
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 24 May 2024
Sec. Neuro-Ophthalmology
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1333091
 Hengli Zhang1†
Hengli Zhang1† Yawen Li1†
Yawen Li1† Yizhen Tang2
Yizhen Tang2 Xiaowei Yan1
Xiaowei Yan1 Yulei Geng1
Yulei Geng1 Weijia Li1
Weijia Li1 Kuitang Shi1
Kuitang Shi1 Guangxian Tang1*
Guangxian Tang1* Hongtao Guo3*
Hongtao Guo3*Introduction: Acute primary angle closure (APAC) is an emergency ophthalmic presentation and a major cause of irreversible blindness in China. However, only a few studies have focused on the characteristics of optic disc hemorrhage (ODH) during an APAC attack, including its shape, depth, location, scope, and duration after intraocular pressure (IOP) control, along with changes in the optic nerve. This study aimed to analyze the characteristics of ODH and optic nerve changes in patients during their first APAC episode.
Methods: This retrospective study involved 32 eyes from 32 patients with APAC who received sequential treatment and analyzed the following parameters: the highest IOP and its duration, ODH, retinal nerve fiber layer thickness (RNFLT), and mean deviation (MD). We compared parameters obtained from the affected eye (ODH group) and contralateral unaffected eye (control group), as well as intragroup comparisons.
Results: The mean IOP in the ODH group was 64.28 ± 10.36 mmHg, with a duration of 4.44 ± 2.35 days. Flame and splinter shapes accounted for 84.38% of the ODH. The mean ODH duration was 4.81 ± 3.25 weeks. ODH during APAC was isolated to one sector in 59.38% of cases, mostly occurring in the temporal superior and temporal inferior (each accounting for 21.88% of the cases). There was a positive correlation between the extent of hemorrhage and the highest IOP duration (p < 0.001). RNFLT was significantly thickened within 72 h post-IOP control but was thinned by 2 weeks. By 6 months, the thinning stabilized, and there was no difference noted between the ODH and control groups at 12 months. MD partly improved at 6 months post-IOP control, and ODH scope significantly affected the MD (p < 0.001). The duration of high IOP was positively correlated to the ODH scope and MD damage.
Discussion: Timely and effective IOP management is essential for recovering visual function following an APAC attack.
Primary angle closure glaucoma (PACG), a serious and irreversible blinding eye disease, is the main type of glaucoma across Asia, with approximately 48% of affected patients based in China (1, 2). Acute primary angle closure (APAC) is an emergency ophthalmic presentation and a major cause of irreversible blindness in China (3, 4). It is characterized by a sudden increase in intraocular pressure (IOP), reduced visual acuity (VA), and ocular pain, eventually leading to severe visual impairment and glaucomatous optic neuropathy development (5). However, high IOP has diverse effects on the optic nerve during APAC attacks (6–8). Studies have reported a high prevalence of APAC in Asian populations, often presenting with more severe and longer duration (1, 9).
Prolonged elevation of IOP mechanically compresses the optic nerve, disrupting the lamina cribrosa continuity, distorting lamina cribrosa pores, impeding axoplasmic flow, and causing optic disc hemorrhage (ODH) (10–12). However, only a few studies have investigated the characteristics of ODH during an APAC attack. Accordingly, the shape, depth, location, scope, and duration of ODH after IOP control, as well as optic nerve changes, remain largely unclear. In this study, we aimed to analyze ODH characteristics and optic nerve changes following an initial APAC attack to reveal their impact on disease progression and prognosis.
In this retrospective study, 32 patients (32 eyes) with ODH during an APAC attack who were admitted to Shijiazhuang People’s Hospital between October 2018 and January 2020 were recruited. All cases presented with a single-eye attack. In each patient, the eye experiencing the acute attack with the highest initial IOP and ODH was categorized into the ODH group, whereas the contralateral unaffected eye, maintaining a controlled IOP and without ODH, served as the control group. All patients underwent routine ophthalmic examinations when the cornea was transparent.
The highest IOP and its duration, ODH characteristics, best-corrected visual acuity (BCVA), RNFLT, and VF were recorded within 72 h and at 2 weeks, 1 month, 3 months, 6 months, and 12 months after IOP control. This study adhered to the principles of the Declaration of Helsinki and received approval from the Ethics Committee of Shijiazhuang People’s Hospital. All patients provided written informed consent.
This study included patients who met the following criteria: (1) aged >18 years; (2) diagnosed using the International Society of Geographic and Epidemiologic Ophthalmology diagnostic criteria (3, 13); (3) experiencing their first APAC attack with no history of intraocular surgery; (4) presenting with eye or periorbital pain, accompanied by ipsilateral headache, nausea, decreased vision, conjunctival congestion, corneal epithelial edema, shallow anterior chamber, mydriasis, and light reflex disappearance; and (5) exhibiting angle closure under dynamic gonioscopy.
The exclusion criteria were as follows: (1) secondary angle closure, such as uveitis and neovascular glaucoma; (2) APAC with a confirmed diagnosis of PACG; (3) multiple APAC attacks; (4) history of intraocular surgery; (5) unreliable OCT and VF examination results; (6) exceeding ±5.0 D and ± 3.0 D refractive astigmatism; and (7) presence of retinopathy or other conditions that damage the optic nerve, such as diabetes and hypertension.
A Snellen chart was used for BCVA assessment, with results expressed using the logarithmic minimum angle of resolution (logMAR). IOP was measured using a calibrated Goldmann applanation tonometer. Gonioscopy, with a single mirror Gonio diagnostic lens, was used to measure the anterior chamber angle, whereas the anterior segment was examined using a slit-lamp microscope. Direct ophthalmoscopic examination and stereophotography were conducted to detect ODH. All examinations were performed by experienced ophthalmologists and technicians.
ODH was defined as a hemorrhage within one disc diameter of the optic nerve (14) and presented in a splinter, flame, or linear shape radially perpendicular to the optic disc edge or as parapapillary hemorrhage (<1 papilla diameter) (15). Using spectral-domain optical coherence tomography (SD-OCT; Spectralis OCT, Heidelberg, Germany) automatic scanning (Figure 1), the ODH scope, which is the extent of ODH distribution within the optic disc, was divided into different sectors as follows: temporal superior (TS), temporal (T), temporal inferior (TI), nasal superior (NS), nasal (N), and nasal inferior (NI). Within 3 months after IOP control, ODH changes were closely monitored using direct ophthalmoscopy and stereo photography (Kowa Nonmyd WX-3D) every 2 weeks, with stereoscopic optic disc imaging.
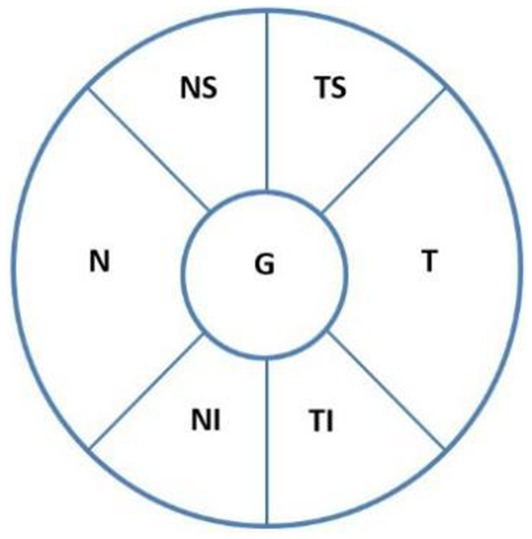
Figure 1. ODH locations according to the optic disc, as revealed on SD-OCT automatic scanning. The sectors are as follows: temporal superior (TS), temporal (T), temporal inferior (TI), nasal superior (NS), nasal (N), and nasal inferior (NI). ODH, optic disc hemorrhage.
The Humphrey-750i Field Analyzer (Carl Zeiss Meditec, Oberkochen, Germany) was used to assess the VF. Before VF testing, the patients underwent slit-lamp and fundus examinations to exclude factors that may influence VF examination, such as corneal abnormalities or retinal and optic neuropathies. The reliability criteria comprised a fixation loss rate of <20% and false-negative and false-positive rates of <15%. The mean deviation (MD) was recorded based on the results of three tests conducted to establish a reliable baseline, and unreliable VFs were examined repeatedly. VF and OCT were performed by experienced doctors within the same month.
The SD-OCT (Spectralis OCT) optic disc examination was performed with the optic disc at the center and a diameter of 3.4 mm for circular scanning. The measurement parameters were as follows: TS, T, TI, NS, N, NI, the average RNFLT (G), and the depth of the ODH.
All data were analyzed using SPSS 20.0 (IBM, Armonk, NY, United States). The measurement values are expressed as mean ± standard deviation, where n represents the number of eyes. The parameters of the ODH and control groups at the same time point were compared using paired t-tests. Repeated measures analysis of variance was used to detect intragroup variations of the RNFLT and MD at different time points after IOP control, and the Student–Newman–Keuls test was used to compare intragroup differences between two time points. Linear regression analysis was used to assess the association between the highest IOP, its duration, and ODH scope, as well as between the ODH scope and MD. p < 0.05 was considered statistically significant.
The patients were aged between 45 and 74 years (65.13 ± 6.91) and included 8 men (8 eyes) and 24 women (24 eyes).
In the ODH group, 28 eyes underwent trabeculectomy, 2 underwent vitreous aspiration and anterior chamber reformation, and 2 underwent phacoemulsification combined with goniosynechialysis. In the control group, surgical and laser peripheral iridectomy procedures were performed in 18 and 14 eyes, respectively.
The average duration of increased IOP during the APAC attack was 4.44 ± 2.35 (range 2–11) days. In the ODH group, the mean IOP during the APAC attack was 64.28 ± 10.36 mmHg (range 56–87 mmHg), which was significantly higher than that of the control group (16.66 ± 2.31 mmHg; t = 25.64, p < 0.001). At 12 months post-IOP control, the mean IOP in the ODH and control groups was 16.38 ± 2.77 and 15.56 ± 1.76 mmHg, respectively, with no statistically significant difference observed (t = 1.34, p = 0. 19). IOP was maintained at the target IOP level in all patients during the follow-up period. At 6 months post-IOP control, two patients with elevated IOP in the ODH group received an IOP-lowering medication, whereas another patient was treated with two IOP-lowering medications to achieve an IOP < 21 mmHg. The changes in IOP during the follow-up period are shown in Table 1.
In the ODH group, the mean 72 h post-IOP control BCVA was 1.48 ± 0.42, which differed significantly from that of the control group (t = 16.53, p < 0.001). The BCVA improved after IOP control, demonstrating a significant intragroup difference (F = 159.4, p < 0.001). There was a significant improvement of BCVA at 2 weeks post-IOP control compared to that within 72 h post-IOP control (q = 22.42, p < 0.001); this improvement was also observed at 1 month compared to that at 2 weeks (q = 5.69, p < 0.001). However, no significant alteration was observed at 6 months post-IOP control compared to that at 1 month post-IOP control (q = 1.65, p > 0.05) (Table 2).
The mean ODH duration was 4.81 ± 3.25 (range: 2–13) weeks. Flame and splinter ODH shapes constituted 84.38% (27 eyes) of all cases, whereas linear shapes accounted for 15.63% (5 eyes). The hemorrhages were located in the superficial layer of the retina; 62.5% (20 eyes) of the hemorrhages were radially perpendicular to the optic disc edge, whereas 37.5% (12 eyes) were parapapillary hemorrhages. Moreover, 12.5% (4 eyes) of the cases were accompanied by a flame-shaped hemorrhage along the blood vessels to the peripheral retina (Figure 2).

Figure 2. Photos of the different types of ODH: (A) linear ODH shapes; (B) splinter ODH shapes; (C,D) flame ODH shapes.
ODH was isolated to one sector in 59.38% (19 eyes); TS, TI, and T accounted for 21.88% (7 eyes), 21.88% (7 eyes), and 15.63% (5 eyes), respectively. Hemorrhages spanned two, three, and four sectors, and the circle accounted for 18.75% (6 eyes), 12.5% (4 eyes), 3.13% (1 eye), and 6.25% (2 eyes), respectively. Among the 32 cases in the ODH group, the overall prevalence of ODH in the TI, TS, T, NS, NI, and N sectors was 50% (16 cases), 46.88% (15 cases), 34.37% (11 cases), 21.88% (7 cases), 18.75% (6 cases), and 12.5% (4 cases), respectively (Figure 3).
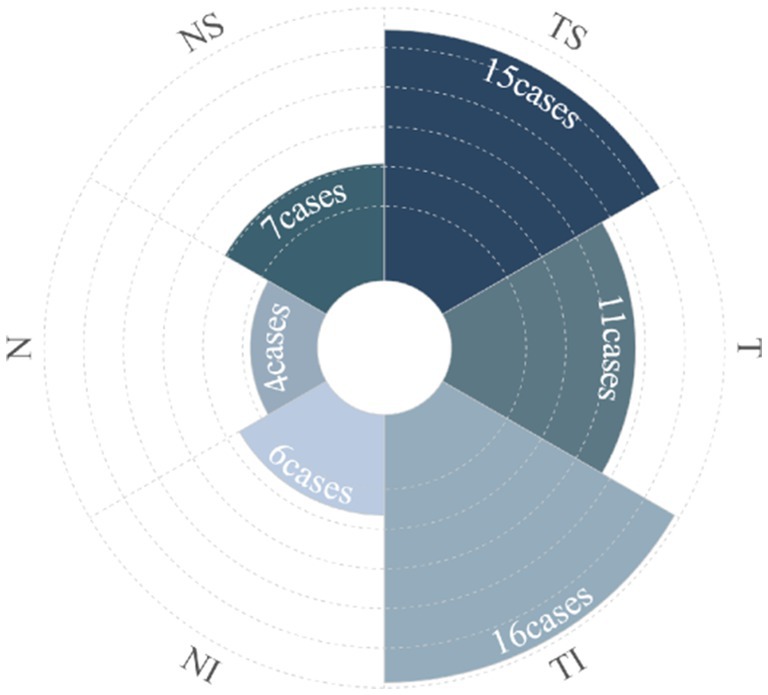
Figure 3. The polar diagram shows the number of cases with disc hemorrhages in relationship to ON sectors.
In the ODH group, the RNFLT in each sector at 72 h post-IOP control was significantly thicker than that of the control group (all p < 0.001). With the exception of the temporal side (p = 0.92), the RNFLT in the other sectors remained significantly thickened at 2 weeks (all p < 0.001). However, it was significantly thinner in each sector from 1 to 12 months of follow-up post-IOP control (all p < 0.05) (Table 3).
In the control group, no significant difference in RNFLT was observed in any sector during the follow-up period (NS: F = 0.75, p = 0.59; N: F = 0.71, p = 0.62; NI: F = 0.27, p = 0.93; TS: F = 0.33, p = 0.90; T: F = 0.58, p = 0.72; TI: F = 0.30, p = 0.91; G: F = 0.35, p = 0.88; Figure 4).
In the ODH group, RNFLT decreased with time (NS: F = 97.36, p < 0.001; N: F = 81.39, p < 0.001; NI: F = 142.2, p < 0.001; TS: F = 289.6 p < 0.001; T: F = 63.56, p < 0.001; TI: F = 188.4, p < 0.001; G: F = 199.2, p < 0.001). At 2 weeks, no significant difference in RNFLT was observed in the nasal sector (p > 0.05). However, the RNFLT at all other time points was significantly thinner (all p < 0.05) than at 72 h post-IOP control. The RNFLT in each sector was thinner after 1 month than at 2 weeks post-IOP control (p < 0.001). No significant difference in temporal RNFLT was observed between 1 and 3 months post-IOP control (p > 0.05), whereas RNFLT in the other sectors decreased (p < 0.05). At the same time, RNFLT in the TS, TI, and G sectors was significantly lower at 6 months post-IOP control than at 3 months post-IOP control (p < 0.05); no significant difference was observed in the other sectors (p > 0.05). RNFLT in each sector within 1 year after IOP control showed no significant change compared to 6 months after IOP control (p > 0.05). The post-IOP control changes in RNFLT in the ODH group are shown in Figure 4.
The RNFLT gradually thinned in ODH located in TS and TI (TS: F = 159.9, p < 0.001; TI: F = 202.6, p < 0.001), and was significantly thinner at 6 months post-IOP control (both p < 0.05). No significant changes were observed between 6 and 12 months post-IOP control (p > 0.05). Intragroup analysis revealed that during the follow-up period, RNFLT in the T sector at each time point differed significantly (F = 127.3, p < 0.001), with a significant reduction in RNFLT at 2 weeks and 1-month post-IOP control compared to that at 72 h post-IOP control (p < 0.01). No significant difference was observed at each time point within 3 months post-IOP control (p > 0.05) (Table 4).
Within the ODH group, RNFLT in the hemorrhages located in TS, TI, and T increased at 72-h post-IOP control; however, the differences were not statistically significant (p = 0.18, 0.14, and 0.06, respectively). No distinct differences were observed at 2 weeks, 1 month, 3 months, 6 months, and 12 months following IOP control (all p > 0.05). RNFLT data from other sectors (NS: 7 eyes; N: 4 eyes; NI: 6 eyes) were not statistically analyzed owing to the small sample sizes (Table 4). Figure 5 shows a representative case.
The MD at each time point post-IOP control in the ODH group was significantly lower than that in the control group (all p < 0.001); however, it increased gradually over time (F = 63.65, p < 0.001). The MD significantly increased at 1-month post-IOP control compared to 72 h (p < 0.001). Within the ODH group, significant differences were observed at all time points from 1 to 6 months post-IOP control (all p < 0.05), whereas no significant difference was observed from 6 to 12 months (p > 0.05). No significant changes in MD over time were observed in the control group (F = 2.33, p = 0.07) (Table 5).
Multiple linear regression analysis revealed a statistically significant association (F = 72.54, p < 0.001), with the duration of the highest IOP exhibiting a significant positive effect on ODH scope (β = 0.53, t = 9.56, p < 0.001); the longer the duration, the greater the scope. However, no significant correlation was observed between the highest IOP and ODH scope (β = 0.00, t = −0.35, p = 0.97).
Linear regression analysis revealed that the regression model was statistically significant (F = 79.23, p < 0.001) and that the ODH scope significantly impacted MD value (β = 1.97, T = −8.90, p < 0.001); the larger the scope, the more severe the damage.
ODH occurs in 57.5% of primary open-angle glaucoma (POAG) cases, 37.5% of normal-tension glaucoma (NTG) (14) cases, and approximately 5% of PACG cases, with lower rates in Asia (16). For POAG and NTG, ODH often indicates disease progression, with corresponding RNFLT thinning and atrophy after hemorrhage absorption. Disease progression can be effectively controlled by reducing IOP (12, 15). Lan et al. (16) reported that ODH in PACG was associated with the progression of optic atrophy and VF defects. However, investigations focusing on ODH in PACG, particularly in APAC, are limited.
This study revealed that ODH predominantly occurred as flame- and splinter-shaped hemorrhages during the first acute attack of APAC (84.38%), located in the superficial layer of the retina, primarily in the TI and TS sectors. The single-sector ODH occurrence rates were 21.88% each in the TI and TS sectors and 15.63% in the T sector, whereas the overall ODH occurrence rate was 50, 46.88, and 34.37% in the TI, TS, and T sectors, respectively. These findings align with the results of previous studies on POAG, ocular hypertension, and NTG disc hemorrhage (14, 17, 18). The mean ODH duration was 4.81 ± 3.25 weeks, similar to that reported by Lan et al. (16). These findings indicate that ODH in APAC attacks mostly occurs at the TI and TS sectors, possibly due to prolonged high IOP exerting pressure on the optic disc, lamina cribrosa (LC), and LC pores. This pressure eventually leads to microvascular ischemia, autoregulatory dysfunction, vascular dysregulation, and axoplasmic transport obstruction in the LC area. This can also influence axonal physiological function and nutrition, causing continuous LC interruption, LC pore deformation, and eventually capillarization through the ruptured area (11, 12). Alternatively, RNFLT in the TI and TS sectors of the optic disc may be thicker because of peak RNFLT distribution along the temporal vascular branches of the retina. Hence, under the same conditions (e.g., elevated IOP), a thicker RNFLT is more likely to be involved in ODH (17, 19).
The present study also found that four eyes (12.5%) were simultaneously accompanied by a flame-shaped hemorrhage along the blood vessels to the peripheral retina—namely, a decompression retinopathy. The highest IOP values recorded in these patients were 81, 78, 87, and 52 mmHg, with highest IOP durations of 10, 11, 8, and 6 days and hemorrhage durations of 12, 13, 10, and 4 weeks, respectively. These patients were otherwise healthy with no history of other diseases. Similar to our results, Nah et al. reported that decompression retinopathy occurred after the IOP decreased in patients experiencing APACG with acute high IOP (20). Moreover, Saricaoglu et al. showed that high preoperative IOP and IOP fluctuations were the main risk factors for decompression retinopathy (21). This phenomenon may be attributed to the sudden reduction in IOP, which decreases retinal arterial resistance, leading to increased flow and leakage through already fragile capillaries, and induces forward movement of the LC, leading to axonal transport blockage and central retinal vein compression, causing hemorrhagic retinopathy similar to central retinal vein occlusion.
The BCVA of all patients experiencing an APAC attack partially improved after IOP control. However, at the last follow-up, the BCVA in the ODH group remained significantly lower than that in the control group (p < 0.001), suggesting that visual function was affected despite IOP control following APAC attacks.
In the control group, no significant difference in RNFLT was observed (p > 0.05) during the follow-up period, indicating that there was no distinct change in RNFLT within 12 months under normal physiological conditions. In the ODH group, the RNFLT in each sector initially thickened predominantly in the early stage post-IOP control but gradually thinned at 2 weeks, eventually stabilizing at 12 months. Previous studies have demonstrated that RNFLT predominantly increases within 2 weeks after IOP control and significantly thins from 1 month to 6 months post-APAC attack compared to that in the contralateral eyes (22, 23). This increase in RNFLT may be due to acute, persistent high IOP mechanically compressing the optic nerve, LC area, and blood vessels, resulting in LC deformation, axoplasmic flow blockage, prolonged ischemia and hypoxia, retinal ganglion cell (RGC) apoptosis, and optic nerve edema (24). Alternatively, post-IOP control-induced ischemia–reperfusion injury may cause intracellular calcium overload and excess oxygen-free radical and inflammatory factor release, exacerbating RGC apoptosis and atrophy (25).
VF assessment revealed significant MD damage, reduced visual sensitivity, and diffuse VF defects within 72-h post-IOP control during APAC. IOP control significantly improved the MD at and VF defects (p < 0.05) at 1 month, and this significant improvement in the MD persisted at 6 months, consistent with the findings of a previous study (26). MD decreased over time and remained stable at 12 months. Compared to the MD in the control group, the MD was still significantly reduced at 12 months in the ODH group, suggesting that acute, persistent high IOP caused visual function damage, which was partly reversed after IOP control, consistent with previous findings (27). This may be attributed to the acute high IOP causing diffuse ischemia and hypoxia in the RNFL, tissue edema, and impaired visual function. After the IOP decreased to normal, the ischemia and hypoxia improved, and the abnormal substances released as a result of the ischemia and ischemia-repercussion injury gradually decreased. The condition naturally improved, RNFL edema subsided, and visual function partially recovered.
Studies have shown that APAC attacks, the duration of the highest IOP, and RNFLT loss are associated with VF damage (26). In the study by Li et al. (28), the IOP during the acute attack was 53.97 ± 13.27 mmHg, the time from symptom appearance to treatment was 6.49 ± 9.81 days, and the proportion of blindness caused by APAC was12.54% post-treatment. This suggests that a high IOP and prolonged TST may lead to increased optic nerve damage and subsequently contribute to a higher proportion of blindness (VA < 3/60) in APAC cases (28). The results of the present study showed that the duration of the highest IOP directly impacted ODH: the longer the duration of the highest IOP, the larger the scope of ODH. In addition, we found that the scope of ODH significantly impacted the MD value (β = 1.97, T = −8.90, p < 0.001), that is, the larger the scope of ODH, the more severe the VF damage. Therefore, early reduction of IOP, minimizing the duration of elevated IOP, and timely effective treatment of APAC are crucial for enhancing patients’ visual function and quality of life and reducing the proportion of glaucoma-induced blindness, particularly in the Asia-Pacific region.
Liu et al. (22) reported that IOP exceeding 40 mmHg could impact the optic nerve. In our study, the average IOP among patients was 64.28 ± 10.36 (range: 56–87) mmHg, which significantly surpassed the threshold value of optic nerve damage. This may explain why the highest IOP showed a relatively weak correlation with ODH and visual function. Additionally, we noted that VF defects mainly manifested as a decrease in diffuse visual acuity.
This study had some limitations. Specifically, the sample size was small, and the follow-up time was limited. Future studies should include large sample sizes and observe ODH characteristics during APAC attacks, changes in ODH and the optic nerve after IOP control, and factors associated with ODH over a long period of time.
This study revealed that ODH, presenting as flame and splinter shapes, mostly occurred in the TI, TS, and T sectors of the optic disc in patients experiencing their first APAC attack. The duration of high IOP was positively correlated with the ODH scope; the larger the ODH scope, the more severe the MD damage. After IOP control, the RNFLT became swollen and then thinner, stabilized 6 months later, and the MD was partly improved. Our findings suggest that timely and effective IOP management is essential for restoring visual function following APAC.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved the Ethics Committee of Shijiazhuang People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
HZ: Writing – original draft, Writing – review & editing. YL: Writing – review & editing, Writing – original draft. YT: Writing – review & editing. XY: Writing – review & editing. YG: Writing – review & editing. WL: Writing – review & editing. KS: Writing – review & editing. GT: Writing – review & editing. HG: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Hebei Province Key Research and Development Program Project (223777110D).
The authors thank Xuejing Zhang, PhD, of Shijiazhuang People’s Hospital and Dr. Mei Han of Beijing University of Chinese Medicine for their comments on the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
APAC, Acute primary angle closure; BCVA, Best-corrected visual acuity; IOP, Intraocular pressure; MD, Mean deviation; N, Nasal; NI, Nasal inferior; NS, Nasal superior; NTG, Normal-tension glaucoma; ODH, Optic disc hemorrhage; PACG, Primary angle closure glaucoma; POAG, Primary open-angle glaucoma; RNFLT, Retinal nerve fiber layer thickness; T, Temporal; TI, Temporal inferior; TS, Temporal superior; VA, Visual acuity; VF, Visual field.
1. Aung, T, Friedman, DS, Chew, PT, Ang, LP, Gazzard, G, Lai, YF, et al. Long-term outcomes in Asians after acute primary angle closure. Ophthalmology. (2004) 111:1464–9. doi: 10.1016/j.ophtha.2003.12.061
2. Zhang, H, Tang, G, and Liu, J. Effects of phacoemulsification combined with goniosynechialysis on primary angle-closure glaucoma. J Glaucoma. (2016) 25:e499–503. doi: 10.1097/IJG.0000000000000297
3. Foster, PJ, Buhrmann, R, Quigley, HA, and Johnson, GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. (2002) 86:238–42. doi: 10.1136/bjo.86.2.238
4. Lee, JW, Wong, BK, Yick, DW, Wong, IY, Yuen, CY, and Lai, JS. Primary acute angle closure: long-term clinical outcomes over a 10-year period in the Chinese population. Int Ophthalmol. (2013) 34:165–9. doi: 10.1007/s10792-013-9806-7
5. Muto, T, Nishimura, T, Sakamoto, M, Inomata, T, and Machida, S. Identification of eyes at risk of acute primary angle-closure in elderly Japanese patients. Clin Ophthalmol. (2019) 13:859–68. doi: 10.2147/OPTH.S190942
6. Zhu, X, Zeng, W, Wu, S, Chen, X, Zheng, T, and Ke, M. Measurement of retinal changes in primary acute angle closure glaucoma under different durations of symptoms. J Ophthalmol. (2019) 2019:5409837. doi: 10.1155/2019/5409837
7. Jin, SW, and Lee, SM. Comparison of longitudinal changes in c ircumpapillary retinal nerve fiber layer and ganglion cell complex thickness after acute primary angle closure: a 12-month prospective study. Jpn J Ophthalmol. (2018) 62:194–200. doi: 10.1007/s10384-017-0557-2
8. Jiang, R, Xu, L, Liu, X, Chen, JD, Jonas, JB, and Wang, YX. Optic nerve head changes after short-term intraocular pressure elevation in acute primary angle-closure suspects. Ophthalmology. (2015) 122:730–7. doi: 10.1016/j.ophtha.2014.11.008
9. Aung, T, Ang, LP, Chan, SP, and Chew, PT. Acute primary angle-closure: long-term intraocular pressure outcome in Asian eyes. Am J Ophthalmol. (2001) 131:7–12. doi: 10.1016/S0002-9394(00)00621-8
10. Kim, YK, Jeoung, JW, and Park, KH. Effect of focal lamina cribrosa defect on disc hemorrhage area in glaucoma. Invest Ophthalmol Vis Sci. (2016) 57:899–907. doi: 10.1167/iovs.15-18389
11. Lee, EJ, Han, JC, and Kee, C. A novel hypothesis for the pathogenesis of glaucomatous disc hemorrhage. Prog Retin Eye Res. (2017) 60:20–43. doi: 10.1016/j.preteyeres.2017.08.002
12. Kim, KE, and Park, KH. Optic disc hemorrhage in glaucoma: pathophysiology and prognostic significance. Curr Opin Ophthalmol. (2017) 28:105–12. doi: 10.1097/ICU.0000000000000345
13. Tan, AM, Loon, SC, and Chew, PT. Outcomes following acute primary angle closure in an Asian population. Clin Experiment Ophthalmol. (2009) 37:467–72. doi: 10.1111/j.1442-9071.2009.02060.x
14. Ozturker, ZK, Munro, K, and Gupta, N. Optic disc hemorrhages in glaucoma and common clinical features. Can J Ophthalmol. (2017) 52:583–91. doi: 10.1016/j.jcjo.2017.04.011
15. Uhler, TA, and Piltz-Seymour, J. Optic disc hemorrhages in glaucoma and ocular hypertension: implications and recommendations. Curr Opin Ophthalmol. (2008) 19:89–94. doi: 10.1097/ICU.0b013e3282f3e6bc
16. Lan, YW, Wang, IJ, Hsiao, YC, Sun, FJ, and Hsieh, JW. Characteristics of disc hemorrhage in primary angle-closure glaucoma. Ophthalmology. (2008) 115:1328–33. doi: 10.1016/j.ophtha.2007.10.041
17. Hsia, Y, Su, CC, Wang, TH, and Huang, JY. Clinical characteristics of glaucoma patients with disc hemorrhage in different locations. Graefes Arch Clin Exp Ophthalmol. (2019) 257:1955–62. doi: 10.1007/s00417-019-04379-y
18. Kim, YK, Park, KH, Yoo, BW, and Kim, HC. Topographic characteristics of optic disc hemorrhage in primary open-angle glaucoma. Invest Ophthalmol Vis Sci. (2014) 55:169–76. doi: 10.1167/iovs.13-13192
19. Hood, DC, Fortune, B, Arthur, SN, Xing, D, Salant, JA, Ritch, R, et al. Blood vessel contributions to retinal nerve fiber layer thickness profiles measured with optical coherence tomography. J Glaucoma. (2008) 17:519–28. doi: 10.1097/IJG.0b013e3181629a02
20. Nah, G, Aung, T, and Yip, CC. Ocular decompression retinopathy after resolution of acute primary angle closure glaucoma. Clin Experiment Ophthalmol. (2000) 28:319–20. doi: 10.1046/j.1442-9071.2000.00325.x
21. Saricaoglu, MS, Kalayci, D, Guven, D, Karakurt, A, and Hasiripi, H. Decompression retinopathy and possible risk factors. Acta Ophthalmol. (2009) 87:94–5. doi: 10.1111/j.1600-0420.2007.01083.x
22. Liu, X, Li, M, Zhong, Y, Xiao, H, Huang, J, and Mao, Z. The damage patterns of retinal nerve fiber layer in acute and chronic intraocular pressure elevation in primary angle closure glaucoma. Eye Sci. (2011) 26:154–60. doi: 10.3969/j.issn.1000-4432.2011.03.006
23. Tsai, JC, Lin, PW, Teng, MC, and Lai, C. Longitudinal changes in retinal nerve fiber layer thickness after acute primary angle closure measured with optical coherence tomography. Invest Ophthalmol Vis Sci. (2007) 48:1659–64. doi: 10.1167/iovs.06-0950
24. Akagi, T, Zangwill, LM, Saunders, LJ, Yarmohammadi, A, Manalastas, PI, Suh, MH, et al. Rates of local retinal nerve fiber layer thinning before and after disc hemorrhage in glaucoma. Ophthalmology. (2017) 124:1403–11. doi: 10.1016/j.ophtha.2017.03.059
25. Bringmann, A, Uckermann, O, Pannicke, T, Iandiev, I, Reichenbach, A, and Wiedemann, P. Neuronal versus glial cell swelling in the ischaemic retina. Acta Ophthalmol Scand. (2005) 83:528–38. doi: 10.1111/j.1600-0420.2005.00565.x
26. Bonomi, L, Marraffa, M, Marchini, G, and Canali, N. Peri metric defects after a single acute angle-closure glaucoma attack. Graefes Arch Clin Exp Ophthalmol. (1999) 237:908–14. doi: 10.1007/s004170050385
27. Sng, CC, See, JS, Ngo, CS, Singh, M, Chan, YH, Aquino, MC, et al. Changes in retinal nerve fibre layer, optic nerve head morphology, and visual field after acute primary angle closure. Eye (Lond). (2011) 25:619–25. doi: 10.1038/eye.2011.31
Keywords: acute primary angle closure, intraocular pressure, optic disc hemorrhage, retinal nerve fiber layer, visual field
Citation: Zhang H, Li Y, Tang Y, Yan X, Geng Y, Li W, Shi K, Tang G and Guo H (2024) Characteristics of optic disc hemorrhage and optic nerve changes following acute primary angle closure. Front. Neurol. 15:1333091. doi: 10.3389/fneur.2024.1333091
Received: 04 November 2023; Accepted: 29 April 2024;
Published: 24 May 2024.
Edited by:
Andrew White, The University of Sydney, AustraliaReviewed by:
Christian Mardin, University of Erlangen Nuremberg, GermanyCopyright © 2024 Zhang, Li, Tang, Yan, Geng, Li, Shi, Tang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangxian Tang, Z3h0eWt5eUAxMjYuY29t; Hongtao Guo, MTc2MDMxMTk2OTdAMTYzLmNvbQ==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.