
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Neurol., 18 January 2024
Sec. Neuromuscular Disorders and Peripheral Neuropathies
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1281953
This article is part of the Research TopicCase Reports in Neuromuscular Disorders and Peripheral Neuropathies, volume III, 2023View all 23 articles
 Martina Rimoldi1†
Martina Rimoldi1† Gloria Romagnoli1†
Gloria Romagnoli1† Francesca Magri1
Francesca Magri1 Sara Antognozzi2
Sara Antognozzi2 Claudia Cinnante3
Claudia Cinnante3 Elena Saccani4
Elena Saccani4 Patrizia Ciscato1
Patrizia Ciscato1 Simona Zanotti1
Simona Zanotti1 Daniele Velardo1
Daniele Velardo1 Stefania Corti1,5
Stefania Corti1,5 Giacomo Pietro Comi2,5
Giacomo Pietro Comi2,5 Dario Ronchi2,5*
Dario Ronchi2,5*Limb-girdle muscular dystrophy autosomal recessive 8 (LGMDR8) is a rare clinical manifestation caused by the presence of biallelic variants in the TRIM32 gene. We present the clinical, molecular, histopathological, and muscle magnetic resonance findings of a novel 63-years-old LGMDR8 patient of Italian origins, who went undiagnosed for 24 years. Clinical exome sequencing identified two TRIM32 missense variants, c.1181G > A p.(Arg394His) and c.1781G > A p.(Ser594Asp), located in the NHL1 and NHL4 structural domains, respectively, of the TRIM32 protein. We conducted a literature review of the clinical and instrumental data associated to the so far known 26 TRIM32 variants, carried biallelically by 53 LGMDR8 patients reported to date in 20 papers. Our proband's variants were previously identified only in three independent LGMDR8 patients in homozygosis, therefore our case is the first in literature to be described as compound heterozygous for such variants. Our report also provides additional data in support of their pathogenicity, since p.(Arg394His) is currently classified as a variant of uncertain significance, while p.(Ser594Asp) as likely pathogenic. Taken together, these findings might be useful to improve both the genetic counseling and the diagnostic accuracy of this rare neuromuscular condition.
The term limb-girdle muscular dystrophy (LGMD) refers to typically non-syndromic childhood- and adult-onset group of muscular dystrophies, affecting primarily skeletal muscles, and usually associated with elevated serum creatine kinase (CK) concentration (1, 2). Patients with LGMD suffer from progressive muscle weakness and wasting, involving proximal more than distal districts, in particular muscles of the shoulder and pelvic girdles (1, 2). However, other muscle groups, such as facial, distal upper and lower limbs, may also be affected (3). In the pre-molecular era, LGMD diagnosis used to be purely clinical, and it could only be confirmed differentially once specific protein testing became available (4, 5) to exclude X-linked recessive neuromuscular disorders, such as Duchenne muscular dystrophy and Becker muscular dystrophy (1). Since the advent of molecular myology, pathogenic variants in 29 genes have been reported in distinct LGMD clinical presentations, classified into dominant (LGMDD) or recessive (LGMDR) forms according to the pattern of inheritance, with some genes presenting both kinds of transmission (1, 2).
LGMDR8 (MIM #254110) represents the subtype 8 of the autosomal recessive type of LGMD, previously known as LGMD2H (1, 2), and initially referred to as Sarcotubular Myopathy when first described in the Hutterite population of Manitoba, in North America (6–9). This subtype of LGMDR can display clinical heterogeneity, with symptom onset ranging between the first and the fourth decade of life (10). The clinical course is progressive, and severity goes from absence of symptoms to muscular weakness with atrophy (11, 12), possibly requiring the use of a wheelchair.
Biallelic variants in the TRIM32 gene, on chromosome 9q33, have been associated with LGMDR8, which is caused by the homonymous protein deficiency (2). TRIM32 encodes the member 32 of the TRIpartite Motif-containing (TRIM) ubiquitin E3 ligase family, which ubiquitinates several muscle substrates, including sarcomeric proteins (13). However, the specific effect on the muscle of the so far identified TRIM32 variants has not been fully clarified yet. Bioinformatic modeling suggests these variants may cause a potential misfolding of the TRIM32 protein, especially at the level of the C-terminal domain, leading to pathogenic consequences on the muscle physiology (14). Beside the first variant – c.1459G > A, p.(Asp487Asn) – identified in the Hutterite population with a founder effect (6, 7, 9), additional 25 variants of TRIM32 have been described to date in other 41 non-Hutterite LGMDR8 patients, worldwide (11, 13, 15–28).
Here, we present the novel case of an Italian 63-years-old man, who experienced progressive muscle weakness since the age of 39, and only received a molecular diagnosis 24 years later. He is the first LGMDR8 case in literature to be identified as compound heterozygous for two TRIM32 missense variants, whose pathogenicity has not been clarified yet. We describe his clinical, histopathological, muscle magnetic resonance imaging (MMRI) and molecular findings, against the backdrop of other 52 LGMDR8 patients harboring biallelic TRIM32 variants (Table 1; Figure 1A), reported across 19 published studies (7, 11, 13, 15–30).
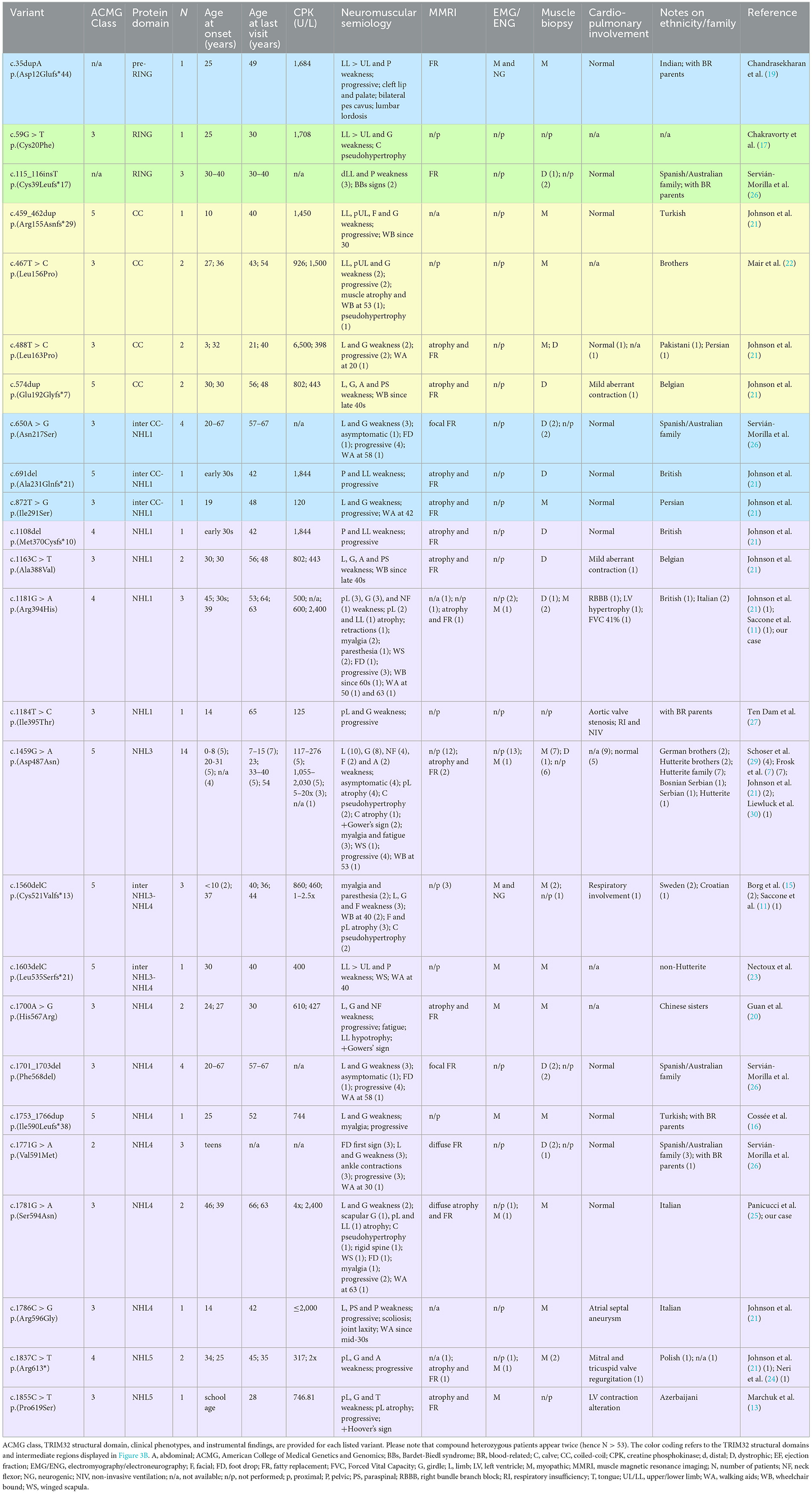
Table 1. Summary of the 26 TRIM32 variants carried biallelically by the 53 LGMDR8 patients (including our case) reported in the literature.
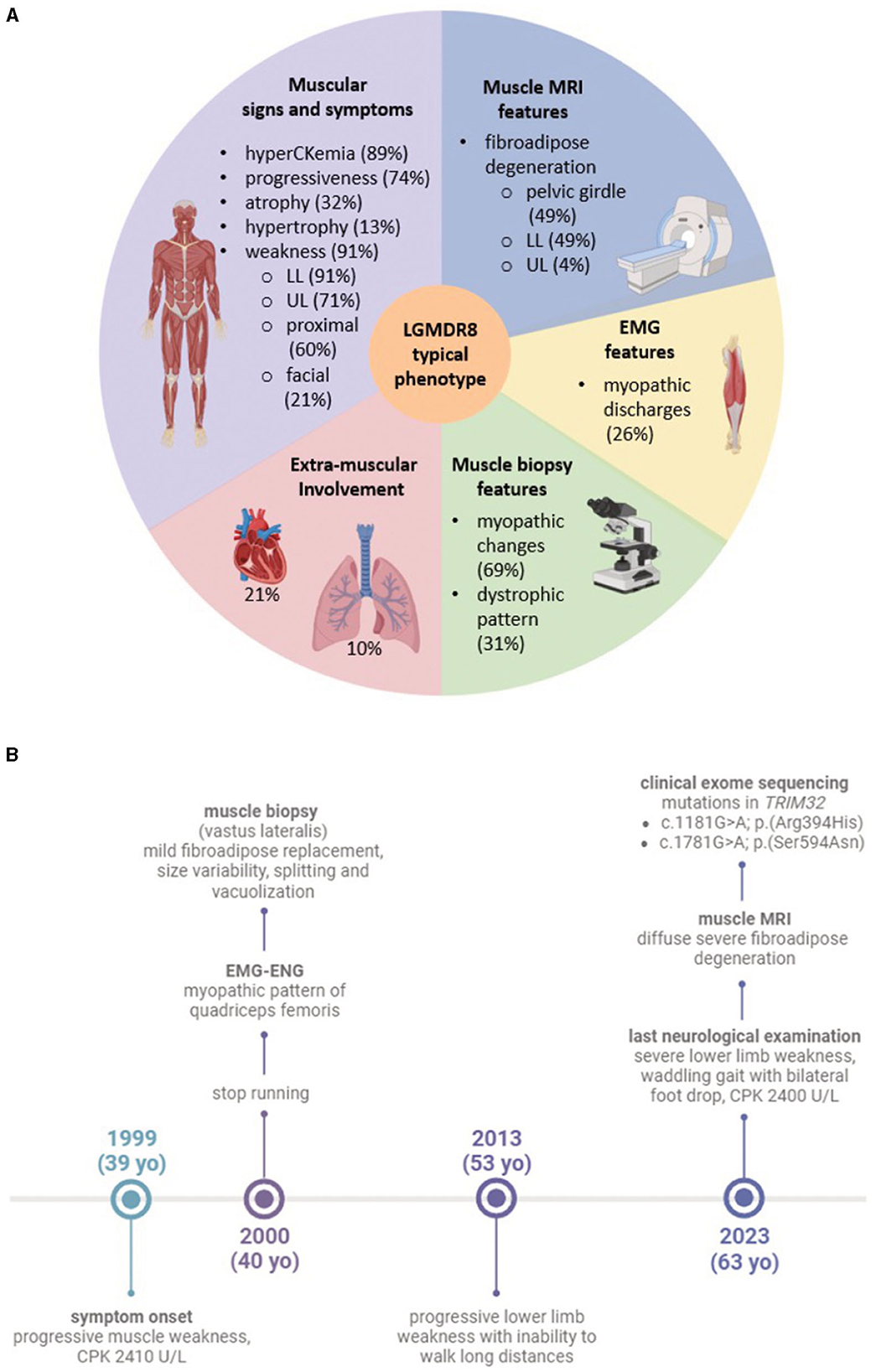
Figure 1. (A) Graphic of the typical LGMDR8 phenotype. The five sections summarize the most frequent clinical and instrumental features, drawn from the analysis of the 53 LGMDR8 TRIM32-mutated patients (including our case) reported in the literature; (B) timeline highlighting our patient's clinical history and diagnostic work-up. Figures (A, B) were created with BioRender.com.
The study was approved by the institutional review board of the Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan (Italy). The patient provided written informed consent for all aspects of the study. All the details related to data collection and analysis are available in Supplementary material. Clinical, histological, immunohistochemical, MMRI, and molecular data from the proband were integrated within our literature review of additional 52 LGMDR8 patients, carrying biallelic TRIM32 variants (Table 1; Figure 1A).
As far as the literature review is concerned, the following terms were searched through PubMed in May 2023, filtering for human studies, abstract and full-text availability in English: “((trim32) AND (lgmdr8)) OR (limb-girdle muscular dystrophy recessive 8).” We included publications reporting patients of any age, and providing clinical, instrumental, and molecular characterization, with the latter clearly specifying the presence of TRIM32 biallelic variants, either carried in homozygosis or compound heterozygosis. We applied exclusion criteria at both molecular and clinical levels: (1) the identification of monoallelic TRIM32 variants led to the exclusion of the heterozygous patients; (2) among the patients carrying TRIM32 variants biallelically, we excluded those cases clearly showing a non-LGMDR8 phenotype, such as the Bardet-Biedl syndrome type 11 (BBS11).
A 63-year-old man came to our attention presenting with progressive muscle weakness and difficulty in climbing the stairs, since the age of 39. The patient was born to non-consanguineous parents, had a typical psychomotor development, and a negative family history of neuromuscular disorders. He stopped running in 12-month time from symptom onset, developing a waddling gait and inability to walk long distances by the age of 53. Serum CK levels were persistently increased over the years, ranging between 600 and 3,000 U/L (with 350 U/L representing the upper normal limit in men); his cardiopulmonary examination was always unremarkable.
A muscle biopsy of the vastus lateralis, collected at the age of 40, revealed dystrophic changes, such as severe fibroadipose replacement, predominance of type 2 fibers, increased fiber size variability, and presence of necrotic fibers with macrophagic invasion. Furthermore, there was evidence of numerous splitting fibers, subsarcolemmal and intracytoplasmic vacuoles containing finely granular material, without the presence of sarcotubular aggregates (Figures 2A–D). Immunohistochemistry showed desmin and myotilin accumulations within muscle fibers, as well as increased p62 detection compared to control muscles (Figures 2E–G). Electromyography (EMG) and electroneurography (ENG), performed at the early stage of the disease, showed a myopathic pattern of the quadriceps femoris, supporting the diagnosis of a pelvic girdle myopathy (clinical timeline is displayed in Figure 1B). The primary hypothesis of a putative involvement of the DMD gene, possibly responsible for Becker muscular dystrophy, was ruled out by Sanger sequencing and MLPA analysis, at the time of symptom onset.
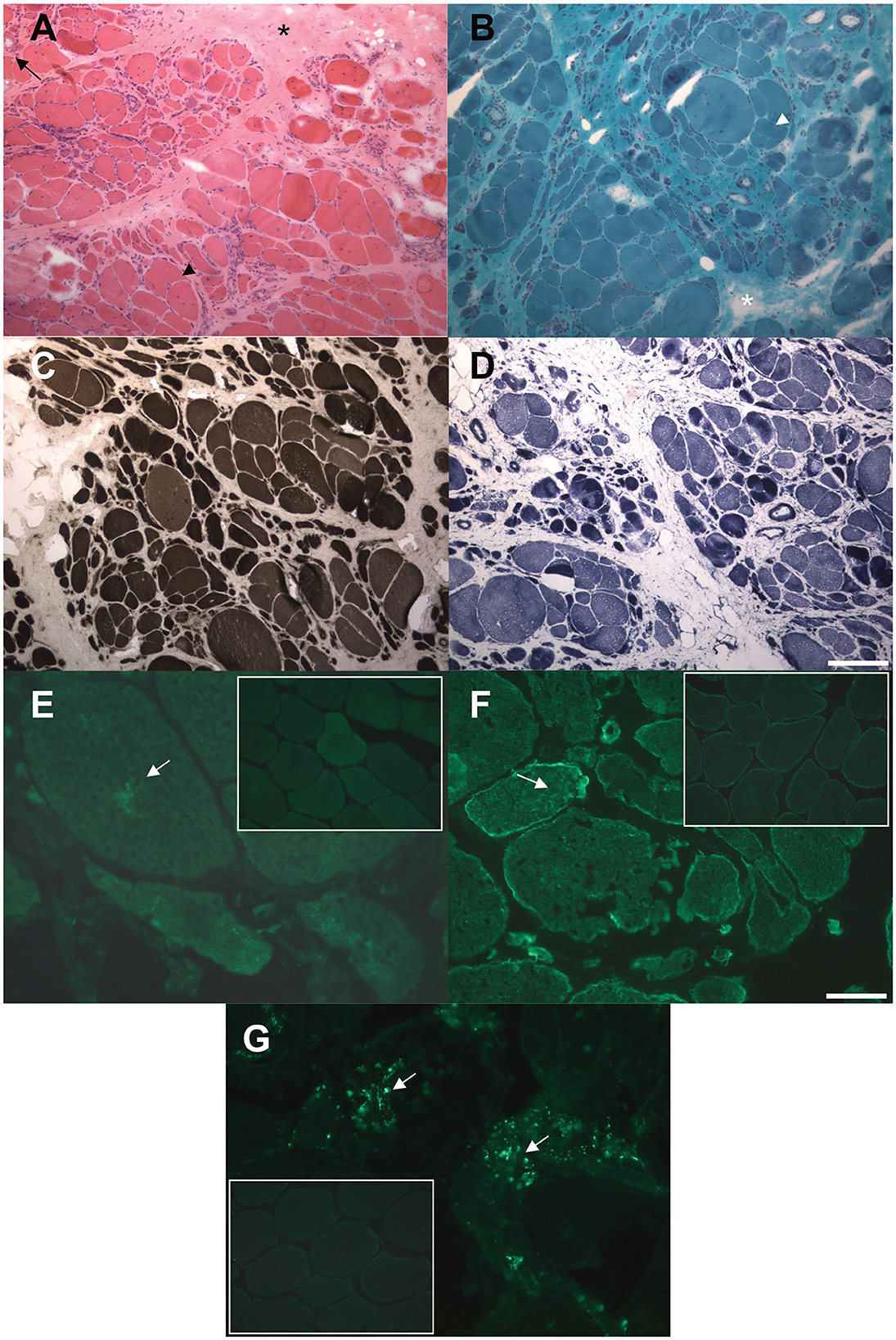
Figure 2. Histological and immunohistochemical findings in muscle biopsy. (A, B) Hematoxylin and eosin (A) and modified Gomori trichrome (B) stains showing markedly increased fiber size variability, central nuclei, severe fibro-adipose replacement (asterisks), splitting fibers (arrowheads), internal vacuoles (arrow). (C) ATPase pH 9.4 stain showing predominant type 2 (darker) fibers atrophy. (D) NADH-TR stain showing preserved oxidative enzymes activity in most of the observed fibers, with darker stain of the atrophic fibers. (E, F) Immunofluorescence assay showing increased intracellular accumulation of myotilin (E) and desmin (F) proteins compared to control muscles (small frames). (G) Immmunofluorescence assay showing increased cytoplasmic p62 signal compared to control muscle (small frame). Scale bars: 100 μm (A–D) 25 μm (E–G).
His last physical examination revealed bilateral hypotrophy of the anterior compartment of the thigh and of the gastrocnemius, winged scapula, and selective weakness of pectoral, upper and lower limb muscles, causing waddling gait and bilateral foot drop. He was able to climb the stairs and stand up from a seated position only with the use of external aids. The patient reported to have recently experienced mild weakness of the upper limbs, and right thigh myalgias, with no cramps nor myoglobinuria. He did not complain of dysphagia or dysphonia. The most recent CK dosage was 2,400 U/L, in line with his previous values.
Following the neuromuscular visit, a muscle MRI scan was performed for the first time in 24 years of disease duration. This revealed diffuse morphological changes in several muscles of the upper and lower limbs (Supplementary Figure 1), with a severe fibroadipose degeneration involving the shoulder and pelvic girdles. In particular, the fatty infiltration pattern of the pelvic girdle and of the thigh muscles showed relative sparing of rectus femoris, gracilis, sartorius, and the short head of biceps femoris. The most affected compartments of the thigh were the medial and the posterior ones, with a diffuse bilateral hypotrophy, which was mostly symmetric. Dystrophic changes were also present at the level of the leg muscles, with relative sparing of the flexor hallucis longus, flexor digitorum longus and tibialis posterior muscles. As for the shoulder girdle, there was diffuse fatty degeneration, with relative sparing of the right supraspinatus, as well as of the trapezius, infraspinatus and pectoralis muscles, bilaterally. At the upper arm level, the most affected muscle was the triceps brachii, followed by the deltoid muscle. In contrast to what shown by the MMRI, clinical examination of the upper limbs was normal, with MRC score being 5 in all districts, except for pectoral muscles, where MRC score was 4 bilaterally.
To make the definitive diagnosis of LGMDR8, a Clinical Exome Sequencing was performed at our center, revealing the presence of two heterozygous TRIM32 variants (Figures 3B, C): c.1181G > A/p.(Arg394His), and c.1781G > A/p.(Ser594Asp). These were already described by Saccone et al. (11) and Panicucci et al. (25), and classified as class 4 (likely pathogenic) and class 3 (of uncertain significance) variants, respectively, according to the ACMG (American College of Medical Genetics and Genomics) criteria (Figure 3). Proband's parents or relatives were not available for segregation testing.
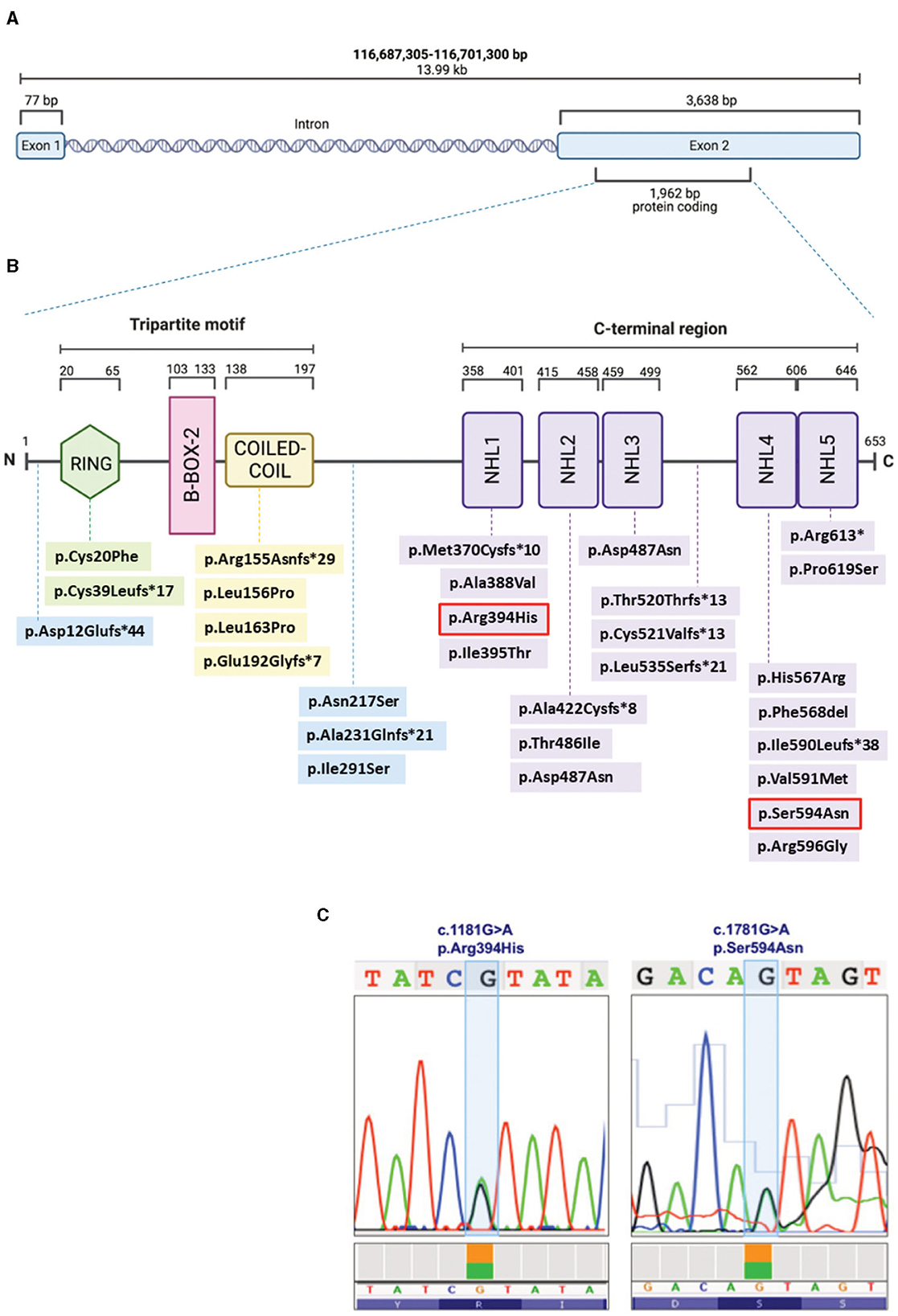
Figure 3. Schematic representation of the TRIM32 gene (A) and TRIM32 protein (B), displaying the structural domains and intermediate regions with the LGMDR8-associated biallelic variants (that are listed with the same color coding in Table 1). The two variants (c.1181G > A, p.Arg394His; c.1781G > A, p.Ser594Asn) carried by our patient are outlined in red (B) and also shown in the chromatograms (C). Figures (A, B) were created with BioRender.com.
We conducted a literature review of the clinical data related to 26 TRIM32 variants, carried biallelically (in either compound heterozygosis or homozygosis) by 53 LGMDR8 patients (included our proband). Clinical and instrumental findings extracted from the 20 extant studies are reported in Table 1, and a visual summary of the LGMDR8 typical phenotype, based on the literature, is displayed in Figure 1A (7, 11, 13, 15–30).
The 91% (48/53) of the LGMDR8 patients described in the literature suffered from muscle weakness, usually self-reported as difficulty in walking/running and climbing the stairs. The upper limbs were involved in 71% of the patients (34/48), whereas proximal muscles (including those of the scapular girdle) were affected in 60% (29/48) of the cases. Other reported muscular symptoms were myalgias (11/53), exercise-intolerance (7/53), fatigue/fatigability (5/53), paresthesia (3/53), ankle contraction (3/53), muscle stiffness (1/53) and cramps (1/53). Physical examination also revealed lower limb (calves or quadriceps) hypertrophy in 13% (7/53) of the patients, and atrophy/hypotrophy (mainly affecting the lower limbs) in 32% (17/53) of them. Scapular winging was also reported in 13% (7/53) of the cases, and one patient presented with a scapuloperoneal phenotype (30). Finally, a small group of patients (5 out of 53), aged 4–33 years, did not complain of any muscle symptoms, and their neurological evaluation showed no signs of muscular impairment (7, 26). The clinical course showed progressiveness in 74% (39/53) of the cases. In this sub-group of progressive LGMDR8, 21% (8/39) of the patients needed unilateral (3/39) or bilateral (5/39) aids for walking, after 5–24 years of disease duration; while 23% (9/39) of them were wheelchair-bound at an age ranging from 30 to 70 years, after 8–40 years of disease progression. An EMG study was performed in 26% (14/53) of the LGMDR8 patients, who typically showed a myopathic pattern; neurogenic electromyographic changes were also reported in one-third of these cases.
Cardiac and respiratory involvement is not a frequent presentation in LGMDR8, but there might be cardiorespiratory alterations in some cases. Cardiological examination (electrocardiography and/or echocardiography) and spirometry testing were reported normal in 77% (30/39) of the patients with available cardiorespiratory data. The remaining 23% (9/39) showed some respiratory (10%, 4/39) and/or cardiac abnormalities (21%, 8/39), such as respiratory insufficiency and violations of myocardial conduction (Table 1).
MMRI studies of the lower limbs were described in 49% (26/53) of the cases [our patient; (13, 19, 21, 24–28)]. Their images showed fibroadipose degeneration, with preferential affection of the posterior thigh compartment and selective sparing of specific muscles. Upper limb MMRI findings have been rarely reported in the LGMDR8 literature [our case; (28)]. Wei at al. (28) described a minimal fatty infiltration without a distinct involvement pattern; conversely, our patient's MMRI showed diffuse fibroadipose replacement of the shoulder girdle, and involvement of the high arm, with selective sparing of specific muscles.
Histological findings were reported in 39 patients: 69% (27/39) of the biopsies showed nonspecific myopathic changes, whereas the remaining 31% (12/39) displayed a clearly dystrophic pattern, with two cases presenting both myopathic and neurogenic features. Immunohistochemical analysis of our patient's biopsy showed accumulations of desmin and myotilin, that are muscle-relevant targets of the TRIM32 ubiquitin ligase activity, alongside dysbindin, actin, α-actinin and tropomyosin (14). Similar findings were also observed in another LGMDR8 patient (25), who was homozygous for the c.1781G > A, p.(Ser594Asn) TRIM32 variant, also carried by our proband. Furthermore, a certain degree of autophagy alteration has been observed in muscle samples from LGMDR8 patients: reduced levels of p62 and LC3II were described by Servián-Morilla et al. (26), whereas an increased p62 signal was detected in the muscle biopsy of our patient. Further investigations are therefore needed to elucidate TRIM32 pathways in autophagy regulation, and whether autophagy is up- or down-regulated in LGMDR8 (14).
The TRIM family includes RING E3 ubiquitin ligases sharing a common evolutionary origin and a similar structure of the N-terminal RING domain as well as of the NHL repeats at the C-terminus.
In the TRIM32 653-amino-acid-long protein (Figure 3B), the N-terminal conserved tripartite motif consists of: (1) a RING structural domain (20–65 amino acids), which confers catalytic activity by interacting with the E2, and by promoting ubiquitin transfer; (2) a single type 2 B-box structural domain (103–133 amino acids), which possibly enhances the E3 RING domain activity (31) and modulates the rate of poly-ubiquitin chain synthesis (32); and (3) the Coiled-Coil structural domain (138–197 amino acids), which mediates dimerization and oligomerization (14). The C-terminus consists of five (or six) NHL repeats of about forty residues each (358–401, 415–458, 459–499, 562–605, 606–646), that are involved in protein binding and in mediating higher order self-association and homomultimerization (14).
Looking at the clinical features and genotypes of the 53 patients from our literature review, a clear genotype-phenotype correlation did not emerge. Interestingly, Guan and coauthors (20) recently analyzed 86 TRIM32-mutated patients from the literature, highlighting a correlation of the TRIM32 variants exclusively impacting on the NHL domains, with a lower age at onset and higher CK levels, compared to subjects carrying variants outside the NHL regions. A gender effect was also observed, with a lower age of symptom onset, and higher CK levels, in males than in females (20). However, their analysis included both LGMDR8 and BBS11 patients, carrying bi- or monoallelic TRIM32 variants (20), and this may explain the different results from our review, which instead specifically focused on LGMDR8-associated TRIM32 biallelic variants.
To date, 50% (13/26) of the TRIM32 variants (Table 1) considered to be causative for LGMDR8 (when present biallelically, either in homozygosis or in compound heterozygosis) are located in one of the five NHL repeats, like in the case of our patient. However, LGMDR8-causing TRIM32 variants have been recently identified also in non-NHL domains (RING and Coiled-Coil), as well as in the intermediate regions outside the structural domains, like the pre-RING region (19), inter-CoiledCoil-NHL1 (26), and inter-NHL3-NHL4 (21). These findings dismantled the initial hypothesis of a selective involvement of the NHL domains in the LGMDR8 pathogenesis (14). In particular, the RING domain was found to be mutated (p.Cys20Phe, p.Cys39Leufs*17) in 4 LGMDR8 patients reported independently by Chakravorty et al. (17) and Servián-Morilla et al. (26). Additional 4 variants have been identified also in the Coiled-Coil domain (p.Arg155Asnfs*29; p.Leu156Pro; p.Leu163Pro; p.Glu192Glyfs*7) of other 7 LGMDR8 patients, described by Johnson et al. (21) and Mair et al. (22). The only TRIM32 structural domain which seems not to be involved in the LGMDR8 pathogenesis is the B-box-2. To date, the only noted B-box-2 variant is p.(Pro130Ser), which is, in fact, associated with BBS11 (33). BBS11 is a multisystemic disorder with no skeletal muscle involvement, characterized by obesity, polydactyly, retinal dystrophy and kidney abnormalities. Interestingly, despite LGMDR8 and BBS11 being two distinct disorders, some of the LGMDR8 patients (26) carrying the truncating variant, p.Cys39Leufs*17, in the RING domain, showed typical BBS11 systemic symptoms in addition to the LGMDR8 muscular presentation. A putative explanation for the presence of such different TRIM32-related phenotypes might be that RING and B-Box-2 domains have less muscular specificity and a more pleiotropic activity than the Coiled-Coil domain and the NHL repeats, that instead seem to mediate more muscle-specific functions.
Our patient carried a likely pathogenic variant in the NHL1 domain, and a variant of uncertain significance in the NHL5 domain. The NHL1 variant, p.(Arg394His), was previously reported in homozygosis in two LGMDR8 patients by Saccone et al. (11) and Johnson et al. (21); whereas the NHL5 variant, p.(Ser594Asp), was described in homozygosis in one patient by Panicucci et al. (25). Clinical, histological, and imaging features of our proband were very similar to the ones described in the patient carrying the NHL5 variant (25). As for the other two cases carrying the same NHL1 variant harbored by our patient, one had analogous muscular presentation and progressiveness of the disease (21), whereas the second patient, unlike our proband, presented with additional cardiorespiratory symptoms (11). Interestingly, two of these three cases were of Italian origins (11, 25), like our patient.
We presented a novel LGMDR8 patient of Italian origins, caused by two TRIM32 missense variants for the first time described in compound heterozygosis, and previously reported in homozygosis in three independent patients (11, 21, 25). This case report provided new evidence in support of the pathogenicity of both p.(Arg394His) and p.(Ser594Asp) variants, that are not yet classified as pathogenic. We also defined the typical LGMDR8 phenotype associated with the so far identified 26 TRIM32 variants, by performing a literature review of clinical and instrumental data related to 53 LGMDR8 patients. All these findings, taken together, might potentially be of help in improving both the diagnostic accuracy and the genetic counseling of this rare neuromuscular disease.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
MR: Data curation, Investigation, Writing—original draft. GR: Data curation, Investigation, Writing—original draft. FM: Investigation, Writing—review & editing. SA: Methodology, Visualization, Writing—original draft. CC: Investigation, Methodology, Writing—original draft. ES: Methodology, Writing—original draft. PC: Methodology, Writing—original draft. SZ: Methodology, Writing—original draft. DV: Methodology, Writing—original draft. SC: Writing—review & editing. GC: Writing—review & editing. DR: Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing—original draft, Writing—review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was (partially) funded by Italian Ministry of Health - Current research IRCCS Ca' Granda Ospedale Maggiore Policlinico and by SEQMD project (IRCCS Cà Granda Ospedale Maggiore Policlinico, PI: GC).
This work was promoted within the European Reference Network (ERN) for Rare Neuromuscular Diseases. We thank the Associazione Centro Dino Ferrari for its support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1281953/full#supplementary-material
1. Angelini C. LGMD. Identification, description and classification. Acta Myol. (2020) 39:207–17. doi: 10.36185/2532-1900-024
2. Straub V, Murphy A, Udd B, group Lws. 229th ENMC international workshop: limb girdle muscular dystrophies - nomenclature and reformed classification Naarden, the Netherlands, 17-19 March 2017. Neuromuscul Disord. (2018) 28:702–10. doi: 10.1016/j.nmd.2018.05.007
3. Magri F, Nigro V, Angelini C, Mongini T, Mora M, Moroni I, et al. The italian limb girdle muscular dystrophy registry: relative frequency, clinical features, and differential diagnosis. Muscle Nerve. (2017) 55:55–68. doi: 10.1002/mus.25192
4. Hoffman EP, Brown Jr RH, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. (1987) 51:919–28. doi: 10.1016/0092-8674(87)90579-4
5. Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C, Kunkel LM. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. (1987) 50:509–17. doi: 10.1016/0092-8674(87)90504-6
6. Frosk P, Weiler T, Nylen E, Sudha T, Greenberg CR, Morgan K, et al. Limb-girdle muscular dystrophy type 2H associated with mutation in TRIM32, a putative E3-ubiquitin-ligase gene. Am J Hum Genet. (2002) 70:663–72. doi: 10.1086/339083
7. Frosk P, Del Bigio MR, Wrogemann K, Greenberg CR. Hutterite brothers both affected with two forms of limb girdle muscular dystrophy: LGMD2H and LGMD2I. Eur J Hum Genet. (2005) 13:978–82. doi: 10.1038/sj.ejhg.5201436
8. Jerusalem F, Engel AG, Gomez MR. Sarcotubular myopathy. A newly recognized, benign, congenital, familial muscle disease. Neurology. (1973) 23:897–906. doi: 10.1212/WNL.23.9.897
9. Shokeir MH, Kobrinsky NL. Autosomal recessive muscular dystrophy in manitoba hutterites. Clin Genet. (1976) 9:197–202. doi: 10.1111/j.1399-0004.1976.tb01568.x
10. Shieh PB, Kudryashova E, Spencer MJ. Limb-girdle muscular dystrophy 2H and the role of TRIM32. Handb Clin Neurol. (2011) 101:125–33. doi: 10.1016/B978-0-08-045031-5.00009-8
11. Saccone V, Palmieri M, Passamano L, Piluso G, Meroni G, Politano L, et al. Mutations that impair interaction properties of TRIM32 associated with limb-girdle muscular dystrophy 2H. Hum Mutat. (2008) 29:240–7. doi: 10.1002/humu.20633
12. Weiler T, Greenberg CR, Zelinski T, Nylen E, Coghlan G, Crumley MJ, et al. A gene for autosomal recessive limb-girdle muscular dystrophy in manitoba hutterites maps to chromosome region 9q31-q33: evidence for another limb-girdle muscular dystrophy locus. Am J Hum Genet. (1998) 63:140–7. doi: 10.1086/301925
13. Marchuk M, Dovbonos T, Makukh H, Semeryak O, Sharhorodska Y. Sarcotubular myopathy due to novel TRIM32 mutation in association with multiple sclerosis. Brain Sci. (2021) 11:1020. doi: 10.3390/brainsci11081020
14. Lazzari E, Meroni G. TRIM32 ubiquitin E3 ligase, one enzyme for several pathologies: from muscular dystrophy to tumours. Int J Biochem Cell Biol. (2016) 79:469–77. doi: 10.1016/j.biocel.2016.07.023
15. Borg K, Stucka R, Locke M, Melin E, Ahlberg G, Klutzny U, et al. Intragenic deletion of TRIM32 in compound heterozygotes with sarcotubular myopathy/LGMD2H. Hum Mutat. (2009) 30:E831–44. doi: 10.1002/humu.21063
16. Cossée M, Lagier-Tourenne C, Seguela C, Mohr M, Leturcq F, Gundesli H, et al. Use of SNP array analysis to identify a novel TRIM32 mutation in limb-girdle muscular dystrophy type 2H. Neuromuscul Disord. (2009) 19:255–60. doi: 10.1016/j.nmd.2009.02.003
17. Chakravorty S, Nallamilli BRR, Khadilkar SV, Singla MB, Bhutada A, Dastur R, et al. Clinical and genomic evaluation of 207 genetic myopathies in the Indian subcontinent. Front Neurol. (2020) 11:559327. doi: 10.3389/fneur.2020.559327
18. Chan GHJ, Ong PY, Low JJH, Kong HL, Ow SGW, Tan DSP, et al. Clinical genetic testing outcome with multi-gene panel in Asian patients with multiple primary cancers. Oncotarget. (2018) 9:30649–60. doi: 10.18632/oncotarget.25769
19. Chandrasekharan SV, Sundaram S, Malaichamy S, Poyuran R, Nair SS. Myoneuropathic presentation of limb girdle muscular dystrophy R8 with a novel TRIM32 mutation. Neuromuscul Disord. (2021) 31:886–90. doi: 10.1016/j.nmd.2021.06.003
20. Guan Y, Liang X, Li W, Lin W, Liang G, Xie H, et al. TRIM32 biallelic defects cause limb-girdle muscular dystrophy R8: identification of two novel mutations and investigation of genotype-phenotype correlation. Skelet Muscle. (2023) 13:10. doi: 10.1186/s13395-023-00319-x
21. Johnson K, De Ridder W, Töpf A, Bertoli M, Phillips L, De Jonghe P, et al. Extending the clinical and mutational spectrum of TRIM32-related myopathies in a non-Hutterite population. J Neurol Neurosurg Psychiatry. (2019) 90:490–3. doi: 10.1136/jnnp-2018-318288
22. Mair D, Biskup S, Kress W, Abicht A, Brück W, Zechel S, et al. Differential diagnosis of vacuolar myopathies in the NGS era. Brain Pathol. (2020) 30:877–96. doi: 10.1111/bpa.12864
23. Nectoux J, de Cid R, Baulande S, Leturcq F, Urtizberea JA, Penisson-Besnier I, et al. Detection of TRIM32 deletions in LGMD patients analyzed by a combined strategy of CGH array and massively parallel sequencing. Eur J Hum Genet. (2015) 23:929–34. doi: 10.1038/ejhg.2014.223
24. Neri M, Selvatici R, Scotton C, Trabanelli C, Armaroli A, De Grandis D, et al. A patient with limb girdle muscular dystrophy carries a TRIM32 deletion, detected by a novel CGH array, in compound heterozygosis with a nonsense mutation. Neuromuscul Disord. (2013) 23:478–82. doi: 10.1016/j.nmd.2013.02.003
25. Panicucci C, Traverso M, Baratto S, Romeo C, Iacomino M, Gemelli C, et al. Novel TRIM32 mutation in sarcotubular myopathy. Acta Myol. (2019) 38:8–12.
26. Servián-Morilla E, Cabrera-Serrano M, Rivas-Infante E, Carvajal A, Lamont PJ, Pelayo-Negro AL, et al. Altered myogenesis and premature senescence underlie human TRIM32-related myopathy. Acta Neuropathol Commun. (2019) 7:30. doi: 10.1186/s40478-019-0683-9
27. Ten Dam L, de Visser M, Ginjaar IB, van Duyvenvoorde HA, van Koningsbruggen S, van der Kooi AJ. Elucidation of the genetic cause in dutch limb girdle muscular dystrophy families: a 27-year's journey. J Neuromuscul Dis. (2021) 8:261–72. doi: 10.3233/JND-200585
28. Wei XJ, Miao J, Kang ZX, Gao YL, Wang ZY, Yu XF. A novel homozygous exon2 deletion of TRIM32 gene in a Chinese patient with sarcotubular myopathy: a case report and literature review. Bosn J Basic Med Sci. (2021) 21:495–500. doi: 10.17305/bjbms.2020.5288
29. Schoser BG, Frosk P, Engel AG, Klutzny U, Lochmüller H, Wrogemann K. Commonality of TRIM32 mutation in causing sarcotubular myopathy and LGMD2H. Ann Neurol. (2005) 57:591–5. doi: 10.1002/ana.20441
30. Liewluck T, Tracy JA, Sorenson EJ, Engel AG. Scapuloperoneal muscular dystrophy phenotype due to TRIM32-sarcotubular myopathy in South Dakota Hutterite. Neuromuscul Disord. (2013) 23:133–8. doi: 10.1016/j.nmd.2012.09.010
31. Micale L, Chaignat E, Fusco C, Reymond A, Merla G. The tripartite motif: structure and function. Adv Exp Med Biol. (2012) 770: 11–25. doi: 10.1007/978-1-4614-5398-7_2
32. Lazzari E, El-Halawany MS, De March M, Valentino F, Cantatore F, Migliore C, et al. Analysis of the Zn-binding domains of TRIM32, the E3 ubiquitin ligase mutated in limb girdle muscular dystrophy 2H. Cells. (2019) 8:254. doi: 10.3390/cells8030254
Keywords: LGMDR8, TRIM32, limb-girdle muscular dystrophy, clinical exome sequencing, tripartite motif-containing proteins
Citation: Rimoldi M, Romagnoli G, Magri F, Antognozzi S, Cinnante C, Saccani E, Ciscato P, Zanotti S, Velardo D, Corti S, Comi GP and Ronchi D (2024) Case report: A novel patient presenting TRIM32-related limb-girdle muscular dystrophy. Front. Neurol. 14:1281953. doi: 10.3389/fneur.2023.1281953
Received: 23 August 2023; Accepted: 19 December 2023;
Published: 18 January 2024.
Edited by:
Edoardo Malfatti, Hôpitaux Universitaires Henri Mondor, FranceReviewed by:
Aravindhan Veerapandiyan, Arkansas Children's Hospital, United StatesCopyright © 2024 Rimoldi, Romagnoli, Magri, Antognozzi, Cinnante, Saccani, Ciscato, Zanotti, Velardo, Corti, Comi and Ronchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dario Ronchi, ZGFyaW8ucm9uY2hpQHVuaW1pLml0
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.