- 1Department of Otorhinolaryngology, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Otorhinolaryngology, Central Hospital Affiliated to Chongqing University of Technology, Chongqing, China
- 3Department of Eye and ENT, Chongqing Maternal and Child Health Care Hospital, Chongqing, China
Background: The association between benign paroxysmal positional vertigo (BPPV) and various mental disorders is still controversial. This study used the Mendelian randomization (MR) method to clarify the correlation between BPPV and seven mental disorders (bipolar disorder, depression, anxiety disorder, schizophrenia, suicidality, neuroticism, and mood swings) to aid in the exploration of BPPV complications and prevention and early treatment of mental disorders.
Methods: The datasets for BPPV and seven mental disorders were obtained from genome-wide association studies (GWASs). Two-sample MR was used to analyze the correlation between exposure (BPPV) and various outcomes (bipolar disorder, depression, anxiety disorder, schizophrenia, suicidality, neuroticism, and mood swings). A reverse MR study was also performed. The inverse variance weighting (IVW) method, the MR–Egger method, the simple mode method, the weighted mode method, and the weighted median method were selected.
Results: The MR analysis and the reverse MR analysis results did not reveal significant associations between BPPV and bipolar disorder, depression, anxiety disorder, schizophrenia, suicidal tendencies, neuroticism, and mood swings. Interestingly, neuroticism (IVW: OR = 1.142, 95% CI: 1.059–1.231, P = 0.001; P-MR-PRESSO adjustment = 0.0002) and mood swings (IVW: OR = 3.119, 95% CI: 1.652–5.884, P = 0.0004) may have a significant association with BPPV. After MR-PRESSO adjustment, there was no horizontal pleiotropy or heterogeneity, and a significant association between neuroticism, mood swings, and BPPV has still been suggested.
Conclusion: We conducted MR analysis on genetic data from European populations and discovered a causal relationship between BPPV and the seven mental disorders. Our research findings suggest that BPPV may not have a significant causal relationship with bipolar disorder, depression, anxiety disorder, schizophrenia, or suicidal tendencies. However, neuroticism and mood swings may be risk factors for BPPV.
1 Introduction
Benign paroxysmal positional vertigo (BPPV) is the most common cause of vertigo, and 24.1% of patients with dizziness/vertigo have BPPV (1). The underlying mechanism of BPPV may be the displacement of degenerate otoliths into the semicircular canal, resulting in increased sensitivity to head movement, which induces paroxysmal positional vertigo (2). The lifetime incidence of BPPV is as high as 2.4% (3), and BPPV seriously affects the quality of life in affected individuals (4), increases their risk of falls, and reduces their walking speed (5). BPPV has caused a significant medical burden worldwide (6). Therefore, exploring the impact of BPPV on the incidence of other diseases would be highly helpful for informing personalized treatment and improving patient prognosis.
At present, increased attention has been given to mental disorders worldwide. Approximately one in five people experience a common mental disorder in a year (7). Mental disorders lead to a serious decline in the participation rate of affected individuals in the social labor force, and the high cost of treatment seriously affects their quality of life in the later stages of the illness (8, 9). Multiple diseases or behaviors are thought to contribute to an increased prevalence of mental disorders (10–12). Therefore, identifying the risk factors for mental disorders could facilitate early intervention for individuals affected, thereby reducing the impact of mental disorders on both patients and society.
Although vertigo caused by BPPV can be resolved by the implementation of repeated canalith repositioning procedure (CRP), symptoms of vertigo and positional nystagmus in the patient often return (13). However, some studies have suggested that the clinical features of paroxysmal vertigo may induce various mental disorders in patients with BPPV. At present, whether BPPV can increase the risk of various mental disorders in patients is still controversial, and related studies are rare. A cohort study from Taiwan, China, suggested that chronic stress due to paroxysmal vertigo may increase the risk of BPPV-related suicide (14). A survey of the incidence of BPPV in all patients with mood disorders in Korea revealed that mood disorders may be significantly associated with BPPV (15). A recent meta-analysis suggested that BPPV may increase the risk of anxiety, but no significant association between BPPV and depression was found. There were few relevant studies included in this meta-analysis, and the sample size was small; therefore, further research is needed to determine the associations between BPPV and anxiety and depression (16). Similar to anxiety and depression, bipolar disorder and schizophrenia are also common mental disorders (17), and no relevant studies have explored the associations between bipolar disorder and schizophrenia and BPPV. A lower neuroticism score and stable emotions play a certain role in mental health (18, 19). However, recurrent progression of vertigo may lead to greater neuroticism and mood swings in patients (20).
Many studies have explored the association between mental disorders and diseases through Mendelian randomization (MR) (11, 21). MR is used to clarify the association between two traits. Genetic variants are included as instrumental variables. Single-nucleotide polymorphisms (SNPs) are identified from independent genome-wide association study (GWAS) datasets and are subjected to association analysis as instrumental variables (22). The advantages of MR include avoiding the limitations of traditional observational research and eliminating the interference of various confounding factors in the study as much as possible so that the research results have greater credibility. MR studies have improved the statistical power to infer causal relationships between diseases (23). This study aimed to analyze the relationship between BPPV and seven mental disorders (bipolar disorder, depression, anxiety disorder, schizophrenia, suicidality, neuroticism, and mood swings) by using the MR method to clarify whether there is a correlation between BPPV and seven mental disorders. Neuroticism and the presence of mood swings are considered risk factors for mental disorders; therefore, these factors were included in this study to explore the correlation between BPPV and neuroticism and mood swings. The association between BPPV and mental disorders is clarified to improve the timeliness and targeting of the prevention and treatment of both conditions.
2 Methods
2.1 Data sources
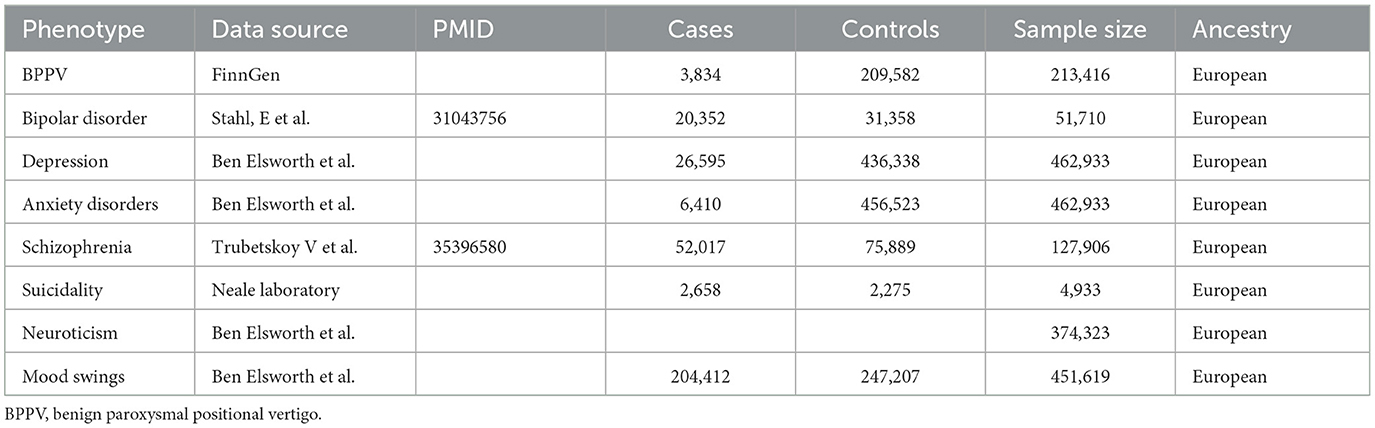
In this study, a two-sample MR analysis was used to analyze the relationship between exposure (BPPV) and various outcomes (bipolar disorder, depression, anxiety disorder, schizophrenia, suicidality, neuroticism, and mood swings). Reverse MR was applied to analyze the correlation between exposure (bipolar disorder, depression, anxiety disorder, schizophrenia, suicidality, neuroticism, and mood swings) and an outcome (BPPV). The GWAS datasets used in this study were all obtained from the IEU GWAS database (https://gwas.mrcieu.ac.uk/), from which the datasets for BPPV and bipolar disorder, depression, anxiety, schizophrenia, suicidality, neuroticism, and mood swings were selected. The BPPV dataset was collected from the FinnGen database, which includes genomic and health data collected from 500,000 Finnish biobanks to determine the genetic basis of the disease. The IEU database has obtained the BPPV dataset from the FinnGen database R5 version. The diagnosis criteria in the FinnGen database are based on the Tenth Revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10). The diagnosis of BPPV requires meeting the diagnostic criteria with the code H81.1 according to the ICD-10. Depression, anxiety disorders, suicidality, neuroticism, and mood swing-related datasets were collected from the UK Biobank, which includes genetic information obtained from more than 500,000 participants from all over the UK. The bipolar disorder and schizophrenia dataset was derived from a GWAS database of patients with bipolar disorder and schizophrenia (24, 25). Detailed information on the GWAS data sources used in our study is provided in Table 1.
2.2 Selection of instrumental variables
The SNPs were selected from the GWAS dataset based on the following conditions: 1. The significance in genome-wide studies to prevent the inclusion of fewer SNPs (P < 5*10−6 was selected as the screening criterion). 2. No linkage disequilibrium was detected between any of the SNPs to preserve SNP independence (r2 <0.001 and 10,000 kb). 3. SNPs with an F-statistic <10 were excluded as they were considered weak instrumental variables. Plus-strand allele inference was then attempted using palindromic allele frequencies.
2.3 Mendelian randomization analysis
The inverse variance weighting (IVW), MR–Egger, simple mode, weighted mode, and weighted median methods were used for data evaluation. IVW obtained a total estimate of the effect of exposure on the outcome by combining the causal estimate of the Wald ratio for each IV, and IVW was used as the primary analysis method (26). The non-zero intercept values shown by the MR–Egger method were mainly used to examine horizontal pleiotropy (27). The weighted median gives an accurate estimate based on the assumption that at least 50% of IVs are effective (28). The simple mode, weighted mode, and weighted median methods were mainly used to verify the reliability and stability of the results. Causality was assessed using the odds ratio (OR) and 95% confidence interval (95% CI) to determine the significance. To strengthen the reliability of this study, the significance was set at 0.05/7 (0.007) according to the Bonferroni correction method.
The MR–Egger method was used to obtain intercept values to evaluate horizontal pleiotropy. The Q-statistic from Cochran's IVW was then used to investigate the impact of heterogeneity. The results of pleiotropic and heterogeneous MR-PRESSO analysis were obtained to remove outlier SNPs from the group and recalculate the MR results.
MR analysis was performed using the TwoSampleMR package in R version 4.2.3 (http://www.r-project.org) (29). The TwoSampleMR package enables online analysis of the association between exposure and outcome datasets through the IEU database.
3 Results
3.1 The results of MR analysis between BPPV and seven mental disorders
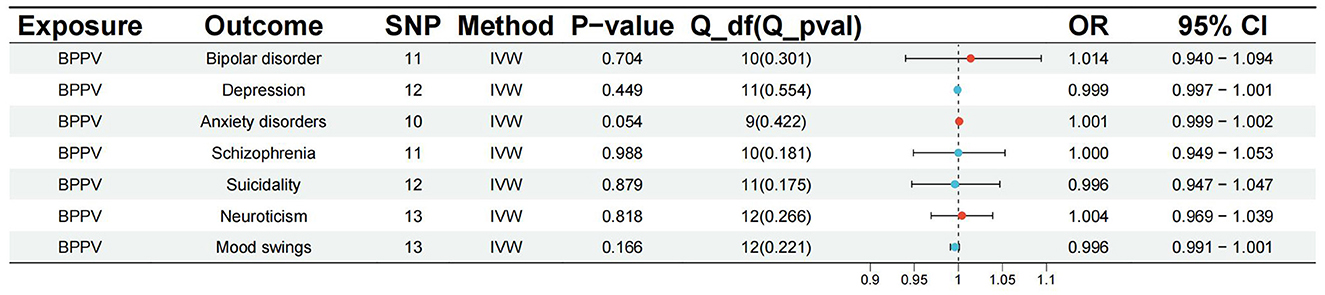
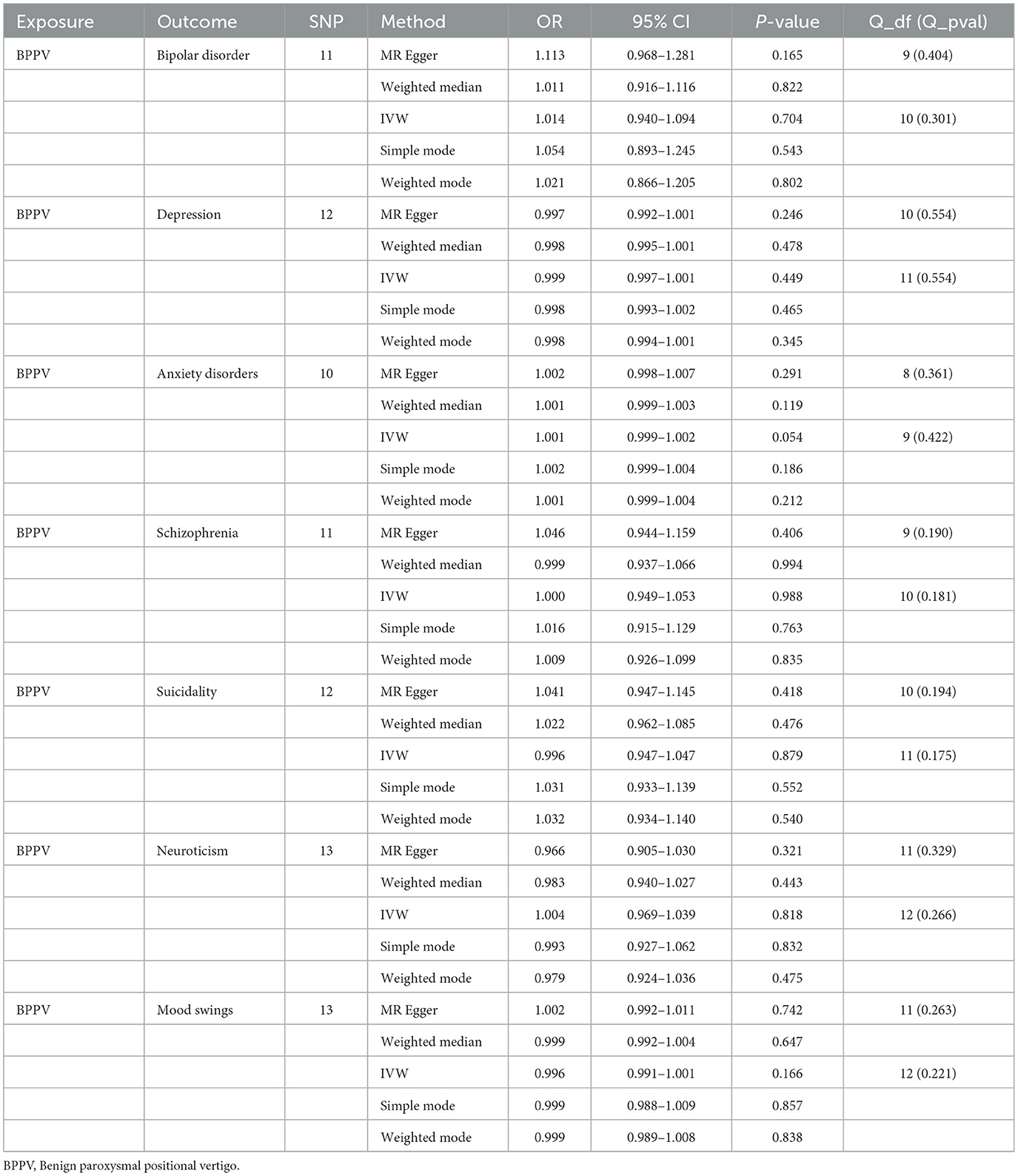
The p-value of <5*10−6 was selected as the screening criterion for BPPV-related SNPs. After screening based on the screening criteria, MR analysis was performed, and the F-statistics of the SNPs included in the analysis were all found to be >10, indicating that they were all strong instrumental variables (Supplementary material). All heterogeneity analyses showed results that p > 0.05, which suggested that there was no heterogeneity in the results. No horizontal pleiotropy was found in any of the MR–Egger analyses (P > 0.05). The results suggested that there was no significant association between BPPV and bipolar disorder (IVW: OR = 1.014, 95% CI: 0.940–1.094, P = 0.704), depression (IVW: OR = 0.999, 95% CI: 0.997–1.001, P = 0.449), anxiety disorders (IVW: OR = 1.001, 95% CI: 0.999–1.002, P = 0.054), schizophrenia (IVW: OR = 1.000, 95% CI: 0.949–1.053, P = 0.988), suicidality (IVW: OR = 0.996, 95% CI: 0.947–1.047, P = 0.879), neuroticism (IVW: OR = 1.004, 95% CI: 0.969–1.039, P = 0.818), and mood swings (IVW: OR = 0.996, 95% CI: 0.991–1.001, P = 0.166) (Figures 1, 2). The detailed analysis results are shown in Table 2.
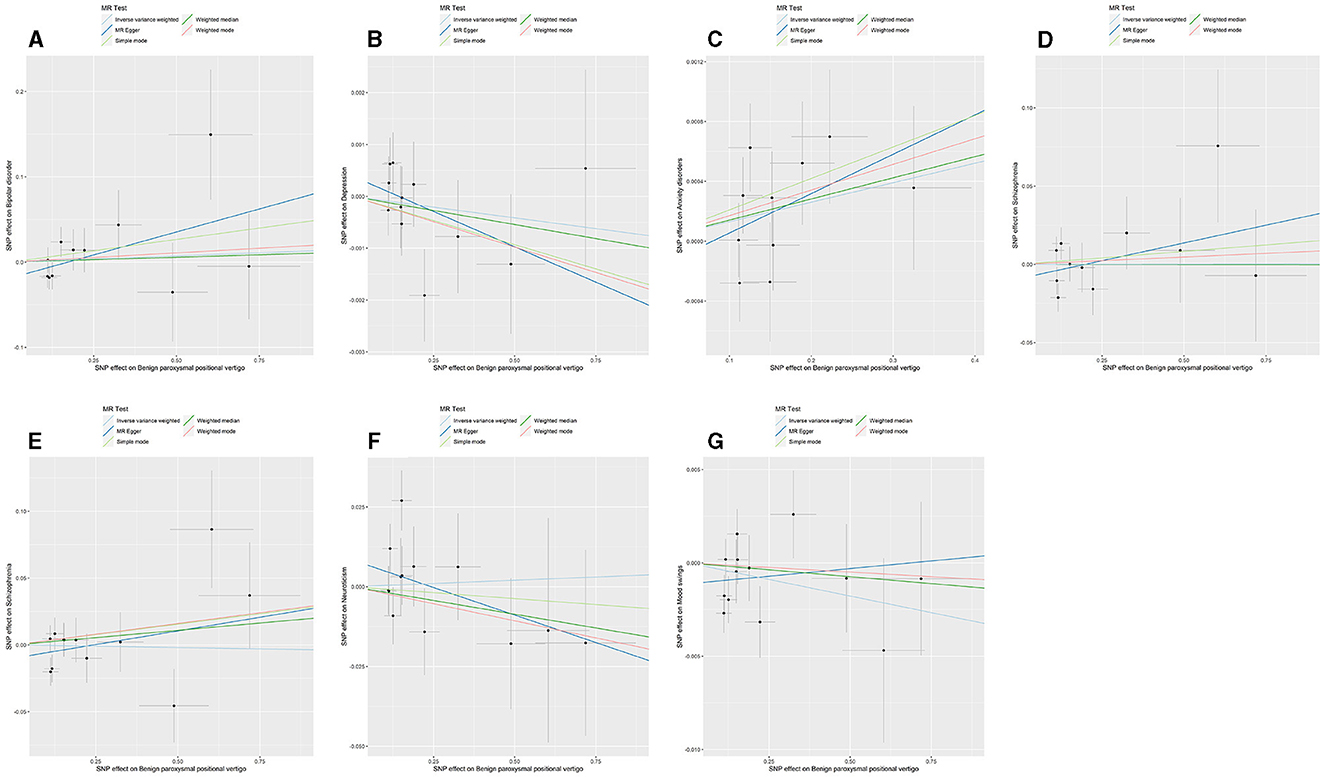
Figure 1. Summary view of the MR images derived from the IVW, MR–Egger, simple mode, weighted median, and weighted mode methods. (A) The MR analysis results for BPPV and bipolar disorder. (B) The MR analysis results for BPPV and depression. (C) The MR analysis results for BPPV and anxiety disorders. (D) The MR analysis results for BPPV and schizophrenia. (E) The MR analysis results for BPPV and suicidality. (F) The MR analysis results for BPPV and neuroticism. (G) The MR analysis results for BPPV and mood swings.
3.2 The results of MR analysis between seven mental disorders and BPPV
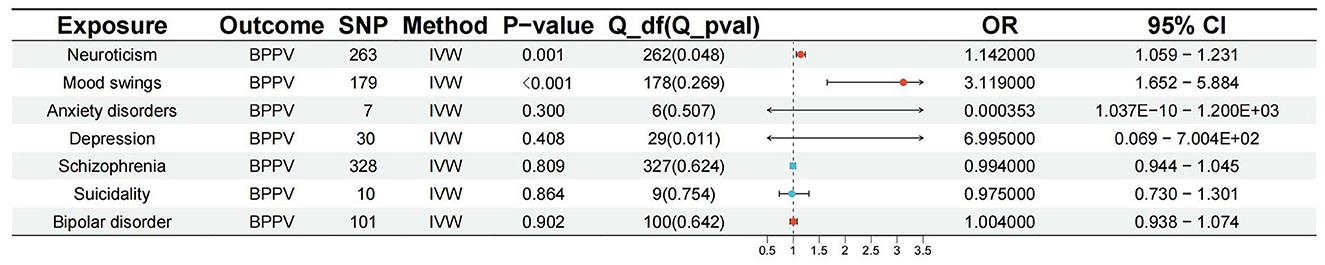
The p-value of <5*10−6 was selected as the screening criterion for seven mental disorder-related SNPs. After screening based on the criteria, MR analysis was performed, and the F-statistics of the SNPs included in the analysis were all found to be >10, indicating strong instrumental variables (Supplementary material). No significant association was found in the reverse MR of bipolar disorder (IVW: OR = 1.004, 95% CI: 0.938–1.074, P = 0.902), depression (IVW: OR = 6.995, 95% CI: 0.069–7.004E+02, P = 0.408, P-MR-PRESSO adjustment = 0.147), anxiety (IVW: OR = 3.529E-04, 95% CI: 1.037E-10-1.200E+03, P = 0.300), schizophrenia (IVW: OR = 0.994, 95% CI: 0.944–1.045, P = 0.809), suicidality (IVW: OR = 0.975, 95% CI: 0.730–1.301, P = 0.864), and BPPV. Neuroticism (IVW: OR = 1.142, 95% CI: 1.059–1.231, P = 0.001; P-MR-PRESSO adjustment = 0.0002) and mood swings (IVW: OR = 3.119, 95% CI: 1.652–5.884, P = 0.0004) were significantly associated with BPPV. Horizontal pleiotropy and heterogeneity were detected in the reverse MR analysis of patients with depression and BPPV, and heterogeneity was detected in the inverse variance MR analysis of patients with neuroticism and BPPV. MR analysis was performed again after MR-PRESSO adjustment, and the results showed a lack of horizontal pleiotropy and heterogeneity (Figures 3, 4). The detailed analysis results are shown in Table 3.
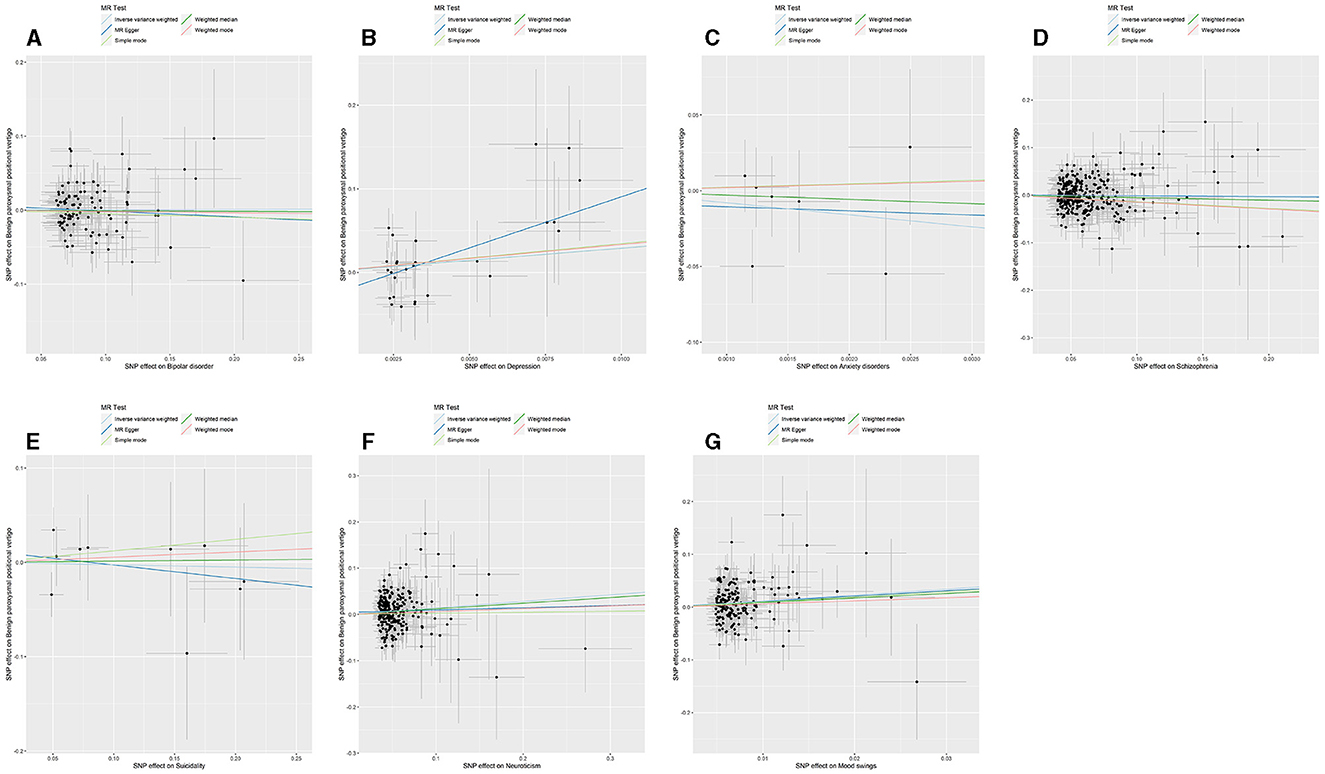
Figure 3. Summary view of the MR images derived from the IVW, MR–Egger, simple mode, weighted median, and weighted mode methods. (MR-PRESSO adjustment) (A) The MR analysis results for bipolar disorder and BPPV. (B) The MR analysis results for depression and BPPV. (C) The MR analysis results for anxiety disorders and BPPV. (D) The MR analysis results for schizophrenia and BPPV. (E) The MR analysis results for suicidality and BPPV. (F) The MR analysis results for neuroticism and BPPV. (G) The MR analysis results for mood swings and BPPV.
4 Discussion
In this study, the MR method was used to assess the association between BPPV and seven mental disorders. The results showed that BPPV was not significantly associated with bipolar disorder, depression, anxiety disorders, schizophrenia, or suicidality. Reverse MR analysis indicated that bipolar disorder, depression, anxiety, schizophrenia, and suicidality were not significantly associated with BPPV, while higher neuroticism scores and mood swings may promote the occurrence and development of BPPV. Analyses of horizontal pleiotropy and heterogeneity after MR-PRESSO adjustment did not reveal significant differences, which suggests the reliability of the results.
In related studies analyzing patients with BPPV in Korea, it was found that the risk of developing mood disorders in BPPV patients was significantly greater than that in healthy people (15). The degree of anxiety and depression may reflect the probability of residual dizziness after canalith repositioning (30). At present, the associations between BPPV and anxiety and depression have been studied the most. A recent meta-analysis of 23 studies and 2,902 patients showed that there was a significant association between BPPV and anxiety, but the association between BPPV and depression still needs to be further studied (16). Yang et al. conducted an analysis of 72,569 patients with peripheral vestibular disorders and 217,707 healthy controls in Taiwan and reported that suicidal attempts were strongly associated with BPPV, Meniere's disease, and vestibular neuritis; however, due to the uncertainty of other suicide risk factors, the association between these conditions needs to be further studied (14), and other studies have shown results similar to those in our analyses. Kalderon et al. analyzed the clinical data of 18 patients with BPPV and 18 healthy controls and reported that there may be no difference in anxiety between patients with BPPV and healthy controls (31). In our research, no relevant clinical studies on BPPV or bipolar disorder or schizophrenia were found, and the association between BPPV and the relevance of bipolar disorder and schizophrenia may require further exploration. Psychological distress has been shown to predict the severity of vestibular dysfunction to a certain extent (32). Neuroticism and mood swings, which are common psychological factors (33), may also have a certain effect on BPPV. Several clinical studies have confirmed our results from other perspectives (20, 34, 35). Our results are inconsistent with the results of several clinical analyses, possibly due to the lack of reliability of the results due to the unmeasured confounding factors that often appear in clinical studies of mental disorders or BPPV. Therefore, the results of clinical studies cannot fully reflect the association between these diseases. We used the MR method at the level of genetic analysis to determine the relationship between the two parameters (mental disorders and BPPV), ruling out various confounding factors, and thus improved the reliability of the results (36).
Due to the influence of various factors on the mechanism of BPPV, there may be no significant association between several mental disorders and this disease. Neuroticism and mood swings are more likely to be the risk factors for BPPV compared to other mental disorders. However, the mechanism by which neuroticism and mood swings, as common psychological distress factors, affect the occurrence and development of BPPV is still unclear, and local inflammation due to abnormal psychology could promote the development of BPPV (37, 38). Psychological stress can trigger a systemic stress response, leading to an inflammatory reaction. This regulation of an inflammatory reaction may serve a protective function in the short term, but sustained chronic inflammation stimulation may affect the functioning of the balance receptors in the inner ear, ultimately promoting the development of BPPV (39). Additionally, neuroticism and mood swings may enhance neural network activity, thereby affecting patients' visual balance control (40). Stable visual perception is crucial for individuals with BPPV (41). Further exploration of the relevant mechanisms is needed in the future. A deeper understanding of these mechanisms will aid in the development of more effective treatment strategies and preventive measures for BPPV.
Although our results suggest that there is no significant association between BPPV and five mental disorders (bipolar disorder, depression, anxiety disorder, schizophrenia, and suicidality), BPPV may have some influence on the occurrence and development of the five mental disorders. The underlying mechanisms of BPPV and mental disorders are complex. It is possible that long-term repeated harmful physical stimuli, such as chronic pain, may lead to emotional changes in patients, which may induce mental disorders (42). It has been suggested that somatic imbalance, spatial orientation disorder, nausea, and vomiting caused by recurrent vertigo attacks lead to secondary psychological distress (43). It has also been hypothesized that the cerebellar and vestibular systems play complementary roles in emotion regulation and that long-term maladaptation to the environment may lead to anxiety and depression (44, 45). Hemispheric lateralization may link vestibular systems to systems that process emotions (46). The chronic physical stress caused by BPPV will also continue to affect the hypothalamic–pituitary–adrenal (HPA) axis (47), and disorders of the HPA axis may affect mood in individuals (48). The exploration of the mechanisms underlying the correlation between neuroticism and mood swings and BPPV merits further study because of the association between BPPV and mental disorders, which may be significant for guiding future research on the underlying mechanisms of the associations between psychological states and BPPV.
To date, no MR study has examined the association between BPPV and mental disorders. We used MR analysis in this study to avoid the bias caused by confounding factors and sample size difficulties that occur in traditional clinical research. The reliability and accuracy of the study were improved. MR analysis strengthened the causal relationship and reduced the probability of confounding and reverse causality. This study has some limitations. Because the datasets were obtained from a public database and the patients were of European ancestry, the results of this study were not necessarily generalizable to other regions or ethnic groups. Although we did not find horizontal pleiotropy after adjustment for MR-PRESSO, we cannot completely rule out that horizontal pleiotropy affected the generalizability of our results. Since the datasets used in this study were obtained from a public database, we cannot classify the sample population by age and sex or analyze their correlation more precisely. Although the findings of the study established a causal relationship between BPPV and neuroticism and mood swings, future research should involve additional design interventions targeting the risk factors for BPPV to aid in the development of better prevention for the recurrence of BPPV.
5 Conclusion
In summary, the results of the two-sample MR analysis revealed that BPPV was not significantly associated with five mental disorders (bipolar disorder, depression, anxiety disorders, schizophrenia, and suicidality). Neuroticism and mood swings are more likely to be the risk factors for BPPV. Therefore, we need to pay more attention to the psychological distress in BPPV patients, and we need to treat BPPV and prevent its recurrence. The association between BPPV and mental disorders is clarified to improve the early prevention and treatment of mental disorders and BPPV in clinical research. The findings of this study will help to improve the comprehensive medical management of patients with mental disorders and BPPV in clinical practice and contribute to further revealing the underlying mechanisms of mental disorders and BPPV.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
SL: Conceptualization, Data curation, Software, Visualization, Writing – original draft. LZ: Investigation, Methodology, Project administration, Supervision, Writing – review & editing. DD: Project administration, Supervision, Visualization, Writing – review & editing, Conceptualization, Funding acquisition. WL: Project administration, Supervision, Visualization, Writing – review & editing.
Funding
The author (s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1310026/full#supplementary-material
References
1. Kim HJ, Lee JO, Choi JY, Kim JS. Etiologic distribution of dizziness and vertigo in a referral-based dizziness clinic in South Korea. J Neurol. (2020) 267:2252–9. doi: 10.1007/s00415-020-09831-2
2. Imai T, Inohara H. Benign paroxysmal positional vertigo. Auris Nasus Larynx. (2022) 49:737–47. doi: 10.1016/j.anl.2022.03.012
3. von Brevern M, Radtke A, Lezius F, Feldmann M, Ziese T, Lempert T, et al. Epidemiology of benign paroxysmal positional vertigo: a population based study. J Neurol Neurosurg Psychiatry. (2007) 78:710–5. doi: 10.1136/jnnp.2006.100420
4. Lindell E, Kollen L, Johansson M, Karlsson T, Ryden L, Falk Erhag H, et al. Benign paroxysmal positional vertigo, dizziness, and health-related quality of life among older adults in a population-based setting. Eur Arch Otorhinolaryngol. (2021) 278:1637–44. doi: 10.1007/s00405-020-06357-1
5. Pauwels S, Casters L, Lemkens N, Lemmens W, Meijer K, Meyns P, et al. Gait and falls in benign paroxysmal positional vertigo: a systematic review and meta-analysis. J Neurol Phys Ther. (2023) 47:127–38. doi: 10.1097/NPT.0000000000000438
6. Kim HJ, Park J, Kim JS. Update on benign paroxysmal positional vertigo. J Neurol. (2021) 268:1995–2000. doi: 10.1007/s00415-020-10314-7
7. Steel Z, Marnane C, Iranpour C, Chey T, Jackson JW, Patel V, et al. The global prevalence of common mental disorders: a systematic review and meta-analysis 1980-2013. Int J Epidemiol. (2014) 43:476–93. doi: 10.1093/ije/dyu038
8. Schofield DJ, Shrestha RN, Percival R, Passey ME, Callander EJ, Kelly SJ. The personal and national costs of mental health conditions: impacts on income, taxes, government support payments due to lost labour force participation. BMC Psychiatry. (2011) 11:72. doi: 10.1186/1471-244X-11-72
9. Christensen MK, Lim CCW, Saha S, Plana-Ripoll O, Cannon D, Presley F, et al. The cost of mental disorders: a systematic review. Epidemiol Psychiatr Sci. (2020) 29:e161. doi: 10.1017/S204579602000075X
10. Howard LM, Oram S, Galley H, Trevillion K, Feder G. Domestic violence and perinatal mental disorders: a systematic review and meta-analysis. PLoS Med. (2013) 10:e1001452. doi: 10.1371/journal.pmed.1001452
11. Cai J, Wei Z, Chen M, He L, Wang H, Li M, et al. Socioeconomic status, individual behaviors and risk for mental disorders: a Mendelian randomization study. Eur Psychiatry. (2022) 65:e28. doi: 10.1192/j.eurpsy.2022.18
12. Sun X, Liu B, Liu S, Wu DJH, Wang J, Qian Y, et al. Sleep disturbance and psychiatric disorders: a bidirectional Mendelian randomisation study. Epidemiol Psychiatr Sci. (2022) 31:e26. doi: 10.1017/S2045796021000810
13. Hilton MP, Pinder DK. The Epley (canalith repositioning) manoeuvre for benign paroxysmal positional vertigo. Cochrane Database Syst Rev. 2014:CD003162. doi: 10.1002/14651858.CD003162.pub3
14. Yang TH, Xirasagar S, Cheng YF, Chen CS, Lin HC. Does peripheral vestibular disorder increase the risk of attempted suicide: a retrospective cohort study. J Affect Disord. (2023) 341:12–6. doi: 10.1016/j.jad.2023.08.110
15. Kim SK, Hong SM, Park IS, Lee HJ, Park B, Choi HG. Mood disorders are associated with increased risk of BPPV: a national sample cohort. Laryngoscope. (2021) 131:380–5. doi: 10.1002/lary.28638
16. Yeo BSY, Toh EMS, Lim NE, Lee RS, Ho RCM, Tam WWS, et al. Association of benign paroxysmal positional vertigo with depression and anxiety-a systematic review and meta-analysis. Laryngoscope. (2023). doi: 10.1002/lary.30957
17. Baxter AJ, Patton G, Scott KM, Degenhardt L, Whiteford HA. Global epidemiology of mental disorders: what are we missing? PLoS ONE. (2013) 8:e65514. doi: 10.1371/journal.pone.0065514
18. Safer DJ. Mood swing and mood stabilizer: how specific are these terms? Bipolar Disord. (2010) 12:685–90. doi: 10.1111/j.1399-5618.2010.00870.x
19. Ormel J, Jeronimus BF, Kotov R, Riese H, Bos EH, Hankin B, et al. Neuroticism and common mental disorders: meaning and utility of a complex relationship. Clin Psychol Rev. (2013) 33:686–97. doi: 10.1016/j.cpr.2013.04.003
20. Trinidade A, Harman P, Stone J, Staab JP, Goebel JA. Assessment of potential risk factors for the development of persistent postural-perceptual dizziness: a case-control pilot study. Front Neurol. (2020) 11:601883. doi: 10.3389/fneur.2020.601883
21. Zhong H, Huan X, Jiao K, He S, Wen Z, Zhao R, et al. Causal relationships between mood instability and autoimmune diseases: a mendelian randomization analysis. Autoimmun Rev. (2023) 22:103214. doi: 10.1016/j.autrev.2022.103214
22. Birney E. Mendelian Randomization. Cold Spring Harb Perspect Med. (2022) 12:a041302. doi: 10.1101/cshperspect.a041302
23. Pierce BL, Burgess S. Efficient design for Mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am J Epidemiol. (2013) 178:1177–84. doi: 10.1093/aje/kwt084
24. Stahl EA, Breen G, Forstner AJ, McQuillin A, Ripke S, Trubetskoy V, et al. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat Genet. (2019) 51:793–803. doi: 10.1038/s41588-019-0397-8
25. Trubetskoy V, Pardinas AF Qi T, Panagiotaropoulou G, Awasthi S, Bigdeli TB, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. (2022) 604:502–8. doi: 10.1038/s41586-022-04434-5
26. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. (2013) 37:658–65. doi: 10.1002/gepi.21758
27. Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. (2017) 32:377–89. doi: 10.1007/s10654-017-0255-x
28. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
29. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. (2018) 7:e34408. doi: 10.7554/eLife.34408
30. Sun J, Ma X, Yang Y, He K, Wang W, Shen J, et al. Associations between cognition, anxiety, depression, and residual dizziness in elderly people with BPPV. Front Aging Neurosci. (2023) 15:1208661. doi: 10.3389/fnagi.2023.1208661
31. Kalderon L, Chaimoff M, Katz-Leurer M. The distinction between state and trait anxiety levels in patients with BPPV in comparison with healthy controls. Front Psychol. (2022) 13:1055467. doi: 10.3389/fpsyg.2022.1055467
32. Probst T, Dinkel A, Schmid-Muhlbauer G, Radziej K, Limburg K, Pieh C, et al. Psychological distress longitudinally mediates the effect of vertigo symptoms on vertigo-related handicap. J Psychosom Res. (2017) 93:62–8. doi: 10.1016/j.jpsychores.2016.11.013
33. Schick RR, Yaksh TL, Roddy DR, Go VL. Release of hypothalamic cholecystokinin in cats: effects of nutrient and volume loading. Am J Physiol. (1989) 256:R248–54. doi: 10.1152/ajpregu.1989.256.1.R248
34. Staab JP, Rohe DE, Eggers SD, Shepard NT. Anxious, introverted personality traits in patients with chronic subjective dizziness. J Psychosom Res. (2014) 76:80–3. doi: 10.1016/j.jpsychores.2013.11.008
35. Wolf J, Sattel H, Limburg K, Lahmann C. From illness perceptions to illness reality? Perceived consequences and emotional representations relate to handicap in patients with vertigo and dizziness. J Psychosom Res. (2020) 130:109934. doi: 10.1016/j.jpsychores.2020.109934
36. Sekula P, Del Greco MF, Pattaro C, Kottgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol. (2016) 27:3253–65. doi: 10.1681/ASN.2016010098
37. Koc A. Benign paroxysmal positional vertigo: is it really an otolith disease? J Int Adv Otol. (2022) 18:62–70. doi: 10.5152/iao.2022.21260
38. Chistyakov DV, Astakhova AA, Sergeeva MG. Resolution of inflammation and mood disorders. Exp Mol Pathol. (2018) 105:190–201. doi: 10.1016/j.yexmp.2018.08.002
39. Rohleder N. Stress and inflammation - The need to address the gap in the transition between acute and chronic stress effects. Psychoneuroendocrinology. (2019) 105:164–71. doi: 10.1016/j.psyneuen.2019.02.021
40. Passamonti L, Riccelli R, Lacquaniti F, Staab JP, Indovina I. Brain responses to virtual reality visual motion stimulation are affected by neurotic personality traits in patients with persistent postural-perceptual dizziness. J Vestib Res. (2018) 28:369–78. doi: 10.3233/VES-190653
41. Nair MA, Mulavara AP, Bloomberg JJ, Sangi-Haghpeykar H, Cohen HS. Visual dependence and spatial orientation in benign paroxysmal positional vertigo. J Vestib Res. (2018) 27:279–86. doi: 10.3233/VES-170623
42. Racine M. Chronic pain and suicide risk: a comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry. (2018) 87(Pt B):269–80. doi: 10.1016/j.pnpbp.2017.08.020
43. Brandt T, Dieterich M. ‘Excess anxiety' and ‘less anxiety': both depend on vestibular function. Curr Opin Neurol. (2020) 33:136–41. doi: 10.1097/WCO.0000000000000771
44. Hilber P. The role of the cerebellar and vestibular networks in anxiety disorders and depression: the internal model hypothesis. Cerebellum. (2022) 21:791–800. doi: 10.1007/s12311-022-01400-9
45. Elyoseph Z, Geisinger D, Zaltzman R, Hartman TG, Gordon CR, Mintz M. The overarching effects of vestibular deficit: imbalance, anxiety, and spatial disorientation. J Neurol Sci. (2023) 451:120723. doi: 10.1016/j.jns.2023.120723
46. Bednarczuk NF, Casanovas Ortega M, Fluri AS, Arshad Q. Vestibulo-cortical hemispheric dominance: the link between anxiety and the vestibular system? Eur J Neurosci. (2018) 47:1517–24. doi: 10.1111/ejn.13948
47. Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. (2016) 6:603–21. doi: 10.1002/cphy.c150015
Keywords: Mendelian randomization, benign paroxysmal positional vertigo, mental disorders, neuroticism, mood swings
Citation: Liu S, Zhang L, Deng D and Luo W (2024) Associations between benign paroxysmal positional vertigo and seven mental disorders: a two-sample Mendelian randomization study. Front. Neurol. 15:1310026. doi: 10.3389/fneur.2024.1310026
Received: 09 October 2023; Accepted: 18 March 2024;
Published: 09 April 2024.
Edited by:
Sulin Zhang, Huazhong University of Science and Technology, ChinaReviewed by:
Jianyong Chen, Shanghai Jiao Tong University, ChinaJun Wang, Huazhong University of Science and Technology, China
Copyright © 2024 Liu, Zhang, Deng and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dan Deng, ODc4NzIwNTc4QHFxLmNvbQ==
 Shihan Liu
Shihan Liu Lingli Zhang2
Lingli Zhang2 Wenlong Luo
Wenlong Luo