
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 28 December 2023
Sec. Neuromuscular Disorders and Peripheral Neuropathies
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1320344
This article is part of the Research Topic New Perspectives in the Treatment of Myasthenia Gravis View all 10 articles
 Yukun Tian1†
Yukun Tian1† Qiqi Shen1†
Qiqi Shen1† Siyang Peng1
Siyang Peng1 Linghao Meng1
Linghao Meng1 Ruiying Fang1
Ruiying Fang1 Anni Xiong1
Anni Xiong1 Shaohong Li2
Shaohong Li2 Yajing Yang3
Yajing Yang3 Weiqian Chang4
Weiqian Chang4 Jinxia Ni5
Jinxia Ni5 Wenzeng Zhu1*
Wenzeng Zhu1*Introduction: Research on myasthenia gravis (MG) has undergone rapid development in recent years. This article aimed to elucidate the characteristics of MG publications over the past 20 years and analyze emerging trends using bibliometric methods.
Methods: Information on MG articles was obtained from the Web of Science Core Collection and stored in Excel for quantitative analyses. Bibliometric analyses were performed using CiteSpace and VOSviewer to visualize publications according to countries/regions, institutions, journals, and authors.
Results: A total of 3,610 publications were included in the analysis. The USA had the highest number of publications (NP) and H-index. Among the institutions, the University of Oxford had the highest NP, followed by the University of Toronto and Duke University. Close cooperation was observed among countries and institutions. The most productive author was Renato Mantegazza, followed by Jan J. Verschuuren, and Amelia Evoli. Muscle & Nerve published the most articles on MG, followed by the Journal of Neuroimmunology and Neuromuscular Disorders. The keyword with the highest strength is “neuromuscular transmission,” followed by “safety” and “rituximab.” Co-citation analysis includes 103 publications cited at least 65 times, categorized into four clusters. Additionally, 123 keywords cited more than 40 times were analyzed and divided into five clusters.
Conclusion: This bibliometric analysis shows the framework of research over the past 20 years by mapping the scholarly contributions of various countries or regions, institutions, journals, and authors in MG. The analysis also explores future trends and prospective directions, emphasizing individualized treatment based on subtypes, novel immunotherapeutic approaches, and thymectomy.
Myasthenia gravis (MG) is an autoimmune disease characterized by skeletal muscle weakness and fatigability. It is caused by antibodies directed against proteins at the neuromuscular junction, including the acetylcholine receptor (AChR), muscle-specific kinase (MuSK), lipoprotein-related protein 4 (LRP4), and the postsynaptic membrane (1). The prevalence of MG is estimated to be 100 to 350 cases per 1 million people, with increasing trends likely due to improved diagnostics, aging populations, and the number of systematic epidemiological studies conducted over the past decade (2–4).
Bibliometric analysis has become an internationally recognized tool since 1958 that not only contributes to important decisions about government budgets (5), but more significantly, also helps understand new research areas in rapidly advancing fields. By analyzing the relationships between journals, citations, keywords, and scholars, it enables the creation of visual maps to represent publications and academic networks. MG research has expanded substantially in recent years. The aim of this study was to use bibliometric techniques to characterize patterns in MG publications over the past 20 years and elucidate trends in this growing area of inquiry.
Data were collected from the Web of Science Core Collection, and the search period was set between 1 January 2003 and 31 December 2022. A literature search was conducted on 9 June 2023. The search terms were as follows: ts = “myasthenia gravis” (ts = topic), and 6,898 publications were identified. The articles and reviews in English were analyzed after removing the meeting abstracts, letters, editorial materials, proceedings, corrections, early accesses, news items, retractions, biographical items, book chapters, and retracted publications. A total of 3,130 articles and 480 reviews were included in the analysis. The collected data included the journal titles, authors, keywords, countries or regions, institutions, and cited references, and the downloaded data were screened by S.Y. Peng to identify the relationships on the topic. The strategy for accessing and searching for articles is shown in Figure 1.
A quantitative analysis of the number of publications (NP) and citations (NC) in the most productive countries, authors, journals, and affiliations was conducted using Microsoft Office Excel 2013. VOSviewer (Centre for Science and Technology Studies, Leiden University, Leiden, the Netherlands) is a computer program used to create scientific maps for co-citation analysis, co-occurrence of keywords, and co-authorship analysis of authors, affiliations, and countries (6). CiteSpace was used to visualize and analyze bursts of references and keywords (7).
An annual analysis of NP directly reflects trends in a specific research field over a given period. The annual publications of MG generally showed a steady growth trend, peaking in 2021, as shown in Figure 2.
Figure 3 displays the world distribution map based on the NP in different countries. In this figure, the depth of the color represents the magnitude of NP. The top 10 countries in NP are presented in Table 1. It is evident that the USA had the highest NP over the past two decades in MG research, followed by China, Japan, the UK, and Italy. The USA had the highest number of total citations after removing self-citations, followed by the UK, Italy, China, and Japan, with H-indices of 68, 49, 45, 37, and 38, respectively. H-indices reflect the general quality of a country’s literature. Figure 4 present a bubble chart of the yearly publications of the top 10 countries in NP is shown in Figure 4, demonstrating an annual increase from 2003 to 2022. The USA led in yearly publications until 2014, after which China took the lead and continued until 2022.
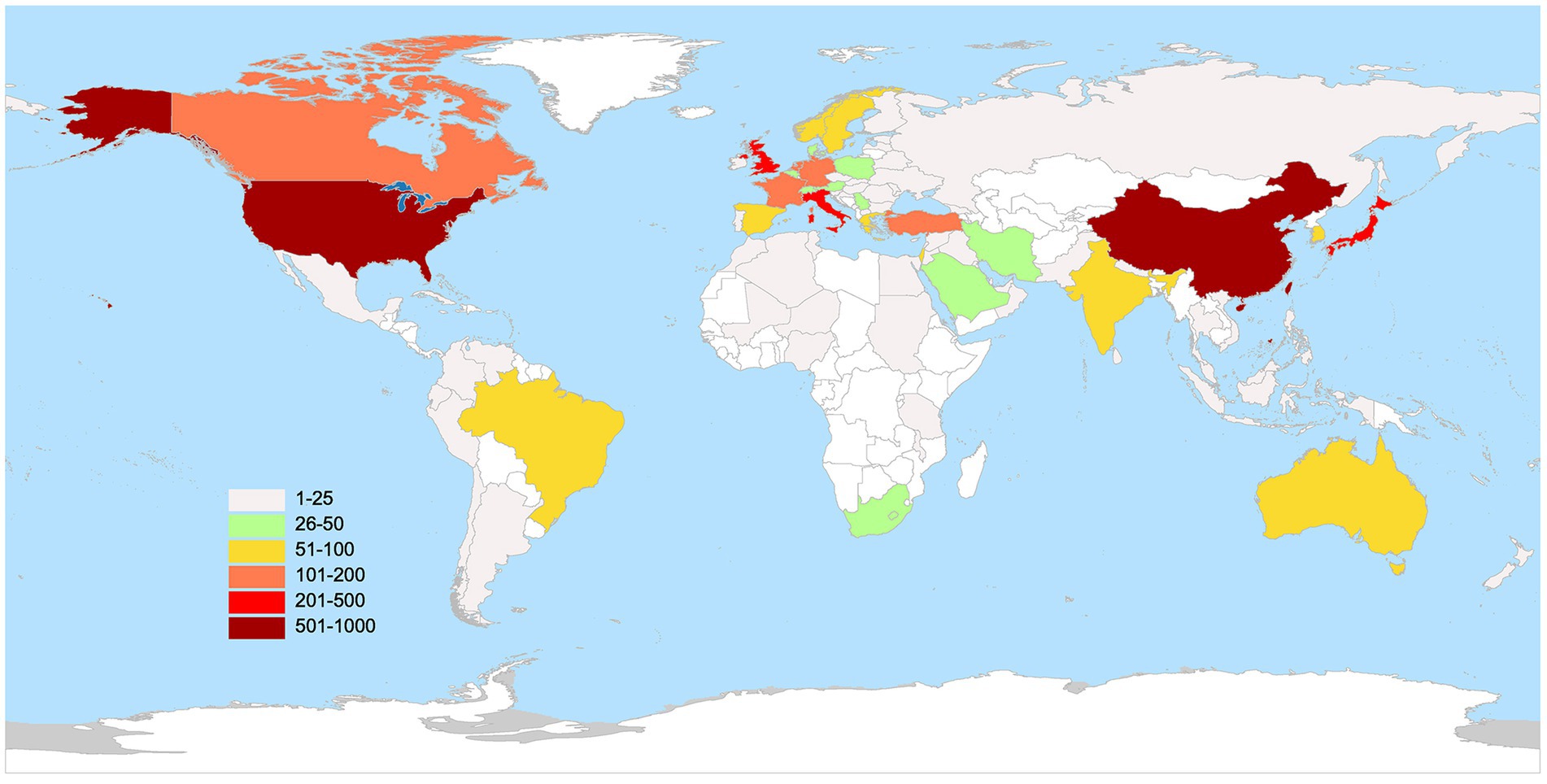
Figure 3. The geography distribution map manifested the number of publications in distinct countries.
In the co-authorship analysis (Figure 5), 55 countries with a minimum of five publications were included. Countries were divided into eight clusters of different colors. Bold lines between countries indicate closer collaborations, and the node size reflects the centrality and influence of the country on associations. Owing to the diversity of scientific research foundations and resources in other countries or regions, a specific collaborative network structure was observed at the national or regional scale. According to this map, cooperation between most countries was close. The USA, China, Japan, the UK, Italy, France, and Canada were the central countries in cooperation, with the USA being the most influential.
The top 10 institutions in the total NP in this research area are listed in Table 2. Oxford University had the highest NP, with 94 publications, followed by the University of Toronto (62) and Duke University (61). One hundred sixty institutions with 10 or more publications were included in VOSviewer for co-authorship analysis. As shown in Figure 6A, the affiliations were divided into nine clusters. Close collaborations among Oxford University, Leiden University, and Duke University were apparent. Meanwhile, Fudan University, Istanbul University, University of Toronto, University of Bergen, and Chiba University were the centers of the collaboration clusters. However, because to geographical distance, a lack of cooperation between several institutions from different countries was observed, whereas cooperation was closer within each cluster because the institutions in a cluster were from the same countries. We also visualized the time overlay for the co-authorship institutional analysis using VOSviewer. As shown in Figure 6B, the institutions were divided into different colors according to the average year of publication; the institutions in yellow were active later than those in blue. The University of Toronto, Capital Medical University, and Shandong University recently appeared more in cooperation, indicating that they were more active in MG research and full of potential in international cooperation.
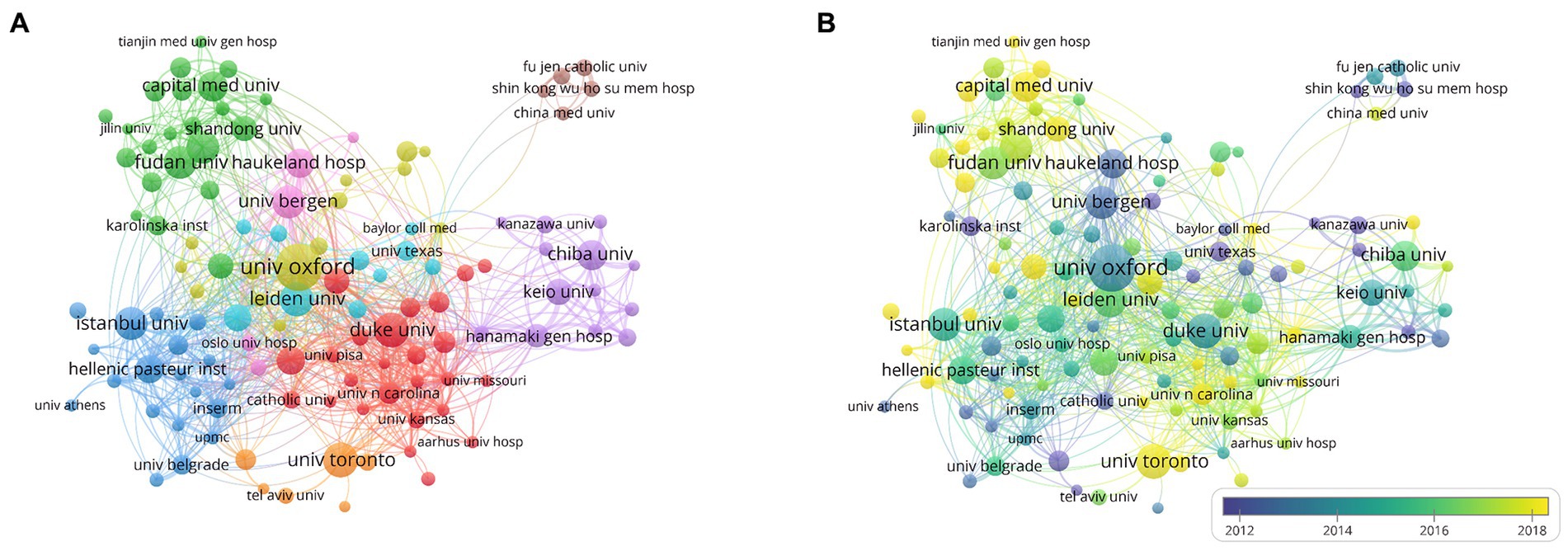
Figure 6. Network visualization of cooperations among institutions. (A) Visualization of institutions divided into nine clusters. (B) Visualization of the institutions network according to the average years of publication.
The influence of a journal is expressed not only by the NP, but also by the NC. In Table 3, we summarized the top 10 journals with the highest NP in MG research. The journal with the highest NP was Muscle & Nerve (n = 224), followed by the Journal of Neuroimmunology (n = 117) and Neuromuscular Disorders (n = 98). Nine of the top 10 journals had an impact factor exceeding 3 and were mostly focused on neuromuscular diseases, neuroimmunology, and neurology.
One hundred five authors with at least 13 publications were included in the co-authorship analysis. The authors were divided into 10 clusters based on their co-authorship, and cooperation among authors was close, as shown in Figure 7A. Based on density visualization (Figure 7B), the central authors were Amelia Evoli, Renato Mantegazza, Vera Bril, Henry Kaminski, Sonia Berrih-Aknin, and Huan Yang. As shown in Table 4, the author with the highest NP was Renato Mantegazza (64), followed by Jan J. Verschuuren (63), and Amelia Evoli (61). Renato Mantegazza is an Italian researcher who has studied a wide range of myopathies and participated in a randomized clinical trial of efgartigimod for the treatment of generalized MG (8). Notably, the author with the highest NC was Amelia Evoli (4,216), followed by Jan J. Verschuuren (3,925) and Angela Vincent (3,865). Amelia Evoli is also from Italy, and by assessing cognitive dysfunction in muscle-specific tyrosine kinase antibody seropositive (MuSK+) passive transfer MG mice, she suggested that recognition memory in the perirhinal cortex of MuSK+ patients with MG could be affected (9). Her team also focuses on immunotherapy in MG (10) and detection methodologies of antibodies in MG (11).
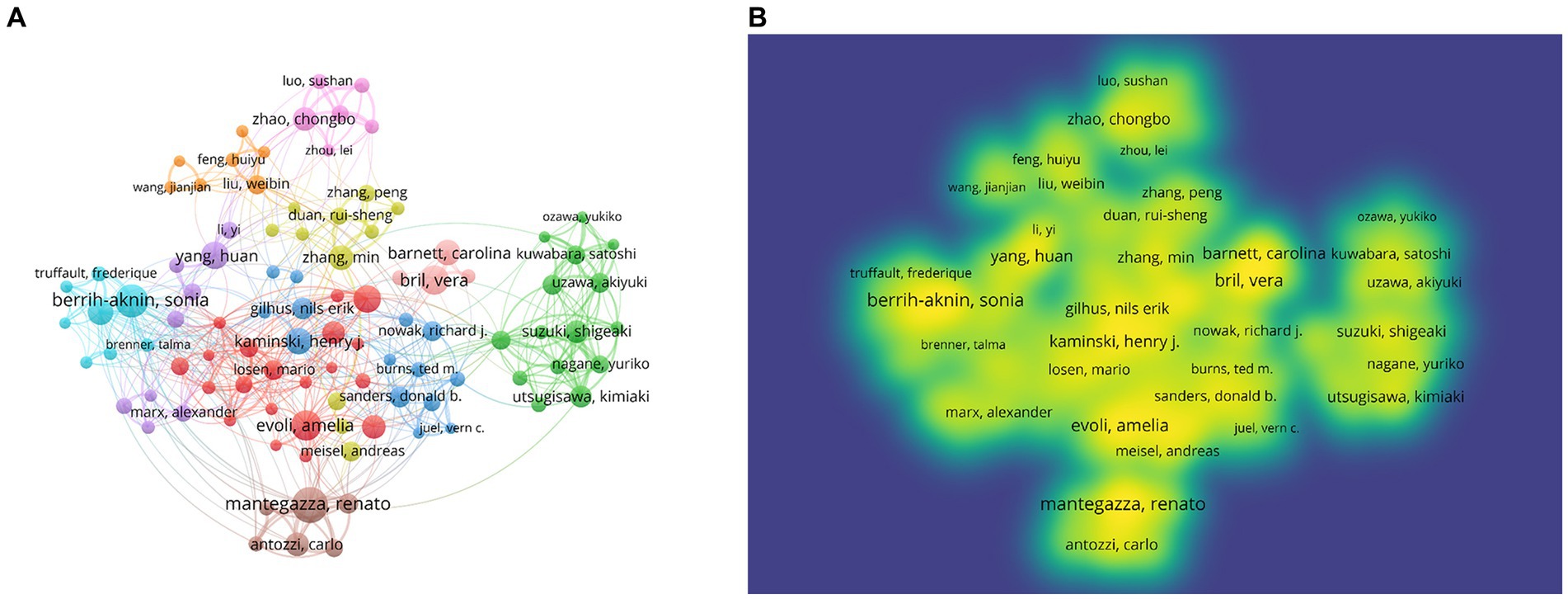
Figure 7. Network visualization of authors. (A) Visualization of authors divided into 10 clusters. (B) Density overlay visualization of the authors network.
The co-cited references were analyzed in VOSviewer, with a threshold of at least 65 co-citation counts, incorporating 103 publications (Figure 8). The NC serves as the most objective and direct measure of a publication’s significance (12). In the figure, the highly cited literature is divided into four clusters corresponding to four colors: red, yellow, blue, and green. The red cluster consists of 34 publications, primarily reviews, that laid the foundation for theory and were conducted before 2010. These contributions standardized the clinical treatment and scientific research of MG (13–15). The green category consists of 27 publications focused on the pathogenic autoimmune antibodies in patients with MG and the immunopathogenesis of MG, providing ideas for therapeutic drug development (16–18). The blue category consists of 24 publications focused on introducing clinical treatment protocols for MG and standardizing management of patients with MG (19–21). The yellow category consists of 18 publications focused on assessing the conditions of patients with MG, such as the design and application of scales, and establishing the clinical assessment and diagnostic criteria of MG (22–24).
The co-occurrence analysis of keywords within the publications was performed using VOSviewer. A minimum occurrence of 40 was set, resulting in 123 keywords for visualization and analysis out of a total of 7,857 keywords (Figure 9A). Cluster analysis of keywords can identify popular research topics that can guide future directions. In the figure, larger circles represent a higher number of keyword occurrences. The lines connecting the circles indicate the frequency of co-occurrence, with different cluster colors signifying distinct research directions. The yellow cluster mainly focuses on thymectomy, featuring keywords such as “thymoma,” “thymectomy,” and “surgery.” The red cluster is associated with the pathogenesis of MG, including keywords such as “acetylcholine receptor,” “b-cells,” and “thymus.” The blue cluster, with keywords such as “autoantibodies,” “musk,” and “protein 4,” explores autoantibodies in patients with MG, revealing the relationship between autoantibodies and MG diagnosis. The green cluster is dominated by therapeutic aspects of MG, featuring keywords such as “rituximab,” “patient,” and “efficacy.” The purple cluster, with keywords such as “prevalence,” “epidemiology,” and “classification,” reflects its theme as epidemiology in MG. Representative terms in MG research, such as “myasthenia gravis,” “thymectomy,” “autoantibodies,” “acetylcholine-receptor,” and “disease,” constitute larger circles in each cluster. Figure 9B depicts the temporal characteristics of keyword co-occurrence from blue to yellow, representing the chronology from 2003 to 2022, with “rituximab,” “safety,” “classification,” “COVID-19,” and “clinical characteristics” as recent research hotspots.

Figure 9. Network visualization of co-occurrence keywords. (A) Visualization of keywords divided into five clusters. (B) Time overlay visualization of the keywords network.
The keyword “outbreak analysis” highlights a sudden growth in keyword citations during a specific period, indicating research hotspots. Figure 10 presents the top 25 keywords with the strongest citation analysis. The red line indicates the time of the outbreak, whereas the blue line indicates the period. Keywords that burst earlier included “acetylcholine receptor antibody,” “IFN gamma,” “lymphocytes,” “alpha subunit,” and “neuromuscular transmission.” Keyword bursts in 2010–2015 that remained in the outbreak period were “safety,” “rituximab,” “efficacy,” “classification,” “eculizumab,” “case report,” “generalized myasthenia gravis,” “neuromuscular disease,” and “prognosis.” The shift in the burst keywords of MG reflects changing research interests in recent years, with “neuromuscular transmission” being the keyword with the highest strength (13.51), followed by “safety” (12.65) and “rituximab” (11.05). These represent current research hotspots.
Figure 11 shows the citation burst analysis of the top 25 cited papers using CiteSpace. This analysis aimed to identify heightened interest during specific periods within the research area, accomplished by examining the temporal characteristics of the cited articles. Over the past two decades, the earliest articles contributing to the burst period were written by W. Hoch, A. Vincent, and A. Jaretzki. Hoch et al. explored the role of MuSK antibodies in MG pathogenesis (16), whereas Vincent et al. provided an overview of the diagnosis and treatment of MG (25). Jaretzki et al. suggested the standardization of clinical trials for MG (13). Publications during the burst phase included articles by G.I. Wolfe, D.B. Sanders, J.F. Howard, and N.E. Gilhus. Sanders et al. made significant contributions to the management of patients with MG (21). Gilhus and colleagues conducted an updated systematic review of MG (26, 27). Additionally, both G.I. Wolfe (28) and J.F. Howard (29) conducted randomized controlled clinical trials that provided substantial evidence supporting thymectomy and eculizumab as effective interventions for MG, respectively.
In this study, we used bibliometrics to visualize publication trends in MG research from 2003 to 2022. We analyzed 3,610 eligible publications using VOSviewer and CiteSpace, exploring patterns in citations, contributing countries and regions, institutions, authors, journals, keywords, and co-citations. Specific bibliometric techniques, including burst hotspot, cluster, and keyword analyses, were conducted to determine the research status and future directions of MG research.
The analysis of the change in annual NP indicated an overall upward trend. The analysis of the top 10 countries in terms of NP revealed that the USA had the highest NP and H-index, which indicates a higher quantity and quality of research in this field. The annual NP in China increased rapidly and exceeded that of the USA in 2014, indicating China’s rapid development in MG research. However, China’s lower citation ranking suggests a need for improved research quality and global impact. Intercountry cooperation is crucial, as seen in the visualization, with most countries collaborating closely. Countries at the periphery face challenges such as geographic distance, and they must strengthen cooperation with other countries, enhance research capacity, and delve into key scientific issues in the field.
Identifying institutions with a solid research base influences the selection of long-term work and cooperation among researchers. In a visual analysis of publishers and institutional collaborations, Oxford University and Duke University stand out, and research institutions must keep pace with these leaders to enhance capacity and explore deeper research. The time overlay analysis revealed that the University of Toronto, Capital Medical University, and Shandong University are up-and-coming institutions in MG research. Hence, it is prudent for government sectors to consider increasing the financial support for these institutions.
According to the visualization of co-authorship, cooperation among authors was close, enhancing research quality. Moreover, authors who play a crucial part in cooperations may have higher quality of research. The core authors of this cooperation were Amelia Evoli, Renato Mantegazza, Vera Bril, Henry Kaminski, Sonia Berrih-Aknin, and Huan Yang. Renato Mantegazza and Amelia Evoli are the most productive and most cited authors, respectively, showing significant influence of their study and outstanding contribution to this field of research. Researchers should read and refer to the publications of these scholars to identify the pivotal and renewed points of MG.
Muscle & Nerve, the Journal of Neuroimmunology, and Neuromuscular Disorders published the most articles, making them suitable outlets for the publication and dissemination of research in this field. Researchers could consider submitting their articles to these journals, and scholars can consult publications from these journals to obtain the latest information on MG.
In the co-citation science map, larger circles indicate articles with more citations, pointing to greater influence. The largest circle features Jaretzki et al.’s article, titled “Myasthenia Gravis: Recommendations for Clinical Research Standards” (13), published in Neurology and cited 918 times. This article addressed challenges in MG clinical trials, including refining the Myasthenia Gravis Foundation of America (MGFA) clinical classification and establishing the Quantitative Myasthenia Gravis (QMG) score, which remains a global scientific standard. Their efforts systematically organized critical details, facilitating uniformity in MG’s clinical research and management processes, explaining the enduring recognition and referencing of this article. The understanding of the MG knowledge base unfolds through the exploration of relevant research domains visualized in the science map generated by the VOSviewer. The four clusters in Figure 7 include topics ranging from the underlying pathogenesis to intricate clinical management and from foundational theoretical aspects to their practical applications. This comprehensive development is reflective of the extensive research conducted on MG from 2003 to 2022.
Upon reviewing citations and keywords, the continuous improvement of precise therapeutic approaches for diverse patient profiles emerged as the hotspot of MG research. This can be categorized into three aspects: MG antibody-related subtype therapy, novel immunotherapeutic approaches, and thymectomy.
The treatment options for MG include cholinesterase inhibitors, thymectomy, immunosuppressive or immunomodulatory medications, and plasma exchange. However, the increasing prevalence of MG demands more effective therapeutic approaches. The keyword “classification” stands out in recent research, especially during the burst period. Notably, an article published in 2015 in Lancet Neurology (19) covering autoantibodies, epidemiology, clinical presentation, and comorbidities holds the highest burst strength (68.11) in reference citation burst detection. It summarizes potential treatments for MG and suggests a future research direction of exploring new immunosuppressive drugs and drug combinations tailored to MG subgroups. This article plays a crucial role in subgroup classification and provides valuable insights for future research in MG. The emphasis on burst keywords and references underscores researchers’ keen interest in MG with different antibodies, emphasizing the crucial role of autoantibodies in MG diagnosis, understanding patient features, and personalizing treatment approaches. Most patients with MG have antibodies targeting AChRs, with fewer having antibodies against MuSK or LRP4 (30). A multicenter study showed that patients with antibodies against LRP4 and/or agrin exhibit more generalized symptoms (69%) than antibody-negative patients, but most of them responded favorably to standard MG therapy (31). This showcases how studying the clinical characteristics of MG with specific antibodies can significantly improve diagnosis and management. MuSK, as an antibody, differs from classic AChR antibodies, impacting clinical manifestations and treatment responses, thus posing challenges to accurate diagnosis and management. MuSK antibody-positive MG is prevalent among females (32), affecting muscles not typically weakened in non-MuSK antibody-positive MG, with increased respiratory weakness in this subgroup (33). Cholinesterase inhibitors often yield unsatisfactory results; therefore, early rituximab administration is recommended (34, 35). Future research will likely focus on treatments for patients with MG who have other specific autoimmune antibodies.
In 1996, a soluble recombinant form of human complement receptor 1 was demonstrated as an additional therapeutic approach for MG (36), suggesting that complement inhibition may be a potential therapeutic approach for MG. A phase 3, randomized, double-blind, placebo-controlled, multicenter study evaluating the efficacy and safety of eculizumab in AChR-positive refractory generalized MG was conducted during the burst period (29). Eculizumab, the first complement-specific drug clinically used for the treatment of paroxysmal nocturnal orphan disease and hemoglobinuria, has bolstered confidence in therapeutic complement inhibition (37) and has gained market approval in countries such as the USA, Japan, and China. As reported in a retrospective study, patients treated with eculizumab were more likely to achieve minimal manifestations than those treated with rituximab, but the risk of crisis was not reduced (38). Eculizumab has also been confirmed to benefit refractory AChR-MG in a real-world experience (39), but one patient reported acute worsening after discontinuation of eculizumab (40). Moreover, the optimal duration of treatment remains unclear. Therefore, further studies are needed on safety and treatment duration. Research to identify biomarkers predicting the response to eculizumab is also necessary. Eculizumab was also a keyword in the burst period, indicating attention to selective immunosuppressants in MG treatment. Ravulizumab is a monoclonal antibody complement inhibitor approved by the Food and Drug Administration (FDA) in April 2022 for AChR-Ab-generalized MG. A phase 3, randomized, double-blind, placebo-controlled, multicenter trial in patients with AChR-Ab-generalized MG indicated improved clinical outcomes for patients treated with ravulizumab, and the drug was well tolerated (41). The latest pharmacokinetics and pharmacodynamics research based on the data from this phase 3 study supports dosing every 8 weeks for immediate, complete, and sustained inhibition of terminal complement C5, and it could reduce the burden on patients (42).
Targeting the neonatal fragment crystallizable receptor (FcRn) is another novel therapeutic approach considered for generalized MG that has failed standard treatment. Meanwhile, efgartigimod, a human IgG1 antibody that reduces IgG recycling and increases IgG degradation by outcompeting endogenous IgG binding (43), was approved by the FDA in July 2022 for the treatment of AChR-Ab-generalized MG in adult patients. A phase 3, randomized, double-blind, placebo-controlled trial in generalized MG revealed better clinical improvement in the efgartigimod arm than in the placebo arm, whereas most patients had AChR-MG (44). A meta-analysis found that anti-FcRn has an advantage in improving the QMG score than complement treatments in patients with generalized MG, and they also proposed the high probability of efgartigimod and rozanolixizumab being the most effective treatment in generalized MG (45).
Rituximab, a keyword with high citation strength and more recent citations, is a monoclonal antibody directed against CD20 antigen on B cells that has been used for years. The clear benefit of refractory MuSK antibody-positive MG has been demonstrated; however, its efficacy in AChR-MG remains controversial (46). A systematic review of 13 studies proposed that the small number of patients with AChR-MG in previous studies may have caused bias in efficacy evaluation and suggested that dosages for different subtypes of MG should be considered in future studies (47). In addition, due to the off-label use of rituximab and the inherent risk of infection associated with continuous B-cell depletion, safety is a crucial factor and should be the focus. For example, infection is the most common side effect of this treatment. A retrospective study of adverse events in treatment of refractory MG with rituximab found that infection was associated with hypogammaglobulinemia and proposed that a standardized monitoring scheme of IgG is necessary (48). To ensure the safety of rituximab in patients with MG and enhance risk control, high-quality randomized controlled trials with large samples should be conducted.
Thymectomy is inevitable in thymomatous MG (49). Moreover, a multicenter, randomized, single-blind trial comparing thymectomy combined with prednisone to prednisone alone, published in the New England Journal of Medicine in 2016 (28), provided a conclusive outcome that thymectomy benefits patients with MGFA clinical class II to IV disease with non-thymomatous AChR-MG. These authors extended the study up to 2 years to investigate the durability of the treatment associated with thymectomy, published in 2019, further affirming the advantages of thymectomy and reversing the treatment decision trends for this procedure (50). Both aforementioned studies were still in the burst period, indicating sustained interest in thymectomy studies. However, the prognosis of thymectomy in certain outcomes can be observed in the worsening or relapse of MG, even after thymectomy. This may be associated with patient heterogeneity, autoantibody profiles, thymic pathology, operation-associated factors, perioperative care, and disease conditions, among other factors (51). To better guide the decisions between conservative treatments or surgery, the pivotal mechanisms of clinical thymectomy in MG need to be elucidated, which may be a promising topic for future research.
The present research has some novel contributions to this field. First, this is the first bibliometric study that not only systematically analyzed research of MG but also paid special attention to research hotpots, especially MG treatment. Therefore, it will be beneficial to scholars who are interested in this subject as well as neurologists who want to catch up with recent advances in a visual manner. Second, based on our study, we proposed some rational recommendations for potential project sponsor and related government sector. Resource integration could be further enhanced, which may stimulate the development of MG research.
This bibliometric analysis has several limitations. First, the NC of recently published articles may not fully reflect their quality, potentially introducing bias into our qualitative assertions (52). Second, as this study exclusively included publications in English, articles in other languages were omitted, potentially introducing bias.
Using VOSviewer and CiteSpace, this study has dynamically clarified the trajectory of MG research, revealing its developmental nuances, hotspots, and future trends. Notably, the USA had the highest NP and NC. Nevertheless, there is potential for more collaboration among countries and institutions. Current MG research shows enthusiasm, especially for individualized treatments based on subtypes, novel immunotherapeutic approaches, and thymectomy. These facets collectively shape ongoing and future research into the intricacies of MG.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
YT: Data curation, Writing – original draft, Writing – review & editing, Formal Analysis, Investigation, Visualization. QS: Data curation, Writing – original draft. SP: Data curation, Writing – review & editing. LM: Writing – review & editing. RF: Data curation, Writing – review & editing. AX: Investigation, Writing – review & editing. SL: Writing – review & editing. YY: Writing – review & editing. WC: Writing – review & editing. JN: Conceptualization, Writing – review & editing. WZ: Conceptualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Innovation Fund of China Academy of Chinese Medical Sciences (CI2021A01309).
The authors acknowledge gratitude to all the staff who participated in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
NC, Number of citations; NP, Number of publications.
2. Santos, E, Coutinho, E, Moreira, I, Silva, AM, Lopes, D, Costa, H, et al. Epidemiology of myasthenia gravis in northern Portugal: frequency estimates and clinical epidemiological distribution of cases. Muscle Nerve. (2016) 54:413–21. doi: 10.1002/mus.25068
3. Gattellari, M, Goumas, C, and Worthington, JM. A national epidemiological study of myasthenia gravis in Australia. Eur J Neurol. (2012) 19:1413–20. doi: 10.1111/j.1468-1331.2012.03698.x
4. Park, S-Y, Lee, JY, Lim, NG, and Hong, Y-H. Incidence and prevalence of myasthenia gravis in Korea: A population-based study using the National Health Insurance Claims Database. J Clin Neurol. (2016) 12:340–4. doi: 10.3988/jcn.2016.12.3.340
5. Thelwall, M. Bibliometrics to webometrics. J Inf Sci. (2008) 34:605–21. doi: 10.1177/0165551507087238
6. van Eck, NJ, and Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84:523–38. doi: 10.1007/s11192-009-0146-3
7. Chen, C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci. (2004) 101:5303–10. doi: 10.1073/pnas.0307513100
8. Howard, JF Jr, Bril, V, Burns, TM, Mantegazza, R, Bilinska, M, Szczudlik, A, et al. Randomized phase 2 study of FcRn antagonist efgartigimod in generalized myasthenia gravis. Neurology. (2019) 92:e2661–73. doi: 10.1212/WNL.0000000000007600
9. Sabre, L, Evoli, A, and Punga, AR. Cognitive dysfunction in mice with passively induced MuSK antibody seropositive myasthenia gravis. J Neurol Sci. (2019) 399:15–21. doi: 10.1016/j.jns.2019.02.001
10. Rodolico, C, Nicocia, G, Damato, V, Antonini, G, Liguori, R, and Evoli, A. Benefit and danger from immunotherapy in myasthenia gravis. Neurol Sci. (2021) 42:1367–75. doi: 10.1007/s10072-021-05077-6
11. Spagni, G, Gastaldi, M, Businaro, P, Chemkhi, Z, Carrozza, C, Mascagna, G, et al. Comparison of fixed and live cell-based assay for the detection of AChR and MuSK antibodies in myasthenia gravis. Neurol Neuroimmunol Neuroinflam. (2023) 10:38. doi: 10.1212/NXI.0000000000200038
12. Pieters, R, and Baumgartner, H. Who talks to whom? Intra-and interdisciplinary communication of economics journals. J Econ Lit. (2002) 40:483–509. doi: 10.1257/jel.40.2.483
13. Jaretzki, A, Barohn, RJ, Ernstoff, RM, Kaminski, HJ, Keesey, JC, Penn, AS, et al. Myasthenia gravis: recommendations for clinical research standards. Neurology. (2000) 55:16–23. doi: 10.1212/WNL.55.1.16
14. Meriggioli, MN, and Sanders, DB. Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol. (2009) 8:475–90. doi: 10.1016/S1474-4422(09)70063-8
15. Carr, AS, Cardwell, CR, McCarron, PO, and McConville, J. A systematic review of population based epidemiological studies in myasthenia gravis. BMC Neurol. (2010) 10:46. doi: 10.1186/1471-2377-10-46
16. Hoch, W, McConville, J, Helms, S, Newsom-Davis, J, Melms, A, and Vincent, A. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med. (2001) 7:365–8. doi: 10.1038/85520
17. Higuchi, O, Hamuro, J, Motomura, M, and Yamanashi, Y. Autoantibodies to low-density lipoprotein receptor–related protein 4 in myasthenia gravis. Ann Neurol. (2011) 69:418–22. doi: 10.1002/ana.22312
18. Leite, MI, Jacob, S, Viegas, S, Cossins, J, Clover, L, Morgan, BP, et al. IgG1 antibodies to acetylcholine receptors in ‘seronegative’ myasthenia gravis†. Brain. (2008) 131:1940–52. doi: 10.1093/brain/awn092
19. Gilhus, NE, and Verschuuren, JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol. (2015) 14:1023–36. doi: 10.1016/S1474-4422(15)00145-3
20. Gilhus, NE, Skeie, GO, Romi, F, Lazaridis, K, Zisimopoulou, P, and Tzartos, S. Myasthenia gravis — autoantibody characteristics and their implications for therapy. Nat Rev Neurol. (2016) 12:259–68. doi: 10.1038/nrneurol.2016.44
21. Sanders, DB, Wolfe, GI, Benatar, M, Evoli, A, Gilhus, NE, Illa, I, et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology. (2016) 87:419–25. doi: 10.1212/WNL.0000000000002790
22. Wolfe, GI, Herbelin, L, Nations, SP, Foster, B, Bryan, WW, and Barohn, RJ. Myasthenia gravis activities of daily living profile. Neurology. (1999) 52:1487–7. doi: 10.1212/WNL.52.7.1487
23. Burns, TM, Conaway, MR, Cutter, GR, and Sanders, DB, Group TMS. Less is more, or almost as much: A 15-item quality-of-life instrument for myasthenia gravis. Muscle Nerve. (2008) 38:957–63. doi: 10.1002/mus.21053
24. Pascuzzi, RM, Coslett, HB, and Johns, TR. Long-term corticosteriod treatment of myasthenia gravis: report of 116 patients. Ann Neurol. (1984) 15:291–8. doi: 10.1002/ana.410150316
25. Vincent, A, Palace, J, and Hilton-Jones, D. Myasthenia gravis. Lancet. (2001) 357:2122–8. doi: 10.1016/S0140-6736(00)05186-2
27. Gilhus, NE, Tzartos, S, Evoli, A, Palace, J, Burns, TM, and Verschuuren, JJGM. Myasthenia gravis. Nat Rev Dis Primers. (2019) 5:1–19. doi: 10.1038/s41572-019-0079-y
28. Wolfe, GI, Kaminski, HJ, Aban, IB, Minisman, G, Kuo, H-C, Marx, A, et al. Randomized trial of Thymectomy in myasthenia gravis. N Engl J Med. (2016) 375:511–22. doi: 10.1056/NEJMoa1602489
29. Howard, JF, Utsugisawa, K, Benatar, M, Murai, H, Barohn, RJ, Illa, I, et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. (2017) 16:976–86. doi: 10.1016/S1474-4422(17)30369-1
30. Vincent, A, Huda, S, Cao, M, Cetin, H, Koneczny, I, Rodriguez-Cruz, P, et al. Serological and experimental studies in different forms of myasthenia gravis. Ann N Y Acad Sci. (2018) 1413:143–53. doi: 10.1111/nyas.13592
31. Rivner, MH, Quarles, BM, Pan, J-X, Yu, Z, Howard, JF Jr, Corse, A, et al. Clinical features of LRP4/agrin-antibody–positive myasthenia gravis: A multicenter study. Muscle Nerve. (2020) 62:333–43. doi: 10.1002/mus.26985
32. Chang, T, Leiite, MI, Senanayake, S, Gunaratne, PS, Gamage, R, Riffsy, MTM, et al. FP59-FR-06 clinical and serological study of myasthenia gravis in a south Asian population using both radioimmunoprecipitation and cell-based assays. J Neurol Sci. (2009) 285:S152. doi: 10.1016/S0022-510X(09)70586-6
33. Guptill, JT, Sanders, DB, and Evoli, A. Anti-musk antibody myasthenia gravis: clinical findings and response to treatment in two large cohorts. Muscle Nerve. (2011) 44:36–40. doi: 10.1002/mus.22006
34. Anderson, D, Phan, C, Johnston, WS, and Siddiqi, ZA. Rituximab in refractory myasthenia gravis: a prospective, open-label study with long-term follow-up. Ann Clin Transl Neurol. (2016) 3:552–5. doi: 10.1002/acn3.314
35. Beecher, G, Anderson, D, and Siddiqi, ZA. Rituximab in refractory myasthenia gravis: extended prospective study results. Muscle Nerve. (2018) 58:452–5. doi: 10.1002/mus.26156
36. Piddlesden, SJ, Jiang, S, Levin, JL, Vincent, A, and Morgan, BP. Soluble complement receptor 1 (sCR1) protects against experimental autoimmune myasthenia gravis. J Neuroimmunol. (1996) 71:173–7. doi: 10.1016/S0165-5728(96)00144-0
37. Ricklin, D, Mastellos, DC, Reis, ES, and Lambris, JD. The renaissance of complement therapeutics. Nat Rev Nephrol. (2018) 14:26–47. doi: 10.1038/nrneph.2017.156
38. Nelke, C, Schroeter, CB, Stascheit, F, Pawlitzki, M, Regner-Nelke, L, Huntemann, N, et al. Eculizumab versus rituximab in generalised myasthenia gravis. J Neurol Neurosurg Psychiatry. (2022) 93:548–54. doi: 10.1136/jnnp-2021-328665
39. Oyama, M, Okada, K, Masuda, M, Shimizu, Y, Yokoyama, K, Uzawa, A, et al. Suitable indications of eculizumab for patients with refractory generalized myasthenia gravis. Ther Adv Neurol Disord. (2020) 13:1756286420904207. doi: 10.1177/1756286420904207
40. Uzawa, A, Ozawa, Y, Yasuda, M, and Kuwabara, S. Severe worsening of myasthenic symptoms after the eculizumab discontinuation. J Neuroimmunol. (2020) 349:577424. doi: 10.1016/j.jneuroim.2020.577424
41. Meisel, A, Annane, D, Vu, T, Mantegazza, R, Katsuno, M, Aguzzi, R, et al. Long-term efficacy and safety of ravulizumab in adults with anti-acetylcholine receptor antibody-positive generalized myasthenia gravis: results from the phase 3 CHAMPION MG open-label extension. J Neurol. (2023) 270:3862–75. doi: 10.1007/s00415-023-11699-x
42. Vu, T, Ortiz, S, Katsuno, M, Annane, D, Mantegazza, R, Beasley, KN, et al. Ravulizumab pharmacokinetics and pharmacodynamics in patients with generalized myasthenia gravis. J Neurol. (2023) 270:3129–37. doi: 10.1007/s00415-023-11617-1
43. Ulrichts, P, Guglietta, A, and Dreier, T, Bragt T van, Hanssens, V, Hofman, E, Vankerckhoven, B, Verheesen, P, Ongenae, N, Lykhopiy, V, et al. Neonatal fc receptor antagonist efgartigimod safely and sustainably reduces IgGs in humans. J Clin Invest (2018) 128:4372–4386. doi: 10.1172/JCI97911
44. Howard, JF, Bril, V, Vu, T, Karam, C, Peric, S, Margania, T, et al. Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): a multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. (2021) 20:526–36. doi: 10.1016/S1474-4422(21)00159-9
45. Saccà, F, Pane, C, Espinosa, PE, Sormani, MP, and Signori, A. Efficacy of innovative therapies in myasthenia gravis: A systematic review, meta-analysis and network meta-analysis. Eur J Neurol. (2023) 30:3854–67. doi: 10.1111/ene.15872
46. Zebardast, N, Patwa, HS, Novella, SP, and Goldstein, JM. Rituximab in the management of refractory myasthenia gravis. Muscle Nerve. (2010) 41:375–8. doi: 10.1002/mus.21521
47. Di Stefano, V, Lupica, A, Rispoli, MG, Di Muzio, A, Brighina, F, and Rodolico, C. Rituximab in AChR subtype of myasthenia gravis: systematic review. J Neurol Neurosurg Psychiatry. (2020) 91:392–5. doi: 10.1136/jnnp-2019-322606
48. Caballero-Ávila, M, Álvarez-Velasco, R, Moga, E, Rojas-Garcia, R, Turon-Sans, J, Querol, L, et al. Rituximab in myasthenia gravis: efficacy, associated infections and risk of induced hypogammaglobulinemia. Neuromuscul Disord. (2022) 32:664–71. doi: 10.1016/j.nmd.2022.06.006
49. Drachman, DB. Myasthenia gravis. N Engl J Med. (1994) 330:1797–810. doi: 10.1056/NEJM199406233302507
50. Wolfe, GI, Kaminski, HJ, Aban, IB, Minisman, G, Kuo, H-C, Marx, A, et al. Long-term effect of thymectomy plus prednisone versus prednisone alone in patients with non-thymomatous myasthenia gravis: 2-year extension of the MGTX randomised trial. Lancet Neurol. (2019) 18:259–68. doi: 10.1016/S1474-4422(18)30392-2
51. Chen, K, Li, Y, and Yang, H. Poor responses and adverse outcomes of myasthenia gravis after thymectomy: predicting factors and immunological implications. J Autoimmun. (2022) 132:102895. doi: 10.1016/j.jaut.2022.102895
52. Roldan-Valadez, E, Salazar-Ruiz, SY, Ibarra-Contreras, R, and Rios, C. Current concepts on bibliometrics: a brief review about impact factor, Eigenfactor score, CiteScore, SCImago journal rank, source-normalised impact per paper, H-index, and alternative metrics. Ir J Med Sci. (2019) 188:939–51. doi: 10.1007/s11845-018-1936-5
Keywords: myasthenia gravis, bibliometric analysis, VOSviewer, CiteSpace, citations, keywords
Citation: Tian Y, Shen Q, Peng S, Meng L, Fang R, Xiong A, Li S, Yang Y, Chang W, Ni J and Zhu W (2023) Mapping current trends and hotspots in myasthenia gravis from 2003 to 2022: a bibliometric analysis. Front. Neurol. 14:1320344. doi: 10.3389/fneur.2023.1320344
Received: 12 October 2023; Accepted: 15 December 2023;
Published: 28 December 2023.
Edited by:
Francesco Saccà, University of Naples Federico II, ItalyReviewed by:
Wladimir Bocca Vieira De Rezende Pinto, Federal University of São Paulo, BrazilCopyright © 2023 Tian, Shen, Peng, Meng, Fang, Xiong, Li, Yang, Chang, Ni and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenzeng Zhu, emh1d2VuemVuZzI2MzBAZ2FteXkuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.