- 1Department of Neurology, The Second Xiangya Hospital, Central South University, Changsha, Hunan, China
- 2China National Clinical Research Center on Mental Disorders, Changsha, China
Introduction: Dural arteriovenous fistula (DAVF) is an uncommon malformation involving an abnormal connection between dural arteries, or the pachymeningeal branches of cerebral arteries, and dural veins. Its exact pathogenesis remains elusive. Known potential triggers for DAVF include cerebral venous sinus thrombosis (CVST), trauma, ear infections, and cranial surgeries. Due to its rarity and diverse clinical presentations, diagnosing DAVF can be a challenge.
Case description: We present a case of DAVF associated with CVST, manifesting as rapidly advancing parkinsonism accompanied by dementia over a month. Brain magnetic resonance imaging (MRI) revealed bilateral symmetric T2 hyperintensities in the basal ganglia and brain stem. Cerebral angiography further confirmed a fistula between the torcular herophili and the transverse-sigmoid sinuses. Despite strong recommendations for transvenous embolization of the fistula, the patient declined the procedure. The anticoagulant therapy and symptomatic treatments administered did not yield any improvement in the patient’s condition. Additionally, we reviewed 27 DAVF-derived parkinsonism and dementia cases.
Conclusion: DAVF must be considered in the differential diagnosis of cases of rapidly progressive parkinsonism with concurrent dementia. Given its potential for treatment and reversibility, timely diagnosis and intervention for DAVF are paramount.
1 Introduction
Dural arteriovenous fistula (DAVF) is a rare vascular anomaly characterized by an abnormal connection between dural arteries, or pachymeningeal branches of cerebral arteries, and dural veins. Typically manifesting later in life, DAVF can be triggered by factors such as cerebral venous sinus thrombosis (CVST), trauma, ear infections, and cranial surgeries (1). It is believed that these factors can lead to venous thrombosis. DAVF patients may exhibit a range of symptoms, from pulsatile tinnitus and ophthalmoplegia to acute confusion, cognitive impairment, parkinsonism, and specific neurological deficits. The clinical and radiographic presentations are largely determined by the pattern of venous drainage. The primary treatment approach is endovascular embolization, but surgical intervention and stereotactic radiosurgery serve as viable alternatives (1). Given that DAVF is treatable and many symptoms are reversible, early diagnosis and intervention can significantly enhance a patient’s prognosis and reduce long-term disabilities. In this context, we present a unique case of a patient with rapidly progressing parkinsonism and dementia attributable to DAVF who was misdiagnosed as having Wernicke’s encephalopathy.
1.1 Case description
A 59-year-old man came in with worsening and consistent gait difficulties and signs of dementia over the past month. He had not experienced any headaches, double vision, nausea, vomiting, or delirium. His medical history was mostly unremarkable, except for a head injury approximately 1 year ago. Additionally, he had a long history of alcohol consumption. He was initially evaluated at a local hospital, where brain magnetic resonance imaging (MRI) revealed bilateral symmetrical lesions in the brain stem and basal ganglia. Due to the patient’s prolonged history of alcohol abuse and progressive cognitive decline, Wernicke’s encephalopathy was initially suspected at the previous hospital. The patient received high-dose thiamin (300 mg/day, administered via intramuscular injection) for 2 weeks. However, his condition continued to deteriorate, leading to his referral to our neurology department.
Upon examination, he took short steps, showed symmetrical rigidity in all four limbs, general slowness of movement, with mild increased muscle tone and normal strength, hyperreflexia, diminished speech, a soft voice, and cognitive decline without lamination (scoring 18/30 on the Mini-Mental State Examination, especially in attention, calculation, and execution ability). He also exhibited urinary incontinence, and a vascular murmur was audible behind his ears. Laboratory tests, including those for blood count, infections, coagulation function, protein C and S levels, lupus anticoagulant, autoimmune markers (anti-neuronal antibodies, anti-nuclear antibodies, antibodies to extractable nuclear antigen, anti-neutrophil cytoplasmic antibody, anti-cardiolipin antibodies, ds-DNA antibodies, and autoimmune encephalitis antibodies), tumor markers, thromboelastogram, anti-aquaporin 4 antibody, anti-myelin oligodendrocyte glycoprotein antibody, anti-glial fibrillary acidic protein antibody, oligoclonal bands, and metabolic screens, all returned normal results. His blood D-dimer level was measured at 0.73 μg/mL (0–0.55 μg/mL). A lumbar puncture showed that the cerebrospinal fluid pressure exceeded 400mmH2O. Analysis of this fluid indicated normal white cell counts, glucose, and chloride levels, with a slightly elevated protein level of 592.3 mg/L. There were no signs of infection, inflammation, or bleeding.
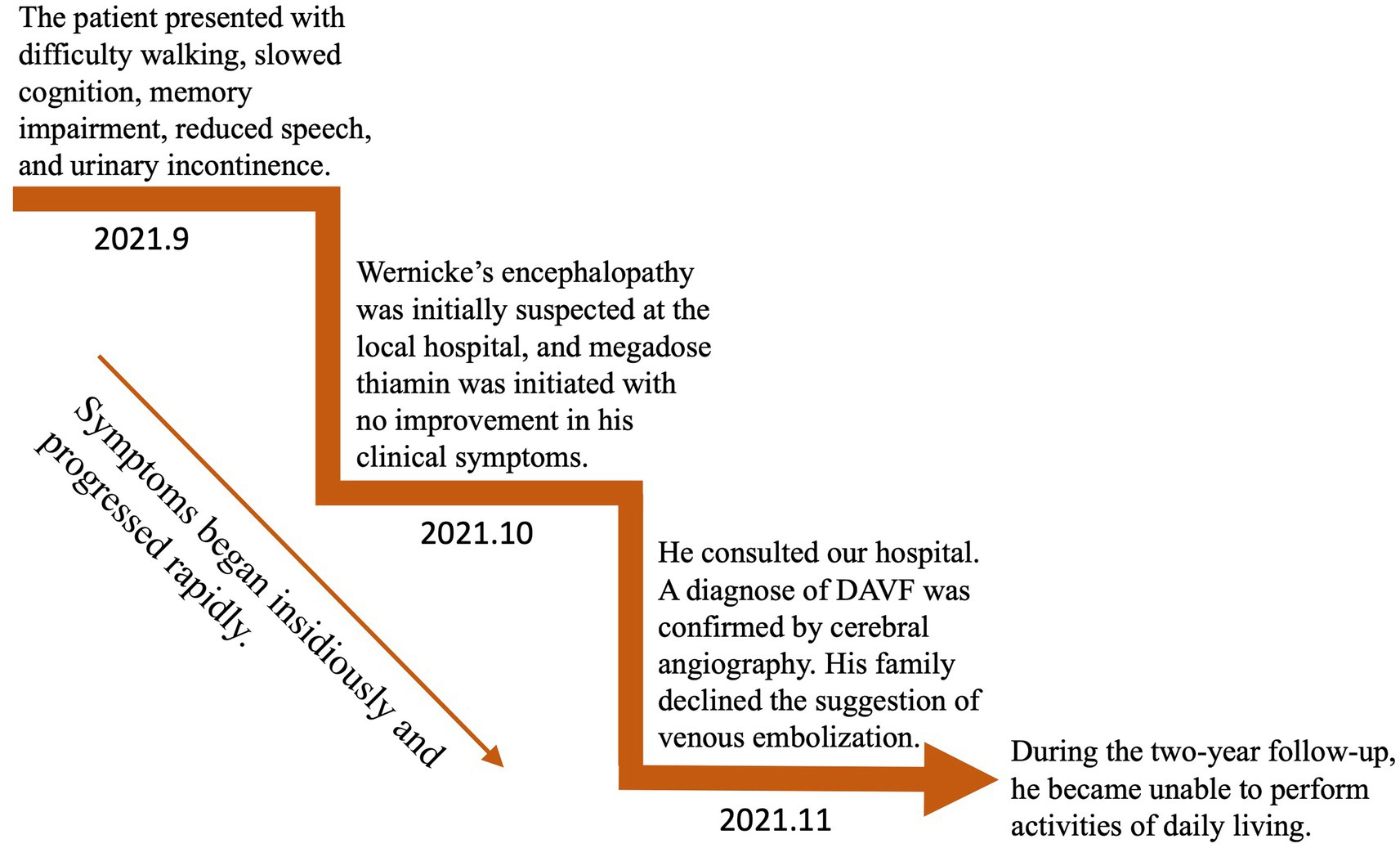
An MRI of the brain revealed bilateral symmetrical lesions in areas such as the pontine, pontine arms, and basal ganglia. These lesions showed restricted diffusion on diffusion-weighted imaging and no enhancement (as seen in Figures 1A–F). Additionally, magnetic resonance venography (MRV) highlighted dilated veins and a filling defect, pointing to possible sinus thrombosis in the superior sagittal sinus (as seen in Figures 1G,H). Cerebral angiography further confirmed thrombosis in the vein of Galen and identified a DAVF between the torcular herophili and transverse-sigmoid sinuses, fed by the occipital artery and arteria cervicalis profunda (as shown in Figure 2). The DAVF drained into the straight sinus, which flowed in a retrograde direction into the vein of Galen, as well as into the internal cerebral veins and basal vein of Rosenthal. Given that DAVFs are treatable and their symptoms can be reversed, it was strongly recommended that the patient undergo transvenous embolization of the fistula. However, the son of the patient declined for personal reasons. Even with subcutaneous injections of low molecular weight heparin (5000AXaIU) every 12 h and symptomatic treatments, including levodopa, there was no improvement in his clinical symptoms. The time course of symptoms, diagnosis, and treatment is shown in Figure 3.

Figure 1. Brain MRI and MRV. The axial section of the T2-weighted image (A,B) and the diffusion-weighted image (C,D) reveal bilateral symmetrical hyper-intensities in the basal ganglia and brain stem (white asterisk). The lesions present with hyperintensity on fluid attenuated inversion recovery (FLAIR) images (E), and no enhancement on enhancing MRI (F). The MRV (G,H) indicates a filling defect in the right transverse, left sigmoid, and superior sagittal sinuses.
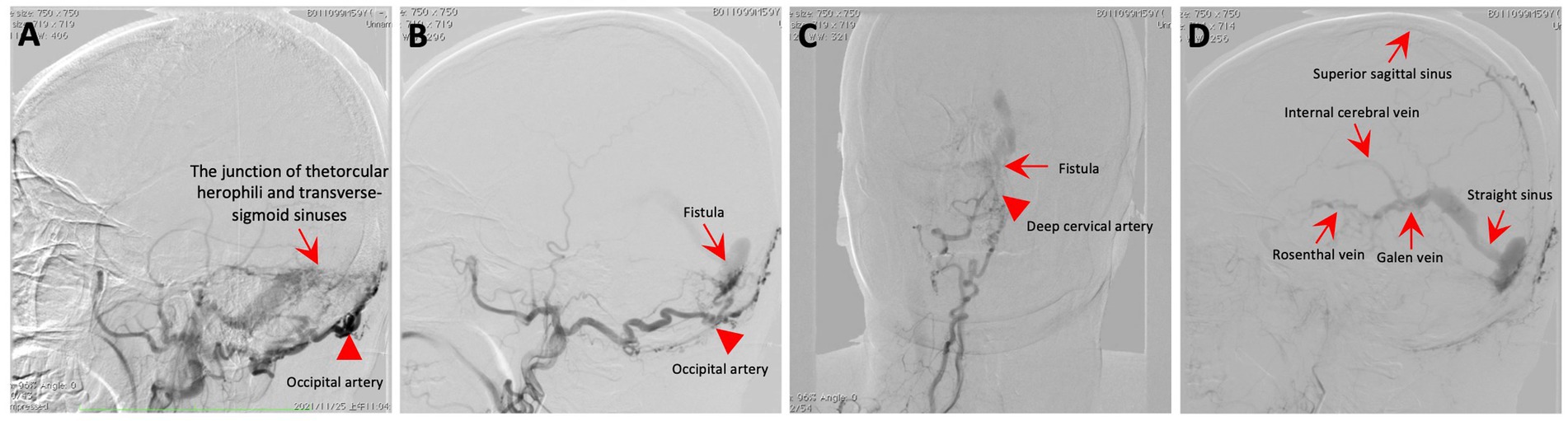
Figure 2. Cerebral angiography. (A–C) The angiogram showcases a fistula (red arrow) connecting the torcular herophili to the transverse-sigmoid sinuses, with blood supply from the occipital artery (red arrowhead) and the deep cervical artery (red arrowhead). (D) The DAVF drained into the straight sinus, which flowed in a retrograde direction into the vein of Galen, as well as into the internal cerebral veins and basal vein of Rosenthal.
2 Discussion
DAVF refers to abnormal shunts between dural arteries and structures such as dural venous sinuses, meningeal veins, or cortical veins. The exact cause of DAVF remains elusive. While many cases appear without any known cause (idiopathic), there are reports suggesting links to CVST, trauma, ear infections, venous hypertension, and prior cranial surgeries. The co-occurrence of DAVF with CVST is relatively rare (2, 3). In our study, we document a case where DAVF is seen alongside CVST. DAVF leads to retrograde blood flow into the straight sinus and the vein of Galen. Thrombosis in the straight sinus and the vein of Galen further exacerbates venous pressure, causing reflux obstruction and insufficient perfusion, subsequently leading to infarction in the corresponding drainage area of brain tissue. Actually, the exact relationship between CVST and DAVF is not well-defined, leading to debates about whether DAVF precipitates CVST or emerges as a consequence of it. Sinus thrombosis can increase venous pressure, which might trigger the development of DAVF by activating dural arteriovenous pathways. This potentially can lead to ischemia because of backward venous flow and blood stagnation. Additionally, the turbulent blood flow observed in DAVF might contribute to thrombus formation (3).
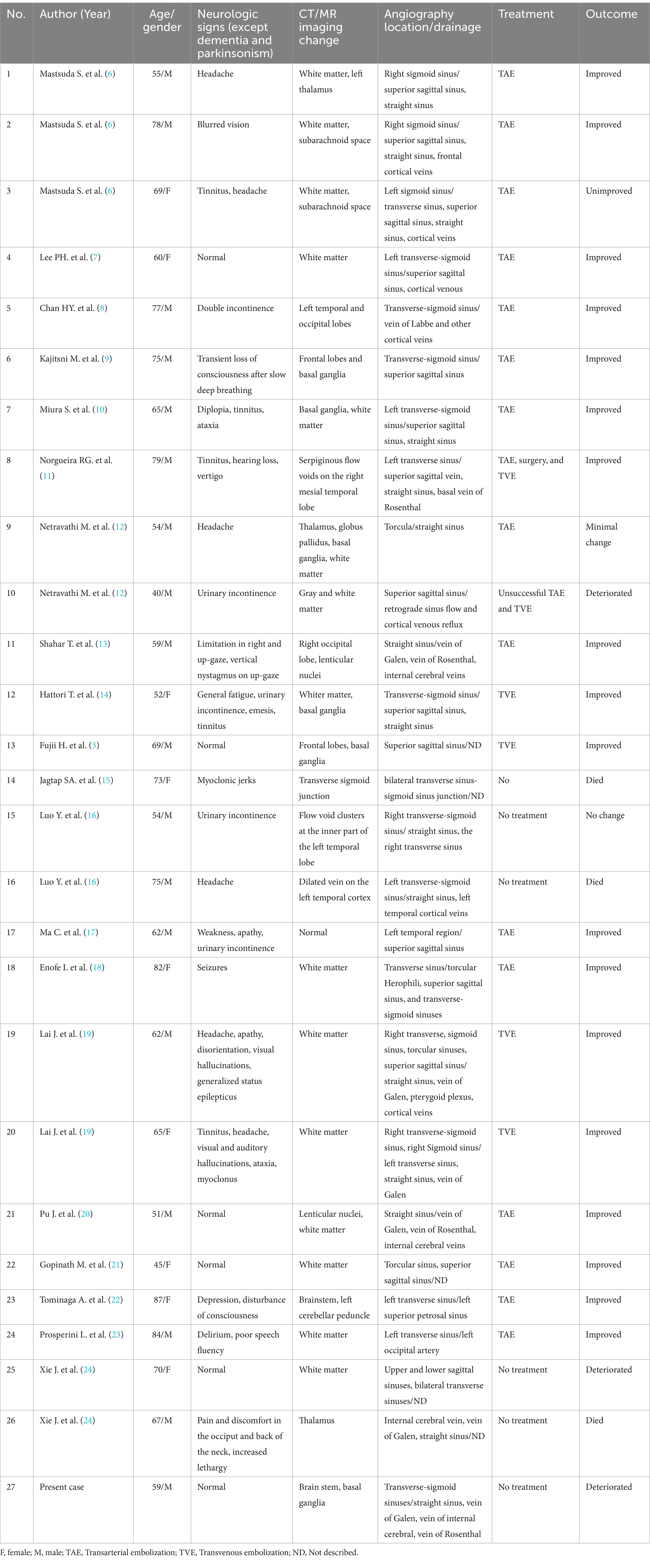
Clinical manifestations linked to DAVF vary widely and are influenced by the pattern of venous drainage. These can range from pulsatile tinnitus and ophthalmoplegia to acute confusion, rapidly advancing dementia, parkinsonism, seizures, cerebellar symptoms, and specific neurological deficits. Notably, DAVF patients with cortical venous drainage and venous ectasia are at a heightened risk of hemorrhage (4). Instances of DAVF patients exhibiting parkinsonism, with or without cognitive dysfunction, are infrequent. Research indicates that parkinsonism in DAVF patients tends to be more prevalent in older male patients, averaging approximately 63 years of age, with cases spanning from 40 to 81 years. The time from initial symptoms to DAVF diagnosis has been documented to range between 1 week and 3 years (5). In this study, we reviewed 27 DAVF patients who have parkinsonism with cognitive dysfunction (Table 1). Of these patients, 18 were male, with an average age of 65 years, ranging from 40 to 87 years old. While the precise mechanism remains unclear, the prevailing theory is that reduced blood flow in the frontal lobes and a perfusion defect in the basal ganglia may be contributing factors to the onset of DAVF-related parkinsonism (5, 7). There is growing evidence suggesting a specific vascular mechanism whereby thalamic damage leads to subacute encephalopathy or swiftly progressing dementia (25). In the case we observed, cognitive impairments included slowed thinking, difficulties with calculations and concentration, memory loss, executive dysfunction, and diminished speech, suggesting dysfunction in the basal ganglia or frontal lobe.
Given the patient’s rapidly advancing parkinsonism and dementia, combined with a long history of alcohol abuse and bilateral symmetrical lesions in the brain stem and basal ganglia as revealed by MRI, various conditions such as Wernicke’s encephalopathy, central pontine myelinolysis, extrapontine myelinolysis, hepatic encephalopathy, Creutzfeldt–Jakob disease, and glioma must be considered (3). Extensive diagnostic efforts were made to rule out these alternatives (as seen in Figure 4). Vitamin B1 levels were not checked because the patient had already been receiving high doses of thiamine for several days. Wernicke’s encephalopathy is more likely to involve the medial thalamus, hypothalamus, periaqueductal gray matter, and the areas surrounding the third and fourth ventricles on MRI. However, the patient does not present with oculomotor paralysis or psychiatric symptoms, and high-dose thiamine treatment has been ineffective, which does not support the diagnosis of Wernicke’s encephalopathy. Extrapontine myelinolysis is more likely to affect the bilateral striatum, and clinical symptoms often outweigh the radiological changes. Additionally, there is no history of electrolyte disturbances in the patient. Non-invasive vascular imaging techniques, including CT and MRI, could offer clues about the presence of DAVF. In this case, retrograde venous drainage can lead to increased venous pressure, resulting in early vascular-origin edema, which may progress to venous infarction characterized by cytotoxic edema. The MRI lesions primarily concentrate in the basal ganglia region, with relatively preserved midbrain areas, correlating with the susceptibility of deep brain tissues to restricted outflow. Moreover, we acknowledge that, in our case, it cannot be conclusively determined whether the involvement of the deep pontine is caused by the known venous thrombus. Cerebral angiography assists in identifying the arterial feeders and venous outflow of the fistula. The Cognard classification system, commonly utilized to categorize DAVF, separates it into five types based on venous drainage patterns (26). In this particular case, according to the angiograph, the DAVF drains into the straight sinus, with filling of the Galen, internal cerebral, and Rosenthal veins, without cortical venous drainage, unveiling a Cognard type IIa DAVF. However, in this classification, there is no detailed subtyping for reflux into deep veins. In future classifications, refinement based on deep venous drainage may have a more positive significance for prognosis assessment and surgical selection.
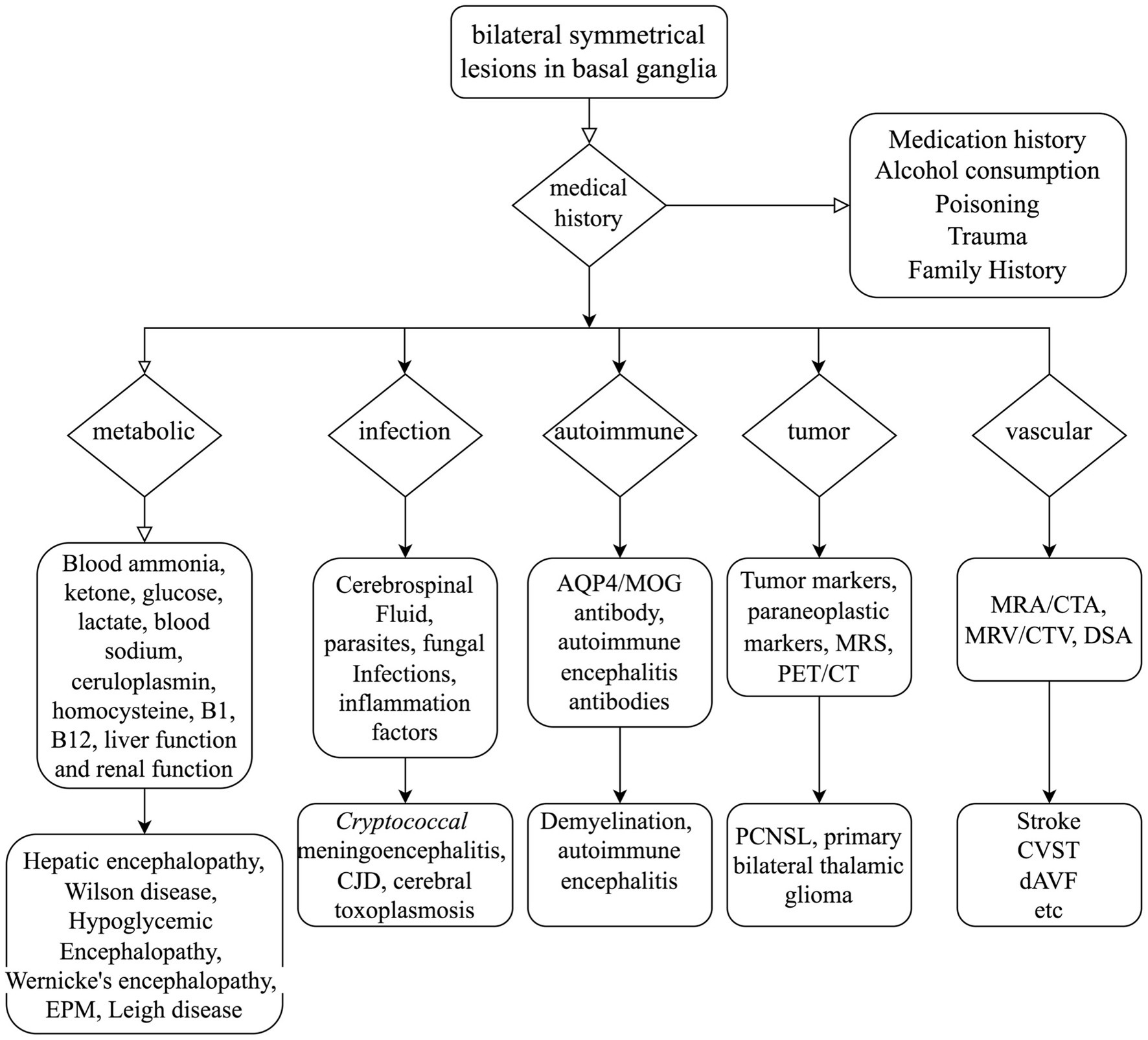
Figure 4. Mind flow chart for the differential diagnosis of bilateral symmetrical lesions in the basal ganglia. AQP4, Aquaporin-4; MOG, myelin oligodendrocyte glycoprotein; MRS, magnetic resonance spectroscopy; PET/CT, positron emission tomography/computed tomography; MRA, magnetic resonance angiography; CTA, computed tomography angiography; MRV, magnetic resonance venography; CTV, computed tomography venography; DSA, digital subtraction angiography; EPM, extrapontine myelinolysis; CJD, Creutzfeldt–Jakob disease; PCNSL, primary lymphomas of the central nervous system; CVST, cerebral venous sinus thrombosis; dAVF, dural arteriovenous fistula.
In the management of non-Parkinson’s disease (PD) patients with parkinsonism, the treatment involves removing precipitating factors, managing the underlying primary disease, and additionally administering medications used in the treatment of PD, such as amantadine, levodopa, dopamine receptor agonists, monoamine oxidase-B inhibitors, and catechol-O-methyl transferase inhibitors, among others. However, these medications may not provide significant improvement in symptoms. Indeed, damage due to edema or ischemia could be reversible. Early intervention through endovascular embolization, aiming for complete obliteration of the fistula, is highly recommended for DAVF patients. Prior research has shown that the vast majority of DAVF patients experienced significant symptom alleviation, with some even achieving total symptom resolution after undergoing endovascular embolization treatments (27). Moreover, clinical improvement correlates with radiographic improvement. Venous sinus thrombosis may further increase venous pressure. Therefore, in patients without surgery, anticoagulant therapy may potentially reduce venous pressure and thus exert a protective effect. However, the role of anticoagulation in untreated DAVF combined with thrombosis remains unclear. In our case, the patient’s family opted against transvenous embolization of the fistula. Consequently, despite receiving anticoagulant therapy and symptomatic treatments, the patient showed no clinical improvement.
We recognize that our case report has certain limitations. First, despite conducting cerebral angiography, we cannot definitively confirm a causal relationship, as the patient did not undergo treatment for the malformation, followed by recovery. Second, our report is limited to a single case. It is important that future reports include a series of cases to shed light on the relationship between CVST and DAVF. Additionally, functional experiments should be conducted to better understand the underlying mechanisms.
In conclusion, we present a classic case of DAVF characterized by rapidly progressing parkinsonism accompanied by dementia. Cerebral angiography was instrumental in identifying the arterial feeders and venous outflow of the fistula. It is crucial to consider DAVF as a potential underlying cause for rapidly progressing parkinsonism with dementia. As the condition is treatable and its effects potentially reversible, prompt diagnosis and intervention for DAVF are of paramount importance.
2.1 Patient perspective
The son of the patient says, “I noticed that over a month ago, my father began to walk slowly, hunching over with a diminished stride, taking small, shuffling steps. Additionally, he has become less responsive, his voice has lowered, and he’s lost control of his bladder and bowels. We sought medical attention at our local hospital and underwent some tests. Given my father’s long history of alcohol consumption, the local hospital treated him for alcohol poisoning. However, not only did his symptoms not improve, they actually worsened. We then consulted the Department of Neurology at the Second Xiangya Hospital. After a cerebral angiogram, the doctor diagnosed him with DAVF and recommended surgery. But the operation can be complicated. We worried about the safety of the surgery as well as a considerable expense, so we could not proceed with the operation.”
Data availability statement
The datasets presented in this article are not readily available because of ethical and privacy restrictions. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
RT: Writing – original draft, Writing – review & editing. QC: Writing – review & editing. LQ: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the National Natural Science Foundation of China (No. 82101342 to LQ), the Natural Science Foundation of Hunan Province (No. 2022JJ30833 to LQ, No. 2022JJ40709 to RT), and the Scientific Research Launch Project for new employees of the Second Xiangya Hospital of Central South University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gandhi, D, Chen, J, Pearl, M, Huang, J, Gemmete, JJ, and Kathuria, S. Intracranial Dural arteriovenous fistulas: classification, imaging findings, and treatment. AJNR Am J Neuroradiol. (2012) 33:1007–13. doi: 10.3174/ajnr.A2798
2. Lindgren, E, Rentzos, A, Hiltunen, S, Serrano, F, Heldner, MR, Zuurbier, SM, et al. Dural arteriovenous fistulas in cerebral venous thrombosis: data from the international cerebral venous thrombosis consortium: data from the international cerebral venous thrombosis consortium. Eur J Neurol. (2022) 29:761–70. doi: 10.1111/ene.15192
3. Parikh, N, Merkler, AE, Cheng, NT, Baradaran, H, White, H, and Leifer, D. Clinical reasoning: an unusual case of subacute encephalopathy. Neurology. (2015) 84:e33–7. doi: 10.1212/wnl.0000000000001233
4. Gross, BA, and Du, R. The natural history of cerebral Dural arteriovenous fistulae. Neurosurgery. (2012) 71:594–602. doi: 10.1227/NEU.0b013e31825eabdb
5. Fujii, H, Nagano, Y, Hosomi, N, and Matsumoto, M. Dural arteriovenous fistula presenting with progressive dementia and parkinsonism. BMJ Case Rep. (2014) 2014:bcr2014203921. doi: 10.1136/bcr-2014-203921
6. Matsuda, S, Waragai, M, Shinotoh, H, Takahashi, N, Takagi, K, and Hattori, T. Intracranial Dural arteriovenous fistula (Davf) presenting progressive dementia and parkinsonism. J Neurol Sci. (1999) 165:43–7. doi: 10.1016/s0022-510x(99)00075-1
7. Lee, PH, Lee, JS, Shin, DH, Kim, BM, and Huh, K. Parkinsonism as an initial manifestation of Dural arteriovenous fistula. Eur J Neurol. (2005) 12:403–6. doi: 10.1111/j.1468-1331.2004.00955.x
8. Chan, HY, Cheng, KM, Lo, MW, Chan, CM, and Cheung, YL. A treatable case of dementia--intracranial dural arteriovenous fistula. Hong Kong Med J. (2006) 12:74–6.
9. Kajitani, M, Yagura, H, Kawahara, M, Hirano, M, Ueno, S, Fujimoto, K, et al. Treatable fluctuating parkinsonism and dementia in a patient with a Dural arteriovenous fistula. Mov Disord. (2007) 22:437–9. doi: 10.1002/mds.21194
10. Miura, S, Noda, K, Shiramizu, N, Muraoka, N, Hirohata, M, Ayabe, M, et al. Parkinsonism and Ataxia associated with an intracranial Dural arteriovenous fistula presenting with Hyperintense basal ganglia in T1-weighted Mri. J Clin Neurosci. (2009) 16:341–3. doi: 10.1016/j.jocn.2008.01.004
11. Nogueira, RG, Baccin, CE, Rabinov, JD, Pryor, JC, Buonanno, FS, and Hirsch, JA. Reversible parkinsonism after treatment of Dural arteriovenous fistula. J Neuroimaging. (2009) 19:183–4. doi: 10.1111/j.1552-6569.2007.00237.x
12. Netravathi, M, Pal, PK, Bharath, RD, and Ravishankar, S. Intracranial Dural arteriovenous fistula presenting as parkinsonism and cognitive dysfunction. J Clin Neurosci. (2011) 18:138–40. doi: 10.1016/j.jocn.2010.04.047
13. Shahar, T, Gadoth, A, Nossek, E, Giladi, N, Ram, Z, and Maimon, S. Reversible freezing of gait caused by Dural arteriovenous fistula and congestion of the Globus pallidus. Mov Disord. (2012) 27:1690–3. doi: 10.1002/mds.25184
14. Hattori, T, Takeuchi, T, Kabeya, R, Ando, K, and Tosaki, F. Transverse-sigmoid sinus Dural arteriovenous fistula presenting with parkinsonism. Neurol Med Chir (Tokyo). (2013) 53:224–7. doi: 10.2176/nmc.53.224
15. Jagtap, SA, Nair, SS, Jain, N, and Nair, MD. Rapidly progressive dementia, parkinsonism and myoclonus: an unusual presentation of Dural arteriovenous fistula. Neurol India. (2014) 62:107–10. doi: 10.4103/0028-3886.128360
16. Luo, Y, Qi, J, Cen, Z, Hu, H, Jiang, B, and Luo, W. Two cases of Dural arteriovenous fistula presenting with parkinsonism and progressive cognitive dysfunction. J Neurol Sci. (2014) 343:211–4. doi: 10.1016/j.jns.2014.05.059
17. Ma, C, Lu, Q, Shi, W, Su, Z, Zhao, Y, Li, C, et al. Diagnosis and treatment of a Dural arteriovenous fistula presenting with progressive parkinsonism and dementia: a case report and literature review. Exp Ther Med. (2015) 9:523–6. doi: 10.3892/etm.2014.2122
18. Enofe, I, Thacker, I, and Shamim, S. Dural arteriovenous fistula as a treatable dementia. Proc. (2017) 30:215–7. doi: 10.1080/08998280.2017.11929592
19. Lai, J, Heran, MKS, Stoessl, AJ, and Gooderham, PA. Reversible parkinsonism and rapidly progressive dementia due to Dural arteriovenous fistula: case series and literature review. Mov Disord Clin Pract. (2017) 4:607–11. doi: 10.1002/mdc3.12480
20. Pu, J, Si, X, Ye, R, and Zhang, B. Straight sinus Dural arteriovenous fistula presenting with reversible parkinsonism: a case report and literature review. Medicine (Baltimore). (2017) 96:e9005. doi: 10.1097/md.0000000000009005
21. Gopinath, M, Nagesh, C, Santhosh, K, and Jayadevan, ER. Dementia and parkinsonism-a rare presentation of intracranial Dural arteriovenous fistulae. Neurointervention. (2017) 12:125–9. doi: 10.5469/neuroint.2017.12.2.125
22. Tominaga, A, Kiura, Y, Kondo, H, Takeshita, S, Magaki, T, Sakoguchi, T, et al. Dural arteriovenous fistula-derived parkinsonism symptoms: a case report with literature review. J Neuroendovasc Ther. (2022) 16:257–64. doi: 10.5797/jnet.cr.2021-0069
23. Prosperini, L, Gentile, M, Ricci, M, Gerace, C, Fabiano, S, Stasolla, A, et al. Dural arteriovenous fistula as a reversible cause of progressive parkinsonism and dementia: a case report. Neurohospitalist. (2022) 12:559–62. doi: 10.1177/19418744221098542
24. Xie, J, Zhang, T, Zhang, Y, Wu, W, Li, P, and Zhang, X. Dural arteriovenous fistula with progressive dementia and parkinsonism: two case reports and a literature review. Medicine (Baltimore). (2023) 102:e35782. doi: 10.1097/md.0000000000035782
25. McKenna, MC, Lope, J, Bede, P, and Tan, EL. Thalamic pathology in frontotemporal dementia: predilection for specific nuclei, phenotype-specific signatures, clinical correlates, and practical relevance. Brain Behav. (2023) 13:e2881. doi: 10.1002/brb3.2881
26. Cognard, C, Gobin, YP, Pierot, L, Bailly, AL, Houdart, E, Casasco, A, et al. Cerebral Dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. (1995) 194:671–80. doi: 10.1148/radiology.194.3.7862961
Keywords: DAVF, parkinsonism, dementia, CVST, Wernicke’s encephalopathy
Citation: Tu R, Chen Q and Qin L (2024) Dural arteriovenous fistula and sinus thrombosis presenting as parkinsonism and dementia: a case report with literature review. Front. Neurol. 15:1336593. doi: 10.3389/fneur.2024.1336593
Edited by:
Osama O. Zaidat, Northeast Ohio Medical University, United StatesReviewed by:
Ronald Antulov, University Hospital of Southern Denmark, DenmarkAhmed Y. Azzam, October 6 University, Egypt
Jinlu Yu, First Affiliated Hospital of Jilin University, China
Li Ma, University of Pittsburgh Medical Center, United States
Copyright © 2024 Tu, Chen and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lixia Qin, cWlubGl4aWExMDI3QGNzdS5lZHUuY24=
 Ranran Tu
Ranran Tu Qihua Chen
Qihua Chen Lixia Qin
Lixia Qin