- Department of Neurology, General Hospital of Northern Theater Command, Shenyang, Liaoning, China
Background and purpose: Blood pressure is associated with outcomes in acute ischemic stroke (AIS) patients receiving intravenous alteplase. The study aimed to explore the effect of sex and age on their association.
Methods: Based on a prospective cohort, we retrospectively enrolled consecutive AIS patients who received intravenous alteplase and had complete blood pressure data, including baseline systolic blood pressure (SBP 01), SBP at 1 h (SBP 02), and SBP at 24 h (SBP 03) after alteplase. Maximum SBP (SBP max), minimum SBP (SBP min), and mean SBP (SBP mean) were calculated. Poor outcome was defined as having a modified Rankin Scale (mRS) score of 2–6 at 90 days. We explored the effect of age and sex on the association of different SBP indicators with the 3-month outcomes.
Results: A total of 1,593 eligible patients were included in the present study. All SBP indicators were found to be higher in patients with poor vs. good outcomes. Multivariate logistic regression analysis showed that all SBP indicators except baseline SBP were associated with poor outcomes with good prediction powers (AUC, 0.762–0.766). More SBP indicators (SBP 02, SBP 03, SBP min, and SBP mean) were associated with poor outcomes in women vs. men, while all SBP indicators after alteplase were associated with poor outcomes in patients aged ≥ 60 years, but none was seen in patients aged < 60 years. Furthermore, all SBP indicators after alteplase were associated with poor outcomes in women aged ≥ 60 years, while only SBP 03 in men aged < 60 years.
Conclusion: Among Chinese stroke patients treated with intravenous alteplase, SBP after alteplase was associated with clinical outcomes, which were affected by age and sex.
Introduction
Intravenous thrombolysis (IVT) is an approved effective treatment for acute ischemic stroke (AIS), but many patients still achieved poor outcomes (1). Many factors were associated with poor outcomes, in which blood pressure was closely associated with poor outcomes (2). Three quarters of AIS patients had elevated blood pressure at presentation with about half of them with a history of hypertension (3). The elevated blood pressure in AIS patients was considered a compensatory response that would increase the perfusion of the ischemic cerebral tissue to save penumbra, whereas excessively elevated blood pressure would worsen cerebral edema and result in hemorrhagic transformation, especially for patients with IVT (4). Some studies suggested that higher mean systolic blood pressure (SBP) (5, 6), greater SBP variability (7–9), and smaller reductions in SBP (8, 9), after IVT were associated with poor outcomes. In AIS patients, a decrease in SBP may reduce the risk of hemorrhagic transformation but decrease perfusion in the penumbra, leading to a poor outcome (10). Current clinical guidelines consistently recommend that SBP should be controlled below 185 mmHg in thrombolysed AIS patients (11).
The age and sex differences in stroke have been widely investigated. Women differed from men in the distribution of risk factors, stroke severity, and outcome, even in lacunar infarcts (12–15). Recent studies found significant sex differences in the clinical outcomes of stroke patients, such as endovascular treatment (16, 17) and intravenous thrombolysis (18, 19). In addition to sex, age was closely related to stroke outcomes, and increased age is often closely linked with poor function recovery (20–22). Collectively, these studies suggest the important effect of sex and age on stroke prevention, prognosis prediction, and treatment strategy. However, the effect of age and sex on the association of SBP with outcomes in IVT-treated patients is not well established.
In this context, we hypothesize that age and sex may influence the association of SBP with clinical outcomes in AIS patients after IVT, which was investigated by a post hoc analysis of the Intravenous Thrombolysis Registry for Chinese Ischemic Stroke within 4.5 h onset (INTRECIS) dataset.
Methods
Study design/patient population
The INTRECIS is a nationwide, multi-center, prospective, and registry study of consecutive adult AIS patients who received intravenous thrombolysis within 4.5 h of the onset of symptoms. The details of the study design have been reported recently (23). From the INTRECIS cohort, patients were included in the current study with the following criteria: (1) consecutive adult AIS patients who received 0.9 mg/kg intravenous alteplase within 4.5 h of a definite time of onset of symptoms; (2) the complete clinical data including SBP at baseline, 1, and 24 h after alteplase. Patients were excluded if they met the following criteria: (1) patients who received urokinase; (2) patients who received a non-standard dose of alteplase; (3) patients who lacked complete clinical data; (4) age > 80 years; (5) patients treated with mechanical thrombectomy. All patients and/or their legally authorized surrogates gave written informed consent.
We collected baseline characteristics of patients including age, sex, current smoker, current drinker, hypertension, diabetes mellitus, history of stroke, coronary heart disease, atrial fibrillation, body mass index, baseline heart rate, symptom onset-to-thrombolysis time (OTT), door-to-needle time (DNT), National Institute of Health Stroke Scale (NIHSS) score, blood pressure data including diastolic blood pressure, baseline SBP (SBP01), immediate SBP after the end of alteplase (SBP02), SBP 24 h after alteplase (SBP03), maximum SBP among three timepoints (SBP max), minimum SBP among three timepoints (SBP min), and average SBP among three timepoints (SBP mean), and mRS at 90 days. Blood pressure was measured using a validated electronic sphygmomanometer while the patient was supine.
Clinical outcomes
In parallel with the INTRECIS study (23), the poor outcome was defined as a mRS score of 2–6 points at 90 days, whereas the good outcome was defined as a mRS score of 0–1 points at 90 days.
Statistical analysis
We performed descriptive statistics for baseline characteristics. Baseline information was compared between favorable and poor outcome groups using the t-test or U-test for continuous variables and the chi-square test or Fisher's exact test for categorical variables. First, we performed the univariate logistic regression analysis to identify the associated variables with poor outcomes. In the multivariate logistic regression analysis, BMI, baseline NIHSS score, baseline heart rate, current drinker, previous stroke, and atrial fibrillation were further adjusted to determine whether different SBP metrics were associated with patient outcomes after thrombolysis. Results are reported as odds ratios (OR) and 95% confidence intervals (CI). Differences with P-values < 0.05 were considered statistically significant in the relevant analytical tests. Second, we used a receiver operating characteristic (ROC) curve to explore the predictive value of SBP via areas under the curve (AUCs). The statistical software SPSS version 26.0 was used for analysis.
Results
Baseline data
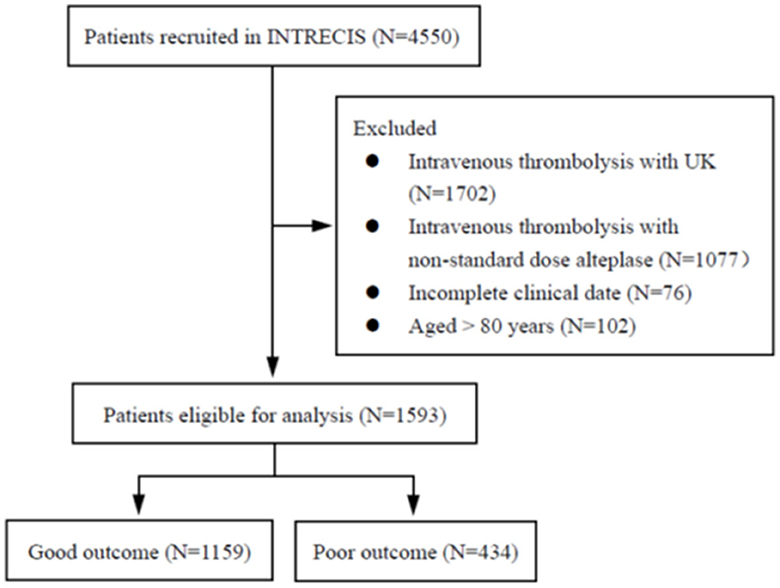
From 4,550 patients enrolled in the INTRECIS cohort between October 2016 and September 2019, 1,593 eligible patients were included in this study: 1,159 (72.8%) in the good outcome group and 434 (27.2%) in the poor outcome group (Figure 1). Table 1 presents baseline characteristics in two groups. Patients with poor outcomes were older (65 years vs. 62 years, P < 0.000), had higher NIHSS score at admission (11 vs. 5, P < 0.000), and higher SBP indicators such as median SBP02 (145 mmHg vs. 141 mmHg, P = 0.010), median SBP03 (142 mmHg vs. 140 mmHg, P < 0.000), median SBP max (156 mmHg vs. 154 mmHg, P = 0.012), and median SBP min (137.5 mmHg vs. 135 mmHg, P = 0.008), and median SBP mean (146.7 mmHg vs. 143.7 mmHg, P = 0.004) (Table 1).
In the univariate logistic regression analysis, age, body mass index, current drinker, history of stroke, atrial fibrillation, baseline heart rate, NIHSS score at baseline, SBP02, SBP03, SBP max, SBP min, and SBP mean were statistically significant (P < 0.05, Table 2). In multivariate logistic regression analysis (Table 2), SBP02, SBP03, SBP max, SBP min, and SBP mean after IVT were associated with a higher likelihood of good outcomes after adjusting for variables with P < 0.05 in univariate regression analysis (per 10 mm Hg higher SBP indicator, adjusted OR 0.87–0.94, all P < 0.05). Furthermore, ROC curve analysis showed these SBP indicators had good prediction power for functional outcomes: SBP02 with an AUC of 0.764 (95% CI = 0.737–0.790), SBP03 with an AUC of 0.766 (95% CI = 0.739–0.792), SBP max with an AUC of 0.762 (95% CI = 0.735–0.789), SBP min with an AUC of 0.764 (95% CI = 0.737–0.790), and SBP mean with an AUC of 0.763 (95% CI = 0.736–0.790) (Figure 2).
Based on sex, patients were divided into two groups: 1113 in the men group and 480 in the women group. After adjusting for BMI, baseline NIHSS score, baseline heart rate, current drinker, previous stroke, and atrial fibrillation, the multivariate logistic regression analysis showed that all SBP indicators after IVT except SBP max were associated with outcome in women, while only SBP03 in men (Table 3).
According to the age, patients were classified into two groups: 962 in the ≥ 60-year-old group and 631 in the < 60-year-old group. After adjusting for BMI, baseline NIHSS score, baseline heart rate, current drinker, previous stroke, and atrial fibrillation, the multivariate logistic regression analysis showed that all SBP indicators after IVT were associated with outcomes in the ≥ 60 years group, but none were associated in the < 60 years group (Table 4). Furthermore, ROC curve analysis showed age had moderate prediction power for functional outcomes: ≥ 60 years with an AUC of 0.572 (95% CI = 0.532–0.611) and < 60 years with an AUC of 0.515 (95% CI = 0.461–0.569) (Figure 3).

Figure 3. Receiver operating characteristic curves for predicting functional outcomes in two age subgroups.
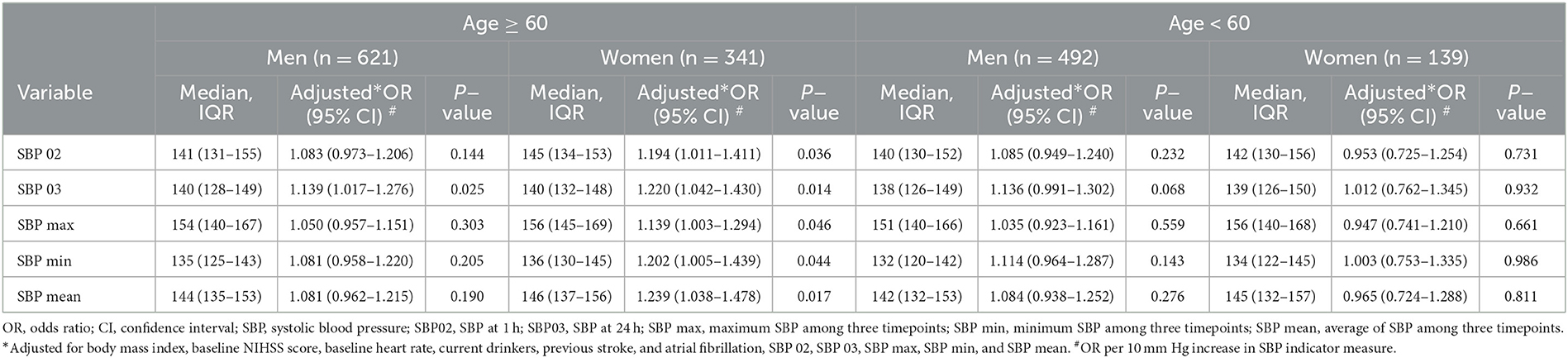
Based on age and sex, we further divided the patients into four subgroups: men ≥60 years, women ≥60 years, men <60 years, and women <60 years. After adjusting for BMI, baseline NIHSS score, baseline heart rate, current drinker, previous stroke, and atrial fibrillation, the multivariate logistic regression analysis showed that all SBP indicators after IVT were associated with outcomes in the ≥60 years women group, while only SBP 03 were associated in the ≥60 years men group (Table 5, Figure 4).

Figure 4. Forest plots of different SBP indicators predicting functional outcomes stratified by age and sex.
Given the close association of SBP indicators with outcomes in the women subgroup with ≥ 60 years, ROC curve analysis was performed. The results showed SBP02 with an AUC of 0.777 (95% CI = 0.725–0.829), SBP03 with an AUC of 0.782 (95% CI = 0.731–0.883), SBP max with an AUC of 0.775 (95% CI = 0.723–0.827), SBP min with an AUC of 0.778 (95% CI = 0.727–0.829), and SBP mean with an AUC of 0.779 (95% CI = 0.727–0.839) (Figure 5).

Figure 5. Receiver operating characteristic curve of prediction of functional outcomes in women patients ≥60 years.
Discussion
In this post hoc analysis, we identified several findings: (1) Among AIS patients who received intravenous alteplase, SBP indicators after IVT, but not baseline SBP were associated with clinical outcomes; (2) more SBP indicators were associated with clinical outcomes in women but only SBP 03 in men; (3) the association of SBP indicators with clinical outcome was found in the ≥ 60 years group but not the < 60 years group; (4) in the ≥ 60 years group, more SBP indicators were associated with clinical outcomes in women but only SBP 03 in men. Collectively, these findings suggest that age and sex should influence the relationship between SBP and outcomes in thrombolysed patients, and the closer association of SBP with outcomes may be in the subgroup of women aged ≥ 60 years.
Prior studies have investigated the relationship between SBP levels and functional outcomes in thrombolysed AIS patients (24), but the results were inconsistent. In our study, there was no difference in SBP at baseline between good and poor outcome groups, but SBP at 1 h and 24 h after IVT was found to be significantly associated with clinical outcomes. The finding was in agreement with a previous study (7), but not with other studies reporting the close association of SBP at the baseline and up to 24 h after thrombolysis with poor prognosis (6, 8, 25). This conflicting reason was not clear. Given the potential effect of stress, bladder pressure, or other transient stimuli on SBP at the stroke onset, the association of baseline SBP with clinical outcomes should be complex. In summary, the current results suggested that increasing SBP after thrombolysis may reduce the likelihood of 3-month good functional outcomes.
The pathophysiological mechanism underlying their associations may be attributed to impaired cerebral autoregulation (CA) function following stroke. Under normal conditions, CA can maintain relatively constant cerebral blood flow (CBF) when arterial blood pressure or cerebral perfusion pressure fluctuates (26, 27). However, CA may be impaired or even vanish after ischemic stroke (28), which may partially explain the association of increased SBP with poor outcomes because impaired CA leads to ischemia-reperfusion injury or reduced cerebral perfusion (29, 30).
In the present study, we found the significant effect of age and sex on the relationship with SBP and functional outcomes in patients with AIS after IVT, and more closer association was identified in old women patients, which was never reported previously. These findings should be plausible. Compared to pre-menopausal women and age-matched men, post-menopausal women were found to have reduced vasomotor reserve, poorer cerebrovascular reactivity, and reduced cerebral blood flow autoregulation (31). Mechanisms underlying these changes may be related to estrogen. For example, estrogen can bind to estrogen receptors on the endothelium of cerebral arteries, which would cause vasodilatation in response to the deficit in cerebral perfusion (32). However, the protective effect of estrogen is lost in post-menopausal women, which makes them more susceptible to changes in blood pressure. As a non-modifiable risk factor for cerebrovascular diseases (33), aging would impair cerebral autoregulation (34). Collectively, these results suggest that aging and estrogen decrease in a synergistic manner cause the impairment of cerebral autoregulation, resulting in poor outcomes in this population.
The main strength of this study was the first attempt to determine the effect of sex and age on the association of SBP indicators with outcomes in patients with AIS after IVT. The results suggested that higher SBP indicators after IVT were associated with poor 3-month functional outcomes, especially in older women patients. However, we recognize several limitations. The main limitation was the retrospective analysis nature, which was subject to selection bias and unexpected confounding factors. Second, the current study was only performed in Chinese ischemic stroke patients, and patients over 80 years old were excluded due to too small sample size, which should limit the generalizability of the results. Third, the detailed data on antihypertensive drugs were not available, which may make it impossible to investigate their effects on the current findings. Fourth, given the sex difference in the distribution of risk factors, stroke severity, functional outcomes (12), the difference in the pathophysiology, prognosis, and clinical features of lacunar vs. non-lacunar stroke (35), and the relationship between SBP, sex, age, and outcomes warrants investigation in patients with lacunar vs. non-lacunar ischemic stroke. Fifth, patients with mild-to-moderate neurologic deficit (median NIHSS score of 6) were enrolled in this study, which limited the generalization in moderate and severe stroke. Sixth, the small absolute difference in SBP indicators in different groups would affect the practical implementation of this finding. Finally, these findings need to be explained with caution due to the exploratory nature of this secondary analysis.
In summary, among Chinese acute ischemic stroke patients, SBP indicators after intravenous thrombolysis were independently associated with outcomes at 3 months, which may be affected by age and sex. The relationship will be worth exploring in different stroke subtypes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of General Hospital of Northern Theater Command (IRB: k2016–11). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
B-JL: Data curation, Formal analysis, Writing—original draft. JL: Formal analysis, Writing—original draft. H-SC: Conceptualization, Funding acquisition, Project administration, Supervision, Writing—review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the Science and Technology Project Plan of Liao Ning Province (2022JH2/101500020).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, et al. Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke-monitoring study (sits-most): an observational study. Lancet. (2007) 369:275–82. doi: 10.1016/S0140-6736(07)60149-4
2. Tikhonoff V, Zhang H, Richart T, Staessen JA. Blood pressure as a prognostic factor after acute stroke. Lancet Neurol. (2009) 8:938–48. doi: 10.1016/S1474-4422(09)70184-X
3. Britton M, Carlsson A, Faire de U. Blood pressure course in patients with acute stroke and matched controls. Stroke. (1986) 17:861–4. doi: 10.1161/01.STR.17.5.861
4. Leonardi-Bee J, Bath PM, Phillips SJ, Sandercock PA. Blood pressure and clinical outcomes in the international stroke trial. Stroke. (2002) 33:1315–20. doi: 10.1161/01.STR.0000014509.11540.66
5. Butcher K, Christensen S, Parsons M, De Silva DA, Ebinger M, Levi C, et al. Postthrombolysis blood pressure elevation is associated with hemorrhagic transformation. Stroke. (2010) 41:72–7. doi: 10.1161/STROKEAHA.109.563767
6. Ahmed N, Wahlgren N, Brainin M, Castillo J, Ford GA, Kaste M, et al. Relationship of blood pressure, antihypertensive therapy, and outcome in ischemic stroke treated with intravenous thrombolysis: retrospective analysis from safe implementation of thrombolysis in stroke-international stroke thrombolysis register (sits-istr). Stroke. (2009) 40:2442–9. doi: 10.1161/STROKEAHA.109.548602
7. Endo K, Kario K, Koga M, Nakagawara J, Shiokawa Y, Yamagami H, et al. Impact of early blood pressure variability on stroke outcomes after thrombolysis: the samurai rt-pa registry. Stroke. (2013) 44:816–8. doi: 10.1161/STROKEAHA.112.681007
8. Berge E, Cohen G, Lindley RI, Sandercock P, Wardlaw JM, Sandset EC, et al. Effects of blood pressure and blood pressure-lowering treatment during the first 24 hours among patients in the third international stroke trial of thrombolytic treatment for acute ischemic stroke. Stroke. (2015) 46:3362–9. doi: 10.1161/STROKEAHA.115.010319
9. Wu W, Huo X, Zhao X, Liao X, Wang C, Pan Y, et al. Relationship between blood pressure and outcomes in acute ischemic stroke patients administered lytic medication in the tims-china study. PLoS ONE. (2016) 11:e0144260. doi: 10.1371/journal.pone.0144260
10. Jauch EC, Saver JL, Adams HP, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. (2013) 44:870–947. doi: 10.1161/STR.0b013e318284056a
11. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. (2018) 49:e46–e110. doi: 10.1161/STR.0000000000000158
12. Arboix A, Blanco-Rojas L, Oliveres M, García-Eroles L, Comes E, Massons J. Clinical characteristics of acute lacunar stroke in women: emphasis on gender differences. Acta Neurol Belg. (2014) 114:107–12. doi: 10.1007/s13760-013-0257-8
13. Appelros P, Stegmayr B, Terent A. Sex differences in stroke epidemiology: a systematic review. Stroke. (2009) 40:1082–90. doi: 10.1161/STROKEAHA.108.540781
14. Ramezankhani A, Parizadeh D, Azizi F, Hadaegh F. Sex differences in the association between diabetes and hypertension and the risk of stroke: cohort of the tehran lipid and glucose study. Biol Sex Differ. (2022) 13:10. doi: 10.1186/s13293-022-00421-7
15. Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990-2010: findings from the global burden of disease study 2010. Lancet. (2014) 383:245–54. doi: 10.1016/S0140-6736(13)61953-4
16. Demel SL, Reeves M, Xu H, Xian Y, Mac Grory B, Fonarow GC. Sex differences in endovascular therapy for ischemic stroke: results from the get with the guidelines-stroke registry. Stroke. (2022) 53:3099–106. doi: 10.1161/STROKEAHA.122.038491
17. Uchida K, Yoshimura S, Sakai N, Yamagami H, Morimoto T. Sex differences in management and outcomes of acute ischemic stroke with large vessel occlusion. Stroke. (2019) 50:1915–8. doi: 10.1161/STROKEAHA.119.025344
18. Spaander FH, Zinkstok SM, Baharoglu IM, Gensicke H, Polymeris A, Traenka C, et al. Sex differences and functional outcome after intravenous thrombolysis. Stroke. (2017) 48:699–703. doi: 10.1161/STROKEAHA.116.014739
19. Lorenzano S, Ahmed N, Falcou A, Mikulik R, Tatlisumak T, Roffe C, et al. Does sex influence the response to intravenous thrombolysis in ischemic stroke?: Answers from safe implementation of treatments in stroke-international stroke thrombolysis register. Stroke. (2013) 44:3401–6. doi: 10.1161/STROKEAHA.113.002908
20. Kim TH, Vemuganti R. Effect of sex and age interactions on functional outcome after stroke. CNS Neurosci Ther. (2015) 21:327–36. doi: 10.1111/cns.12346
21. Ding GY, Xu JH, He JH, Nie ZY. Clinical scoring model based on age, nihss, and stroke-history predicts outcome 3 months after acute ischemic stroke. Front Neurol. (2022) 13:935150. doi: 10.3389/fneur.2022.935150
22. Weimar C, König IR, Kraywinkel K, Ziegler A, Diener HC. Age and national institutes of health stroke scale score within 6 hours after onset are accurate predictors of outcome after cerebral ischemia: development and external validation of prognostic models. Stroke. (2004) 35:158–62. doi: 10.1161/01.STR.0000106761.94985.8B
23. Wang X, Li X, Xu Y, Li R, Yang Q, Zhao Y, et al. Effectiveness of intravenous r-tpa versus uk for acute ischaemic stroke: a nationwide prospective chinese registry study. Stroke Vas Neurol. (2021) 6:603–9. doi: 10.1136/svn-2020-000640
24. Malhotra K, Ahmed N, Filippatou A, Katsanos AH, Goyal N, Tsioufis K, et al. Association of elevated blood pressure levels with outcomes in acute ischemic stroke patients treated with intravenous thrombolysis: a systematic review and meta-analysis. J Stroke. (2019) 21:78–90. doi: 10.5853/jos.2018.02369
25. Wahlgren N, Ahmed N, Eriksson N, Aichner F, Bluhmki E, Dávalos A, et al. Multivariable analysis of outcome predictors and adjustment of main outcome results to baseline data profile in randomized controlled trials: safe implementation of thrombolysis in stroke-monitoring study (sits-most). Stroke. (2008) 39:3316–22. doi: 10.1161/STROKEAHA.107.510768
26. Xiong L, Liu X, Shang T, Smielewski P, Donnelly J, Guo ZN, et al. Impaired cerebral autoregulation: measurement and application to stroke. J Neurol Neurosurg Psychiatry. (2017) 88:520–31. doi: 10.1136/jnnp-2016-314385
27. van Beek AH, Claassen JA, Rikkert MG, Jansen RW. Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly. J Cereb Blood Flow Metab. (2008) 28:1071–85. doi: 10.1038/jcbfm.2008.13
28. Immink RV, van Montfrans GA, Stam J, Karemaker JM, Diamant M, van Lieshout JJ, et al. Dynamic cerebral autoregulation in acute lacunar and middle cerebral artery territory ischemic stroke. Stroke. (2005) 36:2595–600. doi: 10.1161/01.STR.0000189624.06836.03
29. Petersen NH, Ortega-Gutierrez S, Reccius A, Masurkar A, Huang A, Marshall RS, et al. Dynamic cerebral autoregulation is transiently impaired for one week after large-vessel acute ischemic stroke. Cereb Dis. (2015) 39:144–50. doi: 10.1159/000368595
30. Huang Y, Sharma VK, Robinson T, Lindley RI, Chen X, Kim JS, et al. Rationale, design, and progress of the enhanced control of hypertension and thrombolysis stroke study (enchanted) trial: an international multicenter 2 × 2 quasi-factorial randomized controlled trial of low- vs. standard-dose rt-pa and early intensive vs. guideline-recommended blood pressure lowering in patients with acute ischaemic stroke eligible for thrombolysis treatment. Int. J. Stroke Soc. (2015) 10:778–88. doi: 10.1111/ijs.12486
31. Matteis M, Troisi E, Monaldo BC, Caltagirone C, Silvestrini M. Age and sex differences in cerebral hemodynamics: a transcranial doppler study. Stroke. (1998) 29:963–7. doi: 10.1161/01.STR.29.5.963
32. Krause DN, Duckles SP, Pelligrino DA. Influence of sex steroid hormones on cerebrovascular function. J Appl Physio. (2006) 101:1252–61. doi: 10.1152/japplphysiol.01095.2005
33. Boehme AK, Esenwa C, Elkind MS. Stroke risk factors, genetics, and prevention. Circ Res. (2017) 120:472–95. doi: 10.1161/CIRCRESAHA.116.308398
34. Brodie FG, Panerai RB, Foster S, Evans DH, Robinson TG. Long-term changes in dynamic cerebral autoregulation: a 10 years follow up study. Clin Physiol Funct Imaging. (2009) 29:366–71. doi: 10.1111/j.1475-097X.2009.00880.x
Keywords: age, sex, systolic blood pressure, ischemic stroke, thrombolysis, outcome
Citation: Liu B-J, Li J and Chen H-S (2023) Age and sex affect the association of systolic blood pressure with clinical outcomes in thrombolysed stroke patient: a secondary analysis of the INTRECIS study. Front. Neurol. 14:1273131. doi: 10.3389/fneur.2023.1273131
Received: 05 August 2023; Accepted: 25 September 2023;
Published: 18 October 2023.
Edited by:
Matteo Foschi, Azienda Unità Sanitaria Locale (AUSL) della Romagna, ItalyReviewed by:
Adria Arboix, Sacred Heart University Hospital, SpainGang-Yu Ding, Jiading District Central Hospital Affiliated Shanghai University of Medicine and Health Sciences, China
Kais Gadhoumi, Duke University, United States
Copyright © 2023 Liu, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui-Sheng Chen, Y2hzemhAYWxpeXVuLmNvbQ==
 Bai-Jun Liu
Bai-Jun Liu Hui-Sheng Chen
Hui-Sheng Chen