
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 23 January 2024
Sec. Stroke
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1295051
Objective: Some previous studies have suggested a potential link between stroke and gastroesophageal reflux disease (GERD). We used a two-sample bidirectional Mendelian randomization (MR) method to explore the causal relationship between stroke and GERD.
Design: Summary-level data derived from the published genome-wide association studies (GWAS) were employed for analyses. Single-nucleotide polymorphisms (SNPs) as instrumental variables (IVs) for stroke (n = 446,696) and its common subtypes ischemic stroke (IS) (n = 440,328), large vessel stroke (LVS) (n = 410,484), small vessel stroke (SVS) (n = 198,048), and cardioembolic stroke (CES) (n = 413,304) were obtained from the MEGASTROKE consortium. The data on intracerebral hemorrhage (ICH) (n = 721,135) come from the UK Biobank. Instrumental variables (IVs) for lacunar stroke (LS) (n = 474,348) and GERD (n = 602,604) were screened from publicly available genetic summary data. The inverse variance weighted (IVW) method was used as the main MR method. Pleiotropy was detected by the MR-Egger intercept test, MR pleiotropy residual sum and outlier, and leave-one-out analysis. Cochran Q statistics were used as supplements to detect pleiotropy.
Results: We found that GERD can causally increase the risk of stroke [IVW odds ratio (OR): 1.22, 95% confidence interval (CI): 1.13–1.32, p = 1.16 × 10−6] and its common subtypes IS (OR: 1.19, 95% CI: 1.10–1.30, p = 3.22 × 10−5), LVS (OR: 1.49, 95% CI: 1.21–1.84, p = 1.47 × 10−4), and LS (OR: 1.20, 95% CI: 1.001–1.44, p = 0.048). Several important risk factors for stroke have also been implicated in the above causal relationship, including type 2 diabetes, sleep apnea syndrome, high body mass index, high waist-to-hip ratio, and elevated serum triglyceride levels. In reverse MR analysis, we found that overall stroke (OR: 1.09, 95% CI: 1.004–1.19, p = 0.039) and IS (OR: 1.10, 95% CI: 1.03–1.17, p = 0.007) have the causal potential to enhance GERD risk.
Conclusion: This MR study provides evidence supporting a causal relationship between GERD and stroke and some of its common subtypes. We need to further explore the interconnected mechanisms between these two common diseases to better prevent and treat them.
Gastroesophageal reflux disease (GERD) and stroke are two common medical clinical diseases, and their incidence and related health and economic burdens have been increasing in recent years. Stroke is a common cerebrovascular disease caused by neurological deficits associated with acute focal injury to the central nervous system. According to its pathological mechanisms, stroke can be divided into hemorrhagic stroke and ischemic stroke. Ischemic stroke due to blocked arteries and decreased blood flow to the brain accounts for the majority of stroke patients. In recent years, the incidence of stroke, especially ischemic stroke, has gradually increased and has become one of the important causes of death and disability worldwide, causing a severe socioeconomic burden (1). GERD is a digestive system disease that causes various esophageal lesions and related complications due to the reflux of gastric contents (2). The global incidence of gastroesophageal reflux has increased significantly in the past 20 years, and its prevalence in North America (18.1–27.8%) and Europe (8.8–25.9%) is significantly higher than that in East Asia (2.5–7.8%) (3).
Some past studies have found that nearly 50% of stroke patients will develop gastrointestinal complications such as dysphagia and gastrointestinal bleeding after the disease (4). In addition, because of the close connection between the esophagus and the left atrium, GERD is thought to have an intimate role in atrial fibrillation, and some studies have linked GERD to an increased risk of atrial fibrillation, which increases the risk of stroke (5). In recent years, some studies have further suggested that there may be a close relationship between stroke and GERD. A population-based cohort study found that stroke patients had a higher incidence (1.51 times) of gastroesophageal reflux disease than non-stroke subjects (6). A population-based follow-up study suggests that young patients with gastric reflux esophagitis are at higher risk of stroke (7). An analysis of inpatient samples also found that reflux esophagitis increased the incidence of acute stroke in patients with atrial fibrillation (8). It has long been considered that there may be a link between GERD and stroke. However, the causal relationship between GERD and stroke and its subtypes has not been confirmed. Suppose the possible potential causal relationship between GERD and stroke can be clarified, in that case, it will be of great significance to prevent and treat these two common medical clinical diseases.
Mendelian randomization (MR) is an analytical method that uses genetic variants as instrumental variables (IVs) for test exposures to assess the causal role of their associations with clinical outcomes. The application of MR avoids unobserved confounding factors and reverse causation in evaluating the relationship between exposure and outcome, thus becoming a valuable medical research tool (9). We compiled some GWAS data and used MR for the first time to evaluate whether there is a bidirectional causal relationship between stroke and its subtypes and gastroesophageal reflux disease. Based on the two-sample, two-way MR method, we studied the potential bidirectional causal relationship between stroke and its common subtypes, ischemic stroke (IS), large vessel stroke (LVS), small vessel stroke (SVS), cardioembolic stroke (CES), lacunar stroke (LS), intracranial hemorrhage (ICH), and GERD.
We performed a two-sample bidirectional MR study using single-nucleotide polymorphisms (SNPs) as IVs to investigate the causal relationship between stroke and its common subtypes (IS, LVS, SVS, CS, LS, and ICH) and GERD. The workflow of this Mendelian randomization is shown in Figure 1. The design and analysis of MR must be based on the following three key assumptions: (1) correlation: SNPs used as IVs must be associated with exposure; (2) exclusivity: IVs can only affect outcomes through exposure; and (3) independence: IVs cannot influence results through any unmeasured confounding variables. The data used in this MR study are all public data from published studies, so ethical approval and informed consent are not required. The data statistics in this study were conducted using the TwoSampleMR software package and the MR PRESSO software package in R (version 4.3.1). The study design and reporting conformed to using STROBE-MR (10).
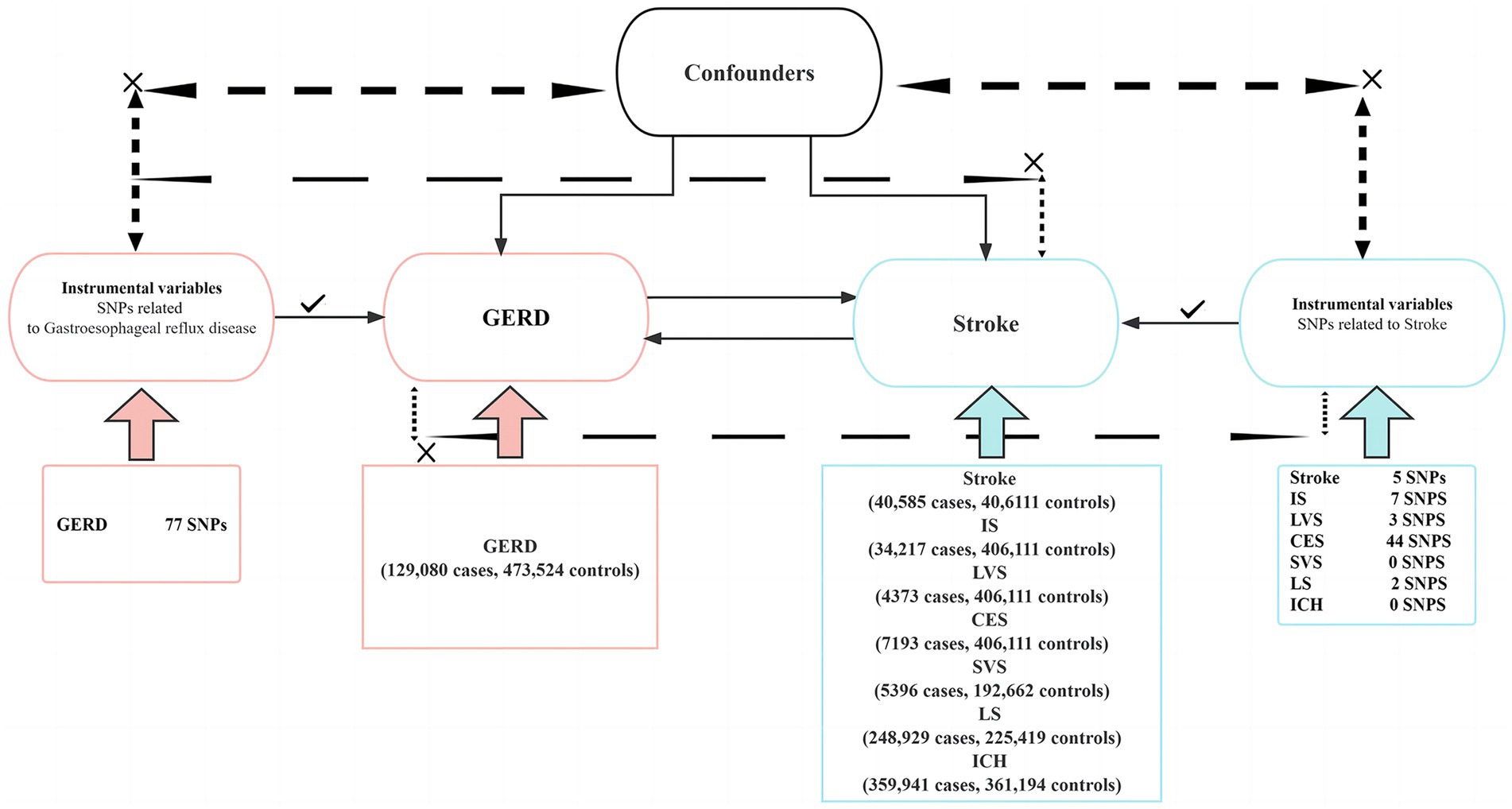
Figure 1. This is a two-sample bidirectional Mendelian randomization experimental design. SNPs, single-nucleotide polymorphisms; GERD, gastroesophageal reflux disease; IS, ischemic stroke; LVS, large vessel stroke; CES, cardioembolic stroke; SVS, small vessel stroke; LS, lacunar stroke; ICH, intracranial hemorrhage.
The summary-level data for stroke and its common subtypes IS, LVS, CES, and SVS were obtained from genome-wide association studies (GWAS) conducted by the MEGASTROKE consortium. The MEGASTROKE consortium performed a meta-analysis of 29 GWAS involving 520,000 European ancestry participants (including 40,5,850 stroke patients and 406,111 controls) and identified a total of 32 stroke risk loci (including 10 previously discovered loci and 22 new loci) (11). The GWAS data of lacunar stroke come from a recent meta-analysis conducted by International Stroke Genetics Consortium (ISGC), including 225,419 subjects of European ancestry (including 6,030 LS patients and 248,929 controls) (12). The summary data for ICH was extracted from the UK Biobank (359,941 cases and 361,194 controls). By excluding SNPs with p > 5 × 10−8 and r2 > 0.01, we extracted 8 SNPs, 9 SNPs, 4 SNPs, 47 SNPs, 3 SNPs, and 1 SNP from the GWAS data of overall stroke, IS, LVS, CES, LS, and ICH, respectively. After that, by removing weak IVs with an F statistic less than 10 and instrumental variables containing confounding factors (by consulting http://www.phenoscanner.medschl.http://cam.ac.uk/), we finally retained 5 SNPs of stroke, 7 SNPs of IS, 3 SNPs of LVS, 3 SNPs of CES, and 2 SNPs of LS. However, there was a lack of IVs of SVS and ICH after excluding them. More details about the trait definition are available in Supplementary Tables S8–S12.
The summary data for GERD phenotypes were obtained from a multi-trait genetic association analysis of GERD. This analysis identified 88 loci associated with GERD by including 129,080 cases and 473,524 controls with a total of 602,604 subjects (13). By excluding SNPs with p > 5 × 10−8 and r2 > 0.01, we extracted 80 SNPs from GWAS data of GERD. By removing weak IVs (F < 10) and instrumental variables containing confounding factors, we finally retained 77 SNPs. More details about the trait definition are available in Supplementary Tables S1–S7. The GWAS data used in this study are detailed in Table 1.
In this study, we adopted inverse-variance weighting (IVW) as the preferred method and conducted a two-sample bidirectional MR analysis to explore the causal relationship between stroke and GERD. Although the IVW method has high statistical power, this method may also treat some IVs that affect the results through other pathways as valid SNPs (14). Therefore, to avoid bias in causality assessment, we used MR-Egger regression, weighted median, weighted mode, and MR pleiotropy residual sum and outlier (MR-PRESSO) approaches for further sensitivity analysis. MR-Egger regression can assess potential horizontal pleiotropy, and MR-PRESSO approaches can identify and remove these pleiotropic outliers (15). The weighted median method enables consistent causal estimation when more than half of the IVs are valid (16). Weighted mode can evaluate the causal relationship between the results and a subset of SNPs with similar causal effects (17). Finally, we also used the leave-one-out method to assess whether omitting a specific SNP would significantly impact the results of MR.
To explore the underlying genetic mechanisms of GERD combined with stroke and stroke combined with GERD, we also analyzed the relationship between GERD and stroke-related risk factors and the relationship between stroke and GERD-related risk factors by referring to the study published by Tao H et al. (18). Based on relevant research data, we included stroke-related risk factors such as hypertension, hyperlipidemia (HDL cholesterol and LDL cholesterol), hypertriglyceridemia (triglyceride), type 2 diabetes, sleep apnea syndrome, and obesity (body mass index and waist–hip ratio) (19). Research shows risk factors for gastroesophageal reflux, including age, smoking, NSAID use, and obesity (20). Therefore, we explored the relationship between stroke and obesity (body mass index and waist–hip ratio). The GWAS data for these risk factors are all from the UK Biobank. The details of each data source are presented in Table 2. We performed MR using GERD or stroke as exposures and the above risk factors as outcome factors. IVW was used as the main analysis method, and a value of p of <0.05 was considered significant.
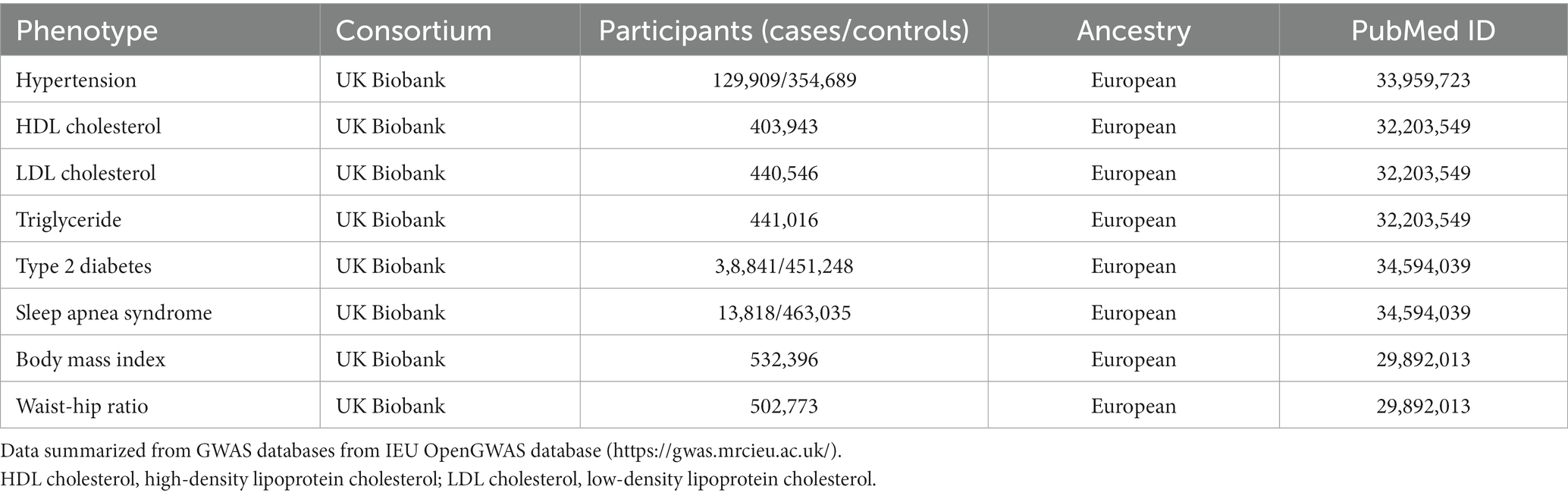
Table 2. GWAS database details of risk factors involved in stroke and gastroesophageal reflux disease.
As revealed by the MR analyses, there was a significant correlation between GERD and increased risk of overall stroke [IVW odds ratio (OR): 1.22, 95% confidence interval (CI): 1.13–1.32, p = 1.16 × 10−6], IS (OR: 1.19, 95% CI: 1.10–1.30, p = 3.22 × 10−5), LVS (OR: 1.49, 95% CI: 1.21–1.84, p = 1.47 × 10−4), and LS (OR: 1.20, 95% CI: 1.001–1.44, p = 0.048). However, IVW method analysis showed no causal relationship between GRED and CES (OR: 1.04, 95% CI: 0.90–1.21, p = 0.563), and ICH (OR: 1.00, 95% CI: 1.00–1.00, p = 0.215). The results of other MR methods (including weighted median, MR-Egger, simple mode, and weighted mode) analyzing the relationship between GERD and overall stroke, IS, LVS, and LSs were consistent with IVW. MR results assessing the causal impact of GERD on the risk of stroke and its common subtypes are shown in Table 3.
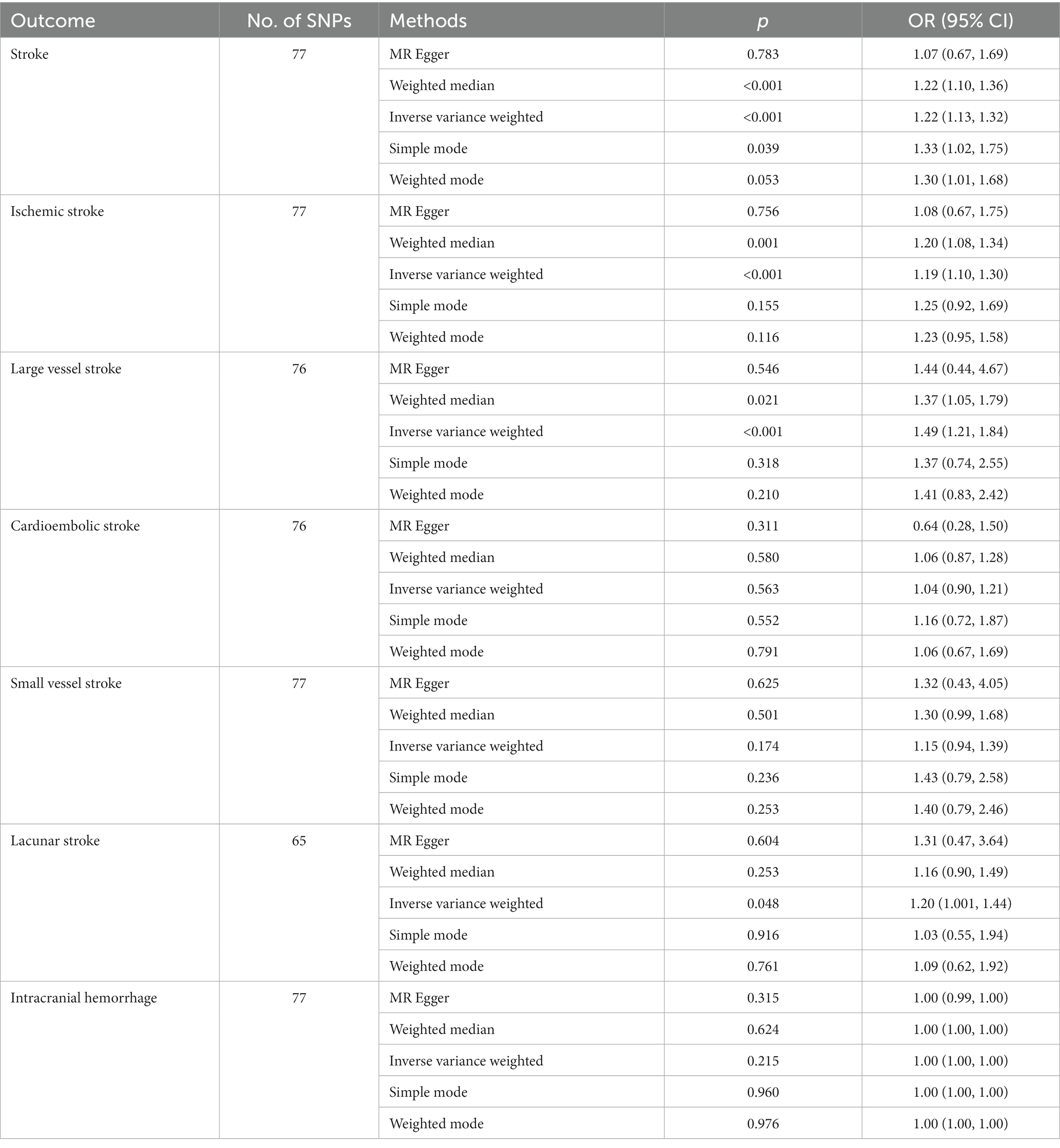
Table 3. MR analysis results of various methods used to assess the causal relationship between GERD and stroke and its common subtypes.
The causal relationship between GERD and overall stroke risk, IS, LVS, and LS can be visually displayed through scatter plots (Figure 2) and forest plots (Supplementary Figure S1).
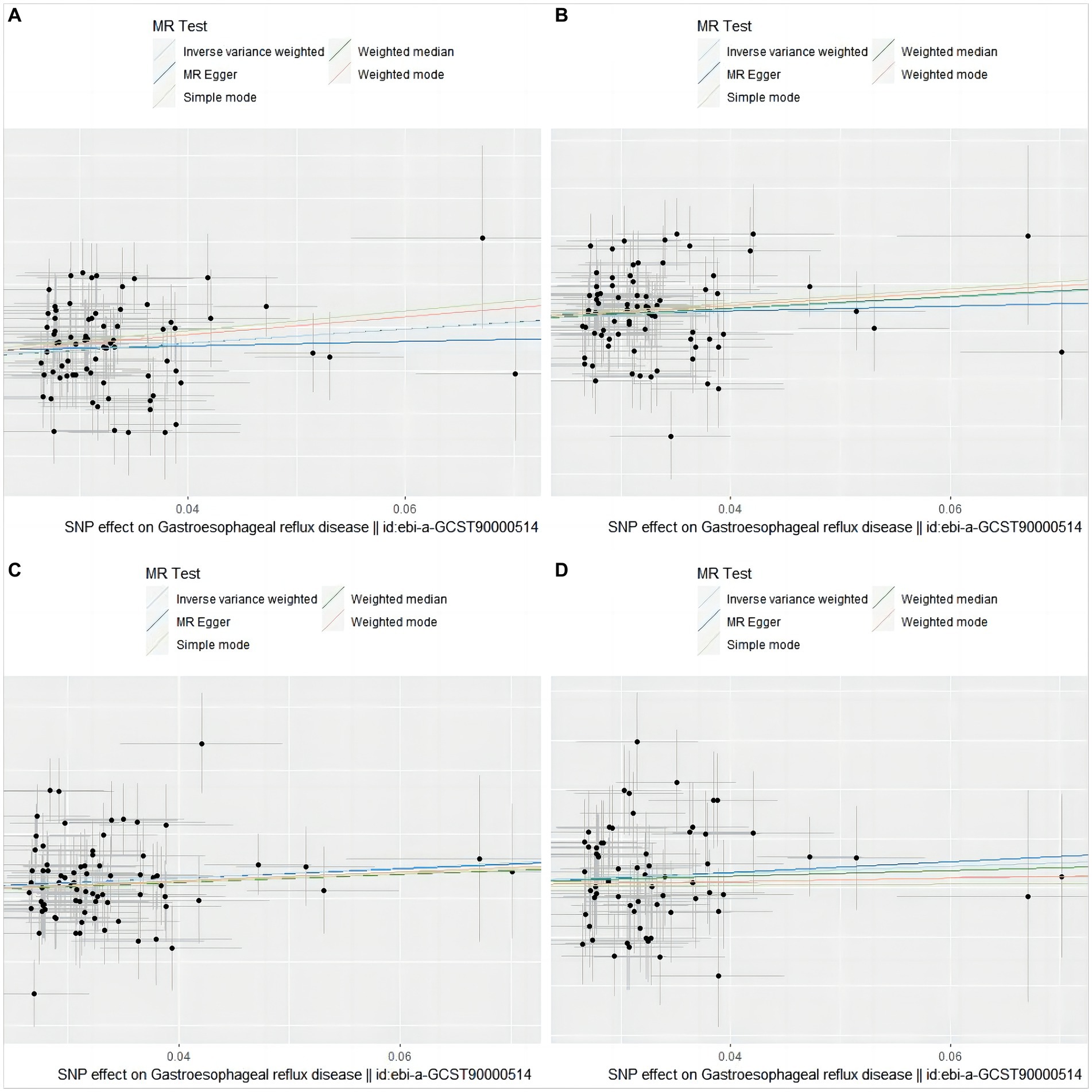
Figure 2. Scatter plot of genetic association between GERD and overall stroke, IS, LVS, and LS. (A) stroke; (B) ischemic stroke; (C) large vessel stroke; (D) lacunar stroke. The slope and direction of the straight line represent the magnitude and direction of the causal relationship.
The MR Egger intercept test found no pleiotropic effect (all p-val > 0.05). The MR PRESSO test of GERD for IS, LVS, CES, SVS, LS, and ICH also did not detect a pleiotropic effect (p-val > 0.05). The Cochran Q test of GERD for IS, LVS, CES, SVS, LS, and ICH did not detect heterogeneity (Q_p-val > 0.05). The Cochran Q test only detected heterogeneity in the overall stroke (Q_p-val = 0.020) (Table 4).
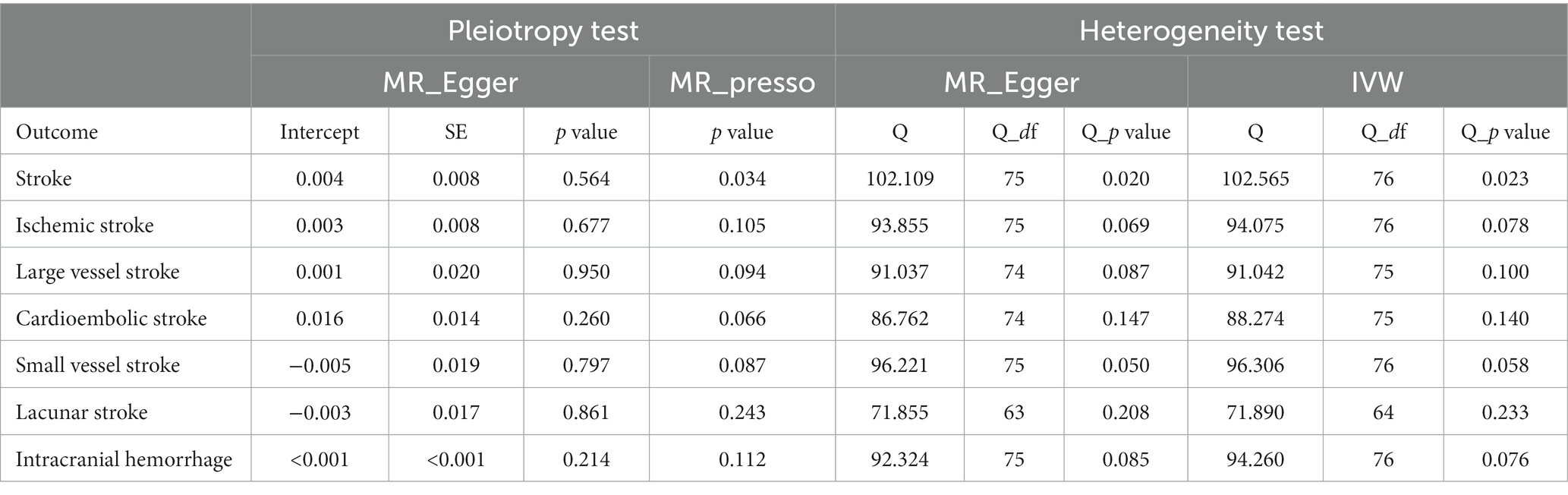
Table 4. Heterogeneity and horizontal pleiotropy analyses between GERD and stroke and its common subtypes.
In the leave-one-out analysis, no single SNP of GERD with a significant effect on MR results was present (Figure 3). Funnel plots (Supplementary Figure S2) also show the distribution of GERD on the risk of stroke, and its common subtypes were roughly symmetrical when a single SNP was used as an IV.
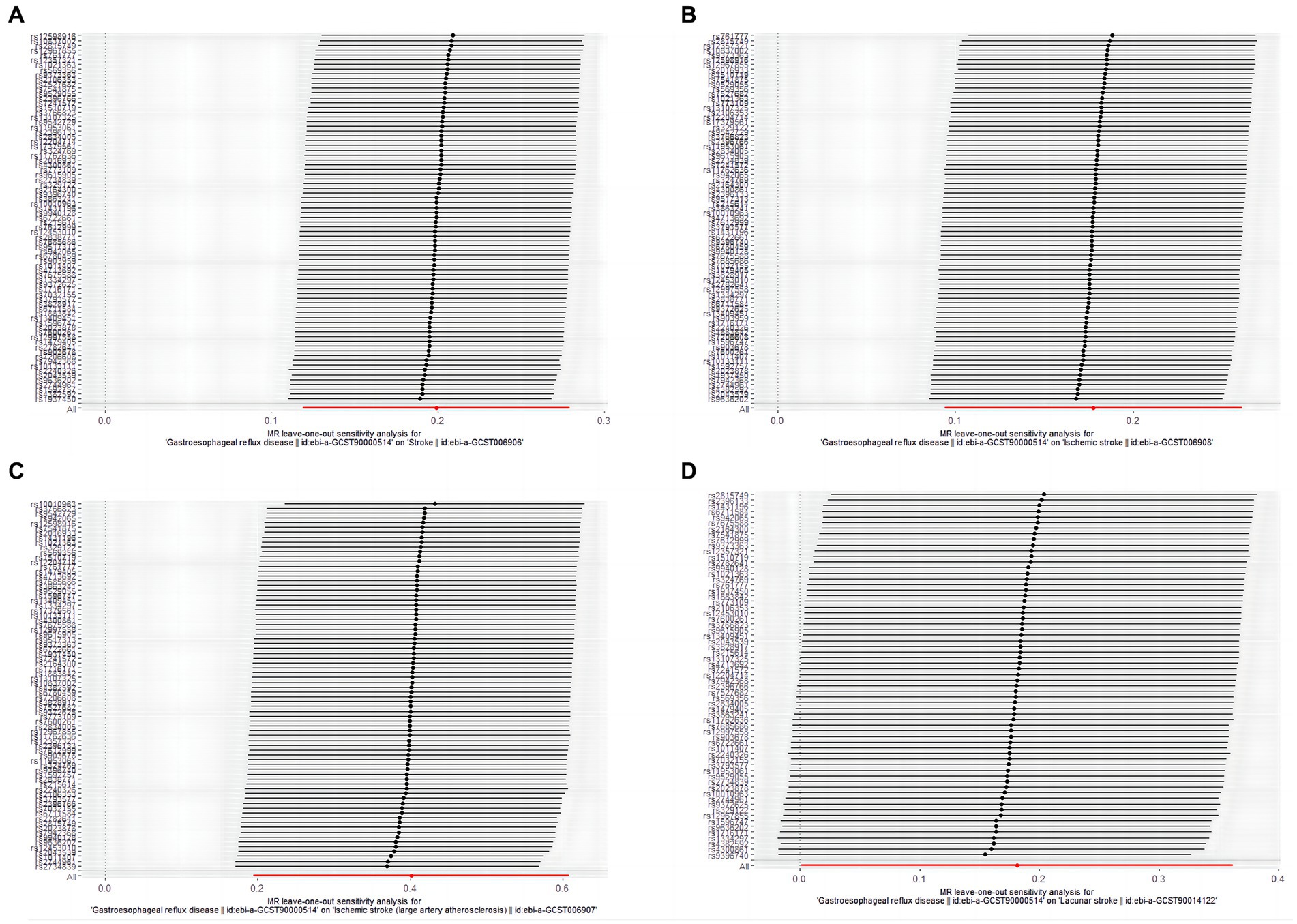
Figure 3. The leave-one-out-sensitivity forest plot of genetic association between GERD and overall stroke, IS, LVS, and LS. (A) stroke; (B) ischemic stroke; (C) large vessel stroke; (D) lacunar stroke.
The IVW result showed that overall stroke (OR: 1.09, 95% CI: 1.004–1.19, p = 0.039) and IS (OR: 1.10, 95% CI: 1.03–1.17, p = 0.007) have an apparent causal effect on the increased risk of GERD. Other complementary MR methods also validated the results obtained from the IVW analysis. However, the results of MR analysis showed no significant causal relationship among LVS (OR: 1.01, 95% CI: 0.96–1.07, p = 0.609), CES (OR: 1.02, 95% CI: 0.98–1.05, p = 0.320), LS (OR: 1.04, 95% CI: 0.99–1.10, p = 0.141), and GERD. Because SVS and ICH lacked sufficient SNPs to be used as IVs, their causal relationship with GERD risk could not be assessed. The MR results evaluating the causal impact of stroke and its common subtypes on the risk of GERD are shown in Table 5.
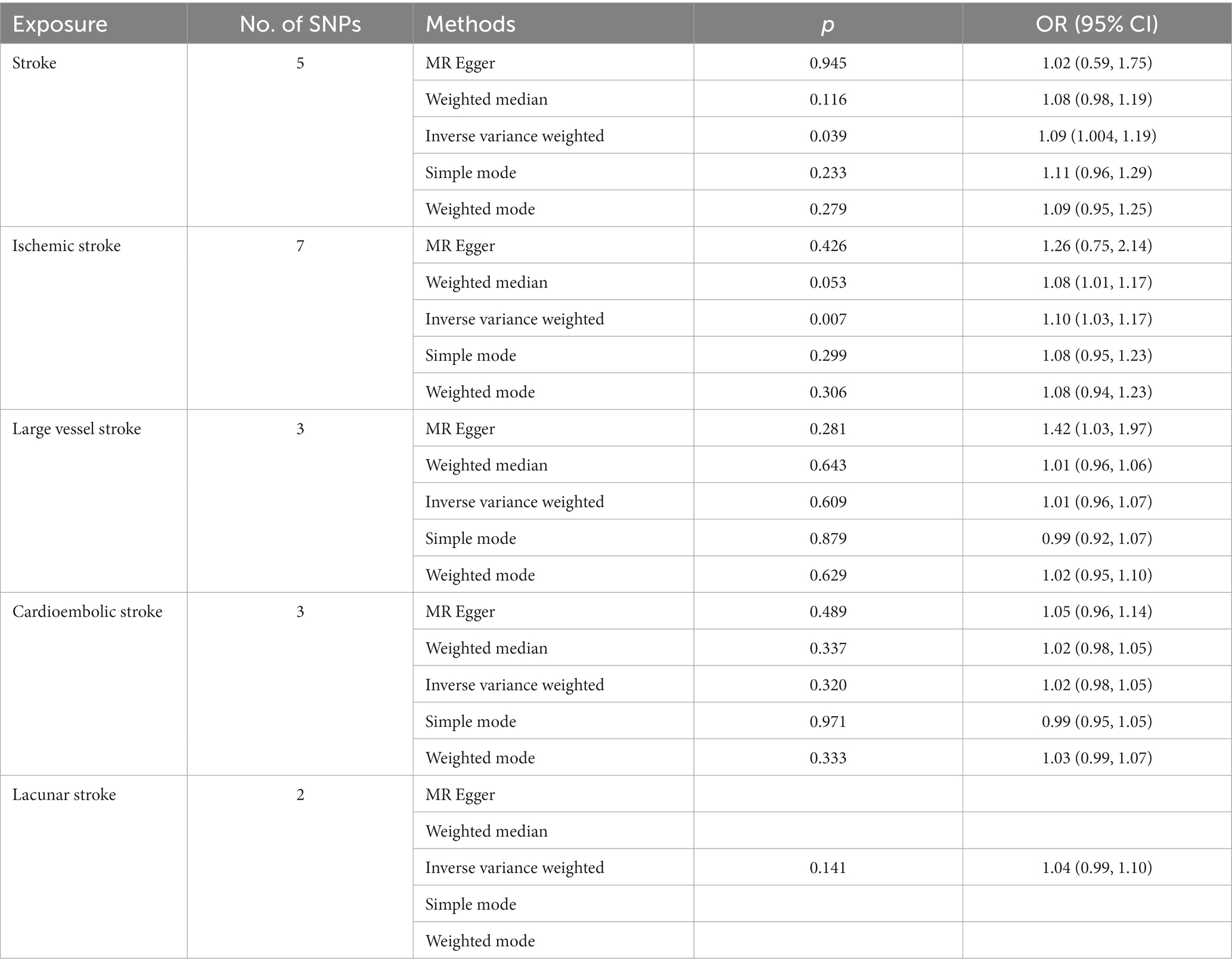
Table 5. MR analysis results of various methods used to assess the causal relationship between stroke and its common subtypes and GERD.
The causal relationship between overall stroke, IS, and the risk of GERD can be visually displayed through scatter plots (Supplementary Figure S3) and forest plots (Supplementary Figure S4).
Cochran Q and MR Egger intercept tests found no heterogeneity or directional pleiotropy between overall stroke, IS, LVS, CES, and GERD (p-val > 0.05). The MR PRESSO test also found no evidence of a pleiotropic effect between overall stroke, IS, CES, and GERD (p-val > 0.05). Although the MR Egger intercept test of causality between LS and GERD found no heterogeneity (p-val > 0.05), the Cochran Q test could not be performed due to the lack of sufficient available SNPs (Table 6).
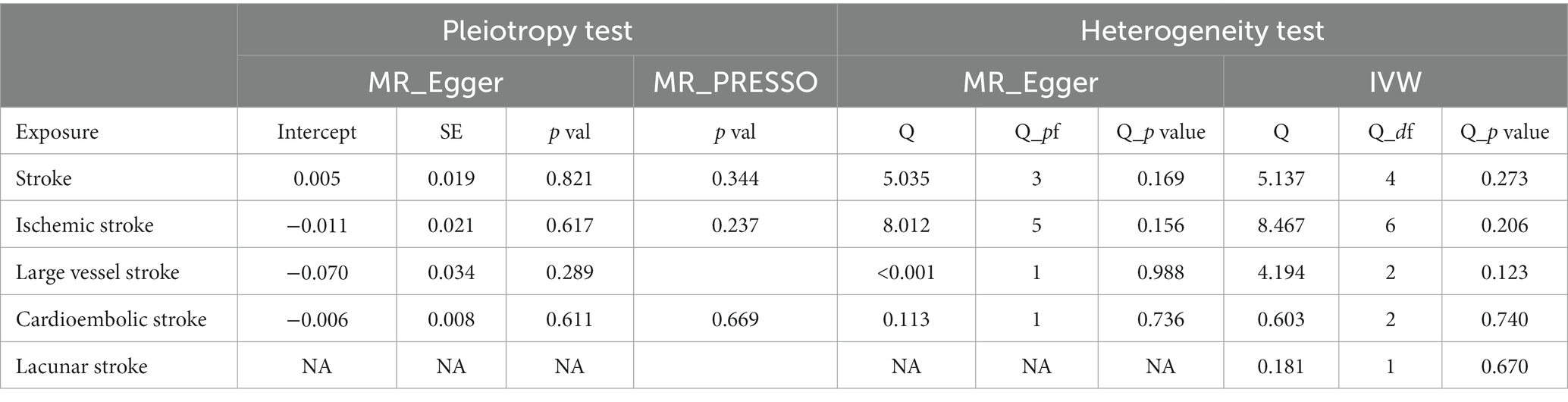
Table 6. Heterogeneity and horizontal pleiotropy analyses between stroke and its common subtypes and GERD.
In the leave-one-out analysis, no single SNP with a significant effect on MR results was present (Supplementary Figure S5).
To further investigate potential mediators of increased risk of GERD and stroke, we used MR analysis to evaluate the causal relationship between GERD on common risk factors for stroke and stroke on the risk factor of obesity for GERD. As shown in Table 7, GERD is causally linked to higher triglyceride levels (OR: 1.20, 95% CI: 1.17–1.23, p = 8.30 × 10−41), type 2 diabetes (OR: 1.58, 95% CI: 1.41–1.78, p = 4.80 × 10−5), higher BMI (OR: 1.44, 95% CI: 1.35–1.54, p = 8.63 × 10−9), greater waist-to-hip ratio (OR: 1.05, 95% CI: 1.02–1.10, p = 0.001), and sleep apnea syndrome (OR: 1.75, 95% CI: 1.54–2.00, p = 3.46 × 10−17). Although there may also be a causal relationship between GERD and lower HDL cholesterol levels (OR: 0.81, 95% CI: 0.77–0.85, p = 8.42 × 10−19) and hypertension (OR: 1.03, 95% CI: 1.01–1.04, p = 0.0004), the MR Egger test of the causal relationship between them found heterogeneity (p-val<0.05). However, no causal relationship was found among stroke, higher BMI, and greater waist-to-hip ratio. According to risk factor analysis, hypertriglyceridemia, type 2 diabetes, obesity, and sleep apnea syndrome may be the reasons for the further increased risk of stroke caused by GERD.
In recent years, as the incidence of GERD and stroke has continued to increase, there has been an increasing overlap between patients with these two common medical clinical diseases. Therefore, further exploration of the potential relationship between the two diseases is increasingly important to prevent and treat them. Several clinical observational studies in recent years have shown that GERD can increase the risk of stroke in different groups of people, such as regular GERD patients, young adult GERD patients, and GERD patients with atrial fibrillation (7, 8, 21). Similarly, a population-based cohort study also found that stroke patients had a higher risk of GERD (6). These studies may show a possible bidirectional correlation between GERD and stroke.
A previous MR study found a causal relationship between GERD and some cardiovascular diseases (CVD) (22). Although this study provides a detailed assessment of the causal relationship between GERD and multiple CVDs, including any stroke, IS and ICH, and numerous CVD risk factors, it was not able to further analyze the causal relationship between GERD and various common subtypes of IS, and the causal association between stroke and its subtypes and GERD risk. Another previous MR study evaluated the causal relationship between stroke and gastrointestinal diseases, including GERD (23). This study found that ICH, especially deep ICH, can causally increase the risk of gastrointestinal disorders such as GERD. This study also found a causal relationship between IS and GERD, but they failed to find a causal relationship between overall stroke and subtypes of IS and GERD. We first used the two-sample MR analysis of bidirectional causality to evaluate the causal relationship between GERD and stroke and its common subtypes (including IS, LVS, SVS, CES, LS, and ICH). Our research results are the first to demonstrate a bidirectional causal relationship between GERD and overall stroke, IS. In addition, GERD will also causally increase the risk of stroke common subtypes such as LVS and LS. However, our study did not find evidence of a causal association between GERD and CES, SVS, and ICH.
The cause of GERD leading to stroke and its common subtypes are not yet precise but may be related to vagal nerve dysfunction. GERD can cause an increased risk of vagal nervous system dysfunction by affecting regular esophageal transport, peristalsis, and normal function of the lower esophageal sphincter (24). Vagal nervous system dysfunction leads to impairment of the cholinergic anti-inflammatory pathway, leading to immune system dysregulation, increased inflammatory mediators in the bloodstream, and exaggerated immune response. The development and progression of atherosclerosis caused by excessive inflammatory response in blood vessels and impairment of cerebrovascular autonomic flow regulation function lead to an increased risk of strokes such as large vessel strokes (25, 26). In addition, studies have shown that esophageal acid reflux can not only cause esophageal peristalsis disorder and vagus nerve damage but also lead to a decrease in coronary blood flow, induce the occurrence of cardiovascular diseases such as angina, and thus increase the risk of cardiogenic stroke (27).
The causal relationship between stroke and the risk of GERD may be attributed to impaired voluntary control of oropharyngeal movements, changes in intestinal mucosal microbiota, and disruption of the gut–brain axis after brain injury. First, dysphagia after IS decreased esophageal sphincter muscle tone, and impairment of normal gastrointestinal motility function are important risk factors leading to the occurrence and aggravation of GERD. In addition, stroke can also exacerbate gastrointestinal symptoms such as dysphagia by affecting nutritional factors and neural centers regulating intestinal motility and appetite, such as the sympathetic and parasympathetic medulla and hypothalamic center, thereby further increasing the risk of GERD (4). Second, recent studies have found that the normal microbiota of the intestinal mucosa of mice is destroyed after brain injury caused by stroke. The main manifestations are the decrease in the level of Prevotellaceae, the increase in the abundance of Clostridium species, and the increase in the level of Myxobacteria (28, 29). A recent study pointed out that the imbalance of intestinal flora is involved in the occurrence and development of GERD (30). Therefore, the destruction of intestinal microbiota by stroke may be one of the critical factors leading to the increased risk of GERD. In addition, ischemic stroke can also lead to dysregulation of immune signal transduction and induce the development of brain–gut complications, such as gut microbiota dysbiosis, thereby further disrupting the normal circulation of the brain–gut axis, leading to the development of gastrointestinal diseases (31). Moreover, the causal relationship between GERD and stroke may also be related to common risk factors such as diet, smoking, and metabolic syndrome (obesity, hypertension, etc.) (32, 33). After we analyzed some related risk factors for stroke and GERD, it was found that GERD is causally responsible for the increased risk of stroke risk factors such as hypertriglyceridemia, type 2 diabetes, obesity, and sleep apnea syndrome.
Stroke and GERD are two common clinical diseases that cause substantial health risks and economic burdens worldwide. Since there is a proven bidirectional causal relationship between the two diseases, clinicians need to guide GERD patients to prevent the occurrence of stroke, and stroke patients should also pay attention to the potential risk of GERD. A population-based study points to a bidirectional causal relationship between GERD and sleep problems (34). Another recent study showed that insomnia increases the risk of stroke by following 31,126 participants for 9 years (35). Similarly, one study analysis found a bidirectional association between GERD and depression (36). Another prospective study found that major depressive episodes significantly increase the risk of stroke (37). Therefore, improving sleep and mood problems in GERD patients may effectively reduce the risk of stroke.
For patients at potential risk of stroke or those following a stroke, the use of drugs such as aspirin, calcium channel blockers, or the use of some non-oral feeding methods such as nasogastric (NG) tubes may lead to a higher risk of stroke. Aspirin is a commonly used drug to prevent ischemic stroke in patients who have experienced a cardiovascular event such as stroke or have risk factors for stroke such as atherosclerosis and antiphospholipid antibody syndrome (38). However, research suggests taking aspirin may lead to an increased risk of gastrointestinal diseases such as GERD. A study shows that long-term use of PA32540 (enteric-coated aspirin 325 mg + immediate-release omeprazole 40 mg) can effectively reduce the risk of gastrointestinal diseases caused by aspirin alone (39). This discovery may provide a new and better medication regimen for patients who use aspirin to prevent the occurrence or recurrence of stroke in the future. In addition, when treating hypertension, a significant risk factor for stroke, attention should also be paid to the gastrointestinal risks caused by antihypertensive drugs such as calcium antagonists (40). Furthermore, for patients who require non-oral feeding methods due to dysphagia after stroke, research has found that compared with NG tube feeding, esophageal (OE) tubes may lead to a smaller risk of developing and developing GERD (41). This discovery may help clinicians better select appropriate non-oral feeding methods for stroke patients.
Our study relied on the two-sample bidirectional MR analysis method to explore the causal relationship between stroke and its common subtypes and GERD. Compared with traditional research, on the one hand, our research effectively enhances the reliability of the research results by avoiding the influence of confounding factors and reverse causality. On the other hand, the instrumental variables selected in our study come from rigorously screened GWAS summary data based on individuals of European ancestry and effectively avoid the adverse effects of population stratification and instrumental variable bias on research results. In addition, the GWAS data on GERD, stroke and its subtypes, and related risk factors we selected are all from different consortiums, and the ancestry of these data is European, so we can rule out the possibility of a high overlap rate of exposure and outcome cohorts.
Honestly, our research still has some flaws: because our data were derived from GWAS data pooled from other studies, the sex and age of each sample were not known, and there may have been overlap in IVs used for exposure and outcomes. Since the samples are all from people of European ancestry, the study’s results may not apply to people of ancestry from other parts of the world, such as East Asia or North America. Our study only found a bidirectional causal relationship among overall stroke, IS, and GERD, so unfortunately, we cannot determine which specific subtypes of stroke can causally increase the risk of GERD. Additionally, due to the lack of available IVs for SVS and ICH, we cannot assess the causal relationship between SVS, ICH, and GERD. These deficiencies may be the focus of future research using MR or other research methods to explore the relationship between stroke and GERD.
In summary, results from this MR study provide evidence for the existence of a causal relationship between GERD and stroke and its common subtypes. The specific mechanism of the causal relationship between the two still needs to be further explored in the future to better guide the diagnosis and treatment of stroke, GERD, and their complications.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
DM: Conceptualization, Data curation, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. XZ: Validation, Writing – review & editing. WY: Validation, Writing – review & editing. GY: Validation, Writing – review & editing. SC: Validation, Writing – review & editing. HL: Supervision, Writing – review & editing. LW: Supervision, Writing – review & editing. FZ: Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors gratefully thank the MEGASTROKE Consortium for providing summary statistics data. All MEGASTROKE Consortium authors are listed online (https://www.megastroke.org/authors.html). The MEGASTROKE project received funding from sources specified at https://www.megastroke.org/acknowledgements.html. The authors thank the METASTROKE consortium, the IEU Open GWAS project, and the UK Biobank for other GWAS summary statistics.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1295051/full#supplementary-material
1. Campbell, B, and Khatri, P. Stroke. Stroke Lancet. (2020) 396:129–42. doi: 10.1016/S0140-6736(20)31179-X
2. Vakil, N, van Zanten, SV, Kahrilas, P, Dent, J, and Jones, R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. (2006) 101:1900–20. doi: 10.1111/j.1572-0241.2006.00630.x
3. El-Serag, HB, Sweet, S, Winchester, CC, and Dent, J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. (2014) 63:871–80. doi: 10.1136/gutjnl-2012-304269
4. Yong, H, Ganesh, A, and Camara-Lemarroy, C. Gastrointestinal dysfunction in stroke. Semin Neurol. (2023) 43:609–25. doi: 10.1055/s-0043-1771470
5. Kunz, JS, Hemann, B, Edwin Atwood, J, Jackson, J, Wu, T, and Hamm, C. Is there a link between gastroesophageal reflux disease and atrial fibrillation. Clin Cardiol. (2009) 32:584–7. doi: 10.1002/clc.20660
6. Chang, CS, Chen, HJ, and Liao, CH. Patients with cerebral stroke have an increased risk of gastroesophageal reflux disease: a population-based cohort study. J Stroke Cerebrovasc Dis. (2018) 27:1267–74. doi: 10.1016/j.jstrokecerebrovasdis.2017.12.001
7. Sheu, JJ, Kang, JH, Lou, HY, and Lin, HC. Reflux esophagitis and the risk of stroke in young adults: a 1-year population-based follow-up study. Stroke. (2010) 41:2033–7. doi: 10.1161/STROKEAHA.110.588558
8. Jiang, Y, Damiris, K, Suero-Abreu, G, Xu, B, and Ahlawat, S. Reflux esophagitis is associated with higher risks of acute stroke and transient ischemic attacks in patients hospitalized with atrial fibrillation: a nationwide inpatient sample analysis. Medicine (Baltimore). (2021) 100:e26502. doi: 10.1097/MD.0000000000026502
9. Sekula, P, Del Greco, MF, Pattaro, C, and Köttgen, A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol. (2016) 27:3253–65. doi: 10.1681/ASN.2016010098
10. Skrivankova, VW, Richmond, RC, Woolf, BAR, Yarmolinsky, J, Davies, NM, Swanson, SA, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. JAMA. (2021) 326:1614–21. doi: 10.1001/jama.2021.18236
11. Malik, R, Chauhan, G, Traylor, M, Sargurupremraj, M, Okada, Y, Mishra, A, et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. (2018) 50:524–37. doi: 10.1038/s41588-018-0058-3
12. Traylor, M, Persyn, E, Tomppo, L, Klasson, S, Abedi, V, Bakker, MK, et al. Genetic basis of lacunar stroke: a pooled analysis of individual patient data and genome-wide association studies. Lancet Neurol. (2021) 20:351–61. doi: 10.1016/S1474-4422(21)00031-4
13. Ong, JS, An, J, Han, X, Law, MH, and Nandakumar, P23 and Me Research team, et al. Multitrait genetic association analysis identifies 50 new risk loci for gastro-oesophageal reflux, seven new loci for Barrett's oesophagus and provides insights into clinical heterogeneity in reflux diagnosis. Gut. (2022) 71:1053–61. doi: 10.1136/gutjnl-2020-323906
14. Lin, Z, Deng, Y, and Pan, W. Combining the strengths of inverse-variance weighting and egger regression in Mendelian randomization using a mixture of regressions model. PLoS Genet. (2021) 17:e1009922. doi: 10.1371/journal.pgen.1009922
15. Verbanck, M, Chen, CY, Neale, B, and Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
16. Bowden, J, Davey Smith, G, Haycock, PC, and Burgess, S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
17. Hartwig, FP, Davey Smith, G, and Bowden, J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. (2017) 46:1985–98. doi: 10.1093/ije/dyx102
18. Tao, H, Fan, S, Zhu, T, You, L, Zheng, D, Yan, L, et al. Psychiatric disorders and type 2 diabetes mellitus: a bidirectional Mendelian randomization. Eur J Clin Investig. (2023) 53:e13893. doi: 10.1111/eci.13893
19. Kleindorfer, DO, Towfighi, A, Chaturvedi, S, Cockroft, KM, Gutierrez, J, Lombardi-Hill, D, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. (2021) 52:e364–364e467. doi: 10.1161/STR.0000000000000375
20. Eusebi, LH, Ratnakumaran, R, Yuan, Y, Solaymani-Dodaran, M, Bazzoli, F, and Ford, AC. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut. (2018) 67:430–40. doi: 10.1136/gutjnl-2016-313589
21. Kristensen, SL, Lindhardsen, J, Ahlehoff, O, Erichsen, R, Lamberts, M, Khalid, U, et al. Increased risk of atrial fibrillation and stroke during active stages of inflammatory bowel disease: a nationwide study. Europace. (2014) 16:477–84. doi: 10.1093/europace/eut312
22. Sun, X, Chen, L, and Zheng, L. A Mendelian randomization study to assess the genetic liability of gastroesophageal reflux disease for cardiovascular diseases and risk factors. Hum Mol Genet. (2022) 31:4275–85. doi: 10.1093/hmg/ddac162
23. Song, J, Chen, W, and Ye, W. Stroke and the risk of gastrointestinal disorders: a Mendelian randomization study. Front Neurol. (2023) 14:1131250. doi: 10.3389/fneur.2023.1131250
24. Cunningham, KM, Horowitz, M, Riddell, PS, Maddern, GJ, Myers, JC, Holloway, RH, et al. Relations among autonomic nerve dysfunction, oesophageal motility, and gastric emptying in gastro-oesophageal reflux disease. Gut. (1991) 32:1436–40. doi: 10.1136/gut.32.12.1436
26. Ko, SH, Song, KH, Park, SA, Kim, SR, Cha, BY, Son, HY, et al. Cardiovascular autonomic dysfunction predicts acute ischaemic stroke in patients with type 2 diabetes mellitus: a 7-year follow-up study. Diabet Med. (2008) 25:1171–7. doi: 10.1111/j.1464-5491.2008.02567.x
27. Chauhan, A, Petch, MC, and Schofield, PM. Cardio-oesophageal reflex in humans as a mechanism for linked angina. Eur Heart J. (1996) 17:407–13. doi: 10.1093/oxfordjournals.eurheartj.a014873
28. Houlden, A, Goldrick, M, Brough, D, Vizi, ES, Lénárt, N, Martinecz, B, et al. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav Immun. (2016) 57:10–20. doi: 10.1016/j.bbi.2016.04.003
29. Stanley, D, Moore, RJ, and Wong, C. An insight into intestinal mucosal microbiota disruption after stroke. Sci Rep. (2018) 8:568. doi: 10.1038/s41598-017-18904-8
30. Rescigno, M . Microbiota in reflux esophagitis and peptic ulcer disease. Surg Infect. (2023) 24:245–9. doi: 10.1089/sur.2023.021
31. Yang, Z, Wei, F, Zhang, B, Luo, Y, Xing, X, Wang, M, et al. Cellular immune signal exchange from ischemic stroke to intestinal lesions through brain-gut Axis. Front Immunol. (2022) 13:688619. doi: 10.3389/fimmu.2022.688619
32. Mohammed, I, Nightingale, P, and Trudgill, NJ. Risk factors for gastro-oesophageal reflux disease symptoms: a community study. Aliment Pharmacol Ther. (2005) 21:821–7. doi: 10.1111/j.1365-2036.2005.02426.x
33. Goldstein, LB, Adams, R, Alberts, MJ, Appel, LJ, Brass, LM, Bushnell, CD, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association stroke council: cosponsored by the atherosclerotic peripheral vascular disease interdisciplinary working group; cardiovascular nursing council; clinical cardiology council; nutrition, physical activity, and metabolism council; and the quality of care and outcomes research interdisciplinary working group: the American Academy of Neurology affirms the value of this guideline. Stroke. (2006) 37:1583–633. doi: 10.1161/01.STR.0000223048.70103.F1
34. Jansson, C, Nordenstedt, H, Wallander, MA, Johansson, S, Johnsen, R, Hveem, K, et al. A population-based study showing an association between gastroesophageal reflux disease and sleep problems. Clin Gastroenterol Hepatol. (2009) 7:960–5. doi: 10.1016/j.cgh.2009.03.007
35. Sawadogo, W, Adera, T, Alattar, M, Perera, R, and Burch, JB. Association between insomnia symptoms and trajectory with the risk of stroke in the health and retirement study. Neurology. (2023) 101:e475–88. doi: 10.1212/WNL.0000000000207449
36. Kim, SY, Kim, HJ, Lim, H, Kong, IG, Kim, M, and Choi, HG. Bidirectional association between gastroesophageal reflux disease and depression: two different nested case-control studies using a national sample cohort. Sci Rep. (2018) 8:11748. doi: 10.1038/s41598-018-29629-7
37. Sun, J, Ma, H, Yu, C, Lv, J, Guo, Y, Bian, Z, et al. Association of Major Depressive Episodes with Stroke Risk in a prospective study of 0.5 million Chinese adults. Stroke. (2016) 47:2203–8. doi: 10.1161/STROKEAHA.116.013512
38. Kernan, WN, Ovbiagele, B, Black, HR, Bravata, DM, Chimowitz, MI, Ezekowitz, MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2014) 45:2160–236. doi: 10.1161/STR.0000000000000024
39. Cayla, G, Collet, JP, Silvain, J, Thiefin, G, Woimant, F, and Montalescot, G. Prevalence and clinical impact of upper gastrointestinal symptoms in subjects treated with low dose aspirin: the UGLA survey. Int J Cardiol. (2012) 156:69–75. doi: 10.1016/j.ijcard.2010.10.027
40. Horikawa, A, Ishii-Nozawa, R, Ohguro, M, Takagi, S, Ohtuji, M, Yamada, M, et al. Prevalence of GORD (gastro-oesophageal reflux disease) in type 2 diabetes and a comparison of clinical profiles between diabetic patients with and without GORD. Diabet Med. (2009) 26:228–33. doi: 10.1111/j.1464-5491.2009.02671.x
Keywords: stroke, gastroesophageal reflux disease, Mendelian randomization, causality, single-nucleotide polymorphisms
Citation: Meng D, Zhang X, Yu W, Yin G, Chen S, Liu H, Wang L and Zhang F (2024) Association between gastroesophageal reflux disease and stroke: a bidirectional Mendelian randomization study. Front. Neurol. 14:1295051. doi: 10.3389/fneur.2023.1295051
Received: 15 September 2023; Accepted: 21 December 2023;
Published: 23 January 2024.
Edited by:
Maurizio Acampa, Siena University Hospital, ItalyReviewed by:
HaoRan Tao, Sun Yat-sen University, ChinaCopyright © 2024 Meng, Zhang, Yu, Yin, Chen, Liu, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fengxia Zhang, Znh6aGFuZzA5ODdAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.