- 1Department of Neurology, Tangdu Hospital, Air Force Medical University, Xi’an, Shaanxi Province, China
- 2Xi’an Medical University, Xi’an, Shaanxi Province, China
Combined central and peripheral demyelination (CCPD) is an extremely rare disease characterized by inflammatory demyelination in both the central and peripheral nervous systems. Herein, we reported case of a 14-year-old teenager who initially presented with the symptoms of acute myelitis (AM). Subsequently, the patient developed symptoms consistent with Guillain-Barré syndrome (GBS), which was supported by nerve conduction studies (NCV) and cerebrospinal fluid (CSF) analysis. Throughout the course of the disease, the patient experienced abdominal pain and abnormal liver function. After a comprehensive evaluation, we determined that the abnormal liver function was a result of hepatitis E virus (HEV) infection, which may have acted as a trigger for GBS. The patient was treated with corticosteroids, intravenous immunoglobulin and Rituximab, resulting in symptom relief and clinical improvement after therapy and follow-up. This case highlights the potential responsiveness and reversibility of CCPD. Given the heterogeneous nature of CCPD, there is currently no standardized diagnostic criteria or clear consensus on its treatment. Therefore, we recommend a thorough assessment of all possibilities and the development of consolidated management guidelines based on available data for this disorder.
Introduction
Inflammatory demyelination diseases encompass a broad range of disorders characterized by demyelinating lesions mediated by inflammation. These disorders typically affect either the central nervous system (CNS) or peripheral nervous system (PNS). Combined central and peripheral demyelination (CCPD) is a rare condition that manifests as simultaneous or temporally separated demyelination in both the CNS and PNS. This particular disease is not commonly encountered in clinical practice (1). Diagnosing CCPD can be challenging due to the limited knowledge available, which is primarily derived from case reports and small case series (2–5). Moreover, some cases exhibited both CNS and PNS impairment were named as Encephalomyeloradiculoneuropathy (EMRN), which is similar with CCPD. Specific biomarkers were identified in CCPD. In 2013, Kawamura et al. found that anti-neurofascin-155 antibodies was positive in some CCPD patients (4). Recent studies reveal some antibodies were also associated with CCPD, such as anti-neurofascin186 antibody, antigalactocerebroside and antilactosylceramide (4, 6, 22, 23). While in EMRN, anti-neutral glycosphingolipid antibodies especially anti-lactosylceramide (LacCer) antibodies were identified (25). In this case report, we present a case report of a patient manifested the symptoms of acute myelitis (AM) and Guillain-Barré syndrome (GBS). Ultimately, she was diagnosed with CCPD.
Case presentation
A 14-year-old girl with no significant medical or past history presented with weakness and tingling paresthesia in both lower extremities for 1 week. Additionally, she experienced stool and urinary retention. Prior to her presentation, she had transient abdominal pain but no fever or diarrhea. She had not received any vaccines within the past month. She was initially diagnosed with acute myelitis at a local hospital and was subsequently admitted to the neurology department of our hospital. The patient had no history of other neurological disorders, fever, weight loss, or night sweats. She also denied any changes in vision, difficulty swallowing or speaking, shortness of breath, chest pain, and loss of smell or taste sensation. Upon admission, the weakness in her lower extremities gradually worsened, while the stool and urinary retention improved. She required a wheelchair and was unable to stand up independently. A thorough examination was conducted. Initial findings and vital signs were within normal limits, and gastrointestinal, respiratory, and cardiovascular examinations were unremarkable. Neurological examination revealed that the patient was conscious, alert, and oriented. She had normal muscle bulk, but the strength of her bilateral lower extremities was graded as 2 on the muscle strength grading scale (with a maximum score of 5), with the right side being more affected than the left. Lower extremity reflexes and plantar reflex were absent, and there was hyperalgesia up to the bilateral groin.
The hemogram, liver, renal, and thyroid functions (TSH, Free T4), electrolytes (Na, K, Ca, P, Mg), muscle enzymes, vitamin B12 levels, as well as glycohemoglobin, were all within normal ranges. Virological tests (HIV, hepatitis B, hepatitis C, COVID-19) yielded negative results or fell within normal ranges. Immunological studies were conducted, and the results showed negative findings for antinuclear antibody, antiphospholipid IgM and IgG, anti-dsDNA, anticardiolipin IgM and IgG, anti-Sjogren antibody SSA and SSB, and extractable nuclear antigen. Visual-evoked potentials (VEPs) demonstrated normal P100 latencies bilaterally (right: 106 ms, left: 108 ms). SEP also exhibited normal latencies bilaterally. The CSF examination revealed normal intracranial pressure, a slightly elevated white blood cell count (20*10^6/L), and an elevated protein level (780 mg/L, normal range: 150–450 mg/L). CSF culture yielded negative results, and CSF cytology showed no malignant cells. Cerebrospinal fluid analysis indicated a normal albumin level (536 mg/L), normal IgG level (75.2 mg/L), and a normal CSF-to-serum albumin ratio (12.3). Serum albumin and serum IgG levels were also within normal ranges. Aquaporin-4, anti-MBP antibodies, and anti-MOG antibodies were not detected. CSF and blood oligoclonal bands were absent.
In the imaging assessments, Magnetic Resonance Imaging (MRI) revealed the absence of brain lesions (Figures 1G–J), while spinal cord resonance imaging and gadolinium-enhanced MRI of the spinal cord demonstrated mild cord edema, but we did not observe obvious enhancement of spinal lesion in enhanced T1-weighted MRI (Figures 1A,B,D,E). The spinal cord MRI did not indicate any manifestations of acute disseminated encephalomyelitis (ADEM) or multiple sclerosis (MS). In the early stages of treatment, the patient received methylprednisolone therapy (1,000 mg once daily) for a duration of 3 days, followed by a gradual reduction of the methylprednisolone dosage over the course of 1 week. Subsequent to the treatment, some improvement in muscle strength was observed (lower limbs: MRC grade: 2).
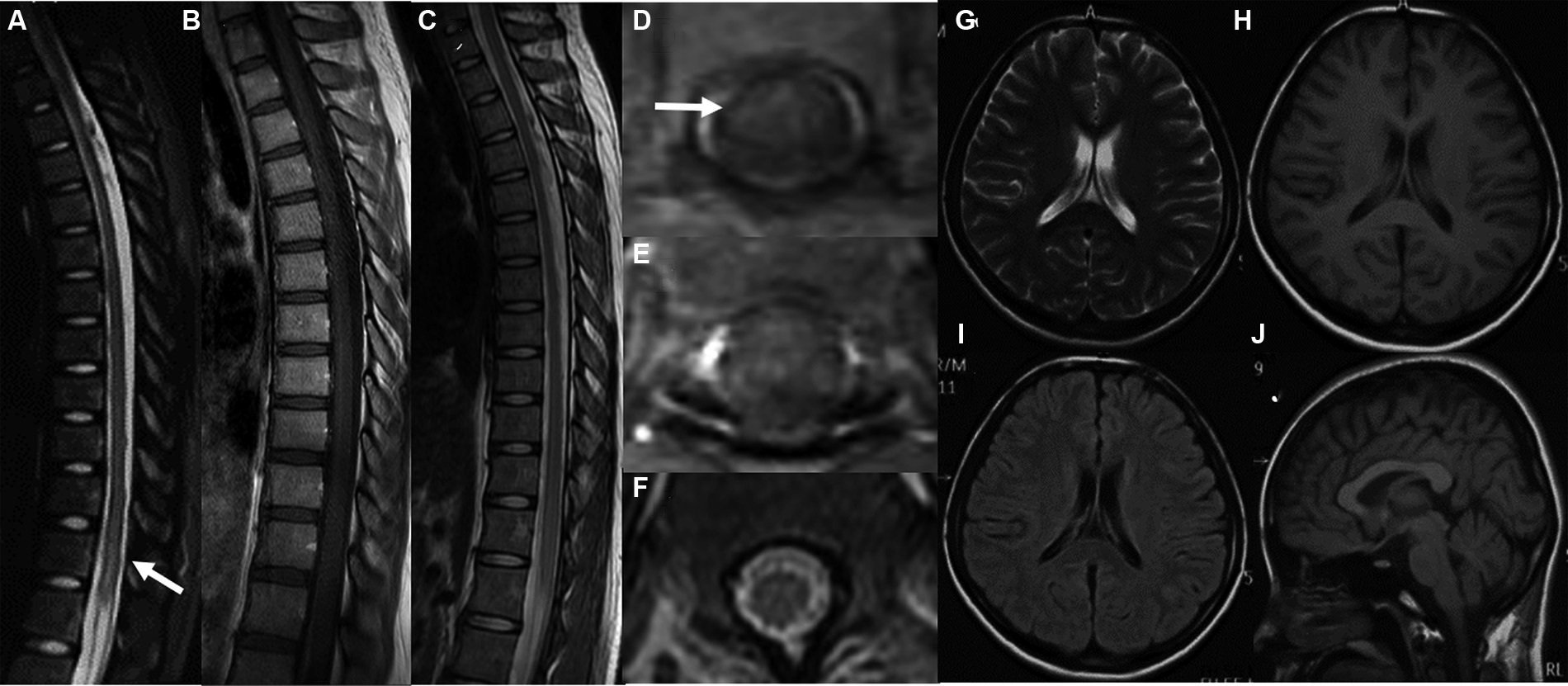
Figure 1. MRI images of the spinal cord both before and after therapy (A,C,D,F). Gadolinium-enhanced axial T1-weighted MRI of the spinal cord (B,E). MRI of the brain (G–J) revealed the absence of brain lesions. The sagittal T1-weighted image (A) and axial T1-weighted image (D) of the thoracic spinal cord before therapy revealed an abnormal signal in the thoracic and lumbar spinal cord (T11-L1). We did not observe obvious enhancement of spinal lesion in enhanced T1-weighted MRI (B,E). After therapy, the sagittal T1-weighted image (C) and axial T1-weighted image (F) demonstrated a significant reduction in the size of the previously observed lesions.
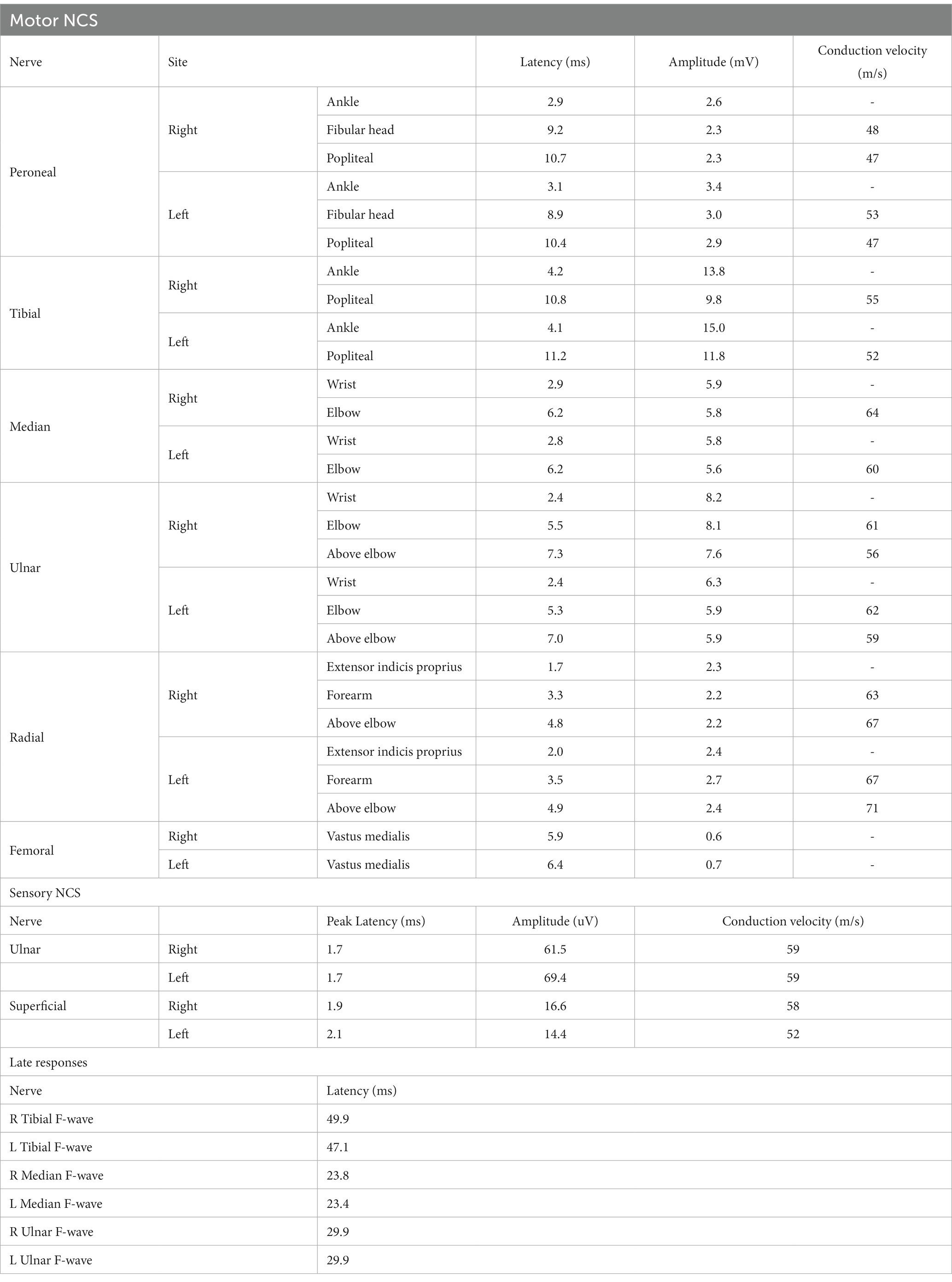
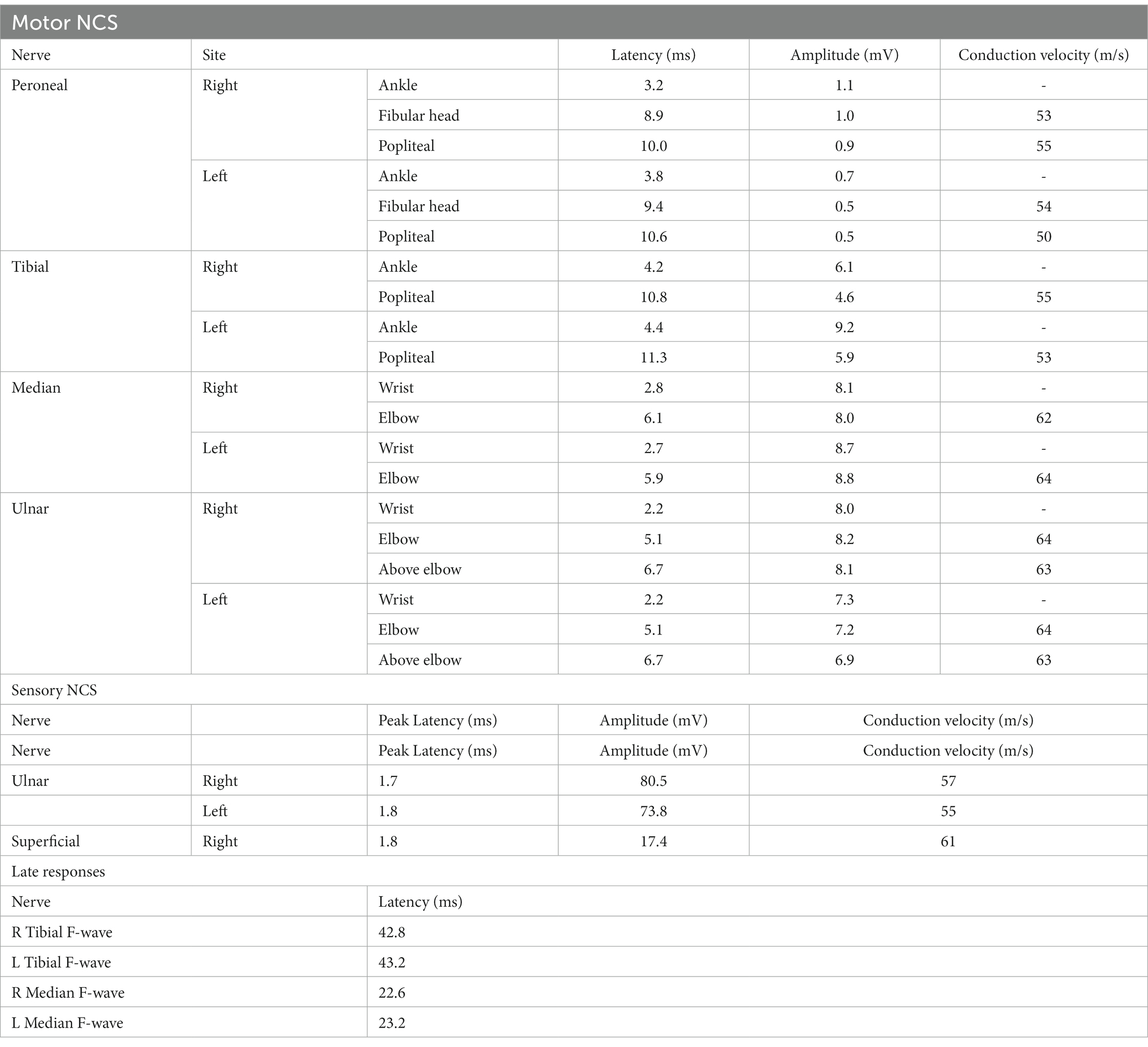
However, during the course of treatment, the patient experienced abdominal pain and exhibited abnormal liver function, as indicated by elevated alanine aminotransferase (ALT) levels of 205 U/L and aspartate aminotransferase (AST) levels of 60 U/L. Notably, muscle enzyme levels remained within the normal range. Screening for Epstein–Barr Virus, Cytomegalovirus, Herpes Simplex Virus, and Herpes Zoster Virus yielded negative results. Even after receiving hepatoprotective therapy, the patient’s ALT and AST levels did not return to normal. Additionally, the patient developed acute weakness in the upper limbs, which rapidly progressed to tetraplegia (bilateral lower limbs: MRC grade 2, left upper limb: MRC grade 2, and right upper limb: MRC grade 3). The patient’s deep tendon reflexes remained hyporeflexic. MRI of the spinal cord revealed no lesions in the cervical spinal cord (Figure 2). Subsequently, the patient underwent a series of electromyograms (EMGs) and nerve conduction studies (NCVs). NCVs revealed peripheral neuropathy (Table 1). CSF analysis showed protein cytological dissociation, with an increased protein level of 1317.5 mg/L and a normal cell count of 4 cells/mm3. The patient was negative for anti-glycolipid (GM1, GM2, GM3, GD1a, GD1b, GD3, GT1b, GQ1b, galactocerebroside). No oligoclonal bands were detected. The patient displayed the classic triad of weakness of extremities, albuminocytologic dissociation was observed in the CSF sample, which strongly supported the diagnosis of Guillain-Barré Syndrome (GBS). In light of the abnormal liver function, a comprehensive examination was conducted, ultimately determining that the patient’s abnormal liver function was a result of HEV infection. Intravenous immunoglobulin (IVIg) was administered at a dose of 0.4 g/kg/day and continued for 5 days. The patient demonstrated some improvement after completing the course of IVIg. She regained full strength (5/5) in all muscle groups in the upper extremities and had a strength of 3/5 in the lower extremities. As liver function continued to improve, the patient received Rituximab (RTX) infusion at a dose of 100 mg, with one-week intervals for two times. At four-month follow-up, her neurological condition is stable. She experienced progressive improvement in muscle strength in all limbs and was able to briefly stand with assistance. Follow-up MRI of the spinal cord revealed evidence of lesion improvement (Figures 1C,F). NCVs indicated improvement in demyelinating polyneuropathy (Table 2).
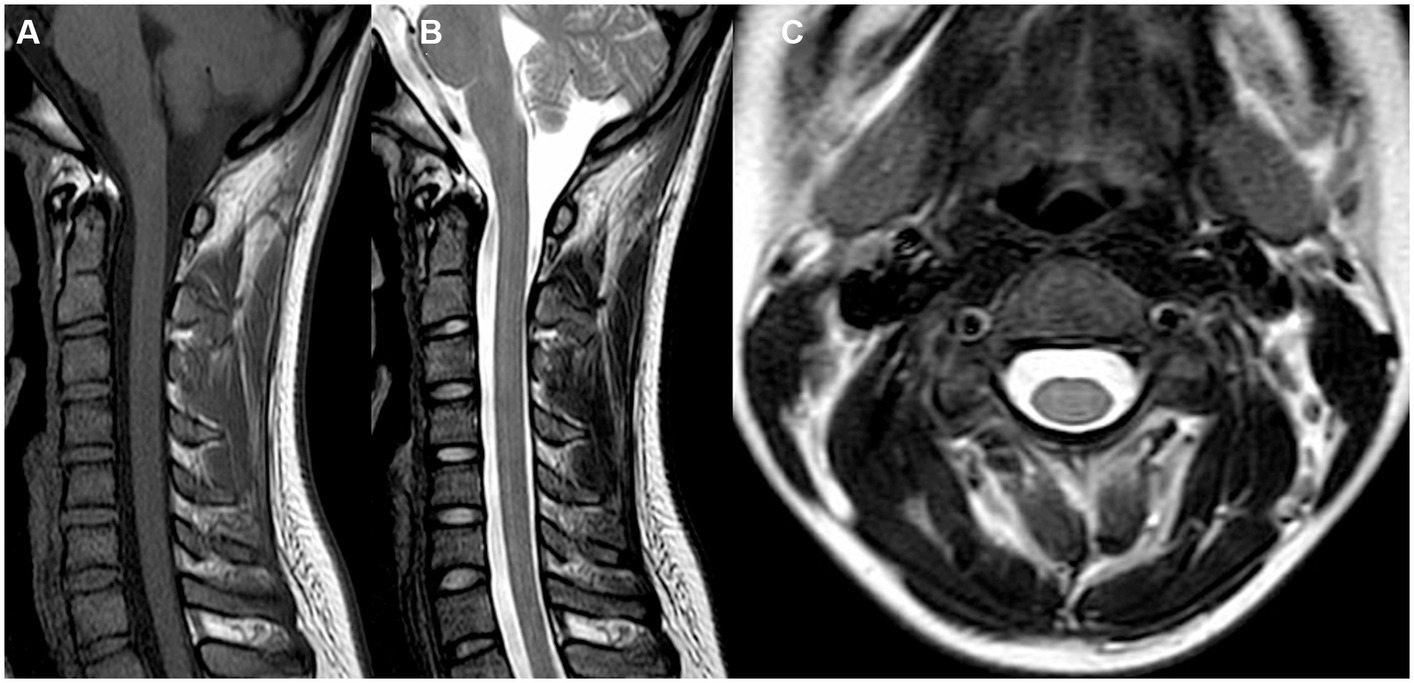
Figure 2. MRI images of the cervical spinal cord (A–C) were obtained. These included sagittal T1-weighted (A), T2-weighted (B), and axial T1-weighted (C) images. Notably, no lesions were observed in the cervical spinal cord.
Discussion
In this report, we present a case of CCPD in a teenage patient. Our case is notable both because the presence of central and peripheral demyelinating processes in one patient is a rare phenomenon. CCPD is characterized by demyelination affecting both the central and peripheral nervous systems. It remains unclear whether CCPD represents a distinct disorder separate from classical demyelinating diseases (6). Currently, there are no standardized criteria for diagnosing CCPD. However, a previous nationwide survey conducted in Japan established criteria for CCPD, which encompassed a diverse range of clinical manifestations involving both the CNS and PNS, such as encephalopathy, cranial neuropathy, myeloradiculoneuropathy, or Guillain-Barré syndrome (6). The synchronous occurrence of GBS and AM in CCPD appears to be rare and variable. Early-stage diagnosis of CCPD, particularly when symptoms involving both the CNS and PNS are present simultaneously, poses challenges. Published literature on cases reporting such concurrent conditions is limited. The underlying pathophysiological mechanism of CCPD remains elusive. It is uncertain whether the same antigenic target exists in both the PNS and CNS, or if there is a cross-reactive immune response to insults affecting either system (3). The immune response elicited by antecedent infections may cross-react with components of the peripheral nerves due to the sharing of common epitopes (7). Overall, CCPD encompasses a spectrum of conditions, including acute, relapsing, and chronic subtypes (4).
Anti-NF155 antibodies is a protein expressed on both central and peripheral myelin. It was first reported in CCPD cases in 2013 (4). Previous study has reported the high prevalence of anti-NF155 antibodies in CCPD (45.5–86%) (4). However, the positivity rates for anti-NF155 antibodies in CCPD differ among studies and ethnicities, some studies revealed the absent of antibody in this disorder. Therefore, we cannot exclude that anti-NF155 antibodies may be present in particular subgroups of patients with CCPD. So far, we are unable to demonstrate a pathophysiologic link between this case of CCPD and anti-NF155 antibody.
The manifestation of CCPD is similar with EMRN, but their pathogenesis remains to be fully elucidated. Although CCPD and EMRN both exhibit CNS and PNS impairments, the detailed pathogenesis of both disorders is still unclear at present. The clinical features of CCPD include a chronic onset, albumin-cytologic dissociation of cerebrospinal fluids, and a low frequency of oligoclonal IgG bands (OCB) positivity. In contrast, EMRN is an acute or subacute progressive disease that causes motor weakness, myelopathy, neuropathy, encephalopathy, and dysautonomia and mild CSF pleocytosis. CCPD is characterized by multifocal acquired inflammatory demyelinating sensory and motor neuropathy. While in EMRN, peripheral neuropathy is axonal, with or without demyelinating neuropathy (23, 24). Some autoantibodies, such as anti-lactosylceramide antibody, which was identified in CCPD was also found in EMRN. However, the specific biomarkers were identified for both disorders: anti-NF155 for CCPD and anti-neutral glycolipid (especially anti-lactosylceramide) for EMRN (6, 24, 27, 28). Our case exhibited some common features of EMRN such as acute motor weakness and dysautonomia, which are not common in CCPD. However, the albumin-cytological dissociation of cerebrospinal fluids and absent of OCB positivity, which is fulfilling the main features of CCPD. Ultimately, we diagnosed the case CCPD based on criteria of Ogata et al. (6). The clinical course of this case highlights the complexity of CCPD. Accurate diagnosis is crucial to ensure the patient receives the most appropriate and effective e treatment. Nerve conduction studies and spinal cord MRI are valuable diagnostic tools. Compared with previous patients with EMRN, our case had a progressing clinical course (25).
Furthermore, the patient presented the symptoms of GBS and we finally revealed HEV infection might be a trigger of GBS. GBS is a heterogeneous disease characterized by acute immune-mediated polyneuropathies, often associated with preceding viral or bacterial infections (7, 8). Previous studies have indicated the most frequently identified infectious triggers for GBS include Campylobacter jejuni infection, influenza-like illnesses, cytomegalovirus, Epstein–Barr virus (EBV), and varicella-zoster virus. Additionally, hepatotropic viruses such as hepatitis A, hepatitis B, and hepatitis C have been implicated as triggering agents (9). Recently, several case reports and studies have reported an association between HEV infection and GBS (10–13). It has been suggested that HEV infection may be responsible for 5 to 11% of sporadic GBS cases in developed countries (14). A study conducted in Bangladesh further confirmed the link between HEV and GBS (15). The exact mechanism underlying GBS associated with HEV infection remains uncertain. One possibility is that HEV directly infects the peripheral nerve roots or the central nervous system, causing neural cell injury, as demonstrated by its ability to replicate in various human neuronal-derived cell lines (16). Another potential mechanism involves molecular mimicry, in which the virus indirectly triggers the disorder by sharing similar antigenic characteristics with components of the peripheral nerves (7). In our patient, who presented with symptoms of GBS and abnormal liver function, we suspected that HEV infection may have triggered the development of GBS based on previous studies (15). Notably, elevated liver enzymes, including alanine or aspartate aminotransferase, are commonly observed in GBS patients with HEV infection, occurring in approximately 75% of cases according to combined series data (17, 18). Confirmation of HEV infection can be established through etiology testing, specifically when serum anti-HEV IgG is positive. As a result, we recommend considering HEV testing in GBS patients.
Due to its rarity, the treatment of CCPD has not been thoroughly investigated. In our case, the patient underwent steroid therapy along with additional administration of IVIg therapy, resulting in a transient response (19). Subsequently, the patient received RTX and exhibited a favorable response. Although similar cases have been reported in previous literature, the optimal therapy for CCPD remains uncertain. Steroids, IVIg, and plasmapheresis have shown efficacy in treating CCPD. Acute or subacute combined CCPD has rarely been observed in adults, with documented successful treatment using plasmapheresis and IV IgG or IV corticosteroids. More recently, Rituximab has also demonstrated positive outcomes in patients with CCPD (1, 20). In another case, RTX was utilized as a third-line therapy following treatment with steroids, IVIg, and Natalizumab, resulting in clinical and radiological improvement without relapses during the 28-month follow-up period (21). Moreover, in a placebo-controlled randomized controlled trial of relapsing–remitting MS (RRMS) patients, RTX was found to drastically decrease the counts of contrast-enhancing lesions as well as volumes of T2 lesions. Furthermore, it reduced the proportion of patients with relapse at 48 weeks (RTX:20.3% vs. placebo: 40.0%) (26). Therefore, aggressive immunomodulation therapy should be considered to induce remission in patients who do not respond to these treatments.
In conclusion, the clinical presentation of CCPD exhibits significant heterogeneity, making the diagnosis challenging in clinical practice. Therefore, it is imperative to establish definitive international criteria for CCPD in larger sample sizes and across multiple centers. We recommend the utilization of MRI and electrophysiological examinations for patients suspected of having CCPD. In cases where both AM and GBS manifestations are observed, we suggest conducting anti-AQP4, MOG, and NCV assessments to achieve a precise diagnosis. Furthermore, it is crucial to exclude HEV infection in patients presenting with GBS symptoms and abnormal liver function. If patients exhibit a poor response to steroids and intravenous immunoglobulin, RTX should be considered as a secondary treatment option.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
XZ: Writing – review & editing, Writing – original draft. AP: Writing – original draft, Writing – review & editing. CL: Writing – original draft, Writing – review & editing. LL: Writing – review & editing. DY: Writing – review & editing. YH: Writing – review & editing. CZ: Writing – review & editing. QY: Writing – review & editing. YL: Writing – review & editing. JL: Writing – review & editing. SL: Writing – review & editing. WNZ: Supervision, Writing – review & editing. YD: Writing – review & editing. WIZ: Writing – review & editing, Supervision.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by research grants from the National Natural Science Foundation of China (81971003, 82171406, and 82271457), Shaanxi Province Health Research and Innovation Team for Cognitive Dysfunction Disease (2023TD-06), Tangdu Hospital Innovation Development Foundation (2018QYTS010, 2019QYTS002), Tangdu Hospital Clinical Research Project (2021LCYJ040), and Tangdu hospital foundation for social recruitment talent (2021SHRC011).
Acknowledgments
We thank the patient for her collaboration.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cortese, A , Franciotta, D , Alfonsi, E , Visigalli, N , Zardini, E , Diamanti, L, et al. Combined central and peripheral demyelination: clinical features, diagnostic findings, and treatment. J Neurol Sci. (2016) 363:182–7. doi: 10.1016/j.jns.2016.02.022
2. Thomas, PK , Walker, RW , Rudge, P , Morgan-Hughes, JA , King, RH , Jacobs, JM, et al. Chronic demyelinating peripheral neuropathy associated with multifocal central nervous system demyelination. Brain. (1987) 110:53–76. doi: 10.1093/brain/110.1.53
3. Zéphir, H , Stojkovic, T , Latour, P , Lacour, A , de Seze, J , Outteryck, O, et al. Relapsing demyelinating disease affecting both the central and peripheral nervous systems. J Neurol Neurosurg Psychiatry. (2008) 79:1032–9. doi: 10.1136/jnnp.2006.108290
4. Kawamura, N , Yamasaki, R , Yonekawa, T , Matsushita, T , Kusunoki, S , Nagayama, S, et al. Anti-neurofascin antibody in patients with combined central and peripheral demyelination. Neurology. (2013) 81:714–22. doi: 10.1212/WNL.0b013e3182a1aa9c
5. Alshamrani, F , Alyami, R , Alghanimi, I , Alajaji, R , Alkhaldi, M , and Alamri, A . A new report of combined central and peripheral demyelination: a case report. Front Neurol. (2021) 12:730129. doi: 10.3389/fneur.2021.730129
6. Ogata, H , Matsuse, D , Yamasaki, R , Kawamura, N , Matsushita, T , Yonekawa, T, et al. A nationwide survey of combined central and peripheral demyelination in Japan. J Neurol Neurosurg Psychiatry. (2016) 87:jnnp-2014-309831–6. doi: 10.1136/jnnp-2014-309831
7. Willison, HJ , Jacobs, BC , and van Doorn, PA . Guillain-Barré syndrome. Lancet. (2016) 388:717–27. doi: 10.1016/S0140-6736(16)00339-1
8. Hahn, AF . Guillain-Barré syndrome. Lancet. (1998) 352:635–41. doi: 10.1016/S0140-6736(97)12308-X
9. Grygorczuk, S , Zajkowska, J , Kondrusik, M , Pancewicz, S , and Hermanowska-Szpakowicz, T . Guillain-Barré syndrome and its association with infectious factors. Neurol Neurochir Pol. (2005) 39:230–6.
10. Kamani, P , Baijal, R , Amarapurkar, D , Gupte, P , Patel, N , Kumar, P, et al. Guillain-Barre syndrome associated with acute hepatitis E. Indian J Gastroenterol. (2005) 24:216.
11. Maurissen, I , Jeurissen, A , Strauven, T , Sprengers, D , and De Schepper, B . First case of anti-ganglioside GM1-positive Guillain-Barré syndrome due to hepatitis E virus infection. Infection. (2012) 40:323–6. doi: 10.1007/s15010-011-0185-6
12. Tse, AC , Cheung, RT , Ho, SL , and Chan, KH . Guillain-Barré syndrome associated with acute hepatitis E infection. J Clin Neurosci. (2012) 19:607–8. doi: 10.1016/j.jocn.2011.06.024
13. Chen, XD , Zhou, YT , Zhou, JJ , Wang, YW , and Tong, DM . Guillain-Barré syndrome and encephalitis/encephalopathy of a rare case of northern China acute severe hepatitis E infection. Neurol Sci. (2014) 35:1461–3. doi: 10.1007/s10072-014-1731-7
14. Stevens, O , Claeys, KG , Poesen, K , Saegeman, V , and Van Damme, P . Diagnostic challenges and clinical characteristics of hepatitis E virus-associated Guillain-Barré syndrome. JAMA Neurol. (2017) 74:26–33. doi: 10.1001/jamaneurol.2016.3541
15. Geurtsvankessel, CH , Islam, Z , Mohammad, QD , Jacobs, BC , Endtz, HP , and Osterhaus, AD . Hepatitis E and Guillain-Barre syndrome. Clin Infect Dis. (2013) 57:1369–70. doi: 10.1093/cid/cit512
16. Drave, SA , Debing, Y , Walter, S , Todt, D , Engelmann, M , Friesland, M, et al. Extra-hepatic replication and infection of hepatitis E virus in neuronal-derived cells. J Viral Hepat. (2016) 23:512–21. doi: 10.1111/jvh.12515
17. van den Berg, B , van der Eijk, AA , Pas, SD , Hunter, JG , Madden, RG , Tio-Gillen, AP, et al. Guillain-Barré syndrome associated with preceding hepatitis E virus infection. Neurology. (2014) 82:491–7. doi: 10.1212/WNL.0000000000000111
18. Fukae, J , Tsugawa, J , Ouma, S , Umezu, T , Kusunoki, S , and Tsuboi, Y . Guillain-Barré and miller fisher syndromes in patients with anti-hepatitis E virus antibody: a hospital-based survey in Japan. Neurol Sci. (2016) 37:1849–51. doi: 10.1007/s10072-016-2644-4
19. Farag, M , Sibtain, N , Burge, J , Chaudhry, V , and Silber, E . Atypical inflammatory demyelinating syndrome with central and peripheral nerve involvement. Mult Scler Relat Disord. (2021) 51:102926. doi: 10.1016/j.msard.2021.102926
20. Pineda, AA , Ogata, K , Osoegawa, M , Murai, H , Shigeto, H , Yoshiura, T, et al. A distinct subgroup of chronic inflammatory demyelinating polyneuropathy with CNS demyelination and a favorable response to immunotherapy. J Neurol Sci. (2007) 255:1–6. doi: 10.1016/j.jns.2007.01.004
21. Granqvist, M , Boremalm, M , Poorghobad, A , Svenningsson, A , Salzer, J , Frisell, T, et al. Comparative effectiveness of rituximab and other initial treatment choices for multiple sclerosis. JAMA Neurol. (2018) 75:320–7. doi: 10.1001/jamaneurol.2017.4011
22. Hoshino, M , Suzuki, Y , Akiyama, H , Yamada, K , Shima, S , Mutoh, T, et al. Efficacy of high-dose steroid pulse therapy for anti-galactocerebroside antibody-positive combined central and peripheral demyelination. Rinsho Shinkeigaku. (2017) 57:747–52. doi: 10.5692/clinicalneurol.cn-000977
23. Saito, K , Toru, S , Shima, S , and Mutoh, T . Anti-neutral glycolipids antibody-positive combined central and peripheral demyelination mimicking encephalomyeloradiculoneuropathy phenotype. Clin Neurol Neurosurg. (2018) 172:90–2. doi: 10.1016/j.clineuro.2018.06.046
24. Shima, S , Kawamura, N , Ishikawa, T , Masuda, H , Iwahara, C , Niimi, Y, et al. Anti-neutral glycolipid antibodies in encephalomyeloradiculoneuropathy. Neurology. (2014) 82:114–8. doi: 10.1212/WNL.0000000000000015
25. Nanaura, H , Kataoka, H , Shima, S , Iwasa, N , Eura, N , Sugie, K, et al. A patient with encephalomyeloradiculoneuropathy exhibiting a relapsing-remitting clinical course: correlation of serum and cerebrospinal fluid anti-neutral glycosphingolipids antibodies with clinical relapse. Front Neurol. (2018) 9:206. doi: 10.3389/fneur.2018.00206
26. Hauser, SL , Waubant, E , Arnold, DL , Vollmer, T , Antel, J , Fox, RJ, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. (2008) 358:676–88. doi: 10.1056/NEJMoa0706383
27. Mutoh, T , Ueda, A , and Niimi, Y . Sphingolipid abnormalities in encephalomyeloradiculoneuropathy (EMRN) are associated with an anti-neutral glycolipid antibody. FEBS Open Bio. (2023) 13:1580–6. doi: 10.1002/2211-5463.13578
Keywords: combined central and peripheral demyelination, acute myelitis, Guillain-Barré syndrome, HEV infection, treatment
Citation: Zhou X, Peng A, Li C, Li L, Yao D, Hao Y, Zhao C, Yan Q, Li Y, Liu J, Liu S, Zhu W, Du Y and Zhang W (2024) Combined central and peripheral demyelination: a case report resembling encephalomyeloradiculoneuropathy. Front. Neurol. 14:1288546. doi: 10.3389/fneur.2023.1288546
Edited by:
Ernestina Santos, University Hospital Center of Porto, PortugalReviewed by:
Tatsuro Mutoh, Fujita Health University, JapanPaulo Victor Sgobbi Souza, Federal University of São Paulo, Brazil
Copyright © 2024 Zhou, Peng, Li, Li, Yao, Hao, Zhao, Yan, Li, Liu, Liu, Zhu, Du and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Du, dGRkdXlpbmdAZm1tdS5lZHUuY24=; Wei Zhang, dGR6d0BmbW11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Xuan Zhou
Xuan Zhou Ali Peng1†
Ali Peng1† Chuan Li
Chuan Li Lin Li
Lin Li Dan Yao
Dan Yao Yunfeng Hao
Yunfeng Hao Chao Zhao
Chao Zhao Qi Yan
Qi Yan Ying Li
Ying Li Juntong Liu
Juntong Liu Shuyu Liu
Shuyu Liu Wenping Zhu
Wenping Zhu Ying Du
Ying Du Wei Zhang
Wei Zhang