
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol. , 05 February 2024
Sec. Neurotrauma
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1301003
 Celine Iswarya Partha Sarathi1
Celine Iswarya Partha Sarathi1 Amil Sinha1
Amil Sinha1 Amir Rafati Fard1
Amir Rafati Fard1 Faheem Bhatti1
Faheem Bhatti1 Tanzil Rujeedawa1
Tanzil Rujeedawa1 Shahzaib Ahmed1
Shahzaib Ahmed1 Melika Akhbari1
Melika Akhbari1 Aniqah Bhatti1
Aniqah Bhatti1 Aria Nouri2
Aria Nouri2 Mark R. Kotter1
Mark R. Kotter1 Benjamin M. Davies1
Benjamin M. Davies1 Oliver D. Mowforth1*
Oliver D. Mowforth1*Introduction: Degenerative cervical myelopathy (DCM) is a form of chronic spinal cord injury, with a natural history of potential for progression over time. Whilst driven by mechanical stress on the spinal cord from degenerative and congenital pathology, the neurological phenotype of DCM is likely to be modified by multiple systemic factors. The role of metabolic factors is therefore of interest, particularly given that ischaemia is considered a key pathological mechanism of spinal cord injury. The objective was therefore to synthesise current evidence on the effect of metabolism on DCM susceptibility, severity, and surgical outcomes.
Methods: A systematic review in MEDLINE and Embase was conducted following PRISMA guidelines. Full-text papers in English, with a focus on DCM and metabolism, including diabetes, cardiovascular disease, anaemia, and lipid profile, were eligible for inclusion. Risk of methodological bias was assessed using the Joanna Briggs Institute (JBI) critical assessment tools. Quality assessments were performed using the GRADE assessment tool. Patient demographics, metabolic factors and the relationships between metabolism and spinal cord disease, spinal column disease and post-operative outcomes were assessed.
Results: In total, 8,523 papers were identified, of which 57 met criteria for inclusion in the final analysis. A total of 91% (52/57) of included papers assessed the effects of diabetes in relation to DCM, of which 85% (44/52) reported an association with poor surgical outcomes; 42% of papers (24/57) discussed the association between cardiovascular health and DCM, of which 88% (21/24) reported a significant association. Overall, DCM patients with diabetes or cardiovascular disease experienced greater perioperative morbidity and poorer neurological recovery. They were also more likely to have comorbidities such as obesity and hyperlipidaemia.
Conclusion: Metabolic factors appear to be associated with surgical outcomes in DCM. However, evidence for a more specific role in DCM susceptibility and severity is uncertain. The pathophysiology and natural history of DCM are critical research priorities; the role of metabolism is therefore a key area for future research focus.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier: CRD42021268814.
Degenerative cervical myelopathy (DCM) is a condition of spinal cord dysfunction secondary to mechanical stress from congenital and/or degenerative changes, such as cervical canal stenosis, intervertebral disc herniation, spondylosis, ligament hypertrophy, calcification and ossification (1). It is estimated to affect as many as 2% of adults (2), and given its association with age, incidence is expected to rise as populations age (3). Patients experience a range of disabilities including pain and stiffness, loss of dexterity, bladder and bowel dysfunction (4, 5). This also has significant impacts on those around them and society as a whole (6).
There remain many clinical research uncertainties in DCM, with two of the most fundamental uncertainties, as established by AO Spine RECODE-DCM (7–9), relating to pathobiology and natural history (10–12). For example, whilst spinal cord compression is considered a pathological hallmark of DCM, its detection on MRI is most commonly an incidental finding (3) and does not correlate with disease severity (13). Moreover, disease trajectory, particularly in the early and milder stages of the disease is heterogenous and unpredictable (14, 15). This suggests that additional factors may play a role in influencing spinal cord damage and disease progression (11). Understanding these factors will be important to better inform clinical care.
Age, smoking status and presence of comorbidities have previously been identified as important predictors of outcomes, however their weight on the progression of DCM and response to medical/surgical treatment remains to be further investigated (16). Cardiovascular disease is a prominent global health problem and is closely associated with altered metabolism in the context of obesity and decreased physical activity (17). The World Health Organisation (WHO) defines the metabolic syndrome as a pathological condition characterised by obesity, insulin resistance, hypertension, and hyperlipidaemia. In practise, metabolism encompasses themes such as diabetes, cardiovascular health, and lipids. Both aberrant metabolism and spinal cord hypoperfusion have been proposed as mechanisms of spinal cord injury in DCM (15, 18).
These comorbidities may have further implications for DCM (19). Firstly, degenerative spinal pathology is more prevalent with obesity; for example, the prevalence of degenerative disc disease is higher in those with the metabolic syndrome (20). Secondly, DCM is treated with surgery and the decision to undergo surgery entails a balance of risks and benefits. Surgical patients with metabolic disorders are at higher risk of a range of adverse outcomes, including death, cardiovascular events, stroke, renal failure, surgical site infections, prolonged hospital stays and have a greater need for post-hospitalisation rehabilitation (21). Whilst in more advanced forms of the disease the benefits of surgery are more certain, in milder forms of the disease this may not necessarily be the case, and decision making needs to be tailored to the individual circumstances (22).
The objective of this review was therefore to assess the current evidence for metabolic dysfunction in DCM, and specifically to synthesise evidence relating metabolic dysfunction to disease onset, severity and surgical outcomes.
A systematic review was conducted with reference to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 checklists (Supplementary Data 1) (23). The protocol was registered on PROSPERO (ID: CRD42021268814).
All primary clinical studies, available in English, considering an aspect of metabolism in the context of DCM were considered eligible for inclusion. Animal studies, case reports, editorials, reviews, opinion articles, corrections and conference papers were excluded. Metabolism was defined as the capability of the body to adapt its endocrine environment according to supply and demand for fuel; such metabolic regulation can be affected by many factors over the course of several years (24). We utilised the WHO definition of metabolic disorders to categorise factors into diabetes and cardiovascular health.
A search of Embase and MEDLINE using Ovid for all papers published until January 2023 was performed using a modified version of a previously published DCM search strategy (25, 26). The full search terms are outlined in Supplementary Data 2.
Title and abstract screening were completed using Rayyan (Rayyan Systems Inc., Cambridge, MA, United States). Studies were independently screened in duplicate by seven authors (CP, FB, AS, MA, AB, SA and TR); a pilot of 100 records were screened by all reviewers to ensure concordance. Discrepancies were settled by discussion and mutual agreement.
Manual data extraction was completed by seven authors (CP, FB, AS, MA, AB, SA and TR) in Microsoft Excel (Version 16.63, Microsoft 365) using a piloted extraction form (Supplementary Data 3). Details of the study design, cohort demographics, intervention(s), metabolic factor(s) and outcomes were extracted.
Risk of methodological bias in individual studies was assessed by two authors (AR and AS) using the Joanna Briggs Institute (JBI) critical assessment tools for cohort or analytical cross-sectional studies depending on study type (Supplementary Data 4) (27).
Due to heterogeneity in study design and data reporting, a qualitative synthesis was performed in accordance with the Synthesis without Meta Analysis (SWiM) guidelines (28). In order to consider the differing implications of metabolic disease on DCM, reported study outcomes were categorised into those relating to spinal column disease (e.g., radiological features of spondylosis), spinal cord disease (e.g., neurological examination, patient-reported outcome measures and recovery rate with treatment) and those relating specifically to the surgical procedure (e.g., adverse events, such as infection). This approach aimed to discern effects of metabolism on spinal cord vulnerability, as opposed to spondylosis or surgical risk (29). Not all studies included outcomes in all three subgroups. Further categorisation for identified metabolic factors was developed to group evidence into diabetes and cardiovascular disease. Studies that considered more than one metabolic factor (i.e., both diabetes and cardiovascular disease) were assigned to both categories. Adverse events of surgery were categorised using the criteria proposed by Tetreault et al. (30).
Confidence in the body of evidence for included studies was assessed using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework (31). A harvest plot was created to provide a visual representation of the GRADE tool results for each paper (Figure 1).
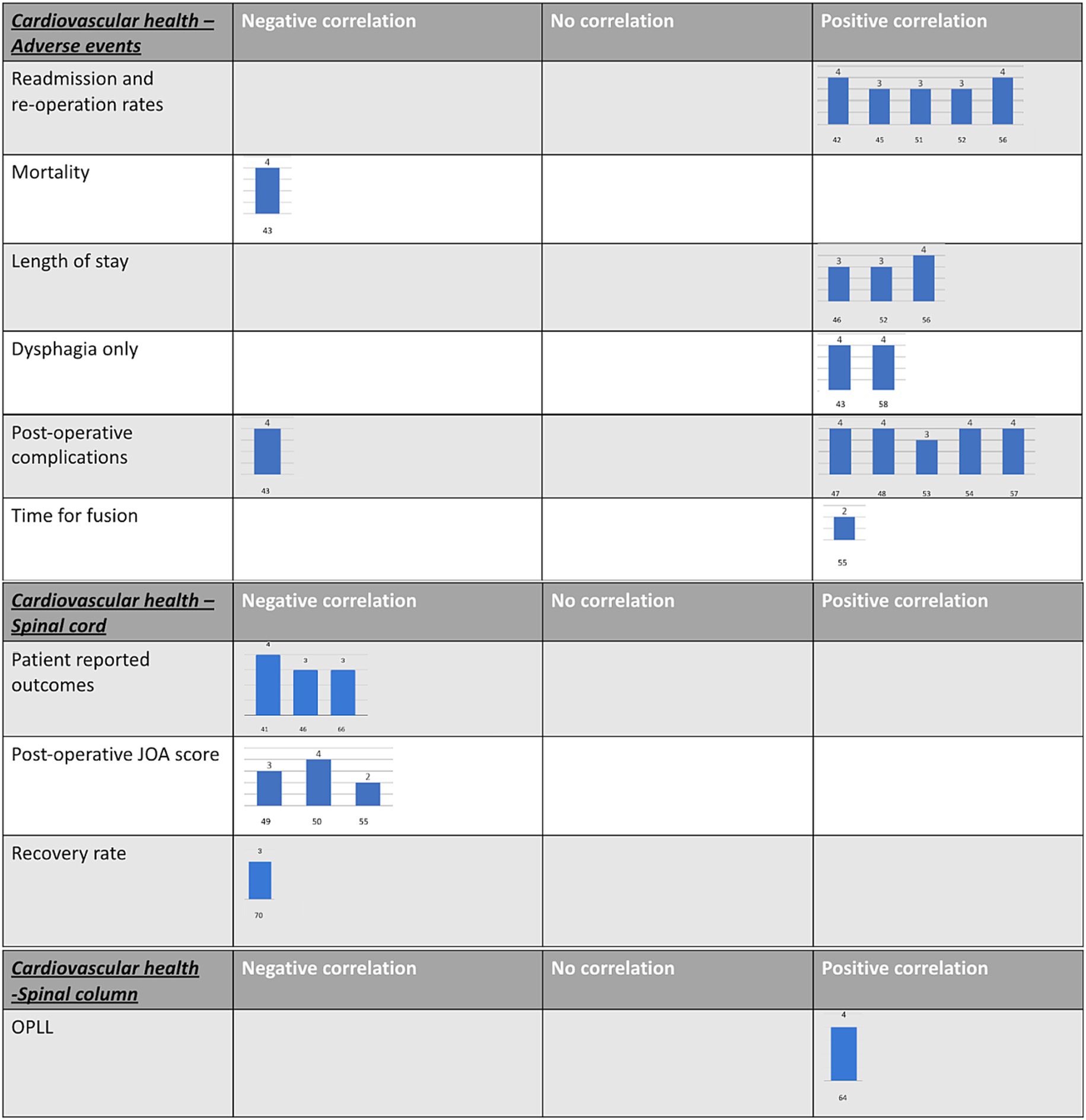
Figure 1. Harvest plots. These were created to provide a visual representation of the GRADE tool results for each paper. (A) A guide on how to interpret the harvest plots. (B) Diabetes harvest plots. (C) Cardiovascular harvest plots.
A total of 8,523 papers were screened, identifying 57 articles focused on metabolic disease in the context of DCM (Figure 2). Of these, 52/57 papers (91%) assessed the effect of diabetes, and 24/57 (42%) papers assessed cardiovascular health. The majority (50/57, 88%) were observational cohort studies (prospective or retrospective), with cohort sizes ranging from 24 to 202,694. The remaining were cross-sectional studies (7/57, 12%). The majority studied surgical cohorts (55/57, 96%). The confidence in the body of evidence from the included studies using the GRADE framework is outlined in Table 1.
A total of 52 papers studied DCM in the context of diabetes (32–83). In total, 44 papers reported that diabetes was associated with poorer surgical outcomes (32–52, 54–76), whilst no association was reported in differing outcome measures across 15 papers (33, 36, 45, 53, 60, 64, 65, 67, 74, 77–83) (Table 1; Figure 1B).
In DCM patients, diabetes was reported to be associated with several other comorbidities, all of which appear more significant with age (45, 49, 50, 58). In addition, a cohort study of 9,071 patients comparing myelopathy and radiculopathy patients found that on average myelopathy patients were older, more likely to be male and had higher rates of diabetes (38).
A retrospective study of 49 patients with ossification of the posterior longitudinal ligament (OPLL), of which eight also had combined diffuse idiopathic skeletal hyperostosis (DISH), reported that patients with a combination of OPLL and DISH were significantly more likely to have diabetes compared to those with only OPLL (56). However, one study of 23 patients with OPLL reported that OPLL occurrence was significantly higher in non-diabetics than in diabetics (55). Furthermore, a study of 39 patients that developed adjacent segment disease (ASD) after anterior cervical discectomy and fusion (ACDF) reported that diabetes was not a significant predictor for the development of ASD after ACDF (81).
A single-centre Singaporean cohort study of 58 patients (29 diabetic vs. 29 non-diabetic) identified that DCM patients were less satisfied following single-level anterior cervical discectomy and fusion (ACDF) if they were diabetic, with surgery more likely to not meet patient expectations, although this difference was not statistically significant (36). A cohort study of 87 patients that had undergone cervical laminoplasty reported that the Japanese Orthopaedic Association (JOA) score improved significantly in both diabetics and non-diabetics; however, the mean post-operative JOA score and mean recovery rate were significantly higher in non-diabetic patients (35). The same study reported that older diabetic patients with a longer history of symptomatic DCM were also more likely to have a poorer recovery rate (35).
A single-centre cohort study of 78 DCM patients undergoing expansive laminoplasty showed that diabetics had poorer recovery of their motor and sensory function in their lower extremities, with a significant negative correlation between pre-operative glycated haemoglobin (HbA1c) and the 6-month recovery rate (33). Two studies reported that post-operative persistence of gait disturbance, hand numbness and bladder dysfunction occurred significantly more in diabetics undergoing cervical laminoplasty (37, 47). One cohort study, consisting of a total of 505 DCM patients, reported that recovery of lower extremity motor and upper extremity sensory function were significantly lower in the diabetic patients (37). The study also reported that the diabetic group had lower pre-and post-operative JOA scores and lower recovery rate of JOA scores (37). However, they also reported that the mean recovery rates of upper extremity motor function after laminoplasty was not significantly different between diabetics and non-diabetic groups (37).
A prospective cohort study of 61 patients showed that JOA scores improved significantly in both diabetic and non-diabetic groups after surgery, with no significant inter-group differences identified (79). However, patients with better control of HbA1c after 12 months had significantly better scores on the Japanese Orthopaedic Association Cervical Myelopathy Evaluation Questionnaire (JOACMEQ), a questionnaire completed by patients to assess the severity of their cervical myelopathy and quality of life. In addition, there were no significant differences in the upper or lower limb function between the two groups (79).
Furthermore, three studies assessed the differences in the reflexes preoperatively between diabetic and non-diabetic patients (41, 47, 54). Diabetics were reported to have a lower prevalence of hyperreflexia and a higher incidence of hyporeflexia (47, 54). Furthermore, a retrospective comparative study of 111 patients that had undergone laminoplasty for DCM reported that Hoffmann’s and Trömner’s reflexes were significantly less common in severely diabetic DCM patients compared to mild diabetics (41). However, the same study also reported no significant difference in the positivity of Babinski’s reflex or the 10-s test (a test of frequency of finger grip and release in 10 s) between those with severe diabetes, mild diabetes and no diabetes (41). Another retrospective study of 438 DCM patients, of which 79 were diabetic, reported no significant difference in Hoffman’s sign between diabetic and non-diabetic patients and found that diabetic patients had a higher incidence of Babinski’s sign (47). Finally, a case–control study of 76 patients reported that diabetic and non-diabetic DCM patients exhibit similar rates of both Hoffmann’s and Babinkski’s sign (54).
Diabetes has been found to be associated with significantly increased rates of reoperation and surgical complications (38, 44). For example, a single-centre cohort study of 105 patients reported that HbA1c levels greater than or equal to 6.5%, and a duration of diabetes of 10 or more years, were significant risk factors for poor surgical outcome; the same study showed that fasting blood glucose did not affect outcomes (39). In a Canadian survey of 916 surgeons, diabetes was identified as the most important comorbidity affecting surgical fusion outcome, risk of reoperation and readmission in DCM patients (63). In addition, diabetes was reported to significantly increase the risk of perioperative dysphagia and dysphonia in DCM patients undergoing anterior cervical surgery (43, 46, 59). Furthermore, there were significantly poorer fusion outcomes after anterior cervical discectomy and fusion in 29 diabetic DCM patients compared to 29 non-diabetic controls at 2 years postoperatively (36).
A multicentre study of 50,000 patients showed that the presence of uncomplicated or complicated diabetes significantly increased the likelihood of perioperative morbidity in DCM patients undergoing surgery (34). Uncontrolled diabetes was shown to significantly increase the likelihood of mortality, cardiac complications, haematoma, post-operative infection and non-routine discharge in cervical myelopathy patients (44, 64), in addition to unplanned intubation, use of a ventilator for more than 48 h, urinary tract infection, deep vein thrombosis and thrombophlebitis (61).
Type 1 diabetics were more likely to suffer from post-operative neurological, cardiovascular, pulmonary, thromboembolic and renal complications (32, 44). Two studies compared the effects of type 1 and type 2 diabetes on DCM surgical outcomes: a multi-centre cohort study of 1,560 cervical corpectomy patients reported a 4-fold higher mortality rate for type 1 diabetes compared with those with no history of diabetes or diet-controlled diabetes (32). Furthermore, a retrospective cohort study of 37,732 cervical spinal fusion patients showed that those with type 1 diabetes had a higher in-hospital mortality rate and longer average length of stay than type 2 diabetics (44).
However, other studies found differing results: despite diabetic patients having a significantly higher prevalence of comorbidities such as hypertension, hyperlipidaemia and anti-coagulant and anti-platelet use, one cohort study of 500 DCM patients reported no statistically significant difference in the follow-up period, operation time, blood loss, postoperative cervical alignment and range of motion (ROM) between diabetics and non-diabetics (37, 48).
A total of 24 papers studied the relationship between cardiovascular health and DCM (34, 38, 43, 45, 46, 48, 50, 51, 53, 58–60, 62, 63, 69–72, 75, 84–88) (Table 1; Figure 1C).
Cardiovascular disease (CVD) was shown to significantly lower post-surgical patient-reported outcomes, including patient quality of life measured with the Short Form-36 scale amongst 154 DCM patients (84).
A survey of 916 surgeons reported a history of angina, coronary artery disease and myocardial infarction as risk factors for surgical complications in DCM patients (63). Coexisting cardiovascular disease (CVD), particularly hypertension, was associated with the greatest risk of all studied comorbidities for post-operative complications in 479 DCM patients undergoing surgery (46), and significantly increased length of hospital stay in 1693 patients undergoing anterior surgeries (62). The five commonest comorbidities that were found to be associated with complications during post-operative rehabilitation included peripheral vascular disease, ischaemic heart disease, stroke, hypertension and diabetes mellitus (58).
Cardiovascular disease was also shown to be a risk factor for the development of perioperative dysphagia in 470 DCM patients undergoing anterior cervical surgery (43). A retrospective cohort study of 3,401 patients following posterior cervical fusion reported pulmonary embolism as a significant predictor for hospital readmission within 30 days after surgery (85). Moreover, in a retrospective national database analysis of 202,694 patients, congestive heart failure and pulmonary circulation disorders were shown to significantly increase the risk of pulmonary aspiration during cervical spine surgery (86).
A multi-centre cohort study of 3,057 showed that patients undergoing posterior surgeries or combined spinal procedures were more likely to be hypertensive than those undergoing anterior approaches (62). Additionally, a case control study of 32 out of 8,250 patients who developed postoperative spinal epidural haematoma (SEH) following spinal decompression reported that, although SEH patients had a higher prevalence of hypertension and coagulopathy than the control group, these differences were not statistically significant (87). However, a multi-centre study of over 54,000 surgical DCM patients reported that hypertension significantly decreased the risk of mortality (34). Currently, expert opinions on anterior vs. posterior surgical approaches tend to be based on cervical sagittal alignment parameters (89). However, the above cardiovascular factors may also have a role in decision making regarding surgical management.
The distribution of assessment of individual items of the JBI critical appraisal tool for cohort studies is depicted in Figure 3A. The similarity of the cohorts used in 10 of the included studies were deemed unclear (32, 35, 36, 49, 52, 56, 58, 60, 61, 86), mainly due to a lack of clear selection criteria. Measurement of exposures was mostly adequate, except in five studies (36, 47, 49, 52, 66), where the reporting of how exposures were measured were not deemed to be detailed enough. Similarly, seven studies did not include sufficient detail to be able to confidently conclude that exposures were measured in a valid and reliable manner (37, 47, 49, 52, 53, 55, 66). Identification of confounding factors (e.g., sex, age and duration of symptoms) was mostly adequate, except in six studies that appeared to miss key confounders (47, 52, 56, 58, 60, 61), most commonly due to lack of complete exclusion criteria, and two studies that appeared to be missing most confounders (32, 49). Strategies to deal with confounders was performed to a lower quality, with 13 studies deemed unclear (38, 39, 50, 52, 53, 55, 58, 60, 61, 66, 73, 77, 79), and four studies deemed inadequate (32, 47, 48, 56), most commonly due to a lack of a multivariate regression analysis. The cohorts were deemed either free of the outcome at the start of all studies or this criteria was not applicable, if for example the outcome was improvement in neurological status. The outcomes were measured in a valid and reliable manner in most studies, except one (49), where the methods section was sparse. The follow-up time was mostly adequate in length and reporting, except in nine studies (34, 44, 47, 49, 55, 58, 61, 73, 86), often due to incomplete reporting. Follow-up was mostly adequate, often owing to the retrospective nature of a significant portion of included studies, or if there was substantial loss to follow-up, this was usually reasonably explored, except in two studies (46, 66). As a result, most studies did not need to provide information on how incomplete follow-up would be handled. All, except for one study (49), appeared to use appropriate statistical analysis.
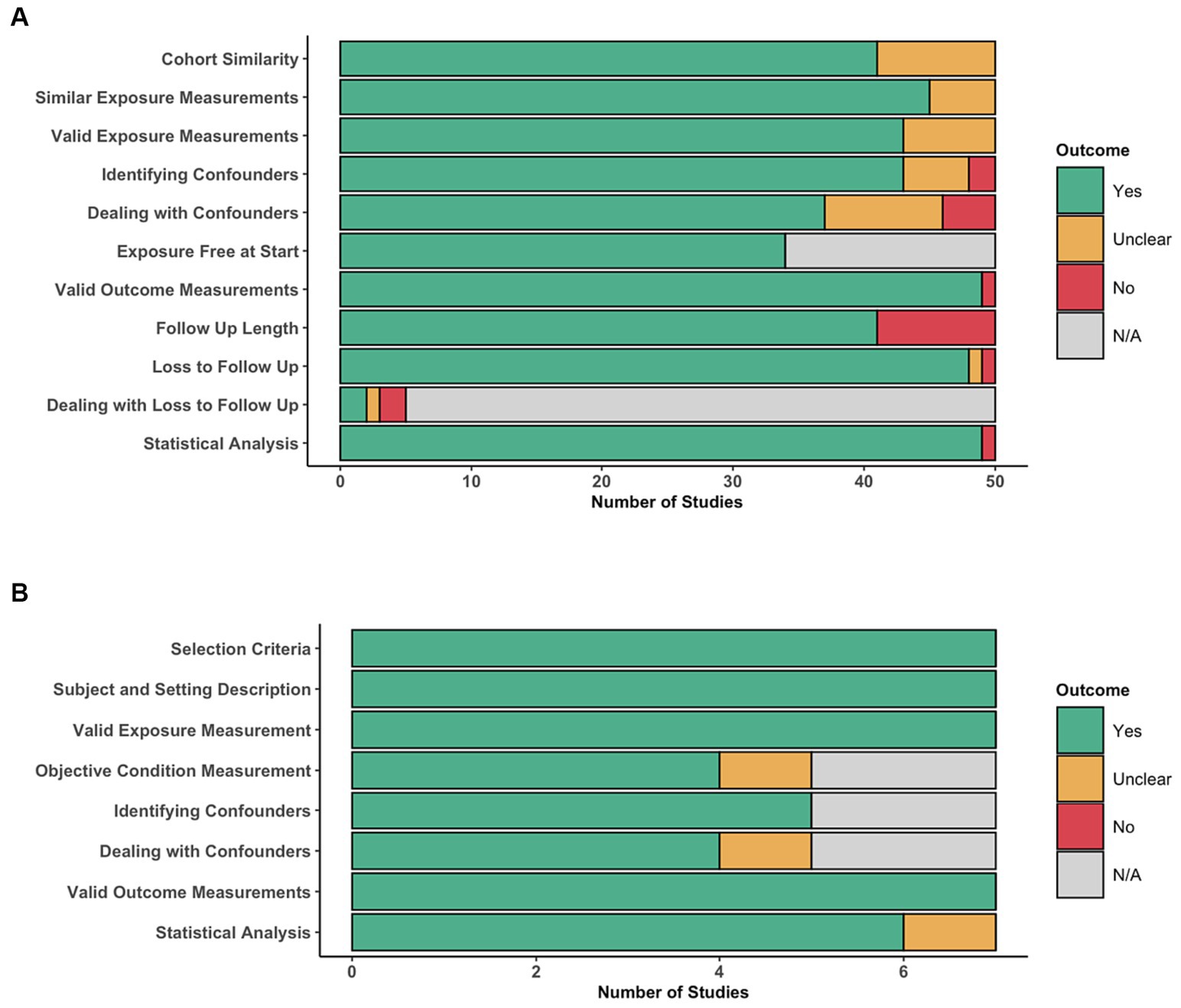
Figure 3. Risk of bias assessment. Distribution of per-item scores for cohort studies, indicating for each item, the number of articles scoring ‘Yes’, ‘Unclear’, ‘No’, or ‘N/A’. Distribution of per-item scores for cross-sectional studies, indicating for each item, the number of articles scoring ‘Yes’, ‘Unclear’, ‘No’, or ‘N/A’.
The distribution of assessment of individual items of the JBI critical appraisal tool for cross-sectional studies is depicted in Figure 3B. On the whole, cross-sectional studies were deemed to have low risk of bias, addressing all of the criteria adequately, except for one study (54), where their strategies to deal with confounding factors and use of statistical analysis were unclear.
The objective of this systematic review was to synthesise the current evidence on metabolic dysfunction in DCM. Our synthesis shows that metabolic factors appear to have an impact on outcomes in DCM. This association is strongest with respect to surgical adverse events, but also exists for spinal cord recovery following surgery. However, studies have not sufficiently evaluated the significance of metabolic factors with respect to the onset of DCM, although they do appear to affect initial clinical assessment. Furthermore, it remains uncertain whether these factors are modifiable. These remain important knowledge gaps and areas for future targeted research (10).
Spinal cord ischaemia is a common feature in pre-clinical models and autopsy specimens of DCM and has been proposed as a final common pathway of spinal cord injury resulting from critical cord compression (11). For example, Ellingson et al. (90) used MRI to evaluate spinal cord perfusion and demonstrated that neurological function using the modified JOA (mJOA) was inversely correlated with oxygen extraction. Although inter-rater reliability of total mJOA and its subscores are useful, mJOA should be interpreted carefully, particularly when near the threshold between severity categories, or when a patient is reassessed for deterioration (91). Moreover, the relationship is likely to be bidirectional, with systemic factors influencing the spinal cord, but also the spinal cord influencing systemic cardiovascular disease. For example, autonomic dysfunction can arise with spinal cord damage including DCM (92–94), and a recent Taiwanese population study identified DCM as an independent risk factor for the occurrence of acute coronary syndromes (95).
Implications for the systemic circulation, and therefore, metabolic disease would seem logical. The aggregated clinical evidence here aligns with this, with studies demonstrating poorer pre-and post-operative neurological function (41, 47, 54). Whilst there is need for further investigation due to existing studies being few in number and generally low in quality, what is clear from the data presented here, is that this line of enquiry will be challenging, due to the significant interaction of these factors. Moreover, the balance of evidence strongly associates metabolic disease with surgical complications, including cardiovascular disease (38, 43, 46, 53, 62, 87) and diabetes (33, 37–39, 44, 46, 47, 56). Whilst this fits with wider surgical experience (96), it will confound the use of post-operative recovery as a surrogate measure to investigate this relationship.
Furthermore, these individual diseases interact and have their own levels of within factor significance. For example, diabetic DCM patients often present with several other confounding conditions, which could worsen their post-operative function (82, 97); these include hypertension, hyperlipidaemia and a procoagulant state (48, 98). Other studies have shown that diabetes and smoking are perhaps the most important risk factors for development of dysphagia (59), but these two factors were shown to coexist with CVD (98). Furthermore, type 1 diabetics were shown to have greater post-operative neurological, cardiovascular, pulmonary, thromboembolic and renal complications than type 2 diabetics (32, 44), and duration of diabetes for over a decade was a significant predictor for poorer surgical outcomes (39). These diseases are also influenced by many unmeasured variables, such as diet and lifestyle (99). So, whilst we might hypothesise that autonomic neuropathy, prevalent and very often subclinical in diabetes, could be a major contributor for these problems, confirming this will be challenging (100).
This complexity is well demonstrated by Badhiwala et al. (84), who used a principal component analysis to explore different clinical phenotypes based on comorbidities and recovery profiles within the AO Spine datasets; they demonstrated that cardiovascular, renal and gastric comorbidities were statistically significant patient characteristics, with ‘eigenvalues >1’, and thus may significantly impact post-surgical outcome in DCM patients. However, once again, the complexity is important to appreciate when making such conclusions.
Whilst the specific impact of metabolic disease on the acquisition of DCM remains theoretical, the burden of cardiovascular disease and implications for surgery, in a condition predominantly treated with surgery, indicate a need to focus research on this question. Across surgery, these factors are considered broadly modifiable or at least suitable for optimisation, either pre-or peri-operatively (101, 102), increasingly termed prehabilitation (103). This is therefore relevant even in context of time constraints, where DCM surgery can be time critical (104, 105).
One additional finding of note was the implications for diabetes on examination findings, albeit inconsistent, in particular the presence or absence pathological reflexes differing between studies (41, 47, 54). Given its restriction to reflexes, these observations may well be driven by a subclinical and co-existent peripheral neuropathy, which is extremely prevalent (~30–50%) amongst diabetics (106). However peripheral diabetic neuropathy is also recognised to manifest other neurological implications, including gait and motor dysfunction (107). This may have implications for diagnosis, where expected examination findings may be mute, or outcome assessments, where measures may be confounded (108, 109). Supporting the former is an AO Spine RECODE DCM research priority1 owing to the significant under, mis-and delayed diagnosis increasing disability and dependence (105, 110–114).
The findings of this study are limited to the existing evidence base, which is low in quality and selective in its focus, largely orientated to surgery, in particular anterior surgery. However overall, the balance of current evidence supports metabolism being important in DCM. What is missing is any proof of causation and elucidation of the fundamental mechanisms. This is especially pertinent since many aforementioned metabolic factors are likely to interact. Contradiction between studies exists for several topics and control of confounding factors has generally been poor, making generalisability of correlations limited. Studies mainly included DCM patients without diabetes or CVD as controls, however, it would also be useful to compare metabolically impaired DCM patients against non-DCM groups, highlighting a knowledge gap that requires further investigation. Given the role of metabolism in DCM may be like that in many other conditions, an initial broad approach focusing on what is known about metabolism in better-researched neurological conditions may also be appropriate. This is likely to require a large, and high-quality dataset, capturing all relevant determinants, including patient demographics such as age, ethnicity, and weight, but also diet and lifestyle.
Metabolic disease increases the risks of adverse events in patients undergoing surgical treatment for DCM (GRADE moderate strength evidence). Given the recognised potential for this to be beneficially modified in other surgical fields, alongside the putative and low-quality clinical evidence indicating a potential significant relationship between metabolic disease and spinal cord function and recovery, this research area merits further investigation. The differing examination findings amongst DCM patients with diabetes is also of relevance to those investigating strategies for earlier diagnosis of DCM. Future directions of DCM management would certainly rely on studies meant to address the knowledge gap on the role of metabolic factors in the decision-making process for DCM (115).
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
CP: Writing – original draft, Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation. AS: Data curation, Writing – review & editing, Formal analysis, Investigation, Methodology. AR: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – review & editing. FB: Data curation, Writing – review & editing. TR: Data curation, Writing – review & editing. SA: Data curation, Writing – review & editing. MA: Data curation, Writing – review & editing. AB: Data curation, Writing – review & editing. AN: Supervision, Writing – review & editing. MK: Supervision, Writing – review & editing. BD: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing. OM: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. BD is supported by an NIHR Clinical Doctoral Research Fellowship. OM is supported by an NIHR Academic Clinical Fellowship. Research in the MK’s laboratory is supported by a core support grant from the Wellcome Trust and MRC to the Wellcome Trust-Medical Research Council Cambridge Stem Cell Institute. MK is supported by a NIHR Clinician Scientist Award, CS-2015-15-023.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
This report is independent research supported by the National Institute for Health and Care Research. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health and Care Research or the Department of Health and Social Care.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1301003/full#supplementary-material
1. Davies, BM, Mowforth, OD, Smith, EK, and Kotter, MR. Degenerative cervical myelopathy. BMJ. (2018) 360:k186. doi: 10.1136/bmj.k186
2. Smith, SS, Stewart, ME, Davies, BM, and Kotter, MRN. The prevalence of asymptomatic and symptomatic spinal cord compression on magnetic resonance imaging: a systematic review and Meta-analysis. Glob Spine J. (2021) 11:597–607. doi: 10.1177/2192568220934496
3. Nouri, A, Tetreault, L, Singh, A, Karadimas, SK, and Fehlings, MG. Degenerative cervical myelopathy: epidemiology, genetics, and pathogenesis. Spine. (2015) 40:E675–93. doi: 10.1097/BRS.0000000000000913
4. Davies, BM, Munro, C, Khan, DZ, Fitzpatrick, SM, Hilton, B, Mowforth, OD, et al. Outcomes of degenerative cervical myelopathy from the perspective of persons living with the condition: findings of a Semistructured interview process with partnered internet survey. Glob Spine J. (2022) 12:432–40. doi: 10.1177/2192568220953811
5. Khan, DZ, Fitzpatrick, SM, Hilton, B, McNair, AG, Sarewitz, E, Davies, BM, et al. Prevailing outcome themes reported by people with degenerative cervical myelopathy: focus group study. JMIR Form Res. (2021) 5:e18732. doi: 10.2196/18732
6. Mowforth, OD, Davies, BM, and Kotter, MR. Quality of life among informal caregivers of patients with degenerative cervical myelopathy: cross-sectional questionnaire study. Interact J Med Res. (2019) 8:e12381. doi: 10.2196/12381
7. Davies, BM, Khan, DZ, Mowforth, OD, McNair, AGK, Gronlund, T, Kolias, AG, et al. RE-CODE DCM (REsearch objectives and common data elements for degenerative cervical myelopathy): a consensus process to improve research efficiency in DCM, through establishment of a standardized dataset for clinical research and the definition of the research priorities. Glob Spine J. (2019) 9:65S–76S. doi: 10.1177/2192568219832855
8. Mowforth, OD, Khan, DZ, Wong, MY, Pickering, GAE, Dean, L, Magee, J, et al. Gathering global perspectives to establish the research priorities and minimum data sets for degenerative cervical myelopathy: sampling strategy of the first round consensus surveys of AO spine RECODE-DCM. Glob Spine J. (2022) 12:8S–18S. doi: 10.1177/21925682211047546
9. Tetreault, L, Mowforth, O, Khan, DZ, Gronlund, T, Garwood, P, Hazenbiller, O, et al. James Lind Alliance priority setting Partnership for Degenerative Cervical Myelopathy [AO spine RECODE-DCM]: An overview of the methodology used to process and short-list research uncertainties. Glob Spine J. (2022) 12:19S–27S. doi: 10.1177/21925682211062501
10. Davies, BM, Kwon, BK, Fehlings, MG, and Kotter, MRN. AO spine RECODE-DCM: why prioritize research in degenerative cervical myelopathy? Glob Spine J. (2022) 12:5S–7S. doi: 10.1177/21925682211035379
11. Davies, BM, Mowforth, O, Gharooni, AA, Tetreault, L, Nouri, A, Dhillon, RS, et al. A new framework for investigating the biological basis of degenerative cervical myelopathy [AO spine RECODE-DCM research priority number 5]: mechanical stress, vulnerability and time. Glob Spine J. (2022) 12:78S–96S. doi: 10.1177/21925682211057546
12. Nouri, A, Tessitore, E, Molliqaj, G, Meling, T, Schaller, K, Nakashima, H, et al. Degenerative cervical myelopathy: development and natural history [AO spine RECODE-DCM research priority number 2]. Glob Spine J. (2022) 12:39S–54S. doi: 10.1177/21925682211036071
13. Tempest-Mitchell, J, Hilton, B, Davies, BM, Nouri, A, Hutchinson, PJ, Scoffings, DJ, et al. A comparison of radiological descriptions of spinal cord compression with quantitative measures, and their role in non-specialist clinical management. PLoS One. (2019) 14:e0219380. doi: 10.1371/journal.pone.0219380
14. Tetreault, LA, Karadimas, S, Wilson, JR, Arnold, PM, Kurpad, S, Dettori, JR, et al. The natural history of degenerative cervical myelopathy and the rate of hospitalization following spinal cord injury: An updated systematic review. Glob Spine J. (2017) 7:28S–34S. doi: 10.1177/2192568217700396
15. Badhiwala, JH, Ahuja, CS, Akbar, MA, Witiw, CD, Nassiri, F, Furlan, JC, et al. Degenerative cervical myelopathy—update and future directions. Nat Rev Neurol. (2020) 16:108–24. doi: 10.1038/s41582-019-0303-0
16. Tetreault, L, Palubiski, LM, Kryshtalskyj, M, Idler, RK, Martin, AR, Ganau, M, et al. Significant predictors of outcome following surgery for the treatment of degenerative cervical myelopathy: a systematic review of the literature. Neurosurg Clin N Am. (2018) 29:115–127.e35. doi: 10.1016/j.nec.2017.09.020
17. Saklayen, MG . The global epidemic of the metabolic syndrome. Curr Hypertens Rep. (2018) 20:12. doi: 10.1007/s11906-018-0812-z
18. Akter, F, and Kotter, M. Pathobiology of degenerative cervical myelopathy. Neurosurg Clin N Am. (2018) 29:13–9. doi: 10.1016/j.nec.2017.09.015
19. Jackson, KL, and Devine, JG. The effects of obesity on spine surgery: a systematic review of the literature. Glob Spine J. (2016) 6:394–400. doi: 10.1055/s-0035-1570750
20. Teraguchi, M, Yoshimura, N, Hashizume, H, Muraki, S, Yamada, H, Oka, H, et al. Metabolic syndrome components are associated with intervertebral disc degeneration: the Wakayama spine study. PLoS One. (2016) 11:e0147565. doi: 10.1371/journal.pone.0147565
21. Patel, N, Bagan, B, Vadera, S, Maltenfort, MG, Deutsch, H, Vaccaro, AR, et al. Obesity and spine surgery: relation to perioperative complications. J Neurosurg Spine. (2007) 6:291–7. doi: 10.3171/spi.2007.6.4.1
22. Fehlings, MG, Tetreault, LA, Riew, KD, Middleton, JW, Aarabi, B, Arnold, PM, et al. A clinical practice guideline for the Management of Patients with Degenerative Cervical Myelopathy: recommendations for patients with mild, moderate, and severe disease and Nonmyelopathic patients with evidence of cord compression. Glob Spine J. (2017) 7:70S–83S. doi: 10.1177/2192568217701914
23. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DGPRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
24. Galgani, J, and Ravussin, E. Energy metabolism, fuel selection and body weight regulation. Int J Obes. (2008) 32:S109–19. doi: 10.1038/ijo.2008.246
25. Davies, BM, Goh, S, Yi, K, Kuhn, I, and Kotter, MRN. Development and validation of a MEDLINE search filter/hedge for degenerative cervical myelopathy. BMC Med Res Methodol. (2018) 18:73. doi: 10.1186/s12874-018-0529-3
26. Khan, MA, Mowforth, OD, Kuhn, I, Kotter, MRN, and Davies, BM. Development of a validated search filter for Ovid Embase for degenerative cervical myelopathy. Health Inf Libr J. (2023) 40:181–9. doi: 10.1111/hir.12373
27. Zeng, X, Zhang, Y, Kwong, JSW, Zhang, C, Li, S, Sun, F, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Med. (2015) 8:2–10. doi: 10.1111/jebm.12141
28. Campbell, M, McKenzie, JE, Sowden, A, Katikireddi, SV, Brennan, SE, Ellis, S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. (2020) 368:l6890. doi: 10.1136/bmj.l6890
29. Pope, DH, Davies, BM, Mowforth, OD, Bowden, AR, and Kotter, MRN. Genetics of degenerative cervical myelopathy: a systematic review and Meta-analysis of candidate gene studies. J Clin Med. (2020) 9:282. doi: 10.3390/jcm9010282
30. Tetreault, L, Lange, SF, Chotai, S, Kryshtalskyj, MT, Martin, AR, Ahuja, CS, et al. A systematic review of definitions for neurological complications and disease progression in patients treated surgically for degenerative cervical myelopathy. Spine. (2019) 44:1318–31. doi: 10.1097/BRS.0000000000003066
31. Guyatt, GH, Oxman, AD, Vist, GE, Kunz, R, Falck-Ytter, Y, Alonso-Coello, P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
32. Boakye, M, Patil, CG, Ho, C, and Lad, SP. Cervical corpectomy: complications and outcomes. Neurosurgery. (2008) 63:295–302. doi: 10.1227/01.NEU.0000327028.45886.2E
33. Dokai, T, Nagashima, H, Nanjo, Y, Tanida, A, and Teshima, R. Surgical outcomes and prognostic factors of cervical spondylotic myelopathy in diabetic patients. Arch Orthop Trauma Surg. (2012) 132:577–82. doi: 10.1007/s00402-011-1449-4
34. Kaye, ID, Marascalchi, BJ, Macagno, AE, Lafage, VA, Bendo, JA, and Passias, PG. Predictors of morbidity and mortality among patients with cervical spondylotic myelopathy treated surgically. Eur Spine J. (2015) 24:2910–7. doi: 10.1007/s00586-015-4010-2
35. Kim, HJ, Moon, SH, Kim, HS, Moon, ES, Chun, HJ, Jung, M, et al. Diabetes and smoking as prognostic factors after cervical laminoplasty. J Bone Joint Surg (Br). (2008) 90-B:1468–72. doi: 10.1302/0301-620X.90B11.20632
36. Liow, MHL, Lee, M, Goh, GSH, Chen, LTJ, Yue, WM, Guo, CM, et al. Poorer fusion outcomes in diabetic cervical Spondylotic myelopathy patients undergoing single-level anterior cervical discectomy and fusion does not compromise functional outcomes and quality of life. Spine. (2018) 43:477–83. doi: 10.1097/BRS.0000000000002395
37. Machino, M, Imagama, S, Ando, K, Kobayashi, K, Hida, T, Ito, K, et al. Characteristics of residual symptoms after Laminoplasty in diabetic patients with cervical Spondylotic myelopathy: a prospective cohort study. Spine. (2017) 42:E708–15. doi: 10.1097/BRS.0000000000001947
38. Park, MS, Ju, YS, Moon, SH, Kim, TH, Oh, JK, Makhni, MC, et al. Reoperation rates after anterior cervical discectomy and fusion for cervical Spondylotic radiculopathy and myelopathy: a National Population-based Study. Spine. (2016) 41:1593–9. doi: 10.1097/BRS.0000000000001590
39. Machino, M, Yukawa, Y, Ito, K, Inoue, T, Kobayakawa, A, Matsumoto, T, et al. Risk factors for poor outcome of cervical laminoplasty for cervical spondylotic myelopathy in patients with diabetes. J Bone Joint Surg Am. (2014) 96:2049–55. doi: 10.2106/JBJS.N.00064
40. Aggarwal, RA, Srivastava, SK, Bhosale, SK, and Nemade, PS. Prediction of surgical outcome in compressive cervical myelopathy: a novel clinicoradiological prognostic score. J Craniovertebr Junction Spine. (2016) 7:82–6. doi: 10.4103/0974-8237.181828
41. Okada, M, Minamide, A, Yoshida, M, Kawakami, M, Enyo, Y, and Endo, T. Observations in the diagnosis of cervical myelopathy in patients suffering from diabetes mellitus. Spinal Cord. (2012) 50:878–81. doi: 10.1038/sc.2012.56
42. Choi, JH, Shin, JJ, Kim, TH, Shin, HS, Hwang, YS, and Park, SK. Does intramedullary signal intensity on MRI affect the surgical outcomes of patients with ossification of posterior longitudinal ligament? J Kor Neurosurg Soc. (2014) 56:121–9. doi: 10.3340/jkns.2014.56.2.121
43. Nagoshi, N, Tetreault, L, Nakashima, H, Arnold, PM, Barbagallo, G, Kopjar, B, et al. Risk factors for and clinical outcomes of dysphagia after anterior cervical surgery for degenerative cervical myelopathy: results from the AOSpine international and North America studies. J Bone Joint Surg Am. (2017) 99:1069–77. doi: 10.2106/JBJS.16.00325
44. Cook, C, Tackett, S, Shah, A, Pietrobon, R, Browne, J, Viens, N, et al. Diabetes and perioperative outcomes following cervical fusion in patients with myelopathy. Spine. (2008) 33:E254–60. doi: 10.1097/BRS.0b013e31816b88ca
45. Nakashima, H, Tetreault, LA, Nagoshi, N, Nouri, A, Kopjar, B, Arnold, PM, et al. Does age affect surgical outcomes in patients with degenerative cervical myelopathy? Results from the prospective multicenter AOSpine international study on 479 patients. J Neurol Neurosurg Psychiatry. (2016) 87:734–40. doi: 10.1136/jnnp-2015-311074
46. Tetreault, L, Tan, G, Kopjar, B, Côté, P, Arnold, P, Nugaeva, N, et al. Clinical and surgical predictors of complications following surgery for the treatment of cervical Spondylotic myelopathy: results from the multicenter, prospective AOSpine international study of 479 patients. Neurosurgery. (2016) 79:33–44. doi: 10.1227/NEU.0000000000001151
47. Houten, JK, and Lenart, C. Diabetes and cervical myelopathy. J Clin Neurosci. (2016) 27:99–101. doi: 10.1016/j.jocn.2015.07.025
48. Machino, M, Yukawa, Y, Ito, K, Inoue, T, Kobayakawa, A, Matsumoto, T, et al. Impact of diabetes on the outcomes of cervical laminoplasty: a prospective cohort study of more than 500 patients with cervical spondylotic myelopathy. Spine. (2014) 39:220–7. doi: 10.1097/BRS.0000000000000102
49. Lee, T, Chacha, PB, and Khoo, J. Ossification of posterior longitudinal ligament of the cervical spine in non-Japanese Asians. Surg Neurol. (1991) 35:40–4. doi: 10.1016/0090-3019(91)90200-S
50. Maeno, T, Okuda, S, Yamashita, T, Matsumoto, T, Yamasaki, R, Oda, T, et al. Age-related surgical outcomes of Laminoplasty for cervical Spondylotic myelopathy. Glob Spine J. (2015) 5:118–23. doi: 10.1055/s-0034-1396759
51. Park, MS, Ju, YS, Moon, SH, Kim, TH, Oh, JK, Makhni, MC, et al. Reoperation rates after surgery for degenerative cervical spine disease according to different surgical procedures: National Population-based Cohort Study. Spine. (2016) 41:1484–92. doi: 10.1097/BRS.0000000000001581
52. Plano, X, Ramírez, M, Matamalas, A, Haddad, S, García de Frutos, A, Casamitjana, JM, et al. 30-Day unplanned surgery in cervical spondylotic myelopathy surgically treated: a single-center experience. Eur Spine J. (2019) 28:1209–16. doi: 10.1007/s00586-019-05892-8
53. Puvanesarajah, V, Hassanzadeh, H, Shimer, AL, Shen, FH, and Singla, A. Readmission rates, reasons, and risk factors following anterior cervical fusion for cervical spondylosis in patients above 65 years of age. Spine. (2017) 42:78–84. doi: 10.1097/BRS.0000000000001663
54. Rhee, JM, Heflin, JA, Hamasaki, T, and Freedman, B. Prevalence of physical signs in cervical myelopathy: a prospective, controlled study. Spine. (2009) 34:890–5. doi: 10.1097/BRS.0b013e31819c944b
55. Sirasanagandla, SR, Al-Kaabi, SA, Al Dhuhli, H, Al-Hinai, G, Al Mushaiqri, M, and Jaju, S. Ossification of posterior longitudinal ligament of cervical spine among Omani patients referred for CT scan at a tertiary Care Hospital in Oman. Oman Med J. (2019) 34:438–43. doi: 10.5001/omj.2019.80
56. Tauchi, R, Lee, SH, Peters, C, Imagama, S, Ishiguro, N, and Riew, KD. Cervical Myeloradiculopathy due to ossification of the posterior longitudinal ligament with versus without diffuse idiopathic spinal hyperostosis. Glob Spine J. (2016) 6:350–6. doi: 10.1055/s-0035-1563722
57. Tetreault, LA, Nouri, A, Singh, A, Fawcett, M, and Fehlings, MG. Predictors of outcome in patients with cervical spondylotic myelopathy undergoing surgical treatment: a survey of members from AOSpine international. World Neurosurg. (2014) 81:623–33. doi: 10.1016/j.wneu.2013.09.023
58. Ullah, S, Qamar, I, Qureshi, AZ, Abu-Shaheen, A, and Niaz, A. Functional outcomes in geriatric patients with spinal cord injuries at a tertiary care rehabilitation hospital in Saudi Arabia. Spinal Cord Ser Cases. (2018) 4:78. doi: 10.1038/s41394-018-0104-5
59. Wang, T, Ma, L, Yang, DL, Wang, H, Bai, ZL, Zhang, LJ, et al. Factors predicting dysphagia after anterior cervical surgery: a multicenter retrospective study for 2 years of follow-up. Medicine (Baltimore). (2017) 96:e7916. doi: 10.1097/MD.0000000000007916
60. Yeung, KKL, Cheung, PWH, and Cheung, JPY. Anterior cervical discectomy and fusion for cervical myelopathy using stand-alone tricortical iliac crest autograft: predictive factors for neurological and fusion outcomes. J Orthop Surg. (2019) 27:2309499019869166. doi: 10.1177/2309499019869166
61. Worley, N, Buza, J, Jalai, CM, Poorman, GW, Day, LM, Vira, S, et al. Diabetes as an independent predictor for extended length of hospital stay and increased adverse post-operative events in patients treated surgically for cervical Spondylotic myelopathy. Int J Spine Surg. (2017) 11:10. doi: 10.14444/4010
62. Passias, PG, Jalai, CM, Worley, N, Vira, S, Hasan, S, Horn, SR, et al. Predictors of hospital length of stay and 30-Day readmission in cervical Spondylotic myelopathy patients: An analysis of 3057 patients using the ACS-NSQIP database. World Neurosurg. (2018) 110:e450–8. doi: 10.1016/j.wneu.2017.11.009
63. Tetreault, L, Nouri, A, Singh, A, Fawcett, M, Nater, A, and Fehlings, MG. An assessment of the key predictors of perioperative complications in patients with cervical Spondylotic myelopathy undergoing surgical treatment: results from a survey of 916 AOSpine international members. World Neurosurg. (2015) 83:679–90. doi: 10.1016/j.wneu.2015.01.021
64. Kawaguchi, Y, Matsui, H, Ishihara, H, Gejo, R, and Yasuda, T. Surgical outcome of cervical expansive laminoplasty in patients with diabetes mellitus. Spine. (2000) 25:551–5. doi: 10.1097/00007632-200003010-00004
65. Nori, S, Nagoshi, N, Yoshioka, K, Nojiri, K, Takahashi, Y, Fukuda, K, et al. Diabetes does not adversely affect neurological recovery and reduction of neck pain after posterior decompression surgery for cervical Spondylotic myelopathy: results from a retrospective multicenter study of 675 patients. Spine. (2021) 46:433–9. doi: 10.1097/BRS.0000000000003817
66. Kimura, A, Takeshita, K, Yoshii, T, Egawa, S, Hirai, T, Sakai, K, et al. Impact of diabetes mellitus on cervical spine surgery for ossification of the posterior longitudinal ligament. J Clin Med. (2021) 10:3375. doi: 10.3390/jcm10153375
67. Nagoshi, N, Watanabe, K, Nakamura, M, Matsumoto, M, Li, N, Ma, S, et al. Does diabetes affect the surgical outcomes in cases with cervical ossification of the posterior longitudinal ligament? A multicenter study from Asia Pacific spine study group. Glob Spine J. (2023) 13:353–9. doi: 10.1177/2192568221996300
68. Kusin, DJ, Ahn, UM, and Ahn, NU. The influence of diabetes on surgical outcomes in cervical myelopathy. Spine. (2016) 41:1436–40. doi: 10.1097/BRS.0000000000001560
69. Liao, X, Jin, Z, Shi, L, Zhao, Y, Zhou, S, Chen, D, et al. Prevalence of ossification of posterior longitudinal ligament in patients with degenerative cervical myelopathy: cervical spine 3D CT observations in 7210 cases. Spine. (2020) 45:1320–8. doi: 10.1097/BRS.0000000000003526
70. Shin, JJ, Jeon, H, Lee, JJ, Kim, HC, Kim, TW, An, SB, et al. Predictors of neurologic outcome after surgery for cervical ossification of the posterior longitudinal ligament differ based on myelopathy severity: a multicenter study. J Neurosurg Spine. (2021) 34:749–58. doi: 10.3171/2020.8.SPINE20504
71. DiMaria, S, Wilent, WB, Nicholson, KJ, Tesdahl, EA, Valiuskyte, K, Mao, J, et al. Patient factors impacting baseline motor evoked potentials (MEPs) in patients undergoing cervical spine surgery for myelopathy or radiculopathy. Clin Spine Surg. (2022) 35:E527–33. doi: 10.1097/BSD.0000000000001299
72. Abudouaini, H, Liu, H, Wang, B, Meng, Y, Yang, Y, Ding, C, et al. Outcome and predictive factors in rapid progressive cervical spondylotic myelopathy: a retrospective case-control study. Clin Neurol Neurosurg. (2020) 198:106226. doi: 10.1016/j.clineuro.2020.106226
73. Wada, K, Tamaki, R, Inoue, T, Hagiwara, K, and Okazaki, K. Postoperative complications and survival rate in hemodialysis-dependent patients undergoing cervical spine surgery. Spine Surg Relat Res. (2022) 6:233–9. doi: 10.22603/ssrr.2021-0173
74. Jiang, J, Sun, K, Lin, F, Lu, M, Huan, L, Xu, X, et al. The effect of diabetes mellitus on the neurological function of patients with cervical Spondylotic myelopathy. Orthop Surg. (2022) 14:3242–50. doi: 10.1111/os.13542
75. Machino, M, Ando, K, Kobayashi, K, Nakashima, H, Kanbara, S, Ito, S, et al. Risk factors for poor outcome of cervical Laminoplasty: multivariate analysis in 505 patients with cervical Spondylotic myelopathy. Spine. (2021) 46:329–36. doi: 10.1097/BRS.0000000000003783
76. Moradi, F, Bagheri, SR, Saeidiborojeni, H, Eden, SV, Naderi, M, Hamid, S, et al. Predictors of poor clinical outcome in patients with cervical spondylotic myelopathy undergoing cervical laminectomy and fusion. Musculoskelet Surg. (2023) 107:77–83. doi: 10.1007/s12306-021-00731-w
77. Nassr, A, Aleem, IS, Eck, JC, Woods, B, Ponnappan, RK, Donaldson, WF, et al. Does resection of the posterior longitudinal ligament impact the incidence of C5 palsy after cervical Corpectomy procedures?: a review of 459 consecutive cases. Spine. (2017) 42:E392–7. doi: 10.1097/BRS.0000000000001806
78. Takahashi, H, Aoki, Y, Saito, J, Nakajima, A, Sonobe, M, Akatsu, Y, et al. Serum oxidative stress influences neurological recovery after surgery to treat acutely worsening symptoms of compression myelopathy: a cross-sectional human study. BMC Musculoskelet Disord. (2019) 20:589. doi: 10.1186/s12891-019-2966-5
79. Tanishima, S, Mihara, T, Tanida, A, Takeda, C, Murata, M, Takahashi, T, et al. Influence of diabetes mellitus on surgical outcomes in patients with cervical myelopathy: a prospective. Multicenter Study Asian Spine J. (2019) 13:468–77. doi: 10.31616/asj.2018.0082
80. Zhang, JT, Meng, FT, Wang, S, Wang, LF, and Shen, Y. Predictors of surgical outcome in cervical spondylotic myelopathy: focusing on the quantitative signal intensity. Eur Spine J. (2015) 24:2941–5. doi: 10.1007/s00586-015-4109-5
81. You, J, Tang, X, Gao, W, Shen, Y, Ding, WY, and Ren, B. Factors predicting adjacent segment disease after anterior cervical discectomy and fusion treating cervical spondylotic myelopathy. Medicine (Baltimore). (2018) 97:e12893. doi: 10.1097/MD.0000000000012893
82. Lee, JC, Lee, SH, Peters, C, and Riew, KD. Risk-factor analysis of adjacent-segment pathology requiring surgery following anterior, posterior, fusion, and nonfusion cervical spine operations: survivorship analysis of 1358 patients. J Bone Joint Surg Am. (2014) 96:1761–7. doi: 10.2106/JBJS.M.01482
83. Nagata, K, Miyahara, J, Nakamoto, H, Kawamura, N, Takeshita, Y, Higashikawa, A, et al. Effect of diabetes on patient-reported outcome measures at one year after laminoplasty for cervical spondylotic myelopathy. Sci Rep. (2022) 12:9684. doi: 10.1038/s41598-022-13838-2
84. Badhiwala, JH, Witiw, CD, Nassiri, F, Jaja, BNR, Akbar, MA, Mansouri, A, et al. Patient phenotypes associated with outcome following surgery for mild degenerative cervical myelopathy: a principal component regression analysis. Spine J. (2018) 18:2220–31. doi: 10.1016/j.spinee.2018.05.009
85. Choy, W, Lam, SK, Smith, ZA, and Dahdaleh, NS. Predictors of 30-Day hospital readmission after posterior cervical fusion in 3401 patients. Spine. (2018) 43:356–63. doi: 10.1097/BRS.0000000000001450
86. Fineberg, SJ, Oglesby, M, Patel, AA, and Singh, K. Incidence, risk factors, and mortality associated with aspiration in cervical spine surgery. Spine. (2013) 38:E1189–95. doi: 10.1097/BRS.0b013e31829cc19b
87. Yamada, K, Abe, Y, Satoh, S, Yanagibashi, Y, Hyakumachi, T, and Masuda, T. Large increase in blood pressure after Extubation and high body mass index elevate the risk of spinal epidural hematoma after spinal surgery. Spine. (2015) 40:1046–52. doi: 10.1097/BRS.0000000000000876
88. Zhong, W, Wang, L, Huang, T, and Luo, X. Risk factors for rapid progressive neurological deterioration in patients with cervical spondylotic myelopathy. J Orthop Surg Res. (2021) 16:75. doi: 10.1186/s13018-021-02227-6
89. Kato, S, Ganau, M, and Fehlings, MG. Surgical decision-making in degenerative cervical myelopathy – anterior versus posterior approach. J Clin Neurosci. (2018) 58:7–12. doi: 10.1016/j.jocn.2018.08.046
90. Ellingson, BM, Woodworth, DC, Leu, K, Salamon, N, and Holly, LT. Spinal cord perfusion MR imaging implicates both ischemia and hypoxia in the pathogenesis of cervical spondylosis. World Neurosurg. (2019) 128:e773–81. doi: 10.1016/j.wneu.2019.04.253
91. Martin, AR, Jentzsch, T, Wilson, JRF, Moghaddamjou, A, Jiang, F, Rienmueller, A, et al. Inter-rater reliability of the modified Japanese orthopedic association score in degenerative cervical myelopathy: a cross-sectional study. Spine. (2021) 46:1063–9. doi: 10.1097/BRS.0000000000003956
92. Manohar, N, Ramesh, VJ, Radhakrishnan, M, and Chakraborti, D. Haemodynamic changes during prone positioning in anaesthetised chronic cervical myelopathy patients. Indian J Anaesth. (2019) 63:212–7. doi: 10.4103/ija.IJA_810_18
93. Ong, ETE, Yeo, LKP, Kaliya-Perumal, AK, and Oh, JYL. Orthostatic hypotension following cervical spine surgery: prevalence and risk factors. Glob Spine J. (2020) 10:578–82. doi: 10.1177/2192568219863805
94. Kalb, S, Zaidi, HA, Ribas-Nijkerk, JC, Sindhwani, MK, Clark, JC, Martirosyan, NL, et al. Persistent outpatient hypertension is independently associated with spinal cord dysfunction and imaging characteristics of spinal cord damage among patients with cervical spondylosis. World Neurosurg. (2015) 84:351–7. doi: 10.1016/j.wneu.2015.03.030
95. Lin, SY, Chen, DC, Lin, CL, Lee, HC, Lin, TC, Wang, IK, et al. Risk of acute coronary syndrome in patients with cervical spondylosis. Atherosclerosis. (2018) 271:136–41. doi: 10.1016/j.atherosclerosis.2018.02.029
96. Gregson, J, Kaptoge, S, Bolton, T, Pennells, L, Willeit, P, Burgess, S, et al. Cardiovascular risk factors associated with venous thromboembolism. JAMA Cardiol. (2019) 4:163–73. doi: 10.1001/jamacardio.2018.4537
97. Kawaguchi, Y, Yasuda, T, Seki, S, Nakano, M, Kanamori, M, Sumi, S, et al. Variables affecting postsurgical prognosis of thoracic myelopathy caused by ossification of the ligamentum flavum. Spine J. (2013) 13:1095–107. doi: 10.1016/j.spinee.2013.03.001
98. Csige, I, Ujvárosy, D, Szabó, Z, Lőrincz, I, Paragh, G, Harangi, M, et al. The impact of obesity on the cardiovascular system. J Diabetes Res. (2018) 2018:1–12. doi: 10.1155/2018/3407306
99. Casas, R, Castro-Barquero, S, Estruch, R, and Sacanella, E. Nutrition and cardiovascular health. Int J Mol Sci. (2018) 19:3988. doi: 10.3390/ijms19123988
100. Bassiouny, SE . Screening of oropharyngeal dysphagia in patients with diabetes mellitus. BJSTR. (2017) 1:9. doi: 10.26717/BJSTR.2017.01.000206
101. Fehlings, MG, Wilson, JR, Kopjar, B, Yoon, ST, Arnold, PM, Massicotte, EM, et al. Efficacy and safety of surgical decompression in patients with cervical spondylotic myelopathy: results of the AOSpine North America prospective multi-center study. J Bone Joint Surg. (2013) 95:1651–8. doi: 10.2106/JBJS.L.00589
102. Soni, S, Shah, S, Chaggar, R, Saini, R, James, E, Elliot, J, et al. Surgical cancellation rates due to peri-operative hypertension: implementation of multidisciplinary guidelines across primary and secondary care. Anaesthesia. (2020) 75:1314–20. doi: 10.1111/anae.15084
103. Carli, F, Baldini, G, and Feldman, LS. Redesigning the preoperative clinic: from risk stratification to risk modification. JAMA Surg. (2021) 156:191–2. doi: 10.1001/jamasurg.2020.5550
104. Tetreault, LA, Côté, P, Kopjar, B, Arnold, P, and Fehlings, MG. AOSpine North America and international clinical trial research network. A clinical prediction model to assess surgical outcome in patients with cervical spondylotic myelopathy: internal and external validations using the prospective multicenter AOSpine north American and international datasets of 743 patients. Spine J. (2015) 15:388–97. doi: 10.1016/j.spinee.2014.12.145
105. Pope, DH, Mowforth, OD, Davies, BM, and Kotter, MRN. Diagnostic delays Lead to greater disability in degenerative cervical myelopathy and represent a health inequality. Spine (Phila Pa 1976). (2020) 45:368–77. doi: 10.1097/BRS.0000000000003305
106. Pfannkuche, A, Alhajjar, A, Ming, A, Walter, I, Piehler, C, and Mertens, PR. Prevalence and risk factors of diabetic peripheral neuropathy in a diabetics cohort: register initiative “diabetes and nerves”. Endocrin Metabol Sci. (2020) 1:100053. doi: 10.1016/j.endmts.2020.100053
107. Fernando, M, Crowther, R, Lazzarini, P, Sangla, K, Cunningham, M, Buttner, P, et al. Biomechanical characteristics of peripheral diabetic neuropathy: a systematic review and meta-analysis of findings from the gait cycle, muscle activity and dynamic barefoot plantar pressure. Clin Biomech. (2013) 28:831–45. doi: 10.1016/j.clinbiomech.2013.08.004
108. Hilton, B, Gardner, EL, Jiang, Z, Tetreault, L, Wilson, JRF, Zipser, CM, et al. Establishing diagnostic criteria for degenerative cervical myelopathy [AO spine RECODE-DCM research priority number 3]. Glob Spine J. (2022) 12:55S–63S. doi: 10.1177/21925682211030871
109. Tetreault, L, Garwood, P, Gharooni, AA, Touzet, AY, Nanna-Lohkamp, L, Martin, A, et al. Improving assessment of disease severity and strategies for monitoring progression in degenerative cervical myelopathy [AO spine RECODE-DCM research priority number 4]. Glob Spine J. (2022) 12:64S–77S. doi: 10.1177/21925682211063854
110. Hilton, B, Tempest-Mitchell, J, Davies, B, and Kotter, M. Route to diagnosis of degenerative cervical myelopathy in a UK healthcare system: a retrospective cohort study. BMJ Open. (2019) 9:e027000. doi: 10.1136/bmjopen-2018-027000
111. Hilton, B, Tempest-Mitchell, J, Davies, B, and Kotter, M. Assessment of degenerative cervical myelopathy differs between specialists and may influence time to diagnosis and clinical outcomes. PLoS One. (2018) 13:e0207709. doi: 10.1371/journal.pone.0207709
112. Behrbalk, E, Salame, K, Regev, GJ, Keynan, O, Boszczyk, B, and Lidar, Z. Delayed diagnosis of cervical spondylotic myelopathy by primary care physicians. Neurosurg Focus. (2013) 35:E1. doi: 10.3171/2013.3.FOCUS1374
113. Radcliff, KE, Curry, EP, Trimba, R, Walker, JB, Purtill, JJ, Austin, MS, et al. High incidence of undiagnosed cervical myelopathy in patients with hip fracture compared with controls. J Orthop Trauma. (2016) 30:189–93. doi: 10.1097/BOT.0000000000000485
114. Davies, BM, Mowforth, O, Wood, H, Karimi, Z, Sadler, I, Tetreault, L, et al. Improving awareness could transform outcomes in degenerative cervical myelopathy [AO spine RECODE-DCM research priority number 1]. Glob Spine J. (2022) 12:28S–38S. doi: 10.1177/21925682211050927
Keywords: cervical cord, myelopathy, spondylosis, stenosis, ossification posterior longitudinal ligament, metabolism, cardiovascular disease, diabetes
Citation: Partha Sarathi CI, Sinha A, Rafati Fard A, Bhatti F, Rujeedawa T, Ahmed S, Akhbari M, Bhatti A, Nouri A, Kotter MR, Davies BM and Mowforth OD (2024) The significance of metabolic disease in degenerative cervical myelopathy: a systematic review. Front. Neurol. 15:1301003. doi: 10.3389/fneur.2024.1301003
Received: 23 September 2023; Accepted: 09 January 2024;
Published: 05 February 2024.
Edited by:
Mario Ganau, Oxford University Hospitals NHS Trust, United KingdomReviewed by:
Kevin Chen, University of Michigan, United StatesCopyright © 2024 Partha Sarathi, Sinha, Rafati Fard, Bhatti, Rujeedawa, Ahmed, Akhbari, Bhatti, Nouri, Kotter, Davies and Mowforth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Oliver D. Mowforth, b20yODNAY2FtLmFjLnVr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.