
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 15 March 2024
Sec. Neuroepidemiology
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1330338
Background: Less research has linked the Systemic Immune Inflammatory Index (SII) with post-stroke depression (PSD). This study aims to look at any potential connections between SII and PSD.
Methods: The National Health and Nutrition Examination Survey (NHANES), conducted in a population that embodied complete SII and stroke data from 2005 to 2020, was used to perform the current cross-sectional survey. A fitted smoothed curve was used to depict the nonlinear link between SII and PSD, and multiple linear regression analysis demonstrated a positive correlation between SII and PSD.
Results: Multiple linear regression analysis showed that SII and PSD were markedly related [1.11(1.05, 1.17)]. Interaction tests showed that the association between SII and PSD was not statistically different between strata, and age, sex, BMI, income poverty ratio, education level, smoking status, diabetes mellitus, coronary heart disease, and heart failure did not have a significant effect on this positive association (p > 0.05 for interaction). In addition, a nonlinear association between SII and PSD was found using a two-stage linear regression model.
Conclusion: The results of our research support the existence of a significant positive correlation between SII levels and PSD. Further prospective trials are required to comprehend SII, which is for the PSD thoroughly.
Stroke is the second leading cause of death globally and the leading cause of disability due to its high morbidity and mortality (1, 2). It is widely known that a wide variety of functional impairments is prompted by stroke, such as cognitive impairment, motor dysfunction, and psychiatric disorders (3, 4).
Depression is the most common and severe neuropsychiatric complication after stroke (5), with a higher prevalence of post-stroke depression (PSD) of about 18–30%, and is considered an essential aspect affecting the recovery process of stroke patients (6). Patients with post-stroke depression are more prone to motor and cognitive dysfunction, thus decreasing the quality of life of patients (7) and hindering their reintegration into society and life (8, 9). Further research is still underway to explore the etiology, pathophysiologic mechanisms, risk factors, management, prevention, and treatment of depression after stroke (10, 11).
Depressive disorders are difficult to explain by a single etiology and mechanism; symptoms vary from person to person, and multiple etiologies and pathophysiologic mechanisms may be involved in different patients. Existing evidence supports that alterations in neurotransmitters, inflammatory responses, neuroendocrine activation, neuronal plasticity, and neurotrophic factors may play a role in identifying disease processes (12).
Immunity and inflammation are critical factors in stroke pathobiology, and inflammation is usually considered a tissue damage response. However, there is a growing view that inflammation affects the brain long after a stroke, especially in strokes caused by arterial occlusion or ischemic stroke (13, 14). Notably, a wealth of studies have shown that increased amounts of some markers in the blood system that respond to the level of inflammation, such as interferon-γ (IFN-γ), IL-2, IL-6, IL-8 and tumour necrosis factor (TNF-α), also play a role in the pathophysiological causes of depression (15). Therefore, several researchers have conducted experiments with animal subjects to verify whether inflammatory processes are involved in the generation of PSD disorders, and this has been supported by evidence (16–18).
In recent years, a new composite index, the Systemic Immune Inflammatory Index (SII), has been pointed out to be sensitive to the inflammatory response as a haematological marker of classical systemic inflammation. The concept of SII was first proposed by Hu et al. (19), who found that SII had a more sensitive predictive ability for hepatocellular carcinoma than one or two cell subsets. The numerator of the formula for SII is platelet count × neutrophil count, and the denominator is lymphocyte count. Inflammation is an essential component of many diseases. SII as an indicator of inflammation is a hot research topic today due to the fact that these cell counts can be obtained in the hospital with a simple lab test. Multiple investigations have additionally established the link between SII and other illnesses such as depression (20), hyperlipidemia (21), coronary heart disease (22), and stroke (23, 24).
A comprehensive review and meta-analysis showed that the SII levels of the groups with poor outcomes, such as mortality and moderate–severe stroke, were significantly greater than those of the groups with positive outcomes, such as survival and minor stroke (23). Yining Xiao and colleagues observed that an increased SII was linked to the severity of cerebral small vessel disease (CSVD) burden, suggesting that a heightened SII might contribute to cognitive impairment by exacerbating the burden of CSVD (25). Several researchers have explored the relationship between SII and mental illness, particularly depression (26, 27). And J. Wang and colleagues, at the core of their research, people with diabetes with depression gain significantly higher levels of SII than people with diabetes without depression. This finding suggests that elevated SII means that people with diabetes are at increased risk for depression. In a prospective stroke cohort study (28), elevated SII, platelet-to-lymphocyte (PLR), derived neutrophil-to-lymphocyte ratio (dNLR), and neutrophil-tolymphocyte (NLR) metrics were associated with the development of PSD and, in particular, were strongly associated with significantly elevated levels of SII on admission. Thus, SII may provide a new diagnostic strategy for early identification of post-stroke depression (29).
As we know, relatively few articles have been published to date exploring the relationship between SII and post-stroke depression. Only one prospective study supports the close correlation between them. However, the study population was relatively small, and further validation based on large samples is still needed in this direction. Therefore, to determine if the SII is related to the beginning, development, and prognosis of patients with PSD in adult US participants, we conducted a cross-sectional investigation with 33683 people. We hypothesized that a strong correlation exists between PSD and SII. SII holds promise as a predictor of involvement in the early identification of PSD, thus helping physicians to make an early diagnosis of PSD and develop rational interventions.
The National Health and Nutrition Examination Survey (NHANES) was sponsored by the Centers for Disease Control and Prevention (CDC) of the United States as a population-based, cross-sectional research study to evaluate the health and nutritional status of adults and children in the country (30). It is free of charge through the Centers for Disease Control and Prevention National Center for Health Statistics (NCHS)1 (31). In this study, data from eight two-year cycles of NHANES (2005–2020) were combined to obtain a total of 76,496 eligible individuals. Firstly, we needed to exclude missing values for exposure factors and outcome variables; in the first step, we screened out 14353 participants with missing SII values and 2131 participants with extreme SII values and excluded them; in the second step, we removed 24172 participants with missing depression scores, and finally, 2157 participants with missing data from the stroke questionnaire were eliminated, leaving 33683 participants in this study. Figure 1 illustrates the process of sample selection.
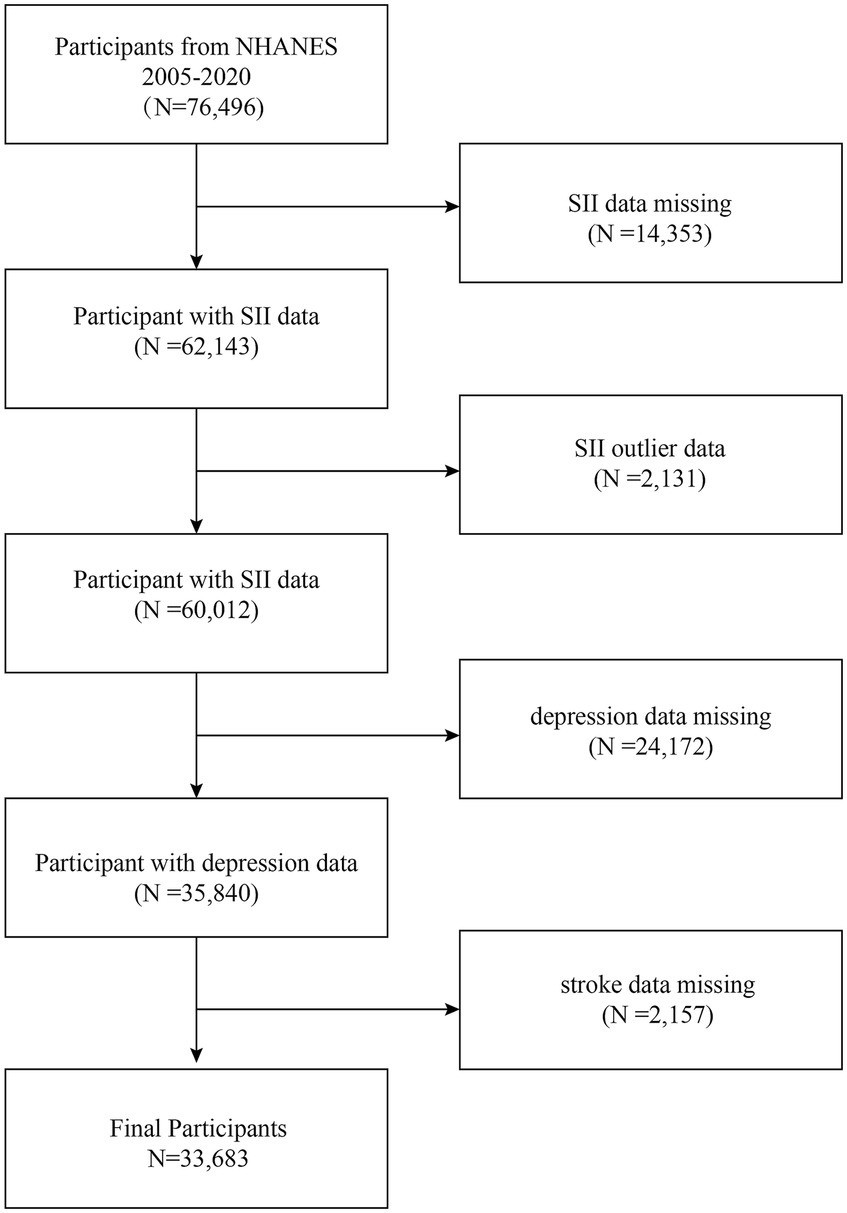
Figure 1. Flowchart of participant selection. NHANES, National Health and Nutrition Examination Survey.
With the help of the Patient Health Questionnaire (PHQ-9), a validated 9-entry patient health questionnaire, depressive symptoms were evaluated (32). It asked how often a person has experienced depressive symptoms in the past two weeks (33). Each item had four response options representing four different levels of response: “not at all,” “a few days,” “half a day,” and “almost every day.” These four responses were scored on a scale of 0 to 3 on a low to high scale, and the final tally of scores for the nine questions ranged from 0 to 27 combined. In this study, the validity and sensitivity of the PHQ-9 was 88%, with scores greater than or equal to 10 indicating a tendency toward depression (34).
The exposure variable in our study was SII. The NHANES data website provided some laboratory data on the values that should be used in the calculation of SII, which were obtained using the laboratory method of complete blood count, which used an automated haematology analyzer to assess the counts of lymphocytes, neutrophils, and platelets in the peripheral blood and is expressed as ×103 cells/μL (19, 35–37). SII was calculated by a simple multiply and divide operation, with the numerator of the formula being the platelet count × neutrophil count and the denominator being the lymphocyte count (38). Several covariates were included in the final analyses including age, sex (male or female), ethnicity (non-Hispanic white, black, Mexican American, other Hispanic, or other ethnicities), educational attainment (less than high school, high school or more than high school), income-to-poverty ratio (0 to 1.5, 1.5 to 3.5, >3.5), body mass index (<18.5, 18.5 to <25, 25 to <30, or ≥ 30 kg/m2) (20), current smoking (yes or no), alcohol use (yes or no), diabetes mellitus (yes or no), hypertension (yes or no), coronary heart disease (yes or no), congestive heart failure (yes or no), and low-density lipoprotein (LDL). LDL (continuous), HDL (continuous), total cholesterol (continuous), triglycerides (continuous), and uric acid (continuous) (21, 23, 29). These covariates might be confounders associated with SII and poststroke depression.
Statistical analyses for this study were performed using R Studio (version 4.2.2) and EmpowerStats (version 2.0). Means are expressed as standard deviation (SD) for continuous variables and as proportions for categorical variables; SII was categorized into quartiles according to their values, arranged from low to high as Q1, Q2, Q3, and Q4, representing different intervals, respectively. When categorized by SII quartiles (continuous variables), differences between subjects were analyzed using weighted t-tests or chi-square tests. We assessed the odds ratios (ORs) and 95% confidence intervals (CIs) for the association of SII with poststroke depression using multivariate logistic regression models. In the multivariate logistic regression analysis model, we constructed three models; model 1 was unadjusted for covariates, and model 2 was adjusted for age, sex, and race. Model 3 adjusted for age, sex, race, income-to-poverty ratio, education level, body mass index, coronary heart disease, congestive heart failure, and diabetes. We were adjusting variables performed simultaneous smoothed curve fitting. It was considered statistically significant when p < 0.05. A weighting strategy was used to reduce the high volatility of the data set.
For this study, a total of 33,683 eligible individuals with a mean age of (49.82 ± 17.74) years were enlisted, of which 50.98%, or nearly 2% more females than males, were female. The majority of participants were non-Hispanics, who made up the majority of the participant population. A mean SII concentration of 493.55 was measured. Additionally, 242 patients (0.72%) reported post-stroke depression.
Table 1 presents the quartiles of SII as a columnar stratification variable, while this table also includes all clinical characteristics associated with the subjects. Differences between SII quartiles were statistically significant (p < 0.05) for income poverty ratios, BMI, alcohol consumption, smoking, diabetes, coronary heart disease, and heart failure. Compared to those representing the lowest quartile of SII levels (Q1), those in the highest quartile (Q4) had income poverty ratios distributed mainly in the range of 1.5–3.5; more people in this study’s population responded to the survey on smoking status that they were still smoking now; and more people had diabetes, coronary heart disease, heart failure, and post-stroke depression. In contrast to higher levels of BMI, PHQ-9 scores, total cholesterol, LDL cholesterol, triglycerides, LSM, and SII, the levels of direct HDL cholesterol and uric acid were lower.
The issue of negligible effect values was resolved by implementing SII/100 to multiply the effect values by 100. The outcomes of the multivariate regression analysis between SII/100 and depressive disorders following a stroke are shown in Table 2. Model 1 (1.11 (1.05, 1.17)), Model 2 (1.11 (1.05, 1.17)), and Model 3 (1.09 (1.03, 1.16)) all exhibited significant relationships between the two. In model 2, sensitivity analyses were performed on SII quartiles, with or of 1.0, 1.13 (0.76, 1.69), 1.20 (0.81, 1.79), 2.06 (1.44, 2.95) for Q1, Q2, Q3 and Q4, respectively. Participants in quartile 4 had an increased risk of post-stroke depression by 106% (p < 0.0001) compared with quartile 1.
Further subgroup analyses showed that the relationship between SII and PSD was inconsistent, as Figure 2 shows. The results showed that SII was strongly linked with post-stroke depression (p < 0.05), with gender, education level, and diabetes mellitus as stratification factors for subgroup analysis. Age, gender, income poverty, education level, body mass index, smoking status, diabetes mellitus, coronary artery disease, and heart failure did not significantly affect this positive correlation, according to interaction tests, which revealed that the relationship between SII and post-stroke depression did not statistically differ between strata (p > 0.05 for interaction tests).
Following that, smoothed curve fitting was used to depict the nonlinear association between SII and PSD (Figure 3). Gender, race, age, income-poverty ratios, education level, diabetes, BMI, coronary artery disease, and heart failure were among the adjusted variables. The nonlinear relationship between SII and post-stroke depression was described by smooth curve fitting.
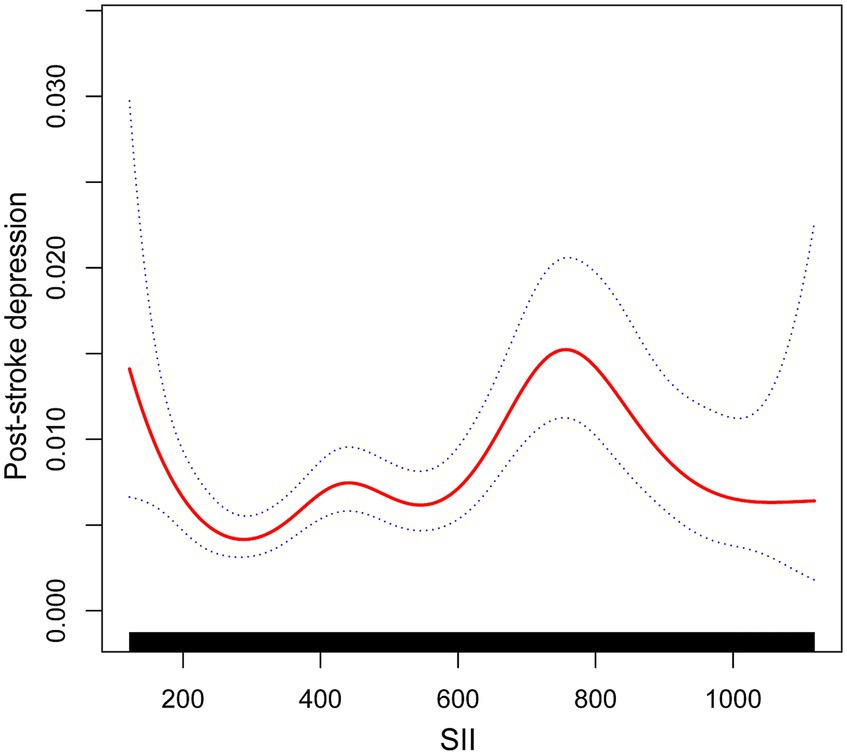
Figure 3. The association between SII and post-stroke depression. The solid red line represents the smooth curve fit between variables. Blue bands represent the 95% confidence interval from the fit.
In a representative study sample of U.S. adults, after cross-sectional analysis, we found that SII levels were positively associated with the development of post-stroke depression. In addition, the highest quartile of SII was associated with an increased incidence of PSD compared with the lowest quartile of SII. The results of subgroup analyses suggest that this association is similar across populations. The study also found a nonlinear relationship between SII and PSD, suggesting that high SII levels may be an independent risk factor for PSD.
As far as we know, this was the first time researchers have conducted an analysis of the connection between SII and post-stroke depression using the NHANES database. Prior to this, according to our findings, some studies have reported an association between SII and stroke prognosis, mood disorders such as depression, and poststroke cognitive impairment (29, 39, 40). A meta-analysis of the association between SII and clinical outcomes in stroke patients noted that in 19 retrospective studies that met the inclusion criteria, the levels of SII were much higher in the poor regression, death, and moderate-to-severe stroke groups than in the excellent regression, survival, and mild stroke groups, respectively (23). SII can be used to predict poor outcomes and high mortality after stroke. Similarly, a prospective cohort study showed that SII was strongly associated with poststroke severity, whereas platelet-albumin-bilirubin was not associated with stroke severity (41).
Many epidemiologic studies have demonstrated the involvement of inflammation in the development of depression. In a multicenter cross-sectional study involving 338 hospitalized TB patients, approximately half of the patients had anxiety and/or depression symptoms. Patients with these symptoms demonstrated a more robust inflammatory response and a worse cellular immune status than those without them (26). Similarly, in a study that included 2566 diabetic patients, 13.3% were diagnosed with depression, and high SII was recognized as an independent risk factor for depression in diabetes. This confirms our finding that high SII levels are associated with an increased risk of depression (28).
Previous research indicated that responses to inflammation in many central and peripheral systems may be biological factors contributing to PSD (42, 43). During the acute phase of ischemic stroke (minutes to hours), infiltration of inflammatory cells, including granulocytes (neutrophils), T cells, monocytes/macrophages, and other cells in the peripheral circulation, infiltrate the ischemic brain areas (44–46). Subsequently, damaged tissues rapidly release oxygen free radicals (ROS) and inflammatory mediators (including cytokines and chemokines). These substances induce the expression of adhesion molecules by endothelial cells and leukocytes in the brain, which prompts peripheral leukocytes to adhere to and traverse the endothelium, directly triggering necrosis and apoptosis in the ischemic region (47, 48). Infiltrating neutrophils release free radicals, cytokines (like IL-6), chemokines (like MCP-1), protein hydrolases, and immunoglobulins during the subacute phase (which lasts for hours to days). They also stimulate/activate matrix metalloproteinase 9 (mostly MMP-9) and protein hydrolases, which worsen the inflammatory response in the brain by causing more broad cellular activation and leukocyte infiltration. In addition, platelets also play an essential role in the inflammatory response after stroke, where platelets aggregate at the site of the lesion. Activated platelets tend to roll and adhere to endothelial cells in the intima of blood vessels from the flowing bloodstream, altering their functional properties and leading to increased neutrophil chemotaxis (49).
These markers of the inflammatory response affect neurotransmission, leading to a legacy of severe dysfunction in the brain, such as mood disorders (depression), cognitive impairment, etc. (50, 51). An out-of-control inflammatory response following stroke can produce damage to the blood–brain barrier, affecting its permeability and making it easy for leukocytes to penetrate it, which has been associated with causing a variety of adverse stroke prognoses in the brain, including pathologic cerebral oedema, hemorrhagic transformation, and higher mortality. A prospective stroke cohort study found that changes in cell counts in the circulating blood of patients with acute ischemic stroke could be represented by composite indices of the inflammatory response, such as SII, dNLR, PLR, and NLR, and found that the development of PSD was inextricably linked to elevated levels of these inflammatory composite indices, exceptionally high SII. Our study observed similar results, suggesting that high SII was positively associated with poststroke depression risk was positively associated (29).
Previous studies have confirmed that early elevation of inflammatory factors such as NLR and PLR measured in peripheral blood on admission to the hospital may provide physicians with some information to facilitate early diagnosis of PSD (52–54). Our study aligns with this perspective, and SII adds a platelet count to the NLR formula, which is more generalized than NLR and PLR.
Currently, clinical treatment of post-stroke depression (PSD) suggests a combination of psychotherapy, medication, and rehabilitation training to achieve optimal results. Although there are no precise research results to support the application of SII in PSD treatment, the correlation between the two suggests that detecting SII levels may guide immunotherapy, anti-inflammatory therapy, and antidepressant therapy for PSD and improve the clinical efficacy of PSD patients. Inflammation may be an essential disease-modifying factor contributing to susceptibility to depression, and controlling inflammation may be beneficial to treatment (55). Some researchers have suggested that long-term use of antidepressant medications may have a therapeutic effect by reducing the appearance of LPS-induced pathological behaviors that resemble depressive symptoms (manifested as reduced activity, such as reduced eating) (56). A meta-analysis of 36 randomized controlled trials (10,000 patients) (57) found antidepressant effects with monotherapy or in combination with anti-inflammatory drugs. Anti-inflammatory drugs such as cytokine inhibitors (58), statins (59), glucocorticoids (60), and nonsteroidal anti-inflammatory drugs (61) have been successfully studied with antidepressants (62). Therefore, antidepressants may also be beneficial in combination with anti-inflammatory medications.
In terms of clinical management of depressed patients after stroke, we can provide early detection and risk stratification of stroke patients based on SII levels and individualized management and treatment based on the degree of inflammatory response. SII levels are also monitored to assess the effectiveness of interventions. Regular monitoring of SII levels during long-term follow-up may also be helpful in early detection of potential recurrence or the emergence of new depressive symptoms, which can lead to timely adjustment of the treatment program. Discovering the correlation between SII and PSD is an essential clinical guide for screening, etiologic research, treatment, and prognostic assessment of PSD. And it provides a more refined and targeted approach to clinical management. Incorporating SII into the evaluation and treatment plan is expected to improve the clinical outcomes of patients. This is a direction well worth in-depth study.
Our study has several limitations; first, it was difficult for us to obtain a clear causal relationship between the exposure factors and the outcome variables due to the cross-sectional study design. Future studies still need more prospective studies to elucidate the causal relationship between SII and PSD. Second, there was no way to include all covariates for analysis because the amount of missing individual covariates was too large. Third, due to the limitations of the NHANES database, we were not able to have self-rating instruments and rater-administered tools such as the Beck Depression Inventory, the Self-Depression Scale (SDS), and the Hamilton Rating Scale for Depression (HRSD), which are more sophisticated depression assessment tools. Using only the PHQ-9 to assess the presence and severity of depression is somewhat different from the assessment of clinical depression. Forth, we should have included the population’s anti-inflammatory drug use history and need to know the use of antiplatelet, which may have affected our results. Despite these limitations, our survey has several strengths. First, the NHANES data, a national population-based sample obtained using standardized protocols. Second, the reliability and representativeness of our study are enhanced by the large sample size and appropriate correction for covariates, as well as subgroup analyses. Third, the potential value of measuring systemic inflammatory biomarkers in identifying individuals at risk for depression in the stroke population is emphasized and has the potential to provide new diagnostic and treatment options for depression.
This cross-sectional study based on the NHANES database demonstrated a significant positive association between SII levels and post-stroke depression. However, the findings do not yet conclusively indicate a causal association between SII and PSD, which should be confirmed in further prospective studies.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by National Center for Health Statistics Ethics Review Board. The patients/participants provided their written informed consent to participate in this study. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
MW: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Software. CP: Conceptualization, Methodology, Writing – original draft, Data curation, Formal analysis. TJ: Formal analysis, Writing – review & editing, Investigation, Methodology. QW: Supervision, Visualization, Writing – review & editing, Methodology, Software. DL: Supervision, Visualization, Writing – review & editing. ML: Conceptualization, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We thank the staff at the National Center for Health Statistics of the Centers for Disease Control for designing, collecting, and collating the NHANES data and creating the public database.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1330338/full#supplementary-material
1. Feigin, VL, Norrving, B, and Mensah, GA. Global Burden of Stroke. Circ Res. (2017) 120:439–48. doi: 10.1161/CIRCRESAHA.116.308413
2. Feigin, VL, Forouzanfar, MH, Krishnamurthi, R, Mensah, GA, Connor, M, Bennett, DA, et al. Global and regional burden of stroke during 1990-2010: findings from the global burden of disease study 2010. Lancet. (2014) 383:245–55. doi: 10.1016/S0140-6736(13)61953-4
3. Mendelson, SJ, and Prabhakaran, S. Diagnosis and Management of Transient Ischemic Attack and Acute Ischemic Stroke: a review. JAMA. (2021) 325:1088–98. doi: 10.1001/jama.2020.26867
4. Ball, EL, Sutherland, R, Squires, C, Mead, GE, Religa, D, Lundström, E, et al. Predicting post-stroke cognitive impairment using acute CT neuroimaging: a systematic review and meta-analysis. Int J Stroke. (2022) 17:618–27. doi: 10.1177/17474930211045836
5. Jørgensen, TSH, Wium-Andersen, IK, Wium-Andersen, MK, Jørgensen, MB, Prescott, E, Maartensson, S, et al. Incidence of depression after stroke, and associated risk factors and mortality outcomes, in a large cohort of Danish patients. JAMA Psychiatry. (2016) 73:1032–40. doi: 10.1001/jamapsychiatry.2016.1932
6. Gillen, R, Tennen, H, McKee, TE, Gernert-Dott, P, and Affleck, G. Depressive symptoms and history of depression predict rehabilitation efficiency in stroke patients. Arch Phys Med Rehabil. (2001) 82:1645–9. doi: 10.1053/apmr.2001.26249
7. Villa, RF, Ferrari, F, and Moretti, A. Post-stroke depression: mechanisms and pharmacological treatment. Pharmacol Ther. (2018) 184:131–44. doi: 10.1016/j.pharmthera.2017.11.005
8. Ayerbe, L, Ayis, S, Wolfe, CDA, and Rudd, AG. Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. Br J Psychiatry. (2013) 202:14–21. doi: 10.1192/bjp.bp.111.107664
9. Medeiros, GC, Roy, D, Kontos, N, and Beach, SR. Post-stroke depression: a 2020 updated review. Gen Hosp Psychiatry. (2020) 66:70–80. doi: 10.1016/j.genhosppsych.2020.06.011
10. Hackett, ML, and Pickles, K. Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke. (2014) 9:1017–25. doi: 10.1111/ijs.12357
11. Hackett, ML, and Anderson, CS. Predictors of depression after stroke: a systematic review of observational studies. Stroke. (2005) 36:2296–301. doi: 10.1161/01.STR.0000183622.75135.a4
12. Ferrari, F, and Villa, RF. The neurobiology of depression: an integrated overview from biological theories to clinical evidence. Mol Neurobiol. (2017) 54:4847–65. doi: 10.1007/s12035-016-0032-y
13. Moskowitz, MA, Lo, EH, and Iadecola, C. The science of stroke: mechanisms in search of treatments. Neuron. (2010) 67:181–98. doi: 10.1016/j.neuron.2010.07.002
14. Iadecola, C, and Anrather, J. The immunology of stroke: from mechanisms to translation. Nat Med. (2011) 17:796–808. doi: 10.1038/nm.2399
15. Dunn, AJ, Swiergiel, AH, and de Beaurepaire, R. Cytokines as mediators of depression: what can we learn from animal studies? Neurosci Biobehav Rev. (2005) 29:891–909. doi: 10.1016/j.neubiorev.2005.03.023
16. Gibney, SM, McGuinness, B, Prendergast, C, Harkin, A, and Connor, TJ. Poly I:C-induced activation of the immune response is accompanied by depression and anxiety-like behaviours, kynurenine pathway activation and reduced BDNF expression. Brain Behav Immun. (2013) 28:170–81. doi: 10.1016/j.bbi.2012.11.010
17. Kang, HJ, Bae, KY, Kim, SW, Kim, JT, Park, MS, Cho, KH, et al. Effects of interleukin-6, interleukin-18, and statin use, evaluated at acute stroke, on post-stroke depression during 1-year follow-up. Psychoneuroendocrinology. (2016) 72:156–60. doi: 10.1016/j.psyneuen.2016.07.001
18. Kim, JM, Kang, HJ, Kim, JW, Bae, KY, Kim, SW, Kim, JT, et al. Associations of tumor necrosis factor-α and interleukin-1β levels and polymorphisms with post-stroke depression. Am J Geriatr Psychiatry. (2017) 25:1300–8. doi: 10.1016/j.jagp.2017.07.012
19. Hu, B, Yang, XR, Xu, Y, Sun, YF, Sun, C, Guo, W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. (2014) 20:6212–22. doi: 10.1158/1078-0432.CCR-14-0442
20. Li, X, Huan, J, Lin, L, and Hu, Y. Association of systemic inflammatory biomarkers with depression risk: Results from National Health and nutrition examination survey 2005-2018 analyses. Front Psych. (2023) 14:1097196. doi: 10.3389/fpsyt.2023.1097196
21. Mahemuti, N, Jing, X, Zhang, N, Liu, C, Li, C, Cui, Z, et al. Association between systemic immunity-inflammation index and hyperlipidemia: a population-based study from the NHANES (2015-2020). Nutrients. (2023) 15:1177. doi: 10.3390/nu15051177
22. Ma, J, and Li, K. (2023). Systemic immune-inflammation index is associated with coronary heart disease: a cross-sectional study of NHANES 2009-2018. Front Cardiovasc Med. 10:1199433.
23. Huang, YW, Yin, XS, and Li, ZP. Association of the systemic immune-inflammation index (SII) and clinical outcomes in patients with stroke: a systematic review and meta-analysis. Front Immunol. (2022) 13:1090305. doi: 10.3389/fimmu.2022.1090305
24. Cheng, W, Bu, X, Xu, C, Wen, G, Kong, F, Pan, H, et al. Higher systemic immune-inflammation index and systemic inflammation response index levels are associated with stroke prevalence in the asthmatic population: a cross-sectional analysis of the NHANES 1999-2018. Front Immunol. (2023) 14:1191130. doi: 10.3389/fimmu.2023.1191130
25. Xiao, Y, Teng, Z, Xu, J, Qi, Q, Guan, T, Jiang, X, et al. Systemic immune-inflammation index is associated with cerebral small vessel disease burden and cognitive impairment. Neuropsychiatr Dis Treat. (2023) 19:403–13. doi: 10.2147/NDT.S401098
26. Liu, X, Bai, X, Ren, R, Tan, L, Zhang, Y, Lan, H, et al. Association between depression or anxiety symptoms and immune-inflammatory characteristics in in-patients with tuberculosis: a cross-sectional study. Front Psych. (2022) 13:985823. doi: 10.3389/fpsyt.2022.985823
27. Cui, S, Li, J, Liu, Y, Yao, G, Wu, Y, Liu, Z, et al. Correlation of systemic immune-inflammation index and moderate/major depression in patients with depressive disorders: a large sample cross-sectional study. Front Psych. (2023) 14:1159889. doi: 10.3389/fpsyt.2023.1159889
28. Wang, J, Zhou, D, Dai, Z, and Li, X. Association between systemic immune-inflammation index and diabetic depression. Clin Interv Aging. (2021) 16:97–105. doi: 10.2147/CIA.S285000
29. Hu, J, Wang, L, Fan, K, Ren, W, Wang, Q, Ruan, Y, et al. The association between systemic inflammatory markers and post-stroke depression: a prospective stroke cohort. Clin Interv Aging. (2021) 16:1231–9. doi: 10.2147/CIA.S314131
30. Xu, Q, Qian, X, Sun, F, Liu, H, Dou, Z, and Zhang, J. Independent and joint associations of dietary antioxidant intake with risk of post-stroke depression and all-cause mortality. J Affect Disord. (2023) 322:84–90. doi: 10.1016/j.jad.2022.11.013
31. Shi, Y, Wang, H, Zhu, Z, Ye, Q, Lin, F, and Cai, G. Association between exposure to phenols and parabens and cognitive function in older adults in the United States: a cross-sectional study. Sci Total Environ. (2023) 858:160129. doi: 10.1016/j.scitotenv.2022.160129
32. Dajpratham, P, Pukrittayakamee, P, Atsariyasing, W, Wannarit, K, Boonhong, J, and Pongpirul, K. The validity and reliability of the PHQ-9 in screening for post-stroke depression. BMC Psychiatry. (2020) 20:291. doi: 10.1186/s12888-020-02699-6
33. Kroenke, K, Spitzer, RL, and Williams, JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
34. Manea, L, Gilbody, S, and McMillan, D. Optimal cut-off score for diagnosing depression with the patient health questionnaire (PHQ-9): a meta-analysis. CMAJ. (2012) 184:E191–6. doi: 10.1503/cmaj.110829
35. Albany, C. Systemic immune-inflammation index in germ-cell tumours: search for a biological prognostic biomarker. Br J Cancer. (2018) 118:761–2. doi: 10.1038/bjc.2018.7
36. Tang, Y, Peng, B, Liu, J, Liu, Z, Xia, Y, and Geng, B. Systemic immune-inflammation index and bone mineral density in postmenopausal women: a cross-sectional study of the national health and nutrition examination survey (NHANES) 2007-2018. Front Immunol. (2022) 13:975400. doi: 10.3389/fimmu.2022.975400
37. Zhao, J, Dong, L, Hui, S, Lu, F, Xie, Y, Chang, Y, et al. Prognostic values of prothrombin time and inflammation-related parameter in acute ischemic stroke patients after intravenous thrombolysis with rt-PA. Clin Appl Thromb Hemost. (2023) 29:10760296231198042. doi: 10.1177/10760296231198042
38. Xie, R, Xiao, M, Li, L, Ma, N, Liu, M, Huang, X, et al. Association between SII and hepatic steatosis and liver fibrosis: a population-based study. Front Immunol. (2022) 13:925690. doi: 10.3389/fimmu.2022.925690
39. Bao, Y, Wang, L, Du, C, Ji, Y, Dai, Y, and Jiang, W. Association between systemic immune inflammation index and cognitive impairment after acute ischemic stroke. Brain Sci. (2023) 13:464. doi: 10.3390/brainsci13030464
40. Huang, L. Increased systemic immune-inflammation index predicts disease severity and functional outcome in acute ischemic stroke patients. Neurologist. (2023) 28:32–8. doi: 10.1097/NRL.0000000000000464
41. Hou, D, Wang, C, Luo, Y, Ye, X, Han, X, Feng, Y, et al. Systemic immune-inflammation index (SII) but not platelet-albumin-bilirubin (PALBI) grade is associated with severity of acute ischemic stroke (AIS). Int J Neurosci. (2021) 131:1203–8. doi: 10.1080/00207454.2020.1784166
42. Robinson, RG, and Jorge, RE. Post-stroke depression: a review. Am J Psychiatry. (2016) 173:221–31. doi: 10.1176/appi.ajp.2015.15030363
43. Das, J, and Rajanikant, GK. Post stroke depression: the sequelae of cerebral stroke. Neurosci Biobehav Rev. (2018) 90:104–14. doi: 10.1016/j.neubiorev.2018.04.005
44. Price, CJS, Menon, DK, Peters, AM, Ballinger, JR, Barber, RW, Balan, KK, et al. Cerebral neutrophil recruitment, histology, and outcome in acute ischemic stroke: an imaging-based study. Stroke. (2004) 35:1659–64. doi: 10.1161/01.STR.0000130592.71028.92
45. Buck, BH, Liebeskind, DS, Saver, JL, Bang, OY, Yun, SW, Starkman, S, et al. Early neutrophilia is associated with volume of ischemic tissue in acute stroke. Stroke. (2008) 39:355–60. doi: 10.1161/STROKEAHA.107.490128
46. Lindsberg, PJ, Carpén, O, Paetau, A, Karjalainen-Lindsberg, ML, and Kaste, M. Endothelial ICAM-1 expression associated with inflammatory cell response in human ischemic stroke. Circulation. (1996) 94:939–45. doi: 10.1161/01.CIR.94.5.939
47. Jin, R, Yang, G, and Li, G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. (2010) 87:779–89. doi: 10.1189/jlb.1109766
48. Darbousset, R, Thomas, GM, Mezouar, S, Frère, C, Bonier, R, Mackman, N, et al. Tissue factor-positive neutrophils bind to injured endothelial wall and initiate thrombus formation. Blood. (2012) 120:2133–43. doi: 10.1182/blood-2012-06-437772
49. Sreeramkumar, V, Adrover, JM, Ballesteros, I, Cuartero, MI, Rossaint, J, Bilbao, I, et al. Neutrophils scan for activated platelets to initiate inflammation. Science. (2014) 346:1234–8. doi: 10.1126/science.1256478
50. Ong, CWM, Pabisiak, PJ, Brilha, S, Singh, P, Roncaroli, F, Elkington, PT, et al. Complex regulation of neutrophil-derived MMP-9 secretion in central nervous system tuberculosis. J Neuroinflammation. (2017) 14:31. doi: 10.1186/s12974-017-0801-1
51. Amantea, D, Nappi, G, Bernardi, G, Bagetta, G, and Corasaniti, MT. Post-ischemic brain damage: pathophysiology and role of inflammatory mediators. FEBS J. (2009) 276:13–26. doi: 10.1111/j.1742-4658.2008.06766.x
52. Chen, H, Luan, X, Zhao, K, Qiu, H, Liu, Y, Tu, X, et al. The association between neutrophil-to-lymphocyte ratio and post-stroke depression. Clin Chim Acta. (2018) 486:298–302. doi: 10.1016/j.cca.2018.08.026
53. Huang, G, Chen, H, Wang, Q, Hong, X, Hu, P, Xiao, M, et al. High platelet-to-lymphocyte ratio are associated with post-stroke depression. J Affect Disord. (2019) 246:105–11. doi: 10.1016/j.jad.2018.12.012
54. Keaton, SA, Madaj, ZB, Heilman, P, Smart, L, Grit, J, Gibbons, R, et al. An inflammatory profile linked to increased suicide risk. J Affect Disord. (2019) 247:57–65. doi: 10.1016/j.jad.2018.12.100
55. Beurel, E, Toups, M, and Nemeroff, CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron. (2020) 107:234–56. doi: 10.1016/j.neuron.2020.06.002
56. Goral, VN, and Yuen, PK. Microfluidic platforms for hepatocyte cell culture: new technologies and applications. Ann Biomed Eng. (2012) 40:1244–54. doi: 10.1007/s10439-011-0453-8
57. Köhler-Forsberg, O, Lydholm, N, Hjorthøj, C, Nordentoft, M, Mors, O, and Benros, ME. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatr Scand. (2019) 139:404–19. doi: 10.1111/acps.13016
58. Tyring, S, Gottlieb, A, Papp, K, Gordon, K, Leonardi, C, Wang, A, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. (2006) 367:29–35. doi: 10.1016/S0140-6736(05)67763-X
59. Haghighi, M, Khodakarami, S, Jahangard, L, Ahmadpanah, M, Bajoghli, H, Holsboer-Trachsler, E, et al. In a randomized, double-blind clinical trial, adjuvant atorvastatin improved symptoms of depression and blood lipid values in patients suffering from severe major depressive disorder. J Psychiatr Res. (2014) 58:109–14. doi: 10.1016/j.jpsychires.2014.07.018
60. DeBattista, C, Posener, JA, Kalehzan, BM, and Schatzberg, AF. Acute antidepressant effects of intravenous hydrocortisone and CRH in depressed patients: a double-blind, placebo-controlled study. Am J Psychiatry. (2000) 157:1334–7. doi: 10.1176/appi.ajp.157.8.1334
61. Müller, N, Schwarz, MJ, Dehning, S, Douhe, A, Cerovecki, A, Goldstein-Müller, B, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. (2006) 11:680–4. doi: 10.1038/sj.mp.4001805
62. Raison, CL, Rutherford, RE, Woolwine, BJ, Shuo, C, Schettler, P, Drake, DF, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. (2013) 70:31–41. doi: 10.1001/2013.jamapsychiatry.4
Keywords: systemic immune-inflammation index (SII), NHANSE, emotional disorders, post-stroke depression (PSD), cross-sectional study
Citation: Wang M, Peng C, Jiang T, Wu Q, Li D and Lu M (2024) Association between systemic immune-inflammation index and post-stroke depression: a cross-sectional study of the national health and nutrition examination survey 2005–2020. Front. Neurol. 15:1330338. doi: 10.3389/fneur.2024.1330338
Received: 30 October 2023; Accepted: 15 February 2024;
Published: 15 March 2024.
Edited by:
Rizgar A. Mageed, Queen Mary University of London, United KingdomReviewed by:
Xiaobin Gu, First Affiliated Hospital of Zhengzhou University, ChinaCopyright © 2024 Wang, Peng, Jiang, Wu, Li and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Lu, bHVtaW5AaHVzdC5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.