- Department of Neurology, Fukuoka University, Fukuoka, Japan
Introduction: Weight loss is one of the non-motor symptoms frequently seen in patients with Parkinson’s disease (PwPD). Weight loss in PwPD is known to be negatively associated with motor and other non-motor symptoms and has been shown to influence the prognosis of PD. In this study, we followed weight change over a 4-year period in PwPD at a single institution and investigated the relationship between weight change and patients’ motor and non-motor symptoms.
Methods: PwPD who visited our hospital from January 2018 to December 2022 were enrolled. Body weights were measured at two points in 2018 (at the start of observation, ‘baseline’) and 2022 (at the end of observation, ‘end date’). In addition, motor symptoms, disease severity, cognitive function, and psychiatric symptoms were evaluated during the same period, and the relationship with weight loss was examined.
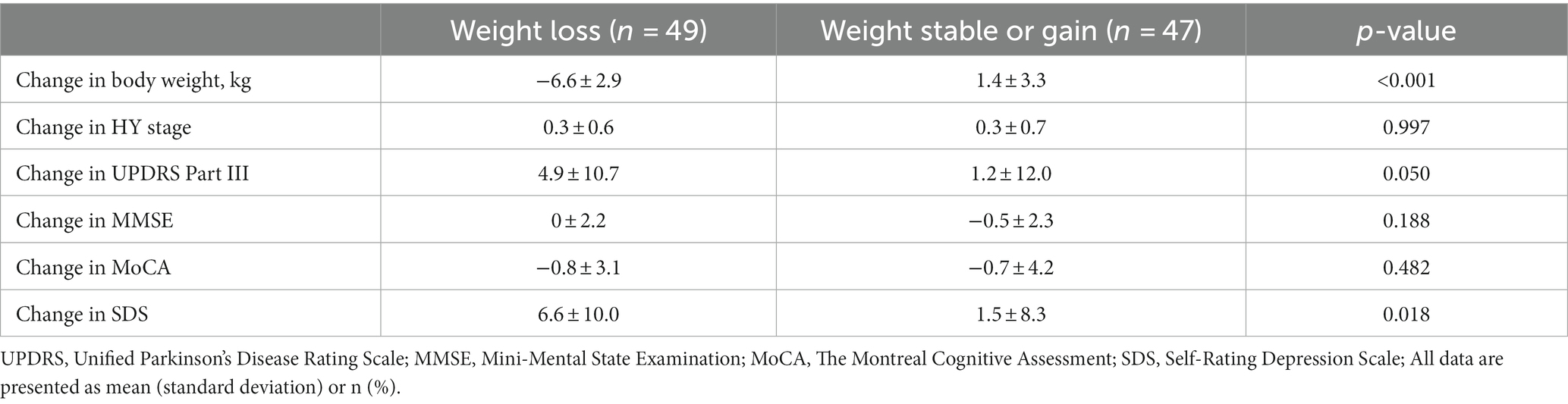
Results: Data of 96 PwPD were available for a 4-year follow-up. At baseline, the mean age was 65.7 ± 10.0 years, the mean disease duration was 6.8 ± 4.0 years, and the mean Hoehn and Yahr stage was 2.4 ± 0.7. Among them, 48 patients (50.0%) had a weight loss of ≥5% from baseline (weight loss group; mean loss was 6.6 ± 2.9 kg). The weight loss group was older (p = 0.031), had a lower Mini-Mental State Examination (MMSE) at baseline (p = 0.019), a significantly lower body mass index (p < 0.001), and a higher Zung Self-Rating Depression Scale (SDS) (p = 0.017) at the end date. There was a negative correlation (γ = −0.349, p < 0.001) between weight change and age, a positive correlation (γ = 0.308, p = 0.002) between weight change and MMSE at baseline, and a negative correlation (γ = −0.353, p < 0.001) between weight change and SDS at the end date. Age-adjusted correlations showed a final negative correlation (γ = −0.331, p = 0.001) between weight change and SDS. MMSE and age-adjusted correlations showed a low negative correlation (γ = −0.333, p = 0.001) between weight change and SDS at the end date.
Conclusion: Weight loss in PwPD in mid-stage was more likely with increasing age, and ≥ 5% weight loss was associated with worsening depression. Further research is needed regarding the significance of weight loss in PwPD.
1 Introduction
Parkinson’s disease (PD) is a chronic progressive neurodegenerative disease characterized by motor symptoms including bradykinesia, resting tremor, rigidity, and postural instability; however, it has been shown that non-motor symptoms precede motor symptoms and significantly affect patients’ quality of life over time (1). Weight loss is one of the most frequent non-motor symptoms, occurring in 48.6–55.6% of patients with Parkinson’s disease (PwPD) (2, 3), and is a symptom that can be present even before the onset of motor symptoms; it can occur at any stage of the disease from early to advanced (4, 5). Such weight loss deserves more attention because it is independently associated with decreased quality of life (6) and mortality (7). Weight loss has also been reported to affect cognitive decline (8), orthostatic hypotension (9), and the appearance of dyskinesia (10). Determinants of weight loss in PwPD are multifactorial; known risk factors include age, duration of disease, total Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), and Hoehn & Yahr (HY) stage (2, 11). To date, few studies have longitudinally followed up on weight loss and clinical symptoms over a long period of time at the same institute. In this study, we investigated the association between weight loss and other factors in PwPD attending our hospital over a 4-year period.
2 Materials and methods
2.1 Protocol approval
All patients were > 20 years old. This study was performed in accordance with the Declaration of Helsinki and approved by the Fukuoka University Medical Ethics Review Board (U20-04-001).
2.2 Patients and study design
This study was conducted as a single-center, longitudinal study in Japan. PwPD received treatment at the Department of Neurology, Fukuoka University Hospital, from January 2018 to December 2022. All patients were examined by a movement disorder specialist and were diagnosed with clinically established PD or probable PD according to the International Parkinson and Movement Disorder Society diagnostic criteria (12).
Demographic and background information such as body weight, body mass index (BMI), age, sex, age at disease onset, duration of disease, dyskinesia, olfactory decline, and hallucinations were extracted from each patient’s medical records. Levodopa-equivalent daily dose (LEDD) was calculated from their medications according to standard assessments (13). Motor symptoms were evaluated by a movement disorder specialist using the HY stage (14) and the MDS-UPDRS part III (15). Cognitive function was assessed with the Japanese version of the Montreal Cognitive Assessment (MoCA) (16, 17) and the Mini-Mental State Examination (MMSE). Depression and apathy were assessed using the Zung Self-Rating Depression Scale (SDS) (18) and the Apathy Scale (19). Patients’ quality of life was assessed using the Parkinson’s Disease Questionnaire-8 (PDQ-8) (15), and summary indexes (PDQ-8 SI) were calculated (20). The 9-symptom Wearing-OFF Questionnaire (WOQ-9) (21) was used to evaluate the phenomenon of “wearing off.” In this study, patients were considered to have worn off if they had two or more positive symptoms on the WOQ-9 and if they improved with dopaminergic therapy. The presence of constipation was diagnosed using the Rome III criteria (22). We performed these evaluations during the patients’ ON state. Data collected in 2018 (baseline) were compared to data collected in 2022 (end date). All participants were measured by their body weight (in kilograms) at baseline and the end date. We weighed all of our patients using the same scale as TANITA Co. (DC-320) at the outpatient clinic in the morning. The shoes, jackets, and heavy clothing were removed, and pockets were emptied at the time of weighing. According to the difference resulting between the baseline and the end-date body weight, subjects were divided into a weight loss group and a weight stable/gain group. There is no uniform definition of significant weight loss. Some previous studies (2, 11, 23) adopted a weight loss of ≥5% as the definition of weight loss for study periods ranging from 6 months to 10 years. Similar to these studies, patients with a weight loss of ≥5% were defined as having significant weight loss in this study.
2.3 Statistics
BMI, age, age at onset, disease duration, LEDD, HY stage, UPDRS part III, MMSE, MoCA, SDS, Apathy Scale, and PDQ-8-SI were analyzed by the Mann–Whitney U-test between the two groups of PwPD with and without ≥5% weight loss. Changes in HY stage, UPDRS part III, MMSE, MoCA, and SDS over 4 years were analyzed by the Mann–Whitney U-test. Sex, wearing off, dyskinesia, hallucinations, olfactory decline, and constipation between the two groups were analyzed by the chi-square test. Correlation coefficients between weight change and age, MMSE, MoCA, and SDS in 2022 and 2018 were analyzed using Pearson’s correlation coefficient. The correlations were interpreted as follows: 0.9–1 indicated a very high correlation; 0.7–0.9 indicated a high correlation; 0.5–0.7 indicated a moderate correlation; 0.3–0.5 indicated a low correlation; and 0–0.3 indicated little to no correlation (24). The correlations between weight change and MMSE, MoCA, and SDS at baseline and end date were analyzed using partial correlation coefficients after controlling for age. All p-values <0.05 were considered statistically significant. Data were analyzed by SPSS v.26 (SPSS Inc., Chicago, IL, United States).
3 Results
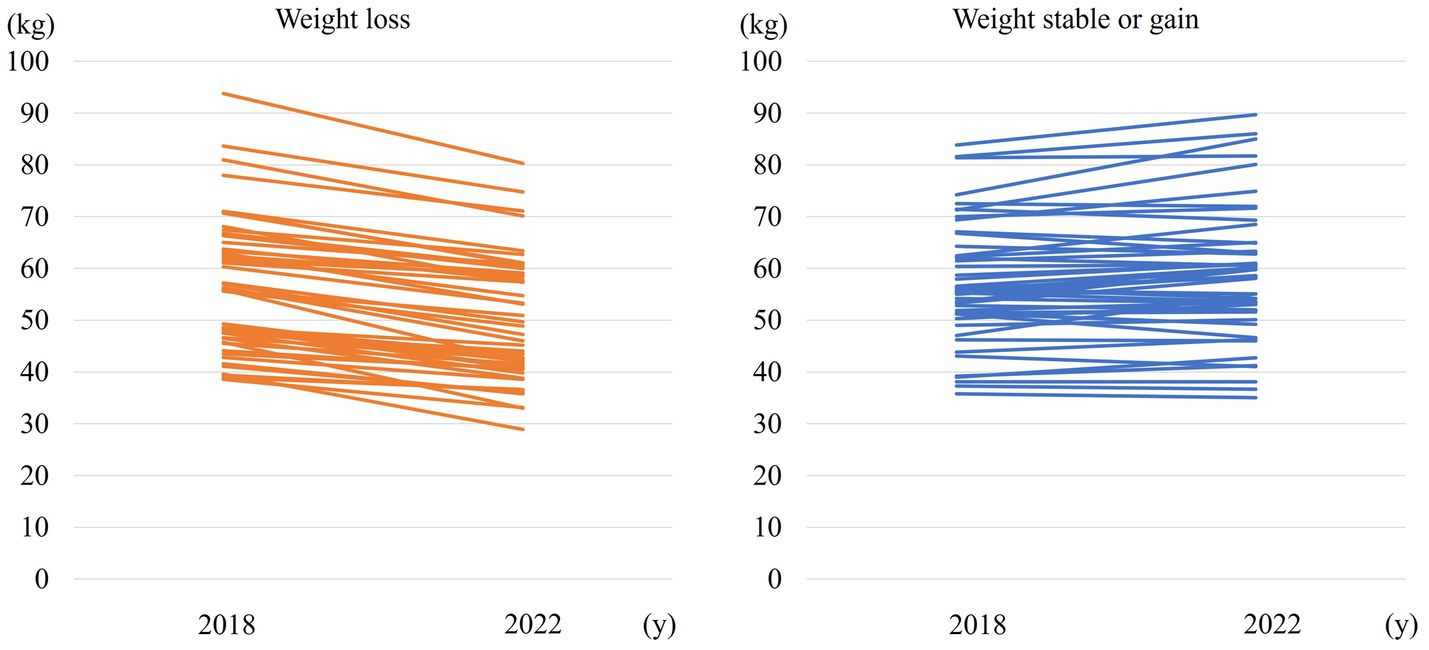
There were 222 PwPD for whom data were collected in 2018. Of these, 96 patients were still attending in 2022, for whom data could be collected. Table 1 shows the clinical characteristics of the patients and comparisons between the two groups: (i) weight loss of ≥5% and (ii) weight stable/gain. There were 37 men and 59 women, with mean age 65.7 ± 10.0 years, mean disease duration 6.8 ± 4.0 years, mean HY stage 2.4 ± 0.7, and mean UPDRS Part III 27.3 ± 12.2. Forty-eight patients (50.0%) had a weight loss of ≥5% (mean 6.6 ± 2.9 kg) (Table 1).
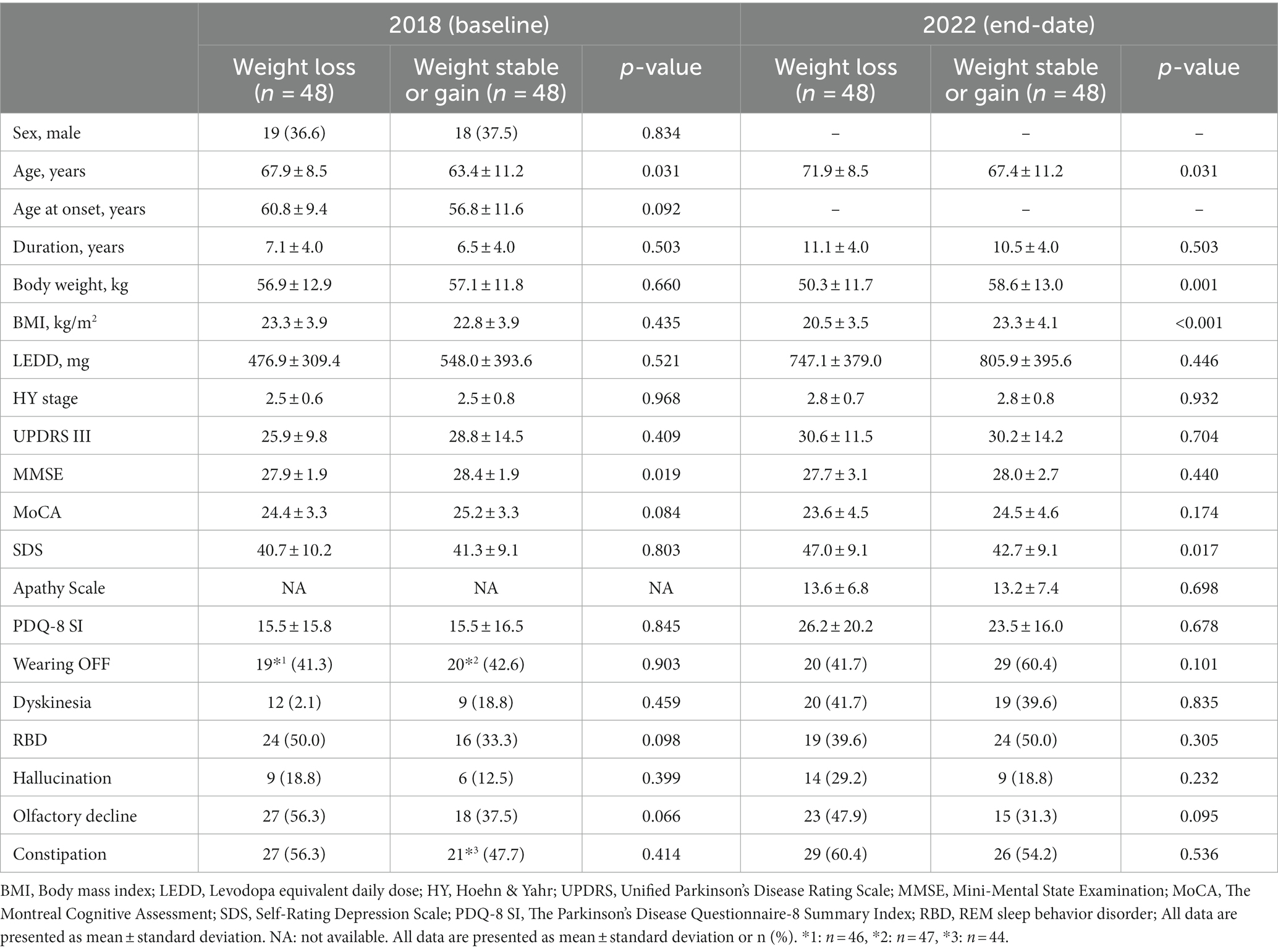
Table 1. Comparison between ≥5% body weight loss and weight stable/gain groups at baseline and end date.
The weight loss group was significantly older (p = 0.031) with a lower MMSE (p = 0.019) in 2018 and had a significantly lower BMI (p < 0.001) and higher SDS (p = 0.017) at the end date (Table 1).
Figure 1 shows weight change over 4 years in the weight loss and weight stable/gain groups.
Table 2 shows a comparison of changes in the HY stage, UPDRS Part III, MMSE, MoCA, and SDS between the weight loss and weight stable/gain groups. The weight loss group showed a significantly greater change in SDS (p = 0.018).
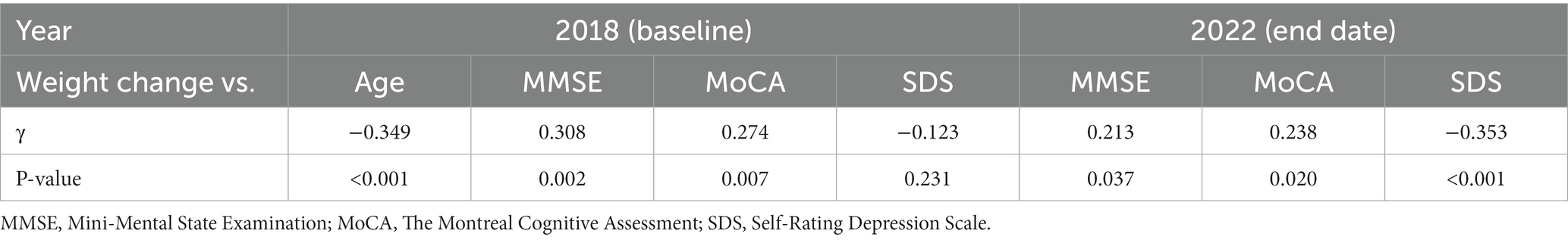
The correlations between weight change and age, MMSE, MoCA, and SDS between baseline and end date are shown in Table 3 and Figure 2.
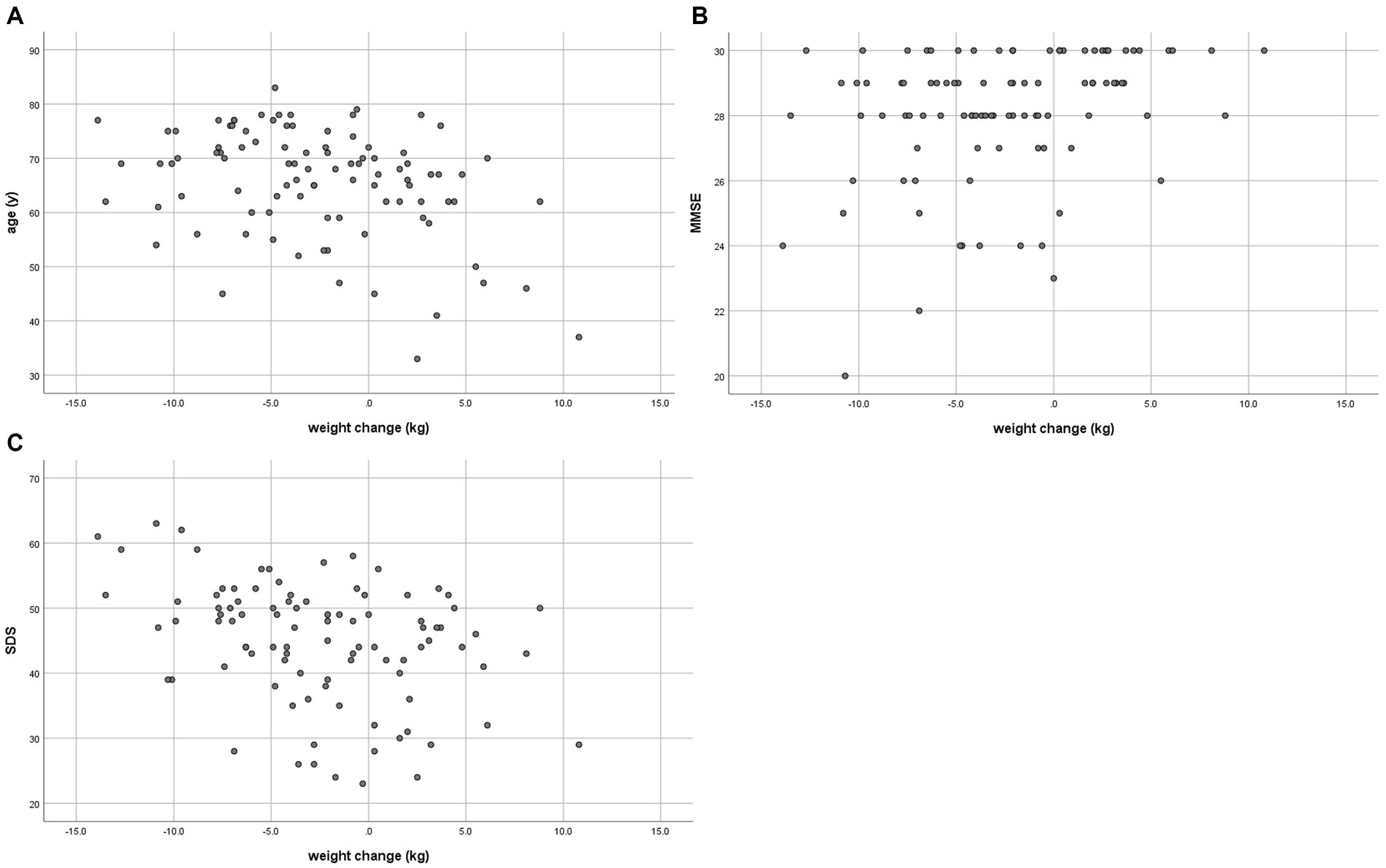
Figure 2. Correlation between weight change and (A) age in 2018, (B) MMSE in 2018, and (C) SDS in 2023.
A low negative correlation (γ = −0.349, p < 0.001) was found between weight change and age. There was a low positive correlation (γ = 0.308, p = 0.002) with MMSE at baseline and a low negative correlation (γ = −0.353, p < 0.001) between weight change and SDS at the end date. Age-adjusted correlations showed a low negative correlation (γ = −0.331, p = 0.001) between weight change and SDS at the end date (Table 4). MMSE and age-adjusted correlations showed a low negative correlation (γ = −0.333, p = 0.001) between weight change and SDS at the end date (Supplementary Table S1).
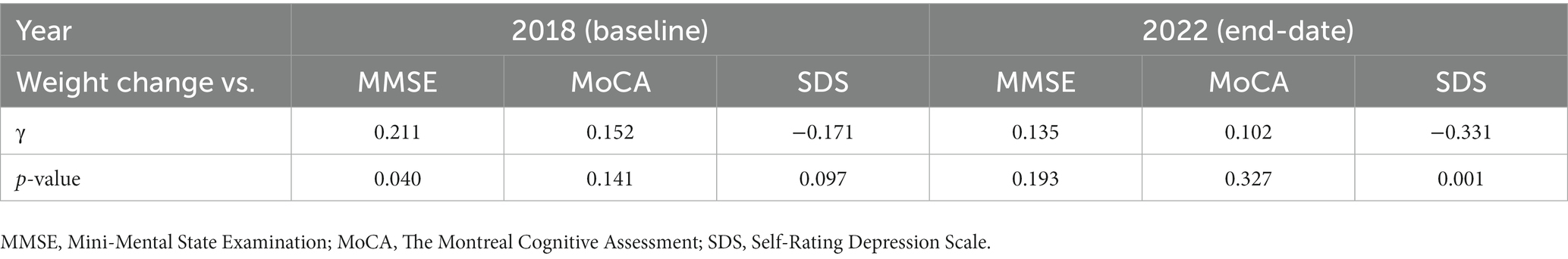
Table 4. Partial correlation coefficient between weight change and other variables after controlling for age.
4 Discussion
Similar to the present study, when using criteria for significant weight loss of ≥5% in PwPD, previous reports have shown that weight loss is associated with age, disease duration, total UPDRS, and HY stage (2, 11, 23). In our study, the weight loss group was significantly older, but there was no significant difference in disease duration or severity of the disease.
Of particular note in this study is the following: the weight loss group had a significantly higher SDS at the end date. Weight change was also negatively correlated with the SDS at the end date, and there were similar results for partial correlation coefficients after controlling for age or MMSE (Table 4, Supplementary Table S1). There was no difference in SDS between the two groups at baseline, suggesting weight loss in PD causes depression, although depressive symptoms and weight loss may interact with each other. Few reports have examined depression and weight loss in PD; one study reported that PwPD with a 3% weight loss showed a significant association with depression (11) and that comorbid depression affected those with a lower BMI in male PwPD (25). An association between depression and malnutrition was reported in PwPD (26). With regard to depression and weight changes, although the patient background is different from this study, it was reported that 40% of depressed patients gained weight and 30% lost weight (27), and that there were differences in activity in several brain regions in response to food stimuli between groups with increased and decreased appetite (28). The present study suggested that weight loss can cause subsequent depression; however, the mechanism by which weight loss causes depression in PD is multifactorial and cannot be determined. We need to be mindful of weight loss as it may cause complications in PwPD and depression (29).
In this study, MMSE was lower in the weight loss group at baseline, and weight change and MMSE were positively correlated. However, partial correlation coefficients after controlling for age showed no correlation. Several reports show that cognitive decline is a factor affecting weight loss in PD (3, 30), and weight changes in early PD increase cognitive decline (8). However, another study did not observe this correlation (31). Although age was a major factor in the current study, further investigation is needed to determine whether cognitive impairment is a risk for weight loss. In this study, there were no significant correlations between weight loss and quality of life or dyskinesia, which has been shown in previous studies (6, 7, 32). Our study and previous studies have shown that weight loss in PwPD could affect the worsening of motor and non-motor symptoms and, in part, quality of life and mortality (6, 7, 33, 34).
The primary limitation of this study was that it involved a single center and was small-scale. Second, it did not take into account each patient’s physical activity or nutritional status. It is reported that PwPD with weight loss increased their energy intake but decreased their activity (30); therefore, further study including data on dietary intake and physical activity is needed. Third, the differentiation of the weight gain group was not considered. In fact, there is evidence that patients with weight gain may follow different trajectories (35). Future studies should consider the fact that the number of participants in our weight gain group was not large: only 17 patients. Hence, it may be worthwhile to conduct more comprehensive investigations into this particular subgroup in future research.
To date, most weight loss studies in PwPD have focused on the early stages of the illness (35, 36), and little is known about the weight loss in the mid-stage that contributes to the alternation of the motor and non-motor symptoms. Our study suggested that in mid-stage PD, a significant weight loss of ≥5% influences psychiatric symptoms, particularly depression. We believe that regular assessments of body weight and nutritional status and intervention if needed should receive more attention in PD management. Previous studies have shown that many PwPD are at risk of malnutrition (37); therefore, they may need to be better assessed for weight changes and nutritional deficiencies. Intervention may be necessary for weight loss in early or mid-stage PwPD, but the establishment of evidence for its efficacy needs to be awaited in future. Dietitians should also be considered members of multidisciplinary PD teams in collaborative PD assessment and nutritional interventions. However, the clinical relevance of underweight and malnutrition in PD needs further investigation. In addition, at present, there is no dietary intervention specific to PwPD, and it is necessary to investigate what kind of diet influences disease progression and prognosis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Fukuoka University-Medical Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because this research is a retrospective study using existing medical information, the research protocol has been made public, and research subjects have been assured of refusal.
Author contributions
KK: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. SF: Data curation, Writing – review & editing. TM: Data curation, Writing – review & editing. YT: Conceptualization, Data curation, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors express their sincere gratitude to the participants in the study. The authors thank Ms. Hitoe Nakashima for her technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1306138/full#supplementary-material
References
1. Yoritaka, A, Shimo, Y, Hatano, T, and Hattori, N. Motor/nonmotor symptoms and progression in patients with Parkinson's disease: prevalence and risks in a longitudinal study. Parkinsons Dis. (2020) 2020:2735361. doi: 10.1155/2020/2735361
2. Cersosimo, MG, Raina, GB, Pellene, LA, Micheli, FE, Calandra, CR, and Maiola, R. Weight loss in Parkinson's disease: the relationship with motor symptoms and disease progression. Biomed Res Int. (2018) 2018:9642524. doi: 10.1155/2018/9642524
3. Uc, EY, Struck, LK, Rodnitzky, RL, Zimmerman, B, Dobson, J, and Evans, WJ. Predictors of weight loss in Parkinson's disease. Mov Disord. (2006) 21:930–6. doi: 10.1002/mds.20837
4. Chen, H, Zhang, SM, Hernán, MA, Willett, WC, and Ascherio, A. Weight loss in Parkinson's disease. Ann Neurol. (2003) 53:676–9. doi: 10.1002/ana.10577
5. Williamson, A, and Hoggart, B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. (2005) 14:798–804. doi: 10.1111/j.1365-2702.2005.01121.x
6. Akbar, U, He, Y, Dai, Y, Hack, N, Malaty, I, McFarland, NR, et al. Weight loss and impact on quality of life in Parkinson's disease. PLoS One. (2015) 10:e0124541. doi: 10.1371/journal.pone.0124541
7. Sharma, JC, and Vassallo, M. Prognostic significance of weight changes in Parkinson's disease: the park-weight phenotype. Neurodegener Dis Manag. (2014) 4:309–16. doi: 10.2217/nmt.14.25
8. Kim, R, Choi, S, Byun, K, Kang, N, Suh, YJ, Jun, JS, et al. Association of early weight change with cognitive decline in patients with Parkinson disease. Neurology. (2023) 100:e232–41. doi: 10.1212/WNL.0000000000201404
9. Nakamura, T, Suzuki, M, Ueda, M, Hirayama, M, and Katsuno, M. Lower body mass index is associated with orthostatic hypotension in Parkinson's disease. J Neurol Sci. (2017) 372:14–8. doi: 10.1016/j.jns.2016.11.027
10. Sharma, JC, Macnamara, L, Hasoon, M, Vassallo, M, and Ross, I. Cascade of levodopa dose and weight-related dyskinesia in Parkinson's disease (LD-WD-PD cascade). Parkinsonism Relat Disord. (2006) 12:499–505. doi: 10.1016/j.parkreldis.2006.07.002
11. Ghourchian, S, Gruber-Baldini, AL, Shakya, S, Herndon, J, Reich, SG, von Coelln, R, et al. Weight loss and weight gain in Parkinson disease. Parkinsonism Relat Disord. (2021) 83:31–6. doi: 10.1016/j.parkreldis.2020.12.018
12. Postuma, RB, Berg, D, Stern, M, Poewe, W, Olanow, CW, Oertel, W, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord. (2015) 30:1591–601. doi: 10.1002/mds.26424
13. Schade, S, Mollenhauer, B, and Trenkwalder, C. Levodopa equivalent dose conversion factors: an updated proposal including opicapone and safinamide. Mov Disord Clin Pract. (2020) 7:343–5. doi: 10.1002/mdc3.12921
14. Hoehn, MM, and Yahr, MD. Parkinsonism: onset, progression and mortality. Neurology. (1967) 17:427–42. doi: 10.1212/wnl.17.5.427
15. Kashihara, K, Kondo, T, Mizuno, Y, Kikuchi, S, Kuno, S, Hasegawa, K, et al. Official Japanese version of the Movement Disorder Society-unified Parkinson's disease rating scale: validation against the original English version. Mov Disord. (2014) 1:200–12. doi: 10.1002/mdc3.12058
16. Nasreddine, ZS, Phillips, NA, Bédirian, V, Charbonneau, S, Whitehead, V, Collin, I, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-54152005.53221.x
17. Fujiwara, Y, Suzuki, H, Yasunaga, M, Sugiyama, M, Ijuin, M, Sakuma, N, et al. Brief screening tool for mild cognitive impairment in older Japanese: validation of the Japanese version of the Montreal cognitive assessment. Geriatr Gerontol Int. (2010) 10:225–32. doi: 10.1111/j.1447-0594.2010.00585.x
18. Zung, WW. Aself-rating depression scale. Arch Gen Psychiatry. (1965) 12:63–70. doi: 10.1001/archpsyc.1965.01720310065008
19. Starkstein, SE, Mayberg, HS, Preziosi, TJ, Andrezejewski, P, Leiguarda, R, and Robinson, RG. Reliability, validity, and clinical correlates of apathy in Parkinson's disease. J Neuropsychiatry Clin Neurosci. (1992) 4:134–9. doi: 10.1176/jnp.4.2.134
20. Jenkinson, C, Fitzpatrick, R, Peto, V, Greenhall, R, and Hyman, N. The PDQ-8: development and validation of a short-form Parkinson's disease questionnaire. Psychol Health. (1997) 12:805–14. doi: 10.1080/08870449708406741
21. Kondo, T, and Takahashi, K. Translation and linguistic validation of the Japanese version of the wearing-off questionnaires (WOQ-19 and WOQ-9). Brain Nerve. (2011) 63:1285–92.
22. Kanazawa, M, Nakajima, S, Oshima, T, Whitehead, WE, Sperber, AD, Palsson, OS, et al. Validity and reliability of the Japanese version of the Rome III diagnostic questionnaire for irritable bowel syndrome and functional dyspepsia. J Neurogastroenterol Motil. (2015) 21:537–44. doi: 10.5056/jnm15016
23. Lankisch, P, Gerzmann, M, Gerzmann, JF, and Lehnick, D. Unintentional weight loss: diagnosis and prognosis. The first prospective follow-up study from a secondary referral Centre. J Intern Med. (2001) 249:41–6. doi: 10.1046/j.1365-2796.2001.00771.x
24. Taylor, R. Interpretation of the correlation coefficient: a basic review. J Diagn Med Sonogr. (1999) 6:35–9. doi: 10.1177/875647939000600106
25. Pilhatsch, M, Kroemer, NB, Schneider, C, Ebersbach, G, Jost, WH, Fuchs, G, et al. Reduced body mass index in Parkinson's disease: contribution of comorbid depression. J Nerv Ment Dis. (2013) 201:76–9. doi: 10.1097/NMD.0b013e31827ab2cc
26. Sheard, JM, Ash, S, Mellick, GD, Silburn, PA, and Kerr, GK. Markers of disease severity are associated with malnutrition in Parkinson's disease. PLoS One. (2013) 8:e57986. doi: 10.1371/journal.pone.0057986
27. Weissenburger, J, Rush, AJ, Giles, DE, and Stunkard, AJ. Weight change in depression. Psychiatry Res. (1986) 17:275–83. doi: 10.1016/0165-1781(86)90075-2
28. Simmons, WK, Burrows, K, Avery, JA, Kerr, KL, Bodurka, J, Savage, CR, et al. Depression-related increases and decreases in appetite: dissociable patterns of aberrant activity in reward and interoceptive neurocircuitry. Am J Psychiatry. (2016) 173:418–28. doi: 10.1176/appi.ajp.2015.15020162
29. Cote, L. Depression: impact and management by the patient and family. Neurology. (1999) 52:S7–9.
30. Lorefält, B, Ganowiak, W, Pålhagen, S, Toss, G, Unosson, M, and Granérus, AK. Factors of importance for weight loss in elderly patients with Parkinson's disease. Acta Neurol Scand. (2004) 110:180–7. doi: 10.1111/j.1600-0404.2004.00307.x
31. Fiszer, U, Michałowska, M, Baranowska, B, Wolińska-Witort, E, Jeske, W, Jethon, M, et al. Leptin and ghrelin concentrations and weight loss in Parkinson's disease. Acta Neurol Scand. (2010) 121:230–6. doi: 10.1111/j.1600-0404.2009.01185.x
32. Sharma, JC, and Lewis, A. Weight in Parkinson's disease: phenotypical significance. Int Rev Neurobiol. (2017) 134:891–919. doi: 10.1016/bs.irn.2017.04.011
33. van der Marck, MA, Dicke, HC, Uc, EY, Kentin, ZH, Borm, GF, Bloem, BR, et al. Body mass index in Parkinson's disease: a meta-analysis. Parkinsonism Relat Disord. (2012) 18:263–7. doi: 10.1016/j.parkreldis.2011.10.016
34. Cumming, K, Macleod, AD, Myint, PK, and Counsell, CE. Early weight loss in parkinsonism predicts poor outcomes: evidence from an incident cohort study. Neurology. (2017) 89:2254–61. doi: 10.1212/WNL.0000000000004691
35. Urso, D, van Wamelen, DJ, Batzu, L, Leta, V, Staunton, J, Pineda-Pardo, JA, et al. Clinical trajectories and biomarkers for weight variability in early Parkinson's disease. NPJ Parkinsons Dis. (2022) 8:95. doi: 10.1038/s41531-022-00362-3
36. Wills, AM, Li, R, Pérez, A, Ren, X, Boyd, J, and Investigators, NINDSNET-PD. Predictors of weight loss in early treated Parkinson's disease from the NET-PD LS-1 cohort. J Neurol. (2017) 264:1746–53. doi: 10.1007/s00415-017-8562-4
Keywords: Parkinson’s disease, weight loss, depression, non-motor symptoms, Zung Self-Rating Depression Scale
Citation: Kurihara K, Fujioka S, Mishima T and Tsuboi Y (2024) Impact of weight loss for depressive symptom in mid-stage patients with Parkinson’s disease: a 4-year follow-up study. Front. Neurol. 14:1306138. doi: 10.3389/fneur.2023.1306138
Edited by:
Antonio Emanuele Elia, IRCCS Carlo Besta Neurological Institute Foundation, ItalyReviewed by:
Daniele Urso, King's College London, United KingdomHiroshi Nagayama, Nippon Medical School, Japan
Copyright © 2024 Kurihara, Fujioka, Mishima and Tsuboi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yoshio Tsuboi, dHN1Ym9pQGNpcy5mdWt1b2thLXUuYWMuanA=
 Kanako Kurihara
Kanako Kurihara Shinsuke Fujioka
Shinsuke Fujioka Takayasu Mishima
Takayasu Mishima Yoshio Tsuboi
Yoshio Tsuboi