- Department of Neurology, Quanzhou Children's Hospital, Quanzhou, Fujian, China
Background: Epidemiological studies have demonstrated a comorbid association between migraine and epilepsy. However, despite the long history of this association, the exact nature of the relationship between migraine and epilepsy remains largely unresolved. Therefore, it is crucial to conduct a meta-analysis in order to thoroughly investigate the relationship between migraine and epilepsy.
Methods: Odds ratios (ORs) or relative risks (RRs) and 95% confidence intervals (CIs) regarding association between migraine and epilepsy were summarized using STATA 12.0 software.
Results: There was an 80% increase in the lifetime prevalence of migraine among patients with epilepsy, compared to those without epilepsy with a random effects model (OR/RR: 1.80, 95% CI: 1.35 to 2.40, I2 = 97.5%, p < 0.001). There was an 80% increase in the lifetime prevalence of epilepsy among patients with migraine, compared to those without migraine with a random effects model (OR/RR: 1.80, 95% CI: 1.43 to 2.25, I2 = 80.6%, p < 0.001).
Conclusions: It is important to note the comorbid association between migraine and epilepsy examined in the study.
1 Introduction
Migraine and epilepsy are two frequently seen neurological disorders, characterized by paroxysmal clinical manifestations (1). Despite the commonly accepted notion that migraine is primarily a neurological disorder with a strong genetic basis (2), it has been found to be frequently co-occurring with a wide array of other medical conditions. Numerous studies have identified a bidirectional relationship between migraine and various disorders, including those affecting the neurological, psychiatric, cardiovascular, cerebrovascular, gastrointestinal, metaboloendocrine, and immunological systems (3). Furthermore, epilepsy, being a complex disorder, is often accompanied by a range of comorbidities. Conditions such as depression, anxiety, dementia, migraine, heart disease, peptic ulcers, and arthritis are up to eight times more prevalent among individuals with epilepsy compared to the general population (4). Since the late nineteenth century, John Hughlings Jackson's observation on the coexistence of migraine and epilepsy has been acknowledged (5). In addition, risk factors, such as positive family history and comorbid affective disorders, along with triggering factors like alcohol consumption, menstruation, and irregular sleep patterns, are commonly observed in both migraine and epilepsy. Moreover, prophylactic medical agents, such as valproic acid and topiramate, also demonstrate similarities in their utilization in migraine and epilepsy. Epidemiological studies have demonstrated varying prevalence rates for migraine and epilepsy in different populations. The frequency of migraine has been reported to range from 5 to 18% in healthy individuals, while in epilepsy patients, the frequency ranges from 8 to 24% (6). Similarly, the prevalence of epilepsy in healthy populations has been reported to be between 0.5 and 1.5%, whereas in migraine patients, it ranges from 1 to 17% (7, 8).
However, despite the long history of this association, the exact nature of the relationship between migraine and epilepsy remains largely unresolved. Brodtkorb et al. (9) and Hesdorffer et al. (10) reported that there was insufficient evidence to establish a definitive correlation between migraine and epilepsy. Therefore, it is crucial to conduct a meta-analysis in order to thoroughly investigate the relationship between migraine and epilepsy.
2 Methods
The present article was performed according to the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guideline (11).
2.1 Search strategy, inclusion criteria, and exclusion criteria
The article searched for literatures published before July 2023 in databases (PubMed, Web of Science, EMBASE, Medline and Google Scholar). We used these search terms: (“migraine” OR “headache”) AND (“epilepsy” OR “seizure” OR “falling sickness”).
The study included studies in accordance with these inclusion criteria as follows: (1) studies investigated migraine; (2) studies investigated epilepsy.
We used these exclusion criteria: (1) studies which did not investigate the association between migraine and epilepsy; (2) studies which did not provide sufficient information to acquire odds ratio (OR) or relative risk (RR) and 95% confidence interval (CI) for the association between migraine and epilepsy; (3) reviews, case reports and meta-analysis. Figure 1 illustrated the flow chart of the study search and selection process.
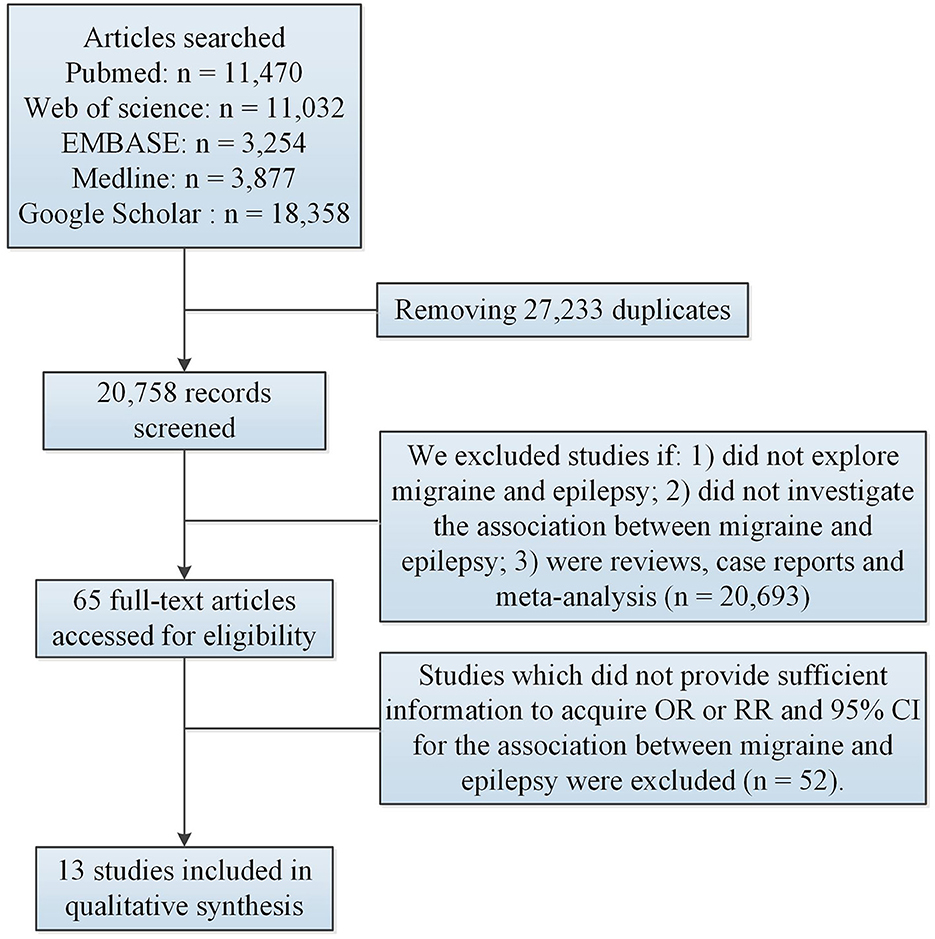
Figure 1. Flow of information through the different phases of meta-analysis. CI, confidence interval; OR, odds ratio; RR, relative risk.
2.2 Data extraction and meta-analysis
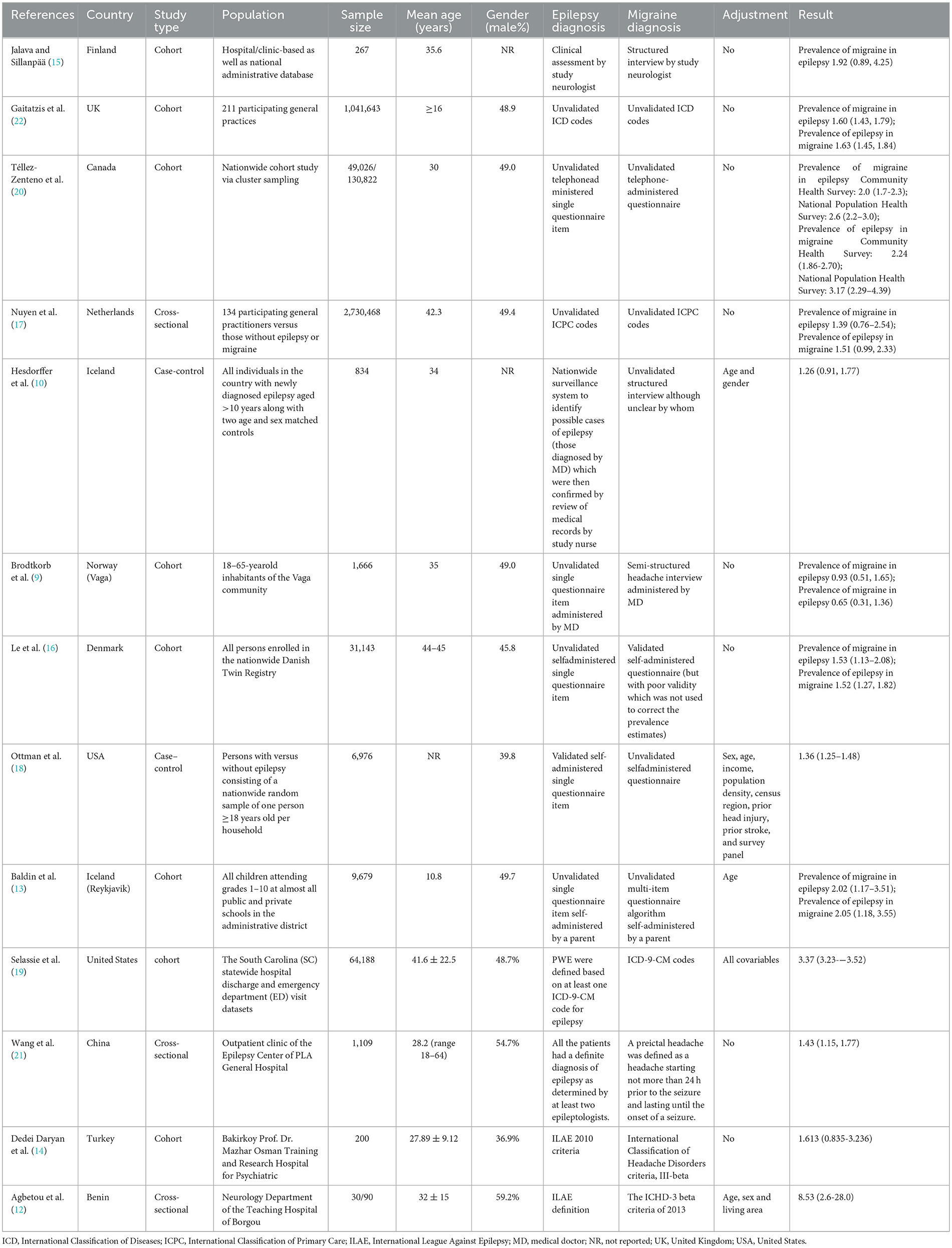
Two researchers extracted data from finally included studies. We extracted these data: Author and publication year, study location, study type, population, sample size, mean age, gender, epilepsy diagnosis, migraine diagnosis, adjusted variables and results.
ORs or RRs and 95% CIs regarding association between migraine and epilepsy were summarized using STATA 12.0 software. When p-value of Q-test was ≤ 0.05 and I2 was higher than or equal to 50%, a random effects model was used to compute all results with the high heterogeneity. When p-value of Q-test was higher than 0.05 and I2 was lower than 50%, a fixed effects model was used to summarize all results with the low heterogeneity. Subgroup studies (for different races, different study types, migraine diagnosed with different methods and adjusted or unadjusted effect estimates) were used to explore the source of heterogeneity. Meta-regression was used to investigate the source of heterogeneity. We used sensitivity analysis to evaluate stabilization of study. We used funnel plot, Begg's test and Egger's test to evaluate publication bias.
3 Results
3.1 Study characteristics
After removing duplications, 27,233 articles remained. Subsequently, 20,693 articles were excluded based on the screening of titles and abstracts. Among 20,693 articles, 18,630 did not explore migraine or epilepsy; 1,952 did not investigate the association between migraine and epilepsy; 46 were reviews, case reports and meta-analysis. Among 65 potentially relevant articles, 52 were excluded because they did not provide sufficient information to acquire OR or RR and 95% CI for the association between migraine and epilepsy. Ultimately, 13 studies (9, 10, 12–22) met the eligibility criteria and were eligible for the comprehensive analysis. Table 1 illustrated study characteristics. These studies investigated the association between epilepsy and the risk of migraine. Among the 13 studies, six studies (9, 13, 16, 17, 20, 22) (including N = 3,863,625 individuals) explored the association between migraine and the risk of epilepsy.
3.2 Association between epilepsy and risk of migraine
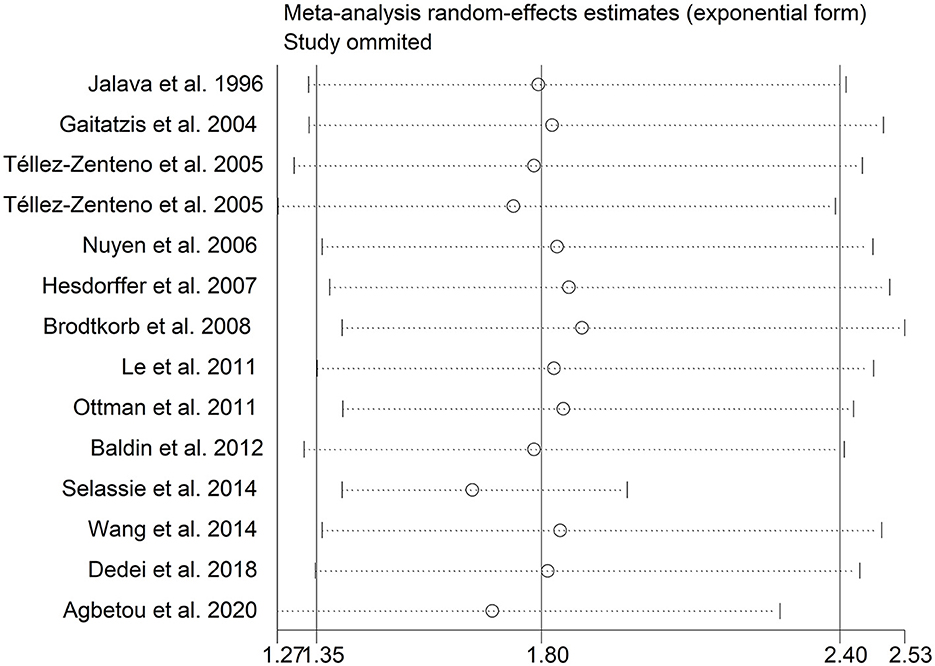
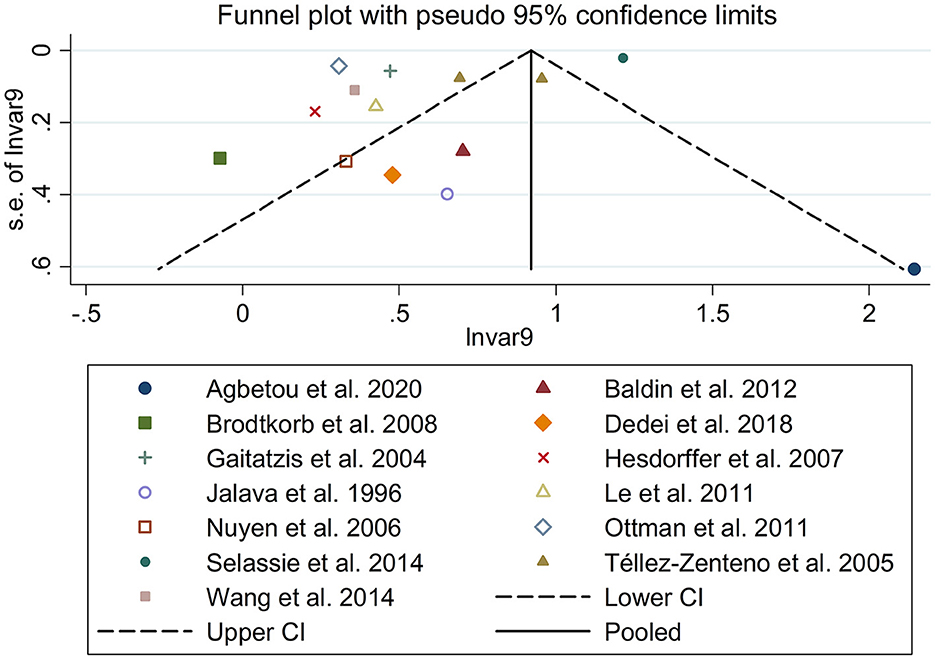
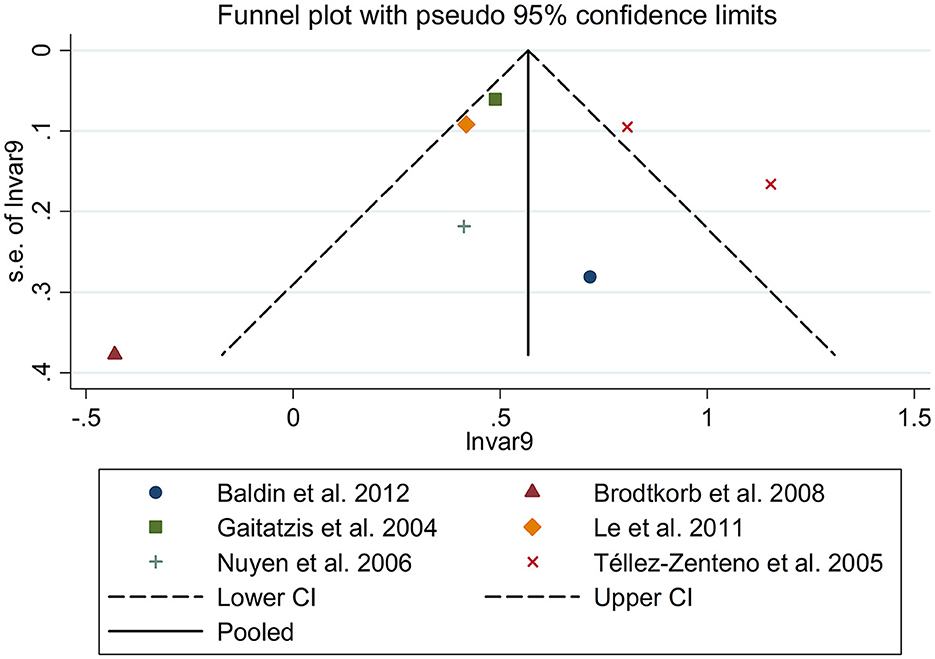
There was an 80% increase in the lifetime prevalence of migraine among patients with epilepsy, compared to those without epilepsy with a random effects model (OR/RR: 1.80, 95% CI: 1.35 to 2.40, I2 = 97.5%, p < 0.001; Figure 2). Subgroup analysis reported a 73% increase in the lifetime prevalence of migraine among patients with epilepsy, compared to those without epilepsy among Caucasian populations (OR/RR: 1.73, 95% CI: 1.28 to 2.35; Supplementary Figure 1). Subgroup analysis reported a significant association between epilepsy and the risk of migraine in cohort and cross-sectional studies (cohort studies: RR: 1.91, 95% CI: 1.41 to 2.59; cross-sectional: OR: 2.03, 95% CI: 1.00 to 4.10; Supplementary Figure 2). In studies where epilepsy was identified by clinical assessment, the pooled OR/RR was 1.40 (95% CI: 1.17, 1.67), whereas the pooled was 2.28 (95% CI: 1.35, 3.84) and 1.71 (95% CI: 1.28, 2.28) when cases were identified using International Classification of Diseases (ICD)/International Classification of Primary Care (ICPC) codes or with a formal questionnaire (Supplementary Figure 3). The overall adjusted OR/RR was 2.18 (95% CI: 1.26, 3.79), whereas the overall unadjusted OR/RR was 1.68 (95% CI: 1.38, 2.05) (Supplementary Figure 4). Meta-regression study reported that publication year, age and gender were not responsible for the heterogeneity across included studies (publication year: p = 0.365; age: p = 0.918; gender: p = 0.361). Sensitivity analysis showed no change in direction of effect when any study was excluded from meta-analysis (Figure 3). Funnel plot, Begg's test and Egger's test showed no significant risk of publication bias (Figure 4; Begg's test: p = 0.090; Egger's test: p = 0.091).
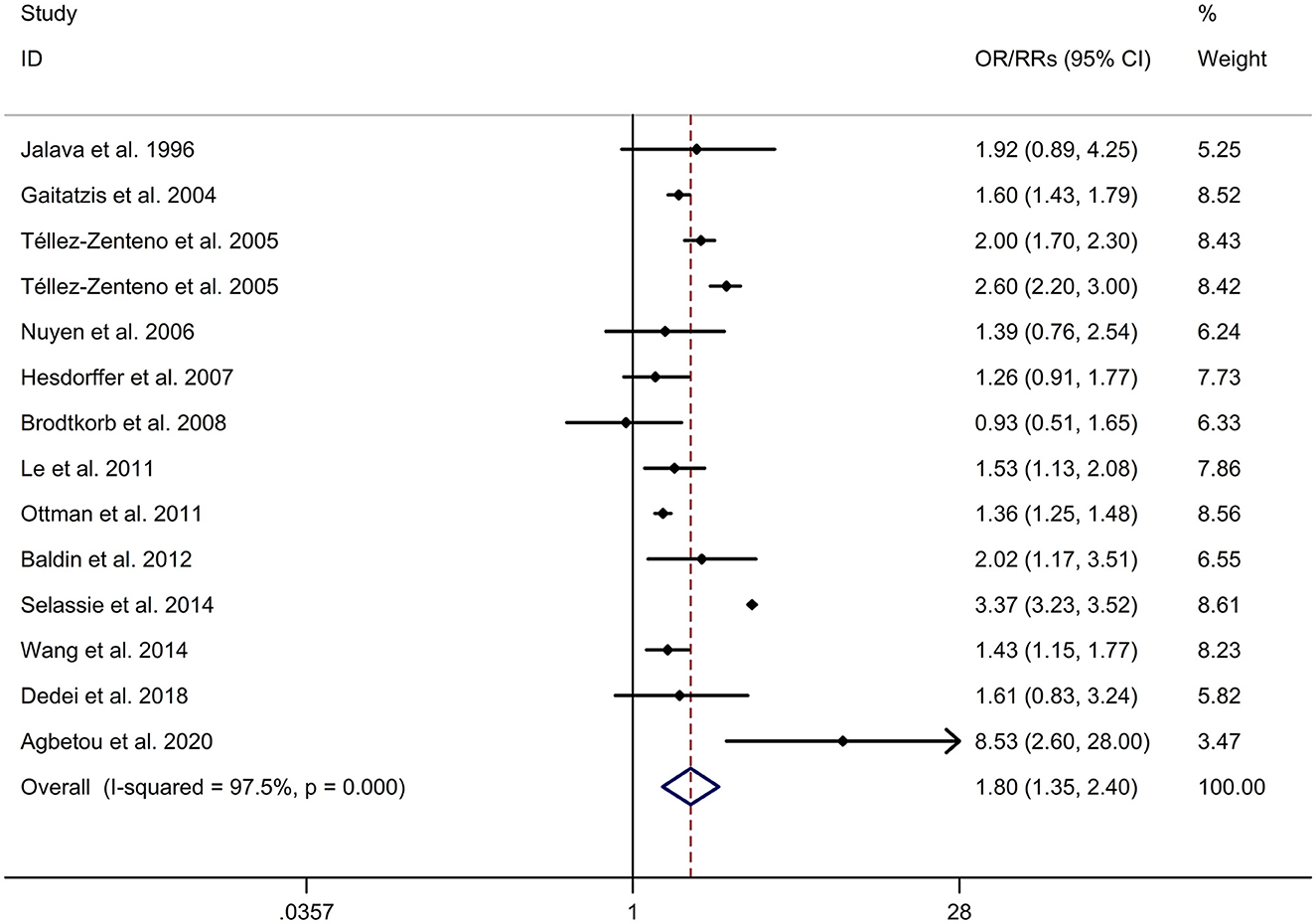
Figure 2. Forest plot for association between epilepsy and risk of migraine. CI, confidence interval; OR, odds ratio; RR, relative risk.
3.3 Association between migraine and risk of epilepsy
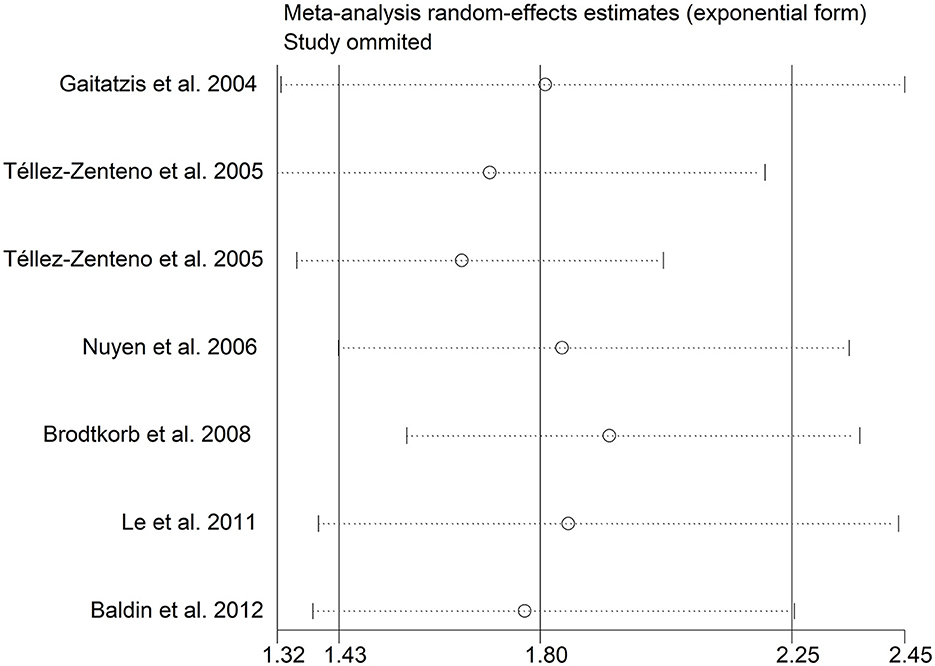
There was an 80% increase in the lifetime prevalence of epilepsy among patients with migraine, compared to those without migraine with a random effects model (OR/RR: 1.80, 95% CI: 1.43 to 2.25, I2 = 80.6%, p < 0.001; Figure 5). Subgroup analysis reported an 84% increase in the lifetime prevalence of epilepsy among patients with migraine, compared to those without migraine in cohort studies (RR: 1.84, 95% CI: 1.43 to 2.35; Supplementary Figure 5). In studies where epilepsy was identified with a formal questionnaire, the pooled OR/RR was 1.86 (95% CI: 1.31, 2.64) (Supplementary Figure 6). The overall unadjusted OR/RR was 1.77 (95% CI: 1.39, 2.25) (Supplementary Figure 7). Meta-regression study reported that publication year, age and gender were not responsible for the heterogeneity across included studies (publication year: p = 0.295; age: p = 0.282; gender: p = 0.376). Sensitivity analysis showed no change in direction of effect when any study was excluded from meta-analysis (Figure 6). Funnel plot, Begg's test and Egger's test showed no significant risk of publication bias (Figure 7; Begg's test: p = 0.881; Egger's test: p = 0.938).
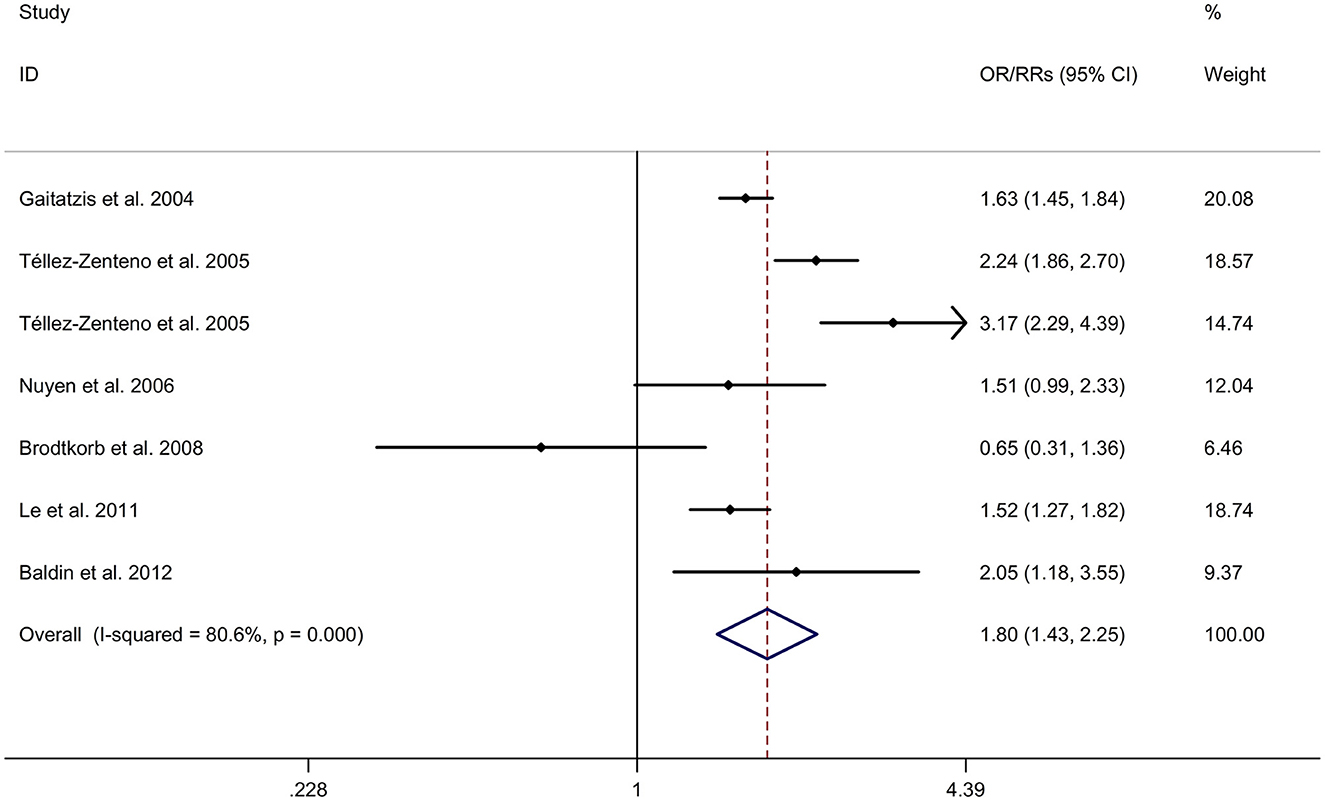
Figure 5. Forest plot for association between migraine and risk of epilepsy. CI, confidence interval; OR, odds ratio; RR, relative risk.
4 Discussion
In our study, migraine was more frequent in patients with epilepsy than in those without epilepsy. The result was consistent with a previous meta-analysis, which reported that there was an overall 52% increase in the prevalence of migraine among patients with epilepsy vs. those without epilepsy (23). In addition, it has been observed that the prevalence of epilepsy is higher among individuals with migraine, compared to those without migraine. The result was consistent with a previous meta-analysis (23), which reported that there was an overall 79% increase in the prevalence of epilepsy among migraineurs vs. those without migraine. Our literature search yielded a comprehensive selection of 13 studies involving a substantial cohort of 3,863,625 participants. This extensive pool of data enabled us to obtain more precise and potentially more accurate estimates of OR and RR compared to the individual primary studies. Additionally, it provided us with the opportunity to explore the potential factors contributing to any observed heterogeneity among these studies. This meta-analysis aims to examine the existing evidence concerning the comorbid association between migraine and epilepsy.
The understanding of the association between migraine and epilepsy remains incomplete due to a lack of fully elucidated underlying mechanisms. It is likely that there are common multifactorial mechanisms that contribute to this association. One possible explanation is neuronal hyperexcitability, which increases the risk of both diseases. This hyperexcitability can be caused by a higher concentration of extracellular glutamate, the main excitatory neurotransmitter, resulting in a cortical spreading depression (CSD) and convulsions (24–26). Migraine is characterized by an initial excess of neuronal activity, leading to CSD and aura, while epilepsy is characterized by neuronal over activity and rhythmic firing of neurons during a seizure (27). Migraine aura may act as a trigger for epileptic seizures (28), which is why some antiepileptic drugs are used in migraine prophylaxis. Channelopathies involving sodium and potassium ions, which can trigger CSD, may be a common pathogenic mechanism in migraine and epilepsy (26). Another focus of research is the hereditary link between epilepsy and migraine. A genetic link is particularly evident in familial hemiplegic migraine, a Mendelian transmission type of migraine. Mutations in genes such as ATP1A2, SCN1A, and CACNA1A, which are associated with familial hemiplegic migraine, have also been implicated in various types of epilepsy and febrile seizures (29, 30). However, the hypothesis of a clear common genetic susceptibility still needs further investigation due to the likely complex polygenic multifactorial inheritance and the role of gene-environment interactions (31). Finally, affective disorders can often accompany both migraine and epilepsy, suggesting a bidirectional relationship (32, 33). Studies have shown a correlation between migraine and depression, anxiety in epilepsy patients (32, 34). Interictal headache is specifically linked to depression, while postictal headache is related to depression and suicidality (12). In summary, the association between migraine and epilepsy is not fully understood, but it is likely that common multifactorial mechanisms, such as neuronal hyperexcitability and channelopathies, play a role. Hereditary factors and affective disorders may also contribute to this association. Further research is needed to fully elucidate the underlying mechanisms and develop targeted therapies for these diseases.
There are a few limitations in the study that should be acknowledged. Firstly, it was inevitable to encounter heterogeneity across the included studies. Although meta-regression and subgroup analyses were performed to identify potential sources of heterogeneity, the exact cause of heterogeneity remains unclear. Further investigation is needed to better understand this issue. Secondly, we could not ignore the possibility of an “overlap” in the rare case of “ictal epileptic headache” in whom an headache could be the sole ictal semeiological manifestations during the ictal phase of an epileptic seizure (35).
5 Conclusions
It is important to note the comorbid association between migraine and epilepsy examined in the study. This association not only has implications for understanding the relationship between the two conditions but also holds therapeutic significance. Certain antiepileptic drugs may have benefits for migraine prophylaxis, and additional considerations should be taken into account when treating individuals with both conditions. Further exploration is warranted to explore the potential of these treatments and their effectiveness in managing comorbid migraine and epilepsy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XW: Formal analysis, Investigation, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing. JZ: Methodology, Project administration, Software, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1276663/full#supplementary-material
References
1. Haut SR, Bigal ME, Lipton RB. Chronic disorders with episodic manifestations: focus on epilepsy and migraine. Lancet Neurol. (2006) 5:148–57. doi: 10.1016/S1474-4422(06)70348-9
2. Maher BH, Griffiths LR. Identification of molecular genetic factors that influence migraine. Mol Genet Genomics. (2011) 285:433–46. doi: 10.1007/s00438-011-0622-3
3. Altamura C, Corbelli I, de Tommaso M, Di Lorenzo C, Di Lorenzo G, Di Renzo A, et al. Pathophysiological bases of comorbidity in migraine. Front Hum Neurosci. (2021) 15:640574. doi: 10.3389/fnhum.2021.640574
4. Keezer MR, Sisodiya SM, Sander JW. Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol. (2016) 15:106–15. doi: 10.1016/S1474-4422(15)00225-2
5. Velioglu SK, Ozmenoglu M. Migraine-related seizures in an epileptic population. Cephalalgia. (1999) 19:797–801. doi: 10.1046/j.1468-2982.1999.1909797.x
6. Marks DA, Ehrenberg BL. Migraine-related seizures in adults with epilepsy, with EEG correlation. Neurology. (1993) 43:2476–83. doi: 10.1212/WNL.43.12.2476
7. Lipton RB, Ottman R, Ehrenberg BL, Hauser WA. Comorbidity of migraine: the connection between migraine and epilepsy. Neurology. (1994) 44:S28–32. doi: 10.1212/WNL.44.11.2105
8. Matias-Guiu J, Galiano L, Vioque J, Falip R, Martin R. A case-control study to evaluate the association of epilepsy and migraine. Neuroepidemiology. (1992) 11:313–4. doi: 10.1159/000110947
9. Brodtkorb E, Bakken IJ, Sjaastad O. Comorbidity of migraine and epilepsy in a Norwegian community. Eur J Neurol. (2008) 15:1421–3. doi: 10.1111/j.1468-1331.2008.02353.x
10. Hesdorffer DC, Lúdvígsson P, Hauser WA, Olafsson E, Kjartansson O. Co-occurrence of major depression or suicide attempt with migraine with aura and risk for unprovoked seizure. Epilepsy Res. (2007) 75:220–3. doi: 10.1016/j.eplepsyres.2007.05.001
11. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
12. Agbetou M, Adouwekonou R, Salanon E, Houehanou C, Kossi O, Hountohotegbe E, et al. Epilepsy and migraine among adolescents and adults in northern benin. Bull Soc Pathol Exot. (2020) 113:209–14. doi: 10.3166/bspe-2020-0142
13. Baldin E, Ludvigsson P, Mixa O, Hesdorffer DC. Prevalence of recurrent symptoms and their association with epilepsy and febrile seizure in school-aged children: a community-based survey in Iceland. Epilepsy Behav. (2012) 23:315–9. doi: 10.1016/j.yebeh.2011.12.012
14. Dedei Daryan M, Güveli BT, Baslo SA, Mulhan K, Sari H, Balçik ZE, et al. Prevalence and clinical characteristics of headache in juvenile myoclonic epilepsy: experience from a tertiary epilepsy center. Neurol Sci. (2018) 39:519–25. doi: 10.1007/s10072-017-3232-y
15. Jalava M, Sillanpää M. Concurrent illnesses in adults with childhood-onset epilepsy: a population-based 35-year follow-up study. Epilepsia. (1996) 37:1155–63. doi: 10.1111/j.1528-1157.1996.tb00547.x
16. Le H, Tfelt-Hansen P, Russell MB, Skytthe A, Kyvik KO, Olesen J. Co-morbidity of migraine with somatic disease in a large population-based study. Cephalalgia. (2011) 31:43–64. doi: 10.1177/0333102410373159
17. Nuyen J, Schellevis FG, Satariano WA, Spreeuwenberg PM, Birkner MD, van den Bos GA, et al. Comorbidity was associated with neurologic and psychiatric diseases: a general practice-based controlled study. J Clin Epidemiol. (2006) 59:1274–84. doi: 10.1016/j.jclinepi.2006.01.005
18. Ottman R, Lipton RB, Ettinger AB, Cramer JA, Reed ML, Morrison A, et al. Comorbidities of epilepsy: results from the epilepsy comorbidities and health (EPIC) survey. Epilepsia. (2011) 52:308–15. doi: 10.1111/j.1528-1167.2010.02927.x
19. Selassie AW, Wilson DA, Martz GU, Smith GG, Wagner JL, Wannamaker BB. Epilepsy beyond seizure: a population-based study of comorbidities. Epilepsy Res. (2014) 108:305–15. doi: 10.1016/j.eplepsyres.2013.12.002
20. Téllez-Zenteno JF, Matijevic S, Wiebe S. Somatic comorbidity of epilepsy in the general population in Canada. Epilepsia. (2005) 46:1955–62. doi: 10.1111/j.1528-1167.2005.00344.x
21. Wang XQ, Lang SY, He MW, Zhang X, Zhu F, Dai W, et al. High prevalence of headaches in patients with epilepsy. J Headache Pain. (2014) 15:70. doi: 10.1186/1129-2377-15-70
22. Gaitatzis A, Carroll K, Majeed A. The epidemiology of the comorbidity of epilepsy in the general population. Epilepsia. (2004) 45:1613–22. doi: 10.1111/j.0013-9580.2004.17504.x
23. Keezer MR, Bauer PR, Ferrari MD, Sander JW. The comorbid relationship between migraine and epilepsy: a systematic review and meta-analysis. Eur J Neurol. (2015) 22:1038–47. doi: 10.1111/ene.12612
24. Baykan B, Ertas M, Karli N, Uluduz D, Uygunoglu U, Ekizoglu E, et al. Migraine incidence in 5 years: a population-based prospective longitudinal study in Turkey. J Headache Pain. (2015) 16:103. doi: 10.1186/s10194-015-0589-2
25. Somjen GG. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol Rev. (2001) 81:1065–96. doi: 10.1152/physrev.2001.81.3.1065
26. Verrotti A, Striano P, Belcastro V, Matricardi S, Villa MP, Parisi P. Migralepsy and related conditions: advances in pathophysiology and classification. Seizure. (2011) 20:271–5. doi: 10.1016/j.seizure.2011.02.012
27. Gameleira FT, Ataíde L, Raposo MC. Relations between epileptic seizures and headaches. Seizure. (2013) 22:622–6. doi: 10.1016/j.seizure.2013.04.016
28. Kramer DR, Fujii T, Ohiorhenuan I, Liu CY. Interplay between cortical spreading depolarization and seizures. Stereotact Funct Neurosurg. (2017) 95:1–5. doi: 10.1159/000452841
29. Nye BL, Thadani VM. Migraine and epilepsy: review of the literature. Headache. (2015) 55:359–80. doi: 10.1111/head.12536
30. Huang Y, Xiao H, Qin X, Nong Y, Zou D, Wu Y. The genetic relationship between epilepsy and hemiplegic migraine. Neuropsychiatr Dis Treat. (2017) 13:1175–9. doi: 10.2147/NDT.S132451
31. Liao J, Tian X, Wang H, Xiao Z. Epilepsy and migraine-Are they comorbidity? Genes Dis. (2018) 5:112–8. doi: 10.1016/j.gendis.2018.04.007
32. Kanner AM, Ribot R, Mazarati A. Bidirectional relations among common psychiatric and neurologic comorbidities and epilepsy: Do they have an impact on the course of the seizure disorder? Epilepsia Open. (2018) 3:210–9. doi: 10.1002/epi4.12278
33. Begasse de Dhaem OAJ, French J, Morrison C, Meador KJ, Hesdorffer DC, Cristofaro S, et al. Migraine comorbidity and cognitive performance in patients with focal epilepsy. Epilepsy Behav. (2019) 97:29–33. doi: 10.1016/j.yebeh.2019.05.008
34. Begasse De Dhaem O, Aldana SI, Kanner AM, Sperling M, French J, Nadkarni SS, et al. Association between migraine comorbidity and psychiatric symptoms among people with newly diagnosed focal epilepsy. J Neuropsychiatry Clin Neurosci. (2022) 34:182–7. doi: 10.1176/appi.neuropsych.21050124
Keywords: epilepsy, migraine, meta-analysis, association, systematic review
Citation: Wu X and Zhuang J (2024) Association between migraine and epilepsy: a meta-analysis. Front. Neurol. 14:1276663. doi: 10.3389/fneur.2023.1276663
Received: 12 August 2023; Accepted: 05 December 2023;
Published: 05 January 2024.
Edited by:
David M. Labiner, University of Arizona, United StatesReviewed by:
Pasquale Parisi, Sapienza University of Rome, ItalyEmel Ur Ozcelik, Istanbul University, Türkiye
Copyright © 2024 Wu and Zhuang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohui Wu, QWloNzc4Mzh1eTJAb3V0bG9vay5jb20=
 Xiaohui Wu
Xiaohui Wu Jiaxin Zhuang
Jiaxin Zhuang