
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol., 14 February 2024
Sec. Neuromuscular Disorders and Peripheral Neuropathies
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1332048
In recent years, artificial intelligence (AI) has undergone remarkable advancements, exerting a significant influence across a multitude of fields. One area that has particularly garnered attention and witnessed substantial progress is its integration into the realm of the nervous system. This article provides a comprehensive examination of AI’s applications within the peripheral nervous system, with a specific focus on AI-enhanced diagnostics for peripheral nervous system disorders, AI-driven pain management, advancements in neuroprosthetics, and the development of neural network models. By illuminating these facets, we unveil the burgeoning opportunities for revolutionary medical interventions and the enhancement of human capabilities, thus paving the way for a future in which AI becomes an integral component of our nervous system’s interface.
Artificial intelligence (AI) in the peripheral nervous system represents a synergy between computational technologies and the complexities of neural networks. It aims to decode the intricacies of neural circuitry and develop advanced therapies to treat neurological disorders and enhance human performance (1, 2). This article explores the integration of AI in the peripheral nervous system, focusing on applications and the potential implications for medical science and neuro-engineering. Therefore, fostering integration among these applications holds the potential to catalyze the emergence of novel ideas and technologies aimed at advancing the field of peripheral nervous system injury management.
Diagnosing PNS disorders is a complex and challenging task, requiring the integration of data from various sources and a comprehensive understanding of nerve function (3). AI, with its ability to process vast amounts of data and identify patterns that might be overlooked by human experts, offers tremendous potential to improve the accuracy and efficiency of PNS diagnostics (4–6). The PNS is composed of an intricate network of nerves that extend throughout the body. Disorders affecting the PNS can lead to a wide range of symptoms, including pain, weakness, numbness, and abnormal reflexes (7). Diagnosing these conditions involves a series of clinical assessments, electrophysiological tests, imaging studies, and sometimes invasive procedures such as nerve biopsies. However, the complex and often subtle nature of PNS disorders can make an accurate diagnosis a formidable challenge for healthcare professionals. Traditionally, PNS diagnostics have been heavily reliant on the expertise of neurologists and other specialists, which can lead to delays in diagnosis and potential misdiagnoses (8). Moreover, the interpretation of test results can be subjective and vary between different practitioners. A review indicated that in PNS-related cases, there was a delay in diagnosis in 82% of patients, largely due to misdiagnoses. This delay often resulted in nerve damage and disability, underscoring the critical nature of timely and accurate diagnosis in PNS disorders (9). In addition, the inherent variability and approximation in dermatome maps, which are used to diagnose PNS conditions, have been identified as factors contributing to delays in diagnosis and potential misdiagnoses. This variability can lead to confusion and inaccuracies in pinpointing neurological issues within the PNS (10). These limitations highlight the need for advanced technological solutions that can augment human expertise and improve the overall diagnostic process (Figure 1).
Figure 1. The integration of AI and precision medicine enhances individual healthcare by optimizing therapy planning and diagnostic methods.
AI’s impact on PNS Image Analysis has been significantly transformed the field of neurology. The analysis of images from techniques like magnetic resonance imaging (MRI), computed tomography (CT), and electromyography (EMG) is critical for diagnosing various PNS disorders, including nerve injuries, neuropathies, tumors, and entrapment syndromes (11–14). Carpal tunnel syndrome (CTS) is caused by compression of the median nerve as it passes through the carpal tunnel in the wrist. AI algorithms can analyze EMG data and aid in identifying CTS with high sensitivity and specificity. Park et al. (15) utilized a machine learning-based methodology to explore the potential of assessing the severity of CTS by considering individual, clinical, and sonographic characteristics. The performance of all machine learning models surpassed a 70% accuracy threshold, with the extreme gradient boosting (XGB) model demonstrating the most impressive results. Moreover, the adoption of a one-versus-rest classification strategy further enhanced accuracy in contrast to the traditional multiclass classification approach.
Traditional manual analysis of these images is labor-intensive, time-consuming, and susceptible to human error. AI, particularly deep learning models, has shown tremendous potential in overcoming these limitations and revolutionizing PNS image analysis (16, 17).
Matsuda et al. (18) developed a method using deep learning models for analyzing images of soma and axons. This method is promising in predicting chemotherapy-induced peripheral neuropathy and understanding the mechanisms of action of different drugs. In the study by Umansky et al. (19), deep learning models were employed as a part of their innovative approach to analyze gait in mice following sciatic nerve injuries. Specifically, they utilized the Visual Gait Lab (VGL) deep learning system for gait analysis. This deep learning approach was used alongside standard manual gait and sensory assays as well as semi-automated analysis methods.
One of the primary challenges in PNS image analysis is the accurate segmentation of neural structures from background tissues. AI-based algorithms can automatically segment and label neural elements in MRI, CT, single photon emission computed tomography (SPECT) and positron emission tomography (PET) scans, allowing for precise anatomical localization (20–22). By identifying nerves, nerve roots, and other structures of interest, AI algorithms streamline the analysis process and provide neurologists with more comprehensive and detailed information for accurate diagnosis (11, 21). Chen et al. (23) explores the automation of quantifying axonal loss in patients with peripheral neuropathies. They developed a deep learning-derived muscle fat fraction (FF) method using a 3D U-Net computational model to segment muscle MRI images for individual muscle FF quantification. This approach significantly improved the efficiency and reduced the labor intensity of manual segmentations. Importantly, the study demonstrated good accuracy and agreement with manual methods. The findings suggest that this automated method can be valuable in the early detection of axonal loss in peripheral neuropathies. A study by Yeh et al. (24) focuses on the real-time automated segmentation of the median nerve in dynamic ultrasonography using deep learning. They developed a lightweight instance segmentation model, SOLOv2-MN, tailored for real-time segmentation of the median nerve in dynamic ultrasonography. This model outperformed several state-of-the-art models in terms of inference speed, while maintaining comparable segmentation accuracy. The study indicates that this model can be potentially integrated into the clinical setting to assist in the real-time diagnosis and evaluation of carpal tunnel syndrome using dynamic ultrasonography (Figure 2).
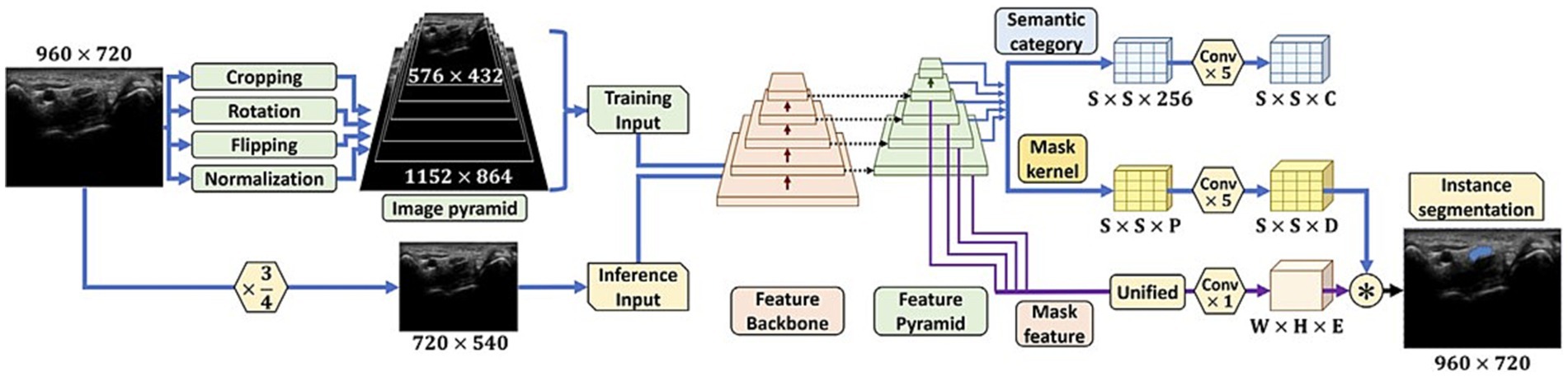
Figure 2. The initial steps for preparing images for analysis, and the layout of the SOLOv2-MN deep learning model. The figure initially shows how images are pre-processed, including normalization and augmentation techniques and provides visual representation of the SOLOv2-MN model’s architecture, critical for their method of automated median nerve segmentation in dynamic ultrasound imaging (24).
Other study explores the application of deep radiomics in diagnosing CTS by focusing on analyzing the deep radiomics features of median nerves using ultrasound images, which could potentially automate and improve the accuracy of CTS diagnosis (25).
AI models can be trained on large datasets of normal and abnormal images to learn subtle patterns and abnormalities in medical images that might go unnoticed by the human eye and assist in identifying and classifying PNS pathologies more accurately (21). This early detection facilitates timely interventions, potentially preventing further nerve damage and enhancing patient outcomes. Zhou et al. (26) present a deep learning framework called “Deep CTS” designed to address the challenges in segmenting the carpal tunnel region from MRI images, a critical aspect in the diagnosis and treatment of CTS. Deep CTS integrates a shape classifier with a simple convolutional neural network (Figure 3A) and a simplified U-Net for segmenting the carpal tunnel region (Figure 3B). This specialized structure is tailored specifically for the carpal tunnel, enabling efficient segmentation and improvement in the accuracy of intersection over union of results (Figure 3C). The article reports that this deep learning framework outperforms other segmentation networks for small objects. The model was trained with 333 images and tested with 82 images, achieving accuracy rate on reached 97.07% and a segmentation efficiency of 0.17 s. These results demonstrate the potential of Deep CTS for clinical application in accurately diagnosing CTS through MRI images. In addition, employing a cluster algorithm for the purpose of feature selection from datasets has yielded a notable degree of purity in the characterization of Guillain–Barré syndrome (GBS) through the application of AI. This outcome underscores a prospective pathway for the realization of computer-assisted GBS diagnosis (27). Furthermore, a study by Preston et al. (28) developed an AI algorithm using deep learning to classify peripheral neuropathy in diabetes and prediabetes. The algorithm achieved high precision, recall, and F1-score for both healthy participants and those with and without neuropathy. In the context of facial nerve paralysis, Song et al. (29) proposed a method for classifying facial nerve paralysis using a convolutional neural network (CNN) trained on clinical images, achieving 97.5% accuracy compared to neurologists’ assessments. While this study is specific to facial nerve paralysis, the approach might be adaptable to other peripheral nerve conditions. For epilepsy diagnosis, Krishnan et al. (30) evaluated DNNs using Gramian Angular Summation Field (GASF) images derived from EEG signals. A custom CNN showed high precision, recall, and F1-score in distinguishing between focal and normal GASF images, suggesting that DNNs could be a promising alternative for epilepsy detection, a condition often related to peripheral nervous system disorders. AI can fuse MRI and diffusion tensor imaging (DTI) data to map neural fiber trajectories and assess nerve integrity in cases of nerve injuries (31, 32). During certain procedures, such as nerve biopsies or nerve decompressions, real-time image analysis can be crucial for ensuring optimal surgical outcomes. DTI is based on the diffusion of water molecules in biological tissues. In peripheral nerves, the anisotropic diffusion of water can be quantified to assess nerve integrity. Key DTI metrics include fractional anisotropy (FA) and apparent diffusion coefficient (ADC), which provide information about nerve density, axonal diameter, and myelination. This technique is increasingly recognized as a valuable tool in peripheral imaging, particularly for assessing nerve abnormalities in conditions like diabetic peripheral neuropathy (DPN). DTI parameters, such FA and ADC, have been found effective in detecting nerve abnormalities in patients with type 2 diabetes and peripheral neuropathy. Decreased FA and increased ADC values are observed in the lumbosacral nerve roots of patients with DPN compared to healthy controls (33, 34). A study reported that acupoint injection of mecobalamin at Zusanli (ST36) could treat DPN and repair damaged nerves, as indicated by increased FA and decreased ADC in DTI (35). In addition, DTI provides reproducible measures of nerve microstructure and is used to assess the “health” of major nerves in the upper limb and its metrics vary with experimental conditions and the age of the subject (36). Furthermore, DTI has been used to evaluate the structure of lower limb muscles in chronic peripheral artery disease, which are not visible on traditional T1- and T2-weighted images (37).
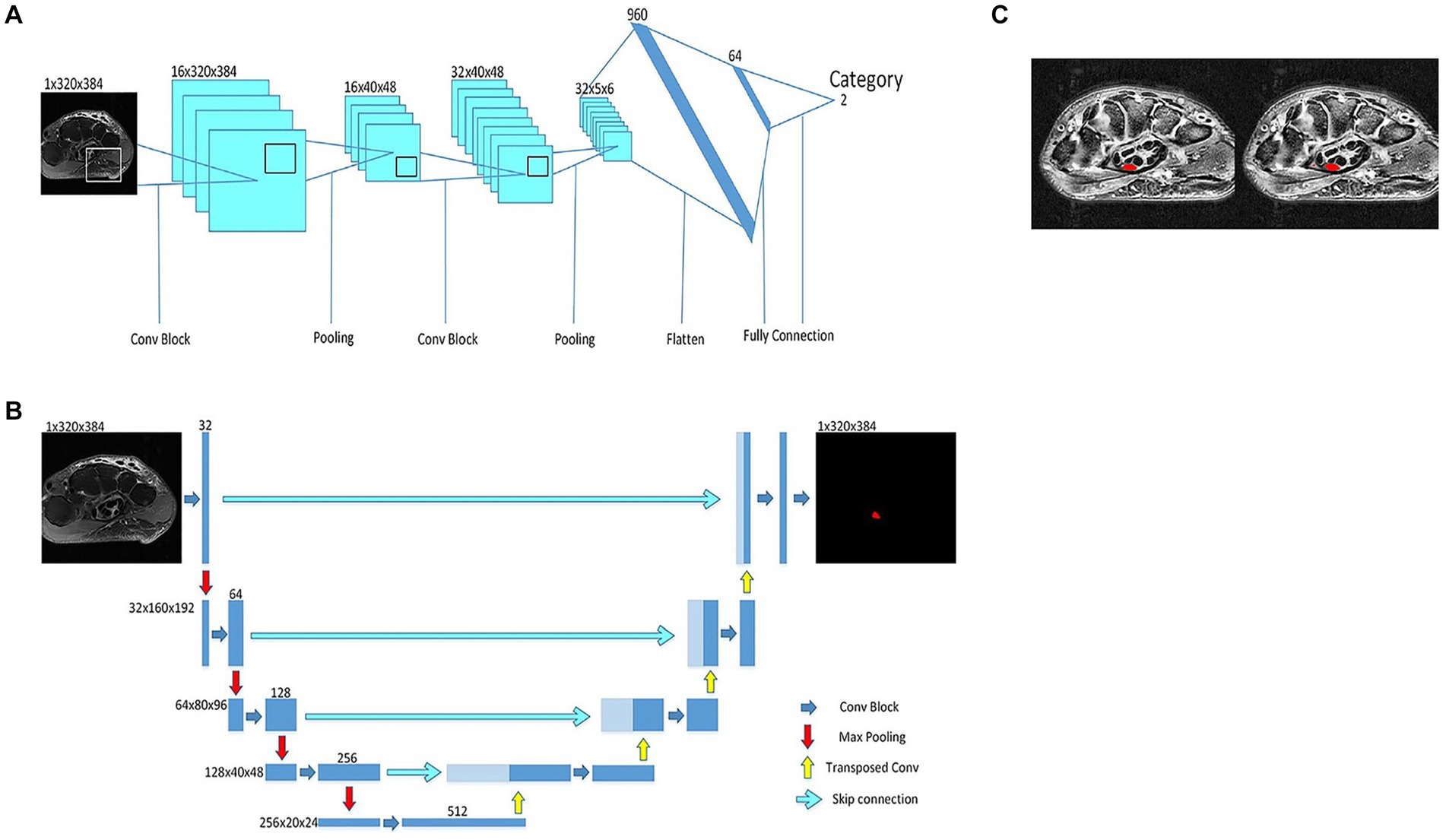
Figure 3. A deep neural network for MRI identification of carpal tunnel syndrome. (A) classification model to determine which MRI category. (B) U-Net for carpal tunnel area segmentation. (C) The result of the new proposed deep neural network, where the marker position (left) and predicted position (right) of a carpal tunnel.
Pain management is a critical aspect of healthcare, particularly when dealing with conditions involving the peripheral nerve system. Neuropathic pain, caused by nerve damage or dysfunction, is a prevalent condition that can significantly impact a patient’s quality of life (38). Over the years, medical advancements, including the use of AI, have revolutionized pain management (39).
AI algorithms have demonstrated remarkable capabilities in pain assessment and diagnosis. These algorithms can analyze vast amounts of patient data, including medical history, imaging results, and sensory feedback, to identify patterns indicative of neuropathic pain (40). Matsangidou et al. (39) conducted a comprehensive systematic review delving into the clinical applications of machine learning (ML) in the context of pain. Their investigation unveiled compelling outcomes, particularly in the domain of pain intensity classification. The efficacy of ML techniques in this regard was substantiated across various medical conditions, encompassing sickle cell disease, spinal cord injury, osteoarthritis, evoked heat pain, low back pain, and thoracic pain, among others. Remarkably, their analysis showcased the versatility of machine learning algorithms in accurately categorizing pain intensity, irrespective of its underlying nature (39, 41–45). With the ability to recognize subtle features that may be overlooked by human clinicians, AI can aid in accurate and timely diagnosis, reducing the risk of misdiagnosis and providing targeted treatment options (40). The study by Coombes et al. (46) presents an eHealth intervention using Personal Activity Intelligence (PAI) for people with DPN. The intervention aimed to enhance physical activity self-management and examine its impact on foot symptoms. It involved weekly sessions with exercise tasters, behavior change counseling, and PAI self-monitoring. The results indicated significant reductions in aching and burning pain in the feet, suggesting the feasibility and potential benefits of the PAI eHealth intervention for managing pain in DPN patients.
Furthermore, AI-powered diagnostic tools can incorporate machine learning techniques, constantly improving their accuracy and efficiency. These tools can assist clinicians in selecting the most appropriate treatment plans tailored to each patient’s specific condition, leading to personalized and more effective pain management strategies (47). Amaya-Rodriguez et al. (48), showed that the use of machine learning is specifically employed in the context of identifying and analyzing druggable sites within the TRPV1 channel. This involves computational approaches that aid in drug discovery and repositioning for pain management. Machine learning algorithms play a pivotal role in understanding the molecular structure of the TRPV1 channel and its interaction with various compounds, thereby informing the development of targeted therapies in pain management. Multiple studies embarked on the creation of innovative models for pain recognition through the utilization of machine learning techniques. And each of these studies achieved remarkable success in accurately detecting instances of pain, showcasing commendable levels of accuracy in their outcomes (49–51). In a separate investigation, a cutting-edge deep-learning model was harnessed to automate pain assessment by analyzing facial expressions, a particularly valuable application in critically ill patients with a high accuracy rate (52). Magoon and Suresh (53) discusses the use of AI in various aspects of perioperative medicine, with a particular focus on pain management. They explore how AI can contribute to objective analgo-scoring and enhance the effectiveness of regional anesthesia. They specifically highlight the role of AI in improving the precision of ultrasound-guided nerve blocks, a critical component in pain management. AI’s predictive capabilities have proved instrumental in determining a patient’s response to different pain management interventions. Fleck et al. (54) examines neurocognitive predictors of adherence to an online pain self-management program for individuals undergoing long-term opioid therapy. The key findings indicate that selective attention and response inhibition/speed are significant predictors of program engagement. Furthermore, the study employs a machine learning approach to enhance the accuracy of predictions. These findings underscore the importance of cognitive factors in the effective management of chronic pain and adherence to self-management programs.
By analyzing historical patient data and treatment outcomes, AI algorithms can predict which interventions are more likely to be effective for specific neuropathic pain conditions. This predictive approach can significantly reduce the trial-and-error process, where patients may undergo various treatments before finding one that works for them (55–57). Moreover, AI can help identify potential side effects or complications associated with certain pain management medications or procedures, enabling clinicians to make informed decisions and minimize risks. This not only enhances patient safety but also leads to more cost-effective healthcare (57–59).
AI plays a pivotal role in precision medicine, especially in the context of pain management. By analyzing genetic and molecular data, AI algorithms can identify specific biomarkers associated with certain neuropathic pain conditions. This information allows clinicians to predict a patient’s susceptibility to particular types of pain and helps design targeted therapies for optimal pain management outcomes (40, 60, 61). Zhao et al. (62) utilized AI in the form of the Weighted Gene Co-expression Network Analysis (WGCNA) algorithm. This method was applied to analyze RNA data, helping to identify modules and RNAs significantly associated with disease characterization in spinal cord injury (SCI) patients. The study also involved constructing co-expression networks and identifying pathways and biomarkers related to neuropathic pain post-SCI. Huang et al. (63) highlights the integration of advanced AI techniques in neuroimaging studies to understand and classify neuropathic pain conditions using specifically machine learning methods to analyze fMRI data of patients with herpes zoster (HZ) and postherpetic neuralgia (PHN). Their approach involved the use of support vector machine (SVM) algorithms for the classification of subjects based on brain activity patterns. The advent of AI in precision medicine opens up new possibilities for developing innovative treatments and breakthroughs in peripheral nerve system pain management. Pain management often involves non-pharmacological interventions to complement medical treatments. Virtual reality (VR) and distraction techniques have shown promise in reducing pain perception by diverting the patient’s attention away from the pain. Dy et al. (64) explored the usability and acceptability of VR for chronic pain management among a diverse group of patients in a safety-net healthcare setting. Using semi-structured interviews and observations of VR usage, the study found that most participants had no prior experience with VR for pain management but were interested in trying it. The results indicated that VR could be a usable and acceptable tool for chronic pain management, with many participants reporting positive experiences and expressing interest in future use. AI-driven VR experiences can be personalized based on a patient’s preferences and pain sensitivity levels (65, 66). Using AI to analyze patient feedback and physiological responses, VR systems can adapt in real-time to deliver content that provides the greatest distraction and pain relief. Additionally, AI can be used to monitor patient progress during VR therapy and adjust the treatment plan accordingly for optimal results (66–68) (Figure 4). Moreover, Suominen et al. (69) contributed to the advancement of pain assessment instruments. They proposed that pain-related textual notes could serve as fertile ground for the development of new pain assessment tools through the integration of human language technology, thereby promising a more refined and nuanced approach to pain evaluation.
Figure 4. Different methods that help the patient to manage the neuropathic pain using artificial algorithms and neural networks.
AI and neuro-prosthetics are rapidly converging fields that hold immense promise in revolutionizing healthcare and improving the quality of life for individuals with neurological impairments (70). Neuro-prosthetics aims to restore lost sensory or motor functions by creating interfaces between the nervous system and external devices (71). Within this realm, advancements in AI have been instrumental in enhancing the efficacy and usability of neuro-prosthetic devices. In particular, AI’s integration into neuro-prosthetics in the PNS has opened up new possibilities for patients with amputations, paralysis, or sensory deficits (70).
For individuals who have suffered injuries or disorders affecting the PNS, controlling and interacting with their limbs becomes challenging, leading to motor impairments or sensory loss (72, 73). Neuro-prosthetics that target the PNS aims to bridge this gap by using external devices to interface with nerves, muscles, or sensory receptors, facilitating communication between the nervous system and the outside world (71).
One of the primary challenges in developing effective neuro-prosthetic devices lies in deciphering the complex neural signals generated by the PNS and translating them into meaningful actions for external prostheses or feedback for sensory restoration (71). This is where AI-powered signal processing techniques come into play. Machine learning algorithms, such as deep learning and neural networks, can process large datasets of neural signals to extract patterns and identify the intended movements or sensations (74).
For instance, AI algorithms have been used to decode neural signals from residual limb muscles in amputees, enabling intuitive and natural control of prosthetic limbs (75). By analyzing muscle activation patterns, AI can predict the intended movement and execute the corresponding action in the prosthetic limb. The study by Li et al. (76) presented a novel approach for planning and controlling a prosthetic arm using muscle-synergy-based methods and neural-adaptive control. The research focused on upper limb prosthetics, aiming to improve the motor control of artificial limbs through a better understanding of muscle synergies. They developed a muscle synergy-based framework that combines intention decoding with motion control, using surface electromyography (sEMG) signals for extracting muscle synergies. Additionally, they implemented a neural network-based control system for the prosthetic arm, demonstrating its effectiveness in practical applications with both healthy participants and an upper limb amputee. This approach has shown significant advancements in dexterity, allowing users to perform complex tasks with more ease and accuracy (77).
Apart from motor control, AI has been pivotal in providing sensory feedback to users of neuro-prosthetic devices. Sensory feedback is crucial for users to perceive the environment, exert the right amount of force, and interact safely. Sensory restoration in neuro-prosthetics involves recording sensory information from the PNS, transmitting it to the brain, and integrating it with other sensory inputs, often in real-time (78, 79). Ghildiyal et al. (80) applied electromyography pattern recognition for controlling prosthetic limbs using various machine learning techniques. They developed a force-controlled prosthetic limb that improves the self-reliance, quality of life, and mental strength of amputees. The study employed machine learning regression approaches, such as support vector regressor (SVR), linear regression, and random forest models, to predict the force required to regulate the voltage for the servomotors in the prosthetic limb. The Random Forest model provided the most accurate prediction for controlling the voltage and, consequently, the limb’s movements. AI algorithms play a central role in interpreting and integrating sensory data. They can recognize and classify different sensory stimuli, such as pressure, temperature, and texture, and relay this information back to the user through the neuro-prosthetic device. This feedback loop facilitates better control and enhances the sense of embodiment, making neuro-prosthetic devices feel like a natural extension of the user’s body (81). Hasse et al. (82) demonstrated the potential of machine learning-based functional electrical stimulation (FES) to evoke complex arm movements, they used FES combined with machine learning to control complex movements in paralyzed upper limbs. In addition, they recorded arm kinematics and electromyographic (EMG) activity from a “trainer” monkey making a range of arm movements. This data was used to train an artificial neural network (ANN) to predict muscle activity patterns. These patterns were converted into stimulus pulses delivered to muscles in paralyzed monkeys.
AI’s adaptability and capacity to learn from user interactions have enabled neuroprosthetic devices to evolve and personalize their functionality (71, 83). Traditional prostheses were often rigid and required manual adjustments to fit individual user needs (84, 85). However, AI-driven neuro-prosthetics can continuously adapt their control mechanisms based on the user’s behavior and preferences. For instance, AI algorithms can learn from a user’s neural signals to optimize control strategies, making the neuro-prosthetic more efficient over time. This adaptability allows users to fine-tune their control, resulting in a more natural and seamless experience with the device (86). Due to the constraints in material selection, sensory synaptic devices can only perform relatively simple functions and lack diverse synaptic characteristics. Consequently, a higher level of device structures must be devised to compensate for this limitation (87). Chun et al. (81) introduced an innovative artificial neural tactile skin system which emulates the intricate human tactile recognition mechanism through the utilization of particle-based polymer composite sensors along with a signal conversion setup. These sensors exhibit discerning responsiveness to pressure and vibration, mirroring the behavior of both slow adaptive and fast adaptive mechanoreceptors found in human skin (Figure 5). This intricate functionality enables the generation of output signal patterns reminiscent of sensory neurons. Moreover, they developed an artificial finger endowed with the capability to acquire the proficiency of categorizing intricate and multifaceted textures. This is achieved by seamlessly integrating the sensor signals with a cutting-edge deep learning technique (81). Luu et al. (88) have introduced a groundbreaking neuro-prosthetic system that showcases a fundamental principle by harnessing the capabilities of AI to translate an amputee’s intended movements via a peripheral nerve interface. Their study reveals that the AI neural decoder, implemented through this nerve interface, exhibits distinct characteristics of robust control over prosthetics. Through this AI agent, individuals with limb amputations can seamlessly command prosthetic upper limbs using their mental intentions, as the AI system deciphers their genuine motor intents (Figure 6). Furthermore, the AI agent demonstrates its potential in enabling intricate hand gestures, effectively decoding multiple degrees of freedom (DOF) simultaneously (88, 89). This aspect becomes particularly significant when coupled with substantial training data, allowing the agent to fully exploit the capabilities of near-anatomic prostheses. This advancement paves the way for diverse hand movements, fulfilling the essential prerequisites for achieving natural hand control. The efficacy of AI agent is substantiated by the hand matching task, underscoring its ability to deliver real-time performance with remarkable accuracy. Notably, the agent attains an impressive prediction accuracy exceeding 99% across all gestures, accompanied by minimal latency of approximately 0.81 s (88). This low latency translates to a substantial information throughput rate of 365.4 beats per minute (bpm), further emphasizing the agent’s efficacy and potential impact.
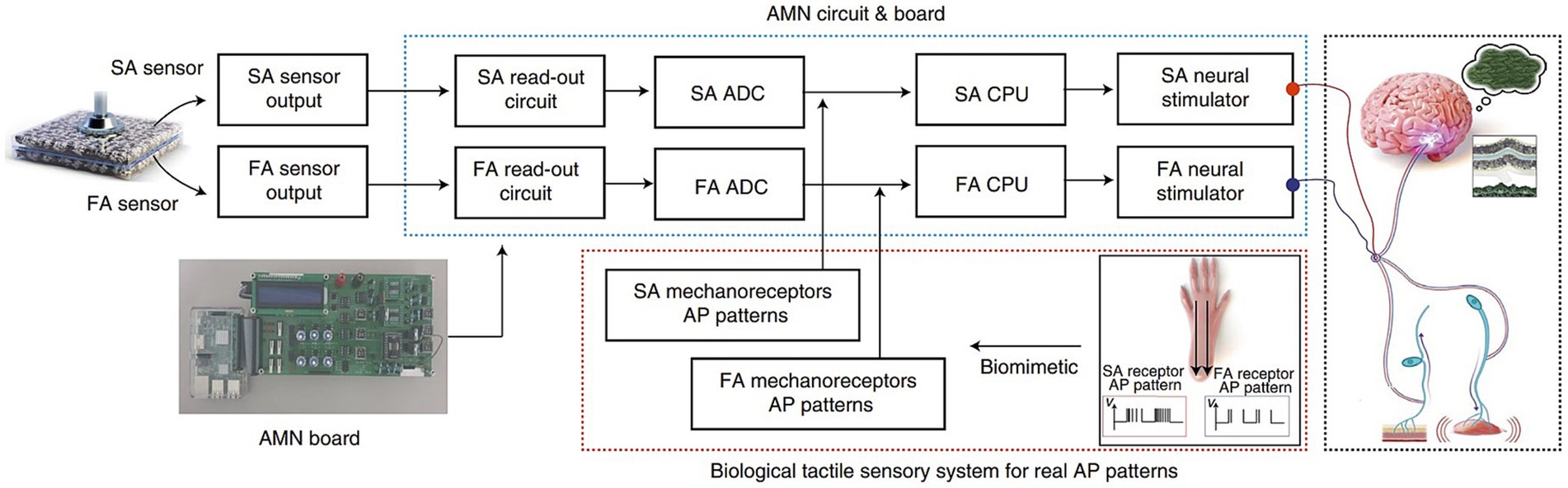
Figure 5. Illustrates the signal-processing procedure in an artificial tactile sensing system. It shows how signals from two types of sensors (SA and FA) are processed through an artificial mechanoreceptor neural board. This processing mimics the responses of real nerve cells to pressure and vibration stimuli, replicating the sensory functions of biological skin (81).
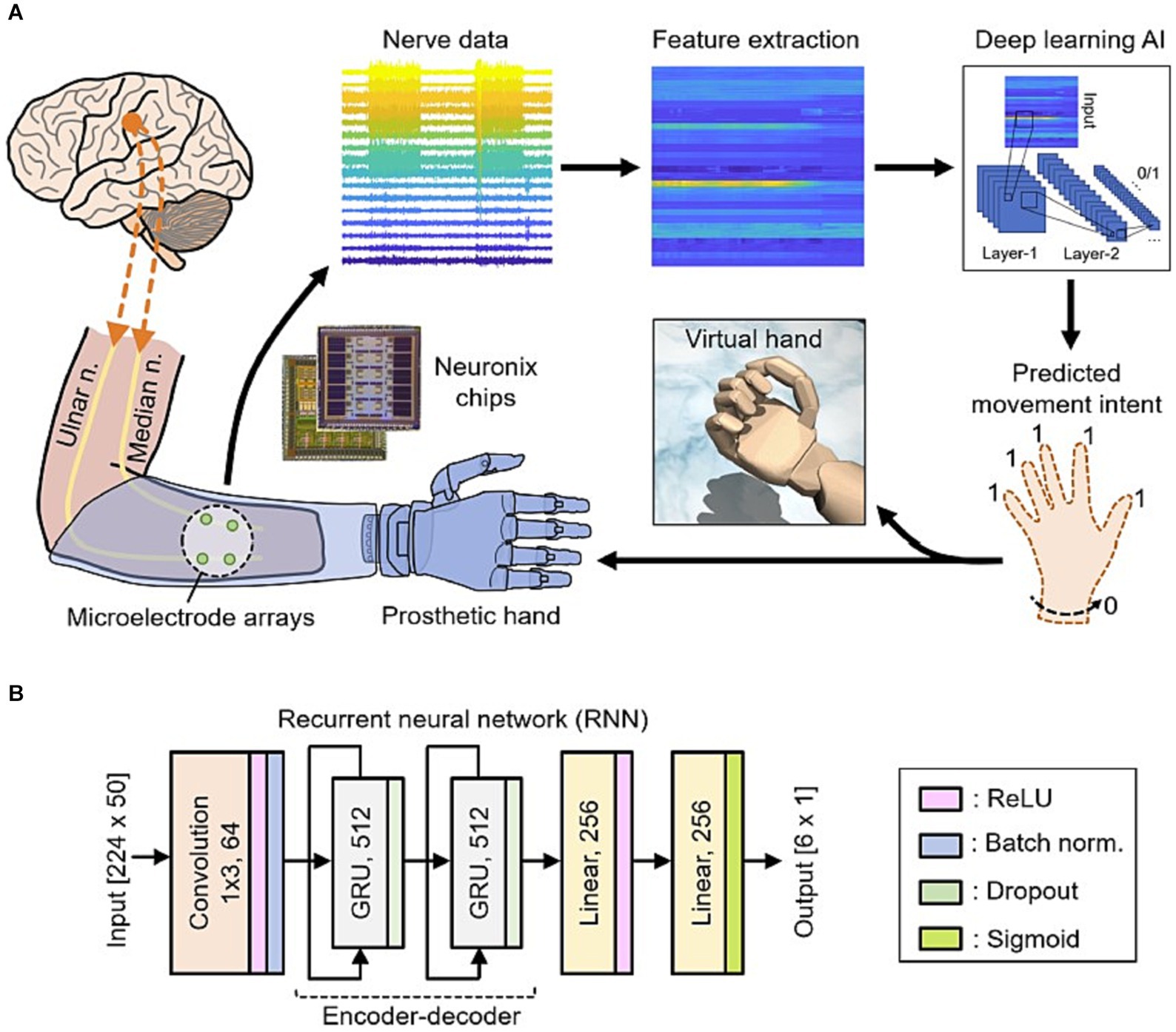
Figure 6. (A) Data from nerves in the individual’s amputated arm are collected using Neuronix neural interface chips. This is followed by the process of extracting key features. Subsequently, a deep learning AI utilizes these features to ascertain the individual’s intention to move multiple degrees of freedom at once. These predictions are then translated into real-time movements of either a virtual hand or a prosthetic hand. (B) The deep learning AI based on the recurrent neural network (RNN) architecture (88).
A pioneering study has conceptualized and demonstrated the inaugural utilization of graphdiyne-based artificial synapse components, boasting both parallel processing capabilities and adept information integration. This innovative approach involves the interconnection of the graphdiyne artificial synapse (GAS) with artificial motor neurons, resulting in the construction of synthetic efferent nerves, which, in turn, propel artificial muscles into motion (90). The GAS component exhibits the remarkable capacity to concurrently process multiple input sets, effectively amalgamating their respective outputs to exert precise control over the degree of artificial muscle flexion. This dynamic integration of parallel processing and output amalgamation holds significant promise for advanced control mechanisms (90, 91).
One significant challenge is the complexity of neural data interpretation. AI algorithms rely on vast datasets for training and validation, but neural signals can be highly variable and noisy (92). Developing AI models that accurately interpret and distinguish between different neural patterns is crucial for reliable diagnosis and treatment. Additionally, the adaptability of AI algorithms to individual variations in the PNS presents a formidable obstacle that demands sophisticated customization and optimization strategies. Data privacy and security also emerge as critical concerns (93). The transmission and storage of sensitive neural information for AI analysis raise ethical questions regarding patient consent, data ownership, and the potential for breaches (94, 95). Safeguarding patient privacy while harnessing the power of AI necessitates the establishment of robust security protocols and transparent regulatory frameworks. Furthermore, the successful integration of AI into the PNS requires interdisciplinary collaboration between medical professionals, neuroscientists, engineers, and computer scientists. Bridging the gap between these fields is essential to ensure seamless communication and effective translation of research findings into clinical applications (96). Transfer learning allows AI models trained on large datasets from one healthcare institution to be fine-tuned on smaller datasets from another institution, thus adapting the model to local patient demographics and variations in image acquisition protocols (39–41). Data augmentation techniques also help improve AI model performance by creating synthetic data variations, thereby increasing the diversity of training data and making the models more robust to unseen cases (42). The preeminence of research articles concentrated on the application of AI in the central nervous system within a prominent citation database highlights the pressing need for expanded research efforts dedicated to the peripheral nervous system. As for future directions, the potential of AI in the PNS is immense. Advances in neural interface technology and machine learning algorithms could lead to more precise and personalized treatments for neurological disorders. Enhanced real-time monitoring and adaptive interventions driven by AI could provide patients with proactive care and symptom management (97). AI algorithms can be deployed on the imaging systems used in the operating room to provide instant feedback to surgeons. By identifying critical structures and avoiding inadvertent damage (15, 98–100). Research efforts should also focus on the long-term effects of AI-PNS interactions. Safety, reliability, and the potential for neural plasticity need to be thoroughly investigated to understand the lasting impact of AI-based interventions on the nervous system (Table 1).
The integration of artificial intelligence (AI) into the peripheral nervous system (PNS) marks a remarkable advancement in the field of medical technology. The utilization of AI in the PNS holds immense promise for diagnosing, monitoring, and treating various neurological disorders, thereby enhancing the quality of life for countless individuals. Through the synergy of AI algorithms and neural interfaces, we are witnessing the development of innovative solutions that bridge the gap between the biological and technological realms. The incorporation of AI enables real-time data analysis, providing healthcare professionals with unprecedented insights into neural activities and patterns. This, in turn, leads to more accurate diagnoses and personalized treatment strategies tailored to each patient’s unique neural response. Moreover, the application of AI in the PNS holds the potential to restore lost sensory or motor functions, offering renewed independence to those with disabilities.
YQ: Data curation, Writing – original draft. AA: Data curation, Writing – original draft. YD: Writing – review & editing. JN: Writing – review & editing. SA: Conceptualization, Investigation, Supervision, Writing – review & editing. HL: Conceptualization, Investigation, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors would like to thank Alibaba group for their supports.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Guo, Y, Sun, L, Zhong, W, Zhang, N, Zhao, Z, and Tian, W. Artificial intelligence-assisted repair of peripheral nerve injury: a new research hotspot and associated challenges. Neural Regen Res. (2024) 19:663–70. doi: 10.4103/1673-5374.380909
2. Narcross, F. Artificial nervous systems-a new paradigm for artificial intelligence. Patterns. (2021) 2:100265. doi: 10.1016/j.patter.2021.100265
3. London, ZN. A structured approach to the diagnosis of peripheral nervous system disorders. Continuum. (2020) 26:1130–60. doi: 10.1212/CON.0000000000000922
4. Sahiner, B, Pezeshk, A, Hadjiiski, LM, Wang, X, Drukker, K, Cha, KH, et al. Deep learning in medical imaging and radiation therapy. Med Phys. (2019) 46:e1–e36. doi: 10.1002/mp.13264
5. Mussigmann, T, Bardel, B, and Lefaucheur, JP. Resting-state electroencephalography (EEG) biomarkers of chronic neuropathic pain. A systematic review. Neuroimage. (2022) 258:119351. doi: 10.1016/j.neuroimage.2022.119351
6. Ganapathy, K. 9—artificial intelligence in neurosciences—are we really there? In: AS Pillai and B Menon, editors. Augmenting neurological disorder prediction and rehabilitation using artificial intelligence : Academic Press (2022). 177–91. doi: 10.1016/B978-0-323-90037-9.00008-4
7. Lanigan, LG, Russell, DS, Woolard, KD, Pardo, ID, Godfrey, V, Jortner, BS, et al. Comparative pathology of the peripheral nervous system. Vet Pathol. (2021) 58:10–33. doi: 10.1177/0300985820959231
8. Broers, MC, Bunschoten, C, Drenthen, J, Beck, TAO, Brusse, E, Lingsma, HF, et al. Misdiagnosis and diagnostic pitfalls of chronic inflammatory demyelinating polyradiculoneuropathy. Eur J Neurol. (2021) 28:2065–73. doi: 10.1111/ene.14796
9. Ekladious, A, and Jiang, B. Motor neuron disease: to identify the mimics and chameleons at the early stage. Int J Biomed Res Pract. (2022) 2:2. doi: 10.33425/2769-6294.1017
10. Apok, V, Gurusinghe, NT, Mitchell, JD, and Emsley, HCA. Dermatomes and dogma. Pract Neurol. (2011) 11:100–5. doi: 10.1136/jnnp.2011.242222
11. Lui, YW, Chang, PD, Zaharchuk, G, Barboriak, DP, Flanders, AE, Wintermark, M, et al. Artificial intelligence in neuroradiology: current status and future directions. AJNR Am J Neuroradiol. (2020) 41:E52–e59. doi: 10.3174/ajnr.A6681
12. Kaka, H, Zhang, E, and Khan, N. Artificial intelligence and deep learning in neuroradiology: exploring the new frontier. Can Assoc Radiol J. (2021) 72:35–44. doi: 10.1177/0846537120954293
13. Li, S, Liu, Q, Zhou, H, Lu, H, and Wang, X. Subtyping of sarcomas based on pathway enrichment scores in bulk and single cell transcriptomes. J Transl Med. (2022) 20:48. doi: 10.1186/s12967-022-03248-3
14. Mun, SK, Wong, KH, Lo, SB, Li, Y, and Bayarsaikhan, S. Artificial intelligence for the future radiology diagnostic service. Front Mol Biosci. (2020) 7:614258. doi: 10.3389/fmolb.2020.614258
15. Park, D, Kim, BH, Lee, SE, Kim, DY, Kim, M, Kwon, HD, et al. Machine learning-based approach for disease severity classification of carpal tunnel syndrome. Sci Rep. (2021) 11:17464. doi: 10.1038/s41598-021-97043-7
16. Barragán-Montero, A, Javaid, U, Valdés, G, Nguyen, D, Desbordes, P, Macq, B, et al. Artificial intelligence and machine learning for medical imaging: a technology review. Phys Med. (2021) 83:242–56. doi: 10.1016/j.ejmp.2021.04.016
17. Martín Noguerol, T, Paulano-Godino, F, Martín-Valdivia, MT, Menias, CO, and Luna, A. Strengths, weaknesses, opportunities, and threats analysis of artificial intelligence and machine learning applications in radiology. J Am Coll Radiol. (2019) 16:1239–47. doi: 10.1016/j.jacr.2019.05.047
18. Matsuda, K, Han, X, Matsuda, N, Yamanaka, M, and Suzuki, I. Development of an in vitro assessment method for chemotherapy-induced peripheral neuropathy (CIPN) by integrating a microphysiological system (MPS) with morphological deep learning of soma and axonal images. Toxics. (2023) 11:848. doi: 10.3390/toxics11100848
19. Umansky, D, Hagen, KM, Chu, TH, Pathiyil, RK, Alzahrani, S, Ousman, SS, et al. Functional gait assessment using manual, semi-automated and deep learning approaches following standardized models of peripheral nerve injury in mice. Biomolecules. (2022) 12:1355. doi: 10.3390/biom12101355
20. Boyle, AJ, Gaudet, VC, Black, SE, Vasdev, N, Rosa-Neto, P, and Zukotynski, KA. Artificial intelligence for molecular neuroimaging. Ann Transl Med. (2021) 9:822. doi: 10.21037/atm-20-6220
21. Vinny, PW, Vishnu, VY, and Padma Srivastava, MV. Artificial intelligence shaping the future of neurology practice. Med J Armed Forces India. (2021) 77:276–82. doi: 10.1016/j.mjafi.2021.06.003
22. Dey, D, Lin, A, Han, D, and Slomka, PJ. Chapter 9—computed tomography and artificial intelligence In: SJ Al’Aref, G Singh, L Baskaran, and D Metaxas, editors. Machine learning in cardiovascular medicine : Academic Press (2021). 211–39. doi: 10.1016/B978-0-12-820273-9.00009-9
23. Chen, Y, Moiseev, D, Kong, WY, Bezanovski, A, and Li, J. Automation of quantifying axonal loss in patients with peripheral neuropathies through deep learning derived muscle fat fraction. J Magn Reson Imaging. (2021) 53:1539–49. doi: 10.1002/jmri.27508
24. Yeh, CL, Wu, CH, Hsiao, MY, and Kuo, PL. Real-time automated segmentation of median nerve in dynamic ultrasonography using deep learning. Ultrasound Med Biol. (2023) 49:1129–36. doi: 10.1016/j.ultrasmedbio.2022.12.014
25. Mohammadi, A, Torres-Cuenca, T, Mirza-Aghazadeh-Attari, M, Faeghi, F, Acharya, UR, and Abbasian Ardakani, A. Deep radiomics features of median nerves for automated diagnosis of carpal tunnel syndrome with ultrasound images: a multi-center study. J Ultrasound Med. (2023) 42:2257–68. doi: 10.1002/jum.16244
26. Zhou, H, Bai, Q, Hu, X, Alhaskawi, A, Dong, Y, Wang, Z, et al. Deep CTS: a deep neural network for identification MRI of carpal tunnel syndrome. J Digit Imaging. (2022) 35:1433–44. doi: 10.1007/s10278-022-00661-4
27. Shang, P, Zhu, M, Wang, Y, Zheng, X, Wu, X, Zhu, J, et al. Axonal variants of Guillain–Barré syndrome: an update. J Neurol. (2021) 268:2402–19. doi: 10.1007/s00415-020-09742-2
28. Preston, FG, Meng, Y, Burgess, J, Ferdousi, M, Azmi, S, Petropoulos, IN, et al. Artificial intelligence utilising corneal confocal microscopy for the diagnosis of peripheral neuropathy in diabetes mellitus and prediabetes. Diabetologia. (2022) 65:457–66. doi: 10.1007/s00125-021-05617-x
29. Song, A, Wu, Z, Ding, X, Hu, Q, and di, X. Neurologist standard classification of facial nerve paralysis with deep neural networks. Future Internet. (2018) 10:111. doi: 10.3390/fi10110111
30. Krishnan, Palani Thanaraj, Parvathavarthini, B., Tanik, U. John, Rajinikanth, V., Kadry, Seifedine, and Kamalanand, K. Implementation of deep neural networks to classify EEG signals using gramian angular summation field for epilepsy diagnosis. arXiv. Available at: https://doi.org/10.48550/arXiv.2003.04534. [Epub ahead of preprint]. (2020)
31. Wang, S, Cao, G, Wang, Y, Liao, S, Wang, Q, Shi, J, et al. Review and prospect: artificial intelligence in advanced medical imaging. Front Radiol. (2021) 1:781868. doi: 10.3389/fradi.2021.781868
32. Ranzenberger, LR, and Snyder, T. Diffusion tensor imaging In: StatPearls. Treasure Island, FL: StatPearls Publishing (2024)
33. Park, H-K, Oh, E, Joon Park, S, Jung Kim, H, and Byun, D. THU347 diffusion tensor imaging of the tibial nerve can detect peripheral neuropathy in type 2 diabetes. J Endocr Soc. (2023) 7:bvad114.780. doi: 10.1210/jendso/bvad114.780
34. Chen, H, Xu, Y, Wang, W, Deng, R, Li, Z, Xie, S, et al. Assessment of lumbosacral nerve roots in patients with type 2 diabetic peripheral neuropathy using diffusion tensor imaging. Brain Sci. (2023) 13:828. doi: 10.3390/brainsci13050828
35. Zhai, Y, Yu, W, and Shen, W. Diffusion tensor imaging evaluates effects of Acupoint injection at Zusanli (ST36) for type 2 diabetic peripheral neuropathy. Med Sci Monit. (2022) 28:e935979. doi: 10.12659/MSM.935979
36. Wade, RG, Lu, F, Poruslrani, Y, Karia, C, Feltbower, RG, Plein, S, et al. Meta-analysis of the normal diffusion tensor imaging values of the peripheral nerves in the upper limb. Sci Rep. (2023) 13:4852. doi: 10.1038/s41598-023-31307-2
37. Mazur, W, Urbańczyk-Zawadzka, M, Banyś, R, Obuchowicz, R, Trystuła, M, and Krzyżak, A. Diffusion tensor imaging as a tool to assess the structure of lower limb muscles invisible on T1- and T2-weighted images in the course of the chronic phase of peripheral artery disease. Postepy Kardiol Interwencyjnej. (2022) 18:446–9. doi: 10.5114/aic.2022.121343
38. Finnerup, NB, Kuner, R, and Jensen, TS. Neuropathic pain: from mechanisms to treatment. Physiol Rev. (2021) 101:259–301. doi: 10.1152/physrev.00045.2019
39. Matsangidou, M, Liampas, A, Pittara, M, Pattichi, CS, and Zis, P. Machine learning in pain medicine: an up-to-date systematic review. Pain Ther. (2021) 10:1067–84. doi: 10.1007/s40122-021-00324-2
40. Zhang, M, Zhu, L, Lin, SY, Herr, K, Chi, CL, Demir, I, et al. Using artificial intelligence to improve pain assessment and pain management: a scoping review. J Am Med Inform Assoc. (2023) 30:570–87. doi: 10.1093/jamia/ocac231
41. Yang, F, Banerjee, T, Narine, K, and Shah, N. Improving pain management in patients with sickle cell disease from physiological measures using machine learning techniques. Smart Health. (2018) 7-8:48–59. doi: 10.1016/j.smhl.2018.01.002
42. Levitt, J, Edhi, MM, Thorpe, RV, Leung, JW, Michishita, M, Koyama, S, et al. Pain phenotypes classified by machine learning using electroencephalography features. Neuroimage. (2020) 223:117256. doi: 10.1016/j.neuroimage.2020.117256
43. Kimura, A, Mitsukura, Y, Oya, A, Matsumoto, M, Nakamura, M, Kanaji, A, et al. Objective characterization of hip pain levels during walking by combining quantitative electroencephalography with machine learning. Sci Rep. (2021) 11:3192. doi: 10.1038/s41598-021-82696-1
44. Rahman, QA, Janmohamed, T, Pirbaglou, M, Clarke, H, Ritvo, P, Heffernan, JM, et al. Defining and predicting pain volatility in users of the manage my pain app: analysis using data mining and machine learning methods. J Med Internet Res. (2018) 20:e12001. doi: 10.2196/12001
45. Lötsch, J, Ultsch, A, Mayer, B, and Kringel, D. Artificial intelligence and machine learning in pain research: a data scientometric analysis. Pain Rep. (2022) 7:e1044. doi: 10.1097/PR9.0000000000001044
46. Coombes, BK, Bisset, L, Sierra-Silvestre, E, Ware, R, Coppieters, M, Coombes, J, et al. Personal activity intelligence eHealth intervention in people with diabetic peripheral neuropathy: a feasibility study. Aust J Gen Pract. (2023) 52:771–7. doi: 10.31128/AJGP-04-23-6797
47. Piette, JD, Newman, S, Krein, SL, Marinec, N, Chen, J, Williams, DA, et al. Artificial intelligence (AI) to improve chronic pain care: evidence of AI learning. Intell Based Med. (2022) 6:100064. doi: 10.1016/j.ibmed.2022.100064
48. Amaya-Rodriguez, CA, Carvajal, KDP, Bustos, D, Alegría-Arcos, M, and Castillo, K. A journey from molecule to physiology in the transient receptor potential vanilloid receptor type 1 (TRPV1) channel and in silico tools for drug discovery. Front. Pharmacol. (2024) 14:1251061. doi: 10.3389/fphar.2023.1251061
49. Kharghanian, R, Peiravi, A, and Moradi, F. Pain detection from facial images using unsupervised feature learning approach. Annu Int Conf IEEE Eng Med Biol Soc. (2016) 2016:419–22. doi: 10.1109/EMBC.2016.7590729
50. Hosseini, E, Fang, R, Zhang, R, Chuah, CN, Orooji, M, Rafatirad, S, et al. Convolution neural network for pain intensity assessment from facial expression. Annu Int Conf IEEE Eng Med Biol Soc. (2022) 2022:2697–702. doi: 10.1109/EMBC48229.2022.9871770
51. Dutta, P, and Nachamai, M. Facial pain expression recognition in real-time videos. J Healthc Eng. (2018) 2018:7961427. doi: 10.1155/2018/7961427
52. Wu, CL, Liu, SF, Yu, TL, Shih, SJ, Chang, CH, Yang Mao, SF, et al. Deep learning-based pain classifier based on the facial expression in critically ill patients. Front Med. (2022) 9:851690. doi: 10.3389/fmed.2022.851690
53. Magoon, R, and Suresh, V. A novel recognition of artificial intelligence in regional anaesthesia. Digit Med. (2023) 9:e00003. doi: 10.1097/DM-2023-00003
54. Fleck, DE, Wilson, M, Lewis, D, Welge, JA, Arya, G, Sathyan, A, et al. Neurocognitive predictors of adherence to an online pain self-management program adjunct to long-term opioid therapy. J Clin Exp Neuropsychol. (2023) 45:242–54. doi: 10.1080/13803395.2023.2221396
55. Allam, A, Feuerriegel, S, Rebhan, M, and Krauthammer, M. Analyzing patient trajectories with artificial intelligence. J Med Internet Res. (2021) 23:e29812. doi: 10.2196/29812
56. Feng, J, Phillips, RV, Malenica, I, Bishara, A, Hubbard, AE, Celi, LA, et al. Clinical artificial intelligence quality improvement: towards continual monitoring and updating of AI algorithms in healthcare. npj Digit Med. (2022) 5:66. doi: 10.1038/s41746-022-00611-y
57. Giordano, C, Brennan, M, Mohamed, B, Rashidi, P, Modave, F, and Tighe, P. Accessing artificial intelligence for clinical decision-making. Front Digit Health. (2021) 3:645232. doi: 10.3389/fdgth.2021.645232
58. Syrowatka, A, Song, W, Amato, MG, Foer, D, Edrees, H, Co, Z, et al. Key use cases for artificial intelligence to reduce the frequency of adverse drug events: a scoping review. Lancet Digit Health. (2022) 4:e137–48. doi: 10.1016/S2589-7500(21)00229-6
59. Bates, DW, Levine, D, Syrowatka, A, Kuznetsova, M, Craig, KJT, Rui, A, et al. The potential of artificial intelligence to improve patient safety: a scoping review. npj Digit Med. (2021) 4:54. doi: 10.1038/s41746-021-00423-6
60. Eldabe, S, Obara, I, Panwar, C, and Caraway, D. Biomarkers for chronic pain: significance and summary of recent advances. Pain Res Manag. (2022) 2022:1940906. doi: 10.1155/2022/1940906
61. Johnson, KB, Wei, WQ, Weeraratne, D, Frisse, ME, Misulis, K, Rhee, K, et al. Precision medicine, AI, and the future of personalized health care. Clin Transl Sci. (2021) 14:86–93. doi: 10.1111/cts.12884
62. Zhao, J, Yang, L, Huang, L, and Li, Z. Screening of disease-related biomarkers related to neuropathic pain (NP) after spinal cord injury (SCI). Hum Genomics. (2021) 15:5. doi: 10.1186/s40246-021-00303-w
63. Huang, J, Li, Y, Xie, H, Yang, S, Jiang, C, Sun, W, et al. Abnormal intrinsic brain activity and neuroimaging-based fMRI classification in patients with herpes zoster and postherpetic neuralgia. Front Neurol. (2020) 11:532110. doi: 10.3389/fneur.2020.532110
64. Dy, M, Olazo, K, Lyles, CR, Lisker, S, Weinberg, J, Lee, C, et al. Usability and acceptability of virtual reality for chronic pain management among diverse patients in a safety-net setting: a qualitative analysis. JAMIA Open. (2023) 6:ooad050. doi: 10.1093/jamiaopen/ooad050
65. Ridout, B, Kelson, J, Campbell, A, and Steinbeck, K. Effectiveness of virtual reality interventions for adolescent patients in hospital settings: systematic review. J Med Internet Res. (2021) 23:e24967. doi: 10.2196/24967
66. Huang, Q, Lin, J, Han, R, Peng, C, and Huang, A. Using virtual reality exposure therapy in pain management: a systematic review and meta-analysis of randomized controlled trials. Value Health. (2022) 25:288–301. doi: 10.1016/j.jval.2021.04.1285
67. Rejula, V, Anitha, J, Belfin, RV, and Peter, JD. Chronic pain treatment and digital health era-an opinion. Front Public Health. (2021) 9:779328. doi: 10.3389/fpubh.2021.779328
68. Thurnheer, SE, Gravestock, I, Pichierri, G, Steurer, J, and Burgstaller, JM. Benefits of mobile apps in pain management: systematic review. JMIR Mhealth Uhealth. (2018) 6:e11231. doi: 10.2196/11231
69. Suominen, H, Lundgrén-Laine, H, Salanterä, S, and Salakoski, T. Evaluating pain in intensive care. Stud Health Technol Inform. (2009) 146:192–6.
70. Lu, Q, Zhao, Y, Huang, L, An, J, Zheng, Y, and Yap, EH. Low-dimensional-materials-based flexible artificial synapse: materials, devices, and systems. Nanomaterials. (2023) 13:373. doi: 10.3390/nano13030373
71. Kansaku, K. Neuroprosthetics in systems neuroscience and medicine. Sci Rep. (2021) 11:5404. doi: 10.1038/s41598-021-85134-4
72. Irimia, A, and Van Horn, JD. Mapping the rest of the human connectome: atlasing the spinal cord and peripheral nervous system. Neuroimage. (2021) 225:117478. doi: 10.1016/j.neuroimage.2020.117478
73. Catala, M, and Kubis, N. Gross anatomy and development of the peripheral nervous system. Handb Clin Neurol. (2013) 115:29–41. doi: 10.1016/B978-0-444-52902-2.00003-5
74. Glaser, JI, Benjamin, AS, Farhoodi, R, and Kording, KP. The roles of supervised machine learning in systems neuroscience. Prog Neurobiol. (2019) 175:126–37. doi: 10.1016/j.pneurobio.2019.01.008
75. Román-Belmonte, JM, Corte-Rodríguez, H, and Rodríguez-Merchán, EC. Artificial intelligence in musculoskeletal conditions. Front Biosci. (2021) 26:1340–8. doi: 10.52586/5027
76. Li, G, Li, Z, Li, J, Liu, Y, and Qiao, H. Muscle-synergy-based planning and neural-adaptive control for a prosthetic arm. IEEE Trans Artif Intell. (2021) 2:424–36. doi: 10.1109/TAI.2021.3091038
77. Smita, N, and Rajesh Kumar, D. Application of artificial intelligence (AI) In: S Volkan, Ö Sinan, and B Pınar Boyraz, editors. Prosthetic and orthotic rehabilitation, in service robotics. Rijeka: IntechOpen (2020)
78. Mastinu, E, Engels, LF, Clemente, F, Dione, M, Sassu, P, Aszmann, O, et al. Neural feedback strategies to improve grasping coordination in neuromusculoskeletal prostheses. Sci Rep. (2020) 10:11793. doi: 10.1038/s41598-020-67985-5
79. Gupta, A, Vardalakis, N, and Wagner, FB. Neuroprosthetics: from sensorimotor to cognitive disorders. Commun Biol. (2023) 6:14. doi: 10.1038/s42003-022-04390-w
80. Ghildiyal, S, Mani, G, and Nersisson, R. Electromyography pattern-recognition based prosthetic limb control using various machine learning techniques. J Med Eng Technol. (2022) 46:370–7. doi: 10.1080/03091902.2022.2062064
81. Chun, S, Kim, JS, Yoo, Y, Choi, Y, Jung, SJ, Jang, D, et al. An artificial neural tactile sensing system. Nat Electron. (2021) 4:429–38. doi: 10.1038/s41928-021-00585-x
82. Hasse, BA, Sheets, DEG, Holly, NL, Gothard, KM, and Fuglevand, AJ. Restoration of complex movement in the paralyzed upper limb. J Neural Eng. (2022) 19:046002. doi: 10.1088/1741-2552/ac7ad7
83. Kabudi, T, Pappas, I, and Olsen, DH. AI-enabled adaptive learning systems: a systematic mapping of the literature. Comput Educ: Artif Intell. (2021) 2:100017. doi: 10.1016/j.caeai.2021.100017
84. Powell, SK, Cruz, RLJ, Ross, MT, and Woodruff, MA. Past, present, and future of soft-tissue prosthetics: advanced polymers and advanced manufacturing. Adv Mater. (2020) 32:e2001122. doi: 10.1002/adma.202001122
85. Turner, S, Belsi, A, and McGregor, AH. Issues faced by prosthetists and physiotherapists during lower-limb prosthetic rehabilitation: a thematic analysis. Front Rehabil Sci. (2022) 2:795021. doi: 10.3389/fresc.2021.795021
86. Fellous, JM, Sapiro, G, Rossi, A, Mayberg, H, and Ferrante, M. Explainable artificial intelligence for neuroscience: behavioral neurostimulation. Front Neurosci. (2019) 13:1346. doi: 10.3389/fnins.2019.01346
87. Zeng, M, He, Y, Zhang, C, and Wan, Q. Neuromorphic devices for bionic sensing and perception. Front Neurosci. (2021) 15:690950. doi: 10.3389/fnins.2021.690950
88. Luu, DK, Nguyen, AT, Jiang, M, Drealan, MW, Xu, J, Wu, T, et al. Artificial intelligence enables real-time and intuitive control of prostheses via nerve interface. IEEE Trans Biomed Eng. (2022) 69:3051–63. doi: 10.1109/TBME.2022.3160618
89. Nguyen, AT, Xu, J, Jiang, M, Luu, DK, Wu, T, Tam, WK, et al. A bioelectric neural interface towards intuitive prosthetic control for amputees. J Neural Eng. (2020) 17:066001. doi: 10.1088/1741-2552/abc3d3
90. Wei, H, Shi, R, Sun, L, Yu, H, Gong, J, Liu, C, et al. Mimicking efferent nerves using a graphdiyne-based artificial synapse with multiple ion diffusion dynamics. Nat Commun. (2021) 12:1068. doi: 10.1038/s41467-021-21319-9
91. Yuan, K, de la Asunción-Nadal, V, Li, Y, Jurado-Sánchez, B, and Escarpa, A. Graphdiyne micromotors in living biomedia. Chemistry. (2020) 26:8471–7. doi: 10.1002/chem.202001754
92. Stein, RB, Gossen, ER, and Jones, KE. Neuronal variability: noise or part of the signal? Nat Rev Neurosci. (2005) 6:389–97. doi: 10.1038/nrn1668
93. Raimundo, R, and Rosário, A. The impact of artificial intelligence on data system security: a literature review. Sensors. (2021) 21:7029. doi: 10.3390/s21217029
94. Chiruvella, V, and Guddati, AK. Ethical issues in patient data ownership. Interact J Med Res. (2021) 10:e22269. doi: 10.2196/22269
95. Redrup Hill, E, Mitchell, C, Brigden, T, and Hall, A. Ethical and legal considerations influencing human involvement in the implementation of artificial intelligence in a clinical pathway: a multi-stakeholder perspective. Front Digit Health. (2023) 5:1139210. doi: 10.3389/fdgth.2023.1139210
96. Bobak, CA, Svoboda, M, Giffin, KA, Wall, DP, and Moore, J. Raising the stakeholders: improving patient outcomes through interprofessional collaborations in AI for healthcare. Pac Symp Biocomput. (2021) 26:351–5.
97. Bajwa, J, Munir, U, Nori, A, and Williams, B. Artificial intelligence in healthcare: transforming the practice of medicine. Future Healthc J. (2021) 8:e188–94. doi: 10.7861/fhj.2021-0095
98. Panesar, SS, Kliot, M, Parrish, R, Fernandez-Miranda, J, Cagle, Y, and Britz, GW. Promises and perils of artificial intelligence in neurosurgery. Neurosurgery. (2020) 87:33–44. doi: 10.1093/neuros/nyz471
99. Buchlak, QD, Esmaili, N, Leveque, JC, Farrokhi, F, Bennett, C, Piccardi, M, et al. Machine learning applications to clinical decision support in neurosurgery: an artificial intelligence augmented systematic review. Neurosurg Rev. (2020) 43:1235–53. doi: 10.1007/s10143-019-01163-8
Keywords: artificial intelligence, peripheral nervous system, neuro-prosthetic, pain management, neural network
Citation: Qian Y, Alhaskawi A, Dong Y, Ni J, Abdalbary S and Lu H (2024) Transforming medicine: artificial intelligence integration in the peripheral nervous system. Front. Neurol. 15:1332048. doi: 10.3389/fneur.2024.1332048
Received: 02 November 2023; Accepted: 01 February 2024;
Published: 14 February 2024.
Edited by:
Jian-Quan Shi, Nanjing Medical University, ChinaReviewed by:
Michel Audette, Old Dominion University, United StatesCopyright © 2024 Qian, Alhaskawi, Dong, Ni, Abdalbary and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Lu, aHVpbHVAemp1LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.