
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 24 April 2024
Sec. Stroke
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1325960
Objective: Inflammation is a central driver of atherogenesis and eventual plaque rupture. This study aimed to evaluate the association between residual inflammatory risk (RIR) and vulnerable plaques in the carotid artery in patients with ischemic stroke.
Methods: Patients with acute ischemic stroke were enrolled from January 2021 to July 2022. They were divided into four groups: RIR only (LDL-C <2.6 mmol/L and hsCRP ≥2 mg/L), residual cholesterol risk (RCR) only (LDL-C ≥2.6 mmol/L and hsCRP <2 mg/L), both risk or residual cholesterol and inflammatory risk (RCIR) (LDL-C ≥2.6 mmol/L and hsCRP ≥2 mg/L), and neither risk (LDL-C <2.6 mmol/L and hsCRP <2 mg/L). Vulnerable plaques were determined if it had a low attenuated plaque CT value of <35 Hounsfield Units (HU) and a remodeling index of >1.1, which indicated a positive remodeling.
Results: Out of the 468 enrolled patients, 157 (33.5%) were detected to have vulnerable plaques. The proportion of patients with neither risk, RIR, RCR, and RCIR were 32.9%, 28.6%, 18.8%, and 19.7%, respectively. Patients with vulnerable plaques exhibited a higher prevalence of hyperlipidemia (P = 0.026), higher proportion of RIR (P = 0.015), a higher ratio of stroke subtypes of large artery atherosclerosis (P = 0.012), and high leukocyte counts (P < 0.001). The logistic regression analysis detected that RIR was associated with vulnerable plaques after adjusted for major confounding factors (OR 1.98, 95% CI 1.13–3.45, P = 0.016), especially in the large artery atherosclerosis subtype (OR 2.71, 95% CI 1.08–6.77, P = 0.034).
Conclusions: In patients with ischemic stroke, RIR is associated with the vulnerability of carotid plaques, especially for those with the large artery atherosclerosis subtype. Therefore, further studies investigating the interventions to modulate inflammation in these patients may be warranted.
Acute ischemic stroke is the most important subtype of stroke and is also one of the major causes of economic burden in China (1). Approximately one fifth of the ischemic stroke is associated with atherosclerosis of the carotid arteries (2). Generally, atherosclerotic plaques can be divided into stable plaques and unstable or vulnerable plaques. Research studies have shown that whether carotid atherosclerotic plaque leads to ischemic stroke is mainly determined by whether the plaque is stable, rather than by the degree of lumen stenosis (2, 3), that is, vulnerable carotid plaque can increase the risk of ischemic stroke (4, 5). Atherosclerosis is a chronic inflammatory disease in which immune mechanisms play a pivotal role, and inflammation may promote the occurrence, development, and rupture of plaques (6–8).
It is well known that low-density lipoprotein cholesterol (LDL-C) is associated with cardiovascular disease. However, even though statins can significantly reduce LDL-C levels, which also suppress inflammation (9), cardiovascular risk remains, which may be associated with high levels of inflammation (10, 11). Inflammation is a central driver of atherogenesis and eventual plaque rupture, and it is an important contributor to residual risk. Therefore, based on the high-sensitivity C-reactive protein (hsCRP) level, hsCRP ≥2 mg/L were generally defined as residual inflammatory risk (RIR) (12). It is significantly associated with myocardial infarction, stroke, and all-cause death (13).
To date, the research on RIR mainly focuses on the clinical prognosis of related diseases. Recent studies have reported that persistent high RIR increases the risk of all-cause death and myocardial infarction after percutaneous coronary intervention (PCI) and increases the risk of recurrence in patients with acute ischemic stroke (14, 15). However, there are no reports of high RIR and specific neuroimaging changes. Therefore, this study aimed to investigate the association between high RIR and vulnerable plaques in the carotid artery in patients with acute ischemic stroke.
Patients with acute ischemic stroke were consecutively enrolled from The First Affiliated Hospital of Anhui University of Science and Technology between January 2021 and July 2022. This study was approved by the ethics committee of The First Affiliated Hospital of Anhui University of Science and Technology, and each patient signed an informed consent form.
The inclusion criteria for this study are as follows: (1) patients had to be aged 18 years or older; (2) have experienced ischemic stroke within the past 7 days; and (3) have undergone completed carotid computed tomography angiography (CTA) scans within 7 days of the index stroke. The exclusion criteria are as follows: (1) had previous carotid endarterectomy or carotid stenting; (2) had active infection; and (3) had malignant tumors or severe heart, liver, or renal failure.
Clinical data including age, sex, height, weight, current smoking and drinking status, medical histories (hypertension, diabetes, atrial fibrillation, coronary heart disease, ischemic stroke, and autoimmune diseases), previous medication (antiplatelet, statins, and anti-inflammatory drug) and National Institutes of Health Stroke Scale (NIHSS) scores were collected from medical records.
Fasting venous blood was collected the next morning after admission. LDL-C and homocysteine levels were measured using a Siemens ADVIA1800 automatic biochemical analyzer, hsCRP was detected using a Mindray BC7500 automatic blood cell analyzer and leukocyte counts were tested using a Sysmex BC7500 automatic blood routine analyzer.
Carotid CTA scans were performed with a dual-source 256 spiral CT scanner (Revolution, GE, USA). A non-ionic iodine contrast agent (iodixanol 350–370 mgI/ml) and normal saline were injected through the cubital vein with a total injection volume of 60 ml, and the injection flow rate was 4 ml/s. The monitoring was delayed for 15 s after injection. The scan was started when the contrast medium reached its peak concentration in the target vessel. The scanning parameters were 125 kV tube voltage, 250 mAs tube current, and 512 × 512 matrix. The scan range was from the lower edge of the aortic arch to the cranial roof, and the scan direction was from the foot to the cephalic side. The original data were processed by digital subtraction using CT workstation, and then the carotid arteries were reconstructed, analyzed, and diagnosed by volume reproduction, maximum density projection, multiplane reconstruction, and advanced vascular analysis.
As our previous research showed, vulnerable plaques were determined if low attenuated plaque CT value of < 35 Hounsfield Units (HU) and a remodeling index of >1.1, which indicated a positive remodeling (16). Low attenuated plaque CT value was measured as the minimum CT value of plaque at least three contentiously cross-sectional images at the interest region and averaged; Remodeling index was calculated as the ratio between the outer vessel area (including both plaque and vessel lumen, roughly equals the external elastic membrane area in intravenous ultrasound) at the site of maximal luminal narrowing and the mean of the proximal and distal reference sites. The image of a vulnerable plaque and a non-vulnerable plaque is shown in Figure 1.
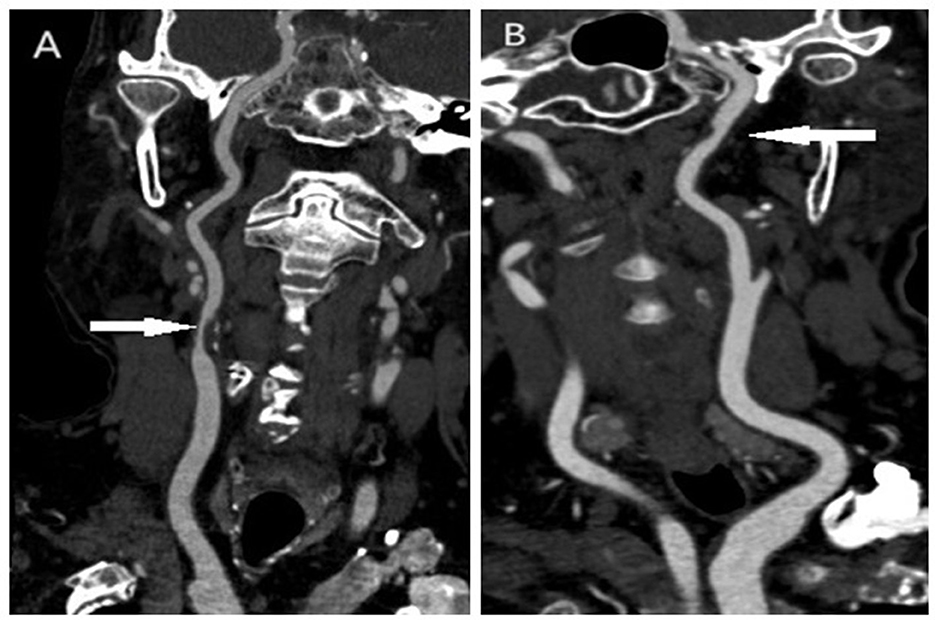
Figure 1. The image of a vulnerable plaque and a non-vulnerable plaque in the carotid artery. (A) Vulnerable plaque in the carotid artery; (B) non-vulnerable plaque in the carotid artery.
According to the degree of carotid artery stenosis, patients were classified into three categories: mild (< 50%), moderate (50%−69%) and severe (≥70%).
SPSS 24.0 (IBM Corp., Armonk, NY, USA) and SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) were used for statistical analysis. The Kolmogorov–Smirnov test was used to analyze whether continuous variables were normally distributed. The data consistent with normal distribution were expressed as mean ± standard deviation, and the comparison between groups was performed using an independent sample t-test. The data that were not normally distributed were showed as median and interquartile range, and the contrast between groups was analyzed using the Mann–Whitney U test. Categorical variables were expressed as frequency and percentage and the Chi-squared test or Fisher's exact test was used for the comparison between groups.
Generally, an LDL-C level of < 2.6 mmol/L has been recommended as a therapeutic target in high-risk individuals, while a high inflammatory status was defined as hsCRP ≥2 mg/L (17, 18). Therefore, we used an LDL-C level of 2.6 mmol/L and an hsCRP level of 2 mg/L as cut-off values, and the patients were divided into four groups:RIR only (LDL-C < 2.6 mmol/L and hsCRP ≥2 mg/L), residual cholesterol risk (RCR) only (LDL-C ≥2.6 mmol/L and hsCRP < 2 mg/L), both risk or residual cholesterol and inflammatory risk (RCIR) (LDL-C ≥2.6 mmol/L and hsCRP ≥2 mg/L), and neither risk (LDL-C < 2.6 mmol/L and hsCRP < 2 mg/L). The association between residual risk and vulnerable plaques in the carotid artery was assessed using the multiple logistic regression model. Model 1 was adjusted for age and sex. Model 2 was adjusted for factors in model 1 and body mass index, smoking, drinking, medical history of hypertension, diabetes, hyperlipemia, coronary heart disease, atrial fibrillation, ischemic stroke and autoimmune diseases, previous usage of antiplatelet agents, statin and anti-inflammatory drugs, baseline NIHSS scores, and the degree of carotid stenosis. Model 3 was adjusted for factors in model 2 and baseline leukocyte and homocysteine counts. The subgroup analysis was further performed according to the different Trial of ORG 10172 in Acute Stroke Treatment (TOAST) subtypes. The results were expressed as odds ratios (OR) and 95% confidence intervals (CI). A significance level of P < 0.05 was considered statistically significant.
A total of 756 patients with acute ischemic stroke were enrolled in this study. Among them, 23 (3.0%) patients with previous carotid endarterectomy or carotid stent implantation, 17 (2.2%) patients with active infection, and 42 (5.5%) patients with malignant tumors or severe heart, liver, or kidney failure were excluded. Of the remaining 674 patients, 282 (41.8%) had intracranial arteriosclerotic stenosis, 468 (69.4%) had significant carotid plaque on at least one side and were eventually included in the analysis.
The median age was 64 (55–72) years and 308 (65.8%) of them were male patients. The median levels of LDL-C and hsCRP were 2.53 mmol/L (1.91–3.02 mmol/L) and 1.66 mg/L (1.29–3.14 mg/L). Based on carotid CTA results, vulnerable plaques were present in 157 patients (33.5%).
Table 1 illustrates baseline characteristics with or without vulnerable plaques in the carotid artery. Patients with vulnerable plaques had a higher prevalence of hyperlipidemia (P = 0.026), a higher proportion of RIR (P = 0.015), a higher ratio of stroke subtypes of large artery atherosclerosis (P = 0.012), and high leukocyte counts (P < 0.001).
Table 2 shows baseline characteristics according to LDL-C and hsCRP levels. The proportion of patients with no residual risk, RIR, RCR, and RCIR were 32.9%, 28.6%, 18.8%, and 19.7%, respectively. Hyperlipidemia (P = 0.039), baseline leukocyte counts (P = 0.010), vulnerable plaques (P = 0.015), and stroke subtypes (P = 0.003) differed among the four groups.
The logistic regression analysis detected that patients with RIR (OR 1.88, 95% CI 1.13–3.13, P = 0.015) and RCIR (OR 2.33, 95% CI 1.34–4.05, P = 0.003) had a higher risk of vulnerable plaques compared to patients with no residual risk (Table 3). After multivariable adjustment (as in model 3), the association remained (OR 1.98, 95% CI 1.13–3.45, P = 0.016; OR 2.14, 95% CI 1.16–3.95, P = 0.015; Table 3).
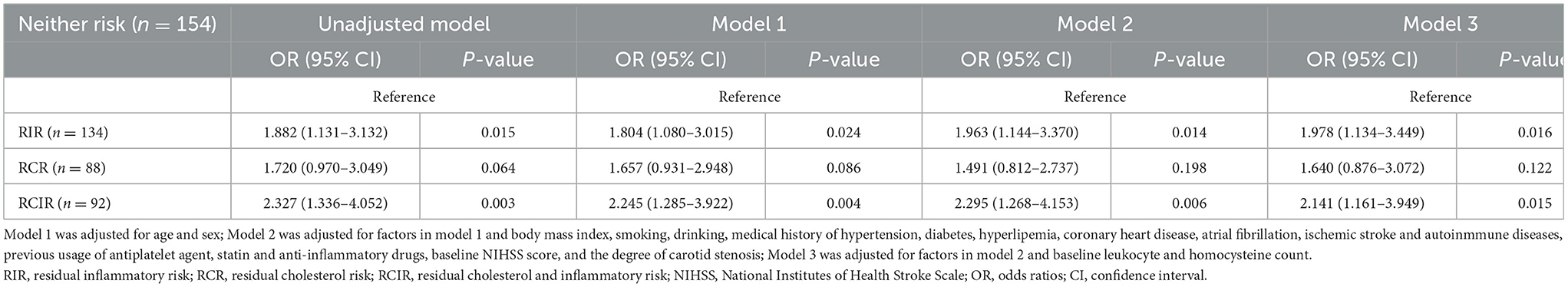
Table 3. Logistic regression analysis for exploring the association between RIR, RCR and RCIR with plaque vulnerability.
Further subgroup analysis showed that, after classifying patients according to the TOAST criteria, those with stroke subtypes of large artery atherosclerosis were more likely to develop vulnerable plaques if experiencing RIR (OR 2.32, 95% CI 1.04–5.16 P = 0.040; Figure 2A) and RCIR (OR 2.86, 95% CI 1.20–6.84, P = 0.018; Figure 2A). After controlling for the potential confounders, results persisted qualitatively similar (OR 2.71, 95% CI 1.08–6.77, P = 0.034; OR 4.46, 95% CI 1.58–12.63, P = 0.005; Figure 2A). There was no significant interaction between RIR and plaque vulnerability in the patients with small vessel occlusion subtype (OR 1.42, 95% CI 0.22–8.97, P = 0.710; Figure 2B) and stroke of other subtypes (OR 2.46, 95% CI 0.77–7.90, P = 0.130; Figure 2C).
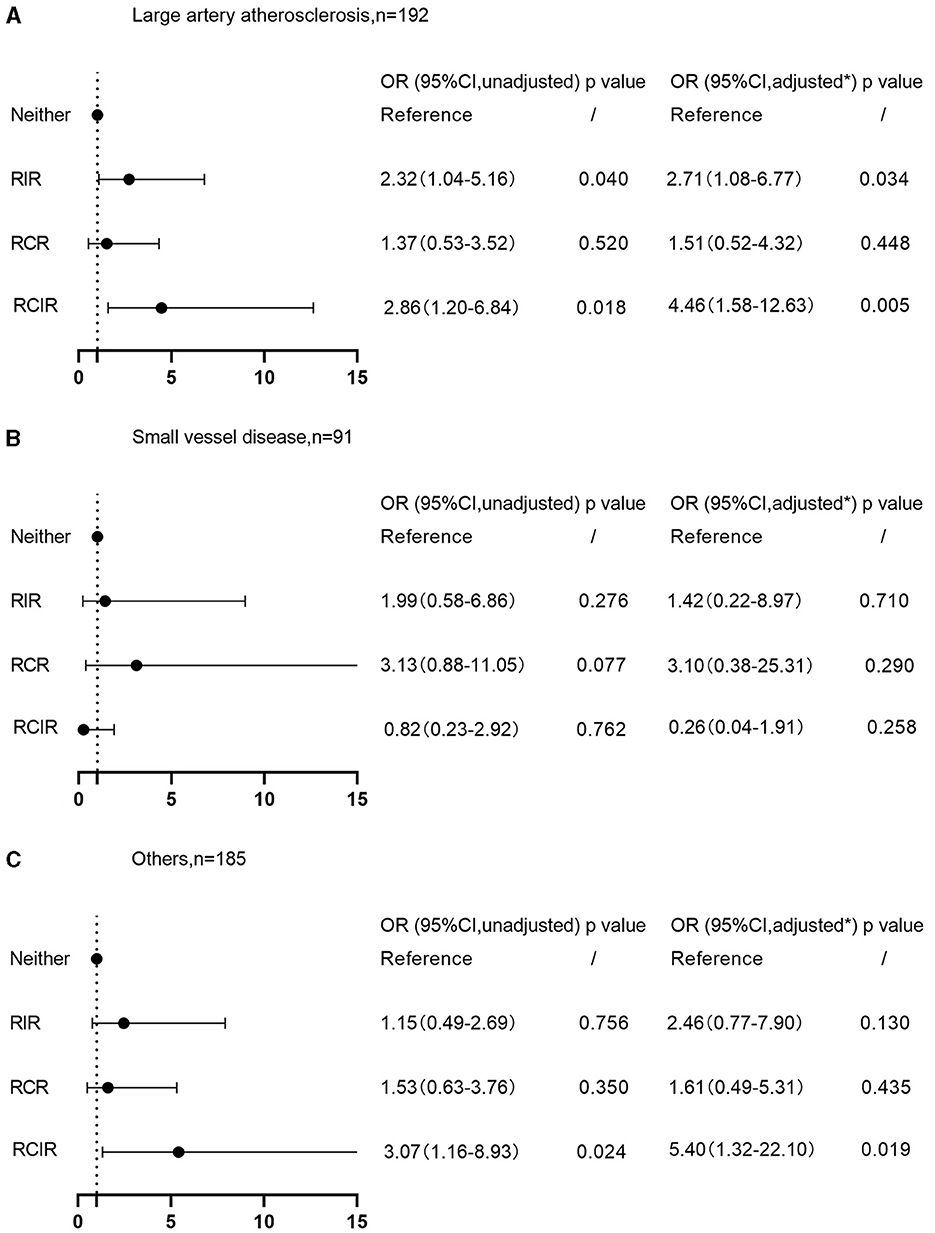
Figure 2. Associations of RIR with plaque vulnerability on TOAST classification. (A) Large artery atherosclerosis subtype; (B) small vessel occlusion subtype; (C) stroke of other subtypes. *Adjusted for age, sex, body mass index, smoking, drinking, medical history of hypertension, diabetes, hyperlipemia, coronary heart disease, atrial fibrillation, ischemic stroke, autoimmune diseases, previous usage of antiplatelet agents, statin and anti-inflammatory drugs, baseline NIHSS scores, the degree of carotid stenosis, and baseline leukocyte and homocysteine counts. RIR, residual inflammatory risk; RCR, residual cholesterol risk; RCIR, residual cholesterol and inflammatory risk; TOAST, Trial of ORG 10172 in Acute Stroke Treatment; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratios; CI, confidence interval.
This study revealed a significant positive association between RIR and vulnerable plaques in the carotid artery in patients with ischemic stroke, especially in the large atherosclerotic subtype, suggesting that these patients may benefit further from initiating anti-inflammatory therapy in addition to lipid-lowering therapy.
Previous research studies have demonstrated that the incidence of RIR varies among different study populations; however, all are so common (19–22). In the large-scale PROVE-IT (Pravastatin or Atorvastatin Evaluation and Infection) study of 3,745 patients with acute coronary syndrome, RIR was present in 29% of those allocated to the atorvastatin 80 mg group (19). Similarly, the IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International) trial revealed that, even in patients treated with either simvastatin 40 mg or the combination of simvastatin 40 mg plus ezetimibe 10 mg daily, the proportion of patients with RIR was still as high as 33% (20). In addition, the VIRGO (Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients) registry study of young patients with coronary heart disease has also shown that 60% of patients had elevated hsCRP levels and RIR presented in 16% of the general population (21). The above analysis of individuals with coronary ischemic heart disease revealed that RIR was universal in the real clinical world even when treated with statins. Moreover, in a recent analysis from China's multicenter cohort study of patients with acute ischemic stroke or transient ischemic attack, Li et al. (22) reported that 23.1% of them were afflicted with RIR. However, in the results of our study, the incidence of RIR was 28.6%, which was slightly higher than that reported in Li et al.'s study. This difference may be due to the cutoff point of hsCRP set at 2 mg/L in this study, which is lower than the cutoff value of 3 mg/L in the study of Li et al.
Carotid plaque vulnerability is a suitable indicator to assess the severity of atherosclerosis in the large arteries. The main characteristics of vulnerable plaques include thin fibrous cap, large lipid core, active inflammation, neovascularization, and dilated remodeling. Pathological neovascularization in plaques can promote the development of atherosclerotic lesions and induce intra-plaque bleeding and plaque rupture, which is an important factor causing increased plaque vulnerability (23). RIR may increase the risk of vulnerable plaques in the carotid artery for reasons that are not entirely clear; however, this association may be attributed due to the following reasons: first, hsCRP is one of the most sensitive inflammatory markers, mainly involved in acute non-specific inflammatory responses. Under the stimulation of inflammatory transmitters, it binds to lipoprotein, activates the complement system to produce a large number of terminal complexes, causing vascular endothelial injury, and then exacerbates the inflammatory response in plaques through the release of tissue factors by inflammatory cells to accelerate the formation of intravascular thrombosis (24). Additionally, hsCRP can induce the secretion of matrix metalloproteinases, which increase the fragility of atherosclerotic plaques by accelerating the degradation of endothelial cells (25). Finally, hsCRP can also promote the formation of new blood vessels within the plaque, increasing the risk of bleeding within the plaque and eventually leading to the formation of vulnerable plaques (25). The association between RIR and vulnerable plaques in the carotid artery was only found in patients with large atherosclerosis. This may be because CRP is involved in the formation of atherosclerotic thrombosis which is the pathogenesis of large atherosclerotic cerebral infarction, through a variety of pathways, including activation of the complement system, induction of apoptosis, vascular cell activation, leukocyte recruitment, lipid accumulation, and platelet aggregation (26). Furthermore, CRP also contributes to plaque instability by inducing the expression of metalloproteinases (MMP) 1, 2, and 9 (27, 28). In addition, we also found a significant association between RCIR and carotid plaque vulnerability in the large atherosclerosis subtype, while no such observation was observed with RCR. This further suggests that inflammation plays an important role in promoting the formation of vulnerable plaques in the carotid artery.
There were several limitations in this study. First, this was a single-center study with a relatively small sample size and is not fully representative of the overall population. Second, for hsCRP, we only examined baseline data at admission and did not monitor its dynamic changes as in some other studies (29, 30). Finally, this was a cross-sectional study and, therefore, could not determine cause and effect. However, no cohort studies have been conducted on the association between RIR and vulnerability of plaques. Thus, further prospective studies are needed.
In patients with acute ischemic stroke, RIR can predict plaque vulnerability in the carotid artery, especially for those with large artery atherosclerosis. Prospective trials should be further explored to investigate inflammation-modulating interventions in these high-risk patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Committee of the First Affiliated Hospital of Anhui University of Science and Technology. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
XG: Writing – original draft, Data curation, Funding acquisition, Investigation, Methodology, Software. CY: Writing – review & editing. ZL: Data curation, Methodology, Writing – original draft. XW: Data curation, Methodology, Writing – original draft. QC: Methodology, Writing – original draft. XC: Methodology, Writing – original draft. JL: Methodology, Writing – original draft.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Anhui Provincial Department of Education Key Project (KJ2021A0434) and Medical Special Cultivation Project of Anhui University of Science and Technology (YZ2023H1C003).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer ZZ declared a shared affiliation with the author XW at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Tu WJ, Zhao Z, Yin P, Cao L, Zeng J, Chen H, et al. Estimated burden of stroke in China in 2020. JAMA Netw Open. (2023) 6:e231455. doi: 10.1001/jamanetworkopen.2023.1455
2. Skagen K, Skjelland M, Zamani M, Russell D. Unstable carotid artery plaque: new insights and controversies in diagnostics and treatment. Croat Med J. (2016) 57:311–20. doi: 10.3325/cmj.2016.57.311
3. Fleg JL, Stone GW, Fayad ZA, Granada JF, Hatsukami TS, Kolodgie FD, et al. Detection of high-risk atherosclerotic plaque: report of the NHLBI Working Group on current status and future directions. JACC Cardiovasc Imaging. (2012) 5:941–55. doi: 10.1016/j.jcmg.2012.07.007
4. Spagnoli LG, Mauriello A, Sangiorgi G, Fratoni S, Bonanno E, Schwartz RS, et al. Extracranial thrombotically active carotid plaque as a risk factor for ischemic stroke. JAMA. (2004) 292:1845–52. doi: 10.1001/jama.292.15.1845
5. Marnane M, Prendeville S, McDonnell C, Noone I, Barry M, Crowe M, et al. Plaque inflammation and unstable morphology are associated with early stroke recurrence in symptomatic carotid stenosis. Stroke. (2014) 45:801–6. doi: 10.1161/STROKEAHA.113.003657
6. Yuki H, Sugiyama T, Suzuki K, Kinoshita D, Niida T, Nakajima A, et al. Coronary inflammation and plaque vulnerability: a coronary computed tomography and optical coherence tomography study. Circ Cardiovasc Imaging. (2023) 16:e014959. doi: 10.1161/CIRCIMAGING.122.014959
7. Liem MI, Kennedy F, Bonati LH, van der Lugt A, Coolen BF, Nederveen AJ, et al. Investigations of carotid stenosis to identify vulnerable atherosclerotic plaque and determine individual stroke risk. Circ J. (2017) 81:1246–53. doi: 10.1253/circj.CJ-16-1284
8. Bäck M, Yurdagul A Jr, Tabas I, Öörni K, Kovanen PT. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol. (2019) 16:389–406. doi: 10.1038/s41569-019-0169-2
9. Satny M, Hubacek JA, Vrablik M. Statins and inflammation. Curr Atheroscler Rep. (2021) 23:80. doi: 10.1007/s11883-021-00977-6
10. Ridker PM. Targeting residual inflammatory risk: the next frontier for atherosclerosis treatment and prevention. Vascul Pharmacol. (2023) 153:107238. doi: 10.1016/j.vph.2023.107238
11. Mohammadnia N, Opstal TSJ, El Messaoudi S, Bax WA, Cornel JH. An update on inflammation in atherosclerosis: how to effectively treat residual risk. Clin Ther. (2023) 45:1055–59. doi: 10.1016/j.clinthera.2023.08.016
12. Ridker PM. How common is residual inflammatory risk? Circ Res. (2017) 120:617–9. doi: 10.1161/CIRCRESAHA.116.310527
13. Everett BM. Residual inflammatory risk: a common and important risk factor for recurrent cardiovascular events. J Am Coll Cardiol. (2019) 73:2410–2. doi: 10.1016/j.jacc.2019.02.056
14. Klingenberg R, Aghlmandi S, Gencer B, Nanchen D, Räber L, Carballo D, et al. Residual inflammatory risk at 12 months after acute coronary syndromes is frequent and associated with combined adverse events. Atherosclerosis. (2021) 320:31–7. doi: 10.1016/j.atherosclerosis.2021.01.012
15. Liu H, Wang M, Xiang X, Pan Y, Li J, Meng X, et al. Association of residual inflammatory risk with stroke recurrence in patients with acute ischaemic stroke or transient ischaemic attack. Eur J Neurol. (2022) 29:2258–68. doi: 10.1111/ene.15344
16. Peng M, Wang L, Xia Y, Tao L, Liu Y, Huang F, et al. High dietary inflammatory index is associated with increased plaque vulnerability of carotid in patients with ischemic stroke. Stroke. (2020) 51:2983–9. doi: 10.1161/STROKEAHA.120.029035
17. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. (2001) 285:2486–97. doi: 10.1001/jama.285.19.2486
18. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. (2017) 377:1119–31. doi: 10.1056/NEJMoa1707914
19. Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. (2005) 352:20–8. doi: 10.1056/NEJMoa042378
20. Bohula EA, Giugliano RP, Cannon CP, Zhou J, Murphy SA, White JA, et al. Achievement of dual low-density lipoprotein cholesterol and high-sensitivity C-reactive protein targets more frequent with the addition of ezetimibe to simvastatin and associated with better outcomes in IMPROVE-IT. Circulation. (2015) 132:1224–33. doi: 10.1161/CIRCULATIONAHA.115.018381
21. Lu Y, Zhou S, Dreyer RP, Spatz ES, Geda M, Lorenze NP, et al. Sex differences in inflammatory markers and health status among young adults with acute myocardial infarction: results from the VIRGO (variation in recovery: role of gender on outcomes of young acute myocardial infarction patients) study. Circ Cardiovasc Qual Outcomes. (2017) 10:e003470. doi: 10.1161/CIRCOUTCOMES.116.003470
22. Li J, Pan Y, Xu J, Li S, Wang M, Quan K, et al. Residual inflammatory risk predicts poor prognosis in acute ischemic stroke or transient ischemic attack patients. Stroke. (2021) 52:2827–36. doi: 10.1161/STROKEAHA.120.033152
23. Michel JB, Martin-Ventura JL, Nicoletti A, Ho-Tin-Noé B. Pathology of human plaque vulnerability: mechanisms and consequences of intraplaque haemorrhages. Atherosclerosis. (2014) 234:311–9. doi: 10.1016/j.atherosclerosis.2014.03.020
24. Choi EY, Yan RT, Fernandes VR, Opdahl A, Gomes AS, Almeida AL, et al. High-sensitivity C-reactive protein as an independent predictor of progressive myocardial functional deterioration: the multiethnic study of atherosclerosis. Am Heart J. (2012) 164:251–8. doi: 10.1016/j.ahj.2012.05.010
25. Ishizu T, Seo Y, Machino T, Kawamura R, Kimura T, Murakoshi N, et al. Prognostic impact of plaque echolucency in combination with inflammatory biomarkers on cardiovascular outcomes of coronary artery disease patients receiving optimal medical therapy. Atherosclerosis. (2011) 216:120–4. doi: 10.1016/j.atherosclerosis.2011.01.048
26. Daigo K, Inforzato A, Barajon I, Garlanda C, Bottazzi B, Meri S, et al. Pentraxins in the activation and regulation of innate immunity. Immunol Rev. (2016) 274:202–17. doi: 10.1111/imr.12476
27. Cimmino G, Ragni M, Cirillo P, Petrillo G, Loffredo F, Chiariello M, et al. C-reactive protein induces expression of matrix metalloproteinase-9: a possible link between inflammation and plaque rupture. Int J Cardiol. (2013) 168:981–6. doi: 10.1016/j.ijcard.2012.10.040
28. Doronzo G, Russo I, Mattiello L, Trovati M, Anfossi G. C-reactive protein increases matrix metalloproteinase-2 expression and activity in cultured human vascular smooth muscle cells. J Lab Clin Med. (2005) 146:287–98. doi: 10.1016/j.lab.2005.07.010
29. Kalkman DN, Aquino M, Claessen BE, Baber U, Guedeney P, Sorrentino S, et al. Residual inflammatory risk and the impact on clinical outcomes in patients after percutaneous coronary interventions. Eur Heart J. (2018) 39:4101–8. doi: 10.1093/eurheartj/ehy633
Keywords: inflammatory risk, atherosclerosis, vulnerable plaque, carotid artery, ischemic stroke
Citation: Gong X, Yu C, Lu Z, Wang X, Cai Q, Cheng X and Lu J (2024) Residual inflammatory risk and vulnerable plaque in the carotid artery in patients with ischemic stroke. Front. Neurol. 15:1325960. doi: 10.3389/fneur.2024.1325960
Received: 26 October 2023; Accepted: 11 March 2024;
Published: 24 April 2024.
Edited by:
Carmen Serna-Candel, Hospital General Universitario de Alicante, SpainReviewed by:
Patricia Simal, Hospital Clinico Universitario San Carlos, SpainCopyright © 2024 Gong, Yu, Lu, Wang, Cai, Cheng and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuanqing Yu, eXVjaHVhbnFpbjE5NjdAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.