- 1Department of Neurology, Wakayama Medical University, Wakayama, Japan
- 2Department of Neurology, Wakayama Rosai Hospital, Wakayama, Japan
- 3Department of Neurology, Shingu Municipal Medical Center, Wakayama, Japan
Introduction: Autoimmune encephalitis/encephalopathy (AE) is a complex and heterogeneous disease, making it difficult to predict the prognosis. The neutrophil-to-lymphocyte ratio (NLR) has emerged as a potential prognostic tool, but its usefulness remains a matter of debate. This study aimed to explore prognostic factors in cases of clinically definite or probable AE, including those with autoantibody-negative, or unknown status.
Methods: Data on patients diagnosed with definite or probable AE, including those with autoantibody-negative, or unknown status, were retrospectively collected from the admission records of our department between January 2013 and December 2022. These patients were then categorized into either a good- or poor-response group, based on their short-term treatment response. Clinical characteristics, auxiliary examinations, and treatments were compared between the two groups. A multivariable logistic regression model was constructed to identify independent predictors of poor short-term treatment response by Akaike information criterion backward stepwise method.
Results: A total of 31 patients were included in the final analysis, with 18 of them included in the poor-response group. In the univariable analysis, the poor-response group had a higher proportion of patients with a modified Rankin Scale (mRS) high score upon admission, female, epileptic seizures, or NLRs of 3.93 or higher than the good-response group (all p < 0.10). Furthermore, the multivariable logistic regression analysis revealed that the mRS score upon admission [OR: 5.51, 95% confidence intervals (CI): 1.29–23.50, p = 0.02], epileptic seizures (OR: 10.01, 95% CI: 1.16–86.66, p = 0.04), and NLRs of 3.93 or higher (OR: 11.37, 95% CI: 1.12–114.68, p = 0.04) were significantly associated with poor short-term treatment response.
Conclusion: The NLR may play a supplementary role in predicting the short-term treatment response in patients diagnosed with definite or probable AE, including those with autoantibody-negative, or unknown status.
Introduction
Acute encephalitis is an inflammatory disorder of the brain. Encephalitis/encephalopathy with an autoimmune mechanism is referred to as autoimmune encephalitis/encephalopathy (AE). The incidence of AE in high-income countries is approximately 5–10 per 100,000 people per year (1). However, the incidence is increasing due to the identification of new autoantibodies and growing awareness of this disease (2). A previous study demonstrated that anti-N-methyl-D-aspartate receptor (anti-NMDAR) encephalitis was diagnosed more frequently (four times) than viral encephalitis in patients aged 30 years or younger (3). Autoimmunity has been recognized as one of the major causes of encephalitis or encephalopathy.
The prognosis for AE is generally poor. However, anti-NMDAR encephalitis has a better prognosis than other forms of AE, with up to 80% of patients achieving a modified Rankin Scale (mRS) score of 0–2 within 24 months (2). Nevertheless, a recent study found that only 61% of patients were able to return to their previous work or school life (4). As a result, diagnostic criteria for early detection of AE have been established (1), and numerous studies have investigated potential prognostic factors for AE. Factors such as age, mRS score upon admission, delay in initiating immunotherapy, cerebrospinal fluid (CSF) examination, and others have been investigated, but the results remain controversial (5–7). Recently, the neutrophil count has also been suggested as a potential prognostic factor for AE. The plasma neutrophil-to-lymphocyte ratio (NLR), calculated by dividing the absolute count of neutrophils by the absolute count of lymphocytes, has been proposed as a marker for systemic inflammation and stress (8). NLR is a simple indicator that can be easily evaluated in any hospital setting. Previous studies have shown that an elevated NLR is associated with poor treatment response and prognosis in patients with autoantibody-positive AE (9–11).
However, measuring various types of autoantibodies can often be challenging due to limitations such as facility availability, technical skills, sample volumes, and the health insurance system. As a result, some patients are tentatively diagnosed with AE as autoantibody-negative or with an unknown autoantibody status. To the best of our knowledge, there have been few studies investigating the association between the NLR and the prognosis of cases of definite or probable AE, including those with autoantibody-negative, or unknown status. One study reported the potential association between the NLR and prognosis in a group of AE cases, including those with autoantibody-negative, or unknown status (12). However, it is worth noting that this study included a significant number of patients with possible AE. The diagnostic criteria for AE recommend reconsidering the diagnosis if autoantibodies are negative and patients do not fulfill the criteria for probable autoimmune encephalitis (1). Therefore, whether the NLR can serve as a prognostic factor in cases of definite or probable AE, including those with autoantibody-negative, or unknown status, is still unclear, and requires further assessment.
We hypothesized that the NLR could be a potential prognostic factor in cases of definite or probable AE, including those with autoantibody-negative, or unknown status. To investigate this, we conducted this study utilizing data from the admission records of our department. The primary objective of this study was to assess the association between the NLR and treatment response in cases of definite or probable AE, including those with autoantibody-negative, or unknown status. In addition, we aimed to investigate the association between clinical features and prognostic factors within the same group of patients.
Materials and methods
Ethical approval
This retrospective cohort study was approved by the Institutional Review Board of Wakayama Medical University (Approval Number 3788). Informed consent was obtained through an opt-out disclosure process.
Study design and patients
In this study, candidate patients were recruited from the admission records of our department between January 2013 and December 2022. Our search strategy involved using keywords such as “limbic,” “encephalitis,” “encephalopathy,” “encephalomyelitis,” “leukoencephalopathy,” or “paraneoplastic”. Patients who were aged 16 years, met the diagnostic criteria for definite or probable AE (1), and had an initial diagnosis received immunotherapy or underwent tumor resection were included in this study. In the initial diagnostic assessment, patients who met the diagnostic criteria for possible AE upon admission were recruited. A diagnosis of possible AE was made based on the following criteria: subacute onset (<3 months) of working memory deficits (short-term memory loss), altered mental status, or psychiatric symptoms and at least one of the following conditions: new focal central nervous system (CNS) findings, seizures that could not be explained by a previously known seizure disorder, CSF abnormalities [white blood cell (WBC) count of >5 cells per mm3], or magnetic resonance imaging (MRI) abnormalities indicative of encephalitis. Subsequently, patients who tested positive for autoantibodies were diagnosed with definite AE. Patients who did not test positive for autoantibodies and who were not diagnosed with other CNS disorders were evaluated to confirm if they met the diagnostic criteria for definite autoimmune limbic encephalitis or probable AE. Patients were diagnosed with definite autoimmune limbic encephalitis if they met the following criteria: subacute onset (<3 months) of working memory deficits, seizures, or psychiatric symptoms indicating the involvement of the limbic system, T2-weighted fluid-attenuated inversion recovery MRI abnormalities (bilateral brain abnormalities highly restricted to the medial temporal lobes), and at least one of the following conditions: CSF abnormalities (WBC count of >5 cells per mm3) or electroencephalogram (EEG) abnormalities (epileptic or slow-wave activity involving the temporal lobes). A diagnosis of probable AE was made if the following criteria were met: rapid progression (<3 months) of working memory deficits (short-term memory loss), altered mental status, or psychiatric symptoms; exclusion of well-defined syndromes of autoimmune encephalitis; and at least two of the following conditions: MRI abnormalities indicative of autoimmune encephalitis, CSF abnormalities (pleocytosis, CSF-specific oligoclonal bands or an elevated CSF IgG index, or both), or brain biopsy showing inflammatory infiltrates and excluding other disorders. Patients were excluded if they tested positive for the anti-aquaporin 4 antibody, were diagnosed with neuromyelitis optica spectrum disorders, were lost to follow-up, had incomplete clinical data, or had other CNS disorders. In addition, two clinical neurology specialists, Shuhei Ogami, and Jinsoo Koh, carefully reviewed the medical records of the patients to determine whether they met the criteria for AE with autoantibody-negative or unknown status (definite autoimmune limbic encephalitis or probable AE) and made decisions regarding their inclusion in the study.
Data collection
Data on demographics, including gender, and age, along with neurological disease severity upon admission, clinical symptoms, and features, disease course, laboratory findings, CSF examination findings, EEG, brain MRI findings, and treatments were collected from the patients' medical records. The medical records were reviewed from admission until 4 weeks after the initiation of immunotherapy or tumor resection. The severity of neurological disease was assessed using the mRS score (13). Clinical symptoms and features included loss of consciousness, memory loss, behavioral changes, a fever of 38°C or higher, epileptic seizures, psychiatric symptoms, movement disorders, autonomic dysfunction, and tumor complications. Laboratory tests included WBC, neutrophil, and lymphocyte counts, as well as NLRs. CSF analysis involved assessing protein levels and cell counts. The initial laboratory and CSF examination findings were retrieved from admission until the initiation of immunotherapy or tumor resection. Treatments included intravenous methylprednisolone pulse (500–1,000 mg) for 3 days, intravenous gamma immunoglobulin (400 mg/kg) for 5 days, plasma exchange, cyclophosphamide, tumor resection, oral prednisolone, antiepileptic drugs, and mechanical ventilation, either alone, or in combination. Patients were categorized into a good- or poor-response group based on their short-term treatment response, as previously reported (14). The good response was defined as sustained improvement and a mRS score of 3 or lower at 4 weeks after the initiation of immunotherapy or tumor resection. The poor response was defined as either the absence of sustained improvement or a sustained mRS score of 4 or higher within 4 weeks after the initiation of immunotherapy or tumor resection.
Statistical analysis
The statistical analysis was conducted using JMP Pro 14.1.0. A two-sided p-value of <0.05 was considered statistically significant. Continuous variables were presented as means and standard deviations or medians and interquartile ranges based on their distribution. The Shapiro–Wilk test was used to check their distribution. Continuous variables were categorized by the mean or median value of all patients based on their distribution. Categorical variables were expressed as counts and percentages. The student's t-test or Mann–Whitney U-test was used to compare continuous variables, while Fisher's exact test was used to compare categorical variables between the two groups. A multivariable logistic regression model was constructed to assess the association between independent variables and poor short-term treatment response. Clinical features and auxiliary examinations in categorized variables with p-values < 0.10 in the univariable analysis were chosen as candidate variables. The Akaike information criterion (AIC) backward stepwise method was used to select the potential independent predictors. However, a mRS score in a continuous variable was defined as the coefficient variable, and was forced to be included in the multivariable logistic regression model when backward stepwise method was used. Odds ratios (OR) and 95% confidence intervals (CI) were used to quantify the strength of these associations. The receiver operating characteristic (ROC) curve was used to estimate the cutoff value for the NLR and short-term treatment response.
Results
Clinical characteristics
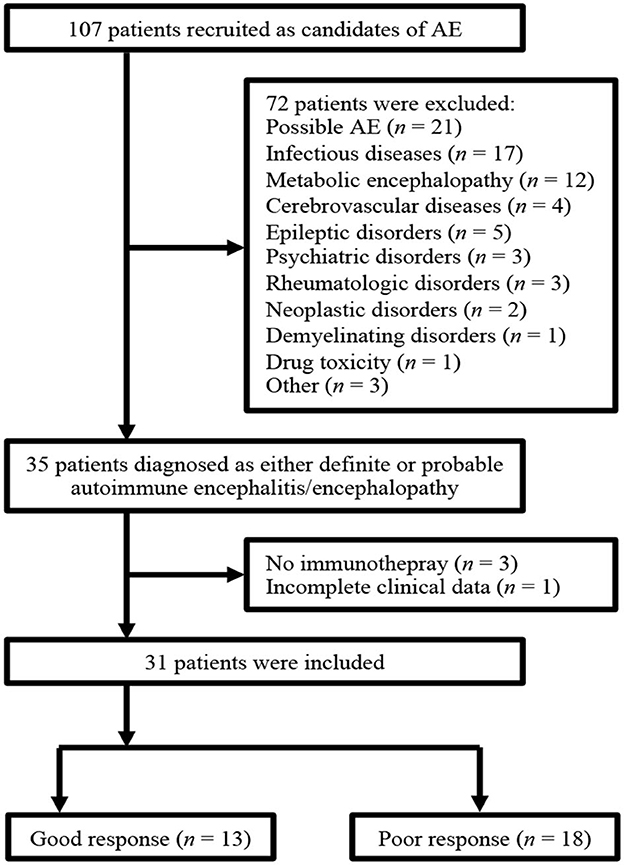
Figure 1 shows the flowchart of patient selection. A total of 107 patients met the diagnostic criteria for possible AE upon admission. Among them, 72 patients were excluded because they were finally diagnosed with possible AE or other CNS disorders. Thirty-five patients fulfilled the diagnostic criteria for either definite AE, definite autoimmune limbic encephalitis, and probable AE. Four patients were excluded from this group for various reasons. Three patients showed improvement before initiating immunotherapy and therefore did not receive it, while one patient had incomplete clinical data. Consequently, 31 patients were included in the final analysis. Out of these, 13 patients were classified into the good-response group, while 18 patients fell into the poor-response group. Further details regarding the final diagnosis are provided in the Supplementary Table 1. Notably, all patients with definite autoimmune limbic encephalitis tested negative for the anti-NMDAR antibody.
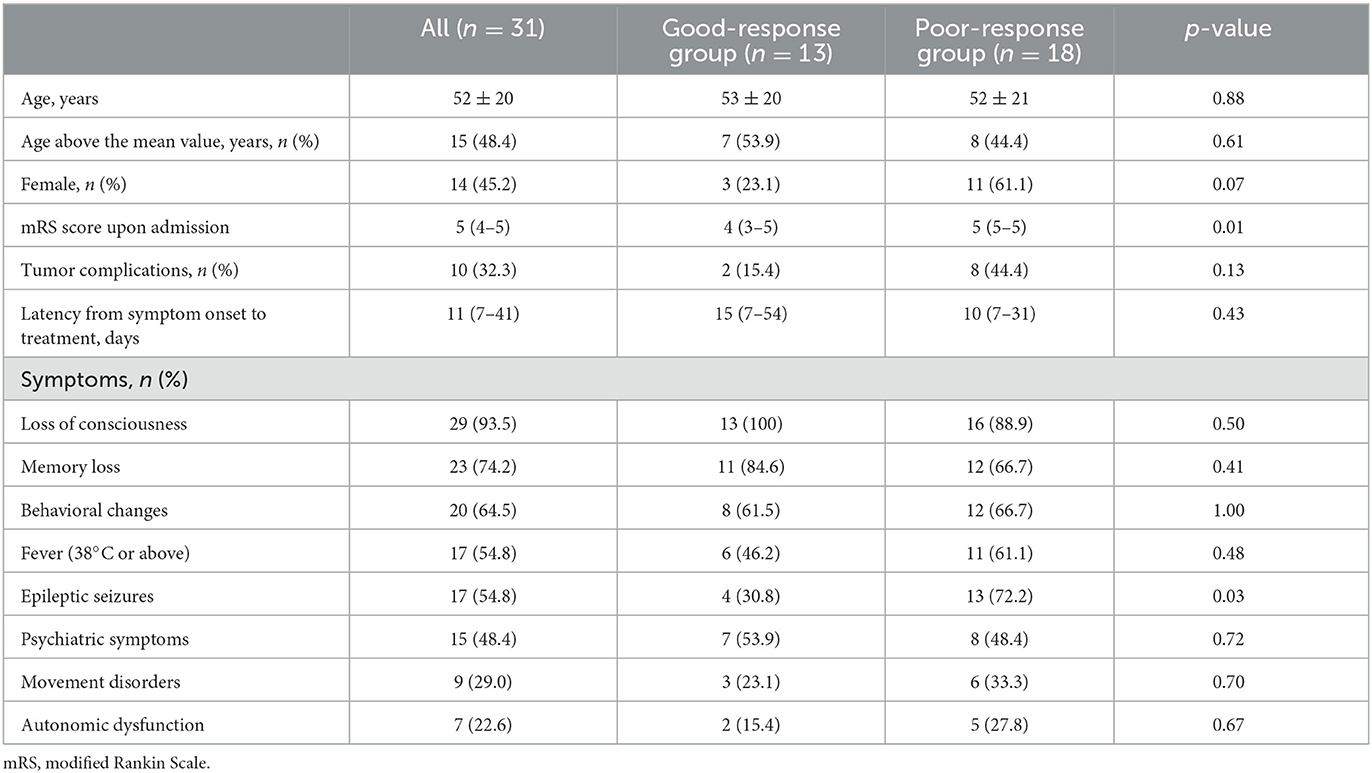
Table 1 summarizes the clinical characteristics of the patients. The mRS score upon admission were significantly higher in the poor-response group than in the good-response group. Furthermore, the poor-response group had a significantly higher proportion of patients with epileptic seizures than the good-response group. However, no significant differences were observed in other variables between the two groups. There were a total of 10 cases with tumor complications. Specifically, there were four cases of mature cystic teratoma and one case of small-cell lung carcinoma in anti-NMDAR encephalitis. In autoimmune glial fibrillary acidic protein astrocytopathy, there was one case of mucinous cystic neoplasm and another case of ovarian teratoma. In addition, there was one case of a retroperitoneal tumor in anti-glutamate receptor encephalitis, one case of papillary renal cell carcinoma in anti-CV2 antibody-associated encephalitis, and one case of lung squamous cell carcinoma in anti-Hu and anti-amphiphysin antibody-associated encephalitis.
Laboratory examinations and treatments
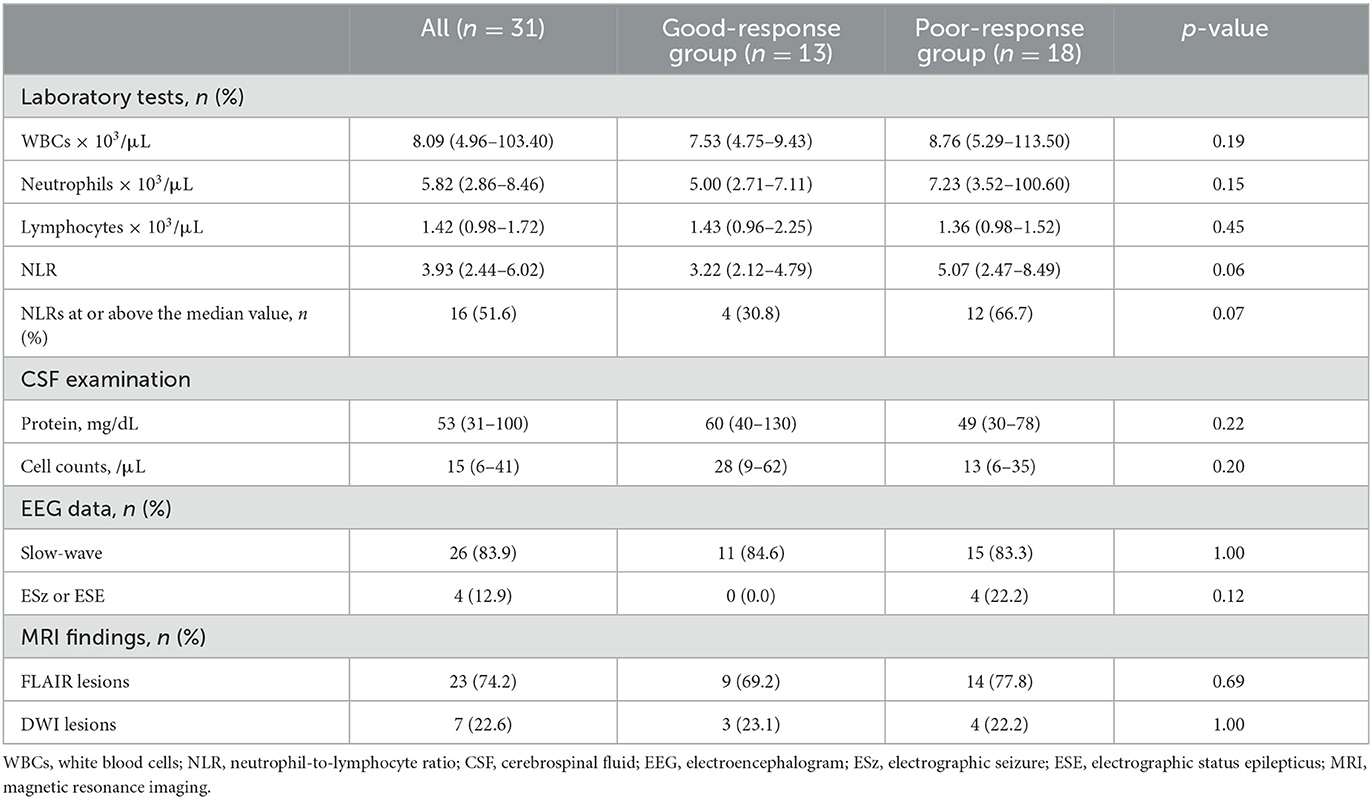
Laboratory, CSF examination, EEG, and brain MRI findings are summarized in Table 2. The median NLR was 3.93, and the proportion of patients with NLRs of 3.93 or higher tended to be higher in the poor-response group than in the good-response group, but the difference was not statistically significant. No other variables also showed statistically significant differences.
There were no significant differences in terms of immunotherapy between the two groups (Table 3). However, 29 patients (93.5%) received methylprednisolone pulse initially, while none received cyclophosphamide until 4 weeks after the initiation of immunotherapy or tumor resection. All four patients who received tumor resection had mature cystic teratoma with anti-NMDAR encephalitis. There was no significant difference in terms of antiepileptic drug induction between the two groups.
Variables associated with poor short-term treatment response
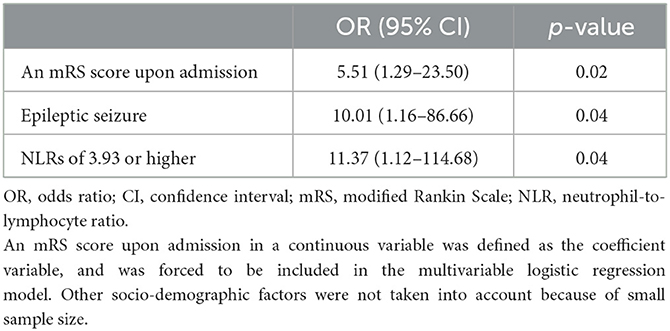
To identify independent predictors for poor short-term treatment response, the mRS score upon admission and categorical variables with p-values < 0.10 (female, epileptic seizures, and NLRs of 3.93 or higher) were included in the AIC backward stepwise method. The AIC backward stepwise method selected epileptic seizures, and NLRs of 3.93 or higher as the final independent predictors in addition to the mRS score upon admission. The multivariable logistic regression analysis revealed that the mRS score upon admission (OR: 5.51, 95% CI: 1.29–23.50, p = 0.02), epileptic seizures (OR: 10.01, 95% CI: 1.16–86.66, p = 0.04), and NLRs of 3.93 or higher (OR: 11.37, 95% CI: 1.12–114.68, p = 0.04) were significantly associated with poor short-term treatment response (Table 4).
Figure 2 shows the ROC curve for the NLR and short-term treatment response. The area under the curve was 0.70, with a sensitivity of 0.67 and a specificity of 0.69 when the median NLR value of 3.93 was set as the cutoff. Using Youden's index to estimate the optimal cutoff value, it was determined to be 4.10, with an area under the curve of 0.70, a sensitivity of 0.67, and a specificity of 0.77.
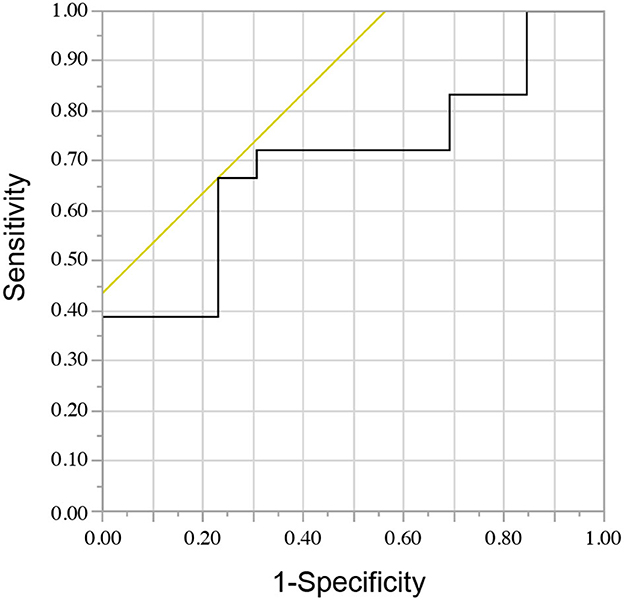
Figure 2. The receiver operating characteristic curve for the neutrophil-to-lymphocyte ratio and short-term treatment response.
Discussion
Our study demonstrated that the NLR can be used to predict short-term treatment response in patients diagnosed with definite or probable AE, including those with autoantibody-negative, or unknown status. The multivariable logistic regression model revealed a significant association between NLRs of 3.93 or higher and poor short-term treatment response. In clinical practice, there is often a need to make a tentative diagnosis and predict the prognosis of AE, particularly when specific autoantibodies are not immediately or fully identified. A previous study highlighted the relationship between NLR and treatment response in patients with AE, including those with autoantibody-negative, or unknown status (12). However, this study included cases of possible AE, potentially encompassing conditions other than AE, which limits the ability to establish a definitive relationship between NLR and AE prognosis. In our study, we observed similar results, particularly in patients diagnosed with definite or probable AE, suggesting that measuring NLR can be used to predict treatment response, even in patients who tentatively meet the diagnostic criteria for definite or probable AE.
A previous study showed that a high NLR was associated with poor prognosis during the final follow-up (15). However, in this study, there was no significant association between NLR and failed first-line treatment. One possible reason is the definition of first-line treatment response. A previous study defined clinical response as improvement in mRS score to ≥1 after 2–4 weeks of the first-line treatment. However, this definition is different from ours. Another potential reason is disease severity. A previous study only included patients with severe autoantibody-positive AE who required mechanical ventilation or admission to the intensive care unit. All patients with severe condition in our study were classified under the poor-response group (Table 3). Patients with severe AE may present with minimal short-term improvement after the first-line treatment, potentially obscuring the statistical difference between the two groups in the previous study.
An optimal NLR cutoff value of 4.10 was considered for predicting poor short-term treatment response (Figure 2). Another report, which included cases of AE with autoantibody-negative or unknown status, revealed that an NLR cutoff value of 4.45 could be used to predict adverse outcomes (12). Taking these factors into consideration, a cutoff value of approximately 4 may indicate a poor prognosis. However, further studies should be conducted to confirm the reproducibility of these findings.
The pathophysiological mechanism related to the association between neutrophils and AE remains uncertain. However, some studies have revealed that neutrophil extracellular traps (NETs) play a role in inflammation in autoimmune diseases (16). If neutrophils are exposed to various stimuli, they release NETs, which activate B cells and macrophages, leading to the production of autoantibodies and the secretion of interleukin (IL)-8, IL-6, and tumor necrosis factor-alpha (TNF-α) (17). Enzymes within NETs can also be a source of autoantigens (18). Neutrophils and NETs may also be associated with multiple sclerosis (MS), an autoimmune disease of the CNS. MS is characterized by immune-associated demyelination of the CNS, resulting in neuronal loss and substantial disability (19). Patients with MS have a high neutrophil count and NET formation marker level (19, 20). In addition, NET-associated proteins can damage the blood–brain barrier in MS (20). Neutrophils may contribute to the pathology of CNS diseases by generating NETs. In a recent study on patients with anti-NMDAR encephalitis, the production of NET increased, and the NET levels were found to be correlated with the levels of IL-8, IL-6, and TNF-α (21). Neutrophils may enhance immune response via NET generation in AE pathology. Therefore, a higher neutrophil count may indicate a less effective response to immunotherapy.
Our study found a significant association between epileptic seizures and short-term treatment response in the multivariate logistic regression model. The association between epileptic seizures and prognosis remains controversial (6, 22). This conflicting result may be attributed to variations in the detection rate of epileptic seizures among different hospitals. To increase the rate of abnormality detection, it is necessary to repeat EEGs or conduct long-term EEG monitoring, as standard EEGs can only identify interictal epileptic abnormalities in 30–50% of cases (23). Our study recruited patients with epileptic seizures based on their medical history and standard EEG results. None of the patients underwent long-term EEG monitoring. Thus, we could have overlooked non-convulsive epileptic seizures in patients experiencing loss of consciousness. The lack of long-term EEG monitoring is one of the limitations of our study, thereby underscoring the need for a standardized protocol for EEG monitoring.
In our analysis, the mRS score upon admission was another prognostic factor. However, a previous study reported no association between the mRS score upon admission and prognosis (24). This conflicting result may be attributed to several reasons. One possible reason is the variation in background diseases across the studies. The previous study had a higher proportion of patients with anti-NMDAR encephalitis than our study. In the subgroup analysis, the mRS score upon admission was not associated with prognosis in patients with anti-NMDAR encephalitis but was associated with anti-leucine-rich glioma inactivated 1 encephalitis (24). Therefore, the impact of the mRS score upon admission on the prognosis may depend on the background disease. Another potential reason for this conflicting result is the reliability of the mRS score itself. While the mRS score is frequently used in clinical research, it is a subjective assessment, and a previous study has highlighted uncertainties regarding its reliability (25). Recently, a more detailed and novel assessment scale for AE called the clinical assessment scale in autoimmune encephalitis (CASE) has been proposed (26). Ideally, disease severity should be assessed using both the mRS score and CASE. However, our study was limited by incomplete clinical data, which prevented us from utilizing CASE. This limitation should be acknowledged as well.
Our study has some limitations that should be acknowledged. First, it was a retrospective study conducted at a single hospital. Second, the small sample size might have led to bias and limited statistical power. Hence, an effort was made to decrease these limitations by adopting strict criteria for selecting potential independent predictors in the multivariate logistic regression model. Continuous variables, except the mRS score, were not selected as candidate independent variables. In addition, the number of independent variables was adjusted. Hence, it was not extremely large relative to the sample size. However, coefficient variables were challenging to control due to small sample size. The number of independent variables was limited to three because the sample size was 31. Hence, we could not choose gender, age, and other variables, except the mRS score, as coefficient variables. Third, approximately half of the patients were lost to follow-up within 1 year, which hindered our analysis of the long-term prognosis and the long-term prognosis could not be analyzed. It would be beneficial to extend the follow-up period to at least 1 year, as previous studies have done. Fourth, the measured antibodies varied among patients. It is important to establish a standardized examination protocol for measuring at least commercially available autoantibodies. Lastly, due to restrictions imposed by the Japanese health insurance system, immunotherapy drugs such as rituximab, bortezomib, mycophenolate mofetil, and methotrexate were not administered. If these drugs become accessible in the future, it is possible that the results may be altered.
Conclusion
Our study has demonstrated that an elevated NLR may be associated with poor short-term treatment response in patients diagnosed with definite or probable AE, including those with autoantibody-negative or unknown status. This suggests that neutrophils may contribute to the pathophysiological mechanism of AE. However, the exact mechanism remains unclear and requires further investigation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of Wakayama Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin because Informed consent was obtained through an opt-out disclosure process.
Author contributions
SO: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing—original draft, Writing—review and editing. JK: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Writing—original draft, Writing—review and editing. KM: Writing—review and editing, Investigation, Supervision, Funding acquisition. MM: Writing—review and editing, Investigation. MT: Investigation, Writing—review and editing. YN: Investigation, Writing—review and editing. MS: Investigation, Writing—review and editing. YH: Investigation, Writing—review and editing. YK: Investigation, Writing—review and editing. HIs: Investigation, Writing—review and editing. HIt: Writing—review and editing, Supervision.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by JSPS KAKENHI (Grant Number JP 22K07498).
Acknowledgments
The authors would like to express their gratitude to Akio Kimura from the Department of Neurology at Gifu University Graduate School of Medicine, Keiko Tanaka from the Department of Neurology at Kanazawa Medical University, Makoto Yoneda from the Faculty of Nursing and Social Welfare Sciences at Fukui Prefectural University, Osamu Watanabe from the Department of Neurology and Geriatrics at Kagoshima University Graduate School of Medical and Dental Sciences, and Yukitoshi Takahashi from the NHO Shizuoka Institute of Epilepsy and Neurological Disorders for their assistance in measuring autoantibodies. The authors would like to express their appreciation to all the patients and their family members for their cooperation throughout the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1284717/full#supplementary-material
References
1. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. (2016) 15:391–404. doi: 10.1016/S1474-4422(15)00401-9
2. Uy CE, Binks S, Irani SR. Autoimmune encephalitis: clinical spectrum and management. Pract Neurol. (2021) 21:412–23. doi: 10.1136/practneurol-2020-002567
3. Gable MS, Sheriff H, Dalmau J, Tilley DH, Glaser CA. The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California Encephalitis Project. Clin Infect Dis. (2012) 54:899–904. doi: 10.1093/cid/cir1038
4. Hirose S, Hara M, Kamei S, Dalmau J, Nakajima H. Characteristics of clinical relapses and patient-oriented long-term outcomes of patients with anti-N-methyl-D-aspartate receptor encephalitis. J Neurol. (2022) 269:2486–92. doi: 10.1007/s00415-021-10828-8
5. Schwarz L, Akbari N, Prüss H, Meisel A, Scheibe F. Clinical characteristics, treatments, outcome, and prognostic factors of severe autoimmune encephalitis in the intensive care unit: standard treatment and the value of additional plasma cell-depleting escalation therapies for treatment-refractory patients. Eur J Neurol. (2023) 30:474–89. doi: 10.1111/ene.15585
6. Broadley J, Seneviratne U, Beech P, Buzzard K, Butzkueven H, O'Brien T, et al. Prognosticating autoimmune encephalitis: a systematic review. J Autoimmun. (2019) 96:24–34. doi: 10.1016/j.jaut.2018.10.014
7. Broadley J, Wesselingh R, Seneviratne U, Kyndt C, Beech P, Buzzard K, et al. Prognostic value of acute cerebrospinal fluid abnormalities in antibody-positive autoimmune encephalitis. J Neuroimmunol. (2021) 353:577508. doi: 10.1016/j.jneuroim.2021.577508
8. Zahorec R. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. (2001) 102:5–14.
9. Zhang X, Wang C, Zhu W, Wang B, Liang H, Guo S. Factors affecting the response to first-line treatments in patients with anti-N-methyl-D-aspartate receptor encephalitis. J Clin Neurol. (2019) 15:369–75. doi: 10.3988/jcn.2019.15.3.369
10. Broadley J, Wesselingh R, Seneviratne U, Kyndt C, Beech P, Buzzard K, et al. Peripheral immune cell ratios and clinical outcomes in seropositive autoimmune encephalitis: a study by the Australian autoimmune encephalitis consortium. Front Immunol. (2020) 11:597858. doi: 10.3389/fimmu.2020.597858
11. Liu F, Zhang B, Huang T, Wang B, Wang C, Hao M, et al. Influential factors, treatment and prognosis of autoimmune encephalitis patients with poor response to short-term first-line treatment. Front Neurol. (2022) 13:861988. doi: 10.3389/fneur.2022.861988
12. Qiu X, Zhang H, Li D, Wang J, Jiang Z, Zhou Y, et al. Analysis of clinical characteristics and poor prognostic predictors in patients with an initial diagnosis of autoimmune encephalitis. Front Immunol. (2019) 10:1286. doi: 10.3389/fimmu.2019.01286
13. Bloch RF. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. (1988) 19:1448. doi: 10.1161/01.STR.19.11.1448
14. Titulaer MJ, McCracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. (2013) 12:157–65. doi: 10.1016/S1474-4422(12)70310-1
15. Wang B, Wang C, Feng J, Hao M, Guo S. Clinical features, treatment, and prognostic factors in neuronal surface antibody-mediated severe autoimmune encephalitis. Front Immunol. (2022) 13:890656. doi: 10.3389/fimmu.2022.890656
16. Gupta S, Kaplan MJ. The role of neutrophils and NETosis in autoimmune and renal diseases. Nat Rev Nephrol. (2016) 12:402–13. doi: 10.1038/nrneph.2016.71
17. Fousert E, Toes R, Desai J. Neutrophil extracellular traps (NETs) take the central stage in driving autoimmune responses. Cells. (2020) 9:915. doi: 10.3390/cells9040915
18. Sadeghi M, Dehnavi S, Jamialahmadi T, Johnston TP, Sahebkar A. Neutrophil extracellular trap: a key player in the pathogenesis of autoimmune diseases. Int Immunopharmacol. (2023) 116:109843. doi: 10.1016/j.intimp.2023.109843
19. Bar-Or A, Li R. Cellular immunology of relapsing multiple sclerosis: interactions, checks, and balances. Lancet Neurol. (2021) 20:470–83. doi: 10.1016/S1474-4422(21)00063-6
20. Manda-Handzlik A, Demkow U. The brain entangled: the contribution of neutrophil extracellular traps to the diseases of the central nervous system. Cells. (2019) 8:1477. doi: 10.3390/cells8121477
21. Qiao S, Sun QY, Zhou P, Zhang SC, Wang ZH, Li HY, et al. Increased formation of neutrophil extracellular traps in patients with anti-N-methyl-d-aspartate receptor encephalitis. Front Immunol. (2022) 13:1046778. doi: 10.3389/fimmu.2022.1046778
22. Gastaldi M, Mariotto S, Giannoccaro MP, Iorio R, Zoccarato M, Nosadini M, et al. Subgroup comparison according to clinical phenotype and serostatus in autoimmune encephalitis: a multicenter retrospective study. Eur J Neurol. (2020) 27:633–43. doi: 10.1111/ene.14139
23. Michel V, Mazzola L, Lemesle M, Vercueil L. Long-term EEG in adults: sleep-deprived EEG (SDE), ambulatory EEG (Amb-EEG) and long-term video-EEG recording (LTVER). Neurophysiol Clin. (2015) 45:47–64. doi: 10.1016/j.neucli.2014.11.004
24. Liu Z, Li Y, Wang Y, Zhang H, Lian Y, Cheng X. The neutrophil-to-lymphocyte and monocyte-to-lymphocyte ratios are independently associated with the severity of autoimmune encephalitis. Front Immunol. (2022) 13:911779. doi: 10.3389/fimmu.2022.911779
25. Quinn TJ, Dawson J, Walters MR, Lees KR. Reliability of the modified Rankin Scale: a systematic review. Stroke. (2009) 40:3393–5. doi: 10.1161/STROKEAHA.109.557256
Keywords: neutrophils, neutrophil-to-lymphocyte ratio, neutrophil extracellular traps, short-term treatment response, definite or probable autoimmune encephalitis/encephalopathy
Citation: Ogami S, Koh J, Miyamoto K, Mori M, Takahashi M, Nakayama Y, Sakata M, Hiwatani Y, Kajimoto Y, Ishiguchi H and Ito H (2023) Predictive value of the neutrophil-to-lymphocyte ratio for treatment response in patients diagnosed with definite or probable autoimmune encephalitis/encephalopathy. Front. Neurol. 14:1284717. doi: 10.3389/fneur.2023.1284717
Received: 29 August 2023; Accepted: 09 October 2023;
Published: 23 October 2023.
Edited by:
Amanda Piquet, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Shuo Wang, Central South University, ChinaChih-Hsiang Lin, Kaohsiung Chang Gung Memorial Hospital, Taiwan
Copyright © 2023 Ogami, Koh, Miyamoto, Mori, Takahashi, Nakayama, Sakata, Hiwatani, Kajimoto, Ishiguchi and Ito. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuhei Ogami, c2h1b2dtQHdha2F5YW1hLW1lZC5hYy5qcA==
 Shuhei Ogami
Shuhei Ogami Jinsoo Koh
Jinsoo Koh Katsuichi Miyamoto
Katsuichi Miyamoto Megumi Mori1
Megumi Mori1 Yoshiaki Nakayama
Yoshiaki Nakayama Yoshinori Kajimoto
Yoshinori Kajimoto