- 1Department of Neurology, Hannover Medical School, Hannover, Germany
- 2Department of Neurology, St Josef Hospital, Ruhr University Bochum, Bochum, Germany
- 3Department of Neurology, Faculty of Medicine and University Hospital of Cologne, University of Cologne, Cologne, Germany
- 4Department of Neurology with Experimental Neurology, Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt Universität, Berlin, Germany
- 5Neuroscience Clinical Research Center, Charité — Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, Berlin, Germany
Introduction: Blood-cerebrospinal fluid (CSF) barrier dysfunction is pivotal for diagnosing immune-mediated neuropathies, especially in spinal nerve root inflammation. Typically, either total CSF protein or the CSF to serum albumin ratio (QAlb) is measured. Total CSF protein measurements have limitations, notably its fixed reference value regardless of age, in contrast to the age-dependent reference for QAlb. Our goal was to evaluate both markers in patients with immune-mediated neuropathies.
Methods: In our multicenter research, we collected retrospective CSF data from patients suffering from immune-mediated neuropathies across four German research centers. These parameters were analyzed in relation to their clinical characteristics.
Results: Out of 419 samples, 36 (8.6%) displayed a notable variation between total CSF protein and QAlb values. A detailed analysis revealed that patients displaying elevated QAlb but normal total CSF protein levels were significantly younger at disease onset (p = 0.01), at the time of diagnosis (p = 0.005), and when undergoing lumbar puncture (p = 0.001) compared to patients with elevated CSF protein and normal QAlb levels. These effects were especially evident for the subgroup of samples derived by female patients.
Discussion: Our work confirms the crucial role of QAlb in diagnosing immune-mediated neuropathies and particularly its efficacy as a marker for evaluating the blood-CSF barrier in patients with an earlier disease onset. Considering the significance of the albumin quotient, its assessment is especially advisable in younger patients of female sex to avoid missing a potential barrier dysfunction that might be falsely negative when using total protein.
1 Introduction
Blood-cerebrospinal fluid (CSF) barrier dysfunction is one of the hallmarks of the local inflammation at the spinal nerve roots that characterizes autoimmune-mediated neuropathies such as Guillain-Barré syndrome (GBS) and chronic inflammatory demyelinating polyneuropathy (CIDP) (1, 2). Information of the blood-CSF barrier function therefore plays a crucial role in the diagnosis and subsequent management of patients with immune-mediated neuropathies. Therefore, CSF analysis is included in the European Academy of Neurology/Peripheral Nerve Society (EAN/ PNS) diagnostic consensus guidelines for CIDP and multifocal motor neuropathy (MMN) (3, 4), as well as in the diagnostic workup of GBS (5).
Albumin, which is solely produced in the liver, is the most abundant protein within the CSF, even though it is not produced or catabolized intrathecally (6). Therefore, the ratio of CSF and serum albumin concentrations (QAlb) has been proposed as the preferred marker of blood-CSF-barrier function (7). But more importantly, the interpretation of QAlb is facilitated by age-dependent upper reference limits (QAlb lim) (8–10).
An alternative and still widely implemented approach to assess the blood-CSF-functionality is represented by the total CSF protein level. Although measurements of total protein in CSF are readily available, this traditional parameter has several disadvantages. The absolute CSF protein concentration is dependent on the serum protein concentration, age, CSF flow rate, and assay type (11). Also, a possible intrathecal synthesis of antibodies can influence total CSF protein values (12). Therefore, the measurement of absolute protein concentration in CSF may show normal results even though blood-CSF-barrier dysfunction is present (13).
Since the identification of a blood-CSF-barrier-dysfunction is needed for the diagnosis of inflammatory polyneuropathies and in particular for CIDP, a sensitive detection method is highly relevant in clinical routine. This is especially important considering that normal reference values for both parameters in healthy populations have been well studied (14–16). Therefore, our aim was to compare the applicability of QAlb and total CSF protein levels in a large number of patients with immune-mediated neuropathies.
2 Methods
2.1 Patient recruitment
In this multicenter study, CSF parameters of patients with autoimmune-mediated neuropathies were retrospectively recorded at four German study sites {Hannover Medical School (MHH), Ruhr-University Bochum [Department of Neurology, University Hospital St. Josef-Hospital] (UK-RUB), Faculty of Medicine and University Hospital of Cologne, Charité- Universitätsmedizin Berlin}, that participate in the German neuritis network “Neuritis Netz” (17). Study inclusion required that patients over the age of 18 years independent of disease duration and severity met the national and European guidelines for diagnosis of immune-mediated neuropathy according to EAN criteria at time of sampling. Only samples with information on total CSF protein and CSF and serum albumin were considered.
2.2 Assessment of CSF/ serum parameters and clinical data
Albumin concentrations in CSF and serum were determined using kinetic nephelometry. At Hannover Medical School, the Beckman Coulter IMMAGE was utilized, at UK-RUB both the Beckman Coulter IMMAGE 800 and Siemens Atellica® NEPH 630 System were used, at the University Hospital of Cologne the BN ProSpec was employed, and in Berlin, turbidimetry with the cobas®8,000-modul 701 was adopted. The CSF total protein levels were measured at MHH using a Beckman Coulter spectrophotometer, at UK-RUB using diasys respons®910, and in Berlin and Cologne by turbidimetry with a Cobas system (Cologne: Cobas c702).
The CSF albumin/serum albumin ratio was expressed as QAlb. The age-dependent upper reference limit for QAlb (QAlb lim) was calculated according to the established formula suggested by Reiber and Trendelenburg: QAlb lim = (age in years/15) + 4 (9, 10). QAlb values higher than QAlb lim were considered as elevated. CSF total protein values >500 mg/L were reported as elevated. Further CSF parameters included cell count, q-IgG, q-IgA, q-IgM, and evidence of oligoclonal bands. In addition, socio-demographic data including baseline information on diagnosis and symptom onset was collected.
At the time of CSF analysis, disease severity and impairment levels were assessed using two metrics. First, the Inflammatory Neuropathy Cause and Treatment (INCAT) disability score was utilized. This score evaluates both upper and lower limb functionality, with a total score range of 0–10 (18). Additionally, the Medical Research Council (MRC) Sum Score was employed for eight distinct muscle groups. These groups included upper extremity abduction, elbow flexion, wrist extension, index finger abduction, hip flexion, knee extension, foot dorsiflexion, and great toe dorsiflexion. The total score for this metric ranges from 0 to 80 (19, 20).
An effect of the contributing centers or of the diagnoses was ruled out for all subgroup analyses.
2.3 Statistical analysis
The data were handled descriptively. Continuous variables were first tested for normal distribution using the Shapiro–Wilk test. Subsequently, parametric variables were described as mean and standard deviation (SD) and non-parametric variables as median and interquartile range (IQR). Subgroup analysis was performed using chi2-test or Fisher’s exact test for categorical data as appropriate and Mann–Whitney-U-test for metric variables without normal distribution. Correlation analysis was performed using Spearman’s rank correlation. Furthermore, logistic regression analysis was performed for candidate variable in a univariable manner and for variables with significant effects additionally in a multivariable manner. value of ps <0.05 were considered statistically significant. Statistical analysis was performed by STATA® V16.1 (StataCorp LLC, Texas, United States).
3 Results
3.1 Patients’ characteristics
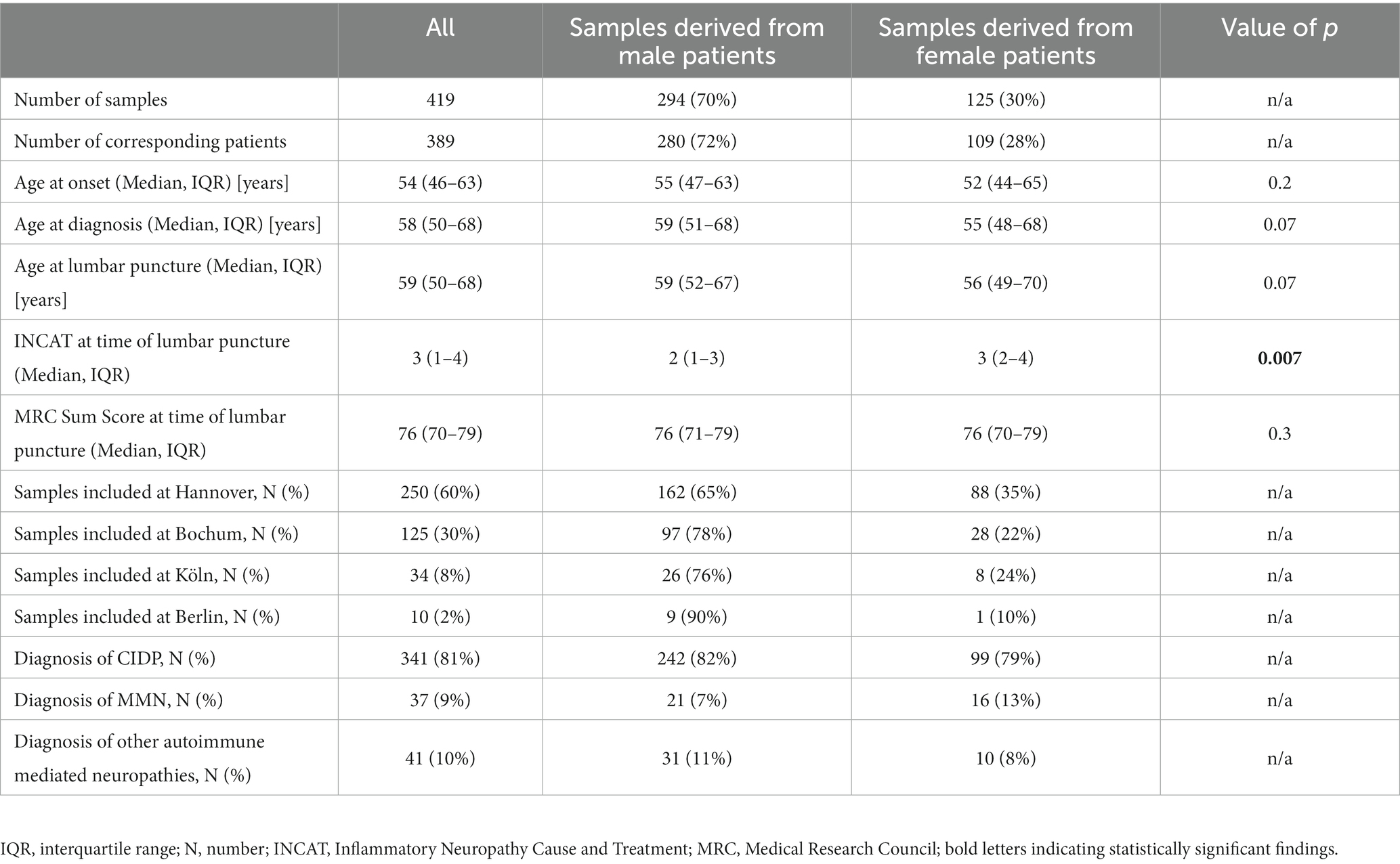
Information on 419 CSF samples was assessed at four study sites in Germany. Based on a repeated lumbar puncture in 30 cases, the samples were derived from a total of 389 included patients with a median age of 59 [IQR 51–68] years of which 72% were of male sex (n = 280) suffering from immune-mediated neuropathies. Samples from therapy-naïve patients had been collected between 04/1996 and 11/2022. Baseline data are shown in Table 1 and the current diagnoses for the included samples are shown in Figure 1. CIDP diagnosis was based on the latest diagnostic criteria available at the time-point of sampling.
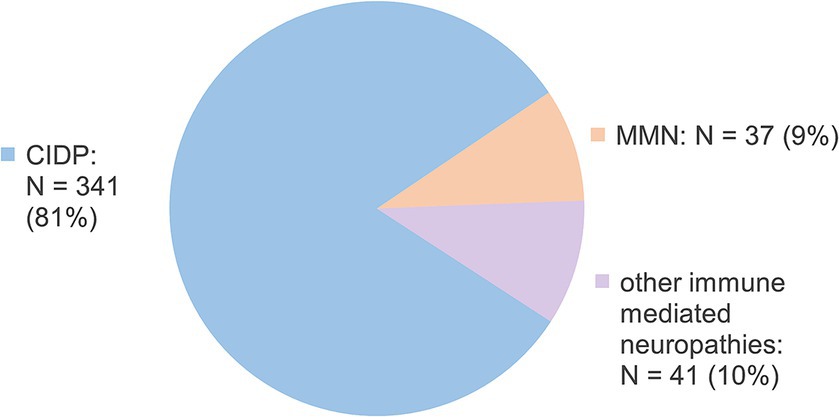
Figure 1. Currently prompted diagnoses for included samples. N, number; CIDP, chronic inflammatory demyelinating polyneuropathy; MMN, multifocal motor neuropathy; GBS, Guillain-Barré syndrome.
3.2 Blood-CSF dysfunction markers
246 samples (58%) showed elevated QAlb values, while 260 samples (62%) showed elevated total CSF protein values. 25 of 419 samples (6%) had elevated total CSF protein levels without elevated QAlb, while QAlb was solely elevated in 11 of 419 samples (2.6%). The detailed analysis is depicted in Figure 2.
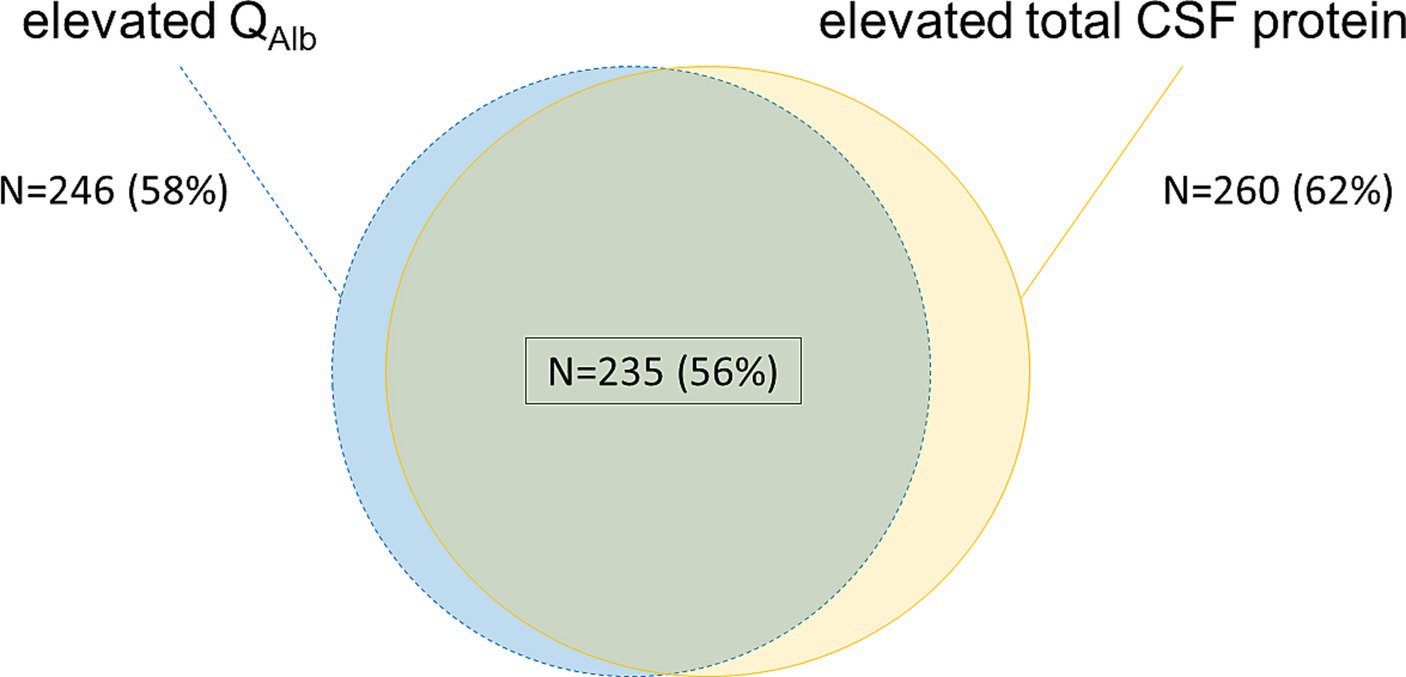
Figure 2. Comparison of the blood-CSF dysfunction-markers QAlb and total CSF protein. Blue/ dotted lines—elevated QAlb calculated by age-dependent Reiber-formula (21); yellow—elevated total CSF protein >500 mg/L; green overlapping background/ black frame—both elevated QAlb and elevated total CSF protein as previously defined. Percentage values refer to the total sample size of 419 samples. N, number; CSF, cerebrospinal fluid; QAlb, ratio of CSF and serum albumin concentrations.
A first subgroup analysis revealed, that samples with elevated QAlb and normal total CSF protein values derived from patients of significantly younger age at onset (p = 0.01), significantly younger age at diagnosis (p = 0.005) and significantly younger age at lumbar puncture (p = 0.001) than samples with elevated total CSF protein values and normal QAlb. The different contributing study sites (p = 0.3 and p = 0.6) or the corresponding diagnoses (p = 1.0 and 0.8) did not prove to be significant confounders for this subgroup analysis.
A second subgroup analysis showed the same significant relations for the comparison of samples with isolated elevated QAlb values versus all samples with elevated total CSF protein values [significantly younger age at onset (p = 0.04), diagnosis (p = 0.03) and at lumbar puncture (p = 0.04)]. In this comparison, INCAT scores at time of lumbar puncture were significantly higher for patients with elevated total CSF protein values independent of QAlb values compared to patients with isolated elevated QAlb.
Clinical characteristics of all the above-mentioned sample subgroups are shown in Table 2.
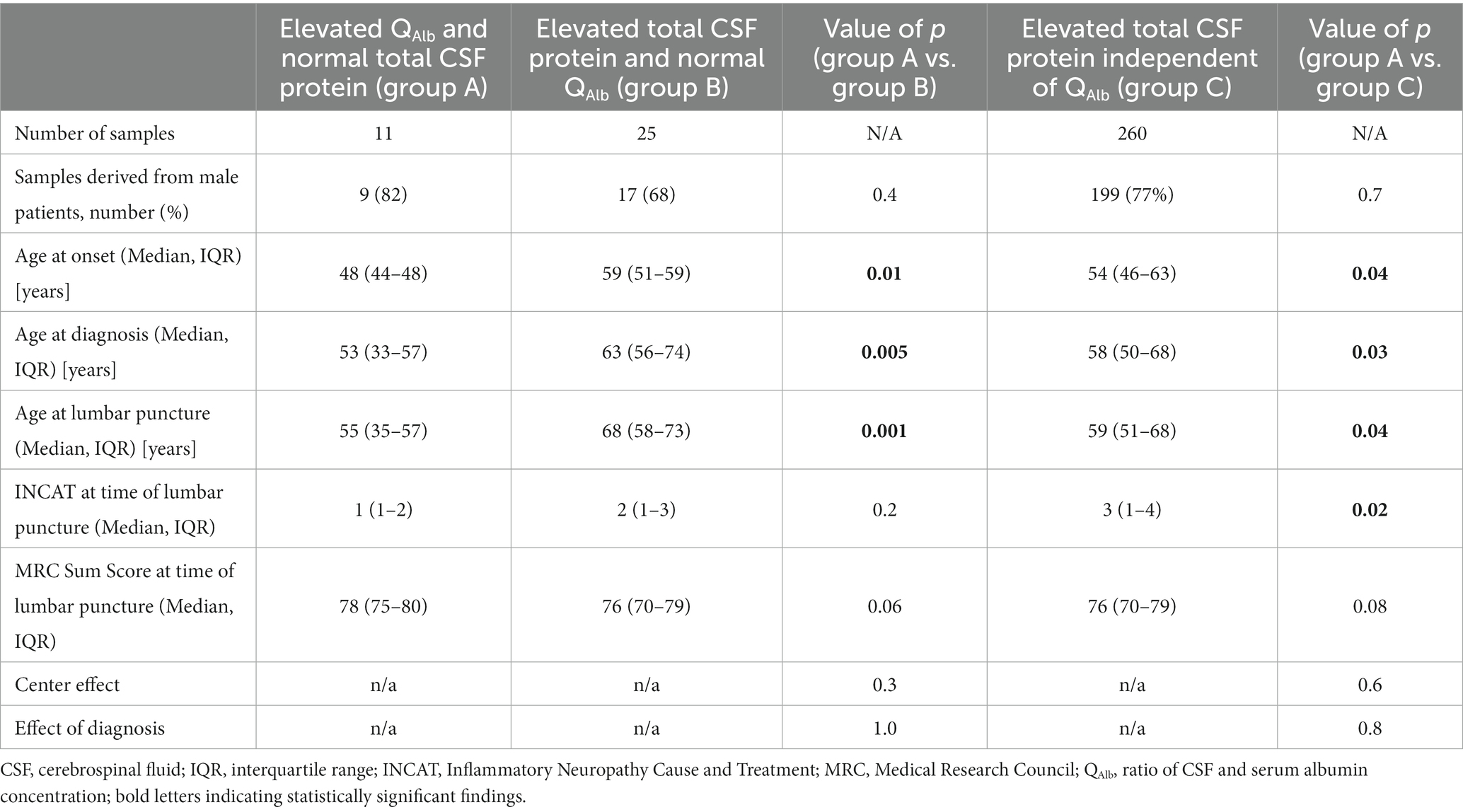
Table 2. Clinical characteristics of samples with isolated pathological changes for either QAlb (group A) or total CSF protein (group B) in comparison with all samples, that showed elevated total CSF protein values.
Even though sex distribution did not differ significantly between the previously described subgroups, there have been many studies implying a sexual bias with significantly higher QAlb and total CSF protein values in male compared to female patients (22). We therefore reassessed both previously conducted subgroup analyses for male and female patients separately: This showed concordant findings for age at diagnosis and at lumbar puncture for male and female patients in the comparison of samples with elevated QAlb and normal total CSF protein values versus samples with elevated total CSF protein values and normal QAlb (significantly younger age at diagnosis (males p = 0.03, females p = 0.04) and at lumbar puncture (males p = 0.02, females p = 0.04). However, the same age dependent effect was only present in samples derived from female patients when comparing samples with isolated elevated QAlb values versus all samples with elevated total CSF protein values [significantly younger age at diagnosis (males p = 0.1, females p = 0.03) and at lumbar puncture (males p = 0.1, females p = 0.03)]. The full workup is included in Table 3.
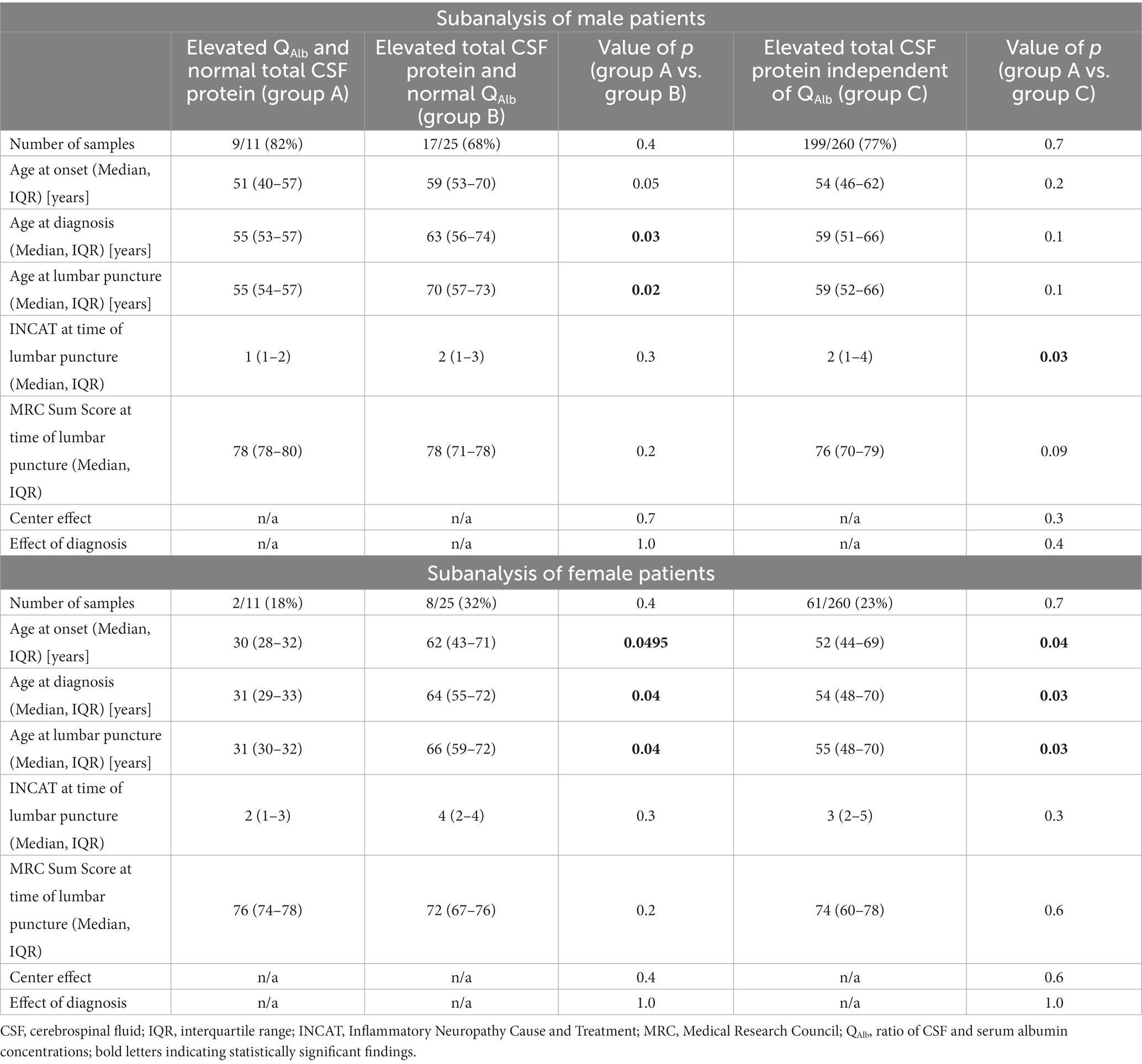
Table 3. Influence of sex on clinical characteristics of samples with isolated pathological changes for either QAlb (group A) or total CSF protein (group B) in comparison with samples, that showed elevated total CSF protein values.
We further conducted a logistic regression analysis for all samples with elevated QAlb and for all samples with elevated total CSF protein values for better understanding of sex-, age- and disability-dependent effects on both markers: Therein, samples from female patients showed significantly less often elevated QAlb (odds ratio [OR] 0.39 [95% confidence interval [CI] 0.26–0.61], p < 0.001) and total CSF protein values (0.46 [95% CI 0.3–0.7], p < 0.001). Also, age at lumbar puncture was significantly younger for samples with elevated QAlb (OR 0.98 [95% CI 0.97–0.996], p = 0.04). The full logistic regression analysis is detailed in Supplementary Table 1.
3.3 Overall interrelation between QAlb and total CSF protein
QAlb and total CSF protein values showed a highly positive correlation (Spearman’s rho coefficient = 0.96, p < 0.0001). The corresponding data are graphically presented in Figure 3.
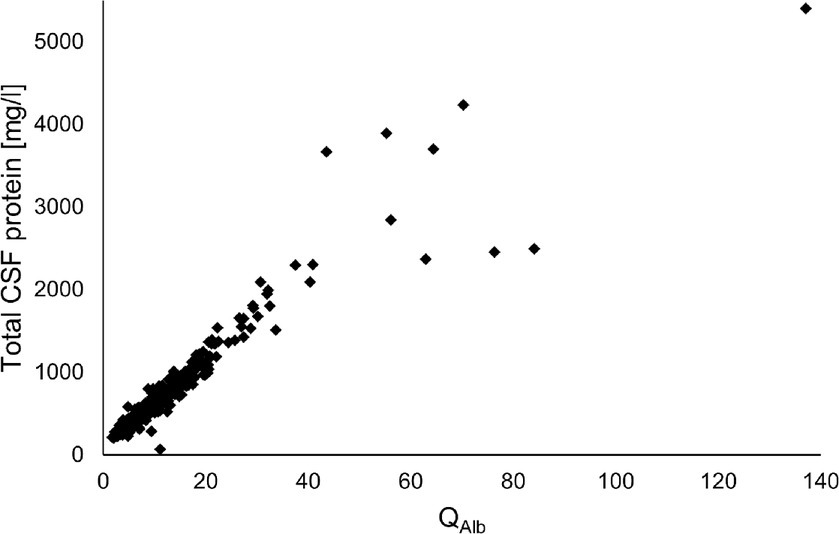
Figure 3. Correlation analysis of QAlb and total CSF protein values (Spearman’s rho coefficient = 0.96, p < 0.0001). CSF, cerebrospinal fluid; QAlb, ratio of CSF and serum albumin concentrations.
Concerning the interrelation between blood-CSF dysfunction and disease severity scores (depicted in Supplementary Figure 1), a marginal inverse correlation was found for QAlb and the MRC Sum score at the time of lumbar puncture (Spearman’s rho coefficient = −0.098, p = 0.048). Further correlation analysis did not reveal a statistical relationship for total CSF protein values with the respective MRC Sum (Spearman’s rho coefficient = −0.08, p = 0.1), or INCAT scores (Spearman’s rho coefficient = 0.06, p = 0.2), as well as for QAlb and the respective INCAT scores (Spearman’s rho coefficient = 0.09, p = 0.08).
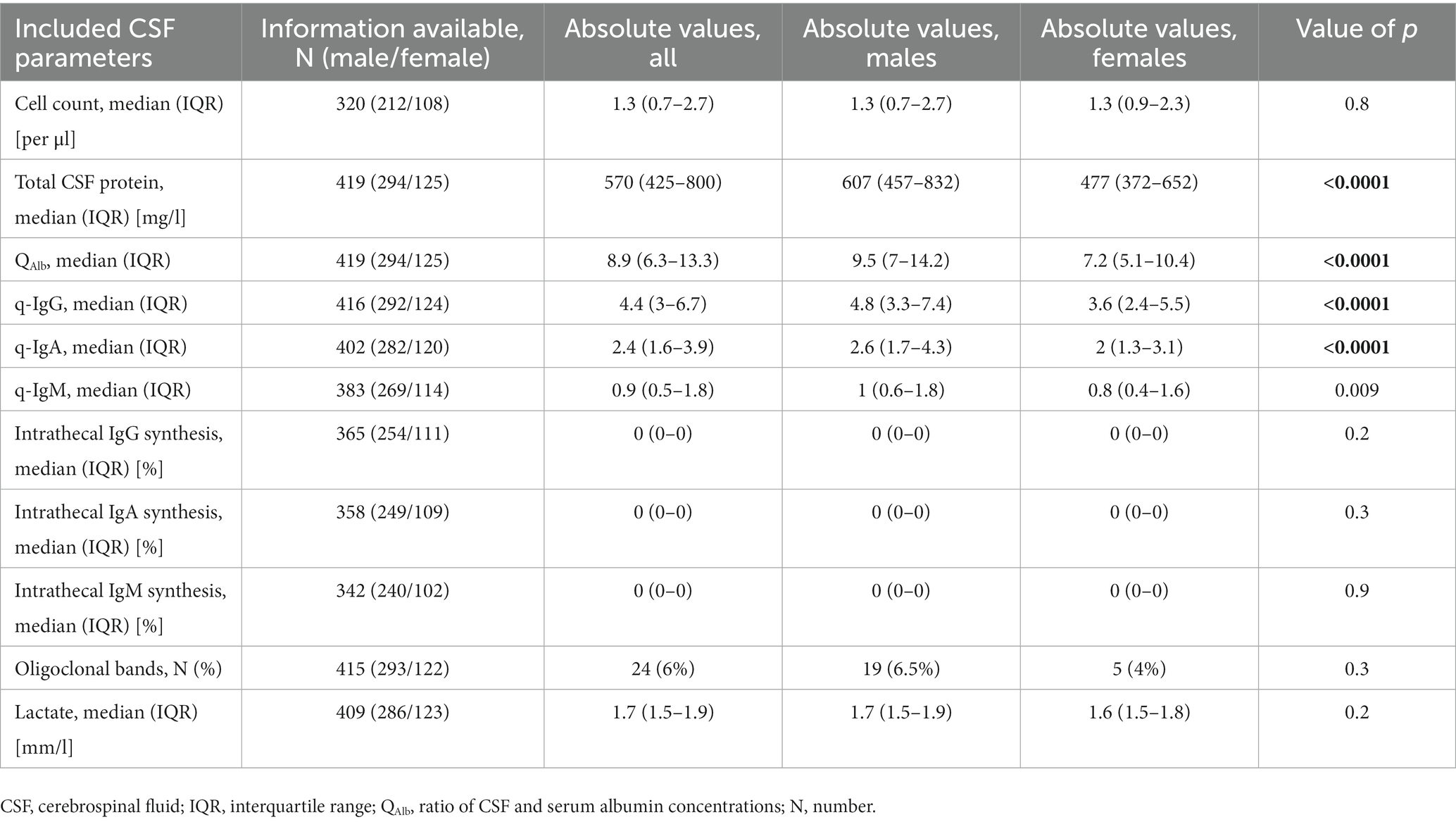
The full workup of the remaining CSF parameters is provided in Table 4.
4 Discussion
In clinical settings, total CSF protein levels remain a popular choice for evaluating the blood-CSF-barrier function. While this method might seem advantageous owing to its straightforwardness and cost-efficiency (only needing one CSF sample instead of both CSF and serum samples for the albumin quotient), it is not without its challenges. A host of confounding factors can complicate the interpretation, making the procedure less reliable than it may initially appear (7).
Evaluating solely the total CSF protein levels without accounting for serum protein concentrations is troubled with inaccuracies. A bulk of CSF proteins have their origin in the plasma. Therefore, when the CSF protein level is interpreted in isolation, without considering the serum protein level, it becomes a source of potential error. Factors such as liver and renal diseases can cause hypoproteinemia or paraproteinemia, and treatments like intravenous immunoglobulins can cause hyperproteinemia, subsequently changing the true total CSF protein levels. This means the straightforward measure of absolute protein concentrations in the CSF could misrepresent the actual situation, potentially indicating a normal scenario even in the face of blood-CSF barrier dysfunction or vice versa.
For example, conditions causing kidney damage, like hemolytic-uremic syndrome, can result in a significant loss of plasma proteins via the urine. Studies have shown that when relying solely on total CSF protein measurements, blood-CSF-barrier dysfunction might go unnoticed due to reduced serum albumin levels (13). However, when adjusting for the serum albumin concentration by determining the QAlb, the barrier dysfunction became evident. In certain cases, patients with renal issues showed only a mild elevation in total CSF protein content, while a much more severe barrier dysfunction was evident upon evaluating the albumin quotient.
Further complicating the interpretation is the fact that the presence of intrathecally produced immunoglobulins can also elevate CSF protein levels. Unfortunately, routine checks for intrathecal immunoglobulin synthesis, crucial for differential diagnosis, are often bypassed. Thus, intrathecally produced immunoglobulins can result in misleadingly elevated total CSF protein readings.
Moreover, the relationship between age and both the QAlb and total CSF protein is another point of contention. Studies by Castellazzi et al. (22) and McCudden et al. (23) indicate a positive correlation between these parameters and age. Yet, in clinical practice, the interpretation of total CSF protein is often done without considering age, given the standard, non-age-dependent cut-off values.
Using the fixed cut-off (usually 500 mg/L) value for total protein is particularly dangerous in potentially misdiagnosing blood-CSF-barrier dysfunction among younger individuals. In such cases, if the total protein is below this threshold, it might yield false negative outcomes. Conversely, elderly patients with a well-functioning blood-CSF-barrier might show a falsely elevated total CSF protein level, leading to false positive results.
In patients with immune-mediated neuropathies, the presence of a blood-liquor barrier dysfunction is important for assessing whether, for example, CIDP is imminent.
CIDP represents a widely known inflammatory neuropathy typically involving symmetrical, sensorimotor neuronal damage and - in contrast to other rapidly progressing entities like the Guillain Barré Syndrome - manifesting with a slowly progressive onset but often leading to severe disability and consecutive reduction in quality of life (24–27). As evidence a blood-liquor barrier dysfunction represents one of the key diagnostic features, a reliable measurement is essential in prompting the correct diagnosis and implementing adequate treatment administration (28).
To investigate the applicability of both QAlb and total CSF protein in this particular context, this multicenter analysis in patients with immune neuropathies was conducted. In our multicenter study encompassing 419 samples, we observed a significant divergence between the CSF total protein and QAlb in 8.6% of cases involving patients with immune-mediated neuropathies. In particular, 2.3% of the patients showed a normal total protein value, although the albumin quotient was elevated and thus showed a blood-CSF barrier dysfunction. As hypothesized, these were especially younger patients in whom a blood-CSF barrier dysfunction would otherwise have been mistakenly overlooked if only the total protein had been examined. To our knowledge, this represents a relevant a novel finding that has been hinted at in only one smaller cohort of 110 patients with GBS (29).
We were also able to confirm the correlation of QAlb and total CSF protein values as previously established (29). Additionally, a marginal inverse correlation of QAlb and MRC Sum Score was also evident in our cohort. This finding most likely expresses the quantitative interrelation of blood-CSF-barrier dysfunction and severity of disease progression of immune-mediated neuropathies. Interestingly, this correlation has not yet been thoroughly investigated and was only negative in patients with amyotrophic lateral sclerosis (30).
An additional finding of our analysis was that samples derived from male patients showed significantly higher values for QAlb and total CSF protein. This finding is in line with previously published studies showing the same effect in different cohorts of neurological and psychiatric background (22, 31, 32).
The consecutive separate subgroup analyses for male and female patients, respectively, showed that especially samples from female patients derived from significantly younger patients at onset, diagnosis and lumbar puncture when comparing those with isolated elevated QAlb (and normal total CSF protein) versus samples with elevated total CSF protein (independent of QAlb values). We therefore deduced that especially female patients of younger age at onset, diagnosis and at lumbar puncture were prone to be underdiagnosed in terms of blood-CSF-barrier-dysfunction when neglecting QAlb as the primary biomarker and focusing on the hitherto established sole total CSF protein levels instead.
The main strengths of this manuscript are the relevant cohort size and the approach in a multicenter manner. However, data interpretation is limited by the retrospective study design and the non-standardized CSF collection.
5 Conclusion
In conclusion, our research supports the known risks of blood-CSF-barrier-evaluation depending solely on total protein measurements, especially in younger cohorts. The pivotal importance of QAlb in diagnostics is evident. Excessive dependence on total protein can mislead interpretations, risking precise and prompt clinical decisions. Our findings strongly support for incorporating QAlb evaluations, crucial for achieving reliable results, particularly in younger patients with immune-mediated neuropathies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by local ethics committees MHH: 8172_BO_K_2018, Ruhr-University Bochum: 18-6534-BR, Cologne: 21–1,079, Berlin: EA4/166/23. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
TSe: Conceptualization, Data curation, Formal analysis, Writing – original draft. SG: Data curation, Writing – review & editing. YG: Data curation, Writing – review & editing. LH: Data curation, Writing – review & editing. BL: Data curation, Writing – review & editing. FK: Data curation, Methodology, Writing – review & editing. HP: Data curation, Writing – review & editing. FS: Data curation, Methodology, Writing – review & editing. JM: Data curation, Writing – review & editing. AF: Data curation, Writing – review & editing. TG: Data curation, Writing – review & editing. KP: Supervision, Writing – review & editing. TSk: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. TSe was funded by the Ellen-Schmidt-Scholarship of the Hannover Medical School. FK is supported by the Cologne Clinician Scientist Program (CCSP) / Faculty of Medicine / University of Cologne funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation; Project no. 413543196).
Conflict of interest
TSe reports support for attending meetings by Abbvie. SG reports research support from Alnylam Pharmaceuticals, CSL Behring, Else Kröner Fresenius Foundation, Deutsche Forschungsgemeinschaft and Hannover Biomedical Research School (HBRS) and consulting and/or speaker honoraria from Alexion, Alnylam Pharmaceuticals, AstraZeneca, GSK, Pfizer and Merck all outside the submitted work. JM reports research grants by Biogen and Deutsche Forschungsgemeinschaft, stock/stock options at Biontech and CureVac, as well as support for attending meetings and/or travel by Biogen, Novartis and Alnylam. JM also received honoraria or lectures/ manuscripts by Biogen. KP reports research grants by Biogen idec, Novartis, Celgene, CSL Behring, Grifols, honoraria for lectures from CSL Behring, Grifols, participation on an Advisory Board 2021 by Celgene and non-paid consultant activity for patients organizations POTS and Dysautonomie e.V. TSk reports honoraria for lectures and travel expenses for attending meetings from Alexion, Alnylam Pharmaceuticals, argenx, Bayer Vital, Biogen, Celgene, Centogene, CSL Behring, Euroimmun, Janssen-Cilag, Merck Serono, Novartis, Pfizer, Roche, Sanofi, Siemens, Swedish Orphan Biovitrum, Teva, Viatris. His research is supported by the German Ministry for Education and Research (BMBF), Bristol-Myers Squibb Foundation for Immuno-Oncology, Claudia von Schilling Foundation for Breast Cancer Research, Else Kröner Fresenius Foundation, Hannover Biomedical Research School (HBRS), Alnylam Pharmaceuticals, CSL Behring, Novartis, Sanofi Genzyme, VHV Foundation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1330484/full#supplementary-material
References
1. Yalachkov, Y, Uhlmann, V, Bergmann, J, Soydas, D, Frisch, S, Behrens, M, et al. Patients with chronic autoimmune demyelinating polyneuropathies exhibit cognitive deficits which might be associated with CSF evidence of blood-brain barrier disturbance. PloS One. (2020) 15:e0228679. doi: 10.1371/JOURNAL.PONE.0228679
2. Ruiz, M, Puthenparampil, M, Campagnolo, M, Castellani, F, Salvalaggio, A, Ruggero, S, et al. Oligoclonal IgG bands in chronic inflammatory polyradiculoneuropathies. J Neurol Neurosurg Psychiatry. (2021) 92:969–74. doi: 10.1136/jnnp-2020-325868
3. Van den Bergh, PYK, van Doorn, PA, Hadden, RDM, Avau, B, Vankrunkelsven, P, Allen, JA, et al. European academy of neurology/peripheral nerve society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force—second revision. J Peripher Nerv Syst. (2021) 26:242–68. doi: 10.1111/JNS.12455/SUPINFO
4. Van Schaik, IN, Léger, JM, Nobile-Orazio, E, Cornblath, DR, Hadden, RDM, Koski, CL, et al. European Federation of Neurological Societies/peripheral nerve society guideline on management of multifocal motor neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the peripheral nerve society--first revision. J Peripher Nerv Syst. (2010) 15:295–301. doi: 10.1111/J.1529-8027.2010.00290.X
5. Leonhard, SE, Mandarakas, MR, FAA, G, Bateman, K, MLB, F, Cornblath, DR, et al. Diagnosis and management of Guillain–Barré syndrome in ten steps. Nat Clin Pract Neurol. (2019) 15:671–83. doi: 10.1038/s41582-019-0250-9
7. Franciotta, D, Gastaldi, M, Zardini, E, and Nobile-Orazio, E. Cerebrospinal fluid total protein determination in acute and chronic inflammatory demyelinating polyneuropathies: a critical reappraisal. J Peripher Nerv Syst. (2018) 23:70–2. doi: 10.1111/JNS.12253
8. Reiber, H . External quality assessment in clinical neurochemistry: survey of analysis for cerebrospinal fluid (CSF) proteins based on CSF/serum quotients. Clin Chem. (1995) 41:256–63. doi: 10.1093/clinchem/41.2.256
9. Reiber, H, Otto, M, Trendelenburg, C, and Wormek, A. Reporting cerebrospinal fluid data: knowledge base and interpretation software. Clin Chem Lab Med. (2001) 39:324–32. doi: 10.1515/CCLM.2001.051
10. Reiber, H, Wildemann, B, and Oschmann, P. Laboratory diagnosis in neurology, chapter 21: reference ranges of Analytes in CSF and serum In:. Laboratory diagnosis in neurology. US: Thieme Verlag (2010)
11. Reiber, H, and Peter, JB. Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J Neurol Sci. (2001) 184:101–22. doi: 10.1016/S0022-510X(00)00501-3
12. Andersson, M, Alvarez-Cermeñio, J, Bernardi, G, Cogato, I, Fredman, P, Frederiksen, J, et al. Cerebrospinal fluid in the diagnosis of multiple sclerosis: a consensus report. J Neurol Neurosurg Psychiatry. (1994) 57:897–902. doi: 10.1136/JNNP.57.8.897
13. Skripuletz, T, Wurster, U, Worthmann, H, Heeren, M, Schuppner, R, Trebst, C, et al. Blood-cerebrospinal fluid barrier dysfunction in patients with neurological symptoms during the 2011 northern German E. coli serotype O104:H4 outbreak. Brain. (2013) 136:e241. doi: 10.1093/BRAIN/AWS361
14. Reiber, H . Flow rate of cerebrospinal fluid (CSF) - a concept common to normal blood-CSF barrier function and to dysfunction in neurological diseases. J Neurol Sci. (1994) 122:189–203. doi: 10.1016/0022-510X(94)90298-4
15. Blennow, K, Fredman, P, Wallin, A, Gottfries, CG, Karlsson, I, Langs Troin, G, et al. Protein analysis in cerebrospinal fluid. II. Reference values derived from healthy individuals 18-88 years of age. Eur Neurol. (1993) 33:129–33. doi: 10.1159/000116919
16. Reiber, H . Proteins in cerebrospinal fluid and blood: barriers, CSF flow rate and source-related dynamics. Restor Neurol Neurosci. (2003) 21:79–96.
17. Fisse, AL, Motte, J, Grüter, T, Kohle, F, Kronlage, C, Stahl, JH, et al. Versorgungssituation von CIDP-Patienten in neun deutschen Zentren des Neuritis Netzes. Nervenarzt. (2023) 94:320–6. doi: 10.1007/S00115-022-01377-0
18. Hughes, R, Bensa, S, Willison, H, Van den Bergh, P, Comi, G, Illa, I, et al. Randomized controlled trial of intravenous immunoglobulin versus oral prednisolone in chronic inflammatory demyelinating polyradiculoneuropathy. Ann Neurol. (2001) 50:195–201. doi: 10.1002/ana.1088
19. van Schaik, IN, Bril, V, van Geloven, N, Hartung, HP, Lewis, RA, Sobue, G, et al. Subcutaneous immunoglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy (PATH): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. (2018) 17:35–46. doi: 10.1016/S1474-4422(17)30378-2
20. Kleyweg, RP, van der Meché, FG, and Schmitz, PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve. (1991) 14:1103–9. doi: 10.1002/mus.880141111
21. Reiber, H . Cerebrospinal fluid--physiology, analysis and interpretation of protein patterns for diagnosis of neurological diseases. Mult Scler. (1998) 4:99–107. doi: 10.1177/135245859800400302
22. Castellazzi, M, Morotti, A, Tamborino, C, Alessi, F, Pilotto, S, Baldi, E, et al. Increased age and male sex are independently associated with higher frequency of blood-cerebrospinal fluid barrier dysfunction using the albumin quotient. Fluids Barriers CNS. (2020) 17:14. doi: 10.1186/S12987-020-0173-2
23. McCudden, CR, Brooks, J, Figurado, P, and Bourque, PR. Cerebrospinal fluid Total protein reference intervals derived from 20 years of patient data. Clin Chem. (2017) 63:1856–65. doi: 10.1373/CLINCHEM.2017.278267
24. Rajabally, YA, Simpson, BS, Beri, S, Bankart, J, and Gosalakkal, JA. Epidemiologic variability of chronic inflammatory demyelinating polyneuropathy with different diagnostic criteria: study of a UK population. Muscle Nerve. (2009) 39:432–8. doi: 10.1002/MUS.21206
25. Lunn, MPT, Manji, H, Choudhary, PP, Hughes, RAC, and Thomas, PK. Chronic inflammatory demyelinating polyradiculoneuropathy: a prevalence study in south East England. J Neurol Neurosurg Psychiatry. (1999) 66:677–80. doi: 10.1136/JNNP.66.5.677
26. Chiò, A, Cocito, D, Bottacchi, E, Buffa, C, Leone, M, Plano, F, et al. Idiopathic chronic inflammatory demyelinating polyneuropathy: an epidemiological study in Italy. J Neurol Neurosurg Psychiatry. (2007) 78:1349–53. doi: 10.1136/JNNP.2007.114868
27. Mahdi-Rogers, M, and Hughes, RAC. Epidemiology of chronic inflammatory neuropathies in Southeast England. Eur J Neurol. (2014) 21:28–33. doi: 10.1111/ENE.12190
28. Querol, L, Crabtree, M, Herepath, M, Priedane, E, Viejo Viejo, I, Agush, S, et al. Systematic literature review of burden of illness in chronic inflammatory demyelinating polyneuropathy (CIDP). J Neurol. (2021) 268:3706–16. doi: 10.1007/S00415-020-09998-8
29. Rath, J, Zulehner, G, Schober, B, Grisold, A, Krenn, M, Cetin, H, et al. Cerebrospinal fluid analysis in Guillain–Barré syndrome: value of albumin quotients. J Neurol. (2021) 268:3294–300. doi: 10.1007/S00415-021-10479-9
30. Verde, F, Ferrari, I, Maranzano, A, Ciusani, E, Torre, S, Milone, I, et al. Relationship between cerebrospinal fluid/serum albumin quotient and phenotype in amyotrophic lateral sclerosis: a retrospective study on 328 patients. Neurol Sci. (2023) 44:1679–85. doi: 10.1007/S10072-023-06604-3
31. Meixensberger, S, Bechter, K, Dersch, R, Feige, B, Maier, S, Schiele, MA, et al. Sex difference in cerebrospinal fluid/blood albumin quotients in patients with schizophreniform and affective psychosis. Fluids Barriers CNS. (2020) 17:67. doi: 10.1186/S12987-020-00223-2
Keywords: QAlb, blood-cerebrospinal fluid-barrier, CSF, barrier-dysfunction, immune-mediated neuropathies
Citation: Seeliger T, Gingele S, Güzeloglu YE, Heitmann L, Lüling B, Kohle F, Preßler H, Stascheit F, Motte J, Fisse AL, Grüter T, Pitarokoili K and Skripuletz T (2024) Comparative analysis of albumin quotient and total CSF protein in immune-mediated neuropathies: a multicenter study on diagnostic implications. Front. Neurol. 14:1330484. doi: 10.3389/fneur.2023.1330484
Edited by:
Juan A. Gallego, Northwell Health, United StatesReviewed by:
Massimiliano Castellazzi, University of Ferrara, ItalyEline A. J. Willemse, University Hospital of Basel, Switzerland
Copyright © 2024 Seeliger, Gingele, Güzeloglu, Heitmann, Lüling, Kohle, Preßler, Stascheit, Motte, Fisse, Grüter, Pitarokoili and Skripuletz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thomas Skripuletz, c2tyaXB1bGV0ei50aG9tYXNAbWgtaGFubm92ZXIuZGU=
 Tabea Seeliger
Tabea Seeliger Stefan Gingele
Stefan Gingele Yunus Emre Güzeloglu
Yunus Emre Güzeloglu Lena Heitmann1
Lena Heitmann1 Felix Kohle
Felix Kohle Hannah Preßler
Hannah Preßler Frauke Stascheit
Frauke Stascheit Jeremias Motte
Jeremias Motte Thomas Grüter
Thomas Grüter Kalliopi Pitarokoili
Kalliopi Pitarokoili Thomas Skripuletz
Thomas Skripuletz