
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 01 February 2024
Sec. Neurological Biomarkers
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1322971
 Mohammed Zawiah1
Mohammed Zawiah1 Amer Hayat Khan2
Amer Hayat Khan2 Rana Abu Farha3
Rana Abu Farha3 Abubakar Usman4
Abubakar Usman4 Fahmi Y. Al-Ashwal5
Fahmi Y. Al-Ashwal5 Mohammed Ahmed Akkaif6*
Mohammed Ahmed Akkaif6*Background: Acute ischemic stroke (AIS) remains a substantial global health challenge, contributing to increased morbidity, disability, and mortality. This study aimed at investigating the predictive value of the neutrophil percentage to albumin ratio (NPAR) in determining intensive care unit (ICU) admission among AIS patients.
Methods: A retrospective observational study was conducted, involving AIS cases admitted to a tertiary hospital in Jordan between 2015 and 2020. Lab data were collected upon admission, and the primary outcome was ICU admission during hospitalization. Descriptive and inferential analyses were performed using SPSS version 29.
Results: In this study involving 364 AIS patients, a subset of 77 (21.2%) required admission to the ICU during their hospital stay, most frequently within the first week of admission. Univariable analysis revealed significantly higher NPAR levels in ICU-admitted ischemic stroke patients compared to those who were not admitted (23.3 vs. 15.7, p < 0.001), and multivariable regression models confirmed that higher NPAR (≥19.107) independently predicted ICU admission in ischemic stroke patients (adjusted odds ratio [aOR] = 4.85, 95% CI: 1.83–12.83). Additionally, lower GCS scores and higher neutrophil-to-lymphocyte ratio (NLR) were also associated with increased likelihood of ICU admission. In terms of predictive performance, NPAR showed the highest accuracy with an AUC of 0.885, sensitivity of 0.805, and specificity of 0.854, using a cutoff value of 19.107. NPAR exhibits an AUC of 0.058, significantly outperforming NLR (Z = 2.782, p = 0.005).
Conclusion: NPAR emerged as a robust independent predictor of ICU admission in ischemic stroke patients, surpassing the predictive performance of the NLR.
Acute ischemic stroke (AIS) remains a substantial global health challenge, contributing to increased morbidity (1, 2), disability (3), and mortality (4, 5). While many cases of AIS can be efficiently treated in regular hospital environments or specialized stroke units, it is worth noting that approximately quarter of AIS patients require admission to an intensive care unit (ICU) (3, 6). These ICU admissions are crucial due to the severity of the condition. Importantly, the reported mortality rates for stroke patients receiving treatment in the ICU exhibit a wide range, varying from 14% to as high as 70% (1, 3–5), underscoring the need for comprehensive and targeted care strategies for this patient population.
Predicting ICU admission in acute ischemic stroke (AIS) patients is crucial for optimizing care and resource allocation. While the National Institutes of Health Stroke Scale (NIHSS) is commonly used for severity assessment, it has limitations. Solely relying on NIHSS for ICU admission may offer no cost or outcome benefits for mild to moderate stroke cases (7). Furthermore, its reliance on detailed neurological assessments, often unavailable in-patient records, could limit its applicability (8) and may not capture specific stroke symptoms’ impact (8). Modifications, especially for posterior circulation stroke, where NIHSS is less effective, may be necessary (9). Additionally, low NIHSS scores in acute ischemic stroke with symptomatic arterial occlusion may not signify mild stroke during the acute phase (10).
Inflammation is part of the initial brain damage after a stroke and contributes to tissue repair in the stroke’s later phases (11). Measuring peripheral leukocytes, particularly neutrophils, provided an affordable and accessible means to evaluate the potential presence of inflammation (12). In the initial phases following a stroke, neutrophils migrate to the brain area affected by the infarction and undergo activation. This activation prompts the release of various substances like reactive oxygen and nitrogen species, chemokines, and neutrophil extracellular traps. These substances have the potential to induce additional inflammation, attracting and activating other immune cells in the process (13). Albumin, a protein of moderate size, has a molecular weight ranging from 66 to 69 kilodaltons, constituting over half of the total composition of proteins within the serum (14). Albumin serves various roles such as osmoregulation, antioxidative action, and anti-inflammatory properties (15, 16).
Recent research has emphasized the utility of objective, cost-effective, and readily available laboratory-based tests like the Neutrophil-to-Lymphocyte Ratio (NLR), recognizing its potential as valuable tool in predicting severity and prognostic outcomes for AIS patients (17, 18). Furthermore, the Neutrophil Percentage-to-Albumin Ratio (NPAR) has recently gained attention for its significant role in predicting post stroke complications and mortality of stroke patients (19, 20). However, despite its recognized importance in stroke prognosis, there has been a notable gap in research regarding its potential utility in predicting the admission of ischemic stroke patients to the ICU. This study aimed to address this gap by investigating the predictive value of the NPAR specifically in determining ICU admission among ischemic stroke patients.
This retrospective cohort study focused on patients with acute ischemic stroke admitted to Jordan University Hospital in Amman, Jordan, spanning from beginning of 2015, to the end of 2020. To be eligible for inclusion, patients needed to be adults aged 18 years and older, possess a complete medical record, and have a confirmed physician diagnosis of acute ischemic stroke based on neurologic examination and radiologic confirmation that aligned with the criteria specified by the World Health Organization (21, 22). Exclusions from the study were made for patients with advanced cancer or haematological diseases, individuals with a history of corticosteroid or immunosuppressant use within the 3 months prior to the stroke, patients with chronic liver diseases, and those who were transferred to another hospital.
In adherence to ethical principles, this study was conducted in accordance with the Helsinki Declaration. The research protocol received approval from the JUH Institutional Review Board under reference number 10-2021-4345. Patient consent requirements were waived because of the retrospective nature of the study, as authorized by the ethical committee approval.
During the study period, a thorough review of electronic medical records was conducted to identify eligible participants. From the eligible records, demographic information such as age, sex, and the duration of hospitalization were firstly reported. Relevant pre-existing risk factors such as current smoking and comorbidities like ischemic heart disease, chronic heart failure, hypertension, diabetes, previous stroke, and atrial fibrillation were meticulously documented. Vital signs and clinical data, including systolic and diastolic blood pressure, respiratory rate, heart rate, oxygen saturation, body temperature, and the Glasgow Coma Scale (GCS), were also recorded. Regarding Laboratory Parameters, within the initial 24 h of admission or emergency visit, a comprehensive set of laboratory data was collected. This included complete blood counts, encompassing neutrophils, lymphocytes, monocytes, eosinophils, basophils, hematocrit (Hct), hemoglobin (Hb), red blood cell (RBC) counts, white blood cell (WBC) counts, and platelet counts. Additionally, levels of albumin, serum creatinine, and blood urea nitrogen were measured. Using these laboratory results, several ratios were computed such as the neutrophil-to-lymphocyte ratio (NLR), and the neutrophil percentage to albumin ratio (NPAR). The NLR is calculated by dividing the absolute neutrophil count by the absolute lymphocyte count in a given blood sample, and the NPAR is derived by dividing total neutrophil count divided by total serum albumin measured in grams per decilitre. The included patients were classified based on the occurrence of the primary outcome of interest, which was admission to the ICU. This was determined by any documented admission to the ICU during the hospitalization.
The statistical analysis was conducted using SPSS version 29. Categorical variables were presented as frequencies and percentages, while continuous variables were expressed as medians with interquartile ranges due to their non-normal distribution. Univariable analyses were conducted with two different groupings: one based on ICU admission and the other based on high and low NPAR levels. Categorical variable associations were investigated using the Pearson Chi-square test, and differences in continuous variables between the two groups were assessed using the Mann–Whitney U test. Variables that showed statistical significance in the univariable analyses (p < 0.05) were subsequently included in a multivariable logistic regression model, using a backward stepwise method, to identify independent predictors of ICU admission in acute ischemic stroke patients. Three distinct multivariable models were developed. The first two utilized logistic regression: one treated NPAR as a continuous variable, while the other categorized NPAR based on a cutoff point of 19.07, determined by maximizing the Youden index. The third model employed categorized NPAR in a time-to-event analysis using Cox regression. To assess the model’s performance and NPAR’s predictive ability, discrimination measures were calculated, including the C-statistics (AUC ROC) with a 95% confidence interval (CI). Additionally, NPAR’s predictive power was compared with other ratios, such as NLR, using the DeLong test to evaluate AUC differences. Optimal cutoff values for these ratios were determined by maximizing Youden’s index (sensitivity + specificity − 1). Model calibration was assessed using the Hosmer-Lemeshow test, where a non-significant p-value (≥0.05) and a smaller chi-square value indicated good model calibration.
Throughout the study period, 364 ischemic stroke patients were included (Figure 1). The baseline sociodemographic and clinical characteristics of the recruited patients illustrated in the Table 1. Overall, the median age was 71 years, with an interquartile range (IQR) spanning from 61 to 77 years. Of the patients, 41.8% were male, and 21.4% of them being smokers. Out of the total patients, 77 (21.2%) required admission to the ICU during their hospital stay, with a median ICU admission time of 3 days. It is noteworthy that the vast majority of ICU admissions (90.7%) occurred within the first week after admission.
Significant differences between ICU-admitted and non-ICU-admitted ischemic stroke patients include age (ICU: 75 years, non-ICU: 70 years, p = 0.001), the prevalence of atrial fibrillation (ICU: 22.1%, non-ICU: 8.4%, p < 0.001), congestive heart failure (ICU: 15.6%, non-ICU: 6.6%, p = 0.012), lower oxygen saturation in ICU patients (ICU: 95%, non-ICU: 96%, p < 0.001), and lower GCS in ICU patients (median GCS score: ICU: 13, non-ICU: 15, p < 0.001). ICU patients had significantly higher levels of RBS, WBC count, Neutrophil percentage, and Basophil percentage but lower levels of Lymphocyte percentage, Hemoglobin, Hematocrit, and Albumin. Moreover, Scr levels, BUN levels, and hospitalization duration were notably higher in ICU patients.
Patients with high NPAR levels (≥19.107), compared to those with low NPAR, were older (median age 75 vs. 68 years), had lower GCS scores (median GCS 14 vs. 15), and a higher prevalence of comorbidities such as atrial fibrillation (19.2% vs. 8.0%) and congestive heart failure (14.4% vs. 6.1%). High NPAR patients also displayed elevated neutrophil percentages (median 74.8% vs. 65.8%) and reduced lymphocyte percentages (median 12.4% vs. 27.5%). They had lower albumin levels (median 3.5 vs. 4.2 g/dL) and higher serum creatinine levels (median 0.97 vs. 0.78 mg/dL). High NPAR patients had longer hospitalizations (median 9 vs. 3 days), signifying a greater need for extended medical care (Table 2).
Initially, univariable logistic analysis revealed significantly higher NPAR levels in ICU-admitted ischemic stroke patients compared to those who were not admitted (23.3 vs. 15.7, p < 0.001) (Figure 2). Subsequently, after accounting for potential confounding factors (identified with p values <0.05 in the univariable analysis), a multivariable regression model (Model 1, Table 3) demonstrated that NPAR remained independently associated with an increased likelihood of ICU admission (B = 0.217, p < 0.001). Moreover, in an additional multivariable regression model (Model 2, Table 3), which incorporated NPAR categorized based on a specific cutoff point (≥19.107), it was confirmed that higher NPAR independently predicted ICU admission in ischemic stroke patients (adjusted odds ratio [aOR] = 4.85, 95% CI: 1.83–12.83) while adjusting for the same confounding variables. Cox regression analysis designated as “model 3,” found that the higher NPAR level (≥19.107) increase the likelihood of admission of ischemic stroke patients to the ICU (adjusted hazard ratio [HR] = 5.06, 95% CI, 2.45–10.44: p value, < 0.001). In addition to the NPAR, higher NLR (B = 0.320, p < 0.001) found to be increased likelihood of ICU admission in both univariable (Figure 3) and multivariable analyses. Furthermore, lower GCS scores (B = −0.525, p < 0.001) was also found to be independently predict the admission to ICU. The Hosmer and Lemeshow test indicated good calibration for both models (Model 1: χ2 = 10.297, p = 0.245; Model 2: χ2 = 6.649, p = 0.575).
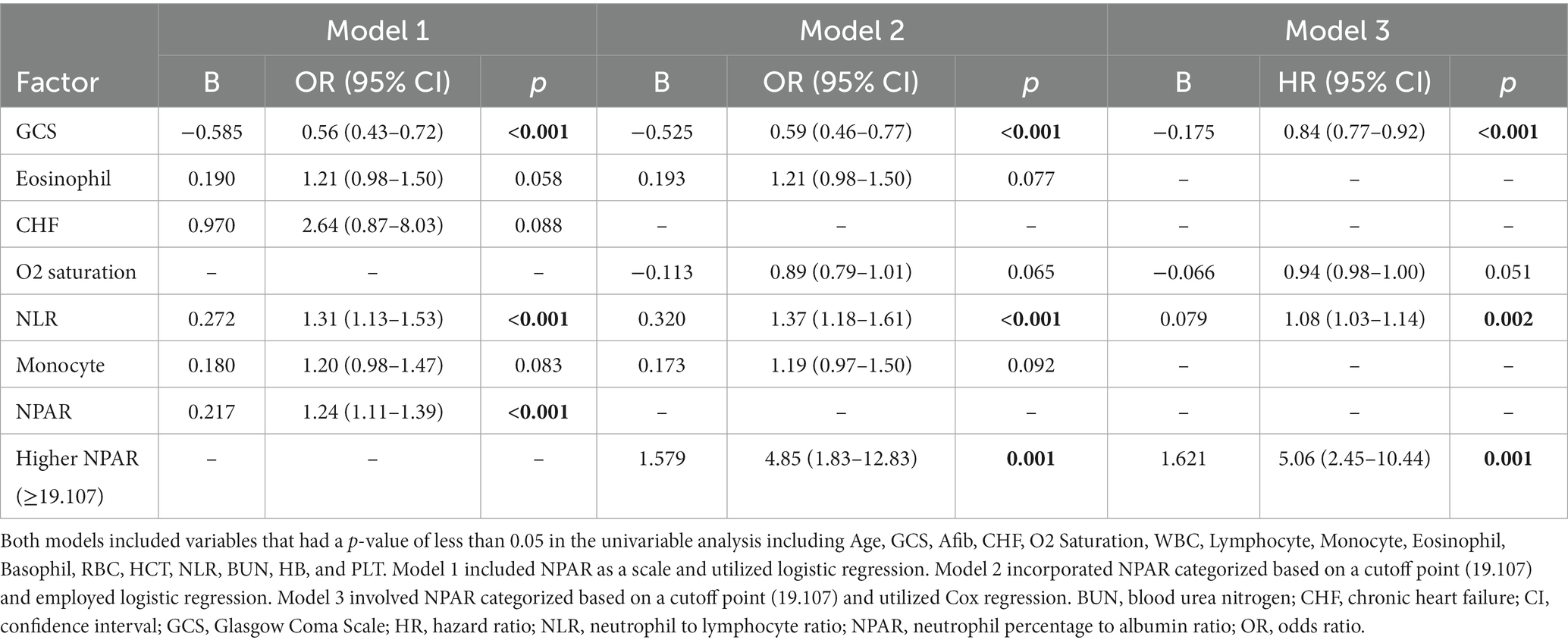
Table 3. Multivariable regression analyses of independent predictors for ICU admission in ischemic stroke patients.
Table 4 and Figures 4, 5 present the predictive performance of various blood-based ratios in ICU admission of ischemic stroke patients. NPAR exhibited the highest performance, with an AUC of 0.885, sensitivity of 0.805, and specificity of 0.854, using a cut-off value of 19.107. NLR also performed well, with an AUC of 0.827, sensitivity of 0.675, and specificity of 0.854 at a cut-off value of 4.524. The DeLong test results (Table 5) demonstrate significant differences in the predictive values between these ratios. NPAR exhibits an AUC of 0.058, significantly outperforming NLR (Z = 2.782, p = 0.005).
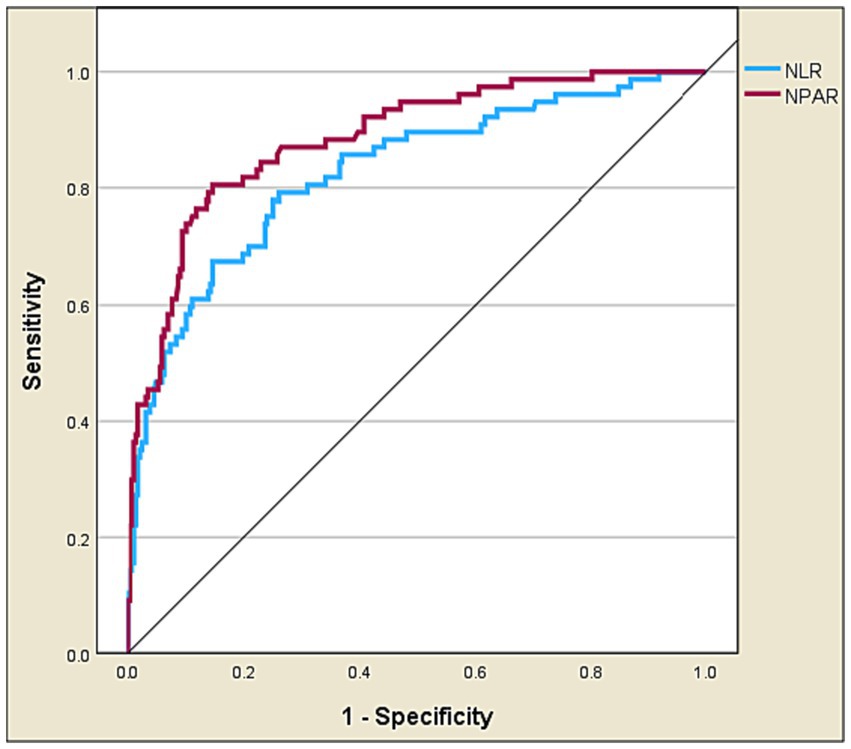
Figure 4. Receiver operating characteristic curve analysis of NPAR vs. NLR in predicting ICU admission of ischemic stroke patients.
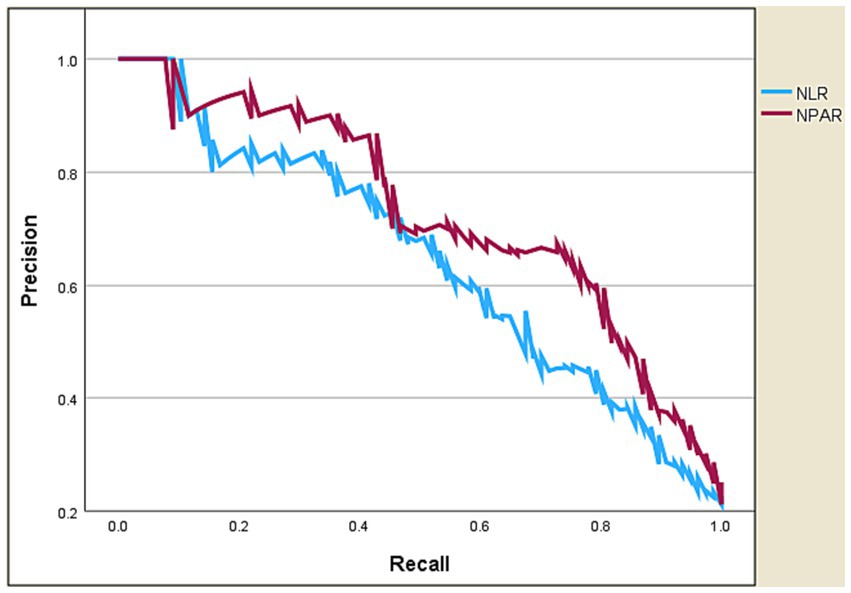
Figure 5. Precision-recall curve of NPAR vs. NLR in predicting ICU admission of ischemic stroke patients.
This is the first study to assess the predictive value of NPAR in determining the need for ICU admission in AIS patients. Our findings revealed important insights. Firstly, NPAR was established as a robust and independent predictor of ICU admission. Secondly, when comparing NPAR and NLR, it became evident that NPAR exhibited significantly higher predictive performance for ICU admission than NLR.
NPAR has been investigated in prior studies and has consistently demonstrated clinical relevance. This study mirrors recent investigations that have shown NPAR to be associated with early neurological deterioration and reflective of the severity of acute ischemic stroke (23, 24). These findings indicate that NPAR could serve as an indicator of the rapidity of neurological decline in stroke patients, which, in turn, could contribute to the need for ICU care. Furthermore, our observation of NPAR’s significance aligns with research linking it to infection incidence, particularly stroke-associated pneumonia which commonly occur in the first week of stroke onset (25, 26). This association suggests that elevated NPAR levels may reflect an underlying inflammatory response triggered by infections, making it a valuable marker for identifying patients at a higher risk of complications, such as those necessitating ICU admission. In essence, the current study provides further validation of the clinical utility of NPAR by demonstrating its superiority, even when compared to the previously studied NLR, in predicting ICU admission. It underscores the comprehensive nature of NPAR as a predictive marker, encompassing crucial aspects of stroke prognosis, and thereby substantiates its value in risk stratification and clinical decision-making for ischemic stroke patients.
The study findings may be underpinned by several mechanistic considerations. Previous studies have shed light on the potential mechanisms that underscore the clinical associations we observed. Firstly, albumin, a major component of the NPAR calculation, is known to exert neuroprotective functions through various pathways (27, 28). It possesses anti-inflammatory properties, which can mitigate the inflammatory response within the brain following ischemic events. Additionally, albumin’s antioxidant characteristics help counteract oxidative stress, a pivotal factor in stroke pathophysiology and severity (14, 29–31). Furthermore, albumin has been shown to inhibit endothelial apoptosis and regulate microvascular permeability, contributing to the maintenance of cerebrovascular integrity (27, 32). Neutrophils, in response to cerebral ischemia, are rapidly recruited to the ischemic site, while microglia become activated (33). Neutrophils release a multitude of substances, including reactive oxygen species, inflammatory mediators, chemokines, cytokines, adhesion molecules, and proteases. This collective release of factors plays a pivotal role in the disruption of the blood–brain barrier (BBB), ultimately exacerbating ischemic damage and contributing to brain edema (34, 35). Furthermore, it is noteworthy that neutrophils are recognized as a significant source of matrix metalloproteinase-9, an enzyme that has been closely associated with BBB breakdown and the occurrence of hemorrhagic transformation (36, 37). This intricate interplay of neutrophil-driven inflammation and its impact on BBB integrity underscores the potential mechanisms that contribute to adverse outcomes in cases of cerebral ischemia.
We could not adjust for the NIHSS in this study due to its unavailability. This limitation has been noted in previous research as well (38, 39). To address this, we used the GCS as alternative way to estimate stroke severity. Moreover, it was carried out at a single center, which may introduce selection bias due to the relatively small sample size. Future research should consider conducting multicentre, prospectively designed studies to address these limitations. Despite these limitations, our study is the first to investigate a new biomarker called NPAR, which could be valuable for predicting ICU admission in ischemic stroke patients.
The findings of our study demonstrated that NPAR is a robust independent predictor of ICU admission in ischemic stroke patients, surpassing the predictive performance of NLR. NPAR’s enhanced predictive power stems from incorporating albumin levels, providing a more comprehensive assessment of inflammatory status and overall health and nutrition. This nuanced approach could improve accuracy in predicting ICU admission, indicating the potential clinical relevance of NPAR over traditional NLR. However, further validation in larger cohorts is necessary to confirm its broader applicability.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by JUH Institutional Review Board under reference number 10-2021-4345. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
MZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Software, Writing – original draft. AK: Investigation, Methodology, Project administration, Validation, Visualization, Writing – review & editing. RF: Data curation, Methodology, Project administration, Software, Validation, Visualization, Writing – review & editing. AU: Data curation, Project administration, Resources, Validation, Writing – review & editing. FA-A: Conceptualization, Data curation, Investigation, Software, Visualization, Writing – review & editing. MA: Project administration, Supervision, Validation, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Deanship of Scientific Research at Northern Border University, grant number NBU-IRC-2024-3503-01.
The authors extend their appreciation to the Deanship of Scientific Research at Northern Border University, Arar, KSA for funding this research work through the project number NBU-IRC-2024-3503-01.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sonneville, R, Gimenez, L, Labreuche, J, Smonig, R, Magalhaes, E, Bouadma, L, et al. What is the prognosis of acute stroke patients requiring ICU admission? Intensive Care Med. (2017) 43:271–2. doi: 10.1007/s00134-016-4553-7
2. Zawiah, M, Hayat Khan, A, Abu Farha, R, Usman, A, Sha’aban, A, Abu Hammour, K, et al. Diagnosis and treatment of stroke associated pneumonia: qualitative exploration of clinicians’ practice. Electron J Gen Med. (2023) 20. doi: 10.29333/ejgm/12849
3. Santos, D, Maillie, L, and Dhamoon, MS. Patterns and outcomes of intensive care on acute ischemic stroke patients in the US. Circ Cardiovasc Qual Outcomes. (2023) 16:e008961. doi: 10.1161/CIRCOUTCOMES.122.008961
4. Golestanian, E, Liou, J-I, and Smith, MA. Long-term survival in older critically ill patients with acute ischemic stroke. Crit Care Med. (2009) 37:3107–13. doi: 10.1097/CCM.0b013e3181b079b2
5. Kortelainen, S, Curtze, S, Martinez-Majander, N, Raj, R, and Skrifvars, MB. Acute ischemic stroke in a university hospital intensive care unit: 1-year costs and outcome. Acta Anaesthesiol Scand. (2022) 66:516–25. doi: 10.1111/aas.14037
6. Coplin, WM. Critical care management of acute ischemic stroke. Continuum (Minneap Minn). (2012) 18:547–59. doi: 10.1212/01.CON.0000415427.53653.1b
7. Briggs, DE, Felberg, RA, Malkoff, MD, Bratina, P, and Grotta, JC. Should mild or moderate stroke patients be admitted to an intensive care unit? Stroke. (2001) 32:871–6. doi: 10.1161/01.STR.32.4.871
8. Bushnell, CD, Johnston, DCC, and Goldstein, LB. Retrospective assessment of initial stroke severity: comparison of the NIH stroke scale and the Canadian neurological scale. Stroke. (2001) 32:656–60. doi: 10.1161/01.STR.32.3.656
9. Siniscalchi, A, Sztajzel, R, Malferrari, G, and Gallelli, L. The National Institutes of Health stroke scale: its role in patients with posterior circulation stroke. Hosp Top. (2017) 95:79–81. doi: 10.1080/00185868.2017.1322888
10. Kim, J-T, Park, M-S, Chang, J, Lee, JS, Choi, K-H, and Cho, K-H. Proximal arterial occlusion in acute ischemic stroke with low NIHSS scores should not be considered as mild stroke. PLoS One. (2013) 8:e70996. doi: 10.1371/journal.pone.0070996
11. Danton, GH, and Dietrich, WD. Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol. (2003) 62:127–36. doi: 10.1093/jnen/62.2.127
12. Mortaz, E, Alipoor, SD, Adcock, IM, Mumby, S, and Koenderman, L. Update on neutrophil function in severe inflammation. Front Immunol. (2018) 9:2171. doi: 10.3389/fimmu.2018.02171
13. Strecker, J-K, Schmidt, A, Schäbitz, W-R, and Minnerup, J. Neutrophil granulocytes in cerebral ischemia–evolution from killers to key players. Neurochem Int. (2017) 107:117–26. doi: 10.1016/j.neuint.2016.11.006
14. Arques, S. Human serum albumin in cardiovascular diseases. Eur J Intern Med. (2018) 52:8–12. doi: 10.1016/j.ejim.2018.04.014
15. Roche, M, Rondeau, P, Singh, NR, Tarnus, E, and Bourdon, E. The antioxidant properties of serum albumin. FEBS Lett. (2008) 582:1783–7. doi: 10.1016/j.febslet.2008.04.057
16. Don, BR, and Kaysen, G. Poor nutritional status and inflammation: serum albumin: relationship to inflammation and nutrition. Semin Dial. (2004) 17:432–7. doi: 10.1111/j.0894-0959.2004.17603.x
17. Fang, Y-N, Tong, MS, Sung, PH, Chen, YL, Chen, CH, Tsai, NW, et al. Higher neutrophil counts and neutrophil-to-lymphocyte ratio predict prognostic outcomes in patients after non-atrial fibrillation-caused ischemic stroke. Biom J. (2017) 40:154–62. doi: 10.1016/j.bj.2017.03.002
18. Zawiah, M, Hayat Khan, A, Abu Farha, R, Usman, A, and Bitar, AN. Neutrophil-lymphocyte ratio, monocyte-lymphocyte ratio, and platelet-lymphocyte ratio in stroke-associated pneumonia: a systematic review and meta-analysis. Curr Med Res Opin. (2023) 39:475–82. doi: 10.1080/03007995.2023.2174327
19. Chen, Z, Xie, D, Li, Y, Dai, Z, Xiang, S, Chen, Z, et al. Neutrophil albumin ratio is associated with all-cause mortality in stroke patients: a retrospective database study. Int J Gen Med. (2022) 15:1–9. doi: 10.2147/IJGM.S323114
20. Zawiah, M, Khan, AH, Abu Farha, R, Usman, A, AbuHammour, K, Abdeen, M, et al. Predictors of stroke-associated pneumonia and the predictive value of neutrophil percentage-to-albumin ratio. Postgrad Med. (2023) 135:681–9. doi: 10.1080/00325481.2023.2261354
21. Bahou, Y, Hamid, H, and Raqab, MZ. Ischemic stroke in Jordan 2000 to 2002: a two-year, hospital-based study. J Stroke Cerebrovasc Dis. (2004) 13:81–4. doi: 10.1016/j.jstrokecerebrovasdis.2004.02.004
22. WHO Task Force. Stroke-1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO task Force on stroke and other cerebrovascular disorders. Stroke. (1989) 20:1407–31. doi: 10.1161/01.str.20.10.1407
23. Cui, T, Wang, C, Zhu, Q, Li, S, Yang, Y, Wang, A, et al. Association between neutrophil percentage-to-albumin ratio and 3-month functional outcome in acute ischemic stroke patients with reperfusion therapy. Front Neurol. (2022) 13:898226. doi: 10.3389/fneur.2022.898226
24. Mao, S, Hu, Y, Zheng, X, Yang, C, Yang, M, Li, X, et al. Correlation analysis of neutrophil/albumin ratio and leukocyte count/albumin ratio with ischemic stroke severity. Cardiol Cardiovasc Med. (2023) 7:32. doi: 10.26502/fccm.92920305
25. Zhang, H, Wu, T, Tian, X, Lyu, P, Wang, J, and Cao, Y. High neutrophil percentage-to-albumin ratio can predict occurrence of stroke-associated infection. Front Neurol. (2021) 12:705790. doi: 10.3389/fneur.2021.705790
26. Lv, X-N, Shen, YQ, Li, ZQ, Deng, L, Wang, ZJ, Cheng, J, et al. Neutrophil percentage to albumin ratio is associated with stroke-associated pneumonia and poor outcome in patients with spontaneous intracerebral hemorrhage. Front Immunol. (2023) 14:1173718. doi: 10.3389/fimmu.2023.1173718
27. Belayev, L, Liu, Y, Zhao, W, Busto, R, and Ginsberg, MD. Human albumin therapy of acute ischemic stroke: marked neuroprotective efficacy at moderate doses and with a broad therapeutic window. Stroke. (2001) 32:553–60. doi: 10.1161/01.STR.32.2.553
28. Idicula, TT, Waje-Andreassen, U, Brogger, J, Naess, H, and Thomassen, L. Serum albumin in ischemic stroke patients: the higher the better. The Bergen stroke study. Cerebrovasc Dis. (2009) 28:13–7. doi: 10.1159/000215938
29. Jena, I, Nayak, SR, Behera, S, Singh, B, Ray, S, Jena, D, et al. Evaluation of ischemia-modified albumin, oxidative stress, and antioxidant status in acute ischemic stroke patients. J Nat Sci Biol Med. (2017) 8:110–3. doi: 10.4103/0976-9668.198346
30. Sugawara, T, Yu, F, Ma, L, Hsia, CJC, and Chan, PH. Delayed treatment with polynitroxyl albumin reduces infarct size after stroke in rats. Neuroreport. (2001) 12:3609–12. doi: 10.1097/00001756-200111160-00047
31. Xie, Y, Guo, H, Wang, L, Xu, L, Zhang, X, Yu, L, et al. Human albumin attenuates excessive innate immunity via inhibition of microglial Mincle/Syk signaling in subarachnoid hemorrhage. Brain Behav Immun. (2017) 60:346–60. doi: 10.1016/j.bbi.2016.11.004
32. Belayev, L, Pinard, E, Nallet, H, Seylaz, J, Liu, Y, Riyamongkol, P, et al. Albumin therapy of transient focal cerebral ischemia: in vivo analysis of dynamic microvascular responses. Stroke. (2002) 33:1077–84. doi: 10.1161/hs0402.105555
33. Kim, JY, Park, J, Chang, JY, Kim, S-H, and Lee, JE. Inflammation after ischemic stroke: the role of leukocytes and glial cells. Exp Neurobiol. (2016) 25:241–51. doi: 10.5607/en.2016.25.5.241
34. Jickling, GC, Liu, D, Ander, BP, Stamova, B, Zhan, X, and Sharp, FR. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J Cereb Blood Flow Metab. (2015) 35:888–901. doi: 10.1038/jcbfm.2015.45
35. Jin, R, Liu, L, Zhang, S, Nanda, A, and Li, G. Role of inflammation and its mediators in acute ischemic stroke. J Cardiovasc Transl Res. (2013) 6:834–51. doi: 10.1007/s12265-013-9508-6
36. Jickling, GC, Liu, DZ, Stamova, B, Ander, BP, Zhan, X, Lu, A, et al. Hemorrhagic transformation after ischemic stroke in animals and humans. J Cereb Blood Flow Metab. (2014) 34:185–99. doi: 10.1038/jcbfm.2013.203
37. Inzitari, D, Giusti, B, Nencini, P, Gori, AM, Nesi, M, Palumbo, V, et al. MMP9 variation after thrombolysis is associated with hemorrhagic transformation of lesion and death. Stroke. (2013) 44:2901–3. doi: 10.1161/STROKEAHA.113.002274
38. Xu, Y, Qiao, H, Yang, S, Zhou, L, Zhao, Y, Xu, Q, et al. 15-Hydroxyprostaglandin dehydrogenase is a predictor of stroke-associated pneumonia. Front Neurol. (2022) 13:893624. doi: 10.3389/fneur.2022.893624
Keywords: acute ischemic stroke, neutrophil percentage to albumin ratio, intensive care unit, neutrophil-to-lymphocyte ratio, predictive value
Citation: Zawiah M, Khan AH, Farha RA, Usman A, Al-Ashwal FY and Akkaif MA (2024) Assessing the predictive value of neutrophil percentage to albumin ratio for ICU admission in ischemic stroke patients. Front. Neurol. 15:1322971. doi: 10.3389/fneur.2024.1322971
Received: 27 October 2023; Accepted: 09 January 2024;
Published: 01 February 2024.
Edited by:
Mohammad Mofatteh, Queen's University Belfast, United KingdomReviewed by:
Sasa R. Vasilijic, Stanford University, United StatesCopyright © 2024 Zawiah, Khan, Farha, Usman, Al-Ashwal and Akkaif. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammed Ahmed Akkaif, YWtrYWlmQGZ1ZGFuLmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.