
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 11 January 2024
Sec. Movement Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1275660
Purpose: Parkinson's disease (PD) patients frequently experience visual hallucinations (VHs) and obstructive sleep apnea (OSA). The aim of this study was to describe the prevalence and clinical correlates of VHs and OSA in the Chinese population with PD.
Materials and methods: A sample of 489 PD patients was recruited for the present study. Patients were categorized as having formed VHs (FVHs) or minor VHs (MVHs) or as non-hallucinators (NVHs) according to the Unified Parkinson's Disease Rating Scale (UPDRS) and an initial questionnaire. Polysomnography (PSG) was used for objective assessment of sleep.
Results: VHs were observed in 143 (29.2%) patients. Among them, 75 of the hallucinators experienced MVHs, and 68 experienced FVHs. The disease duration, UPDRS Part III score, Hoehn and Yahr (H–Y) stage, Pittsburgh Sleep Quality Index (PSQI) score and rapid eye movement (REM) sleep behavior disorder (RBD) score of hallucinators were significantly greater than those of non-hallucinators (P < 0.05). We also observed OSA in 38.7, 54.7, and 63.3% of the NVH, MVH, and FVH groups, respectively. PSG showed that the VH groups had a lower total sleep time, lower sleep efficiency, higher arousal index, lower sleep latency, lower N1%, higher apnea-hypopnea index (AHI), higher average duration of apnea, higher respiratory-related arousal (RRA), and lower values of the lowest O2 and mean O2. The forward binary logistic regression model showed that AHI, N1%, RRA and lowest O2 were independently associated with VHs in PD patients.
Conclusions: Our results confirm the high prevalence of VHs and OSA as well as their relationship in patients with PD.
Parkinson's disease (PD) is the second most prevalent neurodegenerative disorder globally, and it affects ~10 million individuals (1). This disease is a progressive brain disorder that affects elderly individuals and is associated with multiple factors, including mitochondrial dysfunction that leads to dopaminergic system degeneration (2). Although PD is a movement disorder, it is accompanied by a wide range of non-motor symptoms, such as neuropsychiatric manifestations, an abnormal sense of smell, urinary symptoms, sleep disorders, autonomic dysfunction, and sensory disorders (3).
The most prevalent neuropsychiatric symptom of PD is visual hallucinations (VHs) (4). Our previous study including 371 patients with primary PD found that 19.4% of patients had VHs (5). Types of VHs include formed visual hallucinations (FVHs) and minor visual hallucinations (MVHs). FVHs often appear in dim light, especially when an individual is falling asleep, and mainly consist of people and animals, which often appear suddenly in colorful and blurry forms. MVHs consist of presence hallucinations, passage hallucinations, and visual illusions (6). Numerous studies have indicated that VHs are linked with cognitive decline and elevated mortality in PD patients (7).
There is a growing interest in the impact of sleep and sleep disturbances on the brain, particularly in connection with aging and PD. Due to mouth breathing, pharyngeal collapse, motor coordination impairment, and autonomic dysfunction, PD patients have a high prevalence of obstructive sleep apnea (OSA) (8). OSA is characterized by recurrent hypopnea or apnea during sleep due to airway obstruction, accompanied by headache, a dry mouth in the morning, lack of concentration, excessive daytime sleepiness and various neurocognitive, and psychological manifestations (9).
However, the association between VHs and OSA in PD patients is unclear. The objective of this study was to examine the relationship between VHs and OSA through a retrospective cohort study conducted in the Chinese PD population.
Between January 2021 and July 2023, a total of 489 consecutive PD patients were recruited from the Department of Geriatrics, Nanjing Brain Hospital. All patients with PD met the criteria set by the United Kingdom Parkinson's Disease Society Brain Bank (10) (Figure 1). The exclusion criteria were as follows: (1) diagnoses of progressive supranuclear palsy, multiple system atrophy, Lewy body dementia, corticobasal degeneration, or other forms of atypical parkinsonism; (2) severe illness, such as acute systemic disorder or injury, heart failure, acute cerebral ischemia or intracranial tumors; or (3) missing questionnaire or polysomnography (PSG) data.
To obtain the demographic and clinical characteristics of each patient, including age, sex, age of disease onset, disease duration, education level, body mass index (BMI) and use of antiparkinsonian drugs, a structured questionnaire was utilized. Levodopa equivalent doses (LEDs) were calculated based on previously published data (11).
Clinical assessments of the PD patients, including their scores on the Unified Parkinson's Disease Rating Scale (UPDRS) (12) and their Hoehn and Yahr (H-Y) stage, were used to evaluate PD motor symptoms and disease severity. The Montreal Cognitive Assessment (MoCA) was utilized to evaluate cognitive function (13). The Parkinson's Disease Sleep Scale (PDSS) and Pittsburgh Sleep Quality Index (PSQI) were used to evaluate the overall sleep quality of patients (14, 15). The STOP-Bang scale and Epworth Sleepiness Scale (ESS) were used to assess the risk of apnea and daytime drowsiness of patients, respectively (16, 17). The Rapid Eye Movement Sleep Behavior Disorder Screening Questionnaire (RBD-SQ) was used to assess rapid eye movement (REM) sleep behavior disorder (RBD) in PD patients (18). The Hamilton Depression Rating Scale (HAMD), which includes 24 items, and the Hamilton Anxiety Rating Scale (HAMA) were utilized to quantify the severity of depression and anxiety, respectively (19, 20). We used the UPDRS Part 1, Question 2 to conduct preliminary screening for hallucinatory symptoms. This item was scored on the following scale: 0 = absence of VHs, 1 = illusions or non-formed hallucinations (e.g., MVHs), 2 = FVHs without loss of insight, 3 = FVHs with loss of insight, and 4 = delusions or paranoia. We also created a questionnaire that contained 11 items covering the nature and properties of the patients' VHs (5).
The full-night, attended, laboratory-based video PSG data were recorded on a Compumedics Grael-HD 64 (Compumedics Grael-HD, Australia) polygraph. The PSG data were interpreted according to the American Academy of Sleep Medicine (AASM) Sleep Scoring Manual (21). The signal sampling frequency was set to 256 Hz, and the notch frequency was 60 Hz. The bandpass filter was set to 3–30 Hz. The low-pass frequency of the EMG channel was set to 10 Hz. Electrodes were placed according to the international 10–20 system, and data were recorded from a total of 19 channels: 6 EEG channels (F3-M2, F4-M1, C3-M2, C4-M1, O1-M2, O2-M1), 2 electrooculography (EOG) channels, 1 chin electromyography (EMG) channel, 1 snoring channel, 1 oxygen flow channel, 1 nasal thermosensitive channel, 1 chest respiratory movement channel, 1 abdominal respiratory movement channel, 1 oxygen saturation channel, 1 postural channel, and 2 lower limb EMG channels. PSG data were recorded between 23:00 and 07:00. Sleep efficiency (SE), sleep latency (SL), non-REM (NREM) sleep stage 1 (N1) time (min), NREM sleep stage 3 (N3) time (min), total sleep time (TST; min), the periodic limb movement index (PLMI), the apnea-hypopnea index (AHI), mean O2, lowest O2, the average duration of apnea, and respiratory-related arousal (RRA) were all recorded. The density of REMs was determined by counting the number of eye movements per minute during REM sleep over the entire night (22). OSA was defined as upper airway collapse resulting in a decrease (hypopnea) or loss (apnea) of airflow for at least 10 s. The severity of OSA was defined using the AHI proposed by the AASM. The severity levels were categorized as follows: mild (AHI = 5–15), moderate (AHI = 15–30), and severe (AHI >30).
All continuous data are presented as the mean ± standard deviation, and categorical variables are represented as percentages. To compare continuous data, Student's t-test was utilized, while chi-square tests were used to analyze associations between categorical variables. Differences in continuous variables were evaluated by conducting an analysis of variance (ANOVA) with a post-hoc analysis employing Punnett's T3 and Scheffe's tests. To explore the potential clinical factors associated with VHs, a forward binary logistic regression model using VHs as the dependent variable and disease characteristics identified as significant in the univariate analysis as independent covariables was employed for a multivariate analysis. All analyses were conducted using SPSS 19.0 (SPSS Inc., Chicago, IL).
Table 1 presents the demographic characteristics of the patients with and without hallucinations. VHs were observed in 143 patients (29.2%). Regarding the typical VHs, 68 (13.9%) patients described their VHs as a complex visual image (i.e., FVHs) and 75 (15.3%) patients described their VHs as MVHs. No differences were found between patients with and without hallucinations in terms of age, sex, BMI, age of disease onset, or education level. Patients with FVHs had a longer disease duration, more severe motor symptoms, a higher H-Y stage, and a higher LED than patients with MVHs or those without hallucinations (all P < 0.05). We divided PD patients into three groups based on their motor symptoms, namely, the tremor dominant group, akinetic rigid dominant group, and mixed motor group, accounting for 52, 26, and 22% of patients, respectively. We compared VHs among the three groups. We found that the akinetic rigid dominant group and mixed motor group had more frequent and severe hallucinatory symptoms.
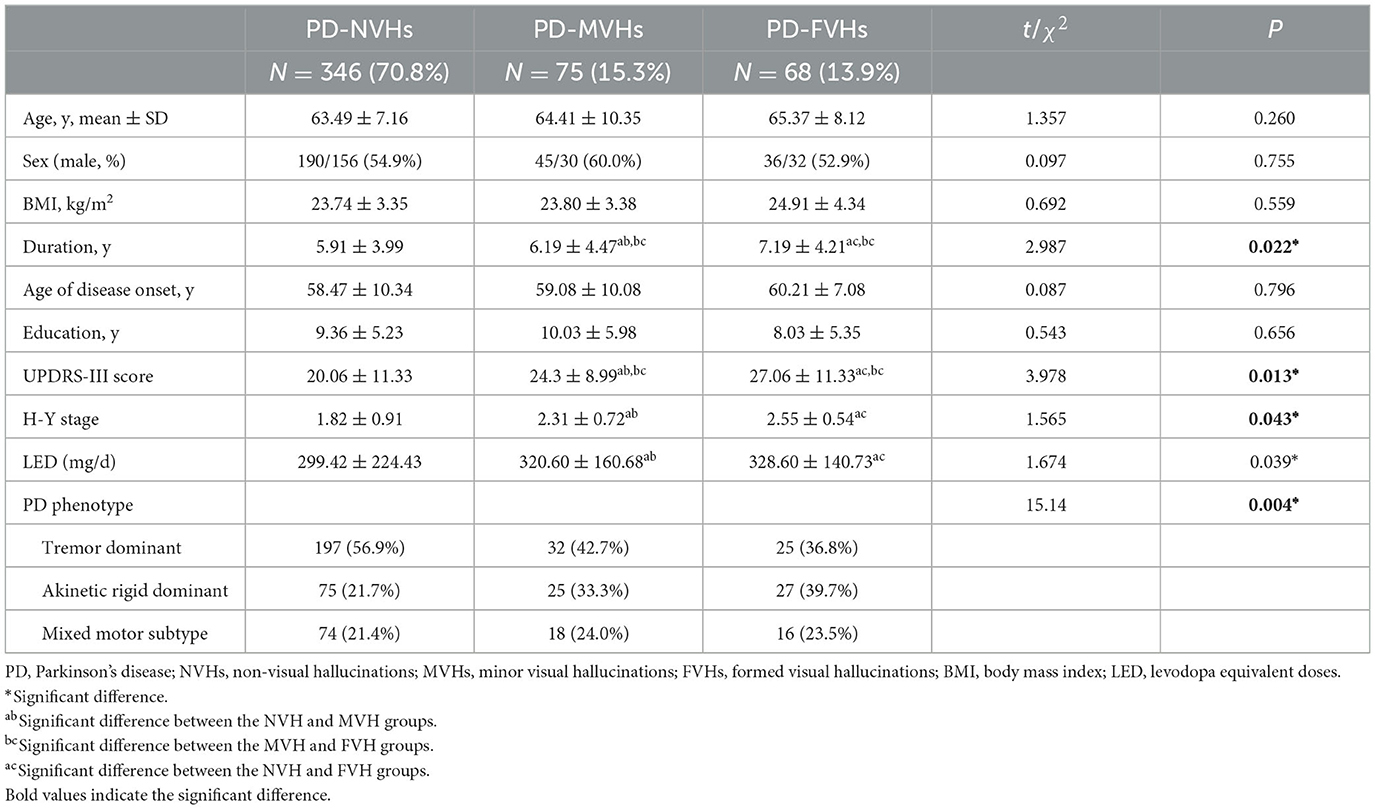
Table 1. Demographic characteristics of enrolled patients with Parkinson's disease according to the presence of VHs.
Non-motor symptoms (NMSs) were also compared between the patients with and without hallucinations. The differences in NMSs are shown in Table 2. Patients with FVHs had significantly higher scores on the STOP-Bang scale, ESS, PSQI, and RBD-SQ (all P < 0.01), indicating that sleep disorders were more common in the patients with hallucinations. The patients with hallucinations had worse scores on the MoCA overall and in the domains of visuospatial/executive function, abstraction and orientation than those of patients with MVHs or patients without hallucinations (all P < 0.05). There were no differences in HAMA or HAMD scores among the three groups.
Between-group differences in parameters are shown in Table 3. Individuals with hallucinations also had a lower TST, a lower SE, a higher arousal index, a longer SL, a higher percentage of time in N1 out of TST (N1%), a lower percentage of time in N3 (N3%), a higher risk of RBD and a higher REM density. This means that patients without hallucinations had deeper and more continuous sleep. The REM sleep latency, percentage of time in N2 (N2%), and REM sleep percentage did not show any statistically significant differences among the three groups.
The prevalence rates of OSA (AHI > 5) in patients with FVHs, MVHs, and without hallucinations were 63.3, 54.7, and 38.7%, respectively (Table 4). A higher proportion of patients with FVHs had moderate to severe OSA (AHI > 15). These patients also experienced more severe hypoxia, a longer average duration of apnea and a higher RRA (all P < 0.05).
The results of the multivariate logistic regression analysis are summarized in Table 5. VHs were independently associated with a higher AHI [odds ratio (OR), 9.382; 95% confidence interval (CI), 4.534–70.796], more severe RRA [OR, 4.382; 95% CI, 2.234–41.420], lower lowest O2 [OR, −2.186; 95% CI, 1.067–4.154], and higher N1% [OR, 2.223; 95% CI, 1.108–5.638].
VHs are the most common neuropsychiatric symptoms in patients with PD. The mechanism behind their formation is complex, and their clinical manifestations are diverse, making it easy for VHs to be overlooked. A previous study showed that between 8 and 40% of patients with PD had VHs (23). In our study, 13.9% of the FVHs reported were complex visual images that typically moved and were of normal size, lasting for seconds to minutes. On the other hand, our findings also indicated that minor or simple hallucinations (e.g., MVHs), which affect 15.9% of all PD patients, are generally less likely to be apparent to informants and hence to be underestimated by them. These minor phenomena typically lasted for a brief period and occurred more than once a week, while insight was completely preserved. We further discovered that patients with FVHs had a longer duration of illness and more severe motor symptoms than those with MVHs and NVHs. The pathophysiology of VHs is a complex matter. It has been suggested that injuries to the dopaminergic, serotonergic, and cholinergic systems in various brain areas may play a role in causing hallucinations (24). As the disease progresses, the loss of these neurotransmitters may worsen hallucinatory symptoms. Nonetheless, we discovered that the severity of PD was not significantly independently associated with VHs, indicating that physicians ought not to rely solely on conventional measures of disease severity to identify patients at risk of developing VHs.
There is still a debate as to whether anti-PD medications contribute to the development of VHs. In our study, we found that patients with VHs had higher LEDs than those without hallucinations. Benzhexol, amantadine, levodopa, catechol-O-methyltransferase inhibitors, and MAO-B inhibitors contributed to VHs. However, anticholinergic agents may cause hallucinations and are not included in LED calculations. Due to the potential for cognitive and urinary side effects as well as other side effects, Parkinson's disease patients seldom use anticholinergic drugs, which is why these drugs were not included in this study. In the future, we will conduct a more detailed calculation of the proportion and dosage of each type of anti-Parkinson's medication used by individuals with and without VHs.
Our study is the first to compare the NMSs among three groups of PD patients (with FVHs, with MVHs and without VHs). The relationship between depression/anxiety symptoms and VHs is still unknown. Fénelon et al. reported a higher prevalence of depression in patients with VHs (25), and Mack et al. (26) found that patients with VHs had severe depressive symptoms. However, some longitudinal studies that focused on VHs did not report a clear relationship with depression, anxiety, or apathy in PD patients (27, 28). We conducted an evaluation using the HAMA and HAMD and found no statistically significant differences in scores on these scales between patients with and without VHs. In the univariate analysis, we found that the PD patients with VHs showed more severe cognitive impairment, especially in visuospatial/executive function, abstraction, and orientation, than the PD patients without VHs, especially those in the FVH group, consistent with Tom's study showing that VHs are probably the major independent predictor of cognitive deterioration (29). However, neither emerged as an independent factor predictive of VHs in our study. The association between cognitive impairment and VHs could be due to a parallel pathophysiological process, and these symptoms may represent a common endpoint of neurodegeneration. It is unclear how the pathologic process is related to independent cognitive changes and VHs.
The relationship between VHs and RBD is still unknown. In this study, we used PSG data to diagnose RBD to investigate this relationship. The prevalence rates of RBD in the group without VHs (the NVH group), MVH group, and FVH group were 36.9, 56.0, and 57.3%, respectively. The prevalence of RBD in patients with VHs was significantly higher than that in the NVH group, but there was no difference between the MVH and FVH groups. An 8-year longitudinal study found that RBD was correlated with the occurrence of hallucinations, regardless of disease duration, motor stage, age or sex (30). An association between VHs and RBD was also demonstrated in a study by Gjerstad et al. (31). Cholinergic dysfunction could be the common pathway for both RBD and VHs (32). We also found that REM density was higher in VH patients, which means that patients with VHs have more active eye movements during REM sleep. The mechanism underlying this difference is not yet clear, but an increased density of REM sleep may lead to greater activation of the motor cortex.
We further examined the correlation between sleep habits and VHs in patients with PD. Our data showed that patients with VHs had worse sleep disruption or poorer sleep quality as assessed by the PDSS, PSQI, and ESS. Consistent with Barnes's study (33), PD patients with hallucinations reported more sleep disturbances and unexpected daytime sleepiness than those without hallucinations. However, this difference was not previously confirmed by PSG data. Our investigation utilizing PSG demonstrated that patients without VHs had a considerably longer TST than those with VHs. Patients with VHs exhibited lower SE, a higher arousal index, more N1 sleep, less N3 sleep, and more periodic limb movements. We also confirmed an independent association between VHs and N1%. This implies that patients experiencing hallucinations also exhibited poor overall sleep quality, sleep continuity, and depth of sleep. Prolonged SL and greater sleep-arousal changes have been associated with a prolonged state of drowsiness, thereby increasing the likelihood of experiencing VHs (34). A deeper understanding of abnormal sleep patterns and VHs could be obtained through a longitudinal investigation of whether sleep disturbances precede VHs or whether VHs cause sleep disturbances.
OSA is a prevalent disorder that is characterized by recurrent upper airway obstruction during sleep, which results in intermittent hypoxemia and sleep fragmentation. It is estimated that 20–60% of PD patients have concomitant OSA (35). OSA patients with PD exhibit a lower desaturation index, lower mean and nadir oxygen saturation values, and longer time with an oxygen saturation below 90% throughout the night than OSA patients without PD (36, 37). Previous studies have shown an association between vascular comorbidities and OSA as well as the severity of motor and cognitive symptoms in PD (38). However, there is a lack of studies assessing the relationship between OSA and VHs. Approximately 44.6% of our PD patients had OSA (AHI>5], consistent with a previous study. We also found that the prevalence rates of OSA were 38.7, 54.7, and 63.3% in PD patients in the NVH, MVH, and FVH groups, respectively. As hallucinatory symptoms worsen, the accompanying OSA also became more severe. Patients with PD frequently exhibit sleep disorders, including drowsiness, insomnia, and RBD. These sleep problems, such as daytime drowsiness, nighttime snoring, and frequent apnea, are similar to the symptoms of OSA. OSA has the potential to worsen motor and cognitive impairments in PD patients by causing hypoxemia and reducing sleep quality, ultimately impacting motor control and neural conduction. Furthermore, the prevalence of OSA among PD patients is high, and it may be associated with muscle tone disorders, laryngeal muscle dysfunction, and abnormalities in the structure of the upper respiratory tract. Additional research is needed to determine the precise correlation between OSA and PD as well as the influence of OSA on PD symptoms.
To our knowledge, this is the first study to use PSG and to explore the association between OSA and VHs in patients with PD. We not only focused on the prevalence of OSA in each group but also explored parameters related to OSA, such as the mean O2, lowest O2, average duration of apnea, and RRA. We found that the AHI, RRA, and lowest O2 were independent risk factors for VHs. Patients with OSA frequently experience cognitive and emotional issues, such as reduced concentration, memory impairment, and emotional instability. These symptoms could be associated with hypoxia in the brain during sleep and its impact on neuronal activity. By utilizing fMRI, it would be possible to observe abnormal brain activity patterns during sleep and identify specific regions associated with cognitive and behavioral issues in OSA patients. For example, OSA patients exhibited a decrease in metabolic rates in the frontal, temporal, and parietal lobes, which are associated with cognitive control, memory, and emotion regulation (39). Furthermore, individuals with OSA may exhibit brain activity associated with abnormal sleep patterns, which may include reduced slow wave sleep and increased REM sleep. These changes in sleep structure may further impact brain function and cognitive performance, potentially resulting in VHs. The intermittent hypoxia and frequent arousal that occur with OSA may also play a significant role in the pathophysiology of psychosis, particularly the development of VHs. PSG or respiratory monitoring should be a routine method for monitoring PD patients with VHs.
This study also has some limitations. First, this study was a retrospective study and lacked follow-up data. We are currently gathering follow-up data for subsequent statistical analysis. Second, this study does not provide a comparison of the occurrence and severity of VHs before and after OSA treatment. In our subsequent studies, we will evaluate the presence of VHs before and after continuous positive airway pressure (CPAP) treatment.
In conclusion, we found that the prevalence of VHs among PD patients was 29.2%. We further discovered that PD patients with FVHs experienced longer illness durations and severe motor symptoms. PD patients with VHs also exhibited NMSs such as cognitive dysfunction and RBD, and this group had a higher percentage of patients with sleep disorders, a lower TST, a lower SE and a longer SL. Our results confirmed that 44.6% of PD patients had OSA. We also demonstrated that the AHI, N1%, lowest O2, and RRA were significant predictors of VHs. Therefore, clinicians should be aware of the importance of screening for and managing OSA in PD patients, and future studies should concentrate on exploring the pathophysiology of these symptoms.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Committee of Brain Hospital Affiliated to Nanjing Medical University (ethics approval number: 2021-KY007-01). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
JZ: Investigation, Methodology, Writing—original draft. YZ: Data curation, Writing—review & editing. YJ: Data curation, Writing—review & editing, Validation. YP: Writing—review & editing. XJ: Writing—review & editing. YW: Writing—review & editing, Conceptualization, Data curation, Investigation. DL: Writing—review & editing. LZ: Funding acquisition, Project administration, Writing—review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The National Natural Science Foundation of China (grant numbers: 82171249 and 82101332) provided funding for the design of the study, collection and data analysis, and for the development of this manuscript.
We thank the patients and their families for their participation in the study. We also acknowledge the effort and dedicated work of all the staff that implemented the evaluation components of the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Fan HX, Sheng S, Zhang F. New hope for Parkinson's disease treatment: targeting gut microbiota. CNS Neurosci Ther. (2022) 28:1675–88. doi: 10.1111/cns.13916
2. Borghammer P. The alpha-synuclein origin and connectome model (SOC model) of Parkinson's disease: explaining motor asymmetry, non-motor phenotypes, and cognitive decline. J Parkinson's Dis. (2021) 11:455–74. doi: 10.3233/JPD-202481
3. Sauerbier A, Jenner P, Todorova A, Chaudhuri KR. Non motor subtypes and Parkinson's disease. Parkinson Relat Disord. (2016) 22(Suppl.1):S41–6. doi: 10.1016/j.parkreldis.2015.09.027
4. Sveinbjornsdottir S. The clinical symptoms of Parkinson's disease. J Neurochem. (2016) 139(Suppl.1):318–24. doi: 10.1111/jnc.13691
5. Zhu J, Shen B, Lu L, Lan W, Pan Y, Zhang L, et al. Prevalence and risk factors for visual hallucinations in Chinese patients with Parkinson's disease. J Neurol Sci. (2017) 372:471–6. doi: 10.1016/j.jns.2016.10.043
6. Eversfield CL, Orton LD. Auditory and visual hallucination prevalence in Parkinson's disease and dementia with Lewy bodies: a systematic review and meta-analysis. Psychol Med. (2019) 49:2342–53. doi: 10.1017/S0033291718003161
7. Aarsland D, Batzu L, Halliday GM, Geurtsen GJ, Ballard C, Ray Chaudhuri K, et al. Parkinson disease-associated cognitive impairment. Nat Rev Dis Prim. (2021) 7:47. doi: 10.1038/s41572-021-00280-3
8. Kawada T. Parkinson's disease and obstructive sleep apnea. Excli J. (2021) 20:490. doi: 10.17179/excli2021-3359
9. Sobreira-Neto MA, Pena-Pereira MA, Sobreira EST, Chagas MHN, Almeida CMO, Fernandes RMF, et al. Obstructive sleep apnea and Parkinson's disease: characteristics and associated factors. Arq Neuropsiquiatr. (2019) 77:609–16. doi: 10.1590/0004-282x20190098
10. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. (1992) 55:181–4. doi: 10.1136/jnnp.55.3.181
11. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. (2010) 25:2649–53. doi: 10.1002/mds.23429
12. Goetz CG, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stebbins GT, et al. Movement disorder society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov Disord. (2007) 22:41–7. doi: 10.1002/mds.21198
13. Hendershott TR, Zhu D, Llanes S, Poston KL. Domain-specific accuracy of the Montreal Cognitive Assessment subsections in Parkinson's disease. Parkinsonism Relat Disord. (2017) 38:31–4. doi: 10.1016/j.parkreldis.2017.02.008
14. Chaudhuri KR, Pal S, DiMarco A, Whately-Smith C, Bridgman K, Mathew R, et al. The Parkinson's disease sleep scale: a new instrument for assessing sleep and nocturnal disability in Parkinson's disease. J Neurol Neurosurg Psychiatry. (2002) 73:629–35. doi: 10.1136/jnnp.73.6.629
15. Uemura Y, Nomura T, Inoue Y, Yamawaki M, Yasui K, Nakashima K. Validation of the Parkinson's disease sleep scale in Japanese patients: a comparison study using the Pittsburgh Sleep Quality Index, the Epworth Sleepiness Scale and Polysomnography. J Neurol Sci. (2009) 287:36–40. doi: 10.1016/j.jns.2009.09.015
16. Gomes T, Benedetti A, Lafontaine AL, Kimoff RJ, Robinson A, Kaminska M. Validation of STOP, STOP-BANG, STOP-BAG, STOP-B28, and GOAL screening tools for identification of obstructive sleep apnea in patients with Parkinson disease. J Clin Sleep Med. (2023) 19:45–54. doi: 10.5664/jcsm.10262
17. Kumar S, Bhatia M, Behari M. Excessive daytime sleepiness in Parkinson's disease as assessed by Epworth Sleepiness Scale (ESS). Sleep Med. (2003) 4:339–42. doi: 10.1016/S1389-9457(03)00105-9
18. Bušková J, Perinová P, Miletínová E, Dušek P, RuŽička E, Šonka K, et al. Validation of the REM sleep behavior disorder screening questionnaire in the Czech population. BMC Neurol. (2019) 19:110. doi: 10.1186/s12883-019-1340-4
19. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. (1959) 32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x
20. Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. (1988) 45:742–7. doi: 10.1001/archpsyc.1988.01800320058007
21. Grigg-Damberger MM. The AASM Scoring Manual four years later. J Clin Sleep Med. (2012) 8:323–32. doi: 10.5664/jcsm.1928
22. Zhu J, Lu L, Zhong M, Jiang X, Wu Z, Dong J, et al. Increased rapid eye movement density in Chinese patients with Parkinson's disease and RBD. Neurol Sci. (2021) 42:961–8. doi: 10.1007/s10072-020-04597-x
23. Barnes J, Boubert L, Harris J, Lee A, David AS. Reality monitoring and visual hallucinations in Parkinson's disease. Neuropsychologia. (2003) 41:565–74. doi: 10.1016/S0028-3932(02)00182-3
24. Zahodne LB, Fernandez HH. Pathophysiology and treatment of psychosis in Parkinson's disease: a review. Drugs Aging. (2008) 25:665–82. doi: 10.2165/00002512-200825080-00004
25. Fénelon G, Mahieux F, Huon R, Ziégler M. Hallucinations in Parkinson's disease: prevalence, phenomenology and risk factors. Brain. (2000) 123:733–45. doi: 10.1093/brain/123.4.733
26. Mack J, Rabins P, Anderson K, Goldstein S, Grill S, Hirsch ES, et al. Prevalence of psychotic symptoms in a community-based Parkinson disease sample. Am J Geriatr Psychiatry. (2012) 20:123–32. doi: 10.1097/JGP.0b013e31821f1b41
27. Gibson G, Mottram PG, Burn DJ, Hindle JV, Landau S, Samuel M, et al. Frequency, prevalence, incidence and risk factors associated with visual hallucinations in a sample of patients with Parkinson's disease: a longitudinal 4-year study. Int J Geriatr Psychiatry. (2013) 28:626–31. doi: 10.1002/gps.3869
28. Lee AH, Weintraub D. Psychosis in Parkinson's disease without dementia: common and comorbid with other non-motor symptoms. Mov Disord. (2012) 27:858–63. doi: 10.1002/mds.25003
29. van Mierlo TJM, Foncke EMJ, Post B, Schmand BA, Bloem BR, van Harten B, et al. Rivastigmine for minor visual hallucinations in Parkinson's disease: a randomized controlled trial with 24 months follow-up. Brain Behav. (2021) 11:e2257. doi: 10.1002/brb3.2257
30. Onofrj M, Thomas A, D'Andreamatteo G, Iacono D, Luciano AL, Di Rollo A, et al. Incidence of RBD and hallucination in patients affected by Parkinson's disease: 8-year follow-up. Neurol Sci. (2002) 23 Suppl 2:S91–4. doi: 10.1007/s100720200085
31. Gjerstad MD, Boeve B, Wentzel-Larsen T, Aarsland D, Larsen JP. Occurrence and clinical correlates of REM sleep behaviour disorder in patients with Parkinson's disease over time. J Neurol Neurosurg Psychiatry. (2008) 79:387–91. doi: 10.1136/jnnp.2007.116830
32. Lenka A, Hegde S, Jhunjhunwala KR, Pal PK. Interactions of visual hallucinations, rapid eye movement sleep behavior disorder and cognitive impairment in Parkinson's disease: a review. Parkinsonism Relat Disord. (2016) 22:1–8. doi: 10.1016/j.parkreldis.2015.11.018
33. Barnes J, Connelly V, Wiggs L, Boubert L, Maravic K. Sleep patterns in Parkinson's disease patients with visual hallucinations. Int J Neurosci. (2010) 120:564–9. doi: 10.3109/00207454.2010.494790
34. Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. (1998) 121:1819–40. doi: 10.1093/brain/121.10.1819
35. da Silva-Júnior FP Jr, do Prado GF, Barbosa ER, Tufik S, Togeiro SM. Sleep disordered breathing in Parkinson's disease: a critical appraisal. Sleep Med Rev. (2014) 18:173–8. doi: 10.1016/j.smrv.2013.04.005
36. Valko PO, Hauser S, Sommerauer M, Werth E, Baumann CR. Observations on sleep-disordered breathing in idiopathic Parkinson's disease. PLoS ONE. (2014) 9:e100828. doi: 10.1371/journal.pone.0100828
37. Nomura T, Inoue Y, Kobayashi M, Namba K, Nakashima K. Characteristics of obstructive sleep apnea in patients with Parkinson's disease. J Neurol Sci. (2013) 327:22–4. doi: 10.1016/j.jns.2013.01.036
38. Meira B, Fernandes M, Salavisa M, Saraiva M, Conceição L, Borbinha C, et al. Obstructive sleep apnea and other vascular risk factors' impact on non-motor symptoms in Parkinson's disease. Mov Disord Clin Pract. (2022) 9:785–98. doi: 10.1002/mdc3.13504
Keywords: Parkinson's disease, visual hallucinations, cognition, sleep, obstructive sleep apnea, apnea-hypopnea index
Citation: Zhu J, Zhao Y, Jiang Y, Pan Y, Jiang X, Wang Y, Li D and Zhang L (2024) The relationship between obstructive sleep apnea and visual hallucinations in PD patients: a polysomnography study. Front. Neurol. 14:1275660. doi: 10.3389/fneur.2023.1275660
Received: 10 August 2023; Accepted: 18 December 2023;
Published: 11 January 2024.
Edited by:
Naima Covassin, Mayo Clinic, United StatesReviewed by:
George D. Vavougios, University of Cyprus, CyprusCopyright © 2024 Zhu, Zhao, Jiang, Pan, Jiang, Wang, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Zhang, bmV1cm9femhhbmdsaUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.