- 1University of Central Florida, Orlando, FL, United States
- 2Roskamp Institute, Sarasota, FL, United States
- 3Orlando VA Healthcare System, Orlando, FL, United States
Background: Frontotemporal lobe disorders (FTD) are amongst the most common brain neurodegenerative disorders. Their relatively covert, frequently subtle presentations and diverse etiologies, pose major challenges in diagnosis and treatments. Recent studies have yielded insights that the etiology in the majority are due to environmental and sporadic causes, rather than genetic in origin.
Aims: To retrospectively examine the cognitive and behavioral impairments in the veteran population to garner the range of differing syndrome presentations and etiological subcategories with a specific focus on frontotemporal lobe disorders.
Methodology: The design is a retrospective, observational registry, case series with the collection of epidemiological, clinical, cognitive, laboratory and radiological data on people with cognitive and behavioral disorders. Inclusion criteria for entry were veterans evaluated exclusively at Orlando VA Healthcare System, neurology section, receiving a diagnosis of FTD by standard criteria, during the observation period dated from July 2016 to March 2021. Frontotemporal disorders (FTD) were delineated into five clinical 5 subtypes. Demographic, cardiovascular risk factors, cognitive, behavioral neurological, neuroimaging data and presumed etiological categories, were collected for those with a diagnosis of frontotemporal disorder.
Results: Of the 200 patients with FTD, further cognitive, behavioral neurological evaluation with standardized, metric testing was possible in 105 patients. Analysis of the etiological groups revealed significantly different younger age of the traumatic brain injury (TBI) and Gulf War Illness (GWI) veterans who also had higher Montreal Cognitive Assessment (MOCA) scores. The TBI group also had significantly more abnormalities of hypometabolism, noted on the PET brain scans. Behavioral neurological testing was notable for the findings that once a frontotemporal disorder had been diagnosed, the four different etiological groups consistently had abnormal FRSBE scores for the 3 principal frontal presentations of (i) abulia/apathy, (ii) disinhibition, and (iii) executive dysfunction as well as abnormal Frontal Behavioral Inventory (FBI) scores with no significant difference amongst the etiological groups. The most common sub-syndromes associated with frontotemporal syndromes were the Geschwind-Gastaut syndrome (GGS), Klüver-Bucy syndrome (KBS), involuntary emotional expression disorder (IEED), cerebellar cognitive affective syndrome (CCA), traumatic encephalopathy syndrome (TES) and prosopagnosia. Comparisons with the three principal frontal lobe syndrome clusters (abulia, disinhibition, executive dysfunction) revealed a significant association with abnormal disinhibition FRSBE T-scores with the GGS. The regression analysis supported the potential contribution of disinhibition behavior that related to this complex, relatively common behavioral syndrome in this series. The less common subsyndromes in particular, were notable, as they constituted the initial overriding, presenting symptoms and syndromes characterized into 16 separate conditions.
Conclusion: By deconstructing FTD into the multiple sub-syndromes and differing etiologies, this study may provide foundational insights, enabling a more targeted precision medicine approach for future studies, both in treating the sub-syndromes as well as the underlying etiological process.
Background
Our understanding of neurodegenerative disease and dementia has evolved rapidly in the last few decades. As recently as the 1970s and 1980’s all dementia was generally considered to be Alzheimer’s disease (AD) (1). Frontotemporal lobe disorders (FTD) are now amongst the most common neurodegenerative disorders, after an approximate 130-year diagnostic hiatus, due largely to the under recognition of Pick’s disease, first described in 1896 (2). The most important reason appears to have been that Pick bodies are found in only 20% of FTD. The pathology in frontotemporal lobe dementia is now known to be due to several associated pathologies such as TDP-43 (A, B, C), tau, FUS (3) and less commonly Pick bodies. In addition to the behavioral and primary progressive aphasia subtypes (semantic aphasia, non-fluent aphasia), cortico-basal degeneration, progressive supranuclear palsy and amyotrophic lateral sclerosis are other recognized clinical variants. In a recent wide-ranging retrospective cohort study, the Frontotemporal Dementia Incidence European Research Study (FRONTIERS) in 9 European countries concluded that the annual incidence rate of 2.36 cases per 100,000 person-years appears more common than formerly appreciated and should be considered irrespective of age (4). In contrast to the frontotemporal dementias, recent studies have yielded insights that the majority may be due to environmental and sporadic causes, rather than being genetic in origin and presenting with mild to moderate behavioral impairment termed frontotemporal disorders or syndromes, rather than dementia (5, 6).
From a diagnostic point of view, much depends on what clinical population is being studied. Cognitive and behavioral neurological disorders are common in the Veteran population as sequelae of traumatic brain injury, post-traumatic stress disorders and neurotoxicological exposure, often presenting as frontotemporal syndromes (7–9). Their relatively covert and frequently subtle presentations and diverse etiologies, pose major challenges in diagnosis and treatments. Yet, these conditions often afflict the most plastic areas of the brain providing potential opportunities for successful interventions. Many, widely differing pathophysiological entities for frontotemporal syndromes have been reported. These include traumatic brain injury (10), vascular causes (11), neurotoxicological syndromes such as Gulf War Illness (primarily a synaptopathy, associated with acute phase lipids, Agent Orange exposure) (9, 12), autoimmune disorders (13), cerebral mechanical aberrations such as sagging brain syndromes (14), infectious causes, including Whipple’s disease (15) and traumatic encephalopathy/chronic traumatic encephalopathy spectrum (16), in addition to the group of frontotemporal lobe dementias. Furthermore, milder forms of FTD with little deterioration over time, such as the mild frontotemporal phenocopy variant have been reported (3). Some of these frontotemporal syndromes may stabilize for decades and even improve, in contradistinction to the traditional dementias such as Alzheimer dementia (AD). Hence timeous and precise diagnosis may allow precision treatment strategies to be implemented.
Mild cognitive impairment occurs years to decades prior to AD and importantly, from a clinical perspective, 30% may have a remediable underlying cause and improve with appropriate treatment (17, 18). Mild behavioral impairment, with recent validated scales, is now also being recognized with similar opportunities for intervention (19). Metabolic syndromes which tend to target the more posterior association areas and the default mode network, often result in the Alzheimer’s spectrum of cognitive dysfunction. The more anterior association cortices of the frontal and anterior temporal lobes are more prone to trauma, toxins and stressors of various kinds, target primarily the salience network and present primarily with an array of behavioral neurological syndromes (20).
Aims
To retrospectively examine the cognitive and behavioral impairments in the veteran population to garner the range of differing syndrome presentations and etiological subcategories with a specific focus on frontotemporal lobe disorders (FTD).
Methodology
The study design was a retrospective observation case series with analysis of veterans with cognitive and behavioral disorders. FTD were specifically documented, which were encountered exclusively at the Orlando VA Healthcare System (OVAMC), neurology service, Orlando, Florida. The data collection comprised of demographic, epidemiological, clinical, cognitive neurological, behavioral neurological, laboratory and neuroradiological data. The inclusion criteria constituted veterans evaluated exclusively at the OVAMC neurology section, receiving a diagnosis of FTD by standard criteria, during the observation period dated from July 2016 to March 2021. Exclusion criteria consisted of veterans with cognitive and behavioral syndromes not amenable for further analysis, due to significant behavioral obstacles, multiple comorbid medical or psychiatric conditions or unwilling to undergo further testing. The high number of antipsychotic medications in this exclusion group typically masqueraded with frequent and significant frontal lobe syndromes (abulia, dysexecutive syndrome) as recognized side effects of this class of medications. After syndrome analysis, further exclusions included AD, Lewy Body dementia (LBD), vascular dementia, and mixed dementia syndromes. The studies involving humans were approved by Orlando VA Institutional Research Board Approval IRBNET ID: 1256151-4 and a finalized the approval date on April 11, 2020.
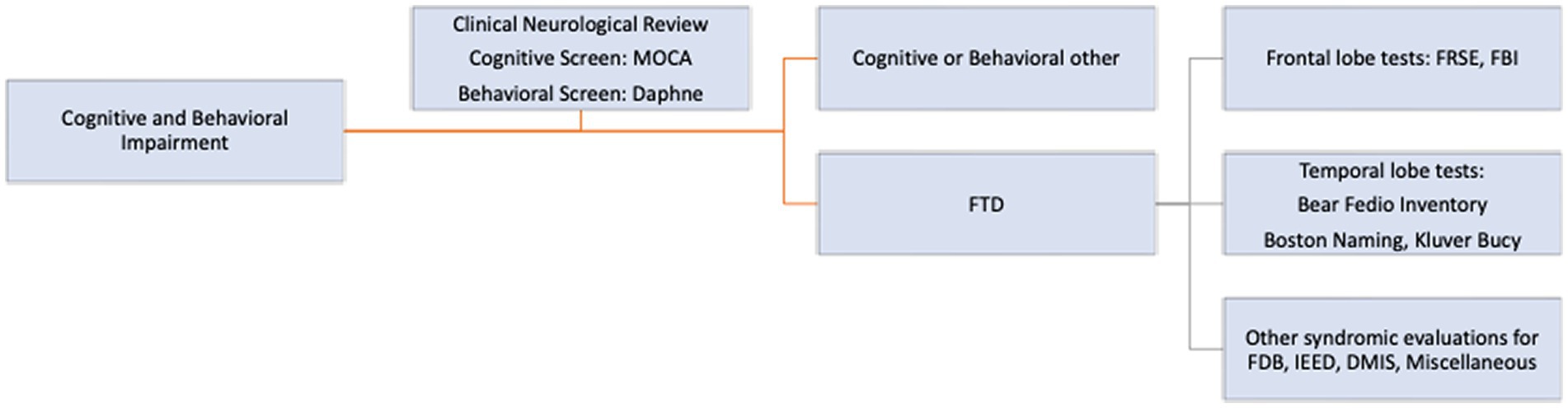
In addition to standard neurological evaluation, a comprehensive screening cognitive and behavioral neurological tool with pre-defined syndromes, according to standard definitions, published elsewhere, was used to guide initial diagnosis (21). Whenever possible the following cognitive and behavioral neurological tests were administered (Figure 1). These included a general cognitive screening test, the MOCA 5-min version (22), a behavioral neurological FTD screening test, the Daphne 6 and 40 (23) and activities of Daily Living using the Katz Disability Scale (24). In addition, specific frontal behavioral tests, the FRSBE (25), Frontal Behavioral Inventory (26) and specific anterior temporal lobe tests and other cognitive syndrome evaluations were used. These included the Boston Naming Test (27), the Bear-Fedio Inventory (BFI) (modified) (28), a Human Klüver Bucy Syndrome Inventory (KBS) (29, 30), a Geschwind-Gastaut inventory (GGS) (31), a delusional misidentification syndrome inventory (DMIS) (32), an involuntary emotional expression disorder (IEED) inventory (33) and a cerebellar cognitive affective syndromes (CCA) inventory (34) (Figure 1). In brief the Human Klüver Bucy syndrome required evidence of any 3 components of (i) visual agnosia, (ii) loss of anger, fear responses with placidity or flattened affect, (iii) altered sexual activity or orientation, (iv) hyperorality or bulimia and hypermetamorphosis (compulsion to manipulate objects in the immediate environment, akin to utilization behavior). The Geschwind-Gastaut syndrome diagnosis was made if any 3 of the following were present; a personality syndrome comprising of: (i) circumstantiality (excessive verbal output, loquacious, hypergraphia, interpersonal viscosity) (ii) Intensified mental life (deepening of emotions, hypermoralism, nascent metaphysical interests, hyper-philosophical), (iii) hyper-religiosity (multiple conversions, deep religious beliefs, mystical states), (iv) altered sexuality (hyposexuality, hyper-sexualism, gender dysphoria, transvestism). DMIS diagnosis was made if a person incorrectly identifies or duplicates persons, places, objects, or even events which may be learned by self-report or substantiated from family members or friends. Many different DMIS have been reported but only 3 types were recorded, including: (i) Capgras syndrome; the belief by the person that a familiar individual or even the person themselves had been replaced by an imposter (hypo-identification), (ii) Fregoli’s syndrome; the belief that an individual familiar to the person is actually impersonating and is presenting themselves as a stranger (hyper-identification) and (iii) intermetamorphosis; two people, both familiar to the person, have interchanged identities with one another. For IEED, item number 6 of FRSBE test was used and was graded on a 5-point Likert scale and if ≥3 was used as positive diagnosis. This delineated a syndrome characterized by spontaneous outbursts of crying, laughing or both, occurring contextually inappropriately. Cerebellar cognitive affective syndromes (CCA) were diagnosed if there was a relevant cerebellar lesion such as stroke or neoplasm with co-occurring onset of cognitive, behavioral or emotional impairment. Traumatic encephalopathy syndrome was diagnosed according to the criteria proposed by the National Comorbidity Survey Replication with at least one of the core criteria and two of the 9 supportive criteria required for diagnosis (35).
Neuroimaging was performed in all patients, including multimodality MRI imaging sequences (GE 3 Tesla) MRI (T1, T2), fluid attenuation inversion recovery (FLAIR) and diffusion weighted imaging (DWI). CT brain scans were a surrogate if MRI contra-indicated and PET brain (FDG, metabolic) scans were performed in selected patients.
Laboratory testing included routine cognitive impairment and dementia related tests. Genetic testing (C9orf72, GRN, and MAPT) was not recommended if there was no family history of frontotemporal dementia. In addition, other factors were considered including the cost factor, patient preference, lack of utility and lack of genetic counseling management if positive.
FTD clinical subtypes were classified into the standard behavioral variant, semantic aphasia, non-fluent aphasia, cortico-basal degeneration variant, progressive supranuclear palsy variant, FTD and amyotrophic sclerosis variant in accordance with currently accepted classification (36). Etiological entities were based on clinical history, cognitive, behavioral, laboratory and imaging analyses and categorized as:
1. Traumatic Brain Injury: Centers for Disease Control and ICD-10 criteria for mild and moderate TBI (37).
2. Frontotemporal lobe degenerations and dementias: Daphne Screening test, FBI test (if the score is ≥27).
3. Vascular Cognitive Disorder and dementia: AHA/ASA criteria (38).
4. Neurotoxicological: Gulf War Illness (GWI), Agent Orange, Camp Lejeune toxin exposure. For GWI Illness: Kansas, Haley or Institute of Medicine criteria (9, 39). In brief, there needed to be least 3 of the 6 symptom domains positive (chronic fatigue, cognitive disorders/headache/mood disorder, dyssomnia, somatic pain, gastrointestinal symptoms, typically chronic diarrhea, respiratory symptoms and skin rashes) for a GWI diagnosis to be made in the context of having been deployed in the 1991 Desert Storm conflict. For Agent Orange and Camp Lejeune toxin exposure, appropriate historical time frame and geographic association was used.
5. Alzheimer’s dementia: NIH Makhann criteria (40) with specific attention to subtype variants of frontal Alzheimer’s, amnestic, visuospatial, logopenic progressive aphasia and posterior cortical atrophy syndrome (Benson’s syndrome).
6. Lewy Body dementia: McKeith criteria (41).
The overall clinical and investigative approach in the study was the delineation of FTD into 3 principal categories, namely by FTD clinical variants, by etiological subtypes, and by a number of common and less common subsyndromes.
Statistical analyses
Baseline, demographic, cardiovascular risk factors, cognitive, behavioral data, neuroimaging and presumed etiological categories, were compared between groups. Analysis of variance (ANOVA) and chi-square tests (for categorical variables) were used to examine whether the means between our groups (TBI, vascular, GWI, and other) were statistically different. In addition, a multivariate linear regression analysis was used to examine factors associated with FRSBE scores. A significance level of p-values below 0.05 was chosen. Statistical analysis was performed using Stata Version 16 MP (StataCorp, College Station, TX, USA) for data management and data analysis. Dispersion and position indices were depicted by interquartile ranges (25th, 50th, 75th).
Results
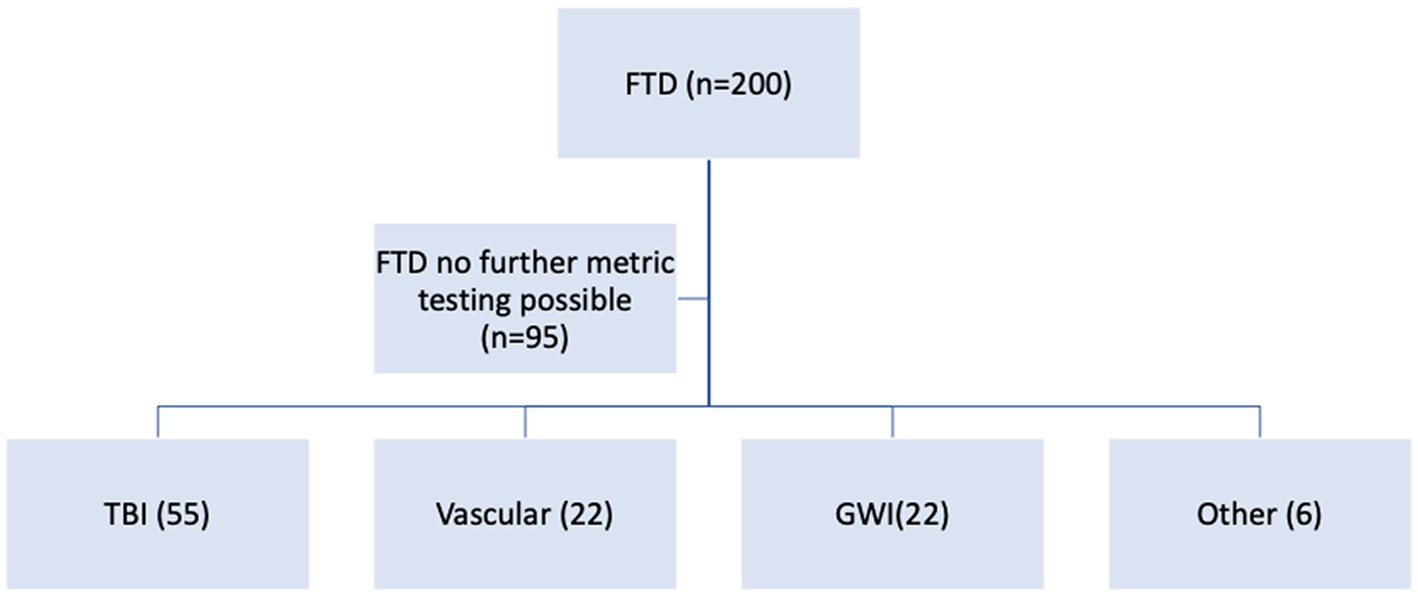
Of the 200 patients with FTD, there were 190 men and 10 women with a mean age of 59.6 years (range 27–90 years) and mean education years of 13.8 (range 8–20 years). Screening cognitive testing with the MOCA tool and frontotemporal behavioral screening with the Daphne test was possible in all patients. This yielded an overall frequency of the generally accepted FTD clinical subtypes in this population, as noted in Figure 2.
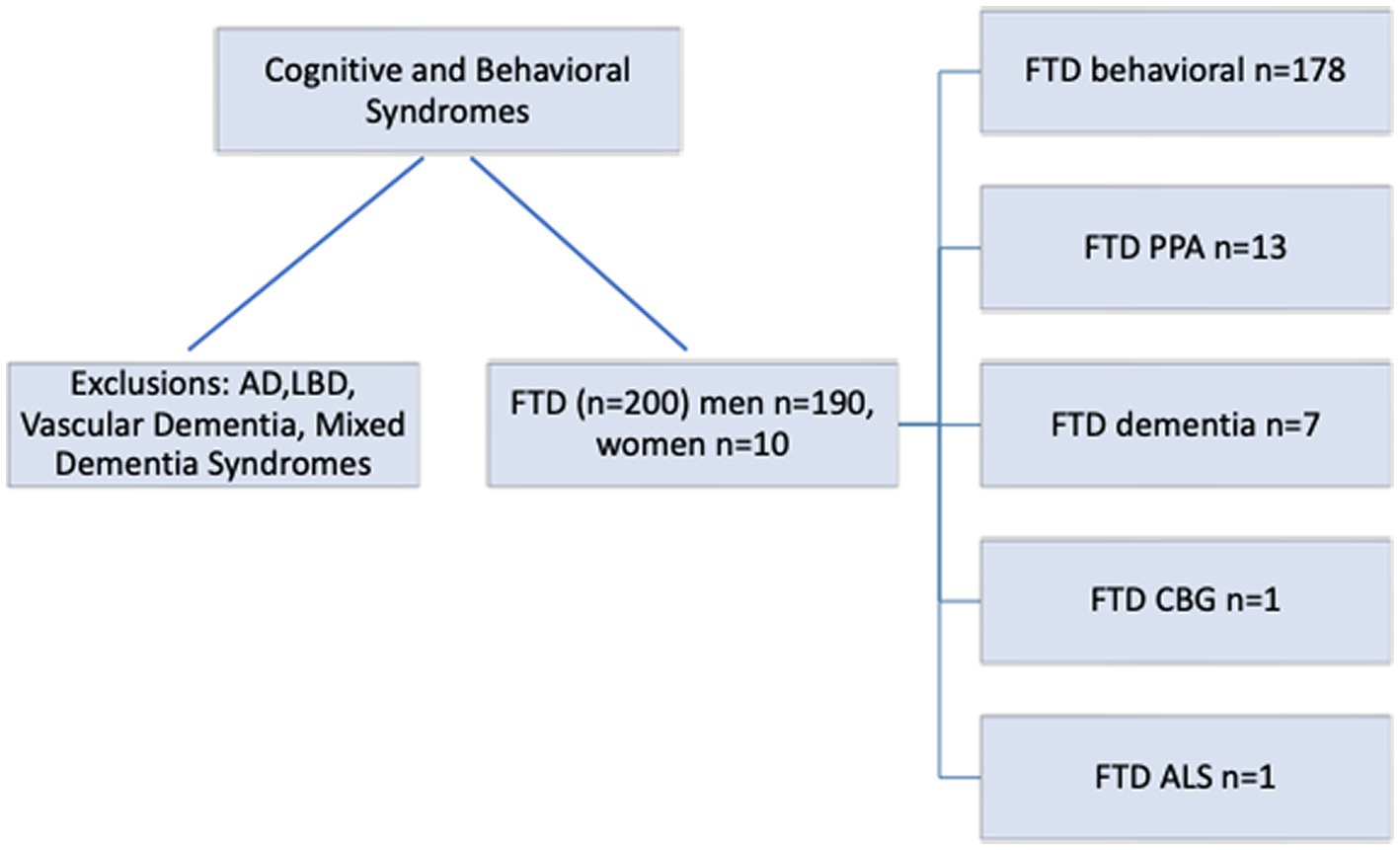
Figure 2. Frontotemporal disorders (FTD) cascade and variants. AD, Alzheimer’s Disease; LBD, Lewy Body Dementia; FTS, Frontotemporal Syndrome; CTE, Chronic Traumatic Encephalopathy.
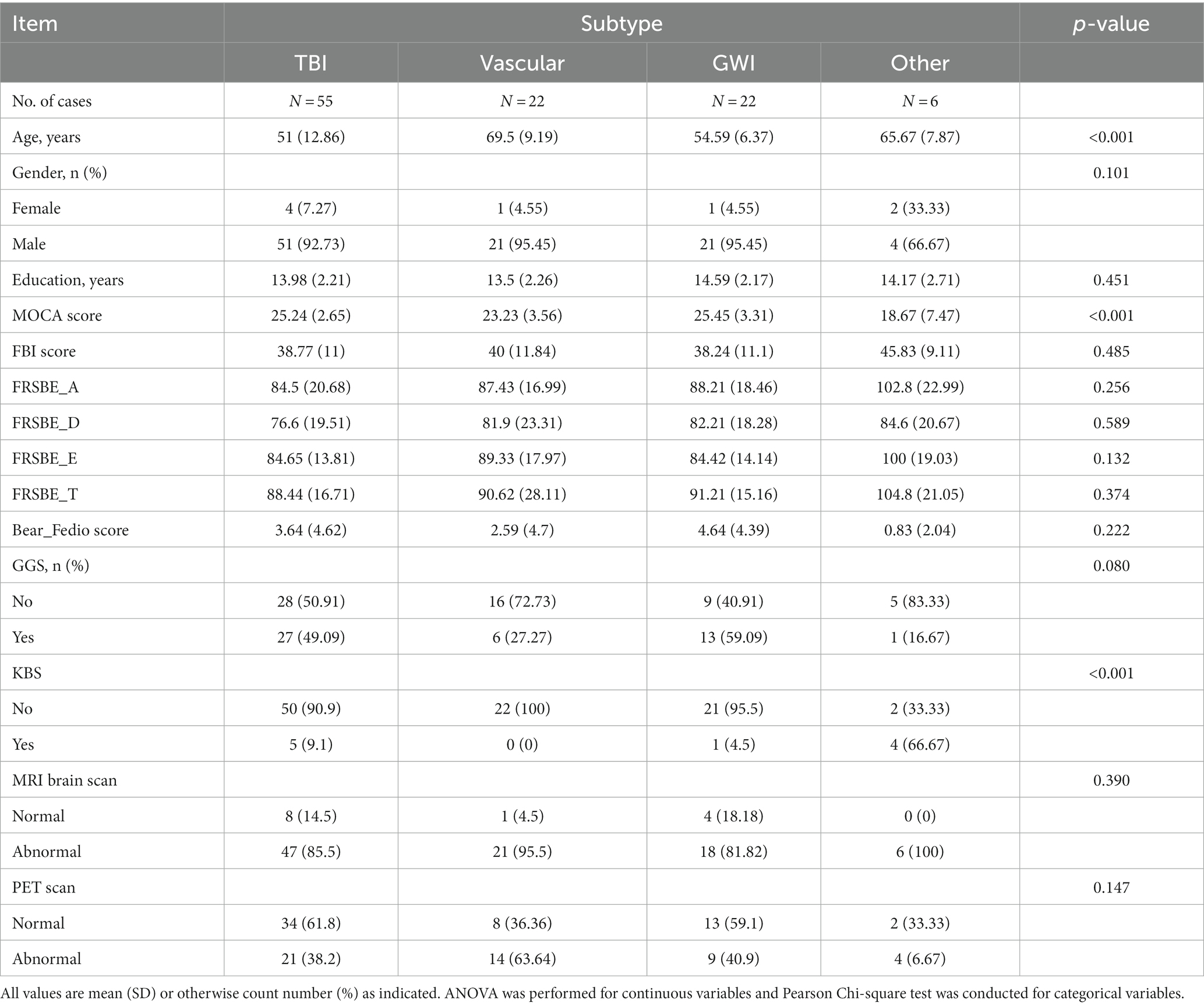
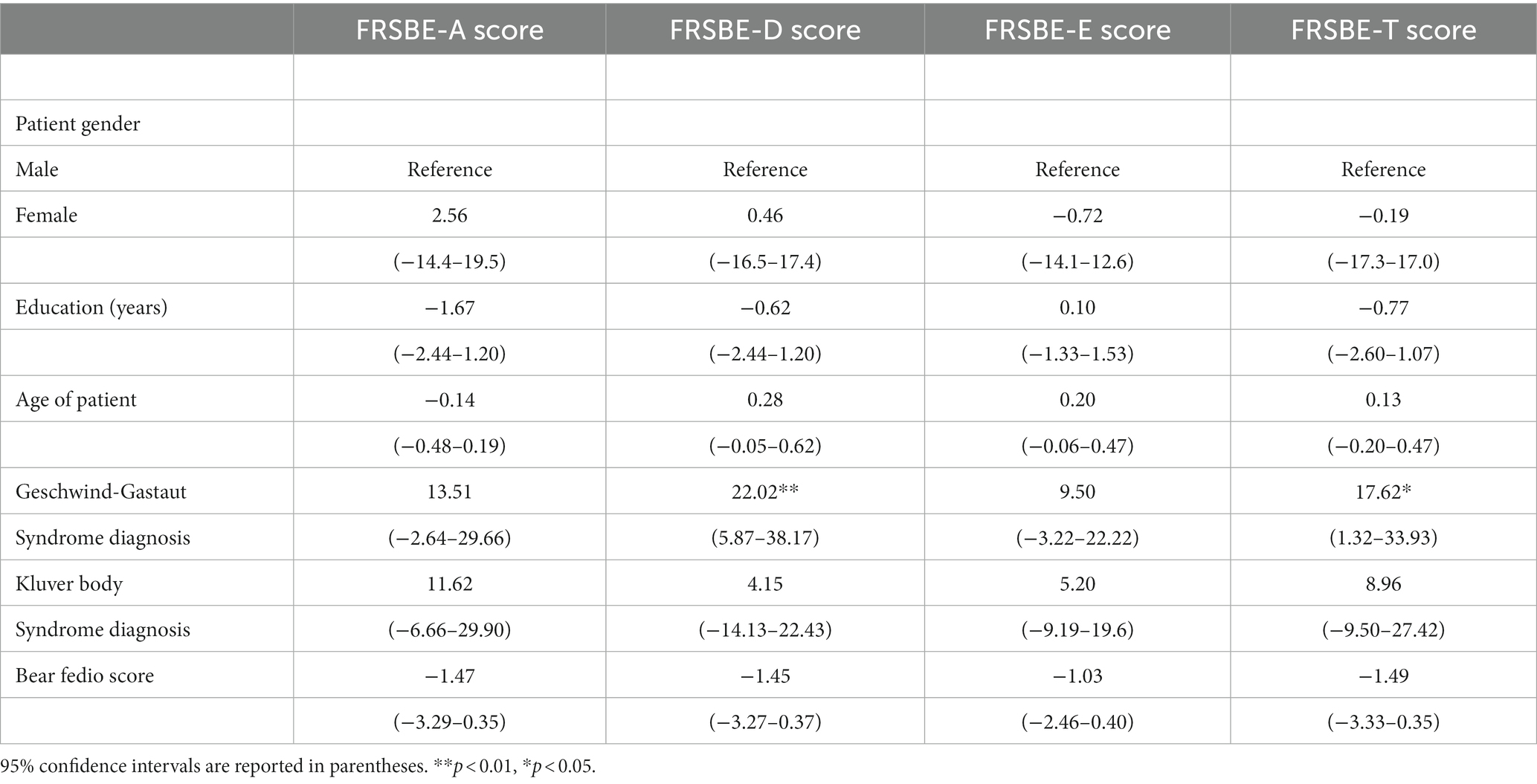
Further behavioral neurological analysis with standardized further metric behavioral neurological evaluation was possible in only 105 of the 200 patients. This was primarily due to inability to perform the tests, lack of follow up, missing data, significant comorbidities or medication related effects hindering reliable testing. Analysis of the etiological groups (Figure 3) revealed significantly different younger age of the TBI and GWI veterans who also had higher MOCA scores, the latter which were overall borderline normal. Both the TBI and GWI groups had significantly different higher MOCA scores compared to the “older” vascular group (Figure 4). The TBI group also had significantly more abnormalities of hypometabolism, noted on the PET brain scans. Table 1 summarizes the means, standard deviations, and p-values from the statistical tests performed for the four different groups. Analysis of the behavioral neurological testing was notable for the findings that once frontotemporal disorder had been diagnosed by the Daphne (or Rascovsky) criteria (42), the four different etiological groups consistently had abnormal FRSBE scores for the 3 principal frontal presentations of (i) abulia/apathy, (ii) disinhibition, and (iii) executive dysfunction as well as abnormal FBI scores. Importantly there was no significant difference amongst the etiological groups (Table 1). This may be regarded as a significant finding underscoring the diagnostic accuracy of the initial FTD diagnosis all of which had similar deficits in the three principal frontal behavioral deficits, namely abulia/apathy, disinhibition and executive dysfunction.
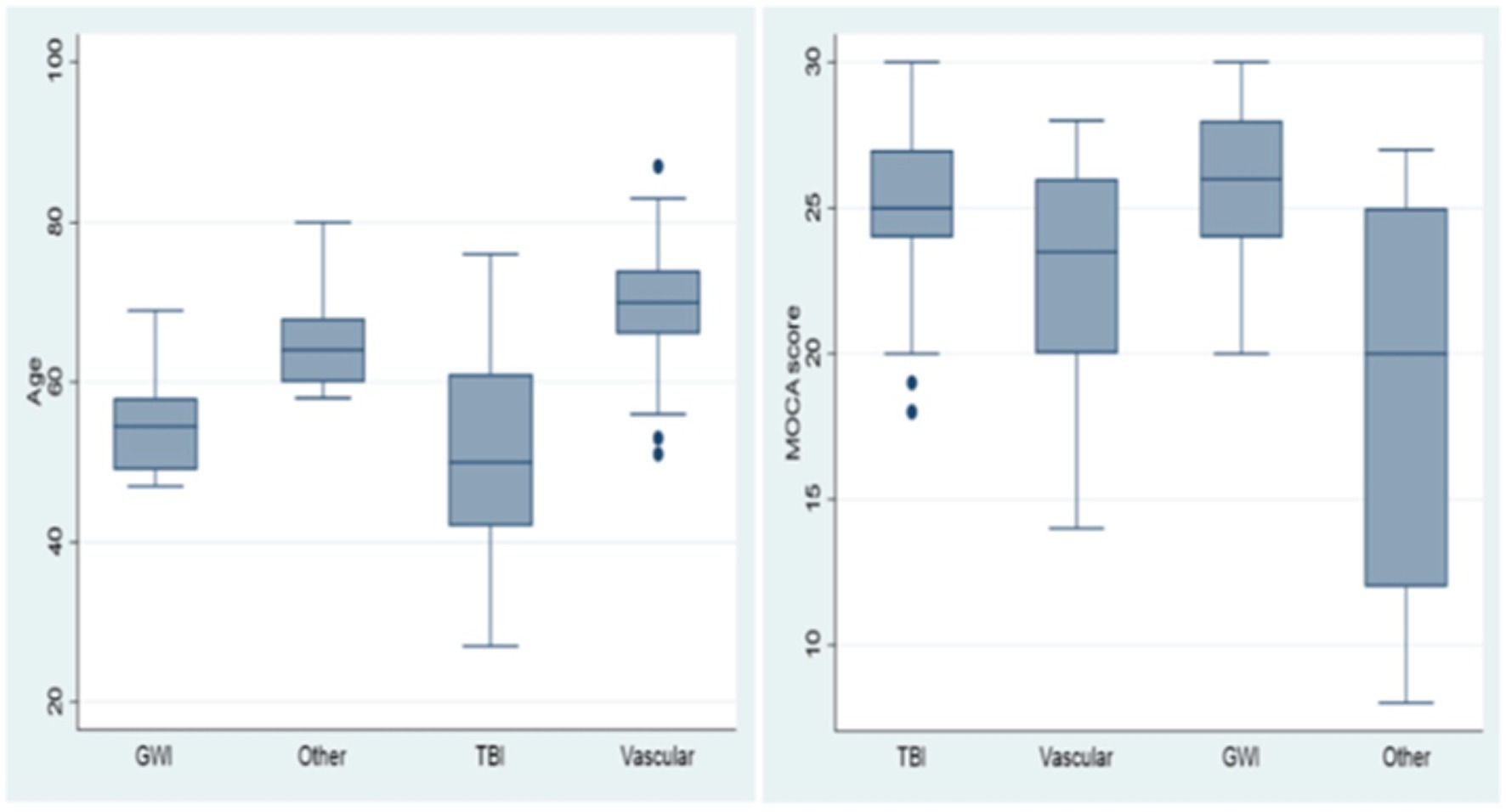
Figure 4. Age and MOCA scores, box and whisker plots. Age and MOCA scores differed significantly in the TBI and GWI groups.
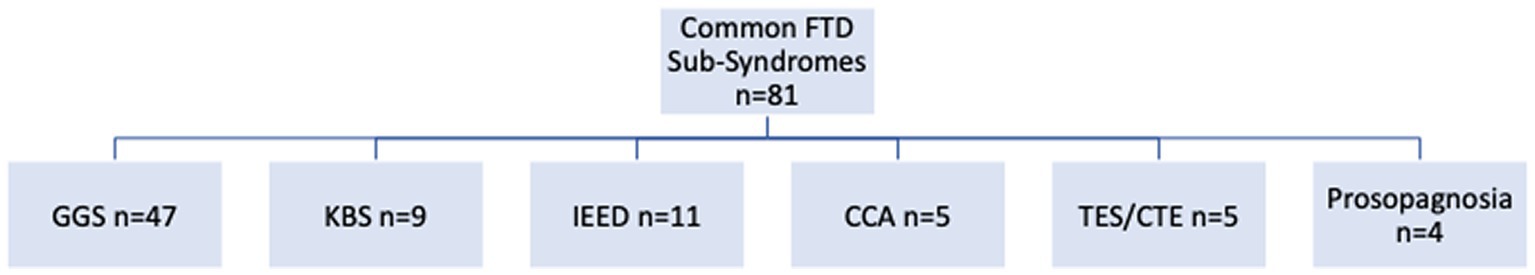
The most common subsyndromes associated with frontotemporal syndromes are depicted in Figure 5. Within this group, GGS was by far the most frequently delineated syndrome. Other relatively common syndromes included KBS, IEED, CCA, TES/CTE and prosopagnosia. Comparisons with the three principal frontal lobe syndrome clusters (abulia, disinhibition, executive dysfunction) revealed a significant association of abnormal disinhibition FRSBE T-scores with the GGS. The regression analysis supported the potential contribution of disinhibition behavior that related to this complex, relatively common behavioral syndrome in this series (Table 2). Linear regression models examining factors associated with FRSBE scores are presented in Table 2. Only patients with GGS had higher FRSBE-D and FRSBE-T scores. The other factors in our models did not have a statistically significant association with FRSBE scores.
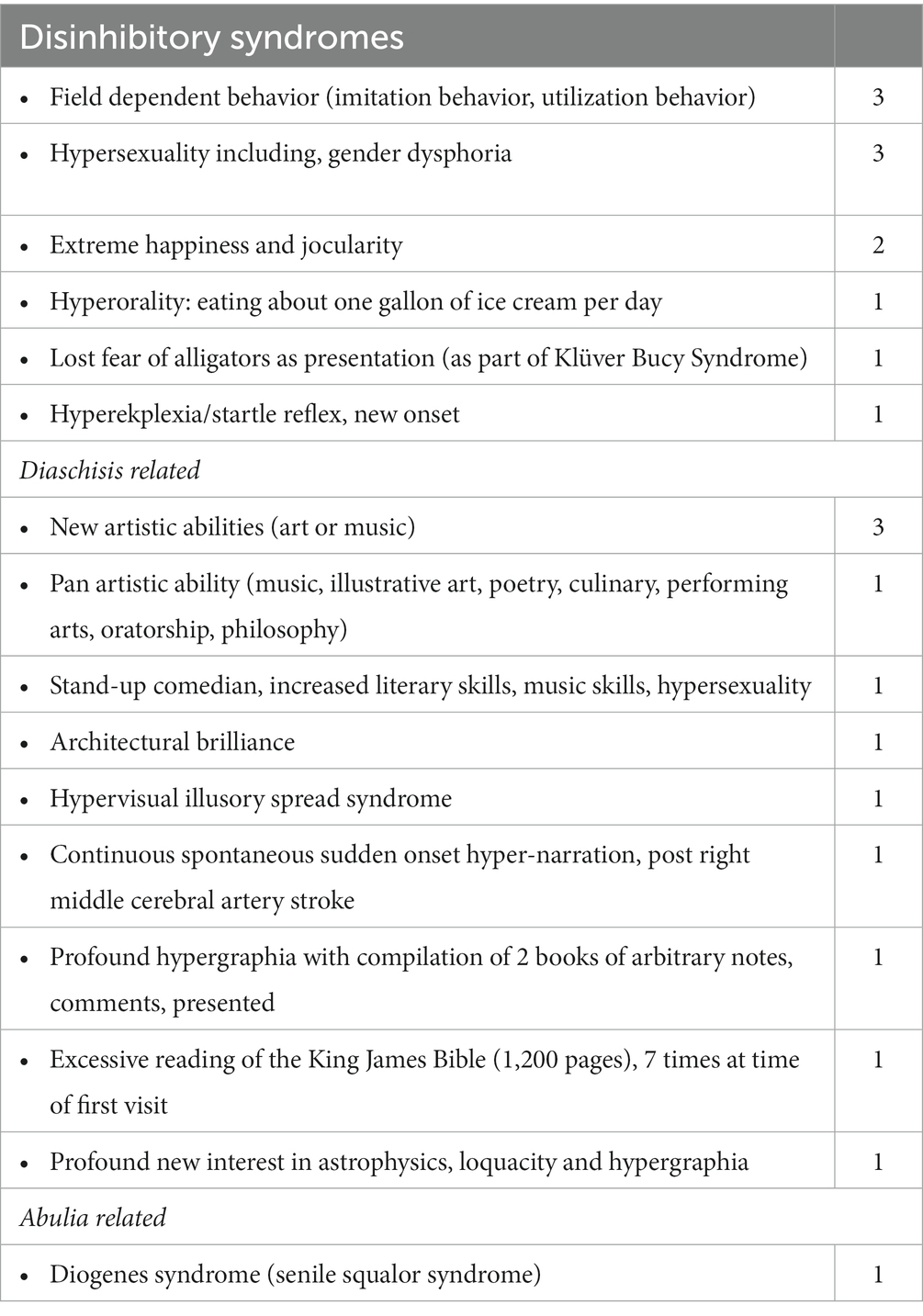
Less common subsyndromes entities are depicted in Table 3. These syndromes, in particular, were notable, as they constituted the initial overriding, presenting symptoms and syndromes characterized into 16 separate conditions. They were conveniently subsumed under the 3 principal frontal syndromes of abulia, disinhibition and executive dysfunction and in addition the presumptive category of diaschisis related syndromes.
Discussion
The main findings of this retrospective analysis from the dedicated OVAMC cognitive neurological clinic included the frequency of FTD and the extensive array of behavioral neurological syndromes and sub-syndromes, embraced under the umbrella of FTD. Importantly, these presented mostly in the context of relatively milder cognitive impairment with the mean MOCA score in the TBI of 25.24 (SD 2.65) and GWI 25.45 (SD3.31), where 26 or greater is regarded as the normal range. In many tertiary medical centers, the MMSE and MOCA are screening tests that triage people with cognitive complaints depending on their scores into further investigations or no investigation at all. In this veteran population, the majority of people with TBI, had FTD, rarely in the dementia category because of relatively preserved basic ADLs and IADLs. Although frontotemporal syndromes may conjure up the more commonly known frontotemporal dementias, this study demonstrates that many of mild to moderate behavioral syndromes, in particular post TBI, post cerebrovascular and post neurotoxicological insults presented with frontotemporal disorders or syndromes and not dementias.
Several additional pertinent findings included the significantly different younger age of the TBI and GWI veterans who also had higher MOCA scores that were overall borderline normal. The MOCA score, the most commonly used cognitive screening study worldwide, being borderline normal in the TBI and GWI groups is important as many members in these groups would have missed being evaluated further in view of the normal MOCA scores, a relatively common practice. The study also presents a remarkable validation of the notable and extensive behavioral neurological repertoire of Andrew Kertesz’s “Banana Lady” exposition (43), wherein he tabulated 17 different frontal network syndromes and based the frontal behavioral inventory test on these findings. Herein we noted a veritable number of 16 differing frontal network syndromes aside from those evaluated by the Frontal Behavioral Inventory evaluation (n = 24). The more common sub-syndromes within the realm of FTD, delineated in this study, included GGS, KBS, DMIS, IEED and prosopagnosia. With regard to GGS, there was a trend toward a greater association with the TBI and GWI categories. At the time of writing, GGS have been reported in association with FTD in only two single case reports (44, 45). However, the remarkable frequency we detected in our retrospective series is noteworthy and warrants further attention as such syndromes are not only important for the person and family to understand, but have potential treatment and management options available. It may also be an important example of cerebral diaschisis with at times increased brain function and even superior or superlative brain functions developing particularly after TBI. Knowing more precise sub-syndromes and their etiologies enables the first step needed to deliver a precision medicine intervention.
From a pathophysiological point of view, the syndromes may be understood, with regards to the perspective of:
1. Anatomical – the particular frontotemporal lesions predilection consequent to TBI.
2. White matter fiber tract level disruption from the anterior temporal lobe to the inferior frontal lobe, the uncinate fasciculus.
3. Network level impairment: salience network.
4. Diaschisis, including subtypes of diaschisis at rest, functional, connectional and connectomal.
The particular predilection of inferior frontal and anterior temporal lobe injury post TBI is worth emphasis as depicted by neuropathological data (Figure 6) (46). This important pathological data has also been confirmed in the neurosurgical literature. Contusion indices in non-missile head injury (n = 151) revealed mean contusion indices (MCI) much more commonly in the frontal (MCI 5.7) and temporal lobes (MCI 5.4) as opposed to the sylvian fissure (MCI 2.7), occipital lobe (MCI1.2), parietal lobe (MCI 0.7) and cerebellum (MCI 0.9) (47).
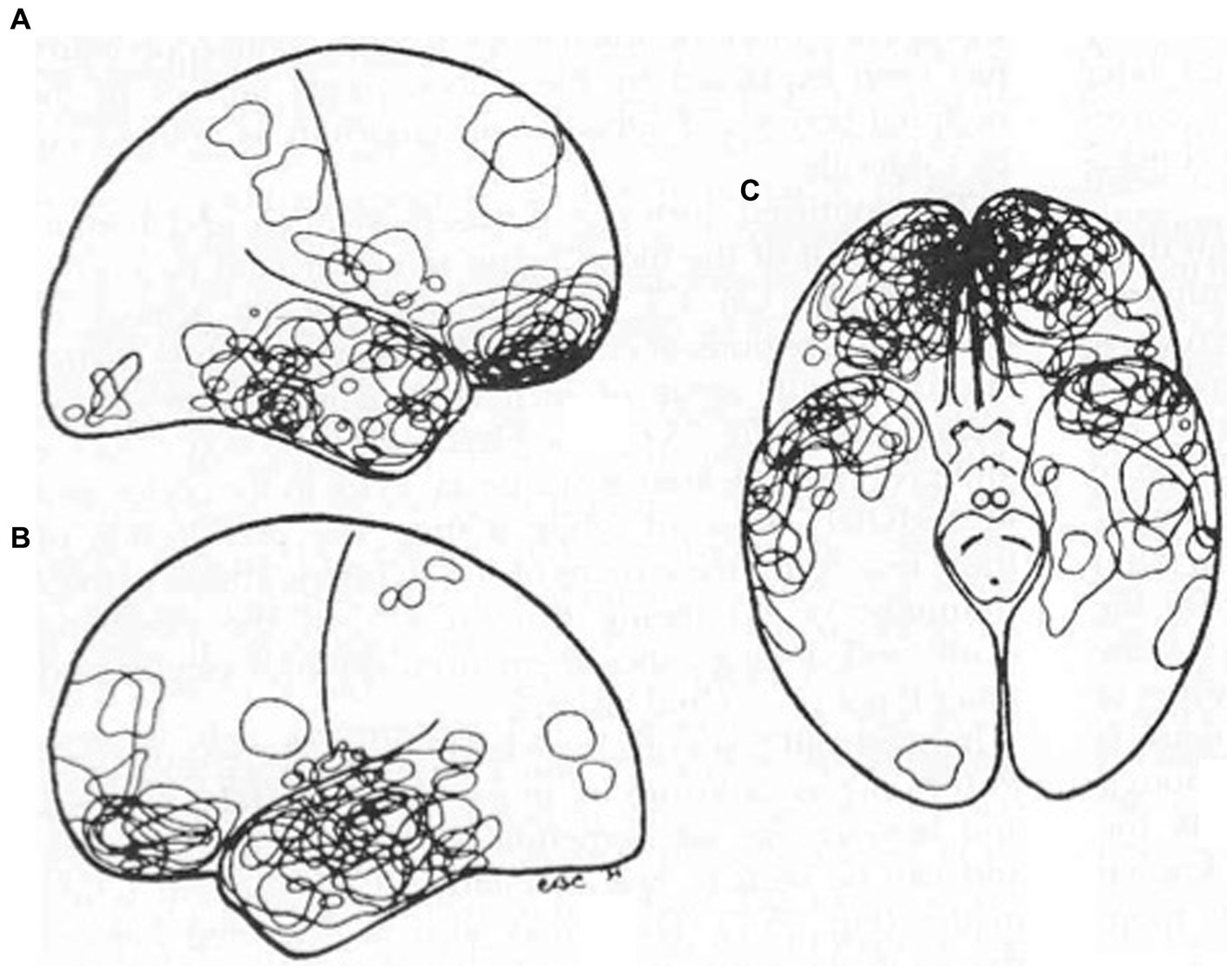
Figure 6. TBI: Fronto-temporal injury predilection distribution of contusions in 40 consecutive autopsy cases. Reproduced from Courville, Pathology of the central nervous system, Part 4 (46).
With regards to GGS, the most common FTD sub-syndrome identified, the underlying abnormality may include: impairment of the anatomical circuit malfunction (uncinate fasciculus), a larger network dysfunction impacting the salience network, or secondary to a right hemisphere lesion, with diaschisis phenomena rendering the syndrome of GGS. The abnormal disinhibition FRSBE T-score association was interesting in that this might be a potential mechanism of diaschisis impacting the complex left temporal lobe cognitive and behavioral processes. An overarching insight premise relates to the extensive array of syndromes that are best explained by the brain network theory of remote injury (von Monokow) in both hodological and hodotopical effects of under-activation and overactivation (48). Brain network science (small world, rich club hubs) and the various hodological effects after lesions with clinically apparent diaschisis syndromes (at rest, functional, connectional) demand that the entire brain be evaluated no matter where the lesion topography (49). These can be accomplished by using metabolic positron emission tomography (PET) and resting state brain network imaging. Using such neuroimaging approaches this may further facilitate more precise diagnoses with more uniform subgroup identification. Furthermore, targeted treatments are then more likely to be successful as the specific brain area or network has been identified. The uncinate fasciculus matures the latest (during the 3rd and 4th decades) of all white matter fiber tracts (50). One may surmise that similar to other tertiary cortical circuitry this major tract may be the most vulnerable of all, to a panoply of neurological insults such as TBI, vascular and neurotoxicological injury. As it links the two of the most significant higher cortical function brain centers (frontal, temporal), the presentation of syndromes such as GGS would not be surprising. Furthermore, the study also illustrates, that, far from only focusing on neurological deficits, neurological hyperfunction in its myriad forms is equally informative and important for treatment prospects. Karl Deisseroth, one of the inventors of optogenetics notably proclaimed that the most accurate and insightful ways of deciphering the human brain is through language and the clinical interview, more so than sophisticated current neuroimaging modalities (51). At the present time, scored questionnaires such as the FRSBE, FBI and BFI are key tools that assist in deciphering these syndromes.
The salience network is a pivotal psychiatric network, linked to the to p-factor (psychopathology factor). The salience network is thought to play an important role, acting as a switch to deploy other major cerebral networks. Hence, lesions that affect this network, as an orchestrator influencing other networks, would have a disproportionate and at the same time may serve as a potential therapeutic target for neuromodulation devices, for example (52).
Behavioral and cognitive disorders are especially prone to failure in the constant stimulation and complicated nature that is part of the information age. This requires sustained attention, effective executive function and insightful reasoning for optimal decision-making, appropriate impulse control and inhibition of responses (53). The extensive networks and highly evolved cells, such as pyramidal, spindle and fork cells, that are characteristic of the association cortices, in particular, are targeted. No effective treatments currently exist, and the pharmaceutical industry has recently de-emphasized research in these areas. The reasons include the complexity, the uniquely significant primate association as opposed to the traditional rodent models, that have minimal association cortices. As FTD syndromes originate in the most plastic areas of the brain, these may also hold the promise of providing opportunities for future successful interventions (54). Recent wars have highlighted two signature syndromes that afflict a significant number of deployed personnel. Both affect brain regions with syndromes that are difficult to diagnose and treat. Mild and moderate traumatic brain injury (mTBI) and GWI fall within the domain of FTD (7, 39, 55). These constitute a panoply of disorders, where the presentation is predominantly behavioral, more so than with cognitive impairments. Decoding these syndromes and their causes, are pivotal to potentially effective treatments.
How to make sense of this extensive panoply of syndrome? Frontotemporal syndromes can be viewed as a generic syndrome that encompasses and at times overlaps with several other neurological and neuropsychiatric conditions. Almost all can be subserved under the three principles frontal syndromes of abulia, disinhibition and executive dysfunction in various combinations (Figure 7). Importantly, FTD commonly co-occur with several others, both neurological and neuropsychiatric syndrome. The study also allows additional insights into these FTD syndrome complexes. For example, suicide rates were reported to be 56% higher in Veterans with TBI when compared to veterans without TBI. Suicide rates in US military veterans increased greater than 10-fold from 2006 to 2020 with presumed associations being more frequent mental health conditions, substance abuse, and firearm related violence gun violence (56). This study provides, perhaps an even more likely possibility. The predilection of TBI for the frontotemporal/uncinate fasciculus/salience network components harbor inhibitory circuitry, impulse control, as well as emotional regulation. A very plausible explanatory factor may related to the dysregulation of this important control circuitry which might explain the propensity for suicidal incidents.

Figure 7. Frontotemporal disorders as a generic disorder encompassing many other associated syndromes. GGS - Geschwind-Gastaut syndrome, KBS - Klüver-Bucy syndrome, DMIS - delusional misidentification syndrome, FDB - field dependent behavior (imitation and utilization behavior), TES/CTE - traumatic encephalopathy/chronic encephalopathy syndrome.
Potential limitations and strengths of the review
The most important shortcoming of this study includes the lack of comparison group. Data collection was often incomplete and there was susceptibility to selection and measurement bias. Another limitation pertains to the imprecise assessment of both PTSD and migraine in this population and this data could not be reliably included. Migraine PTSD/depression/anxiety were commonly encountered in this population group. Although these syndromes were ubiquitous syndromes in the FTD population as a whole, more careful delineation amongst the subgroups were however not possible because of a wide variety of heterogeneous assessment scales. Reports from civilian TBI populations are known to be associated with PTSD in almost half the patients (57). The small number of women represented (n = 10) although all had the same comprehensive evaluations, does not allow further analysis of gender specific differences and is likely due to the predominance of combat related veterans in this analysis.
Scientific analysis may be regarded as having two basic approaches; the traditional hypothesis driven approach or one that uses a data driven approach. The former has a specific prediction based on a proposed hypothesis and is based on intuition. The data driven approach uses extensive data accrued which enables detection of specific patterns allowing a much more veritable hypothesis formulation. A major advantage of a case series, such as the present one, is that it can be regarded as a screening tool for the most plausible hypotheses that merit further investigation. Hence the strengths of this case series include; high external validity, a wide range of patients sample, the study was inexpensive, short in duration and no interference in the treatment process. A case series has specific advantages in generating new hypotheses and treatment efficacy and the external validity of a case series frequently exceeds that of a randomized controlled trial.
Future recommendations: a precision medicine approach to cognitive and dementia care syndrome, etiology, and co-pathology
Increasingly a precision medicine approach is being heralded both from a syndrome and pathological treatment point of view. Recognizing multiple neuropathological entities in people with dementia improves understanding of diagnosis, prognosis, and expected outcomes from therapies (58). Rapidly accruing evidence is emerging for the efficacy of realm of lifestyle/behavioral interventions in most chronic neurological disease including cognitive impairment, Alzheimer’s disease, Parkinsons and more recently frontotemporal dementias (59–61). In addition, a surge of recent neuromodulatory device-based interventions such as magnetic and electrical brain stimulation devices, have shown promise for conditions such as Alzheimer’s, depression, schizophrenia and PTSD for example (62–65). However, a precision medicine type approach is first required to decipher the most likely underlying etiology, whether vascular compromise, biochemical deficiencies or neurotoxicological factors and eliminate these as a first step, if possible. For this reason, one of the objectives of this analysis was to pave the way for the establishment of a registry of people with FTD with deconstructing the FTD syndromes, similar to what Stephen Stahl, amongst others, have long proposed in neuropsychiatric disease (66). Once biochemical, toxins, infections and inflammatory abnormalities have been corrected wherever possible, the lifestyle/behavioral interventions can be initiated and monitored for success. The earliest neurobiological defect of cognitive disorders may well be at the neurovascular level with both clinical and neuroimaging studies supporting impaired cerebrovascular reactivity impairment as the first sign of compromise (67). This underscores the essential role of physical exercise which induces both generalized cardiovascular and cerebrovascular health as well as neurotrophic factors that lead to neurogenesis and augment brain circuitry (68). Physical exercise has particularly potent brain protective effects and has been shown to reduce the incidence of dementia by up to 50%. Healthy diet adherence, such as the various categories of keto type low carbohydrate, MIND diet and Mediterranean-type diet have consistently shown reduction in cardiovascular disease, cancer and dementia (69). Working memory may be regarded as the core frontal lobe function central to all other processes including attention, memory, executive function and inhibition, that improve with cognitive exercises that have been developed, such as Brain HQ and Cogmed computerized programs. Once optimization of lifestyle/behavioral factors has been attained, augmentation of the brain’s plasticity with neuromodulation (t-DCS, TMS, noninvasive vagal stimulation) devices may be implemented in an intervention group and a control group in groups of similar etiology, with greater chance of success. This has recently been confirmed in a pivotal study specifically for frontotemporal degenerations. FTD neurophysiological oscillatory signatures of gamma and theta to alpha wave coupling have been identified, opening the way for both pharmacological targets and neuromodulation interventions (70).
Frontotemporal syndromes, emanating from TBI and GWI pathophysiological processes, occur in the most sensitive and yet most plastic areas of the brain, with the frontal and anterior temporal lobes being preferentially vulnerable to degeneration during a person’s lifespan. However, the emerging concepts that the cerebral networks are impacted, as opposed to only focal lesions, are in support of lifestyle and vascular health promotion that can modify this aging process (71). The brain has tremendous neuroplasticity capability, having 4 x the plasticity of muscles, for example (72). An important recent study in FTD amelioration with physical exercise, emphasizes the efficacy and critical role of physical exercise (59). The accompanying editorial entitled that “diagnosis is not destiny” underscored the pivotal impact of physical exercise in people with FTD despite having potentially disadvantageous genotypes (73).
Conclusion
By deconstructing FTD into the multiple subsyndromes and differing etiologies, this study may provide foundational insights, enabling a more targeted precision medicine approach for future studies, both in treating the sub-syndromes as well as the underlying etiological process.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by VHA Institutional Research Board Approval IRBNET ID: 1256151-4. Approval date: 04/11/2020. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because retrospective analysis of existing data.
Author contributions
MH: Conceptualization, Data curation, Investigation, Methodology, Resources, Supervision, Writing – original draft. FR: Data curation, Investigation, Writing – review & editing. LB: Investigation, Writing – review & editing. CK: Formal analysis, Software, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Orlando VA Healthcare System (Orlando, Florida).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The contents of this publication “do not represent the views of the Department of Veterans Affairs or the United States Government.”
References
1. Bang, J, Spina, S, and Miller, BL. Frontotemporal dementia. Lancet. (2015) 386:1672–82. doi: 10.1016/S0140-6736(15)00461-4
2. Pick, A. Uber die Beziehungen der senilen Hirnatrophie zur Aphasie. Prag Med Wochenschr. (1892) 17:165–7.
3. Kipps, CM, Hodges, JR, and Hornberger, M. Non-progressive, behavioural frontotemporal dementia: recent developments and clinical implications of the 'bv-FTD phenocopy syndrome'. Curr Opin Neurol. (2010) 23:628–32. doi: 10.1097/WCO.0b013e3283404309
4. Logroscino, G, Piccininni, M, Graff, C, Hardiman, O, Ludolph, AC, Moreno, F, et al. Incidence of syndromes associated with frontotemporal lobar degeneration in 9 European countries. JAMA Neurol. (2023) 80:279–86. doi: 10.1001/jamaneurol.2022.5128
5. Kalkonde, YV, Jawaid, A, Qureshi, SU, Shirani, P, Wheaton, M, Pinto-Patarroyo, GP, et al. Medical and environmental risk factors associated with frontotemporal dementia: a case-control study in a veteran population. Alzheimers Dement. (2012) 8:204–10. doi: 10.1016/j.jalz.2011.03.011
6. Wang, HK, Lee, YC, Huang, CY, Liliang, PC, Lu, K, Chen, HJ, et al. Traumatic brain injury causes frontotemporal dementia and TDP-43 proteolysis. Neuroscience. (2015) 300:94–103. doi: 10.1016/j.neuroscience.2015.05.013
7. Greer, N, Ackland, P, Sayer, N, Spoont, M, Taylor, B, MacDonald, R, et al. Relationship of deployment-related mild traumatic brain injury to posttraumatic stress disorder, depressive disorders, substance use disorders, suicidal ideation, and anxiety disorders: a systematic review. Washington, DC: Department of Veterans Affairs (US) (2019).
8. Loignon, A, Ouellet, MC, and Belleville, G. A systematic review and meta-analysis on PTSD following TBI among military/veteran and civilian populations. J Head Trauma Rehabil. (2020) 35:E21–35. doi: 10.1097/HTR.0000000000000514
9. White, RF, Steele, L, O'Callaghan, JP, Sullivan, K, Binns, JH, Golomb, BA, et al. Recent research on gulf war illness and other health problems in veterans of the 1991 gulf war: effects of toxicant exposures during deployment. Cortex. (2016) 74:449–75. doi: 10.1016/j.cortex.2015.08.022
10. Rosso, SM, Landweer, E-J, Houterman, M, Donker Kaat, L, van Duijn, C, and van Swieten, J. Medical and environmental risk factors for sporadic frontotemporal dementia: a retrospective case–control study. J Neurol Neurosurg Psychiatry. (2003) 74:1574–6. doi: 10.1136/jnnp.74.11.1574
11. Torralva, T, Sposato, LA, Riccio, PM, Gleichgerrcht, E, Roca, M, Toledo, JB, et al. Role of brain infarcts in behavioral variant frontotemporal dementia: clinicopathological characterization in the National Alzheimer’s coordinating center database. Neurobiol Aging. (2015) 36:2861–8. doi: 10.1016/j.neurobiolaging.2015.06.026
12. Martinez, S, Yaffe, K, Li, Y, Byers, AL, Peltz, CB, and Barnes, DE. Agent orange exposure and dementia diagnosis in US veterans of the Vietnam era. AMA Neurol. (2021) 78:473–7. doi: 10.1001/jamaneurol.2020.5011
13. Leypoldt, F, Gelderblom, M, Schöttle, D, Hoffmann, S, and Wandinger, KP. Recovery from severe frontotemporal dysfunction at 3 years after N-methyl-d-aspartic acid (NMDA) receptor antibody encephalitis. J Clin Neurosci. (2013) 20:611–3. doi: 10.1016/j.jocn.2012.03.036
14. Wicklund, MR, Mokri, B, Drubah, DA, Boeve, BF, Parisi, JE, Josephs, KA, et al. Frontotemporal brain sagging syndrome. An SIH-like presentation mimicking FTD. Neurology. (2011) 76:1377–82. doi: 10.1212/WNL.0b013e3182166e42
15. Benito-León, J, Sedano, LF, and Louis, ED. Isolated central nervous system Whipple's disease causing reversible frontotemporal-like dementia. Clin Neurol Neurosurg. (2008) 110:747–9. doi: 10.1016/j.clineuro.2008.04.014
16. Heyburn, L, Sajja, VSS, and Long, JB. The role of TDP-43 in military-relevant TBI and chronic neurodegeneration. Front Neurol. (2019) 10:680. doi: 10.3389/fneur.2019.00680
17. Petersen, RC, Lopez, O, Armstrong, MJ, Getchius, TSD, Ganguli, M, Gloss, D, et al. Practice guideline update summary: mild cognitive impairment. Neurology. (2018) 90:126–35. doi: 10.1212/WNL.0000000000004826
18. Li, Z, Heckman, MG, Kanekiyo, T, Martens, YA, Day, GS, Vassilaki, M, et al. Clinicopathologic factors associated with reversion to Normal cognition in patients with mild cognitive impairment. Neurology. (2022) 98:e2036–45. doi: 10.1212/WNL.0000000000200387
19. Ismail, Z, Agüera-Ortiz, L, Brodaty, H, Cieslak, A, Cummings, J, Fischer, CE, et al. The mild behavioral impairment checklist (MBI-C): a rating scale for neuropsychiatric symptoms in pre-dementia populations. J Alzheimers Dis. (2017) 56:929–38. doi: 10.3233/JAD-160979
20. Seeley, WW, Crawford, RK, Zhou, J, Miller, BL, and Greicius, MD. Neurodegenerative disease target large scale human brain networks. Neuron. (2009) 62:42–52. doi: 10.1016/j.neuron.2009.03.024
21. Hoffmann, M, Schmitt, F, and Bromley, E. Comprehensive cognitive neurological assessment in stroke. Acta Neurol Scand. (2009) 119:162–71. doi: 10.1111/j.1600-0404.2008.01101
22. Nasreddine, ZS, Phillips, NA, Bedirian, V, Charbonneau, S, Whitehead, V, Collin, I, et al. The Montreal cognitive assessment (MoCA): a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
23. Boutoleau-Bretonnière, C, Evrard, C, Hardouin, JB, Rocher, L, Charriau, T, Etcharry-Bouyx, F, et al. DAPHNE: a new tool for the assessment of the behavioral variant of frontotemporal dementia. Dement Geriatr Cogn Disord Extra. (2015) 5:503–16. doi: 10.1159/000440859
24. Katz, S. Assessing self-maintenance: activities of daily living, mobility and instrumental activities of daily living. JAGS. (1983) 31:721–7. doi: 10.1111/j.1532-5415.1983.tb03391.x
25. Grace, J, and Malloy, PF. Frontal systems behavior scale (FrSBe): professional manua. Lutz, FL: Psychological Assessment Resources (2001).
26. Kertesz, A, Davidson, W, and Fox, H. Frontal behavioural inventory: diagnostic criteria for frontal lobe dementia. Can J Neurol Sci. (1997) 24:29–36. doi: 10.1017/S0317167100021053
27. Kaplan, E, Goodglass, H, and Weintraub, S. The Boston naming test. 2nd ed. Philadelphia: Lippincott Williams & Wilkins (2001).
28. Bear, DM, and Fedio, P. Quantitative analysis of interictal behavior in temporal lobe epilepsy. Arch Neurol. (1977) 34:454–67. doi: 10.1001/archneur.1977.00500200014003
29. Lilly, R, Cummings, JL, Benson, DF, and Frankel, M. The human Klüver‐Bucy syndrome. Neurology. (1983) 33:1141–5. doi: 10.1212/WNL.33.9.1141
30. Clay, FJ, Kuriakose, A, Lesche, D, Hicks, AJ, Zaman, H, Azizi, E, et al. Klüver-Bucy syndrome following traumatic brain injury: a systematic synthesis and review of pharmacological treatment from cases in adolescents and adults. J Neuropsychiatry Clin Neurosci. (2019) 31:6–16. doi: 10.1176/appi.neuropsych.18050112
31. Trimble, M, and Freeman, A. An investigation of religiosity and the Geschwind-Gastaut syndrome in patients with temporal lobe epilepsy. Epilepsy Behav. (2006) 9:407–14. doi: 10.1016/j.yebeh.2006.05.006
32. Devinsky, O. Delusional misidentification s and duplications: right brain lesions, left brain delusions. Neurology. (2009) 72:80–7. doi: 10.1212/01.wnl.0000338625.47892.74
33. Cummings, JL. Involuntary emotional expression disorder: definition, diagnosis, and measurement scales. CNS Spectr. (2007) 12:11–6. doi: 10.1017/S1092852900025967
34. De Smet, HJ, Paquir, P, and Verhoeven, J. The cerebellum: its role in language and related cognitive and affective functions. Brain Language. (2013) 127:334–42. doi: 10.1016/j.bandl.2012.11.001
35. Montenigro, PH, Baugh, CM, Daneshvar, DH, Mez, J, Budson, AE, Au, R, et al. Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimers Res Ther. (2014) 6:68. doi: 10.1186/s13195-014-0068-z
36. Murley, AG, Coyle-Gilchrist, I, Rouse, MA, Jones, PS, Li, W, Wiggins, J, et al. Redefining the multidimensional clinical phenotypes of frontotemporal lobar degeneration syndromes. Brain. (2020) 143:1555–71. doi: 10.1093/brain/awaa097
37. Centers for Disease Control and Prevention and National Center for Injury Prevention and Control. Report to congress on traumatic brain injury in the United States: epidemiology and rehabilitation. Atlanta, GA: Centers for Disease Control and Prevention (2015).
38. Sachdev, P, Kalaria, R, and O’Brien, J. Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord. (2014) 28:206–18. doi: 10.1097/WAD.0000000000000034
39. Haley, RW, and Kurt, L. Self reported exposure to neurotoxic chemical combinations in the Gulf War. A cross sectional epidemiologic study. JAMA. (1997) 277:231–7.
40. McKhann, GM, Knopman, DS, Chertkow, H, Hyman, Bradley T, Jack, Clifford R Jr, and Kawas, Claudia H, et al. (2011). The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement ; 7, 263–269. doi: 10.1016/j.jalz.2011.03.005
41. McKeith, IG, Dickson, DW, Lowe, J, Emre, M, O'Brien, JT, Feldman, H, et al. Consortium on DLB. Diagnosis and management of dementia with Lewy bodies. Neurology. (2005) 65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1
42. Rascovsky, K, Hodges, JR, Knopman, D, Mendez, MF, Kramer, JH, Neuhaus, J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. (2011) 134:2456–77. doi: 10.1093/brain/awr179
44. Postiglione, A, Milan, G, Pappatà, S, de Falco, C, Lamenza, F, Schiattarella, V, et al. Fronto-temporal dementia presenting as Geschwind’s syndrome. Neurocase. (2008) 14:264–70. doi: 10.1080/13554790802269976
45. Veronelli, L, Makaretz, SJ, Quimby, M, Dickerson, BC, and Collins, JA. Geschwind syndrome in frontotemporal lobar degeneration: neuroanatomical and neuropsychological features over 9 years. Cortex. (2017) 94:27–38. doi: 10.1016/j.cortex.2017.06.003
46. Courville, CB. Pathology of the central nervous system, Part 4. Mountain View, CA: Pacific Press Publishing (1937).
47. Adams, JH, Graham, DI, Scott, G, Parker, LS, and Doyle, D. Brain damage in fatal non-missile head injury. J Clin Pathol. (1980) 33:1132–45. doi: 10.1136/jcp.33.12.1132
48. Carrera, E, and Tononi, G. Diaschisis: past, present and future. Brain. (2014) 137:2408–22. doi: 10.1093/brain/awu101
49. Bullmore, E, and Sporns, O. The economy of brain network organization. Nat Neurosci Rev. (2012) 13:336–49. doi: 10.1038/nrn3214
50. Lebel, C, Walker, L, Leemans, A, Phillips, L, and Beaulieu, C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. (2008) 40:1044–55. doi: 10.1016/j.neuroimage.2007.12.053
52. Downar, J, Blumberger, DM, and Daskalakis, ZJ. The neural crossroads of psychiatric illness: an emerging target for brain stimulation. Trends Cogn Sci. (2016) 20:107–20. doi: 10.1016/j.tics.2015.10.007
53. Arnsten, AFT, and Wang, M. Targeting prefrontal cortical systems for drug development: potential therapies for cognitive disorders. Annu Rev Pharmacol Toxicol. (2016) 56:339–60. doi: 10.1146/annurev-pharmtox-010715-103617
54. Seeley, WW. Selective functional, regional and neuronal vulnerability in frontotemporal dementia. Curr Opin Neurol. (2008) 21:701–7. doi: 10.1097/WCO.0b013e3283168e2d
55. Abdullah, L, Evans, JE, Montague, H, Reed, JM, Moser, A, Crynen, G, et al. Chronic elevations of phosphocholine containing lipids in mice exposes to gulf war agents pyridostigmine bromide and permethrin. Neurotoxicol Teratol. (2013) 40:74–84. doi: 10.1016/j.ntt.2013.10.002
56. Howard, JT, Stewart, IJ, Amuan, ME, Janak, JC, Howard, KJ, Pugh, MJ, et al. Trends in suicide rates among Post-9/11 US military veterans with and without traumatic brain injury from 2006-2020. JAMA Neurol. (2023) 80:1117–9. doi: 10.1001/jamaneurol.2023.2893
57. Iljazi, A, Ashina, H, Al-Khazali, HM, Lipton, RB, Ashina, M, Schytz, HW, et al. Post-traumatic stress disorder after traumatic brain injury—a systematic review and meta-analysis. Neurol Sci. 41:2737–46. doi: 10.1007/s10072-020-04458-7
58. Köbe, T, Witte, AV, Schnelle, A, Lesemann, A, Fabian, S, Tesky, VA, et al. Combined omega-3 fatty acids, aerobic exercise and cognitive stimulation prevents decline in gray matter volume of the frontal, parietal and cingulate cortex in patients with mild cognitive impairment. Neuroimage. (2016) 131:226–38. doi: 10.1016/j.neuroimage.2015.09.050
59. Casaletto, KB, Kornack, J, Paolillo, EW, Rojas, JC, VandeBunte, A, Staffaroni, AS, et al. Association of physical activity with neurofilament light chain trajectories in autosomal dominant frontotemporal lobar degeneration variant carriers. JAMA Neurol. 80:82–90. doi: 10.1001/jamaneurol.2022.4178
60. Ahlskog, JE, Geda, YE, Graff-Radford, NR, and Petersen, RC. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. (2011) 86:876–84. doi: 10.4065/mcp.2011.0252
61. Ngandu, T, Lehtisalo, J, Solomon, A, Levälahti, E, Ahtiluoto, S, Antikainen, R, et al. A 2-year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. (2015) 385:2255–63. doi: 10.1016/S0140-6736(15)60461-5
62. Fregni, F, and Pascual-Leone, A. Technology insight: noninvasive brain stimulation in neurology: perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol. (2007) 3:383–93. doi: 10.1038/ncpneuro0530
63. Lindenberger, U, Wenger, E, and Lövden, M. Towards a stronger science of human plasticity. Nat Rev Neurosci. (2017) 18:261–2. doi: 10.1038/nrn.2017.44
64. Sanders, TH, Weiss, J, Hogewood, L, Chen, L, Paton, C, McMahan, R, et al. Cognition-enhancing vagus nerve stimulation alters the epigenetic landscape. J Neurosci. (2019) 39:3454–69. doi: 10.1523/JNEUROSCI.2407-18.2019
65. Broncel, A, Brocian, R, Kłos-Wojtczak, P, Kulbat-Warycha, K, and Konopacki, J. Vagal nerve stimulation as a promising tool in the improvement of cognitive disorders. Brain Res Bull. (2020) 155:37–47. doi: 10.1016/j.brainresbull.2019.11.011
66. Stahl, SM. Deconstructing psychiatric disorders, part 2: an emerging, neurobiologically based therapeutic strategy for the modern psychopharmacologist. Clin Psychiatry. (2003) 64:1145–6. doi: 10.4088/JCP.v64n1001
67. Fiturria-Medina, Y, Sotero, RC, Toussaint, PJ, Mateos-Pérez, JM, Evans, AC, Weiner, MW, et al. Early role of vascular dysregulation on late onset Alzheimer’s disease based on multifactorial data driven analysis. Nat Commun. (2016) 7:11934. doi: 10.1038/ncomms11934
68. Mendez Colmenares, A, Voss, MW, Fanning, J, Salerno, EA, Gothe, NP, Thomas, ML, et al. White matter plasticity in healthy older adults: the effects of aerobic exercise. Neuroimage. (2021) 239:118305. doi: 10.1016/j.neuroimage.2021.118305
69. Jia, J, Zhao, T, Liu, Z, Liang, Y, Li, F, Li, Y, et al. Association between healthy lifestyle and memory decline in older adults: 10-year, population based, prospective cohort study. BMJ. (2023) 380:e072691. doi: 10.1136/bmj-2022-072691
70. Hughes, LE, Rittman, T, Robbins, TW, and Rowe, JB. Reorganization of cortical oscillatory dynamics underlying disinhibition in frontotemporal dementia. Brain. (2018) 141:2486–99. doi: 10.1093/brain/awy176
71. Bonnelle, V, Ham, TE, Leech, R, Kinnunen, KM, Mehta, MA, Greenwood, RJ, et al. Salience network integrity predicts default mode network function after traumatic brain injury. PNAS. (2020) 109:4690–5. doi: 10.1073/pnas.1113455109
72. Smeets, JSJ, Horstman, AMH, Schijns, OEM, Dings, JTA, Hoogland, G, Gijsen, AP, et al. Brain tissue plasticity: protein synthesis rates of the human brain. Brain. (2018) 141:1122–9. doi: 10.1093/brain/awy015
Keywords: frontotemporal lobe disorders (FTD), etiological categories, veterans, cardiovascular risk factors, behavioral neurological evaluation
Citation: Hoffmann M, Rossi F, Benes Lima L and King C (2024) Frontotemporal disorders: the expansive panoply of syndromes and spectrum of etiologies. Front. Neurol. 14:1305071. doi: 10.3389/fneur.2023.1305071
Edited by:
Mary Jo Pugh, The University of Utah, United StatesReviewed by:
Hari Sagiraju, All India Institute of Medical Sciences, IndiaSamin Panahi, University of Utah Hospital, United States
Eamonn Kennedy, The University of Utah, United States
Copyright © 2024 Hoffmann, Rossi, Benes Lima and King. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Hoffmann, bWljaGFlbC5Ib2ZmbWFubkB1Y2YuZWR1
 Michael Hoffmann
Michael Hoffmann Fabian Rossi1,3
Fabian Rossi1,3 Christian King
Christian King