
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 18 April 2024
Sec. Neurotrauma
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1327871
 Laura Uccella1*
Laura Uccella1* Cristiana Riboni1
Cristiana Riboni1 Francesco Polinelli1
Francesco Polinelli1 Carola Biondi1
Carola Biondi1 Graziano Uccheddu1
Graziano Uccheddu1 Roberta Petrino1
Roberta Petrino1 Pietro Majno-Hurst2
Pietro Majno-Hurst2Background and importance: Mild traumatic brain injury (mTBI) is a frequent presentation in Emergency Department (ED). There are standardised guidelines, the Canadian CT Head Rule (CCHR), for CT scan in mTBI that rule out patients on either anticoagulant or anti-platelet therapy. All patients with these therapies undergo a CT scan irrespectively of other consideration.
Objective: To determine whether standard guidelines could be applied to patients on anticoagulants or anti-platelet drugs.
Design, settings, and participants: 1,015 patients with mTBI and Glasgow Coma Score (GCS) of 15 were prospectively recruited, 509 either on anticoagulant or anti-platelet therapy and 506 on neither. All patients on neither therapy underwent CT scan following guidelines. All patients with mTBI on either therapy underwent CT scan irrespective of the guidelines.
Outcome measure and analysis: Primary endpoint was the incidence of post-traumatic intracranial bleeding in patients either on anticoagulants or anti-platelet drugs and in patients who were not on these therapies. Bayesian statistical analysis with calculation of Confidence Intervals (CI) was then performed.
Main results: Sixty scans were positive for bleeding: 59 patients fulfilled the criteria and 1 did not. Amongst patients with haemorrhage, 24 were on either therapy and only one did not meet the guidelines but in this patient the CT scan was performed before 2 h from the mTBI. Patients on either therapy did not have higher bleeding rates than patients on neither. There were higher bleeding rates in patients on anti-platelet therapy who met the guidelines vs. patients who did not. These rates overlapped with patients on neither therapy, meeting CCHR.
Conclusion: The CCHR might be used for mTBI patients on either therapy. Anticoagulants and anti-platelet drugs should not be considered a risk factor for patients with mTBI and a GCS of 15. Multicentric studies are needed to confirm this result.
Traumatic brain injury (TBI), defined as brain function impairment due to external forces (1, 2) resulting in loss of consciousness, amnesia or disorientation (3) is one of the commonest occurrences at the Emergency Department (ED) worldwide (4).
TBI is classified in severe (GCS ≤ 8), moderate (GCS from 9 to 13) and mild (GCS ≥ 14) (5).
Whilst there is evidence about the need of a head CT scan for patients with a moderate or severe TBI, there is still discussion on when a patient with mild traumatic brain injury (mTBI) should undergo CT. Several guidelines exist, the most important of which is the Canadian CT Head Rule (CCHR) (6, 7), to assist in deciding when further diagnostic investigation is required for mTBI (1). The CCHR for patients with mTBI is 99%–100% sensitive in detecting patients needing a neurosurgical intervention, but is lacking in specificity (39%–51%) (8). The incidence of intracranial bleeding in patients with GCS 15 who meet the criteria for the rule is about 5%–8% (9). The CCHR was derived excluding people on anticoagulant or anti-platelet medication assuming all those with any TBI symptoms (loss of consciousness, amnesia, etc.) would require CT imaging. There was no explicit comment on the need for CT imaging in the context of head injury without clear evidence of TBI in people with anticoagulant and anti-platelet medication. A recent systematic review could not identify robust empirical data to inform recommendations in this population (10, 11).
Historically anticoagulant and anti-platelet drugs have been considered a risk factor in traumatic brain injury. The American College of Emergency Physicians (2008) states that the management of patients on anticoagulants is unclear and gives no specific recommendations (8). The latest update of the NICE Head Injury Guidelines (11) recommend a head CT for any medium risk patient (GCS 15 within 2 h of injury with history of loss of consciousness or amnesia) taking any anticoagulant or anti-platelet regime – excluding aspirin monotherapy. Where no loss of consciousness or amnesia has occurred shared decision making rather than a mandatory CT brain scan is recommended. The consequence is that every patient on either anticoagulants or anti-platelet drugs with a mTBI undergoes a CT scan.
The number of patients on anticoagulant or anti-platelet therapy is increasing. This is mainly due to the increase in the ageing population. The incidence of hospital presentation for mild TBI will also be a significant issue in an ageing population.
The total anticoagulant prescription nearly doubled from 2014 to 2019 in the UK (15.0 million doses vs. 33.0 million doses) (8). Around 43 million adults in the US (19.0%) took aspirin at least three times per week for more than 3 months in 2010. This was an increase of 57% in aspirin use compared with 2005 (12).
The resulting increasing number of patients on these therapies presenting with mTBI makes it necessary for the clinician to weigh the risk of haemorrhage and the risk of irradiating the brain which can lead to radiation-related damage, as well as the costs of performing unnecessary examinations (13–15).
Intracranial bleeding represents the most feared complication in patients under antithrombotic agents, since it is associated with high morbidity and mortality. There is, however, limited evidence of the role of these drugs on mortality after mTBI (4, 16, 17). Literature suggests that anti-platelet and anticoagulant therapy increase the risk for intracranial haematoma and its progression after mTBI (16, 17), but these evidence is based on patients using vitamin K antagonists (VKAs) or anti-platelet drugs. In the last few years, direct oral anticoagulants (DOACs) diffusion has led to the knowledge that these drugs could be safer than VKAs also in the setting of mTBI (18).
The present study aims at determining whether there is a difference in intracerebral haemorrhage rates in patients with GCS 15 taking either antithrombotic or anticoagulant therapy in the mild TBI patients vs. those not on either therapy as an independent risk factor. If this is the case, CCHR could be applied to these patients, reducing the exposure to unneeded radiation. This will help define the management of mTBI in patients under anticoagulant or anti-platelet treatments.
This is a mono-centre prospective cohort observational study, involving patients’ charts data collection from adults presenting with mTBI (that is head trauma resulting in loss of consciousness, amnesia or disorientation) to the Emergency Department from the 29th April 2021 to the 31st June 2022.
The study received the approval of the local Ethics Committee and the participants signed an informed consent. The study was conducted in accordance with the declaration of Helsinki.
A sample size calculation was undertaken. Null hypothesis was that there are no differences in bleeding between patients with mild brain injury and GCS 15 on anticoagulant/anti-platelet therapy vs. patients with mild brain injury and GCS 15 not on anticoagulant/anti-platelet therapy. To test this null hypothesis, we had to assume a difference between patients who were and were not on either therapy. We assumed 5%, as this is the incidence of intracranial bleeding in patients neither on anticoagulants nor on anti-platelet drugs meeting CCHR (5). The sample size was calculated to have 80% power, 95% confidence level and 2% margin of error. This sample size was 457 subjects for each group. Therefore, we planned to recruit at least 914 patients.
We included all the adult patients presenting during the study period with mTBI and GCS 15, both on anticoagulants or anti-platelet drugs and neither meeting criteria for mild traumatic brain injury. Exclusion criteria were: medical cause of head trauma (e.g., syncope, epilepsy…), GCS 2 h after trauma of 14 or less, presence of seizures after injury, pregnancy, delayed presentation (>24 h), not having taken regular anti-platelet or anticoagulant therapy, absence of written consent.
The regularity of taking anticoagulant or anti-platelet therapy was ascertained by interviewing patients, family members and family physicians.
Patients not on therapy were included in the study only when meeting CCHR criteria for performing a head CT scan.
One month after the access to ED, enrolled patients received a follow-up phone call to find out their condition.
Primary endpoint was the incidence of intracranial bleeding in patients either on anticoagulants or anti-platelet drugs and in patients who were not on these therapies.
Secondary endpoints were:
• the need for intervention after post traumatic head bleeding in the two groups;
• the reliability of CT Head Rule in patients on either anticoagulants or anti-platelet drugs;
• to assess whether anticoagulants and/or anti-platelet drugs are a risk factor for patients presenting with mTBI and GCS of 15; and
• to compare mortality and morbidity after mTBI in the two groups with a follow-up period of 1 month.
Two months were selected randomly for data validation. An independent research collaborator was identified to determine the number of patients who should be included in the study within the data collection period and check if any were missed or added unrightfully. This collaborator was not involved in the initial data collection. No differences were detected.
Statistical analysis was performed using the open source packages “Pandas,” “NumPy,” “SciPy,” “Seaborn,” and “PyMC” for Mac Os X versions 1.4.1, 1.21.2, 1.7.3, 0.11.2, and 3.11.14, respectively. Statistical significance was considered achieved based on highly credible intervals of parameter estimates and p < 0.05. Confidence intervals (CI) were calculated at 95%.
Since we needed to compare proportions of haemorrhages in different sub-populations, we performed both a two-tail t-test computation based on classic proportion comparison using Fischer exact test, and a Bayesian estimate of the parameter distribution of a Bernoulli stochastic variable to model bleeding occurrences using a non-informative uniform prior distribution over the interval 0–1. The estimate was obtained by using the Metropolis-Hastings algorithm in a Markov-chain Monte Carlo (MCMC) model, with a burn-in of 5,000 iterations and runs lasting 40,000 iterations. Traces were inspected to verify convergence diagnostics (Geweke plots and Raftery-Lewis analysis). The posterior parameter distribution was then plotted in order to have a graphical overview and confidence intervals were estimated. Furthermore, by sampling the posterior distributions, we were able to estimate both probability that the parameters describing one population would be different from each other as well as the estimate confidence intervals for Relative Risk (19).
Between April 2021 and June 2022, 1,015 patients were enrolled, 509 on either anticoagulants or anti-platelet drugs and 506 on neither.
Personal data, causes of injury, met criteria for CCHR, presence or absence of haemorrhage at CT, need for surgical intervention, reason for anticoagulant/anti-platelet therapy, are summarised in Tables 1, 2.
INR values of 37 out of 52 patients anticoagulated with VKA were recorded. The mean value was 1.78 (range 1.2–6.6), 3 patients had a subtherapeutic value (≤1.5), 18 patients a therapeutic value (1.5 < INR < 2.5) and 16 patients an overtherapeutic value (≥2.5).
Of the 1,015 CT scans performed 60 resulted positive for haemorrhage (5.9%).
We considered a CT scan positive when there was any trace of blood, even the smallest (even one single petechia). Of these positive patients, 24 were patients on either anticoagulants or anti-platelets and 36 on neither. Amongst the 60 patients who resulted positive for haemorrhage at CT scan, only one seemed not to meet the criteria for the CCHR. This was a 74 year old patient on aspirin (in primary prevention) who accidentally fell from her height and hit the back of the head. She arrived at the hospital by ambulance 30 min after the accident and GCS assessment was performed at the arrival (GCS 15). CT scan was performed at 50 min from the fall and was positive for subdural haematoma. Thirty minutes after she became confused and did not recognise her son (GCS 12 E3 V3 M6). She underwent neurosurgical intervention as a consequence of the positive CT scan for subdural haematoma.
The remaining 59 positive patients met CCHR (Table 3) criteria for head CT, had minimal bleeding, remained stable at the next CT check-up and did not require surgery. Anti-platelet and anticoagulant therapy was discontinued (with the exception of one patient on warfarin therapy for a mechanical mitral valve, who was anticoagulated with unfractionated heparin and closely monitored, with no progression of minimal subarachnoid haemorrhage detected on CT).
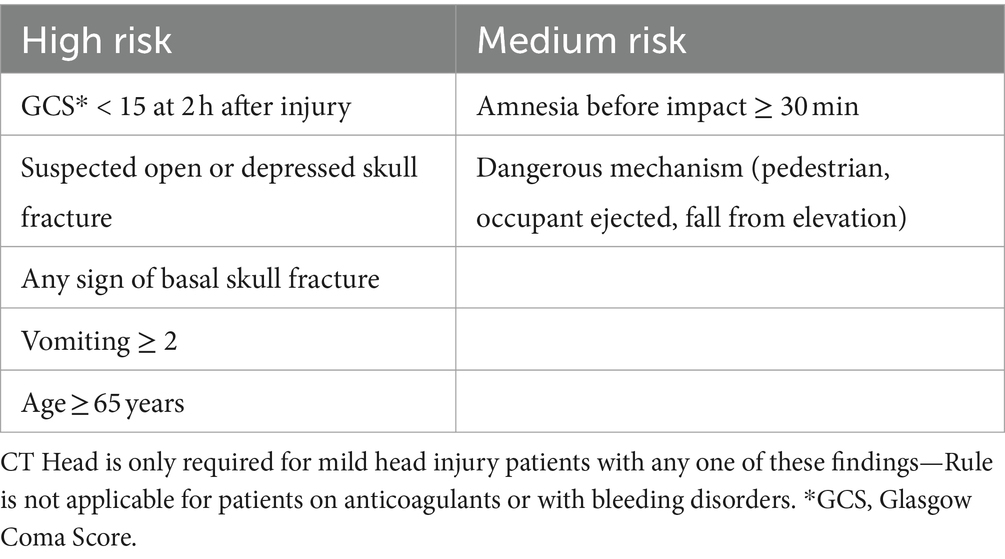
Table 3. Canadian CT head rule criteria—from the work of Stiell et al. (6).
No reversal agents were administered to any patient with a positive scan due to the scarcity of bleeding.
There was no difference in terms of bleeding in the two groups, on anticoagulant/anti-platelet therapy and patients on neither. The two CI greatly overlapped.
At 1 month follow-up we could reach all patients but 20 with a telephone call: 18 did not answer and for two patients phone number was missing. No patient had died or suffered complications following trauma. One patient on DOACs with negative CT scan had suspended rivaroxaban and suffered from ischemic stroke 3 days later. Two patients on neither therapy returned to the ED after 4 and 7 days, reporting headache and neck pain. Investigations revealed no complications and they were discharged home. One last patient on neither drug reported paraesthesias in all four limbs after trauma: an MRI of the spine ruled out major complications.
Of the 509 patients on anticoagulants/anti-platelets, 387 met inclusion criteria for CCHR.
The comparison of patients undergoing either therapy who did and did not fulfil the criteria of the CCHR was statistically significant, as patients who fulfilled the criteria had a higher probability of haemorrhage (p = 0.023, CI 4.0%–9.1% for fulfilled and 1.0%–4.9% for unfulfilled criteria).
Amongst participants who met CCHR criteria, the comparison between patients who did take anticoagulants or anti-platelet drugs and the patients on neither was not statistically significant. The two groups overlapped.
When separating the categories of anti-platelets and anticoagulants, the difference in bleeding rate of those who did and did not meet the criteria for the Rule was statistically significant for anti-platelets (p = 0.013 CI 4.8–13.1% for met criteria and 1.0–6.3% for non-meet criteria; Figure 1).
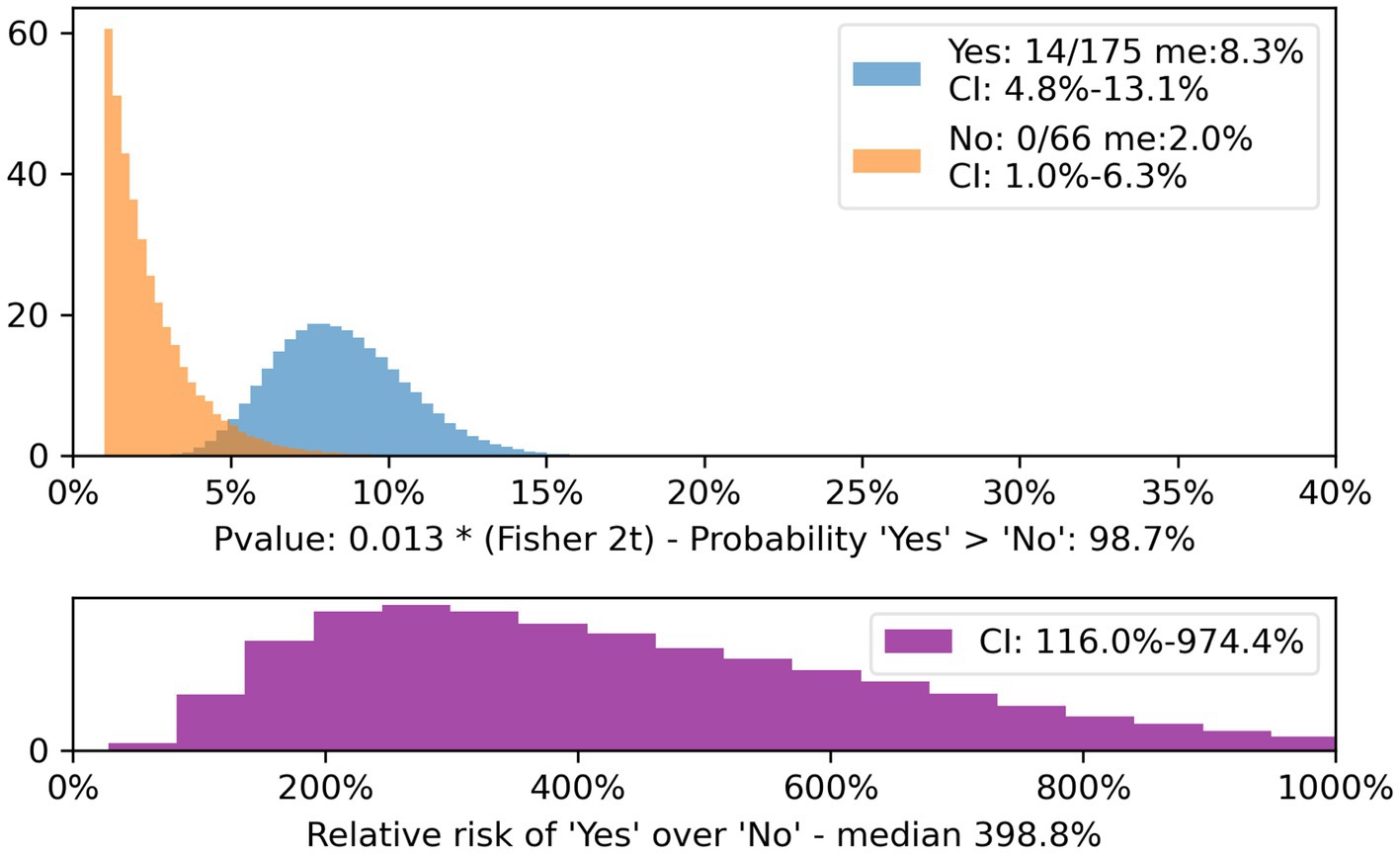
Figure 1. Patients on anti-platelets meeting and non-meeting CT Head Rule, CI and relative risk. The population of patients on anti-platelet drugs who did not meet the CT Head Rule criteria (yellow) is compared with those who did (blue).
The population that met the CCHR criteria had a significantly higher rate of intracranial bleeding and not statistically significant for anticoagulants (CI 2.2%–8.0% for met criteria and 1.1%–6.2% for non-met criteria) with a trend towards more bleeding for patients on anticoagulants who met the criteria.
Comparing anticoagulated patients who met the Rule with patients on anti-platelets who met the Rule, the difference was not statistically significant, with a tendency towards more haemorrhages in patients on anticoagulant drugs.
The comparison between patients anticoagulated with vitamin K antagonists (VKA) and anticoagulated with direct oral anticoagulants (DOACs) was also not statistically significant: the two categories overlapped.
Data from this study are available on https://datadryad.org/stash.
This study shows that anticoagulant or anti-platelet therapies are not an independent risk factor for brain haemorrhage in GCS 15 patients and that the CCHR might be used for patients with mTBI undergoing these treatments.
In the literature mTBI has so far been discussed under the assumption that all patients on anticoagulant or anti-platelet therapy were at high risk, even if they had a GCS of 15 (8, 11, 20). Many retrospective studies evaluated the incidence of bleeding in anticoagulated patients, whilst this paper is giving an answer in a prospective study (4, 16–18).
The results reported are of high importance and likely to impact clinical practice in the ED.
The question is not about whether anticoagulants and anti-platelets are actually a risk factor for haemorrhage in brain injury, even when mild. Indeed, it seems quite clear (although the studies are mostly retrospective) that taking these therapies does carry with it a certain increased risk of developing intracranial bleeding after trauma (21–23).
With this regard, several authors emphasise that anti-platelets vs. anticoagulants, and amongst the latter, VKAs vs. DOACs, are at higher risk (4, 13, 22, 24–26).
The question is whether patients with GCS of 15 within 2 h after trauma should be considered in the same way as other patients. The present work shows that this might be possible. If 2 h after trauma they have maintained an intact neurological state, they should be considered low-risk patients because it is unlikely to be severe damage inside the brain (27). In fact, with regard to patients on either therapy, in this study the probability of bleeding even when meeting the criteria remained comparable to that of patients on neither therapy meeting the same criteria. As for patients on anticoagulants, it appears that those with GCS 15 do not have a higher bleeding rate even when selected using the Rule criteria.
Considering possible confounding factors (median age, percentage of patients > 64 years old, percentage of high-energy incidents, amnesia > 30 min, percentage of low energy traumas), patients on anticoagulant or anti-platelet therapy appear older.
The two groups are also comparable in terms of low energy trauma (with a slight tendency to higher energy trauma for the anticoagulants/anti-platelet group) and amnesia.
We did not proceed with further calculations because, given their older age and similar energy of traumas, they are theoretically at an increased risk of ICH. Since the comparison between anticoagulants/anti-platelets and patients on neither therapy yielded similar results, this reinforces our findings.
In the entire study population, one patient needed surgery for evacuation of a subdural haematoma and she was discharged without neurological sequelae, resuming her normal activity in 1 month. Retrospective analysis of the emergency department management of this patient revealed that head GCS score was registered early. In fact, it had been performed upon the patient’s arrival in the emergency department. At 2 h after the trauma (the time pointed out by CCHR to assess GCS), the patient was no longer GCS 15 and thus theoretically should have been excluded from the study.
With regard to the other 58 patients whose CT was positive for haemorrhage, CT control was stable in all cases. All patients were discharged without neurological sequelae and resumed their normal activity.
Our results are giving an answer to the question whether CCHR is reliable also for patients on anticoagulant and anti-platelet treatment.
More than the immediate symptoms after a mTBI (amnesia, disorientation, transient loss of consciousness), a normal neurological state after 2 h is important, regardless of the treatment the patient is taking.
It is possible to speculate, that the vast majority of CTs performed on GCS 15 patients, even when they meet the Rule’s criteria, are unnecessary, with the exception of patients on anti-platelet therapy.
Our study has some limitations:
• We were not able to measure antiXa activity in the vast majority of patients on DOACs, so we did not actually know their coagulation status, even though the patients we enrolled were regularly taking their therapy. Similarly, we did not assess the platelet function of patients on anti-platelet therapy. Adherence to therapy seemed to us a good surrogate as DOACs level measures are not routinely requested for these therapies. However, this needs further investigation in the context of traumatic intracranial haemorrhage.
• This is a single-centre study that needs confirmation on several sites.
• We were not able to contact 20 patients on follow-up. However, negative outcomes in these 20 individuals are really unlikely (control CT in hospital was stable) and could hardly have changed the outcome of the study.
• This study analysed a group of anticoagulant or anti-platelet medication users combined: bigger studies are needed that analyse anticoagulants alone and anti-platelet drugs alone.
The CCHR could possibly be used for mTBI patients on anticoagulant or anti-platelet therapy, although the number of diagnostic tests requested with the help of this Rule is probably still too high. Multicenter studies are needed to reinforce this opinion.
Anticoagulants and anti-platelet drugs should not be considered per se a risk factor for patients with mTBI and a GCS of 15; the need for CT scan should be weighed against the guidelines used for patients on neither therapy.
Raw data from this study is in the supplementary material.
The studies involving humans were approved by Comitato etico del Canton Ticino. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
LU: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. CR: Conceptualization, Investigation, Methodology, Writing – original draft. FP: Conceptualization, Validation, Writing – original draft. CB: Data curation, Methodology, Writing – review & editing. GU: Data curation, Formal analysis, Writing – review & editing. RP: Data curation, Formal analysis, Writing – review & editing. PM-H: Conceptualization, Project administration, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Thanks to Lorenzo Emilitri: without his unparalleled statistical expertise we would not be able to continue our work. Thanks also to Elena Porro whose support during the preparation of protocol was constant and precious.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1327871/full#supplementary-material
1. Menon, DK, Schwab, K, Wright, DW, and Maas, AI. Demographics and clinical assessment working Group of the International and Interagency Initiative toward common data elements for research on traumatic brain injury and psychological health. Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil. (2010) 91:1637–40. doi: 10.1016/j.apmr.2010.05.017
2. Furlan, JC, Radan, MM, and Tator, CH. A scoping review of registered clinical studies on mild traumatic brain injury and concussion (2000 to 2019). Neurosurgery. (2020) 87:891–9. doi: 10.1093/neuros/nyaa151
3. Saadat, S, Ghodsi, SM, Naieni, KH, Firouznia, K, Hosseini, M, Kadkhodaie, HR, et al. Prediction of intracranial computed tomography findings in patients with minor head injury by using logistic regression. J Neurosurg. (2009) 111:688–94. doi: 10.3171/2009.2.JNS08909
4. Uccella, L, Zoia, C, Bongetta, D, Gaetani, P, Martig, F, Candrian, C, et al. Are antiplatelet and anticoagulants drugs a risk factor for bleeding in mild traumatic brain injury? World Neurosurg. (2018) 110:e339–45. doi: 10.1016/j.wneu.2017.10.173
5. Mena, JH, Sanchez, AI, Rubiano, AM, Peitzman, AB, Sperry, JL, Gutierrez, MI, et al. Effect of the modified Glasgow coma scale score criteria for mild traumatic brain injury on mortality prediction: comparing classic and modified Glasgow coma scale score model scores of 13. J Trauma. (2011) 71:1185–93. doi: 10.1097/TA.0b013e31823321f8
6. Stiell, IG, Wells, GA, Vandemheen, K, Clement, C, Lesiuk, H, Laupacis, A, et al. The Canadian CT head rule for patients with minor head injury. Lancet. (2001) 357:1391–6. doi: 10.1016/s0140-6736(00)04561-x
7. Kavalci, C, Aksel, G, Salt, O, Yilmaz, MS, Demir, A, Kavalci, G, et al. Comparison of the Canadian CT head rule and the New Orleans criteria in patients with minor head injury. World J Emerg Surg. (2014) 9:31. doi: 10.1186/1749-7922-9-31
8. Ho, KH, van Hove, M, and Leng, G. Trends in anticoagulant prescribing: a review of local policies in English primary care. BMC Health Serv Res. (2020) 20:279. doi: 10.1186/s12913-020-5058-1
9. Skandsen, T, Nilsen, TL, Einarsen, C, Normann, I, McDonagh, D, Haberg, AK, et al. Incidence of mild traumatic brain injury: a prospective hospital, emergency room and general practitioner-based study. Front Neurol. (2019) 10:638. doi: 10.3389/fneur.2019.00638
10. Jagoda, AS, Bazarian, JJ, Bruns, JJ Jr, Cantrill, SV, Gean, AD, Howard, PK, et al. American College of Emergency Physicians; Centers for Disease Control and Prevention. Clinical policy: neuroimaging and decisionmaking in adult mild traumatic brain injury in the acute setting. Ann Emerg Med. (2008) 52:714–48. doi: 10.1016/j.annemergmed.2008.08.021
11. NICE . Head injury: assessment and early management Clinical guideline (2023). Available at: https://www.nice.org.uk/guidance/ng232
12. Zhou, Y, Boudreau, DM, and Freedman, AN. Trends in the use of aspirin and nonsteroidal anti-inflammatory drugs in the general U.S. population. Pharmacoepidemiol Drug Saf. (2014) 23:43–50. doi: 10.1002/pds.3463
13. Uccella, L, Zoia, C, Perlasca, F, Bongetta, D, Codecà, R, and Gaetani, P. Mild traumatic brain injury in patients on long-term anticoagulation therapy: do they really need repeated head CT scan? World Neurosurg. (2016) 93:100–3. doi: 10.1016/j.wneu.2016.05.061
14. Van den Brand, CL, Perotti, JR, van der Linden, MC, Tolido, T, and Jellema, K. Effect of the implementation of a new guideline for minor head injury on computed tomography-ratio and hospitalizations in the Netherlands. Eur J Emerg Med. (2020) 27:441–6. doi: 10.1097/MEJ.0000000000000714
15. Cheng, AHY, Campbell, S, Chartier, LB, Dowling, S, Goddard, T, Gosselin, S, et al. Choosing wisely Canada's emergency medicine recommendations: time for a revision. CJEM. (2019) 21:717–20. doi: 10.1017/cem.2019.405
16. Ganti, L, Stead, T, Daneshvar, Y, Bodhit, AN, Pulvino, C, Ayala, SW, et al. GCS 15: when mild TBI isn't so mild. Neurol Res Pract. (2019) 1:6. doi: 10.1186/s42466-018-0001-1
17. Cipriano, A, Park, N, Pecori, A, Bionda, A, Bardini, M, Frassi, F, et al. Predictors of post-traumatic complication of mild brain injury in anticoagulated patients: DOACs are safer than VKAs. Intern Emerg Med. (2021) 16:1061–70. doi: 10.1007/s11739-020-02576-w
18. Cipriano, A, Pecori, A, Bionda, AE, Bardini, M, Frassi, F, Leoli, F, et al. Intracranial hemorrhage in anticoagulated patients with mild traumatic brain injury: significant differences between direct oral anticoagulants and vitamin K antagonists. Intern Emerg Med. (2018) 13:1077–87. doi: 10.1007/s11739-018-1806-1
19. Kruschke, JK . Bayesian estimation supersedes the t-test. J Exp Psychol Gen. (2013) 142:573–603. doi: 10.1037/a0029146
20. Cohen, DB, Rinker, C, and Wilberger, JE. Traumatic brain injury in anticoagulated patients. J Trauma. (2006) 60:553–7. doi: 10.1097/01.ta.0000196542.54344.05
21. Uccella, L, Bongetta, D, Fumagalli, L, Raffa, G, and Zoia, C. Acute alcohol intoxication as a confounding factor for mild traumatic brain injury. Neurol Sci. (2020) 41:2127–34. doi: 10.1007/s10072-020-04313-9
22. Cheng, L, Cui, G, and Yang, R. The impact of preinjury use of antiplatelet drugs on outcomes of traumatic brain injury: a systematic review and Meta-analysis. Front Neurol. (2022) 13:724641. doi: 10.3389/fneur.2022.724641
23. Probst, MA, Gupta, M, Hendey, GW, Rodriguez, RM, Winkel, G, Loo, GT, et al. Prevalence of intracranial injury in adult patients with blunt head trauma with and without anticoagulant or antiplatelet use. Ann Emerg Med. (2020) 75:354–64. doi: 10.1016/j.annemergmed.2019.10.004
24. Alter, SM, Mazer, BA, Solano, JJ, Shih, RD, Hughes, MJ, Clayton, LM, et al. Antiplatelet therapy is associated with a high rate of intracranial hemorrhage in patients with head injuries. Trauma Surg Acute Care Open. (2020) 5:e000520. doi: 10.1136/tsaco-2020-000520
25. Van den Brand, CL, Tolido, T, Rambach, AH, Hunink, MG, Patka, P, and Jellema, K. Systematic review and Meta-analysis: is pre-injury antiplatelet therapy associated with traumatic intracranial hemorrhage? J Neurotrauma. (2017) 34:1–7. doi: 10.1089/neu.2015.4393
26. Turcato, G, Zannoni, M, Zaboli, A, Zorzi, E, Ricci, G, Pfeifer, N, et al. Direct Oral anticoagulant treatment and mild traumatic brain injury: risk of early and delayed bleeding and the severity of injuries compared with vitamin K antagonists. J Emerg Med. (2019) 57:817–24. doi: 10.1016/j.jemermed.2019.09.007
Keywords: anticoagulants, anti-platelet, brain concussion, brain injury, Canadian CT head rule, GCS 15, mild traumatic brain injury
Citation: Uccella L, Riboni C, Polinelli F, Biondi C, Uccheddu G, Petrino R and Majno-Hurst P (2024) Use of the Canadian CT head rule for patients on anticoagulant/anti-platelet therapy presenting with mild traumatic brain injury: prospective observational study. Front. Neurol. 15:1327871. doi: 10.3389/fneur.2024.1327871
Received: 26 October 2023; Accepted: 02 April 2024;
Published: 18 April 2024.
Edited by:
Ansgar J. Furst, War Related Illness and Injury Study Center, United StatesReviewed by:
Adam James Wells, University of Adelaide, AustraliaCopyright © 2024 Uccella, Riboni, Polinelli, Biondi, Uccheddu, Petrino and Majno-Hurst. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence:Laura Uccella, bGF1cmEudWNjZWxsYUBlb2MuY2g=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.