of time and cost (56). And some studies have found limited efficacy against focal lower limb spastic dyskinesia in adults (57). ESWT has been shown to be effective in reducing pain, improving muscle spasm and enhancing limb function in stroke patients. Consider applying ESWT as a complementary therapy after BTX-A injections for sustained anti-spasticity effects.
Previous systematic reviews and meta-analyses only evaluated the efficacy of BTX-A or ESWT alone in PSS (58) or compared the efficacy of the two (33, 59), and no systematic reviews elaborating on the efficacy of the combination were found. In recent years, there has been an increase in the number of randomized clinical trials of BTX-A in combination with ESWT for the treatment of PSS, with two studies in progress (60, 61). This has made it possible to observe the benefits of BTX-A in combination with ESWT in clinical practice. Six randomized controlled trials with a total of 296 patients were included in this study. The differences in MAS scores (p < 0.05), FMA (p < 0.05), MBI scores (p < 0.05), and VAS scores (p < 0.05) were statistically significant after treatment with the combination therapy of BTX-A with ESWT as compared to the control group. Therefore, BTX-A combined with ESWT not only relieved the state of muscle spasm, but also promoted the recovery of limb motor function based on the reduction of muscle tone and alleviated patients’ pain. In addition, some studies have found inconsistencies between BTX-A and ESWT in terms of time to improvement in spasticity and improvement in motor function.
Regarding the degree of spasticity, all articles used the MAS score as an outcome indicator. BTX-A combined with ESWT therapy effectively reduced patients’ MAS scores and relieved the degree of spasticity in the short term (4 W) compared with pre-treatment. Whether compared with conventional rehabilitation or conventional rehabilitation combined with BTX-A or BTX-A combined with ES, BTX-A combined with ESWT relieved spasticity more effectively. In addition to this, the short-term efficacy of the combination therapy was supported by an objective assessment of muscle tone by MyotonPro® by Megna et al. (37). However, there were 2 studies reporting follow-up results, and we found limited long-term (3 M) efficacy of BTX-A combined with ESWT for spasticity relief. This may also be related to the frequency and length of treatment with ESWT. Specifically: Megna et al. (37) performed ESWT on days 1, 4, and 7 after BTX-A injection at a treatment pressure of 1.5 bar. Santamato et al. (38) used an ESWT frequency of 1x/d after BTX-A injection for 5 days at 4 Hz. Another study reporting follow-up results reported different results. Wang et al. (42) showed an increase in spasticity at 4 M follow-up, but still had significant spasticity relief compared to pre-treatment. In this study, ESWT was administered once a week for 4 weeks at a pressure of 2 bar and a frequency of 8 Hz. In addition, Lu et al. used ESWT twice a week for a total of 2 weeks. Duan et al. (39) treated once a day for 1–3 days after BTX-A injection, and then changed to once every 3 days from the second week onwards for a total of 4 weeks. However, both studies only reported short-term efficacy without long-term follow-up. Liang et al. (40) used twice-weekly treatment for a total of 6 weeks. A decrease in spasticity was observed at the endpoint (6 W).
Similarly, BTX-A in combination with ESWT therapy has been shown to be effective in improving patients’ upper or lower extremity mobility. It has also been shown that the combination therapy improves balance function of the lower extremities and increases passive joint mobility. However, Duan et al. (39) found that the combination of BTX-A and ESWT therapy was inconsistent in reducing spasticity and improving motor function over time. BTX-A combined with ESWT therapy did not significantly improve lower extremity motor function and ADL ability on the affected side at 2 weeks. Rather, it was only after 4 weeks of ESWT treatment and regular rehabilitation that the observation group significantly outperformed the control group in terms of motor function and ADL ability. Only one study followed up motor function, which had the same trend as the degree of limb spasticity in Wang et al. et al. (42). And at 4 M post-treatment, motor and ADL abilities were significantly better compared to pre-treatment.
In addition to this, BTX-A combined with ESWT therapy is also effective in mentioning pain relief. However, two studies showed discrepancies in the follow-up results for pain. Megna et al. (37) reported VAS scores (Pre = 8.9 ± 0.3; 3 M = 8.8 ± 0.5) and Santamato et al. (38) reported VAS scores (Pre = 5 ± 1.21; 3 M = 1.87 ± 0.62). Regarding the safety of combination therapy, only the study by Megna et al. (37) reported adverse events. In this study, conventional rehabilitation combined with BTX-A was used in the control group and conventional rehabilitation combined with BTX-A and ESWT was used in the experimental group. Symptoms of muscle weakness were observed in both groups. No related adverse events were reported in other studies. In conclusion BTX-A combined with ESWT therapy has a good safety profile.
Moreover, differences in BTX-A dosage may affect the efficacy analysis of the combination therapy. The specific doses of BTX-A are shown in Table 3. Unfortunately, we were unable to characterize the role of BTX-A dosage in relieving spasticity and improving movement. This is because this review focuses on the efficacy of the combination therapy of BTX-A and ESWT. In some studies, BTX-A was administered in both test and control groups. And the injection site varied from study to study, and the description of the dose was not uniform. In conclusion, the combination therapy of BTX-A and ESWT can effectively relieve spasticity in patients with PSS, improve locomotion and activities of daily living, and relieve patients’ pain.
ESWT has shown promising utility in the clinic, meaningfully reducing spasticity and improving motor function. However, there are no standardized treatment parameters for ESWT for spasticity, including ESWT intensity, frequency, number of pulses, and session duration. To ensure the effectiveness of the treatment, more in-depth clinical studies are needed in the future in order to incorporate ESWT into an effective approach for comprehensive stroke rehabilitation programs and to establish evidence-based guidelines for its application. For patients receiving BTX-A injections, the appropriate timing of ESWT interventions could be further explored. Determining the optimal timing and periodicity of ESWT interventions can help maximize the benefits of ESWT and promote overall stroke recovery. Given that the quality of current studies of combination therapy is not optimal, rigorous study designs, validated spasticity assessment tools, and relevant animal studies are expected to provide more substantial scientific evidence for combination therapy.
5 Limitation
This review has some limitations in terms of the completeness of the literature search. First, the search only included literature in Chinese and English, which may have omitted potentially relevant articles in other languages. Second, the number of included literature was small, and therefore the results of the studies were unstable. The included studies were small and lacked data from large studies to support the efficacy of combination therapy. Also, treatment doses and parameters were not the same across studies. Finally, the method of bias analysis in this paper is somewhat subjective. More clinical trials are needed to supplement this in the future.
6 Conclusion
Management of spasticity states remains challenging. Increasing attention is being paid to the contribution of combining anti-spasticity medications with nonpharmacologic interventions to early and long-term spasticity management. Because high-quality studies of BTX-A in combination with ESWT for spasticity are still insufficient, further studies with well-designed experiments are needed to finalize the most effective management of spasticity status. Future studies could focus on the dose of the combined intervention, frequency of treatment, and treatment period to develop effective spasticity management and improve long-term functional outcomes after stroke. Given the current published evidence, the combination of BTX-A and ESWT is critical for providing long-term, comprehensive rehabilitation for spasticity states after stroke.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
Y-nD: Writing – original draft, Writing – review & editing. YL: Writing – original draft, Writing – review & editing. T-yZ: Writing – original draft, Writing – review & editing. NJ: Writing – original draft, Writing – review & editing. H-yD: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This present study was funded by Science and technology achievements transformation fund of the First Hospital of Jilin University (no. JDYYZH-2022012), Specialized medical health care personnel in Jilin Province (no. JLSWSRCZX2021-059), and the Science and technology research project of the Education Department of Jilin Province (no. JJKH20231222KJ).
Acknowledgments
The authors thank all study participants.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1342545/full#supplementary-material
References
1. Thayabaranathan, T, Kim, J, Cadilhac, DA, Thrift, AG, Donnan, GA, Howard, G, et al. Global stroke statistics 2022. Int J Stroke. (2022) 17:946–56. doi: 10.1177/17474930221123175
2. Zhao, Y, Hua, X, Ren, X, Ouyang, M, Chen, C, Li, Y, et al. Increasing burden of stroke in China: a systematic review and Meta-analysis of prevalence, incidence, mortality, and case fatality. Int J Stroke. (2023) 18:259–67. doi: 10.1177/17474930221135983
3. Yang, L, Tan, JY, Ma, H, Zhao, H, Lai, J, Chen, JX, et al. Warm-needle Moxibustion for spasticity after stroke: a systematic review of randomized controlled trials. Int J Nurs Stud. (2018) 82:129–38. doi: 10.1016/j.ijnurstu.2018.03.013
4. Zeng, H, Chen, J, Guo, Y, and Tan, S. Prevalence and risk factors for spasticity after stroke: a systematic review and Meta-analysis. Front Neurol. (2020) 11:616097. doi: 10.3389/fneur.2020.616097
5. Li, S, Francisco, GE, and Rymer, WZ. A new definition of poststroke spasticity and the interference of spasticity with motor recovery from acute to chronic stages. Neurorehabilit Neural Repair. (2021) 35:601–610.
7. Marciniak, C. Poststroke hypertonicity: upper limb assessment and treatment. Top Stroke Rehabil. (2011) 18:179–94. doi: 10.1310/tsr1803-179
8. Pan, X, Zhao, G, Yang, X, Hua, Y, Wang, J, Ying, Q, et al. Contralateral C7 nerve transfer via the Prespinal route in treatment of spastic paralysis of upper limb after cerebral palsy. Br J Neurosurg. (2023) 37:1292–6. doi: 10.1080/02688697.2020.1859091
9. Langhammer, B, and Lindmark, B. Functional exercise and physical fitness post stroke: the importance of exercise maintenance for motor control and physical fitness after stroke. Stroke Res Treat. (2012) 2012:864835:1–9. doi: 10.1155/2012/864835
10. Li, H, Long, D, Li, B, Liu, H, Ma, T, Wu, T, et al. A clinical study to assess the influence of acupuncture at "Wang's Jiaji" Acupoints on limb spasticity in patients in convalescent stage of ischemic stroke: study protocol for a randomized controlled trial. Trials. (2019) 20:419. doi: 10.1186/s13063-019-3464-7
11. Datta Gupta, A, Visvanathan, R, Cameron, I, Koblar, SA, Howell, S, and Wilson, D. Efficacy of botulinum toxin in modifying spasticity to improve walking and quality of life in post-stroke lower limb spasticity—a randomized double-blind placebo controlled study. BMC Neurol. (2019) 19:96. doi: 10.1186/s12883-019-1325-3
12. Zorowitz, RD, Gillard, PJ, and Brainin, M. Poststroke spasticity: sequelae and burden on stroke survivors and caregivers. Neurology. (2013) 80:S45–52. doi: 10.1212/WNL.0b013e3182764c86
13. Matak, I. Evidence for central Antispastic effect of botulinum toxin type a. Br J Pharmacol. (2020) 177:65–76. doi: 10.1111/bph.14846
14. Jacinto, J, Lysandropoulos, A, Leclerc, M, and Calvi-Gries, F. Experiences of patients with Poststroke spasticity throughout a botulinum toxin treatment cycle: results from a prospective ethnographic study. Front Neurol. (2022) 13:946500. doi: 10.3389/fneur.2022.946500
15. Shafshak, TS, and Mohamed-Essa, A. Phenol Neurolysis for relieving intermittent involuntary painful spasm in upper motor neuron syndromes: a pilot study. J Rehabil Med. (2009) 41:201–2. doi: 10.2340/16501977-0310
16. Natale, M, Mirone, G, Rotondo, M, and Moraci, A. Intrathecal baclofen therapy for severe spasticity: analysis on a series of 112 consecutive patients and future Prospectives. Clin Neurol Neurosurg. (2012) 114:321–5. doi: 10.1016/j.clineuro.2011.10.046
17. Dietz, N, Wagers, S, Harkema, SJ, and D'Amico, JM. Intrathecal and Oral baclofen use in adults with spinal cord injury: a systematic review of efficacy in spasticity reduction, functional changes, dosing, and adverse events. Arch Phys Med Rehabil. (2023) 104:119–31. doi: 10.1016/j.apmr.2022.05.011
18. Waters, RL. Upper extremity surgery in stroke patients. Clin Orthop Relat Res. (1978) 131:30–7. doi: 10.1097/00003086-197803000-00005
19. Zhu, T, Liu, K, Ni, BY, Li, L, Jin, HP, and Wu, W. Effects of extracorporeal shock wave therapy combined with isokinetic strength training on spastic calf triceps in patients after a stroke: a double-blinded randomised controlled trial. Neurol Res. (2023) 45:1019–25. doi: 10.1080/01616412.2023.2255413
20. Hao, M, Fang, Q, Wu, B, Liu, L, Tang, H, Tian, F, et al. Rehabilitation effect of intelligent rehabilitation training system on hemiplegic limb spasms after stroke. Open Life Sci. (2023) 18:20220724. doi: 10.1515/biol-2022-0724
21. Ji, Y, Wang, Y, Lou, H, Zhang, Y, Liu, Y, Zheng, X, et al. Tuina combined with physical therapy for spasticity of Poststroke: a protocol for systematic review and Meta-analysis. Medicine. (2022) 101:e28780. doi: 10.1097/md.0000000000028780
22. Qin, Y, Liu, X, Zhang, Y, Wu, J, and Wang, X. Effects of transcranial combined with peripheral repetitive magnetic stimulation on limb spasticity and resting-state brain activity in stroke patients. Front Hum Neurosci. (2023) 17:992424. doi: 10.3389/fnhum.2023.992424
23. Pizzi, A, Carlucci, G, Falsini, C, Verdesca, S, and Grippo, A. Application of a volar static splint in Poststroke spasticity of the upper limb. Arch Phys Med Rehabil. (2005) 86:1855–9. doi: 10.1016/j.apmr.2005.03.032
24. Picelli, A, Santamato, A, Chemello, E, Cinone, N, Cisari, C, Gandolfi, M, et al. Adjuvant treatments associated with botulinum toxin injection for managing spasticity: an overview of the literature. Ann Phys Rehabil Med. (2019) 62:291–6. doi: 10.1016/j.rehab.2018.08.004
25. Hung, JW, Yen, CL, Chang, KC, Chiang, WC, Chuang, IC, Pong, YP, et al. A pilot randomized controlled trial of botulinum toxin treatment combined with robot-assisted therapy, Mirror therapy, or active control treatment in patients with spasticity following stroke. Toxins. (2022) 14:415. doi: 10.3390/toxins14060415
26. Esquenazi, A, Zorowitz, RD, Ashford, S, Maisonobe, P, Page, S, and Jacinto, J. Clinical presentation of patients with lower limb spasticity undergoing routine treatment with botulinum toxin: baseline findings from an international observational study. J Rehabil Med. (2023) 55:jrm4257. doi: 10.2340/jrm.v55.4257
27. Sun, LC, Chen, R, Fu, C, Chen, Y, Wu, Q, Chen, R, et al. Efficacy and safety of botulinum toxin type a for limb spasticity after stroke: a Meta-analysis of randomized controlled trials. Biomed Res Int. (2019) 2019:8329306. doi: 10.1155/2019/8329306
28. Jia, S, Liu, Y, Shen, L, Liang, X, Xu, X, and Wei, Y. Botulinum toxin type a for upper limb spasticity in Poststroke patients: a Meta-analysis of randomized controlled trials. J Stroke Cerebrovasc Dis. (2020) 29:104682. doi: 10.1016/j.jstrokecerebrovasdis.2020.104682
29. Olver, J, Esquenazi, A, Fung, VS, Singer, BJ, and Ward, AB. Botulinum toxin assessment, intervention and aftercare for lower limb disorders of movement and muscle tone in adults: international consensus statement. Eur J Neurol. (2010) 17:57–73. doi: 10.1111/j.1468-1331.2010.03128.x
30. Francisco, GE, Balbert, A, Bavikatte, G, Bensmail, D, Carda, S, Deltombe, T, et al. A practical guide to optimizing the benefits of post-stroke spasticity interventions with botulinum toxin a: an international group consensus. J Rehabil Med. (2021) 53:2753. doi: 10.2340/16501977-2753
31. Schlomer, BJ. Urologic treatment of nephrolithiasis. Curr Opin Pediatr. (2020) 32:288–94. doi: 10.1097/mop.0000000000000849
32. Wu, YT, Chang, CN, Chen, YM, and Hu, GC. Comparison of the effect of focused and radial extracorporeal shock waves on spastic Equinus in patients with stroke: a randomized controlled trial. Eur J Phys Rehabil Med. (2018) 54:518–25. doi: 10.23736/s1973-9087.17.04801-8
33. Mihai, EE, Popescu, MN, Iliescu, AN, and Berteanu, M. A systematic review on extracorporeal shock wave therapy and botulinum toxin for spasticity treatment: a comparison on efficacy. Eur J Phys Rehabil Med. (2022) 58:565–74. doi: 10.23736/s1973-9087.22.07136-2
34. Kenmoku, T, Ochiai, N, Ohtori, S, Saisu, T, Sasho, T, Nakagawa, K, et al. Degeneration and recovery of the neuromuscular junction after application of extracorporeal shock wave therapy. J Orthop Res. (2012) 30:1660–5. doi: 10.1002/jor.22111
35. Yoldaş Aslan, Ş, Kutlay, S, Düsünceli Atman, E, Elhan, AH, Gök, H, and Küçükdeveci, AA. Does extracorporeal shock wave therapy decrease spasticity of ankle plantar flexor muscles in patients with stroke: a randomized controlled trial. Clin Rehabil. (2021) 35:1442–53. doi: 10.1177/02692155211011320
36. Lee, JH, and Kim, EJ. A comprehensive review of the effects of extracorporeal shock wave therapy on stroke patients: Balance, pain, spasticity. Medicina. (2023) 59:857. doi: 10.3390/medicina59050857
37. Megna, M, Marvulli, R, Farì, G, Gallo, G, Dicuonzo, F, Fiore, P, et al. Pain and muscles properties modifications after botulinum toxin type a (Btx-a) and radial extracorporeal shock wave (Reswt) combined treatment. Endocr Metab Immune Disord Drug Targets. (2019) 19:1127–33. doi: 10.2174/1871530319666190306101322
38. Santamato, A, Notarnicola, A, Panza, F, Ranieri, M, Micello, MF, Manganotti, P, et al. Sbote study: extracorporeal shock wave therapy versus electrical stimulation after botulinum toxin type a injection for post-stroke spasticity-a prospective randomized trial. Ultrasound Med Biol. (2013) 39:283–91. doi: 10.1016/j.ultrasmedbio.2012.09.019
39. Duan, H, Chen, X, Li, H, Liu, X, Liu, N, and Li, Z. Efficacy of botulinum toxin type a combined with low-frequency extracorporeal shock wave in the treatment of post-stroke triceps spasticity of the calf muscle. Chin J Phys Med Rehabil. (2020) 42:992–4. doi: 10.3760/cma.j.issn.0254-1424.2020.11.007
40. Liang, H, Lu, Y, Wu, Z, Cao, W, Guo, J, and Li, R. Clinical study of botulinum toxin type a combined with extracorporeal shock wave in the treatment of post-stroke upper limb spasticity. J Minimally Invas Med. (2021) 16:34–7. doi: 10.11864/j.issn.1673.2021.01.07
41. Lu, Y. Clinical study of myoelectric acoustic feedback instrument-guided botulinum toxin injection combined with extracorporeal shockwave for the treatment of limb spasticity after stroke. Self Care. (2021) 18:81–2.
42. Wang, H, Zhao, C, Yuan, H, Liu, W, Yuan, H, Hui, N, et al. The efficacy of botulinum toxin type a combined with shockwave therapy on the spastic state of the lower limbs after stroke. Chin J Rehabil Med. (2017) 32:773–8. doi: 10.3969/j.issn.1001-1242.2017.07.008
43. Chen, CL, Chen, CY, Chen, HC, Wu, CY, Lin, KC, Hsieh, YW, et al. Responsiveness and minimal clinically important difference of modified Ashworth scale in patients with stroke. Eur J Phys Rehabil Med. (2019) 55:754–60. doi: 10.23736/s1973-9087.19.05545-x
44. Yunhui, X, Hao, GU, Qing, Z, Dejie, LI, Ying, G, Junxing, X, et al. Efficacy of Meridian massage for motor function after a stroke: a systematic review and Meta-analysis. J Tradit Chin Med. (2022) 42:321–31. doi: 10.19852/j.cnki.jtcm.2022.03.001
45. Meseguer-Henarejos, AB, Sánchez-Meca, J, López-Pina, JA, and Carles-Hernández, R. Inter- and intra-rater reliability of the modified Ashworth scale: a systematic review and Meta-analysis. Eur J Phys Rehabil Med. (2018) 54:576–90. doi: 10.23736/s1973-9087.17.04796-7
46. Baunsgaard, CB, Nissen, UV, Christensen, KB, and Biering-Sørensen, F. Modified Ashworth scale and spasm frequency score in spinal cord injury: reliability and correlation. Spinal Cord. (2016) 54:702–8. doi: 10.1038/sc.2015.230
47. Rech, KD, Salazar, AP, Marchese, RR, Schifino, G, Cimolin, V, and Pagnussat, AS. Fugl-Meyer assessment scores are related with kinematic measures in people with chronic hemiparesis after stroke. J Stroke Cerebrovasc Dis. (2020) 29:104463. doi: 10.1016/j.jstrokecerebrovasdis.2019.104463
48. Gladstone, DJ, Danells, CJ, and Black, SE. The Fugl-Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. (2002) 16:232–40. doi: 10.1177/154596802401105171
49. Raghavan, P. Upper limb motor impairment after stroke. Phys Med Rehabil Clin N Am. (2015) 26:599–610. doi: 10.1016/j.pmr.2015.06.008
50. Downs, S, Marquez, J, and Chiarelli, P. The berg balance scale has high intra- and inter-rater reliability but absolute reliability varies across the scale: a systematic review. J Physiother. (2013) 59:93–9. doi: 10.1016/s1836-9553(13)70161-9
51. Ohura, T, Hase, K, Nakajima, Y, and Nakayama, T. Validity and reliability of a performance evaluation tool based on the modified Barthel index for stroke patients. BMC Med Res Methodol. (2017) 17:131. doi: 10.1186/s12874-017-0409-2
52. Shafshak, TS, and Elnemr, R. The visual analogue scale versus numerical rating scale in measuring pain severity and predicting disability in low Back pain. J Clin Rheumatol. (2021) 27:282–5. doi: 10.1097/rhu.0000000000001320
53. Khan, F, Amatya, B, Bensmail, D, and Yelnik, A. Non-pharmacological interventions for spasticity in adults: an overview of systematic reviews. Ann Phys Rehabil Med. (2019) 62:265–73. doi: 10.1016/j.rehab.2017.10.001
54. Marinelli, L, Puce, L, Mori, L, Leandri, M, Rosa, GM, Currà, A, et al. Cannabinoid effect and safety in spasticity following stroke: a double-blind randomized placebo-controlled study. Front Neurol. (2022) 13:892165. doi: 10.3389/fneur.2022.892165
55. Jung, TM, Kim, AR, Lee, Y, Kim, DH, and Kim, DY. Precise muscle selection using dynamic Polyelectromyography for treatment of post-stroke dystonia: a case report. Ann Rehabil Med. (2016) 40:551–5. doi: 10.5535/arm.2016.40.3.551
56. Esquenazi, A, Bloudek, L, Migliaccio-Walle, K, Oliveri, D, Tung, A, Gillard, P, et al. Healthcare resource utilization and costs among patients with post-stroke spasticity before and after spasticity management including Onabotulinumtoxina. J Rehabil Med. (2023) 55:jrm11626. doi: 10.2340/jrm.v55.11626
57. Keam, SJ, Muir, VJ, and Deeks, ED. Botulinum Toxin a (Dysport®): In Dystonias and Focal Spasticity. Drugs. (2011) 71:1043–58. doi: 10.2165/11206340-000000000-00000
58. Liu, DY, Zhong, DL, Li, J, and Jin, RJ. The effectiveness and safety of extracorporeal shock wave therapy (Eswt) on spasticity after upper motor neuron injury: a protocol of systematic review and Meta-analysis. Medicine. (2020) 99:e18932. doi: 10.1097/md.0000000000018932
59. Vidal, X, Martí-Fàbregas, J, Canet, O, Roqué, M, Morral, A, Tur, M, et al. Efficacy of radial extracorporeal shock wave therapy compared with botulinum toxin type a injection in treatment of lower extremity spasticity in subjects with cerebral palsy: a randomized, controlled, cross-over study. J Rehabil Med. (2020) 52:jrm00076. doi: 10.2340/16501977-2703
60. NCT. Does Eswt with Bonta treatment improve outcomes when compared to standard Management for Upper Limb Spasticity Patient. Available at: https://clinicaltrialsgov/show/NCT05226637 (2022).
61. NCT. The effect of extracorporeal shock wave therapy after botulinum toxin type a injection for post-stroke spasticity. Available at: https://clinicaltrialsgov/show/NCT05889026 (2023).
Keywords: botulinum toxin A, extracorporeal shockwave therapy, stroke, spasticity, combination therapy
Citation: Du Y-n, Li Y, Zhang T-y, Jiang N, Wei Y, Cheng S-h, Li H and Duan H-y (2024) Efficacy of botulinum toxin A combined with extracorporeal shockwave therapy in post-stroke spasticity: a systematic review. Front. Neurol. 15:1342545. doi: 10.3389/fneur.2024.1342545
Edited by:
Nasser Kashou, Kash Global Tech, United StatesReviewed by:
Stefania Spina, University of Foggia, ItalyAnjing Zhang, Shanghai First Rehabilitation Hospital, China
Huixian Yu, Capital Medical University, China
Beiyao Gao, China-Japan Friendship Hospital, China
Copyright © 2024 Du, Li, Zhang, Jiang Wei, Cheng, Li and Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao-yang Duan, ZHVhbmh5MTAxMTI5QDE2My5jb20=
†These authors share first authorship
 Ya-nan Du
Ya-nan Du Yang Li
Yang Li Ting-yu Zhang
Ting-yu Zhang Nan Jiang1
Nan Jiang1 Hao-yang Duan
Hao-yang Duan