
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 24 November 2023
Sec. Multiple Sclerosis and Neuroimmunology
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1297709
This article is part of the Research TopicComorbidity in Multiple Sclerosis and Related DisordersView all 9 articles
Introduction: Multiple sclerosis (MS) is a neurodegenerative disease accumulating disabilities over time. However, the mean age of individuals with MS is increasing, consequently elevating their risk of developing comorbidities. Comorbidities' impact on MS is widely debated. Yet very few countries possess population-based registries, which provide unique opportunities for individual-level data linkage. This study aims to assess acute and chronic comorbidities among elderly patients with MS, comparing them to matched controls. Additionally, this study seeks to investigate the influence of chronic comorbidities on all-cause mortality.
Methods: A nationwide register-based study using the Danish Multiple Sclerosis Registry to identify all living patients with MS older than 50 years at the reference date (January 1st, 2022). Patients were matched 1:10 with individuals from the general population. Comprehensive healthcare data within the Danish hospital system were obtained. Chronic comorbidities were classified according to the Charlson Comorbidity Index, while acute comorbidities were based on ICD-10 codes and an “acute” admission type. To investigate all-cause mortality, a Cox regression analysis was conducted.
Results: The study encompassed a total of 8,688 individuals with MS, matched with 86,880 controls. The mean age was 63.5 years, with females constituting 68.3%. Individuals with MS exhibited a higher frequency of acute hospitalizations (OR: 2.1, 95% CI: 1.9–2.2), primarily due to various infectious diseases, along with longer median hospital stays (4 vs. 3 days, p < 0.001). When assessed using the Charlson Comorbidity Index, individuals with MS carried a significantly greater burden of chronic comorbidities (p < 0.001). The most prevalent chronic comorbidity among individuals with MS was “Uncomplicated Diabetes” (20.1%). Notably, while individuals with MS displayed an overall lower 5-year survival rate, this difference ceased to be statistically significant among those with a high Charlson Comorbidity Index score of ≥4 (p = 0.32).
Conclusion: This study highlights a heightened prevalence of both acute and chronic comorbidities among individuals with MS, with chronic comorbidities significantly increasing the risk of mortality. These findings underscore the critical importance of factoring in comorbidities when devising treatment strategies for individuals living with MS.
In recent decades, there has been an increase in the mean age of people with multiple sclerosis (MS), attributed to various contributing factors (1, 2). These factors include the growing incidence of late-onset MS, characterized by the manifestation of initial clinical symptoms after the age of 50 years (1, 2). Additionally, advancements in disease-modifying therapies (DMTs), improved supportive care, developments in diagnostics, and a general rise in life expectancy across the population have played significant roles. However, it is crucial to recognize that advanced age also serves as a risk factor for comorbidities.
Considering that nearly half of the Danish MS population is older than 50 years (median age: 54.9, SD: 14.5, data from the Danish Multiple Sclerosis Registry), it has become important to explore the prevalence and impact of comorbidities on mortality within elderly people with MS. The investigation of comorbidities in people with MS has gained substantial attention in recent years due to its influence on disease activity and other clinical outcomes (3–5). Moreover, a higher burden of concurrent diseases often correlates with poorer prognostic outcomes, and comorbidities contribute to delays in MS diagnosis and can impact the initiation of DMTs (6–9).
The specific characteristics and challenges faced by elderly individuals with MS and comorbidities remain inadequately described. This is primarily due to the exclusion of people older than 55 years in many clinical trials. Furthermore, substantial comorbidities often serve as exclusion criteria, making this group of people more complex to manage and treat within a clinical setting. The issue is further aggravated by the increased risk of polypharmacy with increased concurrent disease conditions, adding further complexity to treatment decisions for these patients.
Although previous studies have reported an elevated risk of specific comorbidities in people with MS, no study has yet investigated the prevalence of the most common acute and chronic comorbidities within the aging Danish MS population. Obtaining such knowledge could facilitate the targeted allocation of resources, allowing for the implementation of prevention strategies tailored to high-risk patients.
The objective of this registry-based study was to investigate the prevalence of common acute and chronic comorbidities among contemporary elderly people with MS, comparing them to matched controls from the general population. Additionally, we aimed to investigate the potential influence of chronic comorbidity on all-cause mortality within this population.
This study is a nationwide population-based study conducted in Denmark. All people with a diagnosis of MS were identified from the Danish Multiple Sclerosis Registry (DMSR) (10). To be eligible for inclusion, people with MS had to meet the following criteria: age above 50 years, residency in Denmark, and alive at the reference date (January 1st, 2022). A 25% random sample of the general Danish population, excluding individuals with MS, was used to select 10 matched controls for each patient. Matching was based on sex, exact age, ethnicity, and geographical region at the reference date. Ethnicity was categorized as immigrants (if people were born outside of Denmark) and descendants (if people were born in Denmark, but both parents were born outside of Denmark) or Danish (all others).
The utilization of the unique personal identification number, assigned to all Danish citizens and individuals with a permanent address residing in the country, enabled cross-linkage between national registries at the individual level (11).
The DMSR is a comprehensive nationwide population-based registry that has been collecting data on all people with MS since 1956. Currently, information is sourced from the 13 MS clinics dispersed throughout the country and is directly entered by clinicians during clinical visits into an online data collection platform. Following the introduction of DMTs in 1996, data entry for treated patients became mandatory. The registry serves as the foundation for national clinical quality indicators, ensuring a high degree of completeness and data validity (12). The DMSR encompasses clinical data on basic personal information, diagnostics, phenotypes, disability status, treatments, imaging, and more.
From the DMSR, we extracted several key variables including age, sex, age at MS onset, onset symptoms, current phenotype, the most recently recorded Expanded Disability Status Scale (EDSS) score within the past 2 years, duration since the last EDSS score record, MRI and clinical visit information, relapse activity, and visit frequency. Disease duration was calculated as the difference in years between the onset of MS and the reference date. Additionally, we created a binary variable termed “lost clinical contact”, indicating whether patients had no recorded clinical visit in an MS clinic, EDSS score, MS-related treatment, or MS-related MRI within the last 10 years. However, it is crucial to note that these patients might have had interactions with the healthcare system concerning other chronic or acute diseases.
The Danish National Patient Registry (DNPR) contains information on admissions to somatic hospital departments since 1977 with the addition of emergency departments and psychiatric departments in 1995 (13). The DNPR uses a Danish adaptation of the coding system “International Classification of Diseases, 10th revision” (ICD-10).
From the DNPR we collected the number of acute hospital admissions in 2021, duration of stay, and the five most overall frequent diagnoses related to acute admission in both people with MS and controls from the general population, allowing overlap. To detect the presence of chronic comorbidities, we searched the last 10 years for diagnosis codes consistent with a disease listed in the Charlson Comorbidity Index or a psychiatric disorder as previously categorized in MS literature (14, 15). The full list of used ICD-10 codes is available in the referenced articles (14, 15).
The characteristics of the people with MS and controls from the general population are presented as frequencies with corresponding percentages for categorical variables, while continuous variables are reported as mean values with standard deviation (SD) or as median values with the 1st and 3rd quartiles.
All statistical comparisons were performed between the two groups (MS or general populations) accounting for the clustering effect of the matched study design. For binary outcomes, odds ratios (OR) with corresponding 95% confidence intervals (95% CI) and p-values were calculated using a generalized estimating equation with a logit link function. The model was adjusted for the clustering of individuals into matched groups, assuming an independent correlation structure within clusters. Exponentiated estimates and joint confidence limits were calculated for the primary predictor. For ordinal outcomes, p-values were assessed using a generalized linear model with a multinomial distribution and a cumulative logit link function. The model accounted for the clustering of individuals within matched groups by introducing a subject-specific random effect, assuming an independent correlation structure within clusters. P-values for the significance of the association were calculated using a joint test. The rate ratio (RR) with the corresponding 95% CI and p-value of acute admission rates was calculated using a generalized linear model with a negative binomial distribution to adjust for overdispersion. The model further accounted for clustering in the dataset by utilizing cluster-robust variance-covariance matrices. The rate ratio and its 95% CI were computed based on the model coefficient and cluster-robust standard errors. To assess differences in length of stay of acute admissions we used a mixed-effects model. The model included a fixed effect group status (case or control) and introduced a random effect for the matched groups to capture the intra-cluster correlation. The empirical variance estimator was used to obtain root-unbiased standard errors.
To assess all-cause mortality, we did a Cox regression analysis without competing risks. We followed participants from January 1st, 2016, until either the event of interest (death from all causes) or censoring [study end (December 31st, 2021) or emigration]. We calculated the Charlson Comorbidity Index score (CCI score) by looking at the previous 4 years (2012–2015) in the DNPR for the presence of an ICD-10 code fulfilling the criteria (14).
We categorized CCI scores into three groups: none or mild comorbidity (0–1), moderate comorbidity (2–3), and high comorbidity (≥4). We used group (MS or general population) and comorbidity categories and added an interaction term to determine whether the effect of comorbidities on mortality differed in the two groups. We accounted for the clustered data by using “the robust sandwich estimator”. By using the CCI groups, we were able to create measures reflecting a pooled analysis considering all chronic diseases together to elucidate the overall difference between people with MS and the general population.
Data management, statistical analyses, and visualizations were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Obtaining informed consent or ethical approval is not mandatory for observational register-based studies in Denmark. This study adheres to the Danish General Data Protection Regulation (GDPR) and is registered with the Knowledge Center for Data Reviews, which serves as the data-responsible entity approved by the Danish Data Protection Agency. Access to the data is available upon submission of a qualified request. To ensure confidentiality, any cells containing information from fewer than three subjects (or neighboring cells enabling cross-cell calculations) are censored to prevent personally identifiable data. The preparation of data was conducted on secure servers provided by Statistics Denmark with the Approved Journal Number 10.123.
Figure 1 shows the disposition chart, and the baseline characteristics of the MS population are presented in Table 1. The study population consisted of 8,688 people with MS and 86,880 matched individuals from the general population. The mean age of the entire population was 63.5 years (SD: 9.0) at the reference date (January 1st, 2022). Of all the included individuals 99.2% had Danish ethnicity. The female-male ratio was 2:1 (68.3% females), and the people with MS had a mean age of 39.3 years (SD: 11.1) at clinical onset, and a mean disease duration of 24.2 years (SD: 12.4) at the reference date. The most frequent onset symptoms were “Sensory” (28.7%), “Pyramidal” (18.0%), and “Optic nerve” (15.6%). The distribution of phenotypes at the reference date among the people with MS was 42.1% RRMS, 25.9% SPMS, 14.2% PPMS, and 17.8% unclassified. The latest EDSS score within 2 years from the reference date had a median of 3.5 (Q1-Q3 = 2.0–6.0) and only 409 (4.7%) had one or more relapses in the last 2 years. The median time since the last visit was 1.1 years (Q1-Q3 = 0.5–9.7). In total 20.9% of the living people with MS older than 50 years were defined as having “lost clinical contact” with the MS clinics.
Table 2 presents the details of acute hospital admissions during 2021. A statistically significant difference was observed in the proportion of people with MS experiencing one or more acute hospital admissions compared to the matched controls from the general population (OR: 2.1, 95% CI: 1.9–2.2). Furthermore, the difference in proportions increased with the number of acute hospital admissions when subdivided into 1, 2, or ≥3 admissions. The calculated rate ratio for acute hospital admission was 2.2 (95% CI: 2.1–2.4). These results represent aggregated outcomes analyzed collectively to assess the overall differences between individuals with MS and the general population.
Among people with MS, “urinary infection” was the most frequent reason for acute hospital admission, with a statistically significantly higher incidence compared to the control group (OR: 10.4, 95% CI: 8.4–12.8). A similar pattern was observed for “urosepsis”, albeit with a lower incidence (OR: 12.5, 95% CI: 9.0–17.3). Additionally, the incidence of “pneumonia,” “bacterial infection, unspecified,” and “unspecified disorder” differed between the MS population and the control group, although these differences were less pronounced (OR: 2.6–6.8). No statistically significant difference between the two groups was found when comparing the incidence of “acute abdominal pain” (OR: 1.0, 95% CI: 0.7–1.7) and “ischemic stroke” (OR: 1.3, 95% CI: 0.8–2.0).
The duration of hospital stays for admitted individuals was both clinically and statistically significantly different in the two groups, with a median of 4 days (Q1-Q3 = 1–9) among people with MS and 3 days (Q1-Q3 = 1–7) among the control group (p < 0.001).
The results of chronic comorbidities used in the Charlson Comorbidity Index and psychiatric diseases are presented in Tables 3, 4, respectively. Upon investigating the comorbidity burden (CCI scores) within the study population, we observed an overall significant difference in favor of the control group when comparing the two groups.
There was no elevated risk of registered “depression,” “anxiety,” or “bipolar disorder” among elderly people with MS compared to the control group with people from the general population, see Table 4.
The interaction term between the group definition (MS or general population) and comorbidity category was highly statistically significant (p < 0.001) and included in the final model; test results are presented in Table 5.
Table 6 presents the estimates for all-cause mortality risks according to the comorbidity categories among people with MS and the control group. The CCI score was positively correlated with an increased hazard ratio (HR) for death in both populations. However, people with MS had higher HR in all three comorbidity categories, though the difference decreased with CCI ≥ 4. Additionally, a higher CCI score corresponded to a lower 5-year survival for both groups. Nevertheless, people with MS experienced worse overall 5-year survival than the general population (88.7 vs. 93.6%). When the two groups were stratified by comorbidity categories the reduced survival among people with MS remained statistically significant among CCI score 0–1 and CCI score 2–3, but this difference was no longer statistically significant among CCI score ≥4. Survival curves are illustrated in Figures 2, 3A–C.
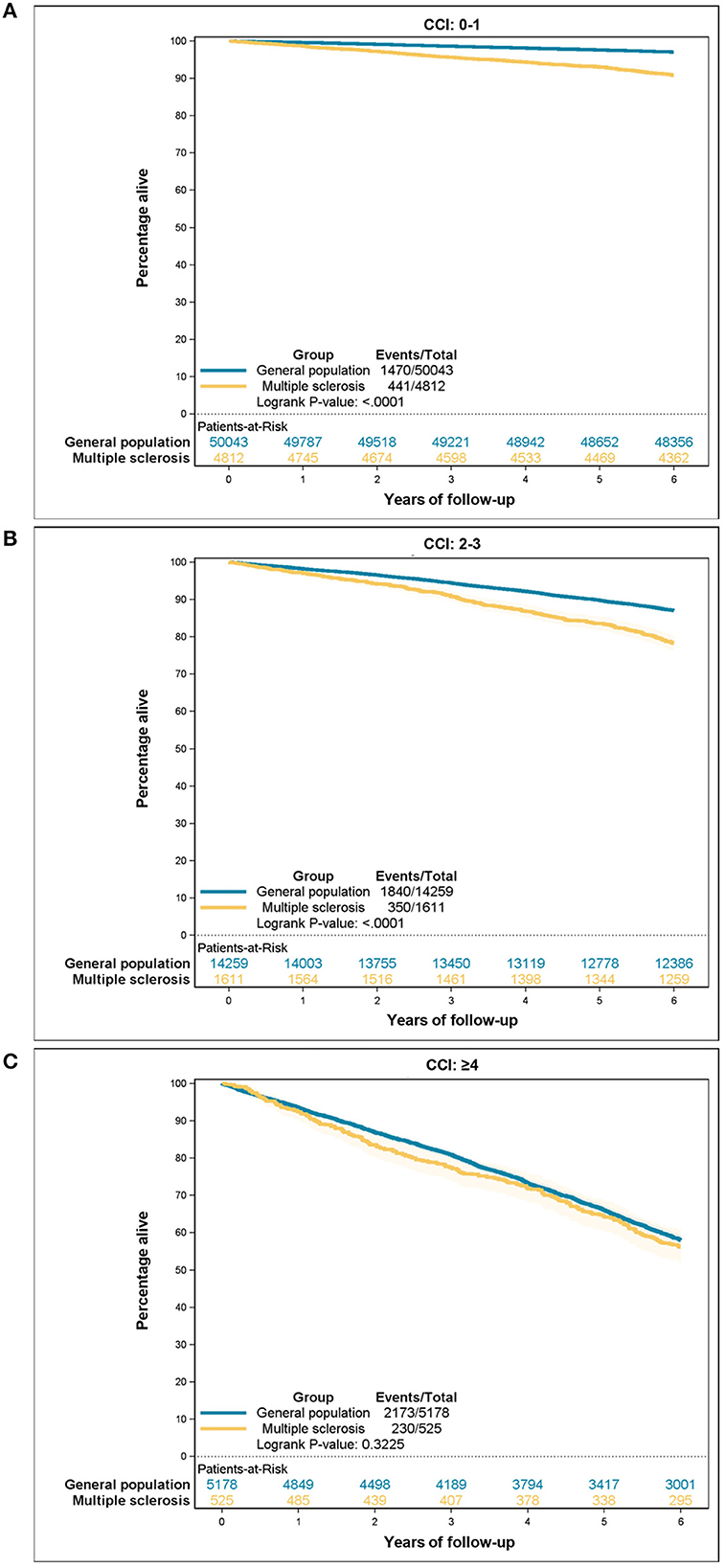
Figure 3. All-cause mortality according to the Charlson Comorbidity Index (CCI) category: (A) CCI 0–1, (B) CCI 2–3, (C) CCI ≥ 4.
This population-based study conducted with nationwide registry data from Denmark revealed significant variations in the prevalence of both acute and chronic comorbidities among older people with MS, as compared to matched controls from the general population.
The results revealing the statistically significant disparity in the frequency of acute hospital admissions indicate an increased burden on the acute healthcare system among people with MS.
Our findings reveal a 10-fold increase in the incidence of urinary tract infections and cases of urosepsis among people with MS. This pronounced difference can be attributed to the well-documented association between MS and bladder dysfunction affecting up to 70% of people with MS, which significantly elevates the susceptibility to urinary tract infections (16, 17). Admission due to pneumonia was also more than three times as frequent among people with MS than in the controls from the general population. This aligns with a study from 2009 that investigated risk factors for pneumonia and found MS to be the strongest (higher than diabetes and chronic respiratory diseases) (18). Our findings also showed an elevated frequency of admitted patients due to bacterial infections like previous studies reporting elevated risk of infections in general among people with MS (19–21).
Thus, our findings are consistent with prior literature demonstrating an elevated risk of various acute infections among individuals with MS. Early detection in the primary health sector resulting in earlier treatment or other prevention strategies might decrease the burden on the acute healthcare system and thereby potentially save time and money (22).
Generally, the literature concerning chronic comorbidities in individuals with MS reveal inconsistent findings, presumably due to different case definition and data sources. To enhance clarity, we decided to only report a subset of disease categories in the subsequent discussion, comparing them with the existing literature.
Our investigation into vascular comorbidities yielded diverse findings. We observed no differences in the prevalence of myocardial infarction or peripheral vascular disease, and a lower prevalence of congestive heart failure, while cerebrovascular disease was more common among people with MS.
We found three studies supporting our results regarding ischemic heart disease, while two other studies and a comprehensive review article all reported an elevated risk of ischemic heart disease (23–28). Three studies and a meta-analysis, including results from a Danish study, reported an elevated risk of cerebrovascular events as observed in the present study, but when one of them excluded the first year of follow-up to avoid ascertainment bias, the elevated risk was no longer statistically significant (24, 25, 27–29). Furthermore, when the British study conducted by Chou et al. subdivided cerebrovascular events in “before” and “after” MS diagnosis, the significant difference was only seen “before” MS diagnosis (25). Misclassified white matter lesions on MRI or relapses mistaken for cerebrovascular insult before MS diagnosis might explain this finding.
Diabetes was the most common chronic comorbidity in our study population. We found a statistically higher prevalence of diabetes among people with MS regardless of whether the condition was with or without complications. Most of the previous studies support our results, but some still contradict them (25, 30–33). The association between MS and diabetes may arise from shared disease mechanisms, genetic factors, or lifestyle changes prompted by an MS diagnosis. Regardless of the underlying cause, it remains crucial to equip healthcare providers with the tools to effectively handle individuals facing both conditions.
When investigating cancer, our study did not demonstrate an increased prevalence among individuals with MS. Our results showed slightly lower odds for “any malignancy” among people with MS. However, it is essential to interpret these findings cautiously, considering the complex and evolving nature of the relationship between MS and cancer, which has yielded conflicting results in previous studies. Six studies supported our finding of no increased risk of cancer, while others in part or completely contradict our findings (34–41).
Our study revealed a higher prevalence of hemiplegia and paraplegia among people with MS. Similarly, dementia without specification was more common in the MS population. However, both diagnoses likely refer to MS symptoms rather than distinct comorbidities.
Unexpectedly we observed a noticeably lower prevalence of psychiatric diseases compared to previously reported rates in MS populations, and we found no differences between our MS cohort and controls from the general population (25, 42, 43). Differences in prevalence observed in other studies may be attributed, at least in part, to the countries investigated. For instance, previous reviews have revealed lower depression prevalence in Europe compared to North America (42, 43). Another potential contributing factor could be the increased incidence and prevalence of depression observed among younger individuals, which would not be observed in our study population consisting of people above 50 years of age (44, 45).
Furthermore, we used diagnoses from the DNPR, which are registered during hospital admissions, which may have led to potential underestimation of psychiatric diagnoses, as most individuals with mild depression or mild anxiety are treated by their general practitioners. Patients admitted to the hospital receive a primary diagnosis code that corresponds to the main reason for their admission. However, it is important to note that additional diagnosis codes may also be assigned, reflecting pre-existing conditions or comorbidities that are relevant to the patient's overall health management, even if they were not the primary reason for the current admission. For instance, if a patient has a previous diagnosis of depression from their general practitioner but has not been admitted to the hospital specifically for depression, the relevant diagnosis code for depression may still be included when they are admitted for a different condition, such as MS, to ensure comprehensive and informed healthcare delivery. We believe that the potential underestimation of psychiatric diseases equally affects both people with MS and controls from the general population. Alternatively, there could be a bias toward relatively higher detection rates among people with MS due to more frequent outpatient hospital visits.
Our study revealed ambiguous results regarding renal disease as people with MS were more likely to have “mild to moderate renal disease”, while “severe renal disease” was less frequent with a borderline statistical significance (p = 0.05).
A review published in 2015 including 6 articles with data on renal disease from 1989 to 2009 reported a prevalence of “renal disease” of 0.74–2.49% and “renal failure” of 0–0.78% among people with MS (46). Only three of the studies included in the review compared people with MS to background populations: two found no difference and one found renal failure to be less common in the MS population (the last-mentioned study was done in 1994 on patients aged ≥65 years). These results were supported by a study from 2019 reporting a hazard ratio of “renal disease” to be 0.9 (95% CI: 0.58–1.38) among people with MS compared to matched controls (25).
Our results might be influenced by closer monitoring of people with MS compared to the general population, explaining the higher number of “mild to moderate” cases because these cases do not necessarily bring otherwise healthy people to the doctor. At the same time, early detection gives the opportunity the intervene and maybe prevent escalation to “severe” disease.
We found no differences regarding liver diseases between people with MS and controls from the general population. This aligns with the existing literature despite the potential liver-related side effects associated with all MS treatments and a suggested association between autoimmune liver diseases and MS (25, 46–49). In terms of peptic ulcer, our results showed a slightly higher prevalence among people with MS, which is partly supported by the literature, despite high heterogeneity in results (25, 46, 47). The use of glucocorticoid treatment in MS might have had some influence on this finding.
We found increased all-cause mortality among people with MS compared to controls from the general population, which is in line with previously reported findings (25). Furthermore, it has been shown that having comorbidities before MS diagnosis increases all-course mortality among people with MS (25). We are, to our knowledge, the first to contribute with knowledge about the relative impact of comorbidity burden on all-course mortality among people with MS. Our findings reveal that coexisting MS at first increases all-course mortality, but when the comorbidity burden gets high enough (CCI score ≥4) people with MS have the same hazard of death as matched controls from the general population.
Certainly, comparing results across different studies that investigate comorbidities can raise difficulties due to a range of factors, including the lack of standardized approaches to categorization and presentation results, as well as differences in study design, populations, data sources, and analytical methods. In the following, we will elaborate further.
Categorizing and presenting results related to comorbidities poses a challenge due to the extensive array of diagnoses, which is further complicated by the divergence in terminology (such as ICD-10, MedDRA, etc.) employed across various countries. Given the myriad of diagnoses, researchers often resort to categorizing comorbidities, albeit through varying approaches, which makes direct comparisons difficult. In this study, we opted to employ the internationally recognized Charlson Comorbidity Index, a tool designed to prognosticate the impact of comorbidities, which aligned with our research objectives (50). Additionally, this index is compatible with ICD-10 codes, the coding system utilized within the Danish healthcare system (14). Importantly, the index does not directly include MS, facilitating a direct comparison of CCI scores between individuals with MS and controls without necessitating alterations to the index. This was pivotal as our investigation aimed to elucidate how the burden of comorbidities influences life expectancy in individuals with MS relative to control subjects.
To illustrate the lack of standardized data collection and presentation, our literature search revealed different approaches to categorization and the resulting impact on findings. Despite the common data source, two British studies reported different results due to variations in their categorization and presentation of comorbidities. Palladino et al. reported an elevated risk of “cardiovascular disease” among people with MS, while Choi et al. found no elevated risk of “myocardial infarction” before or after MS diagnosis (25, 28). These discrepancies highlight the challenges posed by non-standardized approaches to categorization and presentation and emphasize the need for a more unified approach in comorbidity research to facilitate more accurate comparisons.
We chose to focus on comorbidities among older people with MS due to the increased risk of comorbidities with age. Additionally, the elderly MS population is growing, making it increasingly relevant to examine their comorbidities. Including younger patients would probably have resulted in a lower prevalence of comorbidities in both groups, making it more difficult to detect meaningful differences.
Furthermore, the interplay between biological sex, age, and comorbidities presents a multifaceted analytical challenge. Our study did not disaggregate data by sex within different age groups. We prioritized providing a comprehensive overview of comorbidities across the older MS population, rather than conducting a granular analysis by sex and age categories. Nonetheless, we acknowledge the potential impact of these variables and suggest that a detailed analysis that considers these stratifications could be highly informative. This more focused approach represents a valuable direction for future research.
In Denmark, we are fortunate to have a large number of nationwide registers with high data quality, which reduces the risk of selection bias. Furthermore, unique social security numbers (CPR numbers) make it possible to link data on the individual level. Many epidemiological studies utilize administrative health care or insurance data, which may not be as comprehensive or accurate as national disease registries. Additionally, most previous studies do not compare the MS population to controls, thereby failing to provide relative results. Even when comparisons are made, they are often unable to adjust for a range of variables (e.g., ethnicity and geographical region), which limits their ability to control for potential confounding factors that might affect the accuracy and interpretation of the results. Adjusting for ethnicity is important as the general population in Denmark includes 12% immigrants and descendants compared to 0.8% in the Danish MS population. Immigrants and descendants may have different characteristics such as socioeconomic factors like educational level, income, and family structure influencing the risk of comorbidities, but also a different susceptibility for MS and comorbidities in general (51). A study investigating comorbidities found a higher prevalence among immigrants compared to long-term residents underlining the importance of taking ethnicity into account when investigating the consequences of MS (52).
Healthcare data from Denmark benefits uniquely from the Danish universal healthcare system, which provides free and equal services to all citizens regardless of income, effectively reducing the potential for bias arising from high costs. This effectively minimizes potential biases that could distort healthcare data in countries with substantially different healthcare systems.
First, the cross-sectional design used in parts of the study does not allow for the establishment of causal relationships between variables and does not take temporal changes into account. As a result of this, we are not able to project the future trajectory of the observed differences between people with MS and the general population. Second, all comparisons between the two groups are univariate, and thus unadjusted for potential confounders apart from the matching covariates. Third, in the Cox regression, we assumed a non-informative censoring mechanism for emigration. However, one could argue, that individuals able to emigrate indicate a better health status than the average health status of the study population, which could lead to an overestimation of death rates. However, only 0.37 in the general population and 0.16 in the MS population did emigrate, and we consider this potential bias negligible. Fourth, due to the complexity of recent treatment trajectories, we did not include exposure to different DMTs. Finally, our study did not adjust for differences in lifestyle factors such as smoking and alcohol consumption, which could contribute to all-cause mortality. The primary objective of our investigation was to compare health outcomes between individuals with MS and a control group from the general population. The study design was intentionally broad and not equipped to isolate the potential contributory factors to mortality or to dissect the direct impacts of MS vs. other variables not included in the matching process. We aimed to provide an epidemiological overview highlighting the disparities in all-cause mortality in relation to the burden of chronic comorbidities, which are inherently influenced by a variety of factors. While our matching strategy was designed to control for the most substantial known confounders, we recognize that it is not possible to account for all potential confounding factors in observational research.
This study demonstrates a higher occurrence of both acute and chronic comorbidities in people with MS, as well as how comorbidities increase the hazard of death among the studied individuals. Therefore, our results underscore the importance of considering comorbidities when treating people with MS.
The datasets presented in this article are not readily available because access to the used data is only available upon qualified request and approval by the Knowledge Center for Data Reviews (entity responsible for data of the Capital Region of Denmark, approved by the Danish Data Protection Agency) and the Danish Multiple Sclerosis Group (DMSR). Requests to access the datasets should be directed to DMSR, c2NsZXJvc2VyZWdpc3RlcmV0LnJpZ3Nob3NwaXRhbGV0QHJlZ2lvbmguZGs=.
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
RH: Conceptualization, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. MW-H: Data curation, Formal analysis, Investigation, Writing – review & editing. FS: Writing – review & editing. MM: Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was financially supported by the Danish Multiple Sclerosis Society, a non-governmental, patient organization.
The authors would like to acknowledge all Danish Departments of Neurology for assisting in the collection of data, and the Danish Multiple Sclerosis Society for funding the Danish Multiple Sclerosis Registry.
RH has served on a scientific advisory board for Novartis and has received honoraria for lecturing for Novartis and Sanofi. MW-H has served on a scientific advisory board for Sanofi and has received honoraria for lecturing for Novartis and Sanofi. FS has served on scientific advisory boards for, served as a consultant for, received support for congress participation, or received speaker honoraria from Alexion, Biogen, Bristol Myers Squibb, H. Lundbeck A/S, Merck, Novartis, Roche, and Sanofi Genzyme. His laboratory has received research support from Merck, Novartis, Roche, and Sanofi Genzyme. MM has served on scientific advisory boards for Sanofi, Novartis, and Merck, and has received honoraria for lecturing from Biogen, Merck, Novartis, Roche, Sanofi Genzyme, and Bristol Myers Squibb.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Koch-Henriksen N, Thygesen LC, Stenager E, Laursen B, Magyari M. Incidence of MS has increased markedly over six decades in Denmark particularly with late onset and in women. Neurology. (2018) 90:e1954–63. doi: 10.1212/WNL.0000000000005612
2. Solaro C, Ponzio M, Moran E, Tanganelli P, Pizio R, Ribizzi G, et al. The changing face of multiple sclerosis: prevalence and incidence in an aging population. Mult Scler J. (2015) 21:1244–50. doi: 10.1177/1352458514561904
3. Kowalec K, McKay KA, Patten SB, Fisk JD, Evans C, Tremlett H, et al. Comorbidity increases the risk of relapse in multiple sclerosis. Neurology. (2017) 89:2455–61. doi: 10.1212/WNL.0000000000004716
4. Conway DS, Thompson NR, Cohen JA. Influence of hypertension, diabetes, hyperlipidemia, and obstructive lung disease on multiple sclerosis disease course. Mult Scler J. (2017) 23:277–85. doi: 10.1177/1352458516650512
5. Marrie RA, Fisk JD, Fitzgerald K, Kowalec K, Maxwell C, Rotstein D, et al. Etiology, effects and management of comorbidities in multiple sclerosis: recent advances. Front Immunol. (2023) 14:e1197195. doi: 10.3389/fimmu.2023.1197195
6. Binzer S, McKay KA, Brenner P, Hillert J, Manouchehrinia A. Disability worsening among persons with multiple sclerosis and depression. Neurology. (2019) 93:e2216–23. doi: 10.1212/WNL.0000000000008617
7. Thormann A, Sørensen PS, Koch-Henriksen N, Laursen B, Magyari M. Comorbidity in multiple sclerosis is associated with diagnostic delays and increased mortality. Neurology. (2017) 89:1668–75. doi: 10.1212/WNL.0000000000004508
8. Thormann A, Sørensen PS, Koch-Henriksen N, Thygesen LC, Laursen B, Magyari M. Chronic comorbidity in multiple sclerosis is associated with lower incomes and dissolved intimate relationships. Eur J Neurol. (2017) 24:825–34. doi: 10.1111/ene.13297
9. Zhang T, Tremlett H, Leung S, Zhu F, Kingwell E, Fisk JD, et al. Examining the effects of comorbidities on disease-modifying therapy use in multiple sclerosis. Neurology. (2016) 86:1287–95. doi: 10.1212/WNL.0000000000002543
10. Magyari M, Joensen H, Laursen B, Koch-Henriksen N. The Danish multiple sclerosis registry. Brain Behav. (2021) 11:1–10. doi: 10.1002/brb3.1921
11. Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. (2014) 29:541–9. doi: 10.1007/s10654-014-9930-3
12. Magyari M, Koch-Henriksen N, Sørensen P. The Danish multiple sclerosis treatment register. Clin Epidemiol. (2016) 8:549–52. doi: 10.2147/CLEP.S99500
13. Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National patient registry: a review of content, data quality, and research potential. Clin Epidemiol. (2015) 7:449–90. doi: 10.2147/CLEP.S91125
14. Glasheen WP, Cordier T, Gumpina R, Haugh G, Davis J, Renda A. Charlson comorbidity index: ICD-9 update and ICD-10 translation. Am Heal drug benefits. (2019) 12:188–97.
15. McKay KA, Tremlett H, Fisk JD, Zhang T, Patten SB, Kastrukoff L, et al. Psychiatric comorbidity is associated with disability progression in multiple sclerosis. Neurology. (2018) 90:e1316–23. doi: 10.1212/WNL.0000000000005302
16. Al Dandan HB, Coote S, McClurg D. Prevalence of lower urinary tract symptoms in people with multiple sclerosis. Int J MS Care. (2020) 22:91–9. doi: 10.7224/1537-2073.2019-030
17. de Medeiros Junior WLG, Demore CC, Mazaro LP, de Souza MFN, Parolin LF, Melo LH, et al. Urinary tract infection in patients with multiple sclerosis: an overview. Mult Scler Relat Disord. (2020) 46:102462. doi: 10.1016/j.msard.2020.102462
18. Vinogradova Y, Hippisley-Cox J, Coupland C. Identification of new risk factors for pneumonia: population-based case-control study. Br J Gen Pract. (2009) 59:e329–38. doi: 10.3399/bjgp09X472629
19. Persson R, Lee S, Yood MU, Wagner CM, Minton N, Niemcryk S, et al. Infections in patients diagnosed with multiple sclerosis: a multi-database study. Mult Scler Relat Disord. (2020) 41:101982. doi: 10.1016/j.msard.2020.101982
20. Nelson RE, Xie Y, DuVall SL, Butler J, Kamauu AWC, Knippenberg K, et al. Multiple sclerosis and risk of infection-related hospitalization and death in US veterans. Int J MS Care. (2015) 17:221–30. doi: 10.7224/1537-2073.2014-035
21. Luna G, Alping P, Burman J, Fink K, Fogdell-Hahn A, Gunnarsson M, et al. Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. JAMA Neurol. (2020) 77:184. doi: 10.1001/jamaneurol.2019.3365
22. Khan A, Lin P, Kamdar N, Peterson M, Mahmoudi E. Potentially preventable hospitalizations and use of preventive services among people with multiple sclerosis: large cohort study, USA. Mult Scler Relat Disord. (2022) 68:104105. doi: 10.1016/j.msard.2022.104105
23. Marrie RA, Fisk J, Tremlett H, Wolfson C, Warren S, Blanchard J, et al. Differing trends in the incidence of vascular comorbidity in MS and the general population. Neurol Clin Pract. (2016) 6:120–8. doi: 10.1212/CPJ.0000000000000230
24. Roshanisefat H, Bahmanyar S, Hillert J, Olsson T, Montgomery S. Multiple sclerosis clinical course and cardiovascular disease risk - Swedish cohort study. Eur J Neurol. (2014) 21:1353–e88. doi: 10.1111/ene.12518
25. Chou IJ, Kuo CF, Tanasescu R, Tench CR, Tiley CG, Constantinescu CS, et al. Comorbidity in multiple sclerosis: its temporal relationships with disease onset and dose effect on mortality. Eur J Neurol. (2020) 27:105–12. doi: 10.1111/ene.14040
26. Marrie RA, Reider N, Cohen J, Stuve O, Trojano M, Cutter G, et al. A systematic review of the incidence and prevalence of cardiac, cerebrovascular, and peripheral vascular disease in multiple sclerosis. Mult Scler J. (2015) 21:318–31. doi: 10.1177/1352458514564485
27. Thormann A, Magyari M, Koch-Henriksen N, Laursen B, Sørensen PS. Vascular comorbidities in multiple sclerosis: a nationwide study from Denmark. J Neurol. (2016) 263:2484–93. doi: 10.1007/s00415-016-8295-9
28. Palladino R, Marrie RA, Majeed A, Chataway J. Evaluating the risk of macrovascular events and mortality among people with multiple sclerosis in England. JAMA Neurol. (2020) 77:820. doi: 10.1001/jamaneurol.2020.0664
29. Hong Y, Tang HR, Ma M, Chen N, Xie X, He L. Multiple sclerosis and stroke: a systematic review and meta-analysis. BMC Neurol. (2019) 19:139. doi: 10.1186/s12883-019-1366-7
30. Nielsen NM, Westergaard T, Frisch M, Rostgaard K, Wohlfahrt J, Koch-Henriksen N, et al. Type 1 diabetes and multiple sclerosis. Arch Neurol. (2006) 63:1001. doi: 10.1001/archneur.63.7.1001
31. Marrosu MG, Cocco E, Lai M, Spinicci G, Pischedda MP, Contu P. Patients with multiple sclerosis and risk of type 1 diabetes mellitus in Sardinia, Italy: a cohort study. Lancet. (2002) 359:1461–5. doi: 10.1016/S0140-6736(02)08431-3
32. Amiri Z, Azmin M, Amiri S, Akbarisari A, Sahraian MA, Farzadfar F, et al. Prevalence of comorbidities in patients with multiple sclerosis using administrative data from 2007 to 2016 in Iran. Mult Scler Relat Disord. (2023) 74:104693. doi: 10.1016/j.msard.2023.104693
33. Marrie RA, Yu BN, Leung S, Elliott L, Caetano P, Warren S, et al. Rising prevalence of vascular comorbidities in multiple sclerosis: validation of administrative definitions for diabetes, hypertension, and hyperlipidemia. Mult Scler J. (2012) 18:1310–9. doi: 10.1177/1352458512437814
34. Nørgaard M, Veres K, Didden EM, Wormser D, Magyari M. Multiple sclerosis and cancer incidence: a Danish nationwide cohort study. Mult Scler Relat Disord. (2019) 28:81–5. doi: 10.1016/j.msard.2018.12.014
35. Gaindh D, Kavak KS, Teter B, Vaughn CB, Cookfair D, Hahn T, et al. Decreased risk of cancer in multiple sclerosis patients and analysis of the effect of disease modifying therapies on cancer risk. J Neurol Sci. (2016) 370:13–7. doi: 10.1016/j.jns.2016.09.005
36. Ragonese P, Aridon P, Vazzoler G, Mazzola MA, Lo Re V, Lo Re M, et al. Association between multiple sclerosis, cancer risk, and immunosuppressant treatment: a cohort study. BMC Neurol. (2017) 17:155. doi: 10.1186/s12883-017-0932-0
37. Hongell K, Kurki S, Sumelahti M-L, Soilu-Hänninen M. Risk of cancer among Finnish multiple sclerosis patients. Mult Scler Relat Disord. (2019) 35:221–7. doi: 10.1016/j.msard.2019.08.005
38. Alping P, Askling J, Burman J, Fink K, Fogdell-Hahn A, Gunnarsson M, et al. Cancer risk for fingolimod, natalizumab, and rituximab in multiple sclerosis patients. Ann Neurol. (2020) 87:688–99. doi: 10.1002/ana.25701
39. Nørgaard M, Veres K, Sellebjerg FT, Svingel LS, Foch C, Boutmy E, et al. Incidence of malignancy in multiple sclerosis: a cohort study in the Danish Multiple Sclerosis Registry. Mult Scler J Exp Transl Clin. (2021) 7:205521732110539. doi: 10.1177/20552173211053939
40. Grytten N, Myhr K-M, Celius EG, Benjaminsen E, Kampman M, Midgard R, et al. Risk of cancer among multiple sclerosis patients, siblings, and population controls: a prospective cohort study. Mult Scler J. (2020) 26:1569–80. doi: 10.1177/1352458519877244
41. Marrie RA, Reider N, Cohen J, Stuve O, Trojano M, Sorensen PS, et al. A systematic review of the incidence and prevalence of cancer in multiple sclerosis. Mult Scler J. (2015) 21:294–304. doi: 10.1177/1352458514564489
42. Peres DS, Rodrigues P, Viero FT, Frare JM, Kudsi SQ, Meira GM, et al. Prevalence of depression and anxiety in the different clinical forms of multiple sclerosis and associations with disability: a systematic review and meta-analysis. Brain Behav Immun Heal. (2022) 24100484. doi: 10.1016/j.bbih.2022.100484
43. Boeschoten RE, Braamse AMJ, Beekman ATF, Cuijpers P, van Oppen P, Dekker J, et al. Prevalence of depression and anxiety in Multiple Sclerosis: a systematic review and meta-analysis. J Neurol Sci. (2017) 372:331–41. doi: 10.1016/j.jns.2016.11.067
44. Goodwin RD, Dierker LC, Wu M, Galea S, Hoven CW, Weinberger AH. Trends in U.S. depression prevalence from 2015 to 2020: the widening treatment gap. Am J Prev Med. (2022) 63:726–33. doi: 10.1016/j.amepre.2022.05.014
45. Liu Q, He H, Yang J, Feng X, Zhao F, Lyu J. Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. J Psychiatr Res. (2020) 126:134–40. doi: 10.1016/j.jpsychires.2019.08.002
46. Marrie RA, Reider N, Stuve O, Trojano M, Sorensen PS, Cutter GR, et al. The incidence and prevalence of comorbid gastrointestinal, musculoskeletal, ocular, pulmonary, and renal disorders in multiple sclerosis: a systematic review. Mult Scler J. (2015) 21:332–41. doi: 10.1177/1352458514564488
47. Marck CH, Neate SL, Taylor KL, Weiland TJ, Jelinek GA. Prevalence of comorbidities, overweight and obesity in an international sample of people with multiple sclerosis and associations with modifiable lifestyle factors. Ramagopalan S V, editor. PLoS ONE. (2016) 11:e0148573. doi: 10.1371/journal.pone.0148573
48. Biolato M, Bianco A, Lucchini M, Gasbarrini A, Mirabella M, Grieco A. The disease-modifying therapies of relapsing-remitting multiple sclerosis and liver injury: a narrative review. CNS Drugs. (2021) 35:861–80. doi: 10.1007/s40263-021-00842-9
49. Villani R, Serviddio G, Avolio C, Cassano T, D'Amico E. Autoimmune liver disease and multiple sclerosis: state of the art and future perspectives. Clin Exp Med. (2023) 23:3321–38. doi: 10.1007/s10238-023-01128-8
50. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. (1987) 40:373–83. doi: 10.1016/0021-9681(87)90171-8
51. Ward M, Goldman MD. Epidemiology and pathophysiology of multiple sclerosis. Contin Lifelong Learn Neurol. (2022) 28:988–1005. doi: 10.1212/CON.0000000000001136
Keywords: multiple sclerosis, aging, comorbidity, hospital admissions, mortality, patient-centered care
Citation: Holm RP, Wandall-Holm MF, Sellebjerg F and Magyari M (2023) Comorbidity in the aging population with multiple sclerosis: a Danish nationwide study. Front. Neurol. 14:1297709. doi: 10.3389/fneur.2023.1297709
Received: 20 September 2023; Accepted: 08 November 2023;
Published: 24 November 2023.
Edited by:
Viviana Nociti, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Alessio Signori, University of Genoa, ItalyCopyright © 2023 Holm, Wandall-Holm, Sellebjerg and Magyari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rolf Pringler Holm, cm9sZi5wcmluZ2xlci5ob2xtQHJlZ2lvbmguZGs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.