- 1Department of Neurosurgery, Qilu Hospital of Shandong University and Institute of Brain and Brain-Inspired Science, Shandong University, Jinan, China
- 2Key Laboratory of Cardiovascular Remodeling and Function Research, Chinese Ministry of Education, Chinese Ministry of Health and Chinese Academy of Medical Sciences, Qilu Hospital of Shandong University, Jinan, China
- 3State Key Laboratory of Generic Manufacture Technology of Traditional Chinese Medicine, Lunan Pharmaceutical Group Co., Ltd., Linyi, China
- 4School of Basic Medical Sciences, Shandong University, Jinan, China
Background and purpose: Diabetes mellitus (DM) is a well-established cardiovascular risk factor for atherosclerotic disease; however, its effect on the risk of rupture of intracranial aneurysms remains controversial. Herein, we aimed to perform a case–control study to investigate the relationship between DM and aneurysmal subarachnoid hemorrhage (aSAH).
Methods: We retrospectively reviewed the data of patients with ruptured or unruptured aneurysms who were treated between 2013 and 2023. Univariate and multivariate analyses were performed. Propensity score matching (PSM) analysis was conducted to evaluate the relationship between DM and risk of aSAH.
Results: A total of 4,787 patients with 5,768 intracranial aneurysms were included. Among them, 2,957 (61.8%) were females, 1765 (36.9%) had ruptured aneurysms, and 531 (11.1%) presented with DM. Female sex, current drinking, and hypercholesterolemia were associated with a higher risk of aSAH, whereas old age, former smoking, and DM were associated with a lower risk of aSAH in multivariate analysis (p < 0.05). The incidence of DM (13.4%, 406/3022) in the unruptured group was higher than that in the ruptured group (7.1%, 125/1765) (odds ratio, 0.55; 95% confidence interval, 0.444–0.680) (p < 0.001). After propensity score matching, 530 patients with DM were successfully matched, and DM was still associated with a lower risk of aSAH (odds ratio, 0.24; 95% confidence interval, 0.185–0.313) (p < 0.001).
Conclusion: Patients with aSAH have a lower incidence of DM, however, this case-cohort study could not establish a causal relationship. A prospective and large study with long-term follow-up is warranted to establish a causal relationship.
1 Introduction
Subarachnoid hemorrhage (SAH) has an incidence rate of 9 per 100,000 person-years and accounts for approximately 5% of all strokes (1). Nearly 85% of spontaneous SAHs are caused by ruptured intracranial aneurysms (IA) (2). Despite recent advances in medical and surgical treatment, aneurysmal subarachnoid hemorrhage (aSAH) remains an emergency life-threatening cerebrovascular event; up to 40% of patients with SAH die, and 50–66% suffer permanent disability (3). Even survivors with a “good recovery” may present with memory and neurocognitive impairments and cannot return to work (4). With the widespread use of advanced imaging techniques, an increasing number of unruptured IAs have been incidentally discovered. Therefore, identification of the risk factors for IA rupture and prevention of modifiable risk factors are important. The incidence of aSAH is high in Finland and Japan; it increases with age and is common in women (1). Hypertension, smoking, and alcohol abuse are well-established modifiable risk factors for aSAH, and high total serum cholesterol levels and diabetes mellitus (DM) seem to be inversely associated with aSAH (5, 6). However, not all studies reported an inverse association between DM and aSAH (7–9). Previous studies, particularly population-based cohort studies, often included a small number of aSAH cases. Several studies included all spontaneous SAH cases, regardless of the etiology, or SAH cases with unconfirmed aneurysms (10). The control cases in some cohort studies were generally healthy populations without aneurysms rather than patients with unruptured IAs (7–10). Aneurysm formation and rupture are associated with various risk factors and pathophysiologic mechanisms. Furthermore, not all studies balanced the main confounders of aSAH, such as body mass index (BMI) and cholesterol levels, and different studies may have had different adjusted confounders. Chinese people may have different lifestyle habits from those in the West, and the effect of DM on the occurrence of aSAH in Chinese patients has not been well investigated and may differ from that in other parts of the world. Herein, we aimed to perform a case–control study involving a large sample of patients with ruptured and unruptured aneurysms to investigate the relationship between DM and aSAH.
2 Materials and methods
2.1 Patients
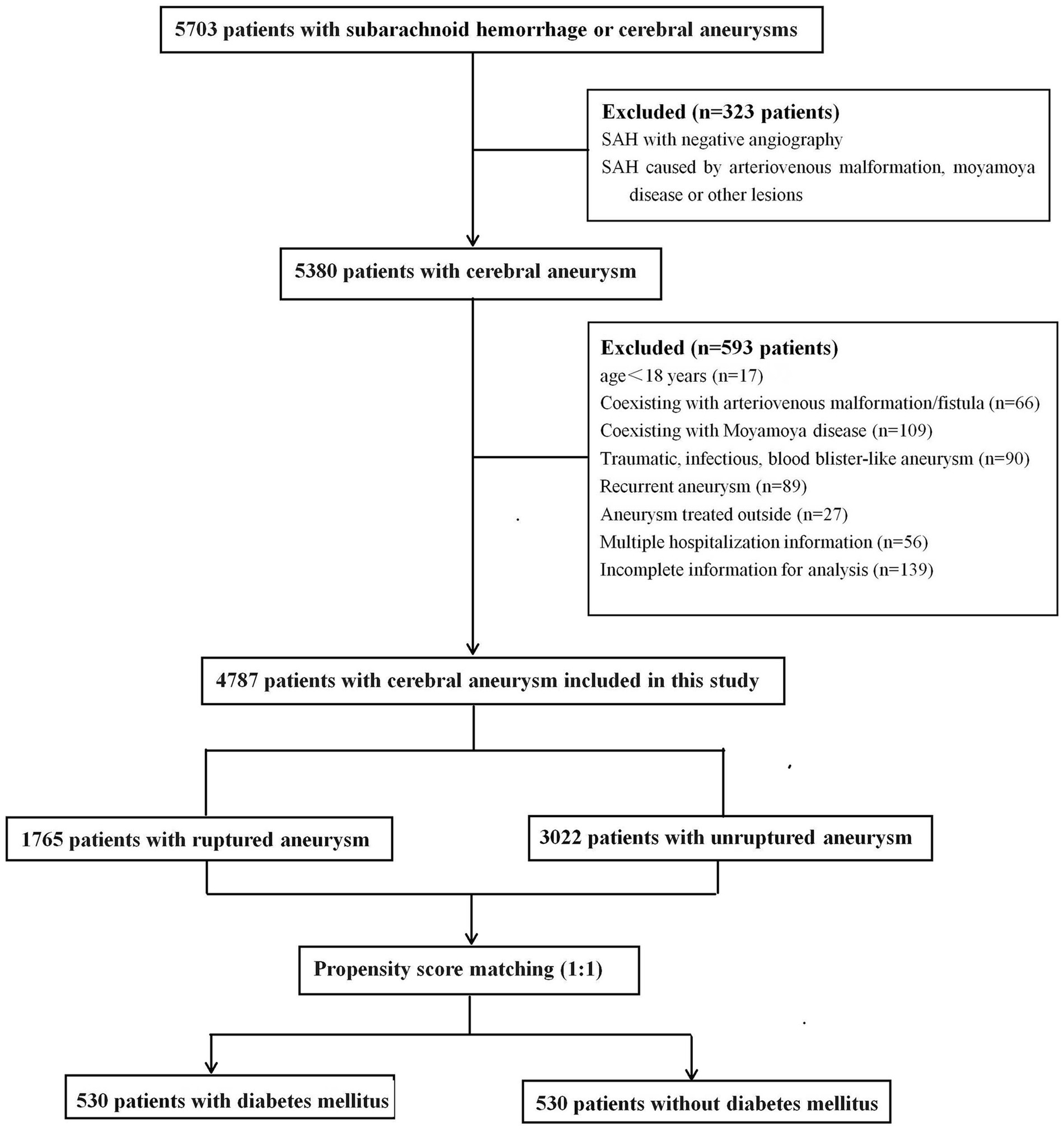
This retrospective study was approved by the Ethics Committee of Qilu Hospital of Shandong University, and the requirement for informed consent was waived. From September 2013 to March 2023, 5,703 consecutive patients diagnosed with SAH or cerebral aneurysms were treated at our tertiary hospital and identified in our prospectively collected data. All IAs were confirmed using computed tomography, magnetic resonance imaging, or digital subtraction angiography. SAH was confirmed using computed tomography or cerebrospinal fluid analysis. For patients who received multiple hospitalization treatments at our hospital, only the initial admission information was collected. The exclusion criteria were as follows: (1) age < 18 years; (2) SAH with negative angiography or SAH caused by trauma, arteriovenous malformation, Moyamoya disease, or other structural lesions; (3) aneurysms related to arteriovenous malformation/fistula or Moyamoya disease; (4) traumatic, infectious, or blood blister-like aneurysm; (5) patients who received aneurysm treatment outside of admission; (6) patients with multiple hospitalizations; and (7) incomplete information for analysis. Based on aneurysm status, the patients were separated into ruptured and non-ruptured groups. Patients with multiple aneurysms were assigned to the ruptured group if they presented with SAH. Finally, 4,787 patients with ruptured or unruptured aneurysms were included in the study (Figure 1).
The following information was collected from all participants: demographic characteristics (sex, age, weight, and height), behavioral history (cigarette smoking and alcohol consumption), comorbidities (diabetes, hypertension, and dyslipidemia), and aneurysm status (ruptured or unruptured). BMI was calculated as weight (in kilograms) divided by the square of height (in meters). BMI was divided into the following four groups: underweight (≤18.5 kg/m2), normal weight (18.5–24 kg/m2), overweight (24–28 kg/m2), and obese (>28 kg/m2). The cigarette smoking status of the patients was divided into never, former, and current smokers (at least five cigarettes per day). Former smokers were defined as those who had smoked at least five cigarettes per day and quit smoking for 3 months. Alcohol consumption status was categorized as never drinker, former drinker, and current drinker (≥150 g of alcohol per week). Former drinkers were defined as those who drank ≥150 g of alcohol per week and had quit smoking for 3 months. DM was defined as treatment with antidiabetic medication or a fasting glucose level of ≥7 mmol/L during a previous health examination. Hypertension was defined as a history of the disorder (a systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥90 mm Hg), regardless of treatment with antihypertension medication. Fasting serum lipid levels were measured on admission. Hyperlipidemia was defined as patients with a history of dyslipidemia treated with lipid-lowering drugs, a fasting plasma cholesterol level of ≥6 mmol/L, a fasting plasma triglyceride level of ≥2 mmol/L, or a fasting plasma low-density lipoprotein level of ≥3.5 mmol/L. Hypercholesterolemia was defined as a serum total cholesterol level of ≥6 mmol/L. In the analyses, we used the normal-weight, never smoker, and never drinker groups as references. Hypertension, DM, and hyperlipidemia were recorded as present or absent. The patients’ age was divided into ≤50, 50–60, 60–70 and > 70 years, and ≤ 50 years old group was the reference.
2.2 Statistical analysis
SPSS (version 23.0; IBM Corp, Armonk, New York, USA) was used for statistical analysis. Continuous variables are presented as means and SD, and categorical variables as numbers (frequency). Continuous variables, such as age and BMI, were transformed into categorical variables, as aforementioned. Fisher’s exact test or Pearson χ2 test was used to determine predictive factors for aSAH. Factors with p < 0.1 in the univariate analysis were considered potential independent variables and subsequently included in the binary logistic regression analysis using an enter process to determine the independent predictors. Statistical significance was set at p < 0.05.
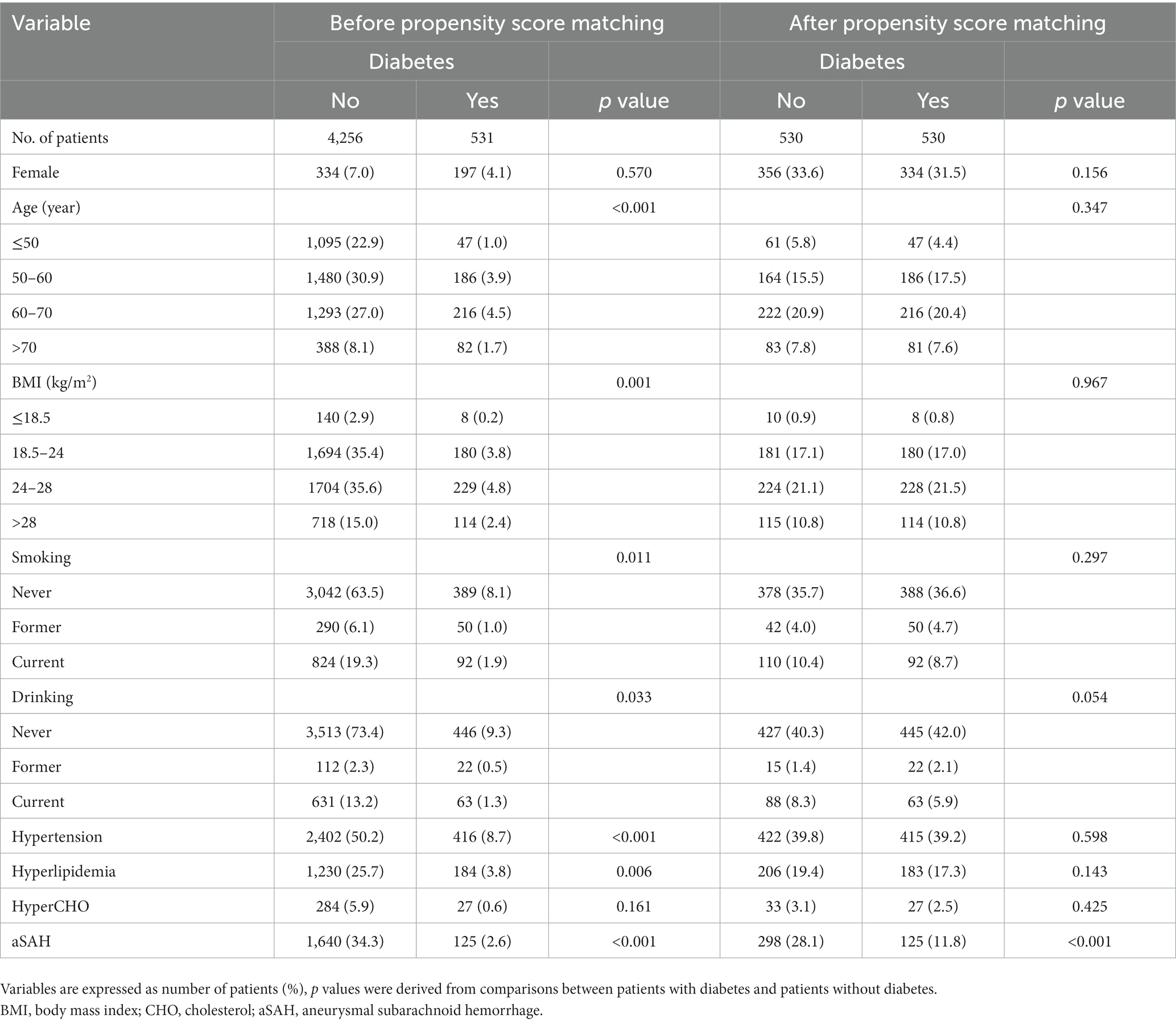
Propensity score matching (PSM) was used to balance confounding factors between patients with and those without DM. PSM was based on sex, age, smoking and drinking histories, BMI, hypertension, hyperlipidemia, and hypercholesterolemia. We conducted a one-to-one PSM analysis using the nearest neighbor method with a caliper value of 0.001 to adjust for the imbalance of the aforementioned baseline characteristics between the two groups.
3 Results
The baseline patient demographics are shown in Table 1. A total of 4,787 patients with 5,768 IAs, including 2,957 (61.8%) females and 1830 (38.2%) males with a mean age of 58 ± 11 years, were included in this study. The mean BMI was 24.8 ± 5.5 kg/m2. Among them, 1765 cases (36.9%) had ruptured aneurysms and 855 cases (11.1%) had multiple aneurysms. A total of 531 (11.1%) patients had diabetes, 2,818 (58.9%) had hypertension, 1,414 (29.5%) had hyperlipidemia, and 311 (6.5%) had hypercholesterolemia.
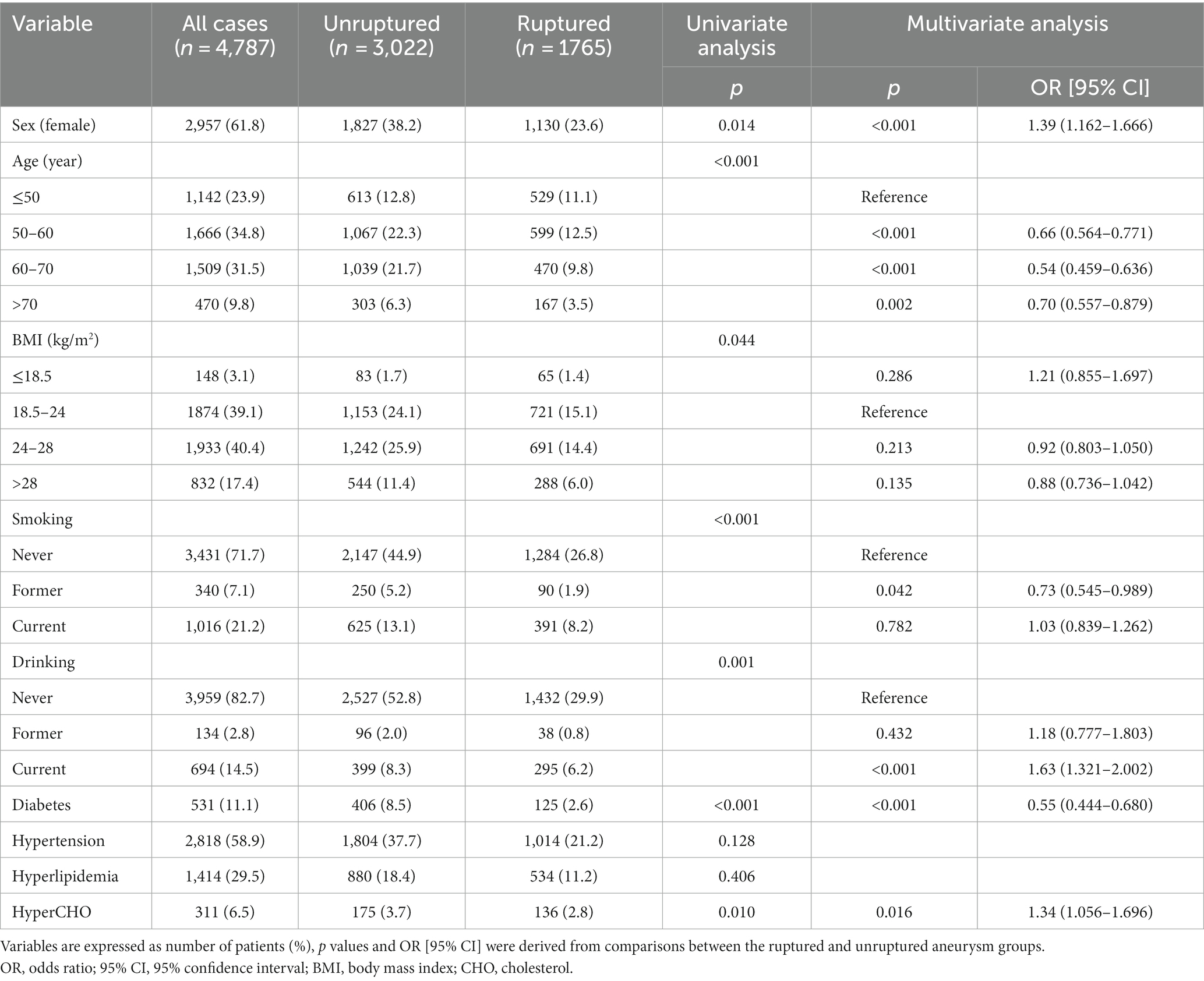
Table 1. Baseline demographic and clinical characteristics of patients with ruptured and unruptured aneurysm.
In ruptured group, there were 1,130(64.0%) females and 635(36.0%) males, with a mean age of 57 ± 11 years and a mean BMI of 24.50 ± 3.64. In total, 481 patients (27.3%) had a history of smoking, 333(18.9%) had drinking, 125(7.1%) had diabetes, 1,014 (57.5%) had hypertension, 534(30.3%) had hyperlipidemia, and 136 (7.7%) had hypercholesterolemia. However; there were 1827(60.5%) females and 1,195(39.5%) males with a mean age of 58 ± 10 years and a mean BMI of 25.00 ± 6.31 in the unruptured group. There were 875 cases of smoking (29.0%), 495 cases of drinking (16.4%), 406 cases of diabetes (13.4%), 1804 cases of hypertension (59.7%), 880 cases of hyperlipidemia (29.1%), and 175 cases of hypercholesterolemia (5.8%) in the unruptured group.
Female sex, current drinking, and hypercholesterolemia were associated with a higher risk of aSAH in univariate and multivariate analyses (p < 0.05; Table 1), whereas old age, former smoking, and diabetes were associated with a lower risk of aSAH in univariate and multivariate analyses (p < 0.05). The unruptured group consisted of 13.4% diabetes cases (406/3022), and the ruptured group consisted of 7.1% diabetes cases (125/1765) (odds ratio, 0.55; 95% confidence interval, 0.444–0.680) (p < 0.001). Other variables, such as BMI, former drinking, current smoking, hypertension, and hyperlipidemia, were not associated with aSAH in the multivariate analysis (p > 0.05). Further analysis revealed that DM was still inversely associated with aSAH in both female and male groups (p < 0.05).
The admission HbA1c levels were only available in 40.1% cases with DM (213/531). Among them, 42 cases (19.7%) had ruptured aneurysm and 171 cases (80.3%) had unruptured aneurysm. Increased HbA1c was noticed in 40.5% cases (17/42) with ruptured aneurysm and 48.0% cases (82/171) with unruptured aneurysm,respectively. Increased HbA1c (>7.0) was not associated with a lower risk of aSAH in univariate analyses in this study (p = 0.384).
In total, 530 patients with DM were successfully matched to 530 patients without DM after PSM. Before PSM, significant differences were found in age, BMI, alcohol consumption, smoking, hypertension, and hyperlipidemia between the patients with and those without DM (Table 2). After PSM, no statistically significant differences were noted in the distribution of these variables. As shown in Table 2, DM was still associated with a lower risk of aSAH after PSM (p < 0.001), and the incidence rate of DM (56.2%) in the unruptured group was still higher than that in the ruptured group (35.7%) (odds ratio, 0.24; 95% confidence interval, 0.185–0.313).
4 Discussion
This case–control study found that the incidence of aSAH was lower among patients with DM than among those without DM; DM was associated with a low risk of aSAH. This inverse association remained significant in our PSM analysis after adjusting for the main confounding factors.
Our result is consistent with a recent literature review study (11), which included 15 case–control studies and three cohort studies with more than 2,000,000 individuals, and indicated that DM reduces the risk of aSAH. A recent population-based cohort study with 421,768 participants also found that the presence of DM is significantly associated with a decreased risk of SAH (10); however, this study could not clearly distinguish SAH cases with or without aneurysm. A recent case–control study of 3,965 patients found an inverse association between them (12). However, these inverse associations are difficult to explain from a pathological perspective. DM is a well-known risk factor for atherosclerosis and can induce atherosclerotic changes and stiffness of cerebral arteries (5). DM is highly correlated with atherosclerotic and stabilized aneurysms based on intraoperative findings (13), and aneurysms with hypertrophied and stiff atherosclerotic walls seem to be more resistant to hemodynamic pressure and are less likely to rupture (13).
However, hyperglycemia can induce vascular endothelial damage and dysfunction and decrease the expression of cerebral tight junction proteins (14). DM can also promote the inflammation process and increase the levels of MMP9 in the arterial walls, all of which can cause aneurysm wall degradation and rupture (15). Clinically, atherosclerotic changes are associated with wall enhancement on magnetic resonance vessel wall imaging, which may indicate rupture-prone aneurysms (16). No association between DM and aneurysm rupture has been reported previously (8). Recent Mendelian randomization studies did not find a causal association between type 2 DMs and aSAH (17); thus, our results should be interpreted with caution. In the real world, patients with DM have a higher risk of dying from other diseases than patients without DM, which reduces the chances of developing aSAH compared with controls. Many patients with diabetes have a higher chance of being evaluated for DM and its complications, such as cerebral infarction and cerebral vascular stenosis, leading to the diagnosis and treatment of cerebral aneurysms before their rupture (8). Patients with DM may modify and maintain a healthy lifestyle (including more exercise, better diet, and less smoking and drinking) and receive medical care to control DM, comorbidities and complications (including hypertension and hyperlipidemia), all of which may lead to a reduced risk of aneurysm rupture (11). Moreover, good and stable glycemic control have been reported to be associated with a reduced risk of IA rupture in patients with DM in previous study (18), and antihyperglycemic agent use was also reported to be associated with a decreased risk of aSAH (19). However, in this study, the exact incidence rate of DM was unknown, and their control group without antihyperglycemic agent use also included patients without diabetes. We could not found an association between blood glucose control status and aneurysms rupture in cases with DM. To determine whether antihyperglycemic agents reduce the risk of aneurysm rupture, a well-designed case–control study is required.
In our study, we found that former smoking but not current smoking was associated with a lower risk of aSAH, and current drinking but not former drinking had a significantly increased risk of aSAH compared with never users (20, 21). Thus, stopping these modifiable risk factors among patients with unruptured IAs is important. Interestingly, hypertension was not associated with aSAH in this study, and a similar result was reported in a previous study (5). This may be because most patients with hypertension undergo antihypertensive treatment and tend to have normal blood pressure after antihypertensive treatment; antihypertensive drugs may further prevent aneurysm rupture through other mechanisms (22). A recent review study including five case–control studies found that hypercholesterolemia is associated with a lower rupture risk of IAs (23). By contrast, in this study, an inverse relationship was observed; hypercholesterolemia increased the risk of aneurysm rupture. A study also found that hypercholesterolemia may increase the risk of SAH particularly in age ≥60 years and female sex (24). No relationship was revealed in other studies (25, 26). Those results should be cautiously explained, because the definition of hypercholesterolemia in other studies includes those cases with lipid-lowering medicaments (23). Statin therapy has been increasingly used not only for lowering blood lipid levels but also for treating cardiovascular and cerebrovascular diseases. Patients with hypercholesterolemia may gain a protective benefit for aneurysm rupture from statin use (23). Further studies with rigorous inclusion and exclusion criteria should be conducted to determine these associations. BMI was not associated with aneurysm rupture in our study. A recent study found that increased BMI is inversely associated with saccular aneurysm rupture in males and patients aged ≥50 years (12). A recent Mendelian randomization study found that a higher BMI increases the risk of both IA and aSAH (17). The association between BMI and aSAH remains uncertain.
4.1 Limitations
A notable strength of this study was its large sample size, including patients with ruptured and unruptured aneurysm and patients with diabetes. The main potential confounding factors were also balanced. However, this study had several limitations. First, it was a single-center, retrospective study. A prospective, multicenter, larger study with long-term follow-up is warranted to establish a causal relationship. However, long-term follow-up of patients with rupture-prone aneurysms does not seem ethical. Second, this study did not distinguish between type 1 and type 2 DM; therefore, a different association may exist between DM and aSAH. The diagnosis of DM was not standardized and was based solely on self-report. Due to the impact of ruptured saccular IA and drug interference, the fasting or random plasma glucose level in patients with ruptured IA is unreliable. Third, HbA1c was not routinely measured, and several patients with undiagnosed diabetes may have been missed in this study. Hyperglycemia control status and the use of hypoglycemic drugs, which was not available in all our cases in this study, may also affect our results. Meanwhile, other treatment for patients with diabetes may also reduce the risk for aSAH. The collected information was not all self-reported, especially in cases of aSAH with a poor clinical condition. Fourth, unobserved confounders, such as a family history of aSAH and the use of antihypertensive agents, statins, and aspirin, were not available. Finally, data of patients who failed to reach the hospital were missing. All the aforementioned factors may have led to biased results. This study focused on the risk factors for aSAH, and further research is needed to evaluate the effects of DM on the incidence and growth of IAs.
5 Conclusion
In conclusion, patients with aSAH have a lower incidence of DM, however this case-cohort study could not establish a causal relationship. The implications of our findings require careful evaluation. A prospective and large study with long-term follow-up is warranted to establish a causal relationship.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the ethics committee of Qilu Hospital of Shandong University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
WZ: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing, Funding acquisition. KC: Data curation, Writing – original draft, Writing – review & editing. ZS: Data curation, Validation, Writing – review & editing. YX: Data curation, Validation, Writing – review & editing. DZ: Data curation, Writing – review & editing. MZ: Formal analysis, Methodology, Writing – review & editing. YW: Writing – review & editing, Data curation. DW: Validation, Writing – review & editing. WS: Validation, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Natural Science Foundation of Shandong Province (grant number: ZR2022MH131).
Conflict of interest
WZ was affiliated with Lunan Pharmaceutical Group Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
DM, diabetes mellitus; SAH, subarachnoid hemorrhage; IA, intracranial aneurysm; BMI, body mass index; PSM, propensity score matching
References
1. de Rooij, NK, Linn, FH, van der Plas, JA, Algra, A, and Rinkel, GJ. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry. (2007) 78:1365–72. doi: 10.1136/jnnp.2007.117655
2. van Gijn, J, Kerr, RS, and Rinkel, GJ. Subarachnoid haemorrhage. Lancet. (2007) 369:306–18. doi: 10.1016/S0140-6736(07)60153-6
3. Boling, B, and Groves, TR. Management of subarachnoid hemorrhage. Crit Care Nurse. (2019) 39:58–67. doi: 10.4037/ccn2019882
4. Al-Khindi, T, Macdonald, RL, and Schweizer, TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke. (2010) 41:e519–36. doi: 10.1161/STROKEAHA.110.581975
5. Inagawa, T. Risk factors for the formation and rupture of intracranial saccular aneurysms in Shimane. World Neurosurg. (2010) 73:155–64. doi: 10.1016/j.surneu.2009.03.007
6. Andreasen, TH, Bartek, J Jr, Andresen, M, Springborg, JB, and Romner, B. Modifiable risk factors for aneurysmal subarachnoid hemorrhage. Stroke. (2013) 44:3607–12. doi: 10.1161/STROKEAHA.113.001575
7. Koshy, L, Easwer, HV, Premkumar, S, Alapatt, JP, Pillai, AM, Nair, S, et al. Risk factors for aneurysmal subarachnoid hemorrhage in an Indian population. Cerebrovasc Dis. (2010) 29:268–74. doi: 10.1159/000275501
8. Lindgren, AE, Kurki, MI, Riihinen, A, Koivisto, T, Ronkainen, A, Rinne, J, et al. Type 2 diabetes and risk of rupture of saccular intracranial aneurysm in eastern Finland. Diabetes Care. (2013) 36:2020–6. doi: 10.2337/dc12-1048
9. Ohkuma, H, Tabata, H, Suzuki, S, and Islam, MS. Risk factors for aneurysmal subarachnoid hemorrhage in Aomori. Japan Stroke. (2003) 34:96–100. doi: 10.1161/01.str.0000048161.57536.42
10. Kim, JH, Jeon, J, and Kim, J. Lower risk of subarachnoid haemorrhage in diabetes: a nationwide population-based cohort study. Stroke Vasc Neurol. (2021) 6:402–9. doi: 10.1136/svn-2020-000601
11. Yao, XY, Jiang, CQ, Jia, GL, and Chen, G. Diabetes mellitus and the risk of aneurysmal subarachnoid haemorrhage: a systematic review and meta-analysis of current evidence. J Int Med Res. (2016) 44:1141–55. doi: 10.1177/0300060516666426
12. Chen, S, Mao, J, Chen, X, Li, Z, Zhu, Z, Li, Y, et al. Association between body mass index and intracranial aneurysm rupture: a multicenter retrospective study. Front Aging Neurosci. (2021) 13:716068. doi: 10.3389/fnagi.2021.716068
13. Song, J, and Shin, YS. Diabetes may affect intracranial aneurysm stabilization in older patients: analysis based on intraoperative findings. Surg Neurol Int. (2016) 7:391–7. doi: 10.4103/2152-7806.183497
14. Ye, X, Chopp, M, Cui, X, Zacharek, A, Cui, Y, Yan, T, et al. Niaspan enhances vascular remodeling after stroke in type 1 diabetic rats. Exp Neurol. (2011) 232:299–308. doi: 10.1016/j.expneurol.2011.09.022
15. Yan, T, Chopp, M, Ning, R, Zacharek, A, Roberts, C, and Chen, J. Intracranial aneurysm formation in type-one diabetes rats. PLoS One. (2013) 8:e67949. doi: 10.1371/journal.pone.0067949
16. Zhong, W, Su, W, Li, T, Tan, X, Chen, C, Wang, Q, et al. Aneurysm wall enhancement in unruptured intracranial aneurysms: a histopathological evaluation. J Am Heart Assoc. (2021) 10:e018633. doi: 10.1161/JAHA.120.018633
17. Karhunen, V, Bakker, MK, Ruigrok, YM, Gill, D, and Larsson, SC. Modifiable risk factors for intracranial aneurysm and aneurysmal subarachnoid hemorrhage: a mendelian randomization study. J Am Heart Assoc. (2021) 10:e022277. doi: 10.1161/JAHA.121.022277
18. Su, SX, Wang, XT, Li, XF, Duan, CZ, Bi, YM, and Zhang, X. Nonlinear association of glycosylated hemoglobin with single intracranial aneurysm rupture in patients with diabetes mellitus: a cross-sectional study. Front Neurol. (2022) 13:854008. doi: 10.3389/fneur.2022.854008
19. Can, A, Castro, VM, Yu, S, Dligach, D, Finan, S, Gainer, VS, et al. Antihyperglycemic agents are inversely associated with intracranial aneurysm rupture. Stroke. (2018) 49:34–9. doi: 10.1161/STROKEAHA.117.019249
20. Can, A, Castro, VM, Ozdemir, YH, Dagen, S, Dligach, D, Finan, S, et al. Alcohol consumption and aneurysmal subarachnoid hemorrhage. Transl Stroke Res. (2018) 9:13–9. doi: 10.1007/s12975-017-0557-z
21. Morel, S, Hostettler, IC, Spinner, GR, Bourcier, R, Pera, J, Meling, TR, et al. Intracranial aneurysm classifier using phenotypic factors: an international pooled analysis. J Pers Med. (2022) 12:1410. doi: 10.3390/jpm12091410
22. Shimizu, K, Imamura, H, Tani, S, Adachi, H, Sakai, C, Ishii, A, et al. Meling TR, et al. Intracranial aneurysm classifier using phenotypic factors: an international pooled analysis. J Pers Med. (2022). 12:1410. doi: 10.1371/journal.pone.0246865
23. Løvik, K, Laupsa-Borge, J, Logallo, N, and Helland, CA. Dyslipidemia and rupture risk of intracranial aneurysms-a systematic review. Neurosurg Rev. (2021) 44:3143–50. doi: 10.1007/s10143-021-01515-3
24. Inagawa, T. Risk factors for aneurysmal subarachnoid hemorrhage in patients in Izumo City, Japan. J Neurosurg. (2005) 102:60–7. doi: 10.3171/jns.2005.102.1.0060
25. Sandvei, MS, Lindekleiv, H, Romundstad, PR, Müller, TB, Vatten, LJ, Ingebrigtsen, T, et al. Risk factors for aneurysmal subarachnoid hemorrhage – BMI and serum lipids: 11-year follow-up of the HUNT and the Tromsø Study in Norway. Acta Neurol Scand. (2012) 125:382–8. doi: 10.1111/j.1600-0404.2011.01578.x
26. Feigin, V, Parag, V, Lawes, CM, Rodgers, A, Suh, I, Woodward, M, et al. Smoking and elevated blood pressure are the most important risk factors for subarachnoid hemorrhage in the Asia-Pacific region: an overview of 26 cohorts involving 306,620 participants. Stroke. (2005) 36:1360–5. doi: 10.1161/01.STR.0000170710.95689.41
Keywords: intracranial aneurysms, subarachnoid hemorrhage, risk factors, diabetes mellitus, propensity score matching
Citation: Zhong W, Chen K, Song Z, Xiao Y, Zhou D, Zhang M, Wang Y, Wang D and Su W (2023) Lower incidence of diabetes mellitus in patients with aneurysmal subarachnoid hemorrhage: a large case–control study with propensity score matching. Front. Neurol. 14:1282486. doi: 10.3389/fneur.2023.1282486
Edited by:
Slaven Pikija, University Hospital Salzburg, AustriaReviewed by:
Tijana Nastasovic, University of Belgrade, SerbiaHua Lu, Nanjing Medical University, China
Copyright © 2023 Zhong, Chen, Song, Xiao, Zhou, Zhang, Wang, Wang and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wandong Su, c3V3YW5kb25nQHNpbmEuY29t
 Weiying Zhong
Weiying Zhong Kai Chen1
Kai Chen1 Donglin Zhou
Donglin Zhou Yunyan Wang
Yunyan Wang