- 1Department of Neurology, Huadong Hospital, Fudan University, Shanghai, China
- 2Department of Neurology, Shanghai East Hospital, Tongji University, Shanghai, China
- 3Department of Neurosurgery, Huadong Hospital, Fudan University, Shanghai, China
- 4Department of Neurosurgery, Huashan Hospital, Fudan University, Shanghai, China
- 5Departments of Anesthesiology, Huadong Hospital, Fudan University, Shanghai, China
Background and purpose: To study the changes of corticocerebral hemodynamics in surgical area and postoperative hyperperfusion syndrome in patients with chronic internal carotid artery occlusion (CICAO) by intraoperative indocyanine green videoangiography (ICGA)-FLOW 800 and CT perfusion after superficial temporal artery (STA)–middle cerebral artery (MCA) bypass surgery.
Methods: From October 2019 to January 2021, 77 patients diagnosed with CICAO underwent direct bypass surgery at Huadong hospital (affiliated with Fudan University) were enrolled. Regions of interest (ROIs) at STA, proximal MCA (PMCA), distal MCA (DMCA), cortical blood capillary (CBC), and cortical vein (CV) were identified after anastomosis by ICGV-FLOW 800 including peak fluorescence intensity (PFI), time to peak (TTP), and area under the time curve (AUC) of fluorescence intensity. All patients underwent perfusion-weighted CT before bypass surgery and those patients with HPS were verified by CTP after bypass.
Results: 14 patients with HPS were verified by perfusion-weighted CT after bypass. In HPS group, the AUCTTP of DMCA was significantly larger (T = −3.301, p = 0.004) and TTP of CBC was shorter (T = −2.929, p = 0.005) than patients in non-HPS group. The larger AUCTTP of DMCA (OR = 3.024, 95%CI 1.390–6.578, p = 0.0050) was an independent risk factor by further multivariate logistic regression analysis.
Conclusion: The hemodynamic changes of cortical vessels during STA-MCA bypass surgery could be recorded accurately by ICGV-FLOW 800. Furthermore, the increased AUCTTP of DMCA and shorter TTP of CBC may be potential risk factors of HPS.
Introduction
Chronic internal carotid artery occlusion (CICAO) is a rare cerebrovascular disease that is characterized by progressive stenosis to occlusion in internal carotid arteries (1). The main treatment for CICAO is revascularization surgery, especially superficial temporal artery (STA)–middle cerebral artery (MCA) bypass surgery, which can directly and efficiently establish sufficient blood flow from the extracranial artery to the intracranial artery to improve cerebral perfusion (2). Hyperperfusion syndrome (HPS) is a common hemodynamics-related complication that may lead to neurological deficits, including cerebral hemorrhage or ischemia, after revascularization (3, 4). However, at present, there are no reliable models and/or methods to predict the occurrence of HPS in patients with CICAO after STA-MCA bypass surgery.
Indocyanine green video-angiography (ICGA) FLOW 800 software (Carl Zeiss Meditec AG) can record time-delayed dynamic video and calculate the hemodynamic parameters of regions of interest (ROIs) at cortical surfaces. ICGA has been gradually introduced in bypass surgery for CICAO because of its high spatial resolution and excellent image quality with reliable real-time parametric variation in cortical vessel flow (5).
In this retrospective cohort study, we investigate the mechanism of HPS in patients with CICAO after STA-MCA bypass surgery and further discuss the determinant by hemodynamic parameters changes in different blood flow periods collected by ICGA-FLOW 800 technology.
Materials and methods
Clinical data
From October 2019 to January 2021, patients diagnosed with CICAO were retrospectively enrolled at Huadong Hospital (affiliated with Fudan University). This study was approved by the Ethics Committee of Huadong Hospital (20190030). All patients signed informed consent forms.
Inclusion criteria: aged between 14 and 75 years old; at II–VI period by Suzuki stage with internal carotid artery occlusion or stenosis (6); no prior intracranial and extracranial vascular anastomosis of any kind; received STA-MCA bypass surgery; after anastomosis, and cortex blood flow tested by ICGA. Exclusion criteria: patients with internal carotid artery or MCA occlusion caused by atherosclerosis without a preoperative and/or postoperative computerized tomography perfusion imaging (CTP) test.
STA-MCA bypass
To perform bypass surgery, the head of the patient was positioned at 60 degrees to the opposite side. The scalp was then incised, and the temporal muscle was isolated from the temporal lobe. After milling the skull, cutting the dura mater, and confirmation of a suitable receptor (MCA-M4 segment, diameter 0.8 mm; Figure 1A), STA was towed through the temporal muscle. After anastomosis, 0.2 mg/kg indocyanine green (Dandong Medical and Pharmaceutical Co., Ltd., China) with 10 mL of normal saline was intravenously injected at a high rate of speed. The ROIs at the STA, the proximal part of MCA (PMCA), distal part of MCA (DMCA), cortical blood capillary (CBC), and the cortical vein (CV) around the anastomoses were selected using fluorescence angiography (OPMI Pentero fluorescence microscope, Carl Zeiss, Germany; Figure 1B). If the fluorescence intensity (PFI) of the blood vessel rises early, the vessel will appear darker red in the composite image, whereas if the PFI rises later, the vessel will appear dark blue (Figure 1C). The coordinate plot of the fluorescence intensity over time and its original data were automatically generated using FLOW-800 microscope (Figure 1D). Time to peak (TTP) indicates the time from the beginning of fluorescence to the strongest fluorescence. The area under the curve (AUC) from the origin of the coordinates to the TTP indicated as AUCTTP, was calculated using GraphPad Prism 8.0 (GraphPad Software, United States). The value of PFI, TTP, and AUCTTP were analyzed.
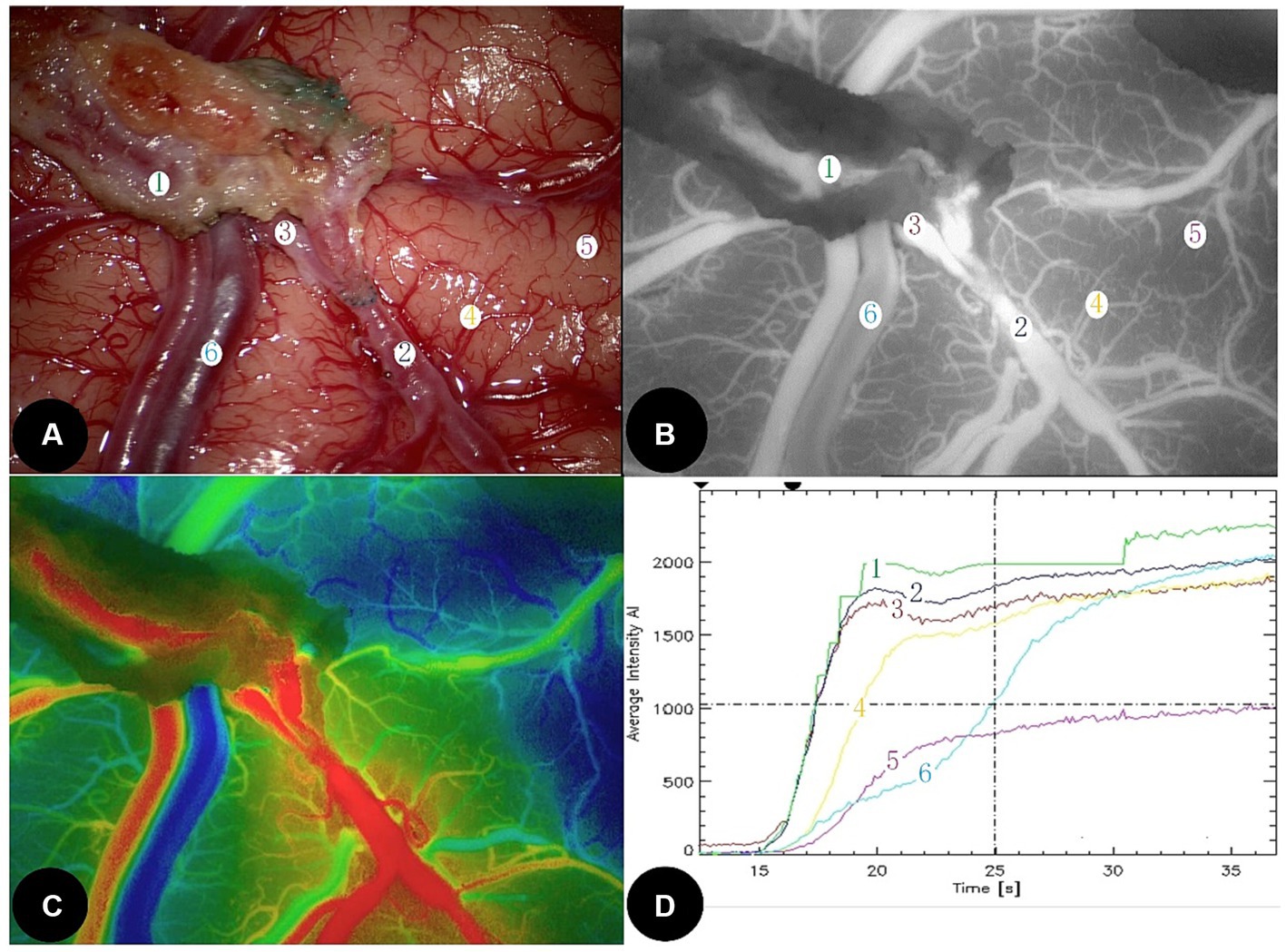
Figure 1. A 56-year-old female developed temporary motor aphasia and dysphagia at 3 days after left STA-MCA direct bypass surgery. (A) Microscopic image during bypass surgery. STA was marked as ①, DMCA as ②, PMCA as ③, CBC as ④ and ⑤, and CV as ⑥. (B) Intraoperative indocyanine green video-angiography image. (C) Schematic diagram of each peak blood flow time; the redder, the peak is the earlier, the bluer, the peak is the later; (D) Fluorescence intensity—time curve at each interest vessels. The value of AUCTTP at STA (mark 1) and DMCA (mark 2) increased significantly. STA, Superficial temporal artery; DMCA, The distal part of middle cerebral artery; PMCA, The proximal part of middle cerebral artery; CBC, Cortical blood capillary; and CA, Cortical vein.
Perioperative management
Maintaining stable blood pressure and ensuring the balance of inflow and outflow, the adverse reactions were recorded in detail. These included the following: (1) persistent headache for more than 72 h; (2) muscle weakness, seizures, and aphasia. Patients with adverse reactions were tested by CT or MRI immediately to exclude intracerebral hemorrhage or a fresh infarction.
Computerized tomography perfusion imaging acquisition and analysis
All patients underwent a CTP test (GE LightSpeed VCT 64 Slice CT Scanner) to determine cerebral perfusion status and not all patients were reviewed for CTP after surgery because of radiation hazards. However, once adverse reaction occurred in patients similar to HPS postoperatively, we would actively review CTP test to determine whether HPS was present. A head retainer was used to keep the patient’s head as stationary as possible during the scan. Ioformol (Hengrui Co. Ltd., China) was administered via the median cubital vein at a flow rate of 5.0 mL/s at a dose of 50 mL. The scan parameters were as follows: tube voltage = 80 kV, tube current = 120–150 mA, layer thickness = 1.25 mm, total scanning time = 60 s; the plateau, inflow, and outflow period were all included. Data were automatically analyzed using brain perfusion CT image processing software (PerfusionGo V1.0; Shukun, Co. Ltd., China) via a singular value decomposition and deconvolution algorithm for dispersion correction (Figure 2).
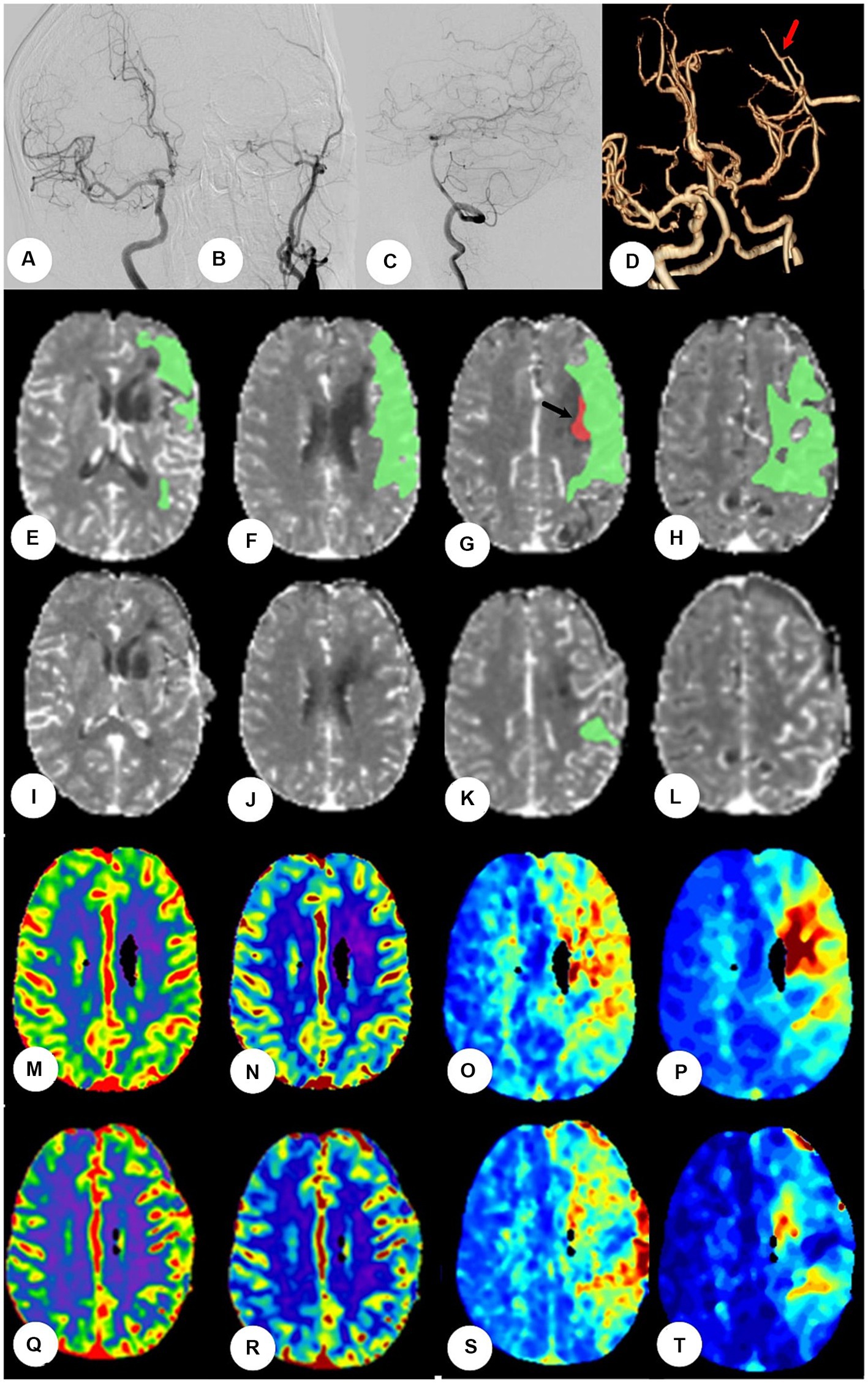
Figure 2. The patients were performed by CTP at the fourth day after bypass surgery. (A–C) Digital subtraction angiography showed left initial internal carotid artery was occluded, right the left internal carotid artery and bilateral vertebral artery systems is normal, but without obvious compensation. (D) Postoperative CT angiography showed the blood flow at anastomosis was patent (red arrow). (E–H): Preoperative CTP showed the mismatch green area (Tmax>6 s) was 93.1 mL and the hypoperfusion red area (rCBF<30%) was 0.6 mL at frontotemporal parietal lobe (black arrow). (I–L) Postoperative CTP showed mismatch area reduced significantly and hypoperfusion area disappeared. (M–P) Figures of CTP showed CBV, CBF, and MTT and Tmax, respectively, before bypass. The values of CBV and CBF were normal, the values of MTT and Tmax lengthened at left frontotemporal parietal lobe. (Q–T) The values of CBV and CBF were still normal, and the values of MTT and Tmax shortened significantly after bypass surgery.
Identification of HPS
If patients show postoperative adverse reactions, CTP should be performed. According to a procedure described in a previous study (7), HPS was confirmed by CTP after bypass as follows: (1) Mismatch area and/or hypoperfusion red area (rCBF < 30%) reduce significantly; (2) CBV and CBF increased or MTT and TTP reduced significantly at the anastomotic region; and (3) Without fresh hemorrhage or infarction.
Statistical analysis
The SPSS v.23.0 statistical software package was used to process the data. Continuous variables were verified using the Kolmogorov–Smirnov normality test. Normal distributional data were represented by mean ± standard deviation ( ±s) and the differences between two groups were detected by an independent t-test. Nonnormal distributional data were compared using the Mann–Whitney U test. Categorical variables were represented as frequencies and compared by the χ2 test. The risk factors for HPS were calculated using multivariate logistic regression. A p value of <0.05 was considered statistically significant.
Results
General information
A total of 77 patients were included in this study (37 males and 40 females), with an average age of 42.2 ± 13.3 years, ranging from 31 to 72 years. According to the clinical classification criteria of Matsushima, 34 patients suffered from transient ischemic attack (TIA), 24 from cerebral infarction, and 19 from cerebral hemorrhage. According to Suzuki staging, 9 cases were at level II, 21 cases were at level III, 14 cases were at IV level, 19 cases were at level V, and 14 cases were at level VI.
Postoperative adverse reactions and HPS syndrome
23 patients had moderate to severe adverse reaction after bypass surgery, 20 received postoperative CTP test, and finally, 14 patients (18.2%) were confirmed to be in the HPS group. The main postoperative adverse events were swallowing dysfunction or salivation (five cases), persistent headache (five cases), epilepsy (two cases), and contralateral muscle strength weakness (two cases; Table 1).
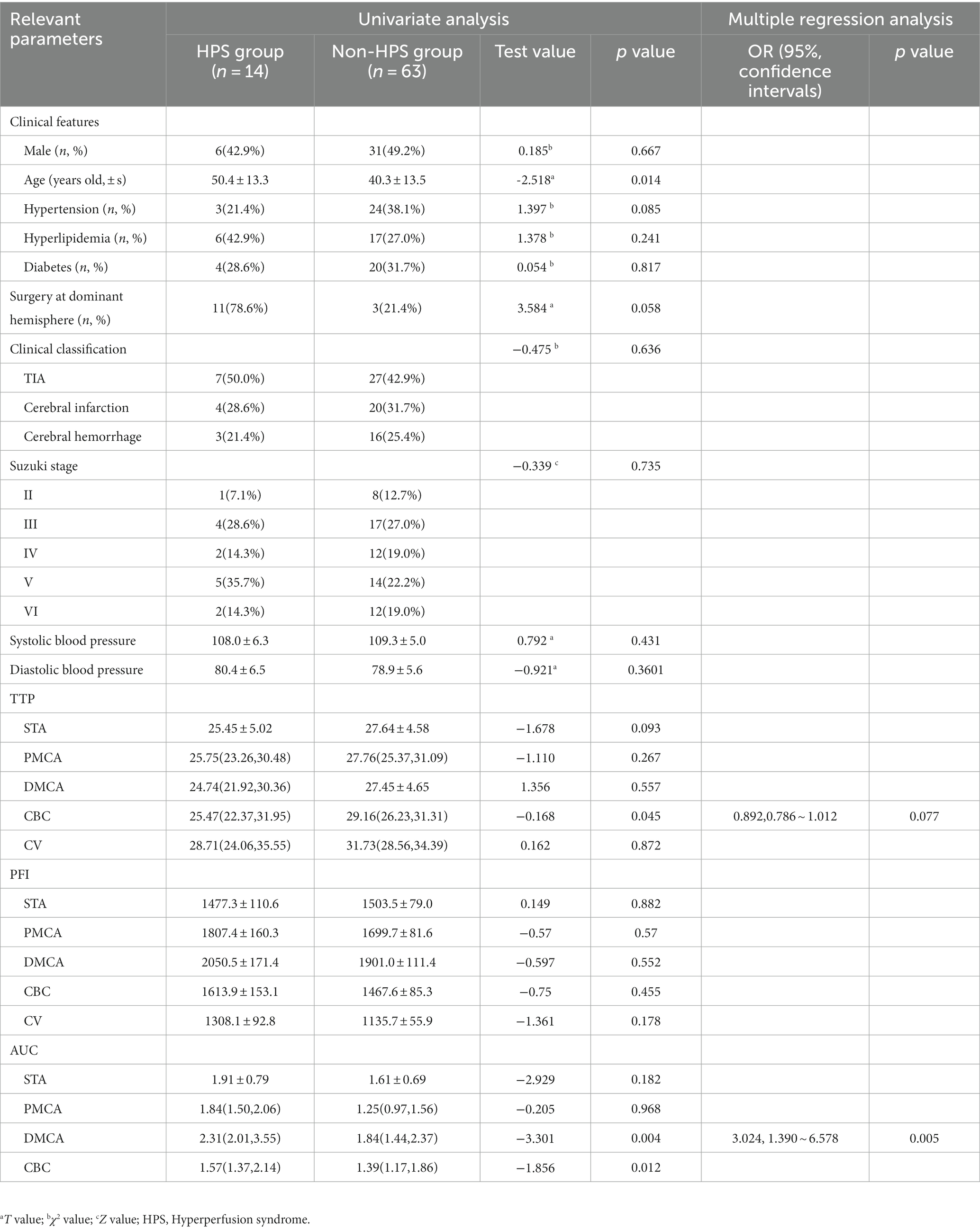
Table 1. Comparison of clinical data between HPS group and non-HPS group of patients with chronic internal carotid artery occlusion.
Risk factors for HPS syndrome
In the HPS group (14 patients), the AUCTTP of the DMCA was significantly larger (T = −3.301, p = 0.004) and the TTP of the CBC was shorter (T = −2.929, p = 0.005) compared to that in the non-HPS group. Multivariate logistic regression analysis indicated that the larger AUCTTP of the DMCA (OR = 3.024, 95% CI: 1.390 ~ 6.578, p = 0.0050) was an independent risk factor for HPS.
Discussion
Chronic internal carotid artery occlusion is associated with a 5–8% annual risk of stroke, even under medical treatment (8, 9). STA-MCA artery bypass, as a low-flow bypass, can reduce the risk of recurrence ipsilateral ischemic stroke, however, would result in HPS-significant focal increase in cerebral blood flow at the site of the anastomosis, which is responsible for postoperative neurological deterioration (10). HPS was first reported by Masaaki in 1998 (11) and the occurrence rate of HPS could be from 4.4% to even 67.5% (12–16). In our cohort, the rate was 18.2%, and the HPS-related symptoms included aphasia, hemiparesis, dysarthria, seizure (17–21). A variety of clinical features have been studied as key risk factors associated with HPS, including preoperative hypertension, weak posterior circulation (22), the hemodynamic sources of the recipient parasylvian cortical arteries (23), and a longer delay time at CTP (24). At present, there are still no reliable models to predict the occurrence of HPS in patients with CICAO after bypass surgery.
ICG fluorescence is a new semiquantitative-visual technique that has been applied for the intraoperative detection of cerebrovascular disease (25–27). Zhang et al. further optimized the parameters of ICG mapping to include peak cerebral blood volume, regional cerebral blood flow, and TTP using FLOW 800 software. Moreover, they found that the differences in peak cerebral blood volume and regional cerebral blood flow before and after bypass were significantly higher in the symptomatic group (28).
According to hemodynamic Hagen–Poiseuille equation (29), Hu put forward the basic principle of the ‘flow-controlled’, the hemodynamic difference between the donor and recipient arteries should reduce after STA-MCA anastomosis (30). Compared with the non-HPS group, the forward volume of the STA in the HPS group was significantly lower, identified using a phase-contrast MRI technique in our previous study (20). However, the obvious flaws of phase-contrast MRI technique include excessively long detection times and fuzzy imaging owing to patient movement, which lead to data bias and difficulty in clinical application (20). In operative practice, we believe that high blood flow in the STA after bypass is an important cause of HPS and follow a principle of matching selection of donor and recipient vessels (31). Using the ICG fluorescence angiography technique, we can directly choose ROIs at the cortical surface, including blood vessels, from the arterial to the capillary and venous stage. Its parameters include TTP and AUC, representing blood flow velocity and blood flow volume, respectively, which are more accurate than the parameters employed in previous hemodynamic research techniques.
A higher STA blood flow results in abnormally high local cerebral perfusion. It was verified that after anastomosis, the circulatory resistance of distal STA suddenly and significantly decreased, and the blood flow of the receptor passively increased (32). If the STA undergoes compensatory thickening after bypass, patients will show higher rates of headache (33). In Figures 1A–C, the blood flow of STA was ejected directly into the MCA-M4 receptor at a high rate of speed, and the fluorescence intensity of the STA was the highest at all cortex blood vessels according to automatic analysis of FLOW-800, which showed that the higher blood flow of the STA appeared in the ultra-early period after anastomosis. According to the Mann–Whitney U-pair analysis, the AUITTP value of the DMCA was significantly larger in the HPS group, confirming that the flow of the DMCA passively increased after bypass.
In contrast, some patients with weaker vascular networks could not withstand changes in blood flow. In this study, the TTP of the CBC was shorter in the HPS group, indicating that the patients with HPS had a weaker buffering function of the CBC than those without. When foreign and reversed STA blood flow is ejected, it may cause further damage to the entire cerebrovascular network, particularly in patients with weaker CBC function. The results of this study proved again that cerebral perfusion around the anastomosis increased after surgery in the HPS group and that if the cerebral perfusion was excessive, postoperative complications could occur (34).
If high HPS risks are found during surgery, several measures will be adopted in our center. Blood pressure should be maintained at <125/85 mmHg by continuous monitoring every 1 h (35). Nimodipine is used routinely in postoperative patients and nicardipine is proposed by intravenous maintenance for those with high blood pressure. Edaravone as a free radical scavenger (36) and Dl-3-n-Butylphthalide approved by Food and Drug Administration of China for treating ischemic stroke (37), are considered to be effective in reducing HPS by our experience. However, the measures for treating HPS also should be assessed by randomized controlled trials for the efficacy and safety.
Limitation
This study was a single-center, small sample retrospective study, underlying HPS risk factors such as age, comorbidities, and stroke severity were not differentiated, intraoperative anesthesia status was also not considered. Some HPS patients may be missed, because not all patients were tested by postoperative CTP. In the future, prospective randomized controlled trials in multicenter are needed to further confirm its reliability.
Conclusion
Intraoperative fluorescein videoangiography FLOW 800 could predict the risk of HPS in patients with CICAO after bypass surgery. A large AUC of DMCA is an important risk factor for HPS.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Huadong Hospital, Fudan University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JD: Writing – original draft. JS: Data curation, Writing – review & editing. JL: Data curation, Writing – original draft, Writing – review & editing. FZ: Funding acquisition, Writing – review & editing. RM: Supervision, Validation, Writing – original draft. YX: Formal Analysis, Methodology, Resources, Writing – review & editing. YD: Conceptualization, Supervision, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding for this study was provided by Natural Science Foundation of China (No. 82071313), China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lall, A, Yavagal, DR, and Bornak, A. Chronic total occlusion and spontaneous recanalization of the internal carotid artery: natural history and management strategy. Vascular. (2021) 29:733–41. doi: 10.1177/1708538120978043
2. Wouters, A, Smets, I, Van den Noortgate, W, Steinberg, GK, and Lemmens, R. Cerebrovascular events after surgery versus conservative therapy for moyamoya disease: a meta-analysis. Acta Neurol Belg. (2019) 119:305–13. doi: 10.1007/s13760-019-01165-9
3. Lin, YH, and Liu, HM. Update on cerebral hyperperfusion syndrome. J Neurointerv Surg. (2020) 12:788–93. doi: 10.1136/neurintsurg-2019-015621
4. Turpin, J, Lynch, DG, White, T, Shah, KA, Yang, K, Katz, JM, et al. Hyperperfusion syndrome after superficial temporal artery-middle cerebral artery bypass for non-moyamoya steno-occlusive disease. J Stroke Cerebrovasc Dis. (2023) 32:107222. doi: 10.1016/j.jstrokecerebrovasdis.2023.107222
5. Saito, M, Saga, T, Hayashi, H, Noro, S, Wada, H, and Kamada, K. Quantitative blood flow assessment by multiparameter analysis of indocyanine green video angiography. World Neurosurg. (2018) 116:e187–93. doi: 10.1016/j.wneu.2018.04.148
6. Suzuki, J, and Takaku, A. Cerebrovascular "moyamoya" disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. (1969) 20:288–99. doi: 10.1001/archneur.1969.00480090076012
7. Delgado, MG, and Bogousslavsky, J. Cerebral Hyperperfusion syndrome and related conditions. Eur Neurol. (2020) 83:453–7. doi: 10.1159/000511307
8. Persoon, S, Luitse, MJ, de Borst, GJ, van der Zwan, A, Algra, A, Kappelle, LJ, et al. Symptomatic internal carotid artery occlusion: a long-term follow-up study. J Neurol Neurosurg Psychiatry. (2011) 82:521–6. doi: 10.1136/jnnp.2010.208330
9. Ma, Y, Gu, Y, Tong, X, Wang, J, Kuai, D, Wang, D, et al. The carotid and middle cerebral artery occlusion surgery study (CMOSS): a study protocol for a randomised controlled trial. Trials. (2016) 17:544. doi: 10.1186/s13063-016-1600-1
10. Thanapal, S, Duvuru, S, Sae-Ngow, T, Kato, Y, and Takizawa, K. Direct Cerebral Revascularization: Extracranial-intracranial Bypass. Asian J Neurosurg. (2018) 13:9–17. doi: 10.4103/ajns.AJNS_76_17
11. Uno, M, Nakajima, N, Nishi, K, Shinno, K, and Nagahiro, S. Hyperperfusion syndrome after extracranial-intracranial bypass in a patient with moyamoya disease--case report. Neurol Med Chir (Tokyo). (1998) 38:420–4. doi: 10.2176/nmc.38.420
12. Fujimura, M, Shimizu, H, Inoue, T, Mugikura, S, Saito, A, and Tominaga, T. Significance of focal cerebral hyperperfusion as a cause of transient neurologic deterioration after extracranial-intracranial bypass for moyamoya disease: comparative study with non-moyamoya patients using N-isopropyl-p-(123)Iiodoamphetamine single-photon emission computed tomography. Neurosurgery. (2011) 68:957–64. doi: 10.1227/NEU.0b013e318208f1da
13. Zhao, M, Deng, X, Zhang, D, Wang, S, Zhang, Y, Wang, R, et al. Risk factors for and outcomes of postoperative complications in adult patients with moyamoya disease. J Neurosurg. (2018) 1:1–12. doi: 10.3171/2017.10.JNS171749
14. Fujimura, M, Inoue, T, Shimizu, H, Saito, A, Mugikura, S, and Tominaga, T. Efficacy of prophylactic blood pressure lowering according to a standardized postoperative management protocol to prevent symptomatic cerebral hyperperfusion after direct revascularization surgery for moyamoya disease. Cerebrovasc Dis. (2012) 33:436–45. doi: 10.1159/000336765
15. Yanagihara, W, Chida, K, Kobayashi, M, Kubo, Y, Yoshida, K, Terasaki, K, et al. Impact of cerebral blood flow changes due to arterial bypass surgery on cognitive function in adult patients with symptomatic ischemic moyamoya disease. J Neurosurg. (2018) 131:1716–24. doi: 10.3171/2018.7.JNS18149
16. Deng, X, Gao, F, Zhang, D, Zhang, Y, Wang, R, Wang, S, et al. Effects of different surgical modalities on the clinical outcome of patients with moyamoya disease: a prospective cohort study. J Neurosurg. (2018) 128:1327–37. doi: 10.3171/2016.12.JNS162626
17. Kim, JE, Oh, CW, Kwon, OK, Park, SQ, Kim, SE, and Kim, YK. Transient hyperperfusion after superficial temporal artery/middle cerebral artery bypass surgery as a possible cause of postoperative transient neurological deterioration. Cerebrovasc Dis. (2008) 25:580–6. doi: 10.1159/000132205
18. Fujimura, M, Kaneta, T, Mugikura, S, Shimizu, H, and Tominaga, T. Temporary neurologic deterioration due to cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in patients with adult-onset moyamoya disease. Surg Neurol. (2007) 67:273–82. doi: 10.1016/j.surneu.2006.07.017
19. Ohue, S, Kumon, Y, Kohno, K, Watanabe, H, Iwata, S, and Ohnishi, T. Postoperative temporary neurological deficits in adults with moyamoya disease. Surg Neurol. (2008) 69:281–6. doi: 10.1016/j.surneu.2007.01.047
20. Gao, F, Zhao, W, Zheng, Y, Li, S, Lin, G, Ji, M, et al. Phase-contrast magnetic resonance imaging analysis of cerebral Hyperperfusion syndrome after surgery in adult patients with Moyamoya disease. World Neurosurg. (2019) 129:e48–55. doi: 10.1016/j.wneu.2019.04.191
21. Yu, J, Zhang, J, Li, J, Zhang, J, and Chen, J. Cerebral Hyperperfusion syndrome after revascularization surgery in patients with Moyamoya disease: systematic review and Meta-analysis. World Neurosurg. (2020) 135:357–366.e4. doi: 10.1016/j.wneu.2019.11.065
22. Chen, Y, Ma, L, Lu, J, Chen, X, Ye, X, Zhang, D, et al. Postoperative hemorrhage during the acute phase after direct or combined revascularization for moyamoya disease: risk factors, prognosis, and literature review. J Neurosurg. (2019) 18:1–10. doi: 10.3171/2019.7.JNS19885
23. Zhang, J, Li, S, Fujimura, M, Lau, TY, Wu, X, Hu, M, et al. Hemodynamic analysis of the recipient parasylvian cortical arteries for predicting postoperative hyperperfusion during STA-MCA bypass in adult patients with moyamoya disease. J Neurosurg. (2019) 134:17–24. doi: 10.3171/2019.10.JNS191207
24. Yao, F, Han, Q, Zhang, Z, and Huang, Y. Assessment of transient cerebral hemodynamic disturbance via whole-brain computed tomography perfusion after extracranial-intracranial bypass in ischemic Moyamoya disease. World Neurosurg. (2021) 146:e112–21. doi: 10.1016/j.wneu.2020.10.046
25. Raabe, A, Beck, J, Gerlach, R, Zimmermann, M, and Seifert, V. Near-infrared indocyanine green video angiography: a new method for intraoperative assessment of vascular flow. Neurosurgery. (2003) 52:132–9. doi: 10.1097/00006123-200301000-00017
26. Takagi, Y, Kikuta, K, Nozaki, K, Sawamura, K, and Hashimoto, N. Detection of a residual nidus by surgical microscope-integrated intraoperative near-infrared indocyanine green videoangiography in a child with a cerebral arteriovenous malformation. J Neurosurg. (2007) 107:416–8. doi: 10.3171/PED-07/11/416
27. Eun, C, Lee, SJ, Park, JC, Ahn, JS, Kwun, BD, and Park, W. Efficacy of intraoperative neuromonitoring (IONM) and intraoperative indocyanine green videoangiography (ICG-VA) during unruptured anterior choroidal artery aneurysm clipping surgery. J Cerebrovasc Endovasc Neurosurg. (2023) 25:150–9. doi: 10.7461/jcen.2023.E2022.10.008
28. Zhang, X, Ni, W, Feng, R, Li, Y, Lei, Y, Xia, D, et al. Evaluation of hemodynamic change by indocyanine green-FLOW 800 videoangiography mapping: prediction of hyperperfusion syndrome in patients with moyamoya disease. Oxidative Med Cell Longev. (2020) 2020:8561609. doi: 10.1155/2020/8561609
29. Holmes, AP, Ray, CJ, Kumar, P, and Coney, AM. A student practical to conceptualize the importance of Poiseuille's law and flow control in the cardiovascular system. Adv Physiol Educ. (2020) 44:436–43. doi: 10.1152/advan.00004.2019
30. Hu, M, Yu, J, Zhang, J, and Chen, J. Designing a flow-controlled STA-MCA anastomosis based on the Hagen-Poiseuille law for preventing postoperative hyperperfusion in adult moyamoya disease. Ther Adv Chronic Dis. (2023) 14:20406223231181492. doi: 10.1177/20406223231181492
31. Zeng, X, Su, K, Tian, X, and Chen, J. Matching selection of donor-recipient vessels in revascularization surgery effectively reduce the incidence of postoperative Hyperperfusion syndrome in adult Moyamoya disease: a retrospective comparison study. Cerebrovasc Dis. (2020) 49:361–8. doi: 10.1159/000509138
32. Li, X, Huang, Z, Wu, MX, and Zhang, D. Effect of adventitial dissection of superficial temporal artery on the outcome of superficial temporal artery-middle cerebral artery bypass in Moyamoya disease. Aging Dis. (2017) 8:384–91. doi: 10.14336/AD.2016.1115
33. Katano, H, Nishikawa, Y, Yamada, H, Shibata, T, Miyachi, S, and Mase, M. Association of Superficial Temporal Artery Dilatation with headache after revascularization in adult Moyamoya disease. World Neurosurg. (2019) 129:e594–606. doi: 10.1016/j.wneu.2019.05.228
34. Noguchi, T, Kawashima, M, Nishihara, M, Egashira, Y, Azama, S, and Irie, H. Noninvasive method for mapping CVR in moyamoya disease using ASL-MRI. Eur J Radiol. (2015) 84:1137–43. doi: 10.1016/j.ejrad.2015.03.011
35. Bouri, S, Thapar, A, Shalhoub, J, Jayasooriya, G, Fernando, A, Franklin, IJ, et al. Hypertension and the post-carotid endarterectomy cerebral hyperperfusion syndrome. Eur J Vasc Endovasc Surg. (2011) 41:229–37. doi: 10.1016/j.ejvs.2010.10.016
36. Ogasawara, K, Inoue, T, Kobayashi, M, Endo, H, Fukuda, T, and Ogawa, A. Pretreatment with the free radical scavenger edaravone prevents cerebral hyperperfusion after carotid endarterectomy. Neurosurgery. (2004) 55:1060–7. doi: 10.1227/01.NEU.0000140838.27450.63
Keywords: chronic internal carotid artery occlusion, fluorescein angiography, cerebral revascularization, regional cerebral blood flow, hyperperfusion syndrome
Citation: Du J, Shen J, Li J, Zhang F, Mao R, Xu Y and Duan Y (2023) Combination of intraoperative indocyanine green video-angiography FLOW 800 and computed tomography perfusion to assess the risk of cerebral hyperperfusion syndrome in chronic internal carotid artery occlusion patients after revascularization surgery. Front. Neurol. 14:1323626. doi: 10.3389/fneur.2023.1323626
Edited by:
Fernando Delgado, Andusian Health Service, SpainReviewed by:
Carlos Pérez-García, San Carlos University Clinical Hospital, SpainTomohiro Inoue, NTT Medical Center, Japan
Copyright © 2023 Du, Shen, Li, Zhang, Mao, Xu and Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yinghua Xu, MTM2NDE4MjE4MjBAMTM5LmNvbQ==; Yu Duan, ZHVhbnl1OTI2QDE2My5jb20=
†These authors share first authorship
‡These authors have contributed equally to this work
 Juan Du1†
Juan Du1† Jian Li
Jian Li Yu Duan
Yu Duan