
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 21 June 2024
Sec. Neuro-Ophthalmology
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1305984
 Rosa Buonamassa1†
Rosa Buonamassa1† Giacomo Boscia1*†
Giacomo Boscia1*† Marida Gaudiomonte1
Marida Gaudiomonte1 Silvana Guerriero1
Silvana Guerriero1 Rita Fischetto2
Rita Fischetto2 Alfonso Montepara1
Alfonso Montepara1 Maria Oliva Grassi1
Maria Oliva Grassi1 Maria Grazia Pignataro1
Maria Grazia Pignataro1 Pasquale Puzo1
Pasquale Puzo1 Ermete Giancipoli3
Ermete Giancipoli3 Marina D’addario1
Marina D’addario1 Giovanni Alessio1
Giovanni Alessio1 Francesco Boscia1‡
Francesco Boscia1‡ Pasquale Viggiano1‡
Pasquale Viggiano1‡Purpose: To compare radial peripapillary capillary (RPC) vascular plexus parameters and peripapillary retinal nerve fiber layer (pRNFL) thickness between Early-Treated Adults with Phenylketonuria (ETPKU) and controls.
Methods: This observational study was a monocentric, case control study including 36 eyes of 36 participants. Among these, 18 were early-treated PKU (ETPKU) and 18 were controls. A SD-OCTA (XR Avanti AngioVue OCTA; Optovue Inc., Fremont, CA) was employed to assess the OCT and OCTA parameters of all the participants. The main outcome measures were the RPC vessels density (VD) %, and the pRNFL thickness.
Results: The average pRNFL thickness was significantly reduced in ETPKU (110.78 ± 12.48 μm) compared to controls (113.22 ± 13.95 μm), p = 0.046. The mean VD% of the small vessels of the RPC plexus was 52.31 ± 2.2 in ETPKU and 50.71 ± 3.2 in controls (p = 0.049), while the VD% of all the radial peripapillary capillary plexus (RPCP) was 58.5 ± 2.2 in ETPKU and 55.08 ± 3.4 in controls (p < 0.001). By contrast, there were no differences in age, sex, and IOP between the two groups.
Conclusion: Through structural OCT and OCTA, we observed thinning of the nerve fibers accompanied by an increase in perfusion of the RPC plexus. Thus, our conclusions suggest that OCTA may serve as a noninvasive method to identify novel retinal biomarkers in ETPKU.
Phenylalanine hydroxylase (PAH) deficiency, conventionally identified as Phenylketonuria (PKU), is an autosomal recessive inherited metabolic disorder marked by various mutations in the gene (1). PAH serves as a catabolic enzyme responsible for converting L-phenylalanine (L-Phe) into L-tyrosine (L-Tyr), a process reliant on molecular oxygen, iron, and tetrahydrobiopterin (BH4) (2). Such deficiency leads to an impaired degradation of L-Phe and its metabolites, resulting in elevated levels in the blood. The hyperphenylalaninaemia and the increased Phe: Tyr ratio leads to Phe and Tyr to compete to cross the blood–brain barrier and tissues of affected individuals, resulting in an accumulation of Phe in the brain, especially in the White Matter (3). The prevalence of PKU varies worldwide, with an incidence of 1:10,000 newborn in Europe (4). As PKU has autosomal recessive inheritance, consanguineous marriage is an important risk factor; thus, high disease incidence is expectable for countries with a high rate of consanguineous marriages (5).
Untreated PKU can lead to a severe neurological impairment and progressive intellectual disability, accompanied by several additional symptoms such as eczematous rash, epilepsy, autism, seizures, and motor deficits (6). The current classification of PKU distinguishes less severe forms of PAH deficiency, referred to as moderate PKU, mild PKU, mild hyperphenylalaninaemia (HPA) or benign HPA, and more severe manifestations referred to as classic PKU (2). The neuropathology is determined by the increased levels of L-Phe, leading to disruptions in neuronal dendritic outgrowth and synaptic connectivity, as well as impaired cerebral glucose metabolism and neurotransmitters deficiency. The primary marker of disease progression includes white matter lesions (WMLs), which correlate with both metabolic control and the patient’s age (2).
To date, the mainstay of the treatment involves limiting dietary phenylalanine intake, coupled with daily supplementation of Phe-free amino acids. Indeed, the introduction of the neonatal screening allowed early intervention for this condition, with a consequent prevention of most of neuropsychological complications and an improved quality of life (3). By consequence, individuals diagnosed with PKU and subjected to lifelong therapy from birth have significantly reduced the incidence of the more severe impairments associated with untreated PKU. This specific group is termed early-treated PKU (ETPKU) (7).
In such a scenario, ocular findings include photophobia, ocular hypopigmentation, cataract and corneal opacities (8), and various functional visual impairment such as a lower Best-corrected visual acuity (BCVA) and a lower contrast sensitivity (9). The introduction of optical coherence tomography (OCT) has facilitated the identification of retinal neurodegeneration across various systemic and ocular conditions (10). Specifically, earlier researchers have directed their attention towards assessing the thickness of the retinal nerve fiber layer (pRNFL) and the ganglion cell layer (GCC), proposing these measurements as potential biomarkers for neurodegeneration (11).
Furthermore, due to the recent advancements in ophthalmic imaging techniques, notably OCTA, the ability to visualize vascular disorders has expanded to encompass various neurodegenerative conditions, including Parkinson’s disease (12) (PD).
Given the retina’s status as an extension of the central nervous system and shares numerous common characteristics with the central nervous system’s vasculature, the potential identification of retinal structural changes through OCT and vascular alterations using OCTA in ETPKU could potentially provide insights into overarching cerebral modifications that manifest during the initial stages of the disease. Consequently, the primary objective of this study was to explore changes in pRNFL thickness and the radial peripapillary capillary (RPC) plexus in individuals with ETPKU when contrasted with controls.
This retrospective observational study was a monocentric, case control study including 36 eyes of 36 participants. Among these, 18 patients had ETPKU and were receiving treatment exclusively through dietary protein restriction, while the remaining 18 participants served as controls. ETPKU patients were recruited from Genetics Department of University of Bari “Aldo Moro” and were addressed for the ophthalmological assessment at the Department of Ophthalmology, University of Bari “Aldo Moro,” Italy, spanning from October 2022 to April 2023. The current investigation was performed in compliance with the tenets of the Declaration of Helsinki for research involving human subjects and approved by the Ethical Committee of this study is retrospective in nature, and in accordance with Italian regulations, ethical approval is not necessary for such studies. Instead, only notification to the ethics committee is required. We reached out to all enrolled individuals and their parents to secure their written informed consent for the retrospective utilization of their clinical information.
All patients underwent a comprehensive ophthalmological examination, including best-corrected visual acuity (BCVA), two consecutive measurements of intraocular pressure (IOP) by means a non-contact tonometer (Topcon CT-1P), slit-lamp biomicroscopy of the anterior segment and dilated fundus examination. All patients were imaged with XR Avanti AngioVue OCTA (Optovue Inc., Fremont, CA). An independent ophthalmologist (GB) was responsible for selecting and exporting high-quality OCT images in accordance with the recommended extension to the OSCAR IB and APOSTEL guidelines for OCT images. Subsequently, the following data were analyzed.
This study enrolled patients who had received a diagnosis of PKU through neonatal screening. The inclusion criteria for participants were as follows: (i) a verified PKU diagnosis from birth, (ii) age 18 years or older, (iii) of Caucasian descent, (iv) ongoing treatment exclusively through dietary protein restriction, (v) a record of consistent compliance, including regular annual check-ups at the adult metabolic center, and (vi) no previous history of other systemic or ocular disorders. It’s worth noting that, in alignment with European guidelines, all enrolled PKU patients were categorized as having “mild PKU” and demonstrated good adherence to dietary (13, 14) restrictions.
The exclusion criteria were (i) ocular comorbidities such as refractive error over ±5.5 diopters of spherical equivalent, previous ocular trauma, history of ocular disease, e.g., macular degeneration and glaucoma, (ii) history of a systemic condition affecting the retina, e.g., diabetes, hypertension, (iii) any other neurological condition (iv) prematurity (<36 weeks) (v), pregnancy (vi) or other medical treatment potentially interfering with HPA.
The control group was carefully matched to the ETPKU patient group in terms of age, ethnicity, and gender, as these are well-known factors that can influence OCT and OCTA results. Regarding the matching by age, we considered an interval of birth of 1–2 years. The inclusion criteria for the control group were as follows: (a) of Caucasian ethnicity, (b) no previous history of systemic or ocular disorders, and (c) age 18 years or older. As for the ETPKU patient group, all case-matched controls underwent a comprehensive ophthalmological examination, including BCVA, two consecutive measurements of IOP by means a non-contact tonometer (Topcon CT-1P), slit-lamp biomicroscopy of the anterior segment and dilated fundus examination.
The main outcome measures were: (i) RPC density [vessels density (VD) %], and (ii) pRNFL thickness. Secondary outcome measures were: (i) BCVA, (ii) IOP. All patients were imaged with an ultrahigh-resolution spectral domain OCT (SD-OCT) system, specifically the AngioVueTM (RTVue-XR Avanti; Optovue Inc., Fremont, CA, USA, software version 2018.0.0.18). The software automatically segmented the scans, and a single, well-trained examiner performed the measurements on the same day. Intraocular pressures were checked by means a non-contact tonometer (Topcon CT-1P).
OCTA imaging was conducted to assess the radial peripapillary capillary (RPC) plexus across the entire image and peripapillary vessel densities in all study participants. The SD-OCTA device employed in this study had an A-scan rate of 70,000 scans per second, utilized a light source centered at 840 nm, and had a full-width at half maximum bandwidth of 45 nm. To minimize motion artifacts, the RTVue-XR Avanti system acquired two consecutive OCTA volume scans (one horizontal and one vertical), each comprising 400 A-scans, which were then combined. Images from five patients captured using OCTA technology did not meet the required quality standards; therefore, these patients were excluded from the analysis. Additionally, to uphold the high quality of the OCTA images, we applied and adhered to the OSCAR-MP criteria (15).
A three-dimensional optic disc scan covering an area of 4.5 × 4.5 mm^2, centered on the optic disc, was captured. Vessel density was quantified as the percentage of the area occupied by vessels. Overall and small vessel density were analyzed (Figure 1). The term “small vessel density” pertains to the assessment of the finest peripapillary vessels, excluding the larger vessels, which are instead taken into account in the evaluation of the “overall vessel density.” This analysis is performed automatically by the software within the device.
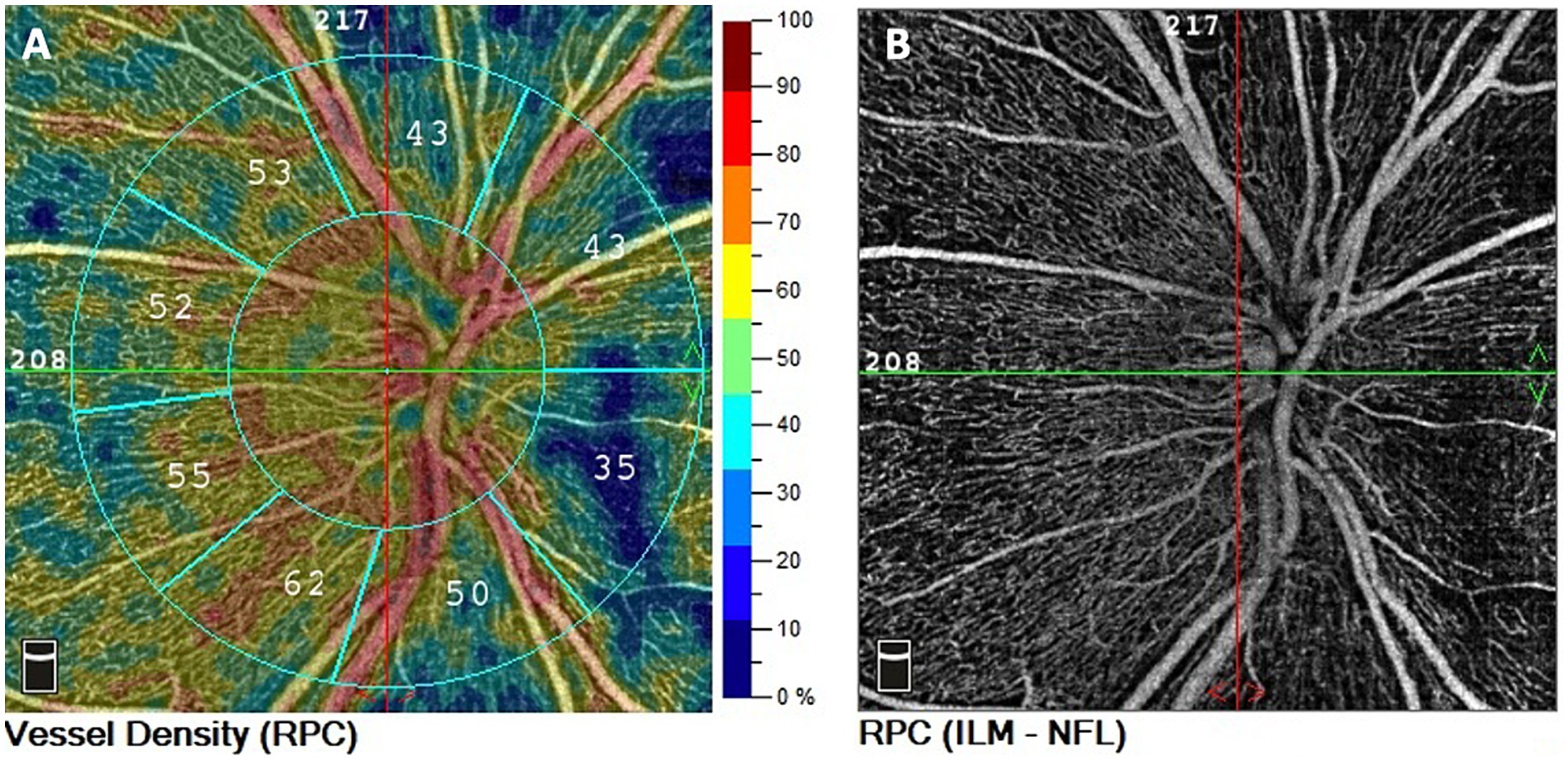
Figure 1. Representation of peripapillary OCT angiography in ETPKU patient. OCTA scan 4.5 × 4.5 mm centered on ONH. (A) Color-coded density maps and automatized vessel density measurements of the RPC. (B) RPC vascular density image.
In cases where segmentation was compromised, manual correction was applied. Additionally, images with poor quality (Signal Strength Index, SSI, less than 6/10), significant motion artifacts, or incorrect segmentation were excluded from the analysis. The pRNFL thickness was obtained using disc map software on the RTvueXR Avanti device, with measurements being repeated until images of at least 9/10 quality were obtained.
All quantitative variables were reported as mean and standard deviation (SD). Data were assessed for normality with Shapiro–Wilk test for all variables. By employing a Sample Size calculator, we determined the power of the sample size to guarantee a 95% confidence level that the true value is within ±5% of the measured/detected value. Differences between the two groups were tested by the independent t test. A Pearson correlation analysis was conducted to examine the correlation between the duration of the disease and the OCT and OCTA parameters. All statistical analyses were performed using Statistical Package for Social Sciences (version 20.0; SPSS Inc., Chicago, IL). The chosen level of statistical significance was p < 0.05.
36 eyes of 36 patients (18 patients with ETPKU and 18 controls) were included in this analysis. Demographic information and biometric characteristic of the study population are summarized in Table 1. In the patient’s group, the mean age was 30.22 ± 4.02 years (range, 25–36; 10 males,8 females), compared to the control group where the mean age was 29.78 ± 2.48 years (range, 27–36; 10 males,8 females). The eye selected for the study was the one with superior imaging quality. All our patients belonged to the Caucasian ethnicity. We excluded from our sample a ETPKU patient belonging to Asian ethnicity because we could not perform a correct match with a control.
The average pRNFL thickness was 113.22 ± 13.95 μm in controls and 110.78 ± 12.48 μm in patients with ETPKU, significantly reduced compared with controls (p = 0.046) (Table 1; Figure 2).
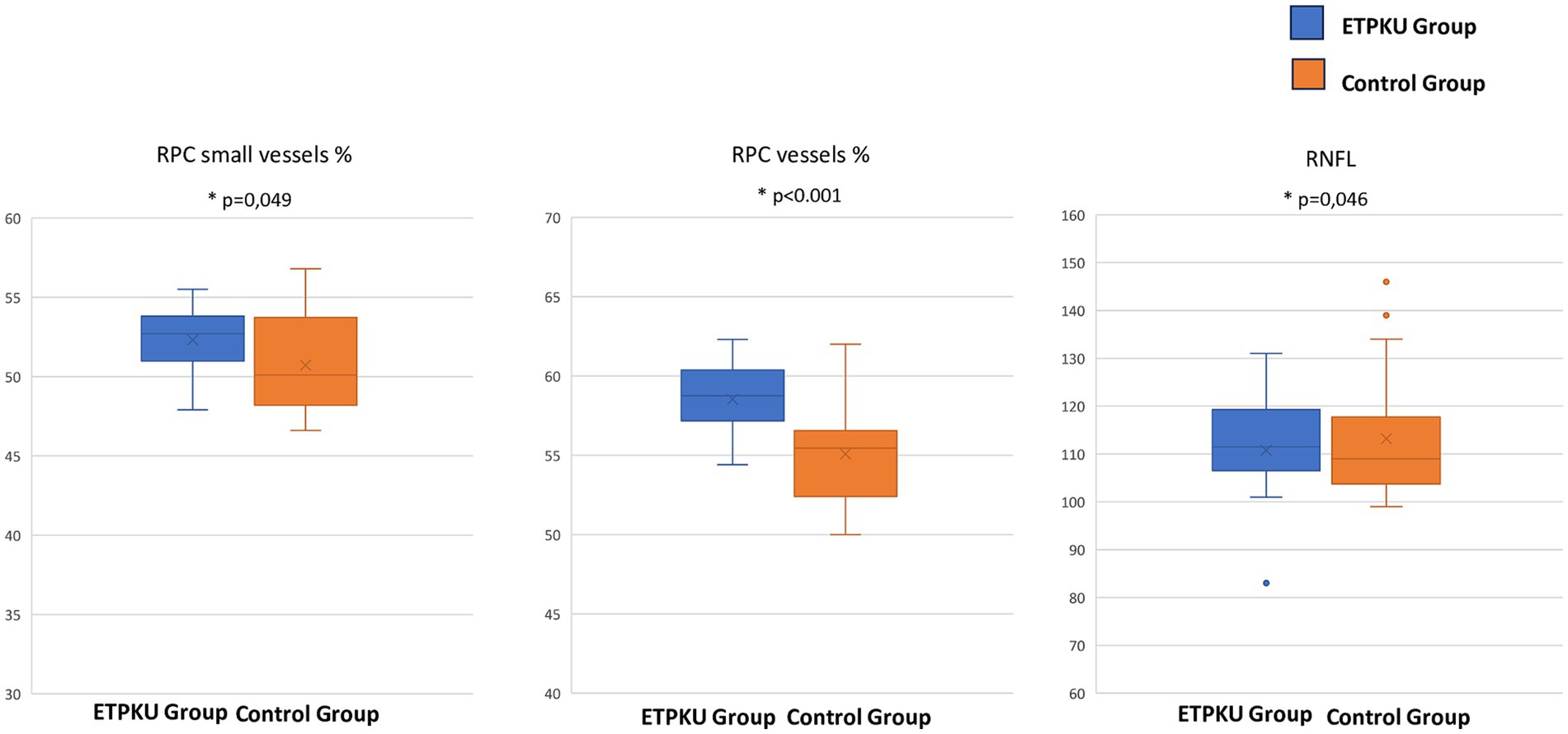
Figure 2. Box and whisker plots showing radial peripapillary capillary plexus vessels density % of the small vessels, of all the plexus and the retinal nerve fiber layer thickness (μm). Each box displays mean (cross within the box), median (central horizontal line) and interquartile range (horizontal extremes of the box) values for each metric. The ends of the whiskers illustrate the minimum and maximum values. Outliers are visualized as dots not included in whiskers. Each graph reports comparisons between the groups for a specific metric (p = 0.049, p < 0.001, and p = 0.046, respectively).
The mean VD% of the small vessels of the RPC was 52.31 ± 2.2 in ETPKU and 50.71 ± 3.2 in controls (p = 0.049), while the VD% of all the RPCP was 58.5 ± 2.2 in ETPKU and 55.08 ± 3.4 in controls (p < 0.001) (Table 1; Figure 2).
Mean BCVA was 0.02 ± 0.04 LogMAR in ETPKU and 0.01 ± 0.03 in controls, while mean IOP was 14.77 ± 2.1 in ETPKU and 14.55 ± 1.8 in controls. There were no differences in age, sex, and IOP between the two groups. There was no correlation between main outcomes and duration of the disease (Table 1).
We investigated the potential association between the duration of the pathology (21.3 ± 2.8 years) and the OCT (pRNFL thickness) and OCTA (RPC density) parameters. Through Pearson correlation analysis, we found no correlation with either pRNFL thickness (r = − 0.104, p = 0.411) or RPC density (r = − 0.089, p = 0.248).
In this observational study, we exanimated the potential role of radial peripapillary capillary density and peripapillary retinal nerve fiber layer as markers of retinal damage in individuals with ETPKU compared to age-matched controls. Our findings align with previous research revealing a significant reduction in pRNFL thickness in ETPKU patients. Interestingly, we also observed that ETPKU patients exhibited a higher density of the total RPC compared to controls, suggesting an increased vascular activity associated with the disease.
ETPKU patients, thanks to neonatal screening and the early initiation of life-long phenylalanine-lowering therapy, benefit from protection against the most severe consequences of the disease (16). Nevertheless, ETPKU patients have more pronounced issues in social functioning and neurocognitive problems (17).
Recently, several researchers explored OCT and OCTA findings in ETPKU patients. For instance, Serfozo and coworkers observed significant thinning of both pRNFL and GCC in ETPKU compared to controls. This suggests that ocular microstructural abnormalities in PKU patients might be influenced by dietary control (13). Subsequently, Lotz-Havla and coworkers corroborated these findings. They also noted a reduction of the inner plexiform layer (IPL) volume, and an inner nuclear layer (INL) swelling strongly correlated with the metabolic control (18). These findings are partly consistent with our results, as we did not measure macular thickness, but we observed a thinning in the pRNFL thickness ETPKU patients who were solely treated with dietary control. Several studies have reported a significant correlation between pRNFL thinning and cerebral neurodegeneration, suggesting that average pRNFL might serve as a suitable biomarker for detecting and monitoring the progression of conditions such as Parkinson’s disease (PD) (19). The pattern of impairment observed in PKU, including ETPKU patients, shares similarities with other conditions characterized by dopamine imbalances and white matter damage such as PD or multiple sclerosis (MS) (20). In a recent review from Lancet, the authors stated that measurements of pRNFL and macular GCL and IPL combined could be an important tool for diagnosis, monitoring and research of MS (20). Specifically, in PKU and ETPKU patients, elevated phenylalanine (Phe) levels in the brain can inhibit the activities of enzymes like tyrosine and tryptophan hydroxylases activities, causing a reduction of dopamine (DA) and serotonin biosynthesis (21).
In the eye, DA is released by amacrine cells, activates dopamine receptors distributed throughout the retina, and covers multiple trophic roles related to circadian rhythmicity, cell survival, and eye growth (22). Consequently, conditions of metabolic dysregulation of DA like PKU, have been showed to be related to a DA imbalance in the retina as well (23). The exact mechanism of neurotoxicity on the retina in PKU is not clearly understood, but, according to one of the hypotheses, altered DA levels and impaired neurotransmission, are discussed to play a role in the retinal damage (21).
In addition to using OCT angiography, this study also evaluated the perfusion of the peripapillary vascular plexus. A previous OCTA study on ETPKU individuals by Serfozo et al. revealed a decrease in parafoveal superficial capillary plexus density in ETPKU compared to controls (24). However, our findings demonstrated a significant increase in RPC perfusion in ETPKU patients.
Given the aforementioned common pathways of retinal damage among DA imbalance diseases, it is not surprising the increased RPC density we found in ETPKU patients compared to controls (25). Indeed, the RPC is a network of capillaries that has a unique anatomic organization since it runs in parallel with retinal nerve fiber layer axon bundles and plays a role in nourishing the axons of retinal ganglion cells in peripapillary area (24).
We speculate that the neuroinflammation and the cellular stress may lead to an increased amount of vasodilatory and vasogenic mediators, resulting in enhanced blood flow to pRNFL axons. A similar mechanism has been proposed in Parkinson’s disease (26). This hypothetical condition might explain the simultaneous increase in RPC density and pRNFL thinning we found. Another potential explanation may be the formation of dysfunctional capillaries similar to the string vessels of Alzheimer’s disease.
The present study has limitations to consider when interpreting our findings. Our sample size was relatively small, which may limit the results generalizability. Furthermore, it’s important to acknowledge that our analysis focused solely on one phenotype of these patients (ETPKU), which may introduce a potential bias in our results. We analyzed OCTA images using spectral domain OCTA which utilizes shorter wavelength light in comparison with swept source OCT angiography. We did not measure the macular thickness and we did not compare the retinal measurements with brain parameters correlated to neurocognitive impairment. Additionally, we did not investigate the correlation between the duration of the pathology and the quantitative OCTA parameters analyzed.
Future randomized prospective case–control trials are necessary to confirm our findings, as well as longitudinal studies are always recommended for establishing the validity of such biomarkers.
In conclusion, our observational study provides evidence of neurovascular impairment in ETPKU patients who were solely treated with dietary control when compared to controls. Through structural OCT and OCT angiography, we observed thinning of the peripapillary nerve fibers accompanied by an increase in perfusion of the peripapillary vascular plexus. We suggest that, similar to other neurodegenerative diseases, increased neuroinflammation may lead to a higher production of vasodilatory mediators, resulting in an augmented vessel density in the RPC. These findings warrant further investigation as they may serve as an additional retinal biomarker in individuals with ETPKU.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants.
RB: Writing – original draft. GB: Formal analysis, Methodology, Supervision, Writing – review & editing. MGa: Data curation, Writing – original draft. SG: Supervision, Writing – original draft. RF: Data curation, Writing – original draft. AM: Conceptualization, Writing – original draft. MGr: Conceptualization, Writing – review & editing. MP: Data curation, Writing – original draft. PP: Conceptualization, Writing – original draft. EG: Conceptualization, Writing – review & editing. MD’a: Data curation, Writing – original draft. GA: Conceptualization, Writing – review & editing. FB: Conceptualization, Writing – review & editing. PV: Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Flydal, MI, and Martinez, A. Phenylalanine hydroxylase: function, structure, and regulation. IUBMB Life. (2013) 65:341–9. doi: 10.1002/iub.1150
2. van Spronsen, FJ, Blau, N, Harding, C, Burlina, A, Longo, N, and Bosch, AM. Phenylketonuria. Nat Rev Dis Primers. (2021) 7:1–19. doi: 10.1038/s41572-021-00267-0
3. Vockley, J, Andersson, HC, Antshel, KM, Braverman, NE, Burton, BK, Frazier, DM, et al. Phenylalanine hydroxylase deficiency: diagnosis and management guideline. Genet Med. (2014) 16:188–200. doi: 10.1038/gim.2013.157
4. Loeber, JG. Neonatal screening in Europe; the situation in 2004. J Inherit Metab Dis. (2007) 30:430–8. doi: 10.1007/s10545-007-0644-5
5. Shoraka, HR, Haghdoost, AA, Baneshi, MR, Bagherinezhad, Z, and Zolala, F. Global prevalence of classic phenylketonuria based on neonatal screening program data: systematic review and meta-analysis. Clin Exp Pediatr. (2020) 63:34–43. doi: 10.3345/kjp.2019.00465
6. Romani, C, Olson, A, Aitkenhead, L, Baker, L, Patel, D, Spronsen, FV, et al. Meta-analyses of cognitive functions in early-treated adults with phenylketonuria. Neurosci Biobehav Rev. (2022) 143:104925. doi: 10.1016/j.neubiorev.2022.104925
7. Enns, GM, Koch, R, Brumm, V, Blakely, E, Suter, R, and Jurecki, E. Suboptimal outcomes in patients with PKU treated early with diet alone: revisiting the evidence. Mol Genet Metab. (2010) 101:99–109. doi: 10.1016/j.ymgme.2010.05.017
8. Zwaan, J. Eye findings in patients with phenylketonuria. Arch Ophthalmol. (1983) 101:1236–7. doi: 10.1001/archopht.1983.01040020238016
9. Gramer, G, Förl, B, Springer, C, Weimer, P, Haege, G, Mackensen, F, et al. Visual functions in phenylketonuria-evaluating the dopamine and long-chain polyunsaturated fatty acids depletion hypotheses. Mol Genet Metab. (2013) 108:1–7. doi: 10.1016/j.ymgme.2012.10.021
10. Vujosevic, S, Parra, MM, Hartnett, ME, O’Toole, L, Nuzzi, A, Limoli, C, et al. Optical coherence tomography as retinal imaging biomarker of neuroinflammation/neurodegeneration in systemic disorders in adults and children. Eye (Lond). (2023) 37:203–19. doi: 10.1038/s41433-022-02056-9
11. Zhou, W-C, Tao, J-X, and Li, J. Optical coherence tomography measurements as potential imaging biomarkers for Parkinson’s disease: a systematic review and meta-analysis. Eur J Neurol. (2021) 28:763–74. doi: 10.1111/ene.14613
12. Tsokolas, G, Tsaousis, KT, Diakonis, VF, Matsou, A, and Tyradellis, S. Optical coherence tomography angiography in neurodegenerative diseases: a review. Eye Brain. (2020) 12:73–87. doi: 10.2147/EB.S193026
13. Guldberg, P, Rey, F, Zschocke, J, Romano, V, François, B, Michiels, L, et al. A European multicenter study of phenylalanine hydroxylase deficiency: classification of 105 mutations and a general system for genotype-based prediction of metabolic phenotype. Am J Hum Genet. (1998) 63:71–9. doi: 10.1086/301920
14. Serfozo, C, Barta, AG, Horvath, E, Sumanszki, C, Csakany, B, Resch, M, et al. Altered visual functions, macular ganglion cell and papillary retinal nerve fiber layer thickness in early-treated adult PKU patients. Mol Genet Metab Rep. (2020) 25:100649. doi: 10.1016/j.ymgmr.2020.100649
15. Wicklein, R, Yam, C, Noll, C, Aly, L, Banze, N, Romahn, EF, et al. The OSCAR-MP consensus criteria for quality assessment of retinal optical coherence tomography angiography. Neurol Neuroimmunol Neuroinflamm. (2023) 10:e200169. doi: 10.1212/NXI.0000000000200169
16. Blau, N, van Spronsen, FJ, and Levy, HL. Phenylketonuria. Lancet. (2010) 376:1417–27. doi: 10.1016/S0140-6736(10)60961-0
17. ten Hoedt, AE, de Sonneville, LMJ, Francois, B, ter Horst, NM, Janssen, MCH, Rubio-Gozalbo, ME, et al. High phenylalanine levels directly affect mood and sustained attention in adults with phenylketonuria: a randomised, double-blind, placebo-controlled, crossover trial. J Inherit Metab Dis. (2011) 34:165–71. doi: 10.1007/s10545-010-9253-9
18. Lotz-Havla, AS, Weiß, K, Schiergens, K, Regenauer-Vandewiele, S, Parhofer, KG, Christmann, T, et al. Optical coherence tomography to assess neurodegeneration in phenylalanine hydroxylase deficiency. Front Neurol. (2021) 12:780624. doi: 10.3389/fneur.2021.780624
19. Chrysou, A, Jansonius, NM, and van Laar, T. Retinal layers in Parkinson’s disease: a meta-analysis of spectral-domain optical coherence tomography studies. Parkinsonism Relat Disord. (2019) 64:40–9. doi: 10.1016/j.parkreldis.2019.04.023
20. Petzold, A, Balcer, LJ, Calabresi, PA, Costello, F, Frohman, TC, Frohman, EM, et al. Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. (2017) 16:797–812. doi: 10.1016/S1474-4422(17)30278-8
21. González, MJ, Gassió, R, Artuch, R, and Campistol, J. Impaired neurotransmission in early-treated phenylketonuria patients. Semin Pediatr Neurol. (2016) 23:332–40. doi: 10.1016/j.spen.2016.11.007
22. Witkovsky, P. Dopamine and retinal function. Doc Ophthalmol. (2004) 108:17–39. doi: 10.1023/b:doop.0000019487.88486.0a
23. Djamgoz, MB, Hankins, MW, Hirano, J, and Archer, SN. Neurobiology of retinal dopamine in relation to degenerative states of the tissue. Vis Res. (1997) 37:3509–29. doi: 10.1016/S0042-6989(97)00129-6
24. Serfozo, C, Barta, AG, Horvath, E, Sumanszki, C, Csakany, B, Resch, M, et al. Reduced macular thickness and macular vessel density in early-treated adult patients with PKU. Mol Genet Metab Rep. (2021) 27:100767. doi: 10.1016/j.ymgmr.2021.100767
25. Robbins, CB, Grewal, DS, Thompson, AC, Soundararajan, S, Yoon, SP, Polascik, BW, et al. Identifying Peripapillary radial capillary plexus alterations in Parkinson’s disease using OCT angiography. Ophthalmol Retina. (2022) 6:29–36. doi: 10.1016/j.oret.2021.03.006
Keywords: early-treated adults with phenylketonuria, phenylketonuria, pRNFL, radial peripapillary capillary plexus, OCTA, retinal imaging
Citation: Buonamassa R, Boscia G, Gaudiomonte M, Guerriero S, Fischetto R, Montepara A, Grassi MO, Pignataro MG, Puzo P, Giancipoli E, D’addario M, Alessio G, Boscia F and Viggiano P (2024) Neurovascular retinal impairment in early-treated adults with phenylketonuria. Front. Neurol. 15:1305984. doi: 10.3389/fneur.2024.1305984
Received: 02 October 2023; Accepted: 04 June 2024;
Published: 21 June 2024.
Edited by:
Enrico Borrelli, Vita-Salute San Raffaele University, ItalyReviewed by:
Ane Murueta-Goyena, University of the Basque Country, SpainCopyright © 2024 Buonamassa, Boscia, Gaudiomonte, Guerriero, Fischetto, Montepara, Grassi, Pignataro, Puzo, Giancipoli, D’addario, Alessio, Boscia and Viggiano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giacomo Boscia, Ym9zY2lhZ2lhY29tb0BnbWFpbC5jb20=
†These authors have contributed equally to this work
‡These authors have contributed equally to this work and share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.