- 1Department of Kinesiology, Indiana University School of Public Health-Bloomington, Bloomington, IN, United States
- 2Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, United States
- 3Program in Neuroscience, The College of Arts and Sciences, Indiana University, Bloomington, IN, United States
- 4Division of Emergency Medicine, Boston Children's Hospital, Boston, MA, United States
- 5Department of Pediatrics, Harvard Medical School, Boston, MA, United States
- 6Department of Emergency Medicine, University of Rochester School of Medicine and Dentistry, Rochester, NY, United States
Traumatic brain injury (TBI), in any form and severity, can pose risks for developing chronic symptoms that can profoundly hinder patients’ work/academic, social, and personal lives. In the past 3 decades, a multitude of pharmacological, stimulation, and exercise-based interventions have been proposed to ameliorate symptoms, memory impairment, mental fatigue, and/or sleep disturbances. However, most research is preliminary, thus limited influence on clinical practice. This review aims to systematically appraise the evidence derived from randomized controlled trials (RCT) regarding the effectiveness of pharmacological, stimulation, and exercise-based interventions in treating chronic symptoms due to TBI. Our search results indicate that despite the largest volume of literature, pharmacological interventions, especially using neurostimulant medications to treat physical, cognitive, and mental fatigue, as well as daytime sleepiness, have yielded inconsistent results, such that some studies found improvements in fatigue (e.g., Modafinil, Armodafinil) while others failed to yield the improvements after the intervention. Conversely, brain stimulation techniques (e.g., transcranial magnetic stimulation, blue light therapy) and exercise interventions were effective in ameliorating mental health symptoms and cognition. However, given that most RCTs are equipped with small sample sizes, more high-quality, larger-scale RCTs is needed.
1 Introduction
Despite nearly a century of dedicated research, traumatic brain injury (TBI) remains a significant public health concern, contributing to both morbidity and mortality on a global scale (1, 2). It is evident that the sequelae of TBI are long-lasting, with symptoms persisting for months to years in a majority of patients with moderate to severe TBI (2). Up to 30% of mild TBI (mTBI)/concussion patients continue to experience symptoms beyond the first month post-injury (3, 4). The timely and accurate diagnosis of TBI is crucial for a prompt recovery, but the impact of appropriate treatment is arguably even more beneficial. Several consensus-based guidelines have been published to guide acute triage and management of TBI (5, 6), but there is a notable absence of universally accepted treatments for the chronic sequelae of TBI, including concussions.
The quality of life remains a vital attribute of individuals grappling with TBI (7). Beyond the evident physical and cognitive disabilities, TBI patients often suffer from psychiatric disorders, encompassing affective, anxiety, post-traumatic stress disorders (PTSD), and sleep disturbances (8, 9). These psychiatric burdens of TBI are largely invisible, yet studies have begun unraveling potential countermeasures for not only physical and cognitive symptoms but also mental health issues stemming from TBI. However, unfortunately, multidisciplinary neurorehabilitation is often inaccessible to the majority of TBI patients. Over the past 3 decades, a myriad of interventions, including pharmacological, stimulation, and exercise-based approaches, have emerged to ameliorate symptoms, such as mental fatigue, and sleep disturbances. For example, Johanson et al. (10) prescribed a four-week course of the stimulant medication (Methylphenidate) to adults experiencing chronic mental fatigue symptoms after a mild to moderate TBI. The results were promising, as Methylphenidate significantly improved mental fatigue symptoms, such as stress, slowness of thinking, difficulty concentrating, and lack of initiative, in a dose-dependent manner. Additionally, other neurostimulants, such as Modafinil (11), Armodafinil (12), and Monoaminergic (13) have demonstrated efficacy as countermeasures for post-TBI fatigue and persistent sleepiness during the post-acute phase of recovery. In a separate randomized controlled trial (RCT), the administration of doxycycline, an antibiotic medication, during the acute phase in TBI patients resulted in significant reductions in the neural injury blood biomarker (neuron-specific enolase) and improvement in the Glasgow Coma Scale over a one-week treatment period compared to a placebo group (14).
In another scope of research, the use of neural stimulation as a therapeutic strategy for TBI has attracted considerable attention. Among these approaches, transcranial magnetic stimulation (TMS) stands out as one of the most extensively explored techniques, designed to elicit repetitive neural activation on the cortical surface with the goal of restoring neural network functionality. Small-scale RCTs implemented repetitive TMS (rTMS) 5 days a week for 2 weeks in patients with mild to moderate TBI. The results indicated that the rTMS intervention was associated with a reduction in pain score and depression symptoms (15, 16), along with improvements in working memory and processing speed (17, 18). However, divergent outcomes emerge from alternative lines of research where the application of rTMS following mild to moderate TBI showed no discernible positive impact on mental health symptoms and demonstrated negligible effects on cognitive function (19) and reducing headaches (20). These conflicting findings, combined with the exploration of other emerging stimulation approach, such as acupuncture and transcranial direct-current stimulation, underscore the necessity for a systematic evaluation of the current landscape of neurostimulant therapy in TBI outcomes, particularly for patients who are living with lingering physical and psychological symptoms.
A groundbreaking study conducted by Dr. John Leddy and his colleagues (21) has played a pivotal role in revolutionizing concussion management through the implementation of a regulated aerobic exercise protocol (22). The early integration of exercise into the recovery process from TBI is thought to stimulate cerebral blood flow (23), trigger the overexpression of neurotrophic factor (e.g., BDNF) to promote neuronal repair (24, 25), and contribute to the regulation of mental health symptoms (26). In a RCT involving adolescents with concussion, individuals assigned to a 20-min daily exercise protocol exhibited significantly faster recovery and a 48% reduced risk of developing persistent post-concussive symptoms (PPCS) compared to their counterparts following a daily stretch protocol (27). These findings were subsequently validated by a recent RCT conducted among adolescents and young adults with concussions (28). While considerable progress has been made in the past decades, leading to promising interventions for TBI, the majority of systematic evaluations for treatment effectiveness has focused on the acute phases of TBI. This underscores a significant knowledge gap and emphasizes the necessity for a systematic review to explore treatment options specifically tailored for the chronic stage of TBI.
This systematic review aims to determine the effectiveness of pharmacological, stimulation, and exercise interventions in alleviating physical, cognitive, and psychiatric symptoms among adult TBI patients experiencing persistent symptoms lasting at least 1-month post-injury. The outcomes of this review aim to offer empirical evidence for individuals grappling with chronic symptoms stemming from various degrees of TBI.
2 Materials and methods
This systematic review was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (29).
2.1 Data sources and search strategy
A systematic review of the current literature was performed by two independent reviewers (DR and CT) using the electronic databases PubMed, EBSCO, and Web of Science. The search timeframe included studies from 1 January 1990 to 1 September 2020. The following keywords were used in different combinations: traumatic brain injury, TBI, concussion, post-concussion syndrome, persistent post-concussive symptom, post-concussive disorder, treatment, and therapy. For the complete list of combinations, see Table 1. Cited papers within articles meeting the selection criteria were also collected. Searches were limited to RCT, human participants, and English language publications. All records of literature search were examined by title and abstract to exclude irrelevant records. All abstracts that are related to TBI involving treatment or therapeutic interventions were selected for a full reading of the article.
2.2 Inclusion and exclusion criteria
This systematic review included RCTs that evaluate the effectiveness and/or efficacy of treatment or therapeutic interventions for TBI. The scope of this review included pharmaceutical, biomedical, and physical aspects of interventions/treatments, whereas vestibulo-ocular rehabilitation was not included in this study because of the recent abundance in available systematic reviews and meta-analysis (30–32). Also, homeopathic medicine was not included in this review, given the ongoing debate surrounding its efficacy and safety. This review focuses on TBI in adult civilian population (>18 years old) because the neurodevelopmental rate varies between pediatric patients, and this neurodevelopmental effect makes an interpretation of therapeutic effectiveness difficult. Furthermore, military TBI are often complicated with emergence of PTSD and other psychiatric issues not originated from brain trauma; thus, this review focused on civilian population. Additional exclusion criteria were foreign language papers other than English, conference abstracts, studies that are not RCT (e.g., cohort studies, case studies, animal studies), editorials, magazine articles, and papers that did not fall within the three main topics. Review articles were considered separately and incorporated into the discussion for context.
After the systematic filtration, papers were categorized into the three main domains, which yielded four subareas of interventions per category.
1. Pharmacological intervention focuses on pharmaceutical treatments that included hormone therapy, anti-inflammatory treatment, and neurotransmitter modulation treatment.
2. Stimulation-based intervention uses electrical- or magnetic-based stimulation techniques to excite or inhibit neural signaling, including stimulation techniques like cutaneous, transcutaneous, optical, and transcranial stimulation.
3. Exercise-based intervention aims to increase blood circulation and promote endogenous healing processes, and the interventions range from independent and dependent activities, virtual reality, to an alteration of body temperature.
2.3 Operational definitions
The chronic phase was operationally defined as patients exhibiting persisting signs and symptoms of TBI after 30 days of injury. In this review, the Glasgow Coma Scale (GCS) was used to categorize the initial severity of TBI. The GCS is based on motor responsiveness, verbal performance, and eye opening to appropriate stimuli with scores ranging from 3 being the most severe to 15 being unaffected. A mTBI/concussion is defined by a score between 13 to 15, a moderate TBI from 9 to 12, and a severe TBI from 3 to 8, which represents when the individual is unresponsive. While the majority of papers used GCS to classify the severity of TBI during an admission, this review is focused on papers describing chronic, lingering aspects of TBI at least 30 days from the initial injury. Therefore, the initial GCS score may not accurately reflect the status of patients at the time of intervention. While the Glasgow Outcome Scale (GOS) is the desirable measure in the chronic stage, there was a very large variability between papers in terms of the assessments of neurological status. Regardless of this limitation, the goal of this systematic review was achievable.
2.4 Data extraction and quality assessment
Two authors (DR and CT) independently performed the identification, screening, eligibility and inclusion of studies, with disagreement settled by the senior author (KK). The following was recorded: first author, year of publication, study design, age and number of patients, time since injury, GCS score, treatment methodology, main outcome. When included studies refer to previous papers for details of their methods, full texts of these references were screened, and available data was extracted. In addition, data listed in supplementary documents will also be extracted to present a fully comprehensive review of the literature.
2.5 Risk of Bias selection
The risk of bias was assessed using the Cochrane Collaboration’s tool for assessing risk of bias (33). The risk of bias was assessed in the context of selection bias (sequence generation and allocation concealment), performance bias (blinding of participants and research personnel), detection bias (blinding of analysis), and reporting bias. The outcomes of the assessment are listed in Supplementary Table 1.
3 Results
3.1 Search outcome
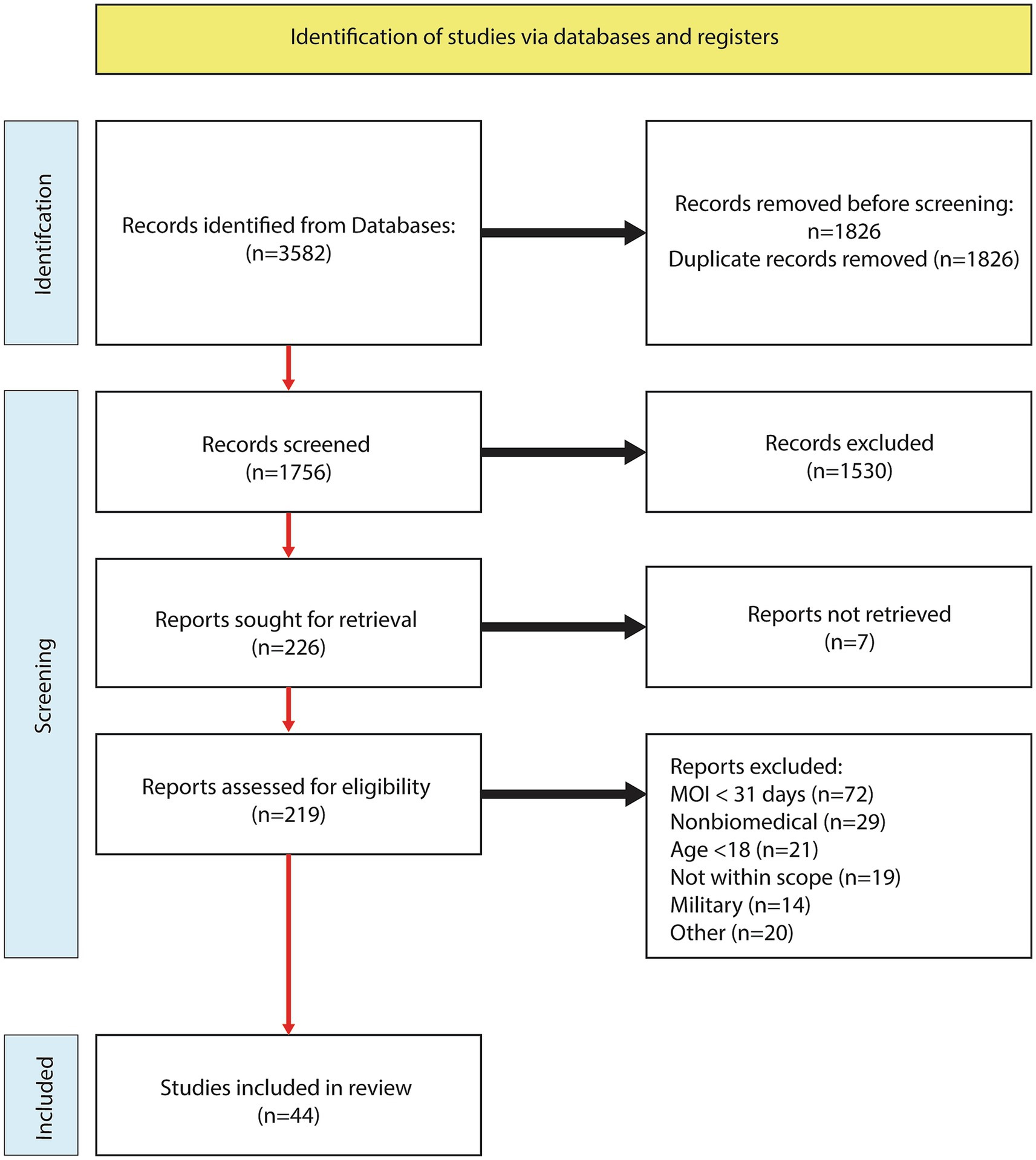
The systematic literature search yielded 1,756 abstracts after filtering for duplicates, and 219 abstracts were assessed and advanced to the full-text review. Following the analysis of 219 full text articles, an additional 175 were excluded. Reasons for exclusions were time-since-injury < 30 days (n = 72), nonbiomedical (n = 29), age < 18 (n = 21), not within scope (n = 19), military (n = 14), and other (n = 19). See the study flow chart for details (Figure 1). A total of 44 articles met the inclusion criteria and further categorized into pharmacological (n = 19: Table 2), stimulation (n = 14: Table 3), and exercise (n = 12: Table 4).
3.2 Pharmacological interventions
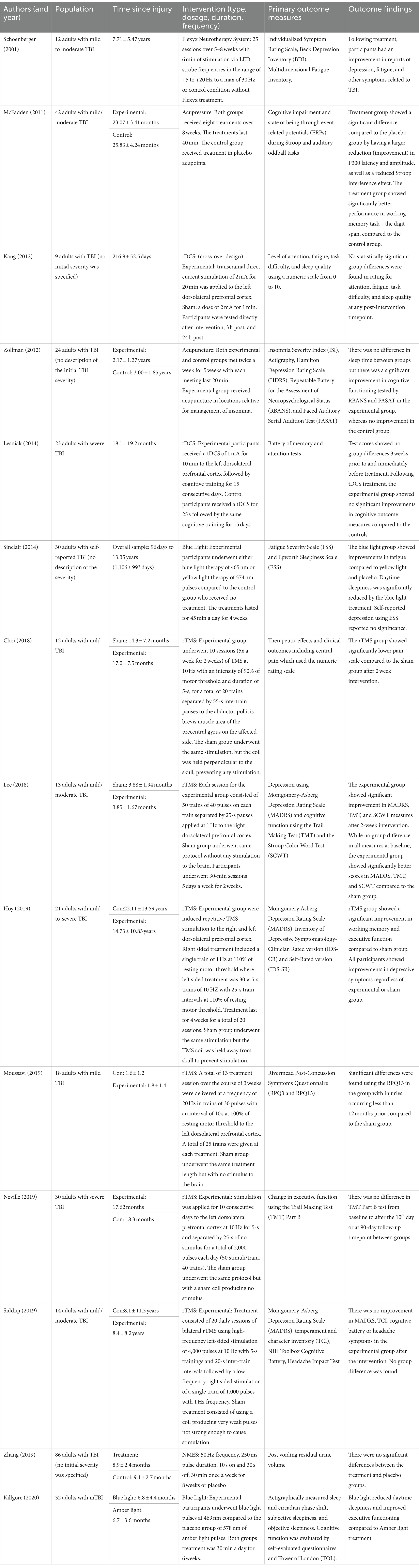
Nineteen studies were examined involving a pharmacological approach (Table 2). Of those, neurotransmitter-modulation treatments (n = 14) were the most abundant intervention, followed by anti-inflammatory/oxidant (n = 2), and hormone therapy (n = 2). The main outcome variable was a cognitive function for 10 papers, including working memory, auditory/visual memory, language, and processing speed, whereas the rest of the papers focused on sleep, physical/mental fatigue, or quality of life. Some of the notable findings are that hormone therapy, such as growth hormone (34) and melatonin (Ramelteon) (35), yielded significant improvement in verbal memory, processing speed, psychomotor functioning, and total sleep duration. Similar improvement in cognitive function was observed in adults with mild to moderate TBI after a 6-month treatment with NeuroAiD II, which has been shown to promote neurite repair and outgrowth (36). Methylphenidate, which is a stimulant medicine typically used to treat attention deficit hyperactivity disorder, has been used in three RCTs (10, 37, 38). Four-week treatment with Methylphenidate was effective in treating mild to moderate TBI patients who suffered from mental fatigue syndrome (10), whereas acute treatment (1 to 2 days) resulted in transient improvements in working memory, attention, and reaction time, yet the beneficial effects disappeared after 2 days (37, 38). Furthermore, fatigue and daytime sleepiness are common lingering symptoms after TBI, and partial improvements in these symptoms were observed in all levels of TBI after several weeks of treatment with Modafinil (neurostimlant) (11), Armodafinil (neurostimlant) (12), or Monoaminergic (neurotransmitter-inducing medicine) (13). Alternatively, doses of Dextroamphetamine (neurostimlant) (39), Atomoxetine (neurostimlant) (40), or Rivastigmine (neurotransmitter-modulating medicine) (41) did not result in improvements in cognitive function or sleep.
3.3 Stimulation-based interventions
The current review yielded 14 articles focusing on stimulation interventions (Table 3), with TMS as the most popular approach (n = 8), followed by transcutaneous and cutaneous stimulations (n = 4) and optical stimulation (n = 2). As with the pharmacological interventions, all studies set outcome measures on cognitive function, sleep, or mental symptoms including fatigue, except for one study examining electrical muscle stimulation effects on urine retention post-TBI. rTMS intervention demonstrated mixed results. Two studies demonstrated improved outcomes after a 2-week treatment of rTMS, with significantly improved depression symptoms, working memory, processing speed (17) as well as decreased headache burden (15) in patients with mild to moderate TBI. Others demonstrated no discernible positive impact on mental health symptoms and demonstrated negligible effects on cognitive function (19) reducing headaches (20). Similarly, transcranial direct-current stimulation (tDCS), despite receiving consecutive treatments for 15 days, did not result in any improvements in cognition, sleep, and fatigue (42, 43).
Optical stimulation therapy, in a form of a 4-to-6 week blue light therapy, has produced significantly reduced daytime sleepiness, fatigue, and depression symptoms compared to controls undergoing yellow or amber light therapy (44, 45). Another type of stimulus, acupressure and acupuncture, for durations of 5 to 8 weeks produced significant improvements in cognitive function, including working memory, comprehension skills, and processing speed, but not sleep duration.
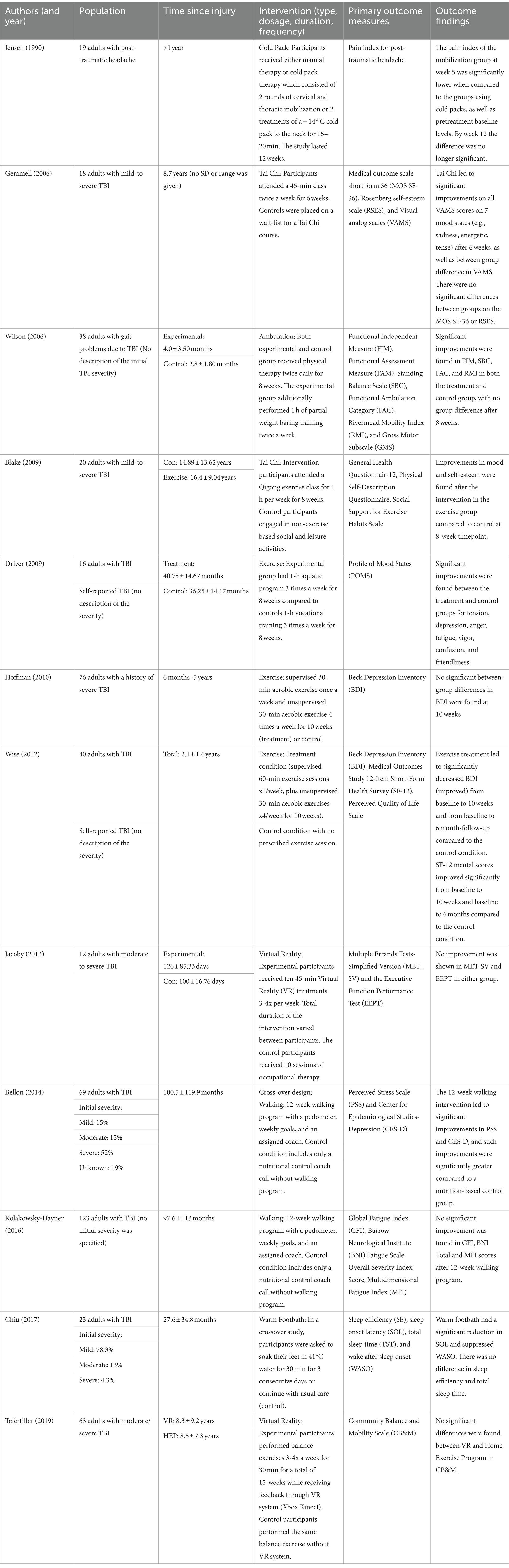
3.4 Exercise interventions
Twelve RCTs using exercise-based therapy investigated the effectiveness of various interventions that aim to promote the healing process by increasing circulation and changes in body temperature (Table 4). There were independent and dependent activity programs (n = 8), virtual reality-based activity (n = 2), and temperature therapy (n = 2). Patients with all severities of TBI and having lingering symptoms for many years benefited from Tai Chi, where 6 to 8 weeks of Tai Chi lessons significantly improved all domains of mood states, such as sadness, energy, happiness, and tension (46, 47). Exercise classes that lasted at least 1 h 3 times a week for 6 to 8 weeks resulted in improvements in symptoms, such as depression, anger, fatigue, vigor, and friendliness in patients with mild to moderate TBI (48, 49). Conversely, a 30-min aerobic exercise for 10 weeks did not alter mental health symptoms in patients with a history of severe TBI. Interventions such as cold-pack therapy, virtual reality, and walking did not improve cognitive function, sleep, or mental health symptoms (50–52).
4 Discussion
Over the past several decades, a vast array of treatments for chronic TBI symptoms have been explored. This systematic review took a novel approach and aimed to evaluate the effectiveness of pharmacological, brain stimulation, and activity-based interventions in the chronic phase (>1-month post) of TBI. There are 4 main findings. First, while many RCTs have used neurostimulant medications to treat physical, cognitive, and mental fatigue, as well as daytime sleepiness, inconsistent results were noted, such that some studies found improvements in fatigue (e.g., Modafinil, Armodafinil) while others failed to realize the improvements after the intervention. Second, in terms of brain stimulation techniques, rTMS showed effectiveness in improving cognitive function and mental health symptoms. Third, blue light therapy induced significant improvements in fatigue and daytime sleepiness in patients with mTBI. Lastly, RCTs outside our scope (e.g., papers published after the search cutoff, 1 September 2020, pediatric TBI) have demonstrated that exercise interventions (e.g., BCTT) may be effective in expediting recovery speed (53–55), especially when an intervention is introduced early on (55). However, RCTs included in this review and using aerobic exercise intervention focused on patients with a history of moderate to severe TBI and indicate that aerobic exercise may not have a strong effect, especially for those who have had a TBI many years ago. Conversely, group exercise like Tai Chi has been shown to improve mental health symptoms.
4.1 Pharmacological interventions
The exploration and characterization of pharmacological interventions have remained a dynamic focus in TBI research since the early 2000s, yielding several medications that effectively enhance cognitive functioning and alleviate fatigue/sleepiness post-TBI. Two articles utilized antioxidative treatments consisting of the administration of MLC901 (NeuroAiD II) (36) and Enzogenol (56). MLC901, rooted in traditional Chinese medicine, exhibited the ability to inhibit cerebral inflammation (57) and promote axonal/dendritic healing and neurogenesis following a TBI (58). Enzogenol is a pine bark extract with potent modulatory factors against neurodegenerative cell signaling (59). Although MLC901 and Enzogenol have not been tested in severe TBI, RCTs in mTBI patients were able to detect a significant improvement in executive function and complex attention skills, suggesting that these antioxidative compounds may contribute to neuronal cellular healing processes at least in the milder spectrum of injury. Another pharmacological intervention with positive effects on cognitive functioning and sleepiness is Ramelteon (35). Ramelteon is an FDA-approved melatonin agonist medication to treat patients with insomnia and has also been used to explore the effects after a TBI. This drug has an affinity to two different G-protein-coupled receptors, MT1 and MT2 in the suprachiasmatic nucleus of the hypothalamus, and regulates the circadian rhythm (60). Following a 3-week nightly dosage of Ramelteon, patients with mild to moderate TBI improved their total sleep time and demonstrated improvements in memory function, psychomotor speed, and cognitive flexibility (35). Of note, in spite of the randomized crossover design, a sample size of 13 patients is underpowered to account for appropriate confounders and potential modulatory factors, warranting a follow-up study with a larger sample size to realize the true effectiveness in post-TBI care.
There were several pharmacological interventions with no promising results, such as Rivastigmine (41) and Atomoxetine (40). Rivastigmine is a cholinesterase inhibitor used to promote mental functioning, including memory and comprehension, and has been used as a treatment for neurodegenerative diseases like Alzheimer’s disease (AD). Silver et al. (41) recruited patients with all severities of TBI in the trial; however, 12 weeks of treatment with Rivastigmine did not improve any aspects of cognitive function compared to a placebo group. This discrepancy between AD and TBI patients is likely due to the level of acetylcholine, such that AD patients often have low acetylcholine levels and benefit from Rivastigmine, whereas acetylcholine levels may not be as depleted after TBI as neurodegenerative conditions. As a result, the RCT by Silver et al. failed to realize the effects of Rivastigmine (41). Atomoxetine is a norepinephrine reuptake inhibitor, often used in patients with attention-deficit hyperactivity disorder (ADHD). This medication is used to increase the levels of norepinephrine in the brain by inhibiting its reuptake into neurons. The abundance of norepinephrine is thought to improve the neural signaling to assist in attention, concentration, and impulse control, which are experienced in some TBI patients. Ripley et al. did not observe Atomoxetine’s positive effects on cognitive function, perhaps due to either the study was conducted in patients who experienced TBI on average 8.2 years ago or not all TBI patients having chief complaint of inattention and impulse control issues.
4.2 Brain stimulation
Brain stimulation techniques have evolved in the past decade, allowing researchers and clinicians to administer magnetic stimulations (rTMS) to elicit neuronal signaling. There is a sound theoretical basis and clinical utility in treating various neurological conditions, including chronic pain and rehabilitation for stroke and movement disorders (61). However, this systematic review revealed unique patterns and effectiveness of rTMS in TBI care. All but one studies using rTMS set the dorsolateral prefrontal cortex (DLPFC) as their target region of interest, and seemingly sooner the rTMS intervention was conducted post-TBI (regardless of the severity of TBI), the greater the benefit may be for cognitive function (17), chronic headaches (15), and overall symptoms (16). Conversely, rTMS may not be effective in patients with severe TBI (19) and if it is applied years after mild to moderate TBI (avg. 8.4 years) (20). These rTMS data suggest that rTMS may be effective in modulating post-TBI cellular activity, and the effectiveness of rTMS may be influenced by the timing of administration and severity of the initial injury. Nonetheless, all RCTs in this domain are underpowered (n = 9 to 30) to inform a protocol used in clinical settings.
Several therapeutic interventions, such as blue light therapy, acupressure, and acupuncture that can impact the connectivity of the neurosenal network, have yielded beneficial effects. Following 30-min blue light therapy sessions for 6 weeks, patients with mTBI greatly benefited in the context of their cognitive functioning, daytime sleepiness, and fatigue (44, 45). Exposure to blue light, as opposed to amber light, has a positive impact on gray matter volume in the posterior thalamus and greater structural and functional connectivity in the prefrontal cortex and thalamus, which has been shown to be associated with improvements in both cognitive performance and sleep/fatigue (44, 62, 63). Likewise, both acupressure (64) and acupuncture (65) reported significant improvements in cognitive function in patients with mild to moderate TBI, which was partly attributed to the modulation in the limbic-paralimbic neocortical network and subcortical gray matter (65), along with an increased relaxation response (64, 65). Taken together, non-invasive neurostimulation interventions have the potency to facilitate healthy brain network connections, and researchers and clinicians should be encouraged to conduct a larger-scale RCT in patients with chronic TBI symptoms.
4.3 Exercise-based intervention
Exercise-based interventions have been spotlighted in TBI care, owing to the pioneer works (BCTT) done by John Leddy et al. (66). Patients with PPCS often report lingering symptoms including depression, anxiety, and decreased quality of life (49). Despite the RCTs in this review focused on patients with a history of moderate to severe TBI, these RCT results replicated ones from newer RCTs focusing on sports-related mTBI and pediatric mTBI. More specifically, light to moderate exercise or walking, when it is frequent (e.g., 1 h for 3 times a week for 6 to 8 weeks), resulted in improvements in mood, stress, and overall quality of life (48, 49, 67), whereas the shorter duration of exercise (e.g., 30-min per session) and self-guided walking without detailed instruction did not improve any TBI symptoms. This dose and intensity-dependent results may suggest that there is a threshold for inducing positive effects. Exercise interventions during recovery from TBI are aimed not only to increase systemic and cerebral blood flows and regulation of autonomic nerve function, but also to improve cortical connectivity and activation and overexpress brain-derived neurotrophic factor (BDNF). While it is not recommended to engage in high-intensity activity to exacerbate TBI symptoms, reaching certain physiological thresholds may be a key to designing an effective treatment protocol for patients. Although self-guided movement activity, such as Tai Chi, does not induce the same physiological reaction as walking or running, two RCTs using Tai Chi have shown consistent improvements in patients’ mood (46, 47), which is likely through the breathing technique inducing participants to relax and relieve tensions.
4.4 Publication bias
It is important to acknowledge the potential publication bias, often encountered in systematic reviews. This bias occurs when studies with positive or significant findings are more likely to be published, whereas negative or non-significant results may face difficulty in publication. This bias is particularly relevant for smaller-scale pilot RCTs using stimulation- and exercise-based interventions because double-blinding is often infeasible and relative easiness of conducting a pilot RCT due to cost-friendly and less logistical hurdles compared to pharmacological-based RCT, which involves additional layers of regulatory clearance (e.g., FDA). As a result, higher risk of bias was noted especially in the exercise-based interventions (Supplementary Table 1).
4.5 Limitations
While there were several promising candidate interventions, some limitations should be noted to propel future clinical trials. Many, but not all, RCTs had small sample sizes of less than 30 patients with TBI. This trend was prominent in relatively newer interventions (e.g., rTMS, Tai Chi, virtual reality); thus, data from underpowered pilot RCTs should be interpreted with caution. Unlike interventions for acute TBI, research focusing on chronic TBI includes a wide range of patients in terms of time since injury (e.g., 1 month to several years). This introduces other confounding factors, such as lifestyle, occupation, and developmental stages after TBI. Regardless of these potential limitations, some interventions were able to elicit beneficial effects on cognition, mood/mental health, and sleep/fatigue. Initial severities of TBI often determine the procedures for acute triage and care. However, severities of TBI begin to intersect after several months to years, with an example of some moderate TBI patients can recover quickly (68), whereas (68), whereas mild TBI does not mean patients recover patients recover rapidly, in fact some patients can develop lingering symptoms, whereas mild TBI does not mean patients recover rapidly, in fact some patients can develop lingering symptoms for months to years (69). Therefore, initial severities of TBI in the selected RCTs may be less relevant when interpreting the effectiveness of interventions for chronic TBI cases.
4.6 Future directions
TBI is a complex condition that can lead to persistent chronic symptoms lasting for months to even years. Given the high prevalence rates of TBI and its potential implications for later-onset neurodegenerative conditions, there is a crucial need for continuous exploration of effective interventions. Research into candidate pharmaceutical interventions, including neurostimulant medications and growth hormone, would significantly benefit from a more rigorous study design. This entails implementing a more uniform inclusion timeline (e.g., within 3–6 months post-TBI), conducting multi-center trials, and extending follow-up time points. Moreover, gaining a deeper mechanistic understanding through preclinical investigations of these medications can provide valuable insights for the clinical care of TBI patients. Brain stimulation interventions, particularly rTMS and blue-light therapy, necessitate larger sample sizes to replicate previous research findings. This approach aims to determine optimal dosage, intensity, and duration that effectively expedite the recovery process. On a parallel note, exercise-based interventions have gained traction across various health domains, including concussion and TBI care. Despite the relative ease and cost-friendliness of implementing such interventions, there is a need for improved research rigor. Standardizing sessions, dosage, and duration of exercise, along with incorporating physiological parameters to monitor the extent of physiological load, would significantly enhance our understanding of the mechanistic link between exercise intervention and improvements in cognitive and physical symptoms after TBI. While this systematic review focuses on the effects of pharmacological, stimulation, and exercise-based interventions on the chronic phases of TBI, it remains imperative to compare and contrast their effectiveness between acute and chronic phases. This consideration is especially crucial in light of a recent systematic review pointing to the pharmacological efficacy in acute TBI (70).
5 Conclusion
This systematic review revealed several key trends. Several hormone-based (growth hormone, melatonin) and anti-inflammatory/oxidant (Enzogenol, MLC901) medications were associated with robust improvements in executive function, but not mental state or overall TBI symptoms. A series of neurotransmitter-modulation medicines and neurostimulants had inconsistent effects on outcomes, regardless of initial TBI severity. rTMS appears to be a beneficial therapy but its effectiveness is influenced by the timing of administration and severity of the initial injury; the milder the TBI severity and the quicker TMS is delivered, the higher the likelihood of a beneficial outcome. However, given the small sample sizes, a large-scale RCT using the TMS technique is needed. Despite being in the early stages of the investigation, blue light therapy, acupressure, and acupuncture show promise for improved outcomes in chronic TBI patients, whereas tDCS does not seem to have a benefit in this population. Various independent exercise protocols have shown potent effects in improving mood, stress, and overall mental health well-being, encouraging follow-up larger-scale RCTs to confirm previous data from pilot RCTs.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
KK: Writing – review & editing, Writing – original draft, Visualization, Supervision, Investigation, Funding acquisition, Conceptualization. DR: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. CT: Writing – review & editing, Writing – original draft, Methodology, Formal analysis, Data curation, Conceptualization. RM: Writing – review & editing, Methodology, Investigation, Conceptualization. JB: Writing – review & editing, Methodology, Investigation, Conceptualization. DD: Writing – review & editing, Supervision, Methodology, Investigation, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was partly supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS) 1R01NS113950 to KK and the Indiana Spinal Cord and Brain Injury Research Fund from the Indiana State Department of Health (SCTBIRF 00055049 to KK).
Conflict of interest
JB reports the interest with Abbott Diagnostics (research support, speaker fees) and BrainScope (research report).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1321239/full#supplementary-material
References
1. Hyder, AA, Wunderlich, CA, Puvanachandra, P, Gururaj, G, and Kobusingye, OC. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation. (2007) 22:341–53. doi: 10.3233/NRE-2007-22502
2. Haarbauer-Krupa, J, Pugh, MJ, Prager, EM, Harmon, N, Wolfe, J, and Yaffe, K. Epidemiology of chronic effects of traumatic brain injury. J Neurotrauma. (2021) 38:3235–47. doi: 10.1089/neu.2021.0062
3. Howell, DR, Zemek, R, Brilliant, AN, Mannix, RC, Master, CL, and Meehan, WP 3rd. Identifying persistent Postconcussion symptom risk in a Pediatric sports medicine clinic. Am J Sports Med. (2018) 46:3254–61. doi: 10.1177/0363546518796830
4. Novak, Z, Aglipay, M, Barrowman, N, Yeates, KO, Beauchamp, MH, Gravel, J, et al. Emergency research Canada predicting persistent Postconcussive problems in pediatrics concussion, Association of Persistent Postconcussion Symptoms with Pediatric Quality of life. JAMA Pediatr. (2016) 170:e162900. doi: 10.1001/jamapediatrics.2016.2900
5. Carney, N, Totten, AM, O'Reilly, C, Ullman, JS, Hawryluk, GW, Bell, MJ, et al. Guidelines for the Management of Severe Traumatic Brain Injury, fourth edition. Neurosurgery. (2017) 80:6–15. doi: 10.1227/NEU.0000000000001432
6. McCrory, P, Meeuwisse, W, Dvorak, J, Aubry, M, Bailes, J, Broglio, S, et al. Consensus statement on concussion in sport-the 5(th) international conference on concussion in sport held in Berlin. Br J Sports Med. (2016) 51:838–47. doi: 10.1136/bjsports-2017-097699
7. Dijkers, MP . Quality of life after traumatic brain injury: a review of research approaches and findings. Arch Phys Med Rehabil. (2004) 85:S21–35. doi: 10.1016/j.apmr.2003.08.119
8. Vasterling, JJ, Jacob, SN, and Rasmusson, A. Traumatic brain injury and posttraumatic stress disorder: conceptual, diagnostic, and therapeutic considerations in the context of co-occurrence. J Neuropsychiatry Clin Neurosci. (2018) 30:91–100. doi: 10.1176/appi.neuropsych.17090180
9. Schwarzbold, M, Diaz, A, Martins, ET, Rufino, A, Amante, LN, Thais, ME, et al. Psychiatric disorders and traumatic brain injury. Neuropsychiatr Dis Treat. (2008) 4:797–816. doi: 10.2147/ndt.s2653
10. Johansson, B, Wentzel, AP, Andrell, P, Odenstedt, J, Mannheimer, C, and Ronnback, L. Evaluation of dosage, safety and effects of methylphenidate on post-traumatic brain injury symptoms with a focus on mental fatigue and pain. Brain Inj. (2014) 28:304–10. doi: 10.3109/02699052.2013.865267
11. Kaiser, PR, Valko, PO, Werth, E, Thomann, J, Meier, J, Stocker, R, et al. Modafinil ameliorates excessive daytime sleepiness after traumatic brain injury. Neurology. (2010) 75:1780–5. doi: 10.1212/WNL.0b013e3181fd62a2
12. Menn, SJ, Yang, R, and Lankford, A. Armodafinil for the treatment of excessive sleepiness associated with mild or moderate closed traumatic brain injury: a 12-week, randomized, double-blind study followed by a 12-month open-label extension. J Clin Sleep Med. (2014) 10:1181–91. doi: 10.5664/jcsm.4196
13. Berginstrom, N, Nordstrom, P, Schuit, R, and Nordstrom, A. The effects of (−)-OSU6162 on chronic fatigue in patients with traumatic brain injury: a randomized controlled trial. J Head Trauma Rehabil. (2017) 32:E46–54. doi: 10.1097/HTR.0000000000000236
14. Mansour, NO, Shama, MA, and Werida, RH. The effect of doxycycline on neuron-specific enolase in patients with traumatic brain injury: a randomized controlled trial. Ther Adv Chronic Dis. (2021) 12:20406223211024362. doi: 10.1177/20406223211024362
15. Choi, GS, Kwak, SG, Lee, HD, and Chang, MC. Effect of high-frequency repetitive transcranial magnetic stimulation on chronic central pain after mild traumatic brain injury: a pilot study. J Rehabil Med. (2018) 50:246–52. doi: 10.2340/16501977-2321
16. Moussavi, Z, Suleiman, A, Rutherford, G, Ranjbar Pouya, O, Dastgheib, Z, Zhang, W, et al. A pilot randomised double-blind study of the tolerability and efficacy of repetitive transcranial magnetic stimulation on persistent post-concussion syndrome. Sci Rep. (2019) 9:5498. doi: 10.1038/s41598-019-41923-6
17. Lee, SA, and Kim, MK. Effect of low frequency repetitive transcranial magnetic stimulation on depression and cognition of patients with traumatic brain injury: a randomized controlled trial. Med Sci Monit. (2018) 24:8789–94. doi: 10.12659/MSM.911385
18. Hoy, KE, McQueen, S, Elliot, D, Herring, SE, Maller, JJ, and Fitzgerald, PB. A pilot investigation of repetitive transcranial magnetic stimulation for post-traumatic brain injury depression: safety, tolerability, and efficacy. J Neurotrauma. (2019) 36:2092–8. doi: 10.1089/neu.2018.6097
19. Neville, IS, Zaninotto, AL, Hayashi, CY, Rodrigues, PA, Galhardoni, R, Ciampi de Andrade, D, et al. Repetitive TMS does not improve cognition in patients with TBI: a randomized double-blind trial. Neurology. (2019) 93:e190–9. doi: 10.1212/WNL.0000000000007748
20. Siddiqi, SH, Trapp, NT, Hacker, CD, Laumann, TO, Kandala, S, Hong, X, et al. Repetitive transcranial magnetic stimulation with resting-state network targeting for treatment-resistant depression in traumatic brain injury: a randomized, controlled, double-blinded pilot study. J Neurotrauma. (2019) 36:1361–74. doi: 10.1089/neu.2018.5889
21. Leddy, JJ, Kozlowski, K, Donnelly, JP, Pendergast, DR, Epstein, LH, and Willer, B. A preliminary study of subsymptom threshold exercise training for refractory post-concussion syndrome. Clin J Sport Med. (2010) 20:21–7. doi: 10.1097/JSM.0b013e3181c6c22c
22. Patricios, JS, Schneider, KJ, Dvorak, J, Ahmed, OH, Blauwet, C, Cantu, RC, et al. Consensus statement on concussion in sport: the 6th international conference on concussion in sport-Amsterdam, October 2022. Br J Sports Med. (2023) 57:695–711. doi: 10.1136/bjsports-2023-106898
23. Tan, CO, Meehan, WP 3rd, Iverson, GL, and Taylor, JA. Cerebrovascular regulation, exercise, and mild traumatic brain injury. Neurology. (2014) 83:1665–72. doi: 10.1212/WNL.0000000000000944
24. Griffin, EW, Mullally, S, Foley, C, Warmington, SA, O'Mara, SM, and Kelly, AM. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol Behav. (2011) 104:934–41. doi: 10.1016/j.physbeh.2011.06.005
25. Stroth, S, Hille, K, Spitzer, M, and Reinhardt, R. Aerobic endurance exercise benefits memory and affect in young adults. Neuropsychol Rehabil. (2009) 19:223–43. doi: 10.1080/09602010802091183
26. Mikkelsen, K, Stojanovska, L, Polenakovic, M, Bosevski, M, and Apostolopoulos, V. Exercise and mental health. Maturitas. (2017) 106:48–56. doi: 10.1016/j.maturitas.2017.09.003
27. Leddy, JJ, Master, CL, Mannix, R, Wiebe, DJ, Grady, MF, Meehan, WP, et al. Early targeted heart rate aerobic exercise versus placebo stretching for sport-related concussion in adolescents: a randomised controlled trial. Lancet Child Adolesc Health. (2021) 5:792–9. doi: 10.1016/S2352-4642(21)00267-4
28. Wingerson, MJ, Hunt, DL, Wilson, JC, Mannix, RC, Meehan, WP, and Howell, DR. Factors associated with symptom resolution after aerobic exercise intervention in adolescent and young adults with concussion. Med Sci Sports Exerc. (2023). doi: 10.1249/MSS.0000000000003358
29. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DGand the PRISMA Group. Reprint—preferred reporting items for systematic reviews and Meta-analyses: the PRISMA statement. Phys Ther. (2009) 89:873–80. doi: 10.1093/ptj/89.9.873
30. Alashram, AR, Annino, G, Raju, M, and Padua, E. Effects of physical therapy interventions on balance ability in people with traumatic brain injury: a systematic review. NeuroRehabilitation. (2020) 46:455–66. doi: 10.3233/NRE-203047
31. Schlemmer, E, and Nicholson, N. Vestibular rehabilitation effectiveness for adults with mild traumatic brain injury/concussion: a Mini-systematic review. Am J Audiol. (2022) 31:228–42. doi: 10.1044/2021_AJA-21-00165
32. Schneider, KJ, Leddy, JJ, Guskiewicz, KM, Seifert, T, McCrea, M, Silverberg, ND, et al. Rest and treatment/rehabilitation following sport-related concussion: a systematic review. Br J Sports Med. (2017) 51:930–4. doi: 10.1136/bjsports-2016-097475
33. Higgins, JP, Savovic, J, Page, MJ, Elbers, RG, and Sterne, JAC. Chapter 8: assessing risk of bias in a randomized trial In: Cochrane handbook for systematic reviews of interventions (2008)
34. Higgins, JPT, Savović, J, Page, MJ, Elbers, RG, and Sterne, JAC. “Chapter 8: Assessing risk of bias in a randomized trial,” in Cochrane Handbook for Systematic Reviews of Interventions version 6.4. Eds. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane (2023).
35. Lequerica, A, Jasey, N, Portelli Tremont, JN, and Chiaravalloti, ND. Pilot study on the effect of Ramelteon on sleep disturbance after traumatic brain injury: preliminary evidence from a clinical trial. Arch Phys Med Rehabil. (2015) 96:1802–9. doi: 10.1016/j.apmr.2015.05.011
36. Theadom, A, Barker-Collo, S, Jones, KM, Parmar, P, Bhattacharjee, R, and Feigin, VL. MLC901 (NeuroAiD II) for cognition after traumatic brain injury: a pilot randomized clinical trial. Eur J Neurol. (2018) 25:1055–e82. doi: 10.1111/ene.13653
37. Kim, YH, Ko, MH, Na, SY, Park, SH, and Kim, KW. Effects of single-dose methylphenidate on cognitive performance in patients with traumatic brain injury: a double-blind placebo-controlled study. Clin Rehabil. (2006) 20:24–30. doi: 10.1191/0269215506cr927oa
38. Dorer, CL, Manktelow, AE, Allanson, J, Sahakian, BJ, Pickard, JD, Bateman, A, et al. Methylphenidate-mediated motor control network enhancement in patients with traumatic brain injury. Brain Inj. (2018) 32:1040–9. doi: 10.1080/02699052.2018.1469166
39. Hart, T, Whyte, J, Watanabe, T, and Chervoneva, I. Effects of dextroamphetamine in subacute traumatic brain injury: a randomized, placebo-controlled pilot study. J Neurosci Res. (2018) 96:702–10. doi: 10.1002/jnr.24102
40. Ripley, DL, Morey, CE, Gerber, D, Harrison-Felix, C, Brenner, LA, Pretz, CR, et al. Atomoxetine for attention deficits following traumatic brain injury: results from a randomized controlled trial. Brain Inj. (2014) 28:1514–22. doi: 10.3109/02699052.2014.919530
41. Silver, JM, Koumaras, B, Chen, M, Mirski, D, Potkin, SG, Reyes, P, et al. Effects of rivastigmine on cognitive function in patients with traumatic brain injury. Neurology. (2006) 67:748–55. doi: 10.1212/01.wnl.0000234062.98062.e9
42. Kang, EK, Kim, DY, and Paik, NJ. Transcranial direct current stimulation of the left prefrontal cortex improves attention in patients with traumatic brain injury: a pilot study. J Rehabil Med. (2012) 44:346–50. doi: 10.2340/16501977-0947
43. Lesniak, M, Polanowska, K, Seniow, J, and Czlonkowska, A. Effects of repeated anodal tDCS coupled with cognitive training for patients with severe traumatic brain injury: a pilot randomized controlled trial. J Head Trauma Rehabil. (2014) 29:E20–9. doi: 10.1097/HTR.0b013e318292a4c2
44. Killgore, WDS, Vanuk, JR, Shane, BR, Weber, M, and Bajaj, S. A randomized, double-blind, placebo-controlled trial of blue wavelength light exposure on sleep and recovery of brain structure, function, and cognition following mild traumatic brain injury. Neurobiol Dis. (2020) 134:104679. doi: 10.1016/j.nbd.2019.104679
45. Sinclair, KL, Ponsford, JL, Taffe, J, Lockley, SW, and Rajaratnam, SM. Randomized controlled trial of light therapy for fatigue following traumatic brain injury. Neurorehabil Neural Repair. (2014) 28:303–13. doi: 10.1177/1545968313508472
46. Blake, H, and Batson, M. Exercise intervention in brain injury: a pilot randomized study of tai chi qigong. Clin Rehabil. (2009) 23:589–98. doi: 10.1177/0269215508101736
47. Gemmell, C, and Leathem, JM. A study investigating the effects of tai chi Chuan: individuals with traumatic brain injury compared to controls. Brain Inj. (2006) 20:151–6. doi: 10.1080/02699050500442998
48. Driver, S, and Ede, A. Impact of physical activity on mood after TBI. Brain Inj. (2009) 23:203–12. doi: 10.1080/02699050802695574
49. Wise, EK, Hoffman, JM, Powell, JM, Bombardier, CH, and Bell, KR. Benefits of exercise maintenance after traumatic brain injury. Arch Phys Med Rehabil. (2012) 93:1319–23. doi: 10.1016/j.apmr.2012.05.009
50. Jacoby, M, Averbuch, S, Sacher, Y, Katz, N, Weiss, PL, and Kizony, R. Effectiveness of executive functions training within a virtual supermarket for adults with traumatic brain injury: a pilot study. IEEE Trans Neural Syst Rehabil Eng. (2013) 21:182–90. doi: 10.1109/TNSRE.2012.2235184
51. Tefertiller, C, Hays, K, Natale, A, O'Dell, D, Ketchum, J, Sevigny, M, et al. Results from a randomized controlled trial to address balance deficits after traumatic brain injury. Arch Phys Med Rehabil. (2019) 100:1409–16. doi: 10.1016/j.apmr.2019.03.015
52. Jensen, OK, Nielsen, FF, and Vosmar, L. An open study comparing manual therapy with the use of cold packs in the treatment of post-traumatic headache. Cephalalgia. (1990) 10:241–50. doi: 10.1046/j.1468-2982.1990.1005241.x
53. Chizuk, HM, Willer, BS, Cunningham, A, Bezherano, I, Storey, E, Master, C, et al. Adolescents with sport-related concussion who adhere to aerobic exercise prescriptions recover faster. Med Sci Sports Exerc. (2022) 54:1410–6. doi: 10.1249/MSS.0000000000002952
54. Micay, R, Richards, D, and Hutchison, MG. Feasibility of a postacute structured aerobic exercise intervention following sport concussion in symptomatic adolescents: a randomised controlled study. BMJ Open Sport Exerc Med. (2018) 4:e000404. doi: 10.1136/bmjsem-2018-000404
55. Leddy, JJ, Haider, MN, Ellis, MJ, Mannix, R, Darling, SR, Freitas, MS, et al. Early subthreshold aerobic exercise for sport-related concussion: a randomized clinical trial. JAMA Pediatr. (2019) 173:319–25. doi: 10.1001/jamapediatrics.2018.4397
56. Theadom, A, Mahon, S, Barker-Collo, S, McPherson, K, Rush, E, Vandal, AC, et al. Enzogenol for cognitive functioning in traumatic brain injury: a pilot placebo-controlled RCT. Eur J Neurol. (2013) 20:1135–44. doi: 10.1111/ene.12099
57. Widmann, C, Gandin, C, Petit-Paitel, A, Lazdunski, M, and Heurteaux, C. The traditional Chinese medicine MLC901 inhibits inflammation processes after focal cerebral ischemia. Sci Rep. (2018) 8:18062. doi: 10.1038/s41598-018-36138-0
58. Quintard, H, Lorivel, T, Gandin, C, Lazdunski, M, and Heurteaux, C. MLC901, a traditional Chinese medicine induces neuroprotective and neuroregenerative benefits after traumatic brain injury in rats. Neuroscience. (2014) 277:72–86. doi: 10.1016/j.neuroscience.2014.06.047
59. Di Pietro, V, Yakoub, KM, Caruso, G, Lazzarino, G, Signoretti, S, Barbey, AK, et al. Antioxidant therapies in traumatic brain injury. Antioxidants. (2020) 9:260. doi: 10.3390/antiox9030260
60. Liu, J, Clough, SJ, Hutchinson, AJ, Adamah-Biassi, EB, Popovska-Gorevski, M, and Dubocovich, ML. MT1 and MT2 melatonin receptors: a therapeutic perspective. Annu Rev Pharmacol Toxicol. (2016) 56:361–83. doi: 10.1146/annurev-pharmtox-010814-124742
61. Eldaief, MC, Press, DZ, and Pascual-Leone, A. Transcranial magnetic stimulation in neurology: a review of established and prospective applications. Neurol Clin Pract. (2013) 3:519–26. doi: 10.1212/01.CPJ.0000436213.11132.8e
62. Bajaj, S, Raikes, AC, Razi, A, Miller, MA, and Killgore, WD. Blue-light therapy strengthens resting-state effective connectivity within default-mode network after mild TBI. J Cent Nerv Syst Dis. (2021) 13:11795735211015076. doi: 10.1177/11795735211015076
63. Raikes, AC, Dailey, NS, Forbeck, B, Alkozei, A, and Killgore, WDS. Daily morning blue light therapy for post-mTBI sleep disruption: effects on brain structure and function. Front Neurol. (2021) 12:625431. doi: 10.3389/fneur.2021.625431
64. McFadden, KL, Healy, KM, Dettmann, ML, Kaye, JT, Ito, TA, and Hernandez, TD. Acupressure as a non-pharmacological intervention for traumatic brain injury (TBI). J Neurotrauma. (2011) 28:21–34. doi: 10.1089/neu.2010.1515
65. Zollman, FS, Larson, EB, Wasek-Throm, LK, Cyborski, CM, and Bode, RK. Acupuncture for treatment of insomnia in patients with traumatic brain injury: a pilot intervention study. J Head Trauma Rehabil. (2012) 27:135–42. doi: 10.1097/HTR.0b013e3182051397
66. Leddy, JJ, and Willer, B. Use of graded exercise testing in concussion and return-to-activity management. Curr Sports Med Rep. (2013) 12:370–6. doi: 10.1249/JSR.0000000000000008
67. Bellon, K, Kolakowsky-Hayner, S, Wright, J, Huie, H, Toda, K, Bushnik, T, et al. A home-based walking study to ameliorate perceived stress and depressive symptoms in people with a traumatic brain injury. Brain Inj. (2015) 29:313–9. doi: 10.3109/02699052.2014.974670
68. Nelson, LD, Temkin, NR, Barber, J, Brett, BL, Okonkwo, DO, McCrea, MA, et al. Functional recovery, symptoms, and quality of life 1 to 5 years after traumatic brain injury. JAMA Netw Open. (2023) 6:e233660. doi: 10.1001/jamanetworkopen.2023.3660
69. Rabinowitz, AR, Li, X, McCauley, SR, Wilde, EA, Barnes, A, Hanten, G, et al. Prevalence and predictors of poor recovery from mild traumatic brain injury. J Neurotrauma. (2015) 32:1488–96. doi: 10.1089/neu.2014.3555
70. Mansour, NO, Elnaem, MH, Abdelaziz, DH, Barakat, M, Dehele, IS, Elrggal, ME, et al. Effects of early adjunctive pharmacotherapy on serum levels of brain injury biomarkers in patients with traumatic brain injury: a systematic review of randomized controlled studies. Front Pharmacol. (2023) 14:1185277. doi: 10.3389/fphar.2023.1185277
Keywords: traumatic brain injury, pharmacology, stimulation, exercise, chronic traumatic encephalopathy
Citation: Kawata K, Rettke DJ, Thompson C, Mannix R, Bazarian JJ and Datta D (2024) Effectiveness of biomedical interventions on the chronic stage of traumatic brain injury: a systematic review of randomized controlled trials. Front. Neurol. 15:1321239. doi: 10.3389/fneur.2024.1321239
Edited by:
Elham Rostami, Uppsala University Hospital, SwedenReviewed by:
Noha O. Mansour, Mansoura University, EgyptDoaa H. Abdelaziz, Future University in Egypt, Egypt
Copyright © 2024 Kawata, Rettke, Thompson, Mannix, Bazarian and Datta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keisuke Kawata, a2thd2F0YUBpbmRpYW5hLmVkdQ==
 Keisuke Kawata
Keisuke Kawata Devin J. Rettke
Devin J. Rettke Christopher Thompson1
Christopher Thompson1 Rebekah Mannix
Rebekah Mannix Jeffrey J. Bazarian
Jeffrey J. Bazarian Dibyadyuti Datta
Dibyadyuti Datta